Abstract
The number of operations performed using the da Vinci Surgical System® (DVSS) has been increasing worldwide in the past decade. We introduced robotic gastrectomy for gastric cancer (GC) in January 2009 to overcome the disadvantage of conventional laparoscopic gastrectomy. Initially, we experienced some troubles in the technical aspect and cost of robotic surgery. After extensive trial and error, we were able to develop the “double bipolar method” and the “da Vinci's plane theory” to use DVSS effectively. We then conducted “Senshiniryo B,” which was a multi‐institutional prospective single‐arm study to determine the safety, feasibility, and effectiveness of robotic gastrectomy for GC in 2014. In that study, we demonstrated that the morbidity rate in the robotic group (2.45%) was significantly lower than that in the historical control group (6.4%). As a consequence of that clinical trial, 12 procedures, including robotic gastrectomy for GC, have been covered under the Japanese national insurance in 2018. An additional seven procedures were newly covered in April 2020. In the first half of this article, we describe the history of robotic surgery in the world and Japan and demonstrate the “double bipolar method” and “da Vinci's plane theory.” In the latter half, we explain the Japanese systems for the safe dissemination of robotic surgery and state our efforts to solve some problems in robotic surgery.
Keywords: gastrectomy, gastric cancer, robotic surgery
In the first half of this article, we describe the history of robotic surgery in the world and Japan and demonstrate the “double bipolar method” and “da Vinci's plane theory.” In the latter half, we explain the Japanese systems for the safe dissemination of robotic gastrectomy and state our efforts to solve some problems in robotic surgery.
![]()
1. INTRODUCTION
Since the first case description of laparoscopic gastrectomy (LG) by Dr Kitano et al in 1994,1 LG has emerged as a widespread procedure for the treatment of gastric cancer (GC) as a minimally invasive surgery worldwide. Several studies have reported that LG is one of the safe and feasible procedures for the treatment of early and advanced GC.2, 3, 4, 5, 6 However, conventional laparoscopic surgery remains technically demanding because of its limited range of motion with long straight forceps, as well as tremors in the hand.7, 8 Therefore, it seems that the operators require a longer learning curve. To overcome these disadvantages of conventional laparoscopic surgery, robotic surgery has been developing. Consequently, there has been a global increase in the number of robotic surgery and related reports (robotic surgery – Search Results – PubMed [nih.gov]). In this article, we describe the history and milestones of robotic surgery associated with its practice in Japan based on our experience and review of literature.
2. HISTORY OF ROBOTIC SURGERY
2.1. Before the merger of Computer Motion and Intuitive Surgical
In the 1990s, Computer Motion was a leading company in the field of medical robotics. The first use of the Automated Endoscopic System for Optimal Positioning (Computer Motion), which was applied for videoscope guidance in minimally invasive mitral valve surgery, was reported in 1998.9 In contrast, the use of the da Vinci telemanipulation system (Intuitive Surgical) for coronary artery disease was reported in 2000. The da Vinci telemanipulation system comprises a remote console where the operating surgeon (master) controls two instrument arms and a central arm to guide the videoscope (slave)10 (Figure 1). In 2000, the Food and Drug Administration (FDA) approved the use of the da Vinci telemanipulation system for general laparoscopic surgical procedures, and it became the first operative surgical robot in the USA. The scope used in the first standard type of the da Vinci telemanipulation system was manufactured by Olympus Corporation. In the following year, Marescaux et al safely conducted remote robot‐assisted cholecystectomy across the Atlantic Ocean (“Operation Lindbergh”) using the ZEUS robotic surgical system (Computer Motion).11
FIGURE 1.

These pictures are a prototype of DVSS, in which master‐slave system was adopted
2.2. After the merger of Computer Motion and Intuitive Surgical
After Intuitive Surgical consolidated with Computer Motion in 2003, there was rapid progress in the development of the da Vinci surgical system (DVSS). The DVSS has three components, including surgeon console, patient cart, and vision cart. It provides three‐dimensional images, ten‐fold magnified view of the operating field, natural hand‐eye coordination, high degree of freedom through its articulating surgical instruments, stabilization of the surgeon's tremor, and scales motion.12 In 2006, the da Vinci S Surgical System (DVSS‐S) obtained FDA approval for use in general laparoscopic procedures. Subsequently, DVSS‐Si and DVSS‐Xi were released in 2009 and 2014, respectively.
2.3. First experience of robotic surgery in Japan before national insurance coverage
In Japan, the DVSS‐Standard was first installed in Keio University and Kyushu University in 2000. Live demonstration of robotic Nissen fundoplication, which was the first robotic surgery live performance in Asia, was performed at the 100th Annual Congress of the Japan Surgical Society in 2000. Hashizume et al reported their early experience in robotic surgeries using DVSS, including distal gastrectomy.13 However, they somehow failed to obtain approval for the use of DVSS in clinical practice based on the Pharmaceuticals and Medical Devices Law, and then, the ice age of robotic surgery occurred in the following decade.
