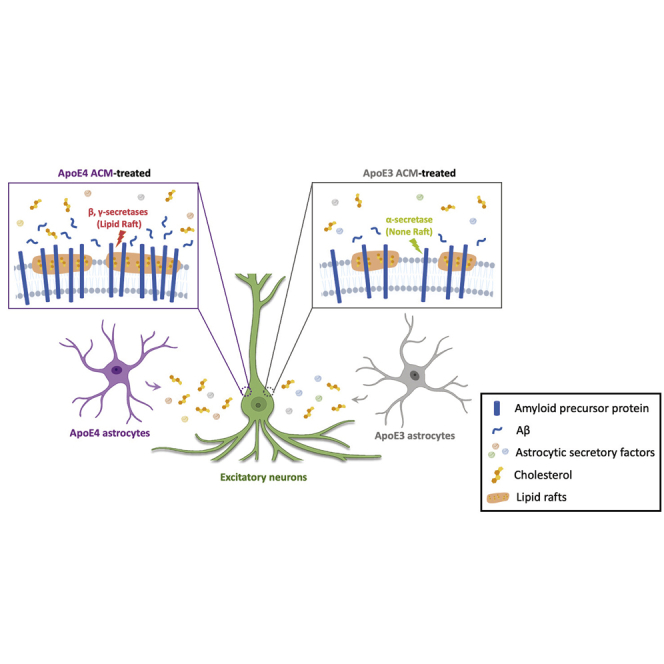
Summary
The ε4 allele of APOE-encoding apolipoprotein (ApoE) is one of the strongest genetic risk factors for Alzheimer’s disease (AD). One of the overarching questions is whether and how this astrocyte-enriched risk factor initiates AD-associated pathology in neurons such as amyloid-β (Aβ) accumulation. Here, we generate neurons and astrocytes from isogenic human induced pluripotent stem cells (hiPSCs) carrying either APOE ε3 or APOE ε4 allele and investigate the effect of astrocytic ApoE4 on neuronal Aβ production. Secretory factors in conditioned media from ApoE4 astrocytes significantly increased amyloid precursor protein (APP) levels and Aβ secretion in neurons. We further found that increased cholesterol secretion from ApoE4 astrocytes was necessary and sufficient to induce the formation of lipid rafts that potentially provide a physical platform for APP localization and facilitate its processing. Our study reveals the contribution of ApoE4 astrocytes to amyloidosis in neurons by expanding lipid rafts and facilitating Aβ production through an oversupply of cholesterol.
Keywords: Alzheimer's disease, human induced pluripotent stem cells, apolipoprotein E, cholesterol, lipid rafts
Graphical abstract
Highlights
-
•
ApoE4 ACM induces neuronal lipid raft expansion and Aβ42 overproduction
-
•
Co-localization of lipid rafts and APP increases in neurons by ApoE4 ACM
-
•
Increasing cholesterol in media recapitulates the effects of ApoE4 ACM on neurons
-
•
Cholesterol depletion in ApoE4 ACM abolishes its effects on neurons
In this report, Seo and colleagues use human iPSCs to generate isogenic neurons and astrocytes carrying either APOE ε3 or APOE ε4 allele and demonstrate that ApoE4 astrocytes secrete more cholesterol to the extracellular space, which is sufficient and necessary to induce lipid raft expansion and Aβ42 overproduction in neurons.
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative brain disorder that accounts for the majority of cases of dementia (2021 Alzheimer's Disease Facts and Figures, 2021). One of the major hallmarks of AD is the accumulation of amyloid-β (Aβ) in the brain (O'Brien and Wong, 2011). Aβ is a fragment peptide derived from amyloid precursor protein (APP), which is highly expressed in neurons and is known to be important for neuronal development and function (O'Brien and Wong, 2011). To maintain a homeostatic environment, generated Aβ is taken up by glial cells, such as astrocytes and microglia, to be degraded (Canter et al., 2016).
Genome-wide association studies have identified novel genetic risk factors associated with AD, even in late-onset AD (LOAD) cases. In contrast with genetic variants in familial cases, many LOAD-associated variants are located in genes known to be enriched in glial cells (Canter et al., 2016; Liu et al., 2017). The precise AD-related phenotypes induced by these variants, and the mechanisms by which they arise, remain to be elucidated.
APOE4 is one of the strongest genetic risk factors for LOAD (Liu et al., 2017). ApoE is an apolipoprotein encoded by the APOE gene and is well known for its function in lipid transport by the formation of lipoprotein complexes. In the central nervous system, ApoE is produced primarily by astrocytes, and its expression is upregulated in microglia under neurodegenerative conditions (Liu et al., 2017). There are three genotypes for APOE in humans, namely APOE2, APOE3, and APOE4. Each genotype produces proteins that are considered to have structural differences according to amino acid sequences at 112 and/or 158 (Liu et al., 2013). Although the difference in their sequence appears subtle, the translated proteins result in a significant difference in the risk for AD. While the ε2 allele is known to be protective, bearing ε4 increases the risk of AD (Liu et al., 2013).
Recent studies using human induced pluripotent stem cells (hiPSCs) from APOE4 carriers suggested that ApoE4 contributes to amyloidosis by increasing Aβ secretion in neurons and decreasing Aβ clearance in astrocytes (Lin et al., 2018; Wang et al., 2018). However, the involvement and mechanisms by which ApoE4 astrocytes contribute to neuronal Aβ production remain to be determined. Here, using hiPSC-derived astrocytes and neurons carrying APOE3 or APOE4, we aimed to investigate whether and how ApoE4 astrocytes regulate neuronal Aβ production.
Results
ApoE4 ACM increased APP expression and Aβ42 secretion in hiPSC-derived neurons
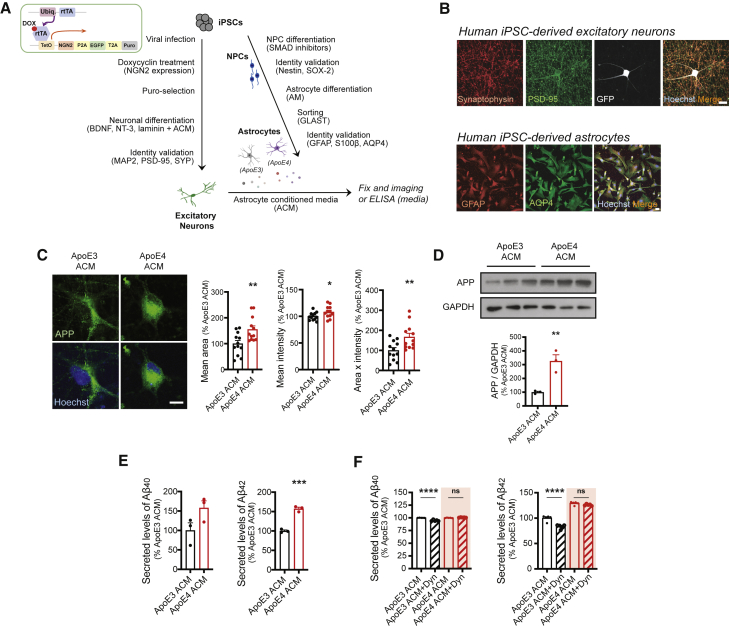
To address the effect of ApoE4 astrocytes on neuronal APP expression and Aβ generation, we utilized an iPSC line derived from healthy individuals carrying the APOE3 allele and its isogenic line in which APOE3 is converted to APOE4 (Lin et al., 2018). Both iPSC lines were differentiated into astrocytes or excitatory neurons (Figure 1A), and their identity was confirmed by immunostaining (Figure 1B). To investigate whether secretory factors from ApoE4 astrocytes could affect neuronal APP expression and its processing to Aβ, we cultured ApoE3 neurons in astrocyte conditioned medium (ACM) from other healthy iPSC-derived astrocytes (APOE3/E4 heterozygote) for 5 weeks, after which the medium was replaced with either ApoE3 or ApoE4 ACM for 4 days (Figure 1A). Immunostaining and immunoblotting analyses in neurons revealed that APP levels were significantly increased by ApoE4 ACM (Figures 1C and 1D). We then directly measured the secreted levels of Aβ40 and Aβ42 and found that only Aβ42 secretion was significantly increased by ApoE4 ACM. These data show that secretory factors from ApoE4 astrocytes positively regulate neuronal APP expression and Aβ secretion. We further investigated whether increased APP processing to Aβ42 occurs through facilitated clathrin-dependent endocytosis, which was shown to be required for activity-dependent APP processing (Das et al., 2013). We treated neurons with Dynasore, which inhibits dynamin activity and thereby abolishes clathrin-mediated endocytosis. Neurons cultured with ApoE3 ACM showed a reduction of Aβ40 and Aβ42; however, elevated levels of Aβ42 in neurons cultured with ApoE4 ACM were not affected by Dynasore treatment. These data suggest that ApoE4 ACM-induced Aβ42 upregulation is mediated by clathrin-independent mechanisms (Figure 1F).
Figure 1.
ApoE4 astrocyte conditioned medium increases amyloid precursor protein expression and Aβ secretion in hiPSC-derived neurons
(A) Schematics for generating astrocytes and excitatory neurons from hiPSCs, and experimental procedure.
(B) Images of hiPSC-derived neurons with synaptic markers, synaptophysin and PSD-95, and hiPSC-derived astrocytes with astrocytic markers, GFAP and AQP4. Scale bars, 10 μm.
(C) Images of amyloid precursor protein (APP) staining from astrocyte conditioned medium (ACM)-treated neurons. Scale bar, 10 μm. Right: APP area, intensity, and area × intensity in neurons (n = 12 images from three experiments).
(D) Western blotting for APP in neurons. Bottom: levels of APP were normalized to GAPