Abstract
Background
Prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shedding has been described in immunocompromised coronavirus disease 2019 (COVID-19) patients, resulting in protracted disease and poor outcome. Specific therapy to improve viral clearance and outcome for this group of patients is currently unavailable.
Methods
Five critically ill COVID-19 patients with severe defects in cellular immune responses, high SARS-CoV-2 viral RNA loads, and no respiratory improvement were treated with interferon gamma, 100 μg subcutaneously, thrice weekly. Bronchial secretion was collected every 48 h for routine diagnostic SARS-CoV-2 RT-PCR and viral culture.
Findings
Interferon gamma administration was followed by a rapid decline in SARS-CoV-2 load and a positive-to-negative viral culture conversion. Four patients recovered, and no signs of hyperinflammation were observed.
Conclusions
Interferon gamma may be considered as adjuvant immunotherapy in a subset of immunocompromised COVID-19 patients.
Funding
A.v.L. and R.v.C. are supported by National Institutes of Health (R01AI145781). G.J.O. and R.P.v.R. are supported by a VICI grant (016.VICI.170.090) from the Dutch Research Council (NWO). W.F.A. is supported by a clinical fellowship grant (9071561) of Netherlands Organization for Health Research and Development. M.G.N. is supported by an ERC advanced grant (833247) and a Spinoza grant of the Netherlands Organization for Scientific Research.
Keywords: COVID-19, SARS-CoV-2, immunocompromised, interferon gamma, immunotherapy
Graphical abstract
Context and significance
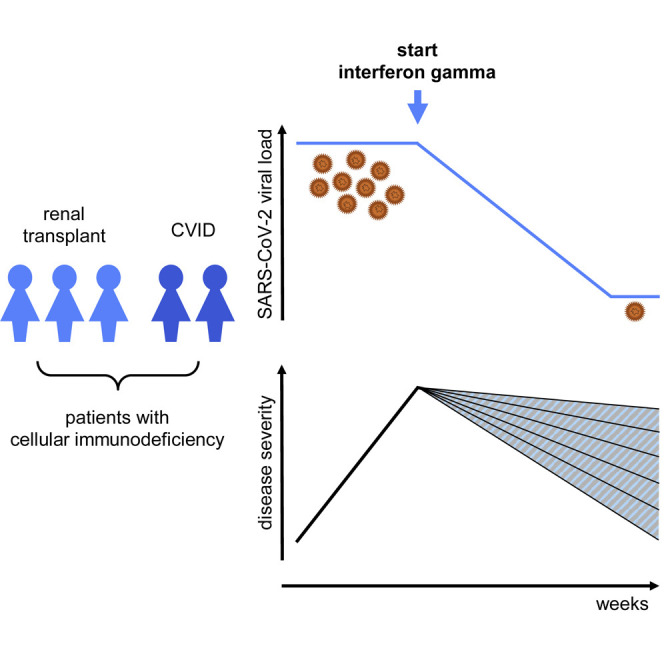
In patients with impaired immune systems, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus causing coronavirus disease 2019 (COVID-19), can persist for a long time. This contributes to a long disease course and poor outcome. Thus far, no therapy is available to help clear the virus. Interferon gamma is a protein that activates cells of the immune system. We applied interferon gamma to five COVID-19 patients with impaired immune systems. These patients were critically ill and did not clear the virus themselves. Interferon gamma therapy was followed by clearance of SARS-CoV-2 in five and clinical recovery in four patients. This report shows that interferon gamma should be considered as a study candidate for immunocompromised COVID-19 patients who are not able to clear the virus themselves.
van Laarhoven et al. report five COVID-19 patients with impaired cellular immunity and persistently high SARS-CoV-2 loads, treated with last-resort interferon gamma immunotherapy, which was followed by viral clearance in five and clinical recovery in four patients. No signs of hyperinflammation were observed.
