Abstract
STUDY QUESTION
Does lifestyle intervention consisting of an energy-restricted diet, enhancement of physical activity and motivational counseling prior to IVF improve embryo utilization rate (EUR) and cumulative live birth rate (CLBR) in women with obesity?
SUMMARY ANSWER
A 6-month lifestyle intervention preceding IVF improved neither EUR nor CLBR in women with obesity in the first IVF treatment cycle where at least one oocyte was retrieved.
WHAT IS KNOWN ALREADY
A randomized controlled trial (RCT) evaluating the efficacy of a low caloric liquid formula diet (LCD) preceding IVF in women with obesity was unable to demonstrate an effect of LCD on embryo quality and live birth rate: in this study, only one fresh embryo transfer (ET) or, in case of freeze-all strategy, the first transfer with frozen-thawed embryos was reported. We hypothesized that any effect on embryo quality of a lifestyle intervention in women with obesity undergoing IVF treatment is better revealed by EUR and CLBR after transfer of all fresh and frozen-thawed embryos.
STUDY DESIGN, SIZE, DURATION
This is a nested cohort study within an RCT, the LIFEstyle study. The original study examined whether a 6-month lifestyle intervention prior to infertility treatment in women with obesity improved live birth rate, compared to prompt infertility treatment within 24 months after randomization. In the original study between 2009 and 2012, 577 (three women withdrew informed consent) women with obesity and infertility were assigned to a lifestyle intervention followed by infertility treatment (n = 289) or to prompt infertility treatment (n = 285).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Only participants from the LIFEstyle study who received IVF treatment were eligible for the current analysis. In total, 137 participants (n = 58 in the intervention group and n = 79 in the control group) started the first cycle. In 25 participants, the first cycle was cancelled prior to oocyte retrieval mostly due to poor response. Sixteen participants started a second or third consecutive cycle. The first cycle with successful oocyte retrieval was used for this analysis, resulting in analysis of 51 participants in the intervention group and 72 participants in the control group. Considering differences in embryo scoring methods and ET day strategy between IVF centers, we used EUR as a proxy for embryo quality. EUR was defined as the proportion of inseminated/injected oocytes per cycle that was transferred or cryopreserved as an embryo. Analysis was performed per cycle and per oocyte/embryo. CLBR was defined as the percentage of participants with at least one live birth from the first fresh and subsequent frozen-thawed ET(s). In addition, we calculated the Z-score for singleton neonatal birthweight and compared these outcomes between the two groups.
MAIN RESULTS AND THE ROLE OF CHANCE
The overall mean age was 31.6 years and the mean BMI was 35.4 ± 3.2 kg/m2 in the intervention group, and 34.9 ± 2.9 kg/m2 in the control group. The weight change at 6 months was in favor of the intervention group (mean difference in kg vs the control group: −3.14, 95% CI: −5.73 to −0.56). The median (Q25; Q75) number of oocytes retrieved was 4.00 (2.00; 8.00) in the intervention group versus 6.00 (4.00; 9.75) in the control group, and was not significantly different, as was the number of oocytes inseminated/injected (4.00 [2.00; 8.00] vs 6.00 [3.00; 8.75]), normal fertilized embryos (2.00 [0.50; 5.00] vs 3.00 [1.00; 5.00]) and the number of cryopreserved embryos (2.00 [1.25; 4.75] vs 2.00 [1.00; 4.00]). The median (Q25; Q75) EUR was 33.3% (12.5%; 60.0%) in the intervention group and 33.3% (16.7%; 50.0%) in the control group in the per cycle analysis (adjusted B: 2.7%, 95% CI: −8.6% to 14.0%). In the per oocyte/embryo analysis, in total, 280 oocytes were injected or inseminated in the intervention group, 113 were utilized (transferred or cryopreserved, EUR = 40.4%); in the control group, EUR was 30.8% (142/461). The lifestyle intervention did not significantly improve EUR (adjusted odds ratio [OR]: 1.36, 95% CI: 0.94–1.98) in the per oocyte/embryo analysis, taking into account the interdependency of the oocytes per participant. CLBR was not significantly different between the intervention group and the control group after adjusting for type of infertility (male factor and unexplained) and smoking (27.5% vs 22.2%, adjusted OR: 1.03, 95% CI: 0.43–2.47). Singleton neonatal birthweight and Z-score were not significantly different between the two groups.
LIMITATIONS, REASONS FOR CAUTION
This study is a nested cohort study within an RCT, and no power calculation was performed. The randomization was not stratified for indicated treatment, and although we corrected our analyses for baseline differences, there may be residual confounding. The limited absolute weight loss and the short duration of the lifestyle intervention might be insufficient to affect EUR and CLBR.
WIDER IMPLICATIONS OF THE FINDINGS
Our data do not support the hypothesis of a beneficial short-term effect of lifestyle intervention on EUR and CLBR after IVF in women with obesity, although more studies are needed as there may be a potential clinically relevant effect on EUR.
STUDY FUNDING/COMPETING INTEREST(S)
The study was supported by a grant from ZonMw, the Dutch Organization for Health Research and Development (50-50110-96-518). A.H. has received an unrestricted educational grant from Ferring pharmaceuticals BV, The Netherlands. B.W.J.M. is supported by an NHMRC Investigator grant (GNT1176437). B.W.J.M. reports consultancy for Guerbet, has been a member of the ObsEva advisory board and holds Stock options for ObsEva. B.W.J.M. has received research funding from Guerbet, Ferring and Merck. F.J.M.B. reports personal fees from membership of the external advisory board for Merck Serono and a research support grant from Merck Serono, outside the submitted work.
TRIAL REGISTRATION NUMBER
The LIFEstyle RCT was registered at the Dutch trial registry (NTR 1530). https://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1530.
Keywords: obesity, lifestyle intervention, embryo quality, embryo utilization rate, cumulative live birth rate
WHAT DOES THIS MEAN FOR PATIENTS?
Women with obesity do not achieve a pregnancy after IVF treatment as easily as normal-weight women. Children born from obese mothers are more likely to have a high birthweight and are more prone to disease in later life. Improving maternal health and reducing body weight before pregnancy is recommended for women with obesity prior to infertility treatment. A lifestyle intervention, meaning changes to how the women live—for example, changes in what and how much they eat and how much exercise they take—is the recommended approach to improve health by reducing body weight. However, studies in obese women that evaluated the effects of lifestyle intervention before IVF treatment have not shown clear results so far. In particular, it is not known how lifestyle intervention may influence the very early development of the embryo that is formed during IVF. In this study, we investigated embryo quality in women who underwent IVF after a 6-month lifestyle intervention. The intervention consisted of diet modification, physical activity enhancement and motivational counseling. We compared women who received the intervention to women who did not. The number of embryos that showed sufficient early development to be transferred back into the womb, the number of live births and the body weight of the babies were compared between these groups.