2.4. Beginning of robotic radical gastrectomy (RG) for GC in Japan
Meanwhile, conventional laparoscopic surgery had prospered in the 2000s, at least partly because it is associated with attenuated blood loss, less pain, and faster recovery compared with open surgery. In the field of stomach surgery, we developed our original methodology known as the outermost layer‐oriented medial approach for improving the safety, efficacy, and reproducibility of suprapancreatic lymph node dissection.14, 15, 16 However, LG did not contribute to either reduction in early postoperative complication except wound infection or improvement in long‐term outcomes.16, 17, 18 We considered that several technical limitations, including limited range of motion with straight forceps and hand tremors, seriously influenced LG outcomes, and consequently led to slight differences in surgical outcomes between LG and open gastrectomy. Therefore, we focused on the high potential of the novel robotic system and expected it to become an ideal tool to overcome these limitations in LG, thereby improving surgical outcomes in patients with GC. Although DVSS‐S was not approved based on the Pharmaceuticals and Medical Devices Law, we personally purchased DVSS‐S for the first time in our country. When we performed an initial case of RG in January 2009, we invited Professor Woo Jin Hyung from Yonsei University in South Korea, regarded as a worldwide leader of RG at that time, and received an intraoperative instruction.19, 20 As a result, the operative time was 398 minutes, and this patient was discharged at 11 days postoperatively without any complications. Thereafter, DVSS‐S was approved based on the Pharmaceuticals and Medical Devices Law in November 2009. Any procedure using the surgical robot had never been covered by the national insurance in Japan, until robotic prostatectomy was insured in April 2012. While robotic stomach and esophageal surgeries were uninsured, patients who agreed to the uninsured use of DVSS underwent robotic surgery, whereas those who hoped for insured medical treatment underwent conventional open, laparoscopic, or thoracoscopic surgery.21, 22
2.5. A prospective study conducted in Japan under the Senshiniryo B system
Our single‐institution retrospective study conducted between 2009 and 2012 demonstrated that the morbidity (overall complication) rate of RG was approximately one‐fifth below the rate of LG,21 and there were no differences in the 3‐year overall survival (OS) and 3‐year recurrence‐free survival between RG and LG.23 In contrast, there have been limited studies on RG from other institutions in both Japan and other countries in this period.24 A meta‐analysis based on retrospective studies demonstrated that RG had a longer operative time, less blood loss, and similar morbidity and hospitalization postoperatively compared to LG.25 Therefore, to confirm the clinical advantage of RG over LG by reproducible results, we conducted a multi‐institutional prospective study between October 2014 and January 2017 to determine the safety, feasibility, and effectiveness of RG for GC.26 This single‐arm study, which was approved for Advanced Medical Technology (“Senshiniryo B”) managed by the Ministry of Health, Labour, and Welfare (MHLW), was designed to confirm the hypothesis that RG reduced the morbidity rate (Clavien‐Dindo classification [C‐D] grade ≥IIIa) of LG to less than half of that in a historical control (6.4%), in which the data consisted of those of three leading institutions of LG, including Kyoto, Saga, and Fujita health universities between 2009 and 2012. A total of 330 patients from 15 institutions were registered in that study. Finally, the morbidity rate in this study was 2.45%; therefore, our hypothesis was successfully confirmed.26
2.6. National insurance coverage and present scenario
Based on the positive results of the trial under the Senshiniryo B system, the MHLW finally decided to recognize 12 robotic procedures, including RG, as part of their corresponding conventional minimally invasive procedures from the standpoint of medical insurance coverage as of April 2018. However, considering its higher cost and lack of evidence for long‐term outcomes, an additional fee was not permitted. An additional seven procedures, including robotic pancreaticoduodenectomy, were insured in April 2020.
3. TECHNICAL ASPECTS
Apart from the previous studies conducted overseas, several Japanese studies have demonstrated the clinical advantage of RG, especially reduction in morbidity rate.24 The reason for this indirectness could be at least partly be because Ichiro Uyama (IU) in collaboration with the Japan Society for Endoscopic Surgery (JSES) had taken the initiative to safely introduce robotic surgery nationwide by sharing our standardized common technical principles in the robotic setup and dissection across institutions and surgeons. In fact, after our initial RG case in January 2009, RG long been penetrated relatively slowly in Japan, because of the following reasons: (a) regulatory approval of DVSS‐S was obtained in November 2009, and DVSS‐S was launched on the Japanese market in March 2010; (b) installation of DVSS was accelerated since robotic prostatectomy was insured in April 2012; (c) RG had long been performed as an uninsured medical treatment until it was insured in 2018 based on the successful outcomes in our prospective trial under the Senshiniryo B system. Because of the same reasons, our institution had been the only institution at which RG was conducted on a daily basis for a considerably long period, during which time we established our original methodologies including the “double bipolar method,” “da Vinci's plane theory,” and “monitor quadrisection theory,” which are universally available for GI and HPB surgeries.16, 21 Thus, IU visited many other Japanese facilities for the proctor, at least 50 institutions and 200 times to introduce and spread the application of RG procedures nationwide.