Introduction
Exuberant immunopathology plays an important role in COVID-19 mortality,1 and indeed, immunosuppressive therapy has demonstrated to improve outcome.2 However, in patients with pre-existent immunodeficiencies, replication-competent SARS-CoV-2 may be shed well after 21 days after disease onset.3 , 4 Convalescent plasma or remdesivir is used in immunocompromised patients, but has not been shown to promote viral clearance. Our patients had conditions reducing their interferon response, which is likely important for viral clearance.4 In addition, interferon-mediated immunity is known to be impaired by COVID-19 itself.5 , 6 Clinical data however failed to demonstrate positive effects of systemic interferon beta-1a in COVID-19 patients,7 while interferon gamma has been thus far avoided due to its potential proinflammatory effects. However, in patients with severe cellular immune defects, therapeutic stimulation of the antiviral host defense may benefit SARS-CoV-2 clearance.
Results
Five immunocompromised COVID-19 patients were treated with adjuvant interferon gamma, 100 μg subcutaneously, thrice weekly. The patients were monitored for SARS-CoV-2 viral load, culture conversion (patients 1–3), and signs of hyperinflammation.
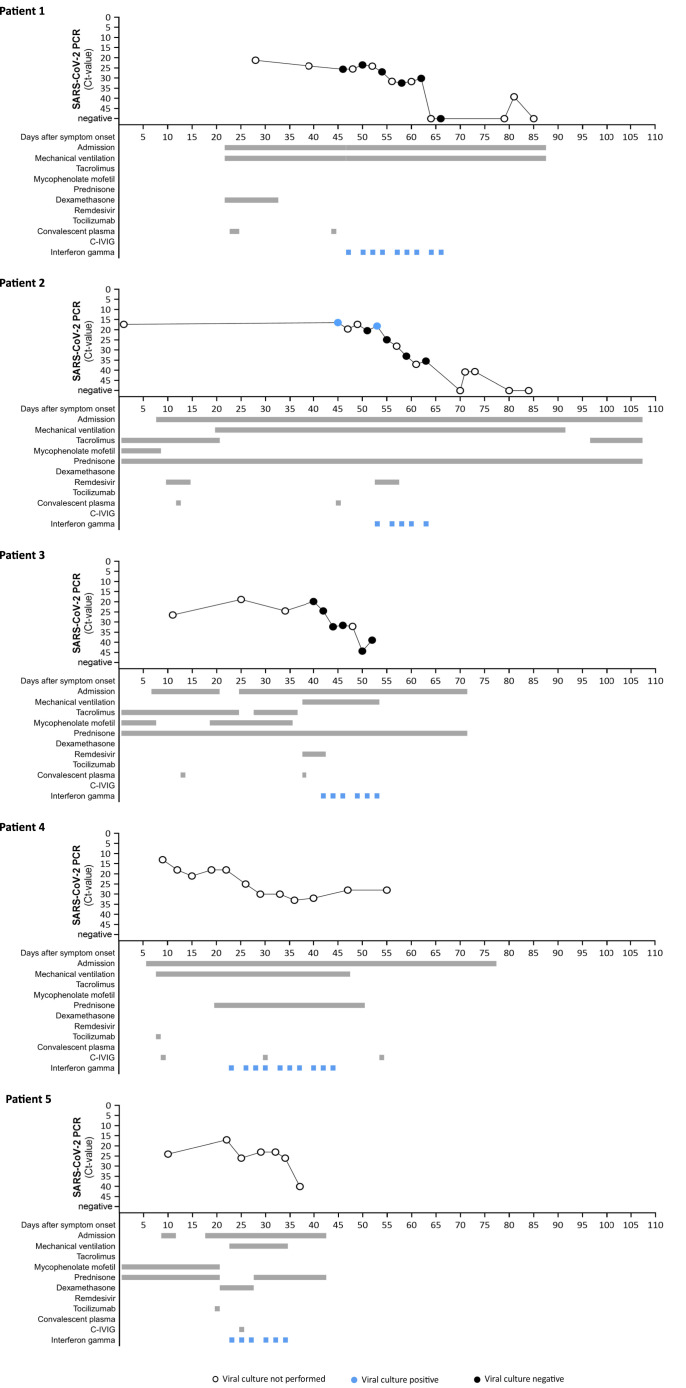
Patient 1
A 50-year-old woman with a history of splenectomy and common variable immunodeficiency (Table 1 ) was admitted to the intensive care unit (ICU). Mechanical ventilation was initiated on day 22 after COVID-19 symptom onset. She had developed an invasive pulmonary Aspergillus nidulans infection and Pseudomonas aeruginosa pneumonia. Because of an absent SARS-CoV-2 antibody response, convalescent plasma was administered (Figure 1 ) without clinical response. Five days after admission, she was transferred to our center because of acute kidney injury and severe acute respiratory distress syndrome. She developed a fulminant herpes simplex stomatitis, with temporary response to acyclovir. Even though lymphocyte counts were only moderately decreased (Table S1), the Aspergillus and herpes virus complications pointed to an impaired T helper 1 (Th1)/interferon gamma response, in addition to her common variable immunodeficiency-related humoral defects. SARS-CoV-2 viral load in bronchial secretion remained high after administration of a third dose of convalescent plasma at day 44. Interferon gamma was initiated on day 47, after which SARS-CoV-2 viral loads decreased. Virus culture was negative at all time points tested, including a sample taken before the initiation of interferon gamma treatment. Although her respiratory status improved, she remained in need of mechanical ventilation as a result of severe ICU-acquired weakness. She died on day 87 after she persisted in her wish to stop supportive treatment.