We compared 51 women who followed the lifestyle intervention to 72 women who did not. We found that the lifestyle intervention did not increase the number of embryos that could be transferred into the womb. There was also no difference between the two groups in the number of babies that were born and in the birthweight of the babies. Further research is necessary to increase our understanding of the complex ways in which lifestyle intervention can influence embryo quality.
Introduction
Obesity is associated with decreased live birth rates following IVF (Luke et al., 2011a,b; Kawwass et al., 2016; Sermondade et al., 2019). Several underlying mechanisms of embryonic origin and endometrial receptivity may lead to this decreased live birth rate. Oocytes retrieved from women with obesity are smaller compared to normal-weight women and they reach the morula stage faster after fertilization (Leary et al., 2015). In women with overweight or obesity, the glucose consumption and endogenous triglyceride levels are disrupted at the blastocyst stage, which leads to abnormal developmental timing and metabolic regulation within embryos (Leary et al., 2015). Additional studies have demonstrated differences in the follicular fluid composition between obese and non-obese women, which might negatively affect oocyte quality and therefore hamper embryo development (Robker et al., 2009; Jungheim et al., 2011; Valckx et al., 2012). Moreover, decreased endometrial receptivity also leads to lower implantation rates and higher miscarriage rates in women with obesity (Bellver et al., 2010, 2013; Provost et al., 2016).
Fetal growth and neonatal outcomes are negatively impacted by maternal obesity, with higher rates of intrauterine fetal death, gestational diabetes, pre-eclampsia, large for gestational age (LGA) babies, congenital anomalies and admission to a neonatal intensive care unit (Leddy et al., 2008). Children born from obese mothers are at higher risk of obesity and metabolic disease later in life (Howell and Powell, 2017). Although there is still a lack of evidence from randomized controlled trials (RCT) evaluating the effectiveness of lifestyle intervention prior to IVF treatment and a debate of the effectiveness of lifestyle intervention prior to IVF treatment is ongoing (Norman and Mol, 2018), up until now optimizing maternal pre-pregnancy health is recommended for women with obesity prior to conception or infertility treatment (Practice Committee of the American Society for Reproductive Medicine, 2015).
Data regarding the effectiveness of lifestyle intervention on oocyte or embryo quality and live birth rate are scarce. In a prospective cohort study, Chavarro et al. (2012) reported that short-term weight loss was associated with a higher percentage of Metaphase II oocytes retrieved but this had no effect on embryo development. An RCT evaluating the efficacy of a low caloric liquid formula diet (LCD) leading to effective weight reduction preceding IVF showed that LCD in women with obesity indicated for IVF did not affect embryo quality (number of good-quality embryos, number of transferred and frozen embryos) and the live birth rate following IVF, although more natural conceptions occurred in the intervention arm (Einarsson et al., 2017). In this study, only one fresh embryo transfer (ET), or in case of a freeze-all strategy, the first transfer with frozen-thawed embryos, was reported. In fresh IVF cycles, the morphologically best embryo(s) are usually chosen for transfer, keeping any remaining good-quality embryos for future frozen-thawed ET when necessary. Therefore, the overall effect of weight loss on embryo quality could be underestimated if frozen-thawed ET(s) following the fresh cycle and pregnancies from these transfers are ignored.
We conducted and published an RCT examining whether lifestyle intervention prior to infertility treatment improves live birth rates in women with obesity (Mutsaerts et al., 2016). Lifestyle intervention prior to infertility treatment did not result in higher rates of a vaginal birth of a singleton at 37 weeks or more within 24 months after randomization compared with prompt infertility treatment. This analysis included all live births resulting from natural conception, ovulation induction (OI), IUI and IVF or ICSI. Embryo quality parameters were not investigated. We now present the evaluation of the effect of lifestyle intervention on embryo quality in a nested cohort of women scheduled for IVF or ICSI during the RCT. The aim of this study was to examine whether a lifestyle intervention improves embryo quality, expressed as embryo utilization rate (EUR) and cumulative live birth rate (CLBR) following the first fresh and subsequent frozen-thawed ET(s). Considering the consecutive processes that lead to pregnancy and eventually childbirth, in which embryo utilization reflects an early stage and live birth encompasses the full process, the effectiveness of lifestyle intervention could be reflected by reporting both of the outcomes.
Materials and methods
Study design
This is a nested cohort study within an RCT. Data for women who underwent IVF or ICSI were selected from the database of the LIFEstyle study (Mutsaerts et al., 2016). Briefly, the original study included 577 women (3 women withdrew informed consent, leaving 574 available for analysis) of reproductive age (18–39 years) with obesity and infertility who were assigned to a 6-month lifestyle intervention followed by 18 months of infertility treatment (n = 289) or to prompt infertility treatment for 24 months (n = 285) between 2009 and 2012. The main goal of the lifestyle intervention was to reduce body weight by at least 5% of the original during the 6-month intervention period or to reduce the BMI to below 29 kg/m2 (Mutsaerts et al., 2010). The intervention consisted of an energy-restricted diet (using an average decrease of 500 kcal below their baseline intake, but not <1200 kcal per day), enhancement of physical activity (using a step counter to increase up to 10 000 steps a day and moderate physical activities lasting at least 30 min for two to three times a week) and motivational counseling.
We included infertile women with a BMI ≥29 kg/m2. Infertility was defined as the failure to become pregnant within 12 months of unprotected intercourse. All participants received explorative fertility investigation before randomization to determine the type of infertility and the concomitant infertility treatment. Participants were scheduled for OI, IUI or IVF/ICSI based on the type of infertility, physical examination, medical history and the Hunault prediction model (Hunault et al., 2004). Subsequently, participants in the intervention group started the lifestyle intervention, while participants in the control group had care as usual, including immediate infertility treatment if needed. The follow-up time was 24 months after randomization.
Participants from the LIFEstyle study who received IVF treatment were eligible for the current analysis. The first complete IVF cycle leading to a successful oocyte retrieval, including subsequent frozen-thawed ET(s) in case no live birth occurred after the fresh ET after randomization, was included.
Oocyte/embryo culture and embryo outcome parameters
IVF or ICSI with controlled ovarian stimulation (COS) or modified nature cycle (MNC) (Pelinck et al., 2008) was performed according to the local protocols of each participating hospital. In general, ∼4 h after follicular aspiration, oocytes were either inseminated with motile spermatozoa (IVF) or injected with one spermatozoon (ICSI) based on the semen quality. The next morning, the presence of two pronuclei (2PN) was checked for each oocyte. Embryonic development was checked on a daily basis. ET was performed on Day 2, 3 or 4 after follicle aspiration. Strategy for the number of embryos to transfer differed for each participating hospital, but no more than two embryos were transferred. Supplementary embryos were cryopreserved if embryo quality was sufficient according to standards of the participating IVF centers. After the pregnancy test was positive, vaginal sonography was performed ∼4 weeks after ET. In case no pregnancy occurred in the fresh cycle, frozen embryos were thawed and transferred if available until the last surviving embryo was transferred. Cycles were prepared using either a natural cycle evaluated with sonography to induce ovulation using hCG when a dominant follicle reached at least 18 mm or an artificial cycle using orally administered estradiol and vaginal progesterone (600 mg divided into three dosages a day).