3.1. Port placement
In the introduction period of DVSS, we placed two ports on the right side of a patient and a port on the left side, according to a previously reported method.27 However, this style was suitable for left‐handed dominant surgeons and not for right‐handed surgeons, including Japanese surgeons. Therefore, to improve a familiar setting, we exchanged the port position to enable control of the two arms with the right hand of a surgeon. Specifically, we placed two ports on the left side of a patient opposite from the introduction cases21 (Figure 2).
FIGURE 2.
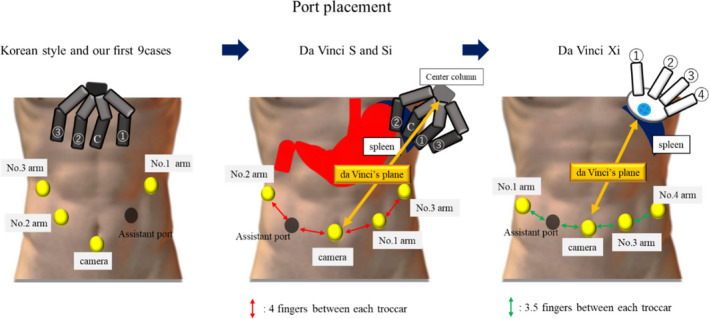
The transition of port placement. In the introduction period of DVSS, we placed two ports on the right side of a patient and a port on the left side. After that, we placed two ports on the left side of a patient
3.2. Double bipolar method
In our initial nine cases of RG, we used the personally imported HARMONIC® (Johnson & Johnson) with the left hand for resecting and coagulating the tissue, especially in suprapancreatic lymph node dissection, tracing the Korean RG style of those days. To complete suprapancreatic dissection using this straight device, left‐handed manipulation of the HARMONIC® was more suitable especially for No. 11p dissection due to matching of the device axis with the appropriate dissected line. However, the HARMONIC® was difficult to purchase via official sales agencies and had no articulating function. Moreover, we considered that handling by the nondominant hand became the obstacle for precise and reproducible dissection and could not facilitate the operators to fully utilize the advantages of the DVSS. Therefore, we stopped its use.
Instead, we developed the so‐called “double bipolar method,”16, 21 in which Maryland bipolar forceps and fenestrated bipolar forceps (Intuitive Surgical Inc.) were used with the operating surgeon's right and left hands, respectively (Figure 3). First, Maryland bipolar forceps were connected to a VIO 300D electrosurgical generator (Erbe) with forced‐coagulation mode effect 2 at 90 W using the external foot pedal. After the version up to VIO3, the bipolar cut effect 5.5 using an external foot pedal was used. After several twists and turns, we have been using a ForceTriad™ Energy Platform (Medtronic plc) with the bipolar macro mode at 60 W (Figure 4). This macro mode enables the set value of the electronic output to be constantly maintained, irrespective of the electrical resistivity of the target tissues, leading to sharp cutting by a momentary high voltage. Furthermore, owing to the upgradation of DVSS, known as P8, we could control the bipolar forceps using the foot pedals in the surgeon console.
FIGURE 3.
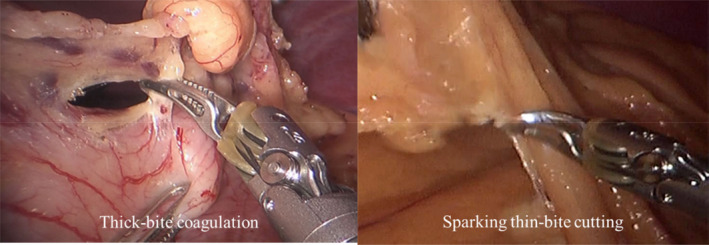
When these bipolar forceps are used with a thick bite, the bitten tissue could be coagulated (left). In contrast, the bitten tissue could be cut by a spark when these forceps are used with a thin bite (right)
FIGURE 4.