Table 1.
Patient characteristics
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Sex | female | female | female | female | female |
| Age at admission (years) | 50 | 32 | 45 | 55 | 21 |
| BMI (kg/m2) | 19 | 27 | 18 | 23 | 19 |
| Comorbidity | |||||
| Cardiovascular disease | aortic prosthesis (18 years prior) after aortitis | no | no | yes | no |
| Diabetes mellitus | no | no | No | no | no |
| Systemic hypertension | no | no | no | no | no |
| Pulmonary disease | bronchiectasis | no | no | bronchiectasis | no |
| Other | common variable immunodeficiency based on a NFKB1-mutation; splenectomy (25 years prior); primary sclerosing cholangitis; recurrent sinopulmonary infections | renal transplant (9 months before admission) | renal transplant (1 month before admission) | common variable immunodeficiency; splenomegaly; immune thrombocytopenic purpura; auto-immune hemolytic anemia; recurrent pulmonary infections | renal transplant (15 years before admission); Mayer Rokitansky Mullerian duct anomalies (MDA) type 1 GJA5 mutation |
| Immunosuppressive medication | none | alemtuzumab at the time of transplantation and 9 days prior to COVID-19 onset; maintenance immunosuppression with tacrolimus mycophenolate mofetil, and prednisone | rituximab, immunoadsorption, intravenous immune globulin, and basiliximab at the time of transplantation; maintenance immunosuppression with tacrolimus, mycophenolate mofetil, and prednisone | none | alemtuzumab 17 months before admission; maintenance immunosuppression with mycophenolate mofetil and prednisone |
| Time from COVID-19 symptom onset (days) | |||||
| to first hospital admission | 22 | 8 | 7 | 6 | 9 |
| to mechanical ventilation | 22 | 20 | 38 | 8 | 23 |
| COVID-19 immunomodulatory treatment | |||||
| Dexamethasone | yes | no | no | yes | yes |
| Prednisone | no | yes | yes | yes | yes |
| Tocilizumab | no | no | no | yes | yes |
| Outcome | deceased following patient-requested policy limitations | tracheostomy; extubated; discharged | extubated; discharged | weaning through tracheostomy; transferred to other hospital; extubated | extubated; discharged |
BMI, body mass index.
Figure 1.
Clinical course and SARS-CoV-2 viral loads
SARS-CoV-2 RNA was measured in bronchial secretions by routine PCR against the E gene, expressed as Ct-value. Interferon gamma was administered as 100 μg subcutaneously, thrice weekly. Convalescent plasma and C-IVIG were provided by the Dutch national blood supply Sanquin and were administered intravenously in doses of approximately 200 mL (plasma) and 50–100 mL (C-IVIG). Remdesivir was administered intravenously with a 200 μg loading dose, followed by daily doses of 100 μg for four days. Tocilizumab was administered intravenously in a dose of 400 mg. Filled circles indicate time points at which virus culture was performed. Blue circles represent samples that were virus culture positive; black circles are samples that were virus culture negative.