Oocyte/embryo parameters included the number of oocytes retrieved, number of oocytes inseminated/injected, number of oocytes fertilized, number of normal fertilized embryos (defined as the presence of 2PN), day of ET and number of transferred embryos, number of cryopreserved embryos and EUR. In the current analysis, EUR was defined as the proportion of inseminated/injected oocytes per cycle that was transferred or cryopreserved as an embryo. In case the first cycle was cancelled prior to oocyte retrieval owing to poor response or anticipation of ovarian hyperstimulation syndrome, the subsequent cycle with successful oocyte retrieval was used for the analysis.
Pregnancy and neonatal data collection
Research nurses recorded data on weight at baseline, at 3 and 6 months after randomization or at the end of lifestyle intervention in case, the target weight was reached, details of the IVF treatment and pregnancy outcomes after ET in a web-based digital form. Data on the course of pregnancy and childbirth were also recorded when a woman conceived within 24 months after randomization but childbirth occurred after the 24-month follow-up. Clinical pregnancy was defined as a pregnancy diagnosed by visualization of one or more intra-uterine gestational sacs. Ongoing pregnancy was defined as a viable intrauterine pregnancy of at least 12 weeks duration confirmed on an ultrasound scan. Miscarriage was defined as the loss of an intra-uterine pregnancy after a clinical pregnancy was registered. Live birth was defined as newborns, after 22 weeks’ gestation, that exhibits any sign of life. CLBR was defined as the percentage of participants with at least one live birth from the first fresh and subsequent frozen-thawed ET(s). Only the first live birth counted per participant. Neonatal outcomes were neonatal birthweight, gestational age, gender and small for gestation age (SGA) or large for gestation age (LGA), defined as birthweight below the 10th or above the 90th percentile, respectively, according to the Dutch reference curves (Visser et al., 2009).
Statistical analysis
SPSS Statistics 25 (IBM, Chicago, IL, USA) was used to perform the analysis. A P-value of <0.05 was considered to indicate statistically significant difference. Normality testing was performed with histograms, normal probability plots (Q–Q plots) combined with the Kolmogorov–Smirnov (K–S) test. Data are presented as mean ± SD (normally distributed) or median with interquartile (Q25 and Q75, non-normally distributed) for continuous variables. Categorical variables are presented as the proportion (percent).
Baseline characteristics of included participants were compared between the intervention and the control group using Fisher’s exact test or chi-square test for categorical variables and Student’s T-test or Mann–Whitney U test for continuous variables. In addition, we compared baseline characteristics between participants with least one oocyte retrieved (participants included in the analysis) and participants with unsuccessful IVF/ICSI treatment (participants not included in the analysis).
To compare the characteristics of oocytes and embryos, analysis was performed per cycle and per oocyte/embryo. For the per cycle analysis, linear regression was used for continuous outcomes and binary (multinomial) logistic regression was used for categorical outcomes. Unadjusted and adjusted odds ratio (OR) and 95% CI or mean difference and 95% CI are presented. Analyses were adjusted for type of infertility (unexplained and male factor) and smoking based on their difference between groups at baseline. For the per oocyte/embryo analysis, a generalized estimation equations model with an exchangeable or independent correlation matrix was used to analyze the effect of the lifestyle intervention on oocyte/embryo parameters, taking the dependency of oocytes from the same women into consideration. Afterward, type of infertility (unexplained and male factor) and smoking were added to the model as potential confounders.
In the per cycle analysis, EUR was first calculated per participant as the number of utilized embryos (transferred or cryopreserved) divided by the number of inseminated or injected oocytes. Then the average rate was calculated and presented for the intervention and the control group. In the per oocyte/embryo analysis, utilization of each individual embryo was recorded and EUR per group was calculated across all individual embryos. The denominator was the total number of inseminated or injected oocytes per group. The proportion and 95% CI were presented for the intervention and the control group.
Pregnancy outcomes (clinical pregnancy, ongoing pregnancy, miscarriage and live birth after the fresh ET and frozen-thawed ET and cumulative outcomes per participant) and singleton neonatal birthweight, birthweight in Z-score, gestational age, gender, LGA or SGA were compared using linear regression or logistic regression where relevant, adjusted for potential confounders as mentioned above. The Z-score for singleton neonatal birthweight was calculated after adjusting for gestational age, offspring gender and parity (Land, 2006).
In an MNC, only one oocyte is retrieved. Thus, EUR in the per cycle analysis is either 0% or 100% for these cycles. A sensitivity analysis excluding MNC was performed to evaluate the influence of these cycles on our overall results.
Results
Study population
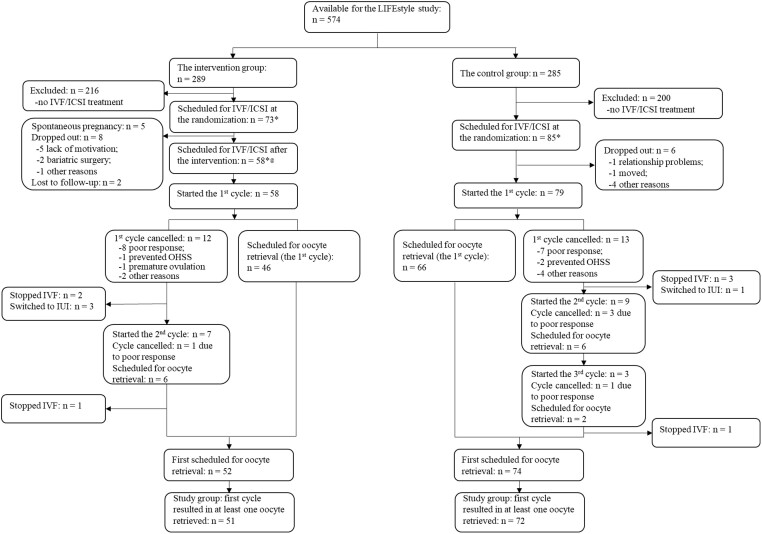
The flowchart of the selection of current study groups is shown in Fig. 1. In total, 158 participants were scheduled for IVF/ICSI treatment at randomization. The flowchart shows the details of women that conceived naturally, dropped out, lost to follow-up, or had cancelled cycles and hence the consecutive cycle was used for the analysis, leaving 51 cycles of women in the intervention group, and 72 in the control group.
Figure 1.
Flowchart of the selection of the study group. *Including participants who were indicated for other treatments and then switched to IVF/ICSI treatment. @Including participants who did not complete the lifestyle intervention. OHSS: ovarian hyperstimulation syndrome.