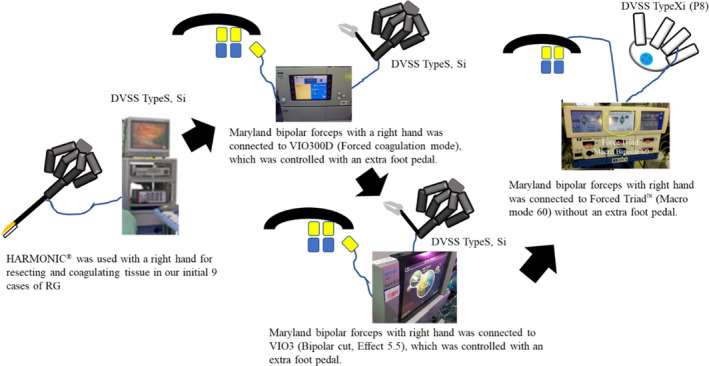
The transition of connection lines between robotic forceps and electrosurgical generator
3.3. da Vinci's plane theory
RG requires a wider range of surgical field than intrapelvic surgery such as prostatectomy and hysterectomy, which could be performed within a limited surgical field; hence, extracorporeal arm collision rarely occurs. Theoretically, robotic arms and forceps never collide with each other as long as the arms are arranged coaxially to the sagittal plane toward the center column of the patient cart, which we refer to as ‘‘da Vinci's plane,’’ and surgical manipulation is performed on the plane.21 Because we replaced two arms of the DVSS on the left side of a patient, the maneuverable area was much greater toward the left of da Vinci's plane than toward the right. Considering that DVSS‐S and DVSS‐Si have some limitations in arm mobility, including axis limitation and thick head of the instruments, to prevent extracorporeal collision between the robotic arms, the da Vinci's plane was determined by the line between the rightmost anatomy toward the surgical field (lower pole of the spleen for RG) and camera port16 (Figure 2). DVSS‐Xi significantly improved the limitations of arm mobility, overhead boom rotation without axis limitation, narrower arm, and longer instrument shaft, which provide better operating environment to surgeons. Moreover, patient clearance of DVSS‐Xi is effective in maintaining a distance of one fist between the joints of an arm to prevent collision between robotic arms.
4. REGULATION FOR THE OPERATOR OF ROBOTIC SURGERY IN JAPAN
4.1. The recommendation from the JSES in 2011
In 2010, in Japan, a patient who underwent RG died on postoperative day 5 because of multiorgan dysfunction. The report of the Accident Investigation Committee concluded that the cause of death was intraoperative pancreas compression by the robotic arm, which induced intraoperative pancreas injury like handlebar injury. To prevent further complications of robotic surgery performed by immature operators and safely introduce robotic surgery into an inexperienced institution, the JSES proposed seven suggestions for introducing robotic surgery in 2011 (Figure 5). A major suggestion was that an operator should be a surgeon qualified by the JSES endoscopic surgical skill qualification system (ESSQS).
FIGURE 5.
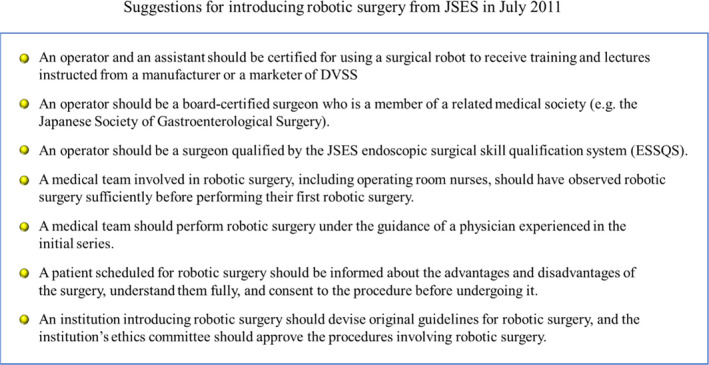
Suggestions for introducing robotic surgery from JSES in July 2011
4.2. Institution criteria founded after the approval of 12 robotic procedures for national medical insurance in 2018
Since the approval of 12 robotic procedures, including RG, for national medical insurance coverage in April 2018, several institutional criteria were formulated to accept national insurance coverage for every procedure. The institutional criteria concerning RG are presented in Figure 6. It was expected that these regulations could restrict rough‐and‐ready introduction of robotic surgery in an inexperienced institution for robotic or conventional laparoscopic surgery.
FIGURE 6.

Standards for medical facilities to introduce robotic gastrectomy under national insurance are shown in http://2020.mfeesw.net/x206/x32/x135/xtk7272/ in Japanese
4.3. Registry system
After the approval of the national insurance coverage in April 2018, the prospective registry system for patients who were planned to undergo robotic surgery before the operation using the National Clinical Database was launched in October 2018 under the leadership of the MHLW and JSES. This registry system aimed to accurately comprehend the real‐time nationwide status regarding the performance of robotic surgery in Japan and create a large prospective database, which could provide evidence to confirm the clinical advantages of robotic surgery.
4.4. Proctor certification system
Before gastroenterological surgery using DVSS was covered by the national insurance, proctor surgeons were selected based on private approval by Intuitive Surgical Inc. However, with the rapidly increasing demands of proctor surgeons after the approval of the national insurance coverage, this proctor system has been officially certified by the JSES since December 2019. A summary of these criteria is presented in Figure 7. Through this proctor system and with the surgeons sharing common surgical concepts and technical principles, it is expected that the learning curve of not only the surgeons but also the surgical team for RG would be shortened.
FIGURE 7.

Eligibility for proctor certification is shown in Japanese the website of JSES, which is http://www.jses.or.jp/pdf/3_RobotAssistedSurgeryProctorCertificationSystem.pdf
4.5. Latest guideline for robotic surgery from JSES changed in 2020
The recommendation for robotic surgery from the JSES was revised in March 2020 for further widespread dissemination and more safe performance of robotic surgery. A major revision was that the presence of a board‐certified surgeon in the Japanese Society of Gastroenterological Surgery still remained essential, whereas the presence of the ESSQS qualification was excluded from the essential criteria. When surgeons with an experience of ≥20 robotic surgeries as an assistant could perform robotic surgery under the guidance of a certified proctor. Furthermore, to perform robotic surgery independently, medical teams should include an ESSQS‐qualified surgeon, and an experience of ≥10 robotic surgeries under the guidance of a certified proctor was required before the first case. The latest revisions suggest that the JSES emphasizes the formation and growth of the entire medical team for robotic surgery, rather than the surgeon's own skill.
5. CURRENT STATUS OF RG
From September 2014, when the prospective study under the Senshiniryo B system was initiated, to April 2020, 19 studies that compared RG with conventional LG could be found after excluding duplicate studies, those with <50 cases in the RG group, those published in non‐English languages, animal studies, and review articles.21, 44 Some prospective studies have analyzed the feasibility of RG in Japan, including two multicenter prospective studies26, 42 and one single‐center prospective study.43 In these studies, the incidence rate of C‐D grade IIIa or higher morbidity rate was reported to be as low as 2.45%‐5.8%. In our institution, with similar favorable short‐term outcome as that of the Senshiniryo B trial26 and the initial series,21 we had successfully demonstrated that RG significantly reduced the incidence rate of intra‐abdominal infectious complications compared with LG using propensity score matched analysis (2.5% vs 5.9%, respectively; P = .038),28 although it was a retrospective study. That study also identified the non‐use of the robotic system as an independent risk factor by multivariate analysis.28 Similarly, a recent study examined patients with cT1 GC in Japan and demonstrated that the incidence of intra‐abdominal infectious complications (C‐D grade IIIa or higher) tended to be lower in the RG group than in the LG group (1.8% and 5.0%; P = .209) but not significantly different.37 In contrast, some studies from other countries have compared RG with LG,29, 30, 31, 32, 33, 34, 35, 36, 39, 41, 44 including a multi‐institutional nonrandomized prospective study.44 A summary of these studies indicates that RG has longer operative time, less intraoperative blood loss, and comparable mortality, morbidity, and postoperative hospitalization compared with LG.24 Several retrospective studies that focused on patients with advanced GC using a propensity score matched analysis were found,33, 34, 35, 40, 41, 42 and one of them showed that RG significantly reduced the morbidity rate compared with LG.34
These findings suggest that the clinical benefit of RG in reducing complications has been more remarkably demonstrated in Japan than in other countries. We consider that this favorable outcome in Japan was due to the proctor system in which the expert RG surgeon (IU) who had introduced RG in Japan played a fundamental role. After the introduction of DVSS in 2009, IU and his colleagues established the common technical methodologies and principles on robotic setup and dissection to safely and reproducibly perform RG. Through the proctor system, all Japanese gastric surgeons who participated in the prospective study under the Senshiniryo B system could share these methodologies and principles, which lead to successful achievement of the primary endpoint of this trial. Therefore, we consider that these speculations would help in the safe and widespread dissemination of RG and improve the surgical outcomes of RG by fully utilizing its potential in the world and in Japan.
6. FUTURE PERSPECTIVES
The use of robotic surgery has been rapidly increasing worldwide. However, it has several limitations and problems to be resolved. Here, we raise three points as future perspectives of robotic surgery, including RG.
First, there is little evidence to confirm the clinical advantages of RG. Prospective surveillance programs, including the 3‐year OS analysis after the prospective study under the Senshiniryo B system and short‐term outcome analysis based on the preoperative registry system, are ongoing and will provide strong evidence from Japan. We expect that the oncological outcomes could be at least equivalent or superior to LG because previous studies have demonstrated that postoperative intra‐abdominal infectious complications, including pancreatic fistula and anastomotic leakage, could be a major independent prognostic factor for long‐term survival in surgery for GC.45, 46
Second, the surgical robotic system has a few disadvantages, including its high cost, large‐sized machine, physical stress to surgeons, and lack of haptic feedback. To solve these problems, we established a collaborative laboratory for research and development of advanced surgical technology in Fujita Health University. One of the major purposes in this collaborative laboratory is to develop a novel surgical robot, the hinotori™ Surgical Robot System, in collaboration with Medicaroid Inc. (Figure 8). This novel robotic system was approved by the Pharmaceuticals and Medical Devices Agency in August 2020. In the near future, the current surgical robotic system will be further improved by development and competition between several companies, which could help in overcoming the disadvantages of the current robotic surgical system.
FIGURE 8.
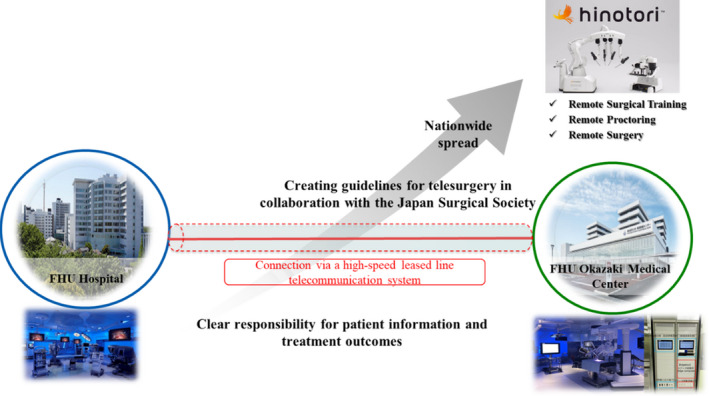
Strategy for realizing the practical use of telesurgery or remote surgery between a primary hospital and another hospital, using the hinotori™ Surgical Robot System, in collaboration with Medicaroid Inc.
Third, to realize the practical use of telesurgery or remote surgery between a primary hospital and another hospital (Figure 8). Although there are several technical problems, we are currently planning for three promising projects on telesurgery. The first is to link the operating rooms between Fujita Health University Hospital and Okazaki Medical Center using a high‐speed secure leased line. By doing so, we can share clinical information and real‐time operating videos of patients undergoing robotic surgery, which can thus potentially provide remote surgical education from an expert surgeon to a nonexpert surgeon. The second is to collaborate with the Japan Surgical Society Committee on the promotion of telesurgery and create guidelines for the practical use of telesurgery. The third is to extend these telesurgery network systems to other institutions beyond Fujita Health University.
In conclusion, we believe that further progression of medical technology would enable surgeons to perform RG more comfortably and safely and would consequently help in achieving less invasive surgery, including reduction of surgical complications and preservation of organ function for patients in the near future.
DISCLOSURE
Conflict of Interest: Author K Kikuchi belonged to an endowed chair by Medicaroid Inc. Author K Suda and T Tanaka belong to an endowed chair by Medicaroid Inc. Author K Suda and I Uyama have advisor position in Medicaroid Inc. Author I Uyama received lecture fee from Intuitive Surgical.
ACKNOWLEDGEMENTS
The authors would like to thank MARUZEN‐YUSHODO Co., Ltd. (https://kw.maruzen.co.jp/kousei‐honyaku/) for the English language editing.
Kikuchi K, Suda K, Shibasaki S, Tanaka T, Uyama I. Challenges in improving the minimal invasiveness of the surgical treatment for gastric cancer using robotic technology. Ann Gastroenterol Surg. 2021;5:604–613. 10.1002/ags3.12463
REFERENCES
- 1.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy‐assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4(2):146–8. [PubMed] [Google Scholar]
- 2.Inokuchi M, Nakagawa M, Tanioka T, Okuno K, Gokita K, Kojima K. Long‐ and short‐term outcomes of laparoscopic gastrectomy versus open gastrectomy in patients with clinically and pathological locally advanced gastric cancer: a propensity‐score matching analysis. Surg Endosc. 2018;32(2):735–42. [DOI] [PubMed] [Google Scholar]
- 3.Zou Z‐H, Zhao L‐Y, Mou T‐Y, Hu Y‐F, Yu J, Liu H, et al. Laparoscopic versus open D2 gastrectomy for locally advanced gastric cancer: a meta‐analysis. World J Gastroenterol. 2014;20(44):16750–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H‐H, Hyung WJ, Cho GS, Kim MC, Han S‐U, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy. Ann Surg. 2010;251(3):417–20. [DOI] [PubMed] [Google Scholar]
- 5.Katai H, Sasako M, Fukuda H, Nakamura K, Hiki N, Saka M, et al. JCOG Gastric Cancer Surgical Study Group. Safety and feasibility of laparoscopy‐assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer. 2010;13(4):238–44. [DOI] [PubMed] [Google Scholar]
- 6.Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, et al. Short‐term surgical outcomes from a phase III study of laparoscopy‐assisted versus open distal gastrectomy with nodal dissection for clinical stageIA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2017;20(4):699–708. [DOI] [PubMed] [Google Scholar]
- 7.Jin S‐H, Kim D‐Y, Kim H, Jeong IH, Kim M‐W, Cho YK, et al. Multidimensional learning curve in laparoscopy‐assisted gastrectomy for early gastric cancer. Surg Endosc. 2007;21(1):28–33. [DOI] [PubMed] [Google Scholar]
- 8.Vinuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta‐analysis of randomized controlled trials and high‐quality nonrandomized studies. Ann Surg. 2012;255(3):446–56. [DOI] [PubMed] [Google Scholar]
- 9.Falk V, Walther T, Autschbach R, Diegeler A, Battellini R, Mohr FW. Robot assisted minimally invasive solo mitral valve operation. J Thorac Cardiovasc Surg. 1998;115(2):470–1. [DOI] [PubMed] [Google Scholar]
- 10.Falk V, Diegeler A, Walther T, Banusch J, Brucerius J, Raumans J, et al. Total endoscopic computer enhanced coronary artery bypass grafting. Eur J Cardio Thorac Surg. 2000;17(1):38–45. [DOI] [PubMed] [Google Scholar]
- 11.Marescaux J, Leroy J, Gagner M, Rubino F, Mutter D, Vix M, et al. Transatlantic robot‐assisted telesurgery. Nature. 2001;413(6854):379–80. [DOI] [PubMed] [Google Scholar]
- 12.Nakauchi M, Uyama I, Suda K, Muhran M, Nakamura T, Shibasaki S, et al. Robotic surgery for the upper gastrointestinal tract: current status and future perspectives. Asian J Endosc Surg. 2017;10(4):354–63. [DOI] [PubMed] [Google Scholar]
- 13.Hashizume M, Shimada M, Tomikawa M, Ikeda Y, Takahashi I, Abe R, et al. Early experiences of endoscopic procedures in general surgery assisted by a computer‐enhanced surgical system. Surg Endosc. 2002;16(4):1187–91. [DOI] [PubMed] [Google Scholar]
- 14.Kanaya S, Haruta S, Kawamura Y, Yoshimura F, Inaba K, Hiramatsu Y, et al. Video: laparoscopy distinctive technique for suprapancreatic lymph node dissection: medial approach for laparoscopic gastric cancer surgery. Surg Endosc. 2011;25(12):3928–9. [DOI] [PubMed] [Google Scholar]
- 15.Uyama I, Suda K, Satoh S. Laparoscopic surgery for advanced gastric cancer: current status and future perspectives. J Gastric Cancer. 2013;13(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suda K, Nakauchi M, Inaba K, Ishida Y, Uyama I. Robotic surgery for upper gastrointestinal cancer: current status and future perspectives. Dig Endosc. 2016;28(7):701–13. [DOI] [PubMed] [Google Scholar]
- 17.Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, et al. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc. 2012;27(1):286–94. [DOI] [PubMed] [Google Scholar]
- 18.Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A prospective randomized study comparing open versus laparoscopy‐assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2011;28(5–6):331–7. [DOI] [PubMed] [Google Scholar]
- 19.Isogaki J, Haruta S, Man‐i M, Suda K, Kawamura Y, Yoshimura F, et al. Robot‐assisted surgery for gastric cancer: experience at our institute. Pathobiology. 2011;78(6):328–33. [DOI] [PubMed] [Google Scholar]
- 20.Uyama I, Kanaya S, Ishida Y, Inaba K, Suda K, Satoh S. Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg. 2012;36(2):331–7. [DOI] [PubMed] [Google Scholar]
- 21.Suda K, Man IM, Ishida Y, Kawamura Y, Satoh S, Uyama I. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc. 2015;29(3):673–85. [DOI] [PubMed] [Google Scholar]
- 22.Suda K, Ishida Y, Kawamura Y, Inaba K, Kanaya S, Teramukai S, et al. Robot‐assisted thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve for esophageal squamous cell carcinoma in the prone position: technical report and short‐term outcomes. World J Surg. 2012;36(7):1608–16. [DOI] [PubMed] [Google Scholar]
- 23.Nakauchi M, Suda K, Susumu S, Kadoya S, Inaba K, Ishida Y, et al. Comparison of the long‐term outcomes of robotic radical gastrectomy for gastric cancer and conventional laparoscopic approach: a single institutional retrospective cohort study. Surg Endosc. 2016;30(12):5444–52. [DOI] [PubMed] [Google Scholar]
- 24.Shibasaki S, Suda K, Obama K, Yoshida M, Uyama I. Should robotic gastrectomy become a standard surgical treatment option for gastric cancer? Surg Today. 2020;50(9):955–65. [DOI] [PubMed] [Google Scholar]
- 25.Hyun MH, Leeb CH, Kim HJ, Tong Y, Park SS. Systematic review and meta‐analysis of robotic surgery compared with conventional laparoscopic and open resections for gastric carcinoma. Br J Surg. 2013;100(12):1566–78. [DOI] [PubMed] [Google Scholar]
- 26.Uyama I, Suda K, Nakauchi M, Kinoshita T, Noshiro H, Takiguchi S, et al. Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: a multi‐institutional prospective single‐arm study. Gastric Cancer. 2019;22(2):377–85. [DOI] [PubMed] [Google Scholar]
- 27.Hyung WJ, Woo Y, Noh DH. Robotic surgery for gastric cancer: a technical review. J Robot Surg. 2011;5(4):241–9. [DOI] [PubMed] [Google Scholar]
- 28.Shibasaki S, Suda K, Nakauchi M, Nakamura K, Kikuchi K, Inaba K, et al. Non‐robotic minimally invasive gastrectomy as an independent risk factor for postoperative intra‐abdominal infectious complications: a single‐center, retrospective and propensity score‐matched analysis. World J Gastroenterol. 2020;26(11):1172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roh CK, Choi S, Seo WJ, Cho M, Choi YY, Son T, et al. Comparison of surgical outcomes between integrated robotic and conventional laparoscopic surgery for distal gastrectomy: a propensity score matching analysis. Sci Rep. 2020;10:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Xi H, Qiao Z, Li J, Zhang K, Xie T, et al. Comparison of robotic‐ and laparoscopic‐assisted gastrectomy in advanced gastric cancer: update short‐ and long‐term results. Surg Endosc. 2019;33(2):528–34. [DOI] [PubMed] [Google Scholar]
- 31.Ye S‐p, Shi J, Liu D‐n, Jiang Q‐g, Lei X, Qiu H, et al. Robotic‐assisted versus conventional laparoscopic‐assisted total gastrectomy with D2 lymphadenectomy for advanced gastric cancer: short‐term outcomes at a mono‐institution. BMC Surg. 2019;19(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong Y, Cao S, Liu X, Li Z, Wang L, Lu C, et al. Short‐term clinical outcomes after laparoscopic and robotic gastrectomy for gastric cancer: a propensity score matching analysis. J Gastrointest Surg. 2020;24(3):531–9. [DOI] [PubMed] [Google Scholar]
- 33.Yang C, Shi Y, Xie S, Chen J, Zhao Y, Qian F, et al. Short‐term outcomes of robotic‐ versus laparoscopic‐assisted total gastrectomy for advanced gastric cancer: a propensity score matching study. BMC Cancer. 2020;20(1):669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye S‐p, Shi J, Liu D‐n, Jiang Q‐g, Lei X, Tang B, et al. Robotic‐ versus laparoscopic‐assisted distal gastrectomy with D2 lymphadenectomy for advanced gastric cancer based on propensity score matching: short‐term outcomes at a high‐capacity center. Sci Rep. 2020;10(1):6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Li J, Li B, Bai B, Liu Y, Lian B, et al. Robotic versus laparoscopic gastrectomy with D2 lymph node dissection for advanced gastric cancer: a propensity score‐matched analysis. Cancer Manag Res. 2018;10:705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang K‐H, Lan Y‐T, Fang W‐L, Chen J‐H, Lo S‐S, Li AF‐Y, et al. Comparison of the operative outcomes and learning curves between laparoscopic and robotic gastrectomy for gastric cancer. PLoS One. 2014;9(10):e111499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hikage M, Tokunaga M, Makuuchi R, Irino T, Tanizawa Y, Bando E, et al. Comparison of surgical outcomes between robotic and laparoscopic distal gastrectomy for cT1 gastric cancer. World J Surg. 2018;42(6):1803–10. [DOI] [PubMed] [Google Scholar]
- 38.Junfeng Z, Yan S, Bo T, Yingxue H, Dongzhu Z, Yongliang Z, et al. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short‐term outcomes. Surg Endosc. 2014;28(6):1779–87. [DOI] [PubMed] [Google Scholar]
- 39.Yang SY, Roh KH, Kim Y‐N, Cho M, Lim SH, Son T, et al. Surgical outcomes after open, laparoscopic, and robotic gastrectomy for gastric cancer. Ann Surg Oncol. 2017;24(7):1770–7. [DOI] [PubMed] [Google Scholar]
- 40.Obama K, Kim Y‐M, Kang DR, Son T, Kim H‐I, Noh SH, et al. Long‐term oncologic outcomes of robotic gastrectomy for gastric cancer compared with laparoscopic gastrectomy. Gastric Cancer. 2018;21(2):285–95. [DOI] [PubMed] [Google Scholar]
- 41.Wang W‐J, Li H‐T, Yu J‐P, Su L, Guo C‐A, Chen P, et al. Severity and incidence of complications assessed by the Clavien‐Dindo classification following robotic and laparoscopic gastrectomy for advanced gastric cancer: a retrospective and propensity score‐matched study. Surg Endosc. 2019;33(10):3341–54. [DOI] [PubMed] [Google Scholar]
- 42.Okabe H, Obama K, Tsunoda S, et al. Feasibility of robotic radical gastrectomy using a monopolar device for gastric cancer. Surg Today. 2019;49(10):820–7. [DOI] [PubMed] [Google Scholar]
- 43.Tokunaga M, Makuuchi R, Miki Y, Tanizawa Y, Bando E, Kawamura T, et al. Late phase II study of robot‐assisted gastrectomy with nodal dissection for clinical stage I gastric cancer. Surg Endosc. 2016;30(8):3362–7. [DOI] [PubMed] [Google Scholar]
- 44.Kim H‐I, Han S‐U, Yang H‐K, Kim Y‐W, Lee H‐J, Ryu KW, et al. Multicenter prospective comparative study of robotic versus laparoscopic gastrectomy for gastric adenocarcinoma. Ann Surg. 2016;263(1):103–9. [DOI] [PubMed] [Google Scholar]
- 45.Kubota T, Hiki N, Sano T, Nomura S, Nunobe S, Kumagai K, et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol. 2014;21(3):891–8. [DOI] [PubMed] [Google Scholar]
- 46.Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra‐abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20(5):1575–83. [DOI] [PubMed] [Google Scholar]