Patient 2
A 32-year-old woman using tacrolimus, mycophenolate mofetil, and prednisone because of a renal transplantation 9 months earlier, developed COVID-19 symptoms 9 days after alemtuzumab treatment of graft rejection. She was admitted with respiratory distress, transient renal insufficiency, and diarrhea another 8 days later. Mycophenolate mofetil was stopped that day and tacrolimus at day 21 after symptom onset. She was treated with remdesivir and convalescent plasma because of an absent SARS-CoV-2 antibody response. Mechanical ventilation was initiated when high-flow nasal oxygen therapy failed at day 20. She was treated for a suspected bacterial pulmonary superinfection at day 42 and remained severely lymphopenic, with high SARS-CoV-2 loads and no signs of clinical improvement. Interferon gamma and a second course of remdesivir were started at day 53. Viral loads subsequently declined and viral culture became negative. Although treated for bacteriemia with extended spectrum beta-lactamase Klebsiella pneumoniae at day 57 and day 90, she recovered with preserved renal function (eGFR CKD-EPI > 90 mL/min/1.73 m2) and started weaning from mechanical ventilation through a tracheostomy tube and was discharged at day 107. After discharge, the creatinine steadily increased, for which a kidney biopsy was performed on day 211, showing signs of an antibody-mediated rejection. It cannot be excluded that this is related to the discontinuation of the mycophenolate mofetil and tacrolimus and the treatment with interferon gamma during the hospital admission for COVID-19.
Patient 3
A 45-year-old woman was admitted 7 days after COVID-19 symptom onset. One month earlier, a blood-group-incompatible renal transplantation had been performed, for which she had received rituximab, immunoadsorption, intravenous immune globulin, and basiliximab. She used tacrolimus, mycophenolate mofetil, and prednisone as maintenance therapy, and immune suppression was adjusted during admission. She developed end-stage renal failure with thrombotic microangiopathy and tubular damage, but no signs of rejection on biopsy. Because of negative SARS-CoV-2 serology, she received convalescent plasma at day 12. High-flow nasal oxygen therapy could be stopped at day 20, and she was discharged at her own request despite persistent requirement for oxygen therapy. At day 25, she was re-admitted and at day 30, a suspected bacterial pneumonia was treated. On day 38, renal replacement therapy and mechanical ventilation were initiated, a second dose of convalescent plasma was administered, and remdesivir was started without clinical improvement. At day 42, the persistent lymphopenia, high SARS-CoV-2 load, and lack of clinical improvement prompted us to start interferon gamma, after which the viral load swiftly declined and respiratory function improved. Virus culture was negative before initiation of interferon gamma treatment and remained negative at other time points tested. She was extubated at day 53 and discharged at day 71. Circulating cytokines did not show signs of hyperinflammation in patients 1–3 (Figure S1).
Patient 4
A 55-year-old woman with common variable immunodeficiency was admitted 6 days after COVID-19 symptom onset and 17 days after receiving her first mRNA COVID-19 vaccination. Mechanical ventilation was started 8 days after COVID-19 symptom onset. She received tocilizumab and hyperimmune anti-COVID-19 intravenous immunoglobulin (C-IVIG) at day 8 and day 9, respectively. She developed portal hypertension and was treated with high-dose steroids for pulmonary fibrosis and with acyclovir for herpes simplex virus pneumonia. Because of persistent high SARS-CoV-2 loads, interferon gamma was initiated at day 23 after which the viral load declined. She received a second and third dose of C-IVIG at, respectively, day 30 and day 54, after symptom onset. Her respiratory function improved, and she started weaning through tracheostomy at day 41. Still weaning, she was transferred to another hospital on day 57.