Baseline characteristics
The baseline characteristics of included participants in the current study are shown in Table I. The overall mean age was 31.6 years in both groups. There was no statistically significant difference in maternal age at the start of the IVF treatment between the intervention group and the control group (32.5 vs 32.2 years, P = 0.74). The mean BMI was 35.4 ± 3.2 kg/m2 in the intervention group, and 34.9 ± 2.9 kg/m2 in the control group. Type of infertility (unexplained, male factor) and smoking habit differed significantly between the two groups. In the intervention group, there were more smokers than in the control group (35.3% vs 18.1%, respectively P = 0.03). The differences between participants included in the analysis and participants not included are shown in Supplementary Table SI.
Table I.
Baseline characteristics of participants with an indication for IVF and with at least one successful oocyte retrieval.
| IVF/ICSI group with LIFEstyle intervention (n = 51) | IVF/ICSI control group without LIFEstyle intervention (n = 72) | P-value | |
|---|---|---|---|
| Maternal age (years) | 31.6 ± 4.6 | 31.6 ± 4.5 | 1.00 |
| Maternal age when starting IVF (years) | 32.5 ± 4.5 | 32.2 ± 4.5 | 0.74 |
| BMI (kg/m2) | 35.4 ± 3.2 | 34.9 ± 2.9 | 0.33 |
| Smoking | 18 (35.3%) | 13 (18.1%) | 0.03 |
| History of previous pregnancies | |||
| Live birth | 14 (27.5%) | 15 (20.8%) | 0.39 |
| Miscarriage | 9 (17.6%) | 10 (13.9%) | 0.57 |
| Ectopic pregnancy | 3 (5.9%) | 3 (4.2%) | 0.69 |
| Duration of infertility (years) | 2.33 (1.67; 4.00) | 2.17 (1.35; 4.38) | 0.57 |
| Primary infertility | 30 (58.8%) | 50 (69.4%) | 0.22 |
| Type of infertility# | |||
| Anovulation | 4 (7.8%) | 9 (12.5%) | 0.41 |
| Unexplained | 5 (9.8%) | 21 (29.2%) | 0.01 |
| Male factor | 38 (74.5%) | 39 (54.2%) | 0.02 |
| Tubal factor | 8 (15.7%) | 9 (12.5%) | 0.61 |
Data are presented as mean ± SD or median with interquartile (Q25; Q75) or proportion (percent). The differences between two groups were compared using Fisher’s exact test or chi-square test for categorical variables, and Student’s T-test or Mann–Whitney U test for continuous variables.
Some women had more than one diagnosis, an overall P-value was unavailable.
Weight change, medication usage, and oocyte/embryo characteristics
After the 6-month lifestyle intervention, the mean weight change in 51 women in the intervention group selected for this analysis was −3.95 kg, while it was −0.80 kg in 72 women in the control group. The mean difference in weight change between the intervention and control groups was statistically significant (mean difference in kg: −3.14, 95% CI: −5.73 to −0.56) in favor of the intervention group (Table II). The stimulation method (COS or MNC), COS regimes (antagonist or agonist) and FSH dosage were not significantly different between the two groups (Table II). The median (Q25; Q75) number of oocytes retrieved was 4.00 (2.00; 8.00) in the intervention group versus 6.00 (4.00; 9.75) in the control group, and was not significantly different, as was the number of oocytes inseminated/injected (4.00 [2.00; 8.00] vs 6.00 [3.00; 8.75]), and the number of normal fertilized embryos (2.00 [0.50; 5.00] vs 3.00 [1.00; 5.00]). Twenty participants in the intervention group (39.2%) had at least one cryopreserved embryo versus 37.5% (27/72) in the control group. The median number of cryopreserved embryos was 2.00 (1.25; 4.75) in the intervention group and 2.00 (1.00; 4.00) in the control group without significant difference (adjusted B: 0.77, 95% CI: −0.58 to 2.13). The median (Q25; Q75) EUR was 33.3% (12.5%; 60.0%) in the intervention group and 33.3% (16.7%; 50.0%) in the control group in the per cycle analysis (adjusted B: 2.7%, 95% CI: −8.6% to 14.0%). In the per oocyte/embryo analysis, in total, 280 oocytes were injected or inseminated in the intervention group, of which 113 were utilized (EUR = 40.4%); in the control group, EUR was 30.8% (142/461). The lifestyle intervention did not significantly improve EUR (crude OR: 1.35, 95% CI: 0.94–1.94; adjusted OR: 1.36, 95% CI: 0.94–1.98) in the per oocyte/embryo analysis.
Table II.
Weight change, medication usage, and oocyte/embryo characteristics.
| Analysis per cycle | ||||
|---|---|---|---|---|
| Characteristics | IVF/ICSI group with LIFEstyle intervention (n = 51) | IVF/ICSI control group without LIFEstyle intervention (n = 72) | Unadjusted OR (95% CI) or mean difference (95% CI) | Adjusted OR (95% CI) or B (95% CI) |
| Weight change at 6 months after intervention (kg) | −3.95 ± 6.98 | −0.80 ± 4.10 | −3.14 (−5.73 to −0.56)* | −3.12 (−5.63 to −0.60)* |
| BMI change at 6 months after intervention (kg/m2) | −1.42 ± 2.53 | −0.31 ± 1.39 | −1.11 (−2.04 to −0.19)* | −1.12 (−2.01 to −0.23)* |
| Stimulation method | ||||
| MNC | 9 (17.6%) | 10 (13.9%) | 1.33 (0.50–3.55) | 1.20 (0.43–3.36) |
| COS | 42 (82.4%) | 62 (86.1%) | Reference | Reference |
| COS regimes | ||||
| Antagonist | 3 (7.1%) | 4 (6.5%) | 1.12 (0.24–5.26) | 1.88 (0.32–11.0) |
| Agonist | 39 (92.9%) | 58 (93.5%) | Reference | Reference |
| Total dose of FSH (IU) | 2152 ± 1242 | 2126 ± 1177 | 26.21 (−417.9 to 470.3) | −13.45 (−482.3 to 455.4) |