Patient 5
A 21-year-old woman using mycophenolate mofetil and prednisone because of a renal transplantation 15 years earlier, and who received alemtuzumab treatment for graft rejection 17 months prior, developed COVID-19 symptoms 4 months after asymptomatic COVID-19 and 7 days after receiving her second mRNA COVID-19 vaccination. She was admitted with renal insufficiency, metabolic acidosis, and diarrhea 9 days after symptom onset, and she was discharged 2 days later. At 18 days after symptom onset, she was readmitted because of renal insufficiency, metabolic dysregulation, and diarrhea. Computed tomography (CT)-thorax showed signs of a pneumomediastinum, and she received low-flow oxygen therapy. Prednisone was switched for dexamethasone. Mycophenolate mofetil was stopped at 20 days after symptom onset, and tocilizumab was administered once. Mechanical ventilation was initiated when high-flow nasal oxygen therapy failed at day 23. Because of clinical deterioration and persistent high SARS-CoV-2 load, interferon gamma was started at day 23 and C-IVIG was administered at day 25. Viral loads declined and her respiratory function improved. She was extubated at day 34 and discharged at day 42. Her renal transplant status remained poor.
Discussion
We report five critically ill immunocompromised COVID-19 patients, with persistent failure of respiratory improvement accompanied by sustained high SARS-CoV-2 viral loads for a prolonged period of time, despite repeated convalescent plasma or C-IVIG administration and remdesivir as adjuvant therapy. The persistent high SARS-CoV-2 viral loads and infectious complications suggested severe immune dysfunction in these patients. Last-resort adjuvant treatment with interferon gamma was well tolerated, followed by a rapid decline in SARS-CoV-2 viral loads, and four patients have subsequently recovered.
Immunotherapeutic trials in COVID-19 have shown benefit of inhibition of exaggerated inflammation.2 However, no therapy is established to augment viral clearance, including convalescent plasma therapy that was safely used in small series of immunocompromised patients. Monoclonal neutralizing antibodies may have a role in seronegative hospitalized patients,8 but an effect on mortality in severe COVID-19 patients later in the course of disease has not yet been shown. Remdesivir has not demonstrated a clinical antiviral effect; thereafter, it is doubtful whether its administration in two of our patients contributed to viral clearance. In critically ill COVID-19 cases, type I interferon pathways are impaired,6 and although these mediators are likely important for viral clearance,4 subcutaneous interferon beta-1a was not associated with survival benefit.7 Because of the lack of effectivity of adjuvant type I interferons, and because type I interferons can have suppressive effects on Th function,9 we deemed administration of type I interferons undesirable in our patients, as Th1 immunity was already impaired in our patients as illustrated by their Aspergillus and herpes simplex virus infections.
Type II interferons are less well studied in COVID-19. Although circulating concentrations are not altered in COVID-19 patients, the interferon gamma production upon ex vivo stimulation is reduced,5 , 10 as is the interferon-gamma-responsive gene signature in critically ill COVID-19 patients.6 Interferon gamma immunotherapy has been applied previously in patients with pulmonary tuberculosis11 and pulmonary aspergillosis,12 but no published data were available for use in COVID-19. We considered application of adjuvant interferon gamma therapy a logical choice of last-resort therapy because of its potent immunostimulatory effects including on tissue macrophages that are likely important for COVID-19 immunity.1 In our patients, interferon gamma administration was indeed followed by viral clearance and clinical improvement in four of five patients. A theoretical adverse effect of interferon gamma administration could be the development of hyperinflammation in the spectrum of a macrophage activation syndrome or hemophagocytic lymphohistiocytosis in which pathophysiology interferon gamma is causally involved.13 Favorably though, follow-up samples showed stable concentrations of plasma C-reactive protein, serum ferritin (Table S1), as well as monocyte-derived proinflammatory cytokines involved in hyperinflammation such as interleukin-1β (IL-1β), IL-6, and IL-18. Only the lymphocyte-stimulating IL-12 showed an increase during interferon gamma treatment in patients 1–3, which might have contributed to viral clearance (Figure S1). No early or cell-mediated anti-allograft immunity was observed in the patients with a functioning renal allograft, but it cannot be excluded that the interferon gamma administration played a role in the antibody-mediated rejection that was later identified in patient 2. A series of seven renal transplant patients with invasive fungal infections treated with adjuvant interferon gamma showed that among the four patients with stable graft function before interferon gamma initiation, the graft function remained stable.12 Future studies on interferon gamma immunotherapy in COVID-19 could include patients with impaired cellular immunity caused by either immunosuppressive medication, primary immunodeficiencies, or malignancies. For patients with auto-immune or auto-inflammatory disorders, extra caution is warranted to monitor for signs of hyperinflammation.
We thus propose interferon gamma immunotherapy as a study candidate for adjuvant therapy in severely immunocompromised COVID-19 patients with persistent high SARS-CoV-2 loads and lack of clinical improvement.
Limitations of the study
Inherent to a case series, limitations of the study include the small sample size of five patients with the specific phenotype of high SARS-CoV-2 viral loads and impaired cellular immunity. Moreover, because interferon gamma was used off-label as a last-resort therapy instead of in a randomized setting, we cannot attribute the viral clearance with certainty to interferon gamma immunotherapy. Lastly, functional immunological data were not available for these patients.
Star★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bronchial secretion from COVID-19 patients | This paper | N/A |
| Critical commercial assays | ||
| Olink Explore 1536 platform | Olink Proteomics, Uppsala, Sweden | N/A |
| Experimental models: Cell lines | ||
| Vero clone E6 cells | ATCC | CRL-1586 |
| Oligonucleotides | ||
| SARS-CoV-2 E gene forward primer (E_Sarbeco_F): 5′-ACAGGTACGTTA ATAGTTAATAGCGT-3′ |
Wölfel et al.14 | N/A |
| SARS-CoV-2 E gene reverse primer (E_Sarbeco_R): 5′-ATATTGCAGCAGTACGCACACA-3′ | Wölfel et al.14 | N/A |
| Software and algorithms | ||
| GraphPad Prism 8.0.2 | GraphPad | N/A |
| Other | ||
| Immukine (Interferon Gamma) | Clinigen Healthcare Ltd | N/A |
Resource availability
Lead contact
Further information and requests should be directed to and will be fulfilled by the lead contact, Arjan van Laarhoven (Arjan.vanLaarhoven@radboudumc.nl).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Experimental model and subject details
Five female patients (age range 21-55), with impaired cellular immunity and severe COVID-19, have been described. Additional interventions and therapies provided to the patients have been discussed.
Ethics approval
Off-label interferon gamma immunotherapy was initiated by the responsible ICU-physician after multidisciplinary consultation, with consent from the patients’ relatives. Institutional ethic approval was therefore not sought.
Consent for publication
Written informed consent was obtained from the patients or relatives for publication of this case series and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Method details
Patients were admitted to the ICU department and received additional treatment before and during interferon gamma therapy as discussed. Interferon gamma (Immukine, Clinigen Healthcare Ltd) was administered as 100 μg subcutaneously, thrice weekly. During interferon gamma immunotherapy, bronchial secretion was collected every 48 hours for routine diagnostic SARS-CoV-2 RT-PCR using commercially available systems targeting the E gene, and in patient 1-3 for inoculation of Vero E6 cells using methods adapted from Wölfel et al.14 Bronchial secretion was diluted and mixed 1:1 in viral transport medium, of which 100 μl was used to infect Vero E6 cells, which were seeded in 24 well plates at a density of 2.5x105 cells/well. After 1 hour, the inoculum was replaced with 1 mL of Dulbecco’s Modified Eagle Medium (DMEM, GIBCO) containing 2% fetal bovine serum (Sigma), 100 μg/ml gentamycin (GIBCO), 2.5 μg/ml amphotericin-B (GIBCO), 100 μg/ml streptomycin and 100 U/ml penicillin (GIBCO) and culture medium was collected directly and at 48 hours and at 96 hours post infection for RNA isolation and RT-qPCR using primers targeting the E gene,14 as described previously.15 A positive viral culture was defined as increased levels of SARS-CoV-2 over time. Patients were monitored clinically for signs of hyperinflammation and inflammatory parameters were measured every 48 h. Circulating cytokines were measured in serum samples of patient 1-3 using the Olink Explore 1536 platform a multiplex proximity extension assay technique (Olink Proteomics, Uppsala, Sweden) and cytokines involved in hyperinflammation and T cell function were analyzed.
Quantification and statistical analysis
No statistical analyses were undertaken. GraphPad Prism version 8.0.2. was used for graphical analysis of data.
Acknowledgments
The authors thank Bart van den Bosch for SARS-CoV-2 PCR measurements and Dr. Maarten Cobussen for clinical support. A.v.L. and R.v.C. are supported by National Institutes of Health for project “Using tryptophan metabolism and response to corticosteroids to define new therapeutic targets for tuberculosis meningitis: Integration of large scale clinical, metabolomic, and genomic data” (R01AI145781). G.J.O. and R.P.v.R. are supported by a VICI grant (016.VICI.170.090) from the Dutch Research Council (NWO). W.F.A. is supported by Clinical Fellowship grant (90715610) of Netherlands Organization for Health Research and Development. M.G.N. is supported by an ERC advanced grant (833247) and a Spinoza grant of the Netherlands Organization for Scientific Research.
Author contributions
All authors contributed to the conception of the work and interpretation of the data. Clinical data were acquired by L.K. G.J.O. performed virus culture experiments under supervision of R.P.v.R. The paper was drafted by L.K., A.v.L., P.P., and M.G.N., and all authors have read and revised the paper and approved the submitted version and take full responsibility for its content. L.K. and A.v.L. had unrestricted access to all data.
Declaration of interests
The authors declare no competing interests.
Published: September 21, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.medj.2021.09.003.
Supplemental information
Data and code availability
This study did not generate any unique datasets or code.
References
- 1.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avanzato V.A., Matson M.J., Seifert S.N., Pryce R., Williamson B.N., Anzick S.L., Barbian K., Judson S.D., Fischer E.R., Martens C., et al. Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell. 2020;183:1901–1912.e9. doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen N.A.F., Grondman I., de Nooijer A.H., Boahen C.K., Koeken V.A.C.M., Matzaraki V., Kumar V., He X., Kox M., Koenen H.J.P.M., et al. Dysregulated Innate and Adaptive Immune Responses Discriminate Disease Severity in COVID-19. J. Infect. Dis. 2021;223:1322–1333. doi: 10.1093/infdis/jiab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., Abdool Karim Q., Alejandria M.M., Hernández García C., Kieny M.P., Malekzadeh R., et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group. Horby P.W., Mafham M., Peto, L, Campbell M., Pessoa-Amorim G., Spata E., Staplin N., Emberson J.R., Prudon B., et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. MedRxiv. 2021 doi: 10.1101/2021.06.15.21258542. [DOI] [Google Scholar]
- 9.McNab F., Mayer-Barber K., Sher A., Wack A., O’Garra A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rother N., Yanginlar C., Lindeboom R.G.H., Bekkering S., van Leent M.M.T., Buijsers B., Jonkman I., de Graaf M., Baltissen M., Lamers L.A., et al. Hydroxychloroquine Inhibits the Trained Innate Immune Response to Interferons. Cell Rep. Med. 2020;1:100146. doi: 10.1016/j.xcrm.2020.100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson R., Condos R., Tse D., Huie M.L., Ress S., Tseng C.-H., Brauns C., Weiden M., Hoshino Y., Bateman E., et al. Immunomodulation with Recombinant Interferon-γ1b in Pulmonary Tuberculosis. PLoS ONE. 2009;4:e6984. doi: 10.1371/journal.pone.0006984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong-James D., Teo I.A., Shrivastava S., Petrou M.A., Taube D., Dorling A., Shaunak S. Exogenous interferon-γ immunotherapy for invasive fungal infections in kidney transplant patients. Am. J. Transplant. 2010;10:1796–1803. doi: 10.1111/j.1600-6143.2010.03094.x. [DOI] [PubMed] [Google Scholar]
- 13.Brisse E., Wouters C.H., Matthys P. Hemophagocytic lymphohistiocytosis (HLH): A heterogeneous spectrum of cytokine-driven immune disorders. Cytokine Growth Factor Rev. 2015;26:263–280. doi: 10.1016/j.cytogfr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 15.Varghese F., van Woudenbergh E., Overheul G., Eleveld M., Kurver L., van Heerbeek N., van Laarhoven A., Miesen P., den Hartog G., de Jonge M., et al. Berberine and Obatoclax Inhibit SARS-Cov-2 Replication in Primary Human Nasal Epithelial Cells In Vitro. Viruses. 2021;13:282. doi: 10.3390/v13020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate any unique datasets or code.