| FSH duration (days) | 12.1 ± 5.29 | 12.5 ± 6.36 | −0.44 (−2.63 to 1.75) | −0.73 (−3.05 to 1.59) |
| Fertilization method | ||||
| IVF | 13 (25.5%) | 26 (36.1%) | 0.61 (0.27–1.34) | 0.74 (0.24–2.25) |
| ICSI | 38 (74.5%) | 46 (63.9%) | Reference | Reference |
| Number of oocytes retrieved | 4.00 (2.00; 8.00) | 6.00 (4.00; 9.75) | −1.04 (−2.81 to 0.73) | −1.61 (−3.44 to 0.22) |
| Number of oocytes inseminated/injected | 4.00 (2.00; 8.00) | 6.00 (3.00; 8.75) | −0.91 (−2.58 to 0.75) | −1.38 (−3.09 to 0.32) |
| Number of oocytes fertilized | 3.00 (1.00; 7.00) | 4.00 (1.00; 7.00) | −0.34 (−1.81 to 1.13) | −0.90 (−2.42 to 0.63) |
| Number of normal fertilized embryos (2PN) | 2.00 (0.50; 5.00) | 3.00 (1.00; 5.00) | −0.24 (−1.42 to 0.94) | −0.62 (−1.86 to 0.63) |
| Fresh ET performed | ||||
| Yes | 40 (78.4%) | 63 (87.5%) | 0.52 (0.20–1.37) | 0.40 (0.14–1.16) |
| No | 11 (21.6%) | 9 (12.5%) | Reference | Reference |
| Day of ET | ||||
| 2 | 23 (57.5%) | 29 (46.0%) | 2.91 (0.73–11.7) | 3.51 (0.83–14.9) |
| 3 | 14 (35.0%) | 23 (36.5%) | 2.23 (0.53–9.41) | 1.95 (0.44–8.65) |
| 4 | 3 (7.5%) | 11 (17.5%) | Reference | Reference |
| Number of transferred embryos | ||||
| 1 | 35 (87.5%) | 48 (76.2%) | 2.19 (0.73–6.58) | 2.40 (0.74–7.79) |
| 2 | 5 (12.5%) | 15 (23.8%) | Reference | Reference |
| Cryopreservation performed | ||||
| Yes | 20 (39.2%) | 27 (37.5%) | 1.08 (0.51–2.25) | 1.03 (0.47–2.25) |
| No | 31 (60.8%) | 45 (62.5%) | Reference | Reference |
| Number of cryopreserved embryos | 2.00 (1.25; 4.75) | 2.00 (1.00; 4.00) | 1.03 (-0.29–2.35) | 0.77 (-0.58–2.13) |
| Number of cryopreserved embryos categorized | ||||
| 0 | 31 (60.8%) | 45 (62.5%) | 0.95 (0.34–2.63) | 0.93 (0.33–2.64) |
| 1 | 5 (9.8%) | 11 (15.3%) | 0.63 (0.16–2.52) | 0.70 (0.17–2.91) |
| 2 | 7 (13.7%) | 5 (6.9%) | 1.93 (0.45–8.33) | 2.00 (0.45–8.87) |
| More than 2 | 8 (15.7%) | 11 (15.3%) | Reference | Reference |
| EUR1 | 33.3% (12.5%; 60.0%) | 33.3% (16.7%; 50.0%) | 1.7% (−8.9% to 12.3%) | 2.7% (−8.6% to 14.0%) |
| Analysis per oocyte/embryo | ||||
| Characteristics |
Oocyte retrieved (n = 304) |
Oocyte retrieved (n = 504) | Unadjusted GEE-OR (95% CI) | Adjusted GEE-OR (95% CI) |
| Injected/inseminated oocytes2 | 280 (92.1%) | 461 (91.5%) | 1.17 (0.62–2.19) | 1.32 (0.68–2.55) |
| Embryo transferred3 | 45 (16.1%) | 78 (16.9%) | 0.94 (0.68–1.31) | 0.99 (0.72–1.38) |
| Embryo cryopreserved3 | 68 (24.3%) | 64 (13.9%) | 1.57 (0.89–2.80) | 1.49 (0.84–2.66) |
| Embryo utilized (EUR)4 | 113 (40.4%) | 142 (30.8%) | 1.35 (0.94–1.94) | 1.36 (0.94–1.98) |
Data are presented as mean ± SD or median with interquartile (Q25; Q75) or proportion (percent). To compare the characteristics of oocytes and embryos, analysis was performed per cycle and per oocyte/embryo. For the per cycle analysis, linear regression or binary (multinomial) logistic regression was used. For the per oocyte/embryo analysis, GEE was used. Data were adjusted for type of infertility (unexplained and male factor) and smoking. Number of cryopreserved embryos categorized were adjusted for smoking due to failure to converge in the fully-corrected model.
COS: controlled ovarian stimulation; ET: embryo transfer; EUR: embryo utilization rate; GEE: generalized estimating equations; MNC: modified nature cycle.
In the per cycle analysis, EUR was first calculated per subject, then averaged per group.
Expressed as proportion of the number of oocytes retrieved.
Expressed as proportion of the number of oocytes injected or inseminated.
In the per oocyte/embryo analysis, utilization of each individual embryo was used. The denominator was the total number of inseminated or injected oocytes per group.
P < 0.05.
Pregnancy and neonatal outcomes
Clinical pregnancy, ongoing pregnancy, miscarriage and live birth resulting from fresh and frozen-thawed ET(s) are shown in Table III. After the first fresh ET, there were 27.5% (14/51) clinical pregnancies and 25.5% (13/51) live births in the intervention group, and 27.8% (20/71) and 18.1% (13/72) in the control group. There were no significant differences in clinical pregnancy rates (adjusted OR: 0.93, 95% CI: 0.40–2.16) and live births rates (adjusted OR: 1.21, 95% CI: 0.48–3.06) between the two groups. Among 38 women not having a live birth after the first ET in the intervention group, there were 14 participants who had at least one cryopreserved embryo. Among 59 women not having a live birth after the first ET in the control group, 20 participants had at least one cryopreserved embryo. All cryopreserved embryos from those 34 women were thawed and transferred if they survived, leading to 1 and 3 live births, respectively, in the intervention and the control group. CLBR was 27.5% in the intervention group versus 22.2% in the control group (crude OR: 1.32, 95% CI: 0.58–3.03; adjusted OR: 1.03, 95% CI: 0.43–2.47). The singleton neonatal birthweight was 3371 ± 606.4 g (n = 13) and 3540 ± 588.5 g (n = 16) in the intervention group and the control group, respectively (adjusted B: −136.6 g, 95% CI: −634.4 to 361.3). No significant differences between groups were found in gender and LGA or SGA of the neonates.
Table III.
Pregnancy and neonatal outcomes.
| IVF/ICSI group with LIFEstyle intervention (n = 51) | IVF/ICSI control group without LIFEstyle intervention (n = 72) | Unadjusted OR (95% CI) or mean difference (95% CI) | Adjusted OR (95% CI) or B (95% CI) | |
|---|---|---|---|---|
| Fresh IVF/ICSI cycle1 | ||||
| Clinical pregnancy | 14 (27.5%) | 20 (27.8%) | 0.98 (0.44–2.20) | 0.93 (0.40–2.16) |
| Ongoing pregnancy | 13 (25.5%) | 15 (20.8%) | 1.30 (0.56–3.04) | 1.03 (0.42–2.52) |
| Miscarriage | 1 (7.1%) | 7 (35.0%) | 0.14 (0.02–1.33) | 0.19 (0.01–3.05) |
| Live births | 13 (25.5%) | 13 (18.1%) | 1.55 (0.65–3.71) | 1.21 (0.48–3.06) |
| Frozen-thawed cycles2 | ||||
| Clinical pregnancy | 2 (14.3%) | 4 (20.0%) | 0.67 (0.10–4.26) | 0.51 (0.08–3.49) |
| Ongoing pregnancy | 1 (7.1%) | 3 (15.0%) | 0.44 (0.04–4.69) | 0.34 (0.03–4.05) |
| Miscarriage3 | 1 (50.0%) | 1 (25.0%) | 3.00 (0.08–107) | NA# |
| Live births4 | 1 (7.1%) | 3 (15.0%) | 0.44 (0.04–4.69) | 0.34 (0.03–4.05) |
| Fresh IVF/ICSI cycle and subsequent frozen-thawed cycles3 | ||||
| Cumulative live births | 14 (27.5%) | 16 (22.2%) | 1.32 (0.58–3.03) | 1.03 (0.43–2.47) |
| Cumulative multiple births | 1 (7.1%) | 0 (0.0%) | NA@ | NA@ |
| Neonatal outcomes4 | ||||
| Neonatal birthweight (g) | 3371 ± 606.4 | 3540 ± 588.5 | −169.4 (−626.4 to 287.6) | −136.6 (−634.4 to 361.3) |
| Birth weight Z-score | 0.28 ± 0.90 | 0.64 ± 1.11 | −0.36 (−1.14 to 0.42) | −0.23 (−1.09 to 0.64) |
| Gestational age (weeks) | 38.8 ± 1.9 | 39.1 ± 1.9 | −0.34 (−1.83 to 1.14) | −0.52 (−2.08 to 1.04) |
| Gender | ||||
| Male | 6 (46.2%) | 7 (43.8%) | 1.10 (0.25–4.80) | 0.89 (0.18–4.38) |
| Female | 7 (53.8%) | 9 (56.3%) | Reference | Reference |
| LGA | 2 (15.4%) | 5 (31.3%) | 0.40 (0.06–2.52) | 0.37 (0.05–2.67) |
| SGA | 1 (7.7%) | 0 (0.0%) | NA@ | NA@ |
Data are presented as mean ± SD or proportion (percent). Data were compared between groups using linear regression or logistic regression. Data were adjusted for type of infertility (unexplained and male factor) and smoking.
LGA: large for gestational age; SGA: small for gestational age.
Expressed as proportion of participants included (miscarriage rates are expressed as proportion of clinical pregnancies result from fresh ET).
Expressed as proportion of participants who had at least one cryopreserved embryo if no pregnancy occurred from fresh ET (miscarriage rates are expressed as proportion of clinical pregnancies result from frozen-thawed ET).
Expressed as proportion of participants included (cumulative multiple birth rates are expressed as proportion of cumulative live births result from fresh or subsequent frozen-thawed ET).
Only singletons were included.
No adjusted model was performed due to the small sample size.
No OR was calculated since there was no event in the control group.
Sensitivity analysis
Exclusion of 19 MNC cases did not affect the main results of EUR and CLBR (Supplementary Tables SII and SIII).
Discussion
This nested cohort study within an RCT showed that, compared to prompt IVF, a 6-month lifestyle intervention preceding IVF did not significantly influence EUR and CLBR in the first cycle leading to successful oocyte retrieval. Moreover, birthweight of the offspring was not affected. Nevertheless, our findings indicate that there may potentially be a clinically relevant effect on EUR, but further studies in larger populations are required to confirm this.
Little is known about the effect of a lifestyle intervention on embryo quality in women with obesity who receive IVF treatment. We hypothesized that a lifestyle intervention could have a beneficial effect on oocytes, leading to a better embryo quality expressed as higher EUR and CLBR. We found that a 6-month lifestyle intervention did not improve EUR or CLBR, despite significantly more weight loss in the intervention group compared to the control group. Einarsson et al. (2017) have also reported similar embryo quality (expressed as the number of oocytes fertilized, the number of good-quality embryos on Day 2 and the number of transferred and frozen embryos) in the intervention and the control group in their RCT investigating the efficacy of LCD prior to IVF. However, there are important differences between Einarsson’s study and ours. First of all, the study by Einarsson was an RCT whereas our study is a post hoc analysis of an RCT. Their group had, on average, a lower BMI at inclusion, ranging between 30 and 35 kg/m2, whereas in our study the average BMI for women indicated for IVF at inclusion was ∼35 kg/m2. Second, different intervention strategies were applied. While Einarsson used LCD with a daily intake of 880 kcal to reduce body weight, we focused on healthy food choices and moderate calorie restriction, i.e. maintaining more than 1200 kcal per day. We wanted to avoid rapid weight loss achieved by crash diets, which is suspected to be detrimental to oocyte quality and fertilization rates (Tsagareli et al., 2006). The study by Einarrsson resulted in an average weight loss of 9 kg in the LCD intervention arm compared to 4 kg weight loss in the intervention group in our study. Moreover, our intervention protocol also aimed to increase moderate physical activity (van Elten et al., 2018), while no intervention on physical activity was included in the RCT reported by Einarsson. Additionally, a recent exploratory retrospective study illustrated that the number of good-quality embryos did not significantly differ between women who underwent bariatric surgery resulting in a mean BMI of 28.9 kg/m2 and women with a mean BMI of 37.7 kg/m2 who did not have surgery (Grzegorczyk-Martin et al., 2020). Although the differences between these studies complicate comparisons of the results, all studies point in the same direction: after intervention with diet in combination with exercise, or LCD, or bariatric surgery to reduce body weight in women with obesity, no improvement was found regarding embryo quality or EUR as proxy for embryo quality. In addition to EUR in our study, CLBR and neonatal birthweight in Z-score were not significantly different between the intervention and the control group. This is consistent with the existing evidence from a meta-analysis on this topic (Lan et al., 2017).
Recent murine studies indicate that mitochondrial dysfunction is the primary underlying mechanism leading to obesity-induced fertility problems (Grindler and Moley, 2013). The effects on oocyte quality of resumption of regular chow diet after a high-fat diet have been explored in a murine model of obesity induced by high-fat diet (Reynolds et al., 2015). Although it was observed that an 8-week switch from a high-fat diet to a regular diet lowered body weight and reduced cholesterol and glucose levels, oocytes from the intervention group exhibited higher rates of meiotic spindle, lipid accumulation and mitochondrial defects than oocytes from control mice on regular chow diet (Reynolds et al., 2015). The exact mechanism is unclear, but these mice studies suggest that resumption of regular diet might not reverse mitochondrial dysfunction in high-fat diet induced obesity in a short period (Reynolds et al., 2015). Results from another murine study showed that voluntary exercise independent of diet did not benefit weight loss and failed to reverse the rate of oocytes with deformed spindles and meiotic maturation. However, lipid metabolism improved, and exercise exposure had potential beneficial effects on mitochondrial ultrastructure (Boudoures et al., 2016). We have shown that participants in the intervention group indeed had more healthy food choices with a reduction in soda, salty and sweet snack intake in the short term (van Elten et al., 2018). However, our current analyses suggest that this did not result in improvement of EUR. In our study, the lifestyle intervention consisted of a combined strategy of caloric restriction, physical enhancement and behavior modification, which makes it difficult to examine the effect of each factor independently. Furthermore, follicles develop for several months before ovulation (McGee and Hsueh, 2000; Baerwald et al., 2012), so the duration of our lifestyle intervention might have been too short to measure its effect on EUR. Of note, although there was no statistically significant difference in EUR between the two groups, the lifestyle intervention prior to IVF resulted in a relative increase of EUR by 35%, and patients may benefit significantly as a result. More studies or pooled analyses are needed to verify these potentially clinically relevant effects of lifestyle intervention.
We observed that for fresh cycles, there were fewer miscarriages in the intervention group (7.1%) than in the control group (35.0%). Since our numbers are small, this may be a chance finding. As suggested, the role of the endometrium may also be critical in this respect (Bellver et al., 2007). In a previous lifestyle intervention study, a 3-month combined diet and exercise lifestyle intervention up-regulated endometrium insulin signaling in women with obesity and polycystic ovary syndrome (Ujvari et al., 2014). The lifestyle intervention positively altered glucose homeostasis and might, thus, restore the endometrium function (Bellver et al., 2011; Ujvari et al., 2014). The importance of endometrium was also illustrated by a large cohort study including 9587 first egg donation cycles, which showed a detrimental effect of obesity of the recipients on the live birth rate. Parameters, such as fertilization rate, embryo fragmentation and number of blastomeres, did not differ significantly between recipient groups with various BMI levels. These results suggest that obesity negatively alters uterine receptivity rather than embryo quality, thereby affecting the live birth rate (Bellver et al., 2013). Future clinical studies could further investigate the role of lifestyle intervention in the improvement of endometrium function in women with obesity.
There are several strengths of our study. Since obesity is associated with increased cycle cancellation rates (Luke et al., 2011a,b), we evaluated outcomes based on cycles with at least one successful oocyte retrieval rather than started cycles, which increased our number of oocytes and embryos that could be analyzed. Analysis per oocyte retrieval rather than per started cycle allowed us to evaluate the effect of lifestyle intervention on oocyte/embryo quality directly. We had detailed prospective data of the study population, which made it possible to adjust for potential confounders. Although our study constitutes a significant contribution to the scarce evidence, there are limitations in our study. It is a post hoc analysis, not supported by a power calculation, and, thus, Type II errors are more likely to occur. The randomization was not stratified for indicated treatment, therefore, our analysis was not a randomized comparison. To account for this, we adjusted our analysis for baseline differences and potential confounders although residual confounding may still occur. Moreover, the relatively homogeneous group of study participants with obesity limits generalizability of the results. The limited absolute weight loss and the short duration of the lifestyle intervention might be insufficient to affect EUR and CLBR. However, given that proposed prolonged lifestyle intervention in infertile patients is difficult in clinical practice, we believe our current study is a good representation of a realistic practical intervention and therefore provides potential guidance for clinical application. Furthermore, a limited number of participants achieved the goal (to reduce body weight by at least 5% or to reduce BMI to below 29 kg/m2) either at 3 or 6 months after randomization, irrespective of randomization group. Thus, performing a subgroup or mediation analysis was not appropriate owing to the relatively small sample size. Finally, not all participating hospitals provided individual parameters of oocyte/embryo. As a result, the number of top-quality embryos could not be reported in this analysis. EUR is an alternative indicator of embryo quality and an even better parameter to evaluate the overall effect of lifestyle intervention for women with obesity since all recovered embryos that could be transferred and cryopreserved should at least be morphologically normally developed embryos.
In conclusion, a 6-month lifestyle intervention preceding IVF did not improve EUR and CLBR in women with obesity. Our data do not support the hypothesis of a beneficial short-term effect of lifestyle intervention on embryo quality in women with obesity, expressed as EUR and CLBR. Further research is necessary to increase our understanding of the complex mechanisms underlying the effects of lifestyle intervention in women with obesity on embryo quality and endometrial receptivity.
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Acknowledgments
The authors thank all the women who participated in this study. The authors thank all participating hospitals and their staff for their contribution to this study, and the lifestyle coaches, research nurses, research midwives and office members of the Dutch Consortium (www.studies-obsgyn.nl) for their hard work and dedication.
The following persons are members of the LIFEstyle study group: Anne M. van Oers, Meike A.Q. Mutsaerts, Henk Groen, Walter K. Kuchenbecker, Ron van Golde, Gerrit J.E. Oosterhuis, Niels E.A. Vogel, Ben W.J. Mol, Annemieke Hoek, Denise A.M. Perquin, Carolien A.M. Koks, Eugenie M. Kaaijk, Frank J. Broekmans, Cornelis B. Lambalk, Fulco van der Veen, Nicole F. Klijn, Patricia E.A. M. Mercelina, Yvonne M. van Kasteren, Annemiek W. Nap, Egbert A. Brinkhuis, Robert J.A.B. Mulder, Ed T.C.M. Gondrie, Jan P. de Bruin and Marko J. Sikkema.
Authors’ roles
All authors were involved in the conduct of the trial and revision for intellectual content and approved the final submitted version. J.V.E.-A. was responsible for the design of the trial. Z.W. and A.H. drafted the manuscript. Z.W. and H.G. carried out the statistical analyses. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, writing of the report or the decision to submit the article for publication.
Funding
Grant from ZonMw, the Dutch Organization for Health Research and Development (50-50110-96-518). ZonMw had no role in data collection, analysis, interpretation of data or writing the report.
Conflict of interest
A.H. has received an unrestricted educational grant from Ferring pharmaceuticals BV, The Netherlands. B.W.J.M. is supported by an NHMRC Investigator grant (GNT1176437). B.W.J.M. reports consultancy for Guerbet, has been a member of the ObsEva advisory board and holds Stock options for ObsEva. B.W.J.M. has received research funding from Guerbet, Ferring and Merck. F.J.M.B. reports personal fees from membership of the external advisory board for Merck Serono and a research support grant from Merck Serono, outside the submitted work.
References
- Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: a committee opinion. Fertil Steril 2015;104:1116–1126. [DOI] [PubMed] [Google Scholar]
- Baerwald AR, Adams GP, Pierson RA.. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update 2012;18:73–91. [DOI] [PubMed] [Google Scholar]
- Bellver J, Ayllon Y, Ferrando M, Melo M, Goyri E, Pellicer A, Remohi J, Meseguer M.. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil Steril 2010;93:447–454. [DOI] [PubMed] [Google Scholar]
- Bellver J, Martínez-Conejero JA, Labarta E, Alamá P, Melo MA, Remohí J, Pellicer A, Horcajadas JA.. Endometrial gene expression in the window of implantation is altered in obese women especially in association with polycystic ovary syndrome. Fertil Steril 2011;95:2335–2341, 2341.e2331–2338. [DOI] [PubMed] [Google Scholar]
- Bellver J, Melo MA, Bosch E, Serra V, Remohi J, Pellicer A.. Obesity and poor reproductive outcome: the potential role of the endometrium. Fertil Steril 2007;88:446–451. [DOI] [PubMed] [Google Scholar]
- Bellver J, Pellicer A, Garcia-Velasco JA, Ballesteros A, Remohi J, Meseguer M.. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertil Steril 2013;100:1050–1058. [DOI] [PubMed] [Google Scholar]
- Boudoures AL, Chi M, Thompson A, Zhang W, Moley KH.. The effects of voluntary exercise on oocyte quality in a diet-induced obese murine model. Reproduction 2016;151:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Ehrlich S, Colaci DS, Wright DL, Toth TL, Petrozza JC, Hauser R.. Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertil Steril 2012;98:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsson S, Bergh C, Friberg B, Pinborg A, Klajnbard A, Karlstrom PO, Kluge L, Larsson I, Loft A, Mikkelsen-Englund AL. et al. Weight reduction intervention for obese infertile women prior to IVF: a randomized controlled trial. Hum Reprod 2017;32:1621–1630. [DOI] [PubMed] [Google Scholar]
- Grindler NM, Moley KH.. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod 2013;19:486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzegorczyk-Martin V, Freour T, De Bantel Finet A, Bonnet E, Merzouk M, Roset J, Roger V, Cedrin-Durnerin I, Wainer R, Avril C. et al. IVF outcomes in patients with a history of bariatric surgery: a multicenter retrospective cohort study. Hum Reprod 2020;35:2755–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KR, Powell TL.. Effects of maternal obesity on placental function and fetal development. Reproduction 2017;153:r97–r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunault CC, Habbema JD, Eijkemans MJ, Collins JA, Evers JL, Te Velde ER.. Two new prediction rules for spontaneous pregnancy leading to live birth among subfertile couples, based on the synthesis of three previous models. Hum Reprod 2004;19:2019–2026. [DOI] [PubMed] [Google Scholar]
- Jungheim ES, Macones GA, Odem RR, Patterson BW, Lanzendorf SE, Ratts VS, Moley KH.. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil Steril 2011;95:1970–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawwass JF, Kulkarni AD, Hipp HS, Crawford S, Kissin DM, Jamieson DJ.. Extremities of body mass index and their association with pregnancy outcomes in women undergoing in vitro fertilization in the United States. Fertil Steril 2016;106:1742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L, Harrison CL, Misso M, Hill B, Teede HJ, Mol BW, Moran LJ.. Systematic review and meta-analysis of the impact of preconception lifestyle interventions on fertility, obstetric, fetal, anthropometric and metabolic outcomes in men and women. Hum Reprod 2017;32:1925–1940. [DOI] [PubMed] [Google Scholar]
- Land JA. How should we report on perinatal outcome? Hum Reprod 2006;21:2638–2639. [DOI] [PubMed] [Google Scholar]
- Leary C, Leese HJ, Sturmey RG.. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod 2015;30:122–132. [DOI] [PubMed] [Google Scholar]
- Leddy MA, Power ML, Schulkin J.. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol 2008;1:170–178. [PMC free article] [PubMed] [Google Scholar]
- Luke B, Brown MB, Missmer SA, Bukulmez O, Leach R, Stern JE; Society for Assisted Reproductive Technology Writing Group. The effect of increasing obesity on the response to and outcome of assisted reproductive technology: a national study. Fertil Steril 2011a;96:820–825. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R; A SART Writing Group. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod 2011b;26:245–252. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJW.. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000;21:200–214. [DOI] [PubMed] [Google Scholar]
- Mutsaerts MA, van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WK, Perquin DA, Koks CA, van Golde R, Kaaijk EM, Schierbeek JM. et al. Randomized trial of a lifestyle program in obese infertile women. N Engl J Med 2016;374:1942–1953. [DOI] [PubMed] [Google Scholar]
- Mutsaerts MAQ, Groen H, ter Bogt NCW, Bolster JHT, Land JA, Bemelmans WJE, Kuchenbecker WKH, Hompes PGA, Macklon NS, Stolk RP. et al. The LIFESTYLE study: costs and effects of a structured lifestyle program in overweight and obese subfertile women to reduce the need for fertility treatment and improve reproductive outcome. A randomised controlled trial. BMC Womens Health 2010;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RJ, Mol BWJ.. Successful weight loss interventions before in vitro fertilization: fat chance? Fertil Steril 2018;110:581–586. [DOI] [PubMed] [Google Scholar]
- Pelinck MJ, Knol HM, Vogel NE, Arts EG, Simons AH, Heineman MJ, Hoek A.. Cumulative pregnancy rates after sequential treatment with modified natural cycle IVF followed by IVF with controlled ovarian stimulation. Hum Reprod 2008;23:1808–1814. [DOI] [PubMed] [Google Scholar]
- Provost MP, Acharya KS, Acharya CR, Yeh JS, Steward RG, Eaton JL, Goldfarb JM, Muasher SJ.. Pregnancy outcomes decline with increasing recipient body mass index: an analysis of 22,317 fresh donor/recipient cycles from the 2008–2010 Society for Assisted Reproductive Technology Clinic Outcome Reporting System registry. Fertil Steril 2016;105:364–368. [DOI] [PubMed] [Google Scholar]
- Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH.. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev 2015;27:716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, Lane M, Norman RJ.. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab 2009;94:1533–1540. [DOI] [PubMed] [Google Scholar]
- Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M, Fréour T.. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update 2019;25:439–451. [DOI] [PubMed] [Google Scholar]
- Tsagareli V, Noakes M, Norman RJ.. Effect of a very-low-calorie diet on in vitro fertilization outcomes. Fertil Steril 2006;86:227–229. [DOI] [PubMed] [Google Scholar]
- Ujvari D, Hulchiy M, Calaby A, Nybacka Å, Byström B, Hirschberg AL.. Lifestyle intervention up-regulates gene and protein levels of molecules involved in insulin signaling in the endometrium of overweight/obese women with polycystic ovary syndrome. Hum Reprod 2014;29:1526–1535. [DOI] [PubMed] [Google Scholar]
- Valckx SD, De Pauw I, De Neubourg D, Inion I, Berth M, Fransen E, Bols PE, Leroy JL.. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum Reprod 2012;27:3531–3539. [DOI] [PubMed] [Google Scholar]
- van Elten TM, Karsten MDA, Geelen A, van Oers AM, van Poppel MNM, Groen H, Gemke R, Mol BW, Mutsaerts MAQ, Roseboom TJ; LIFEstyle Study Group et al. Effects of a preconception lifestyle intervention in obese infertile women on diet and physical activity; a secondary analysis of a randomized controlled trial. PLoS One 2018;13:e0206888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser GHA, Eilers PHC, Elferink-Stinkens PM, Merkus HMWM, Wit JM.. New Dutch reference curves for birthweight by gestational age. Early Hum Dev 2009;85:737–744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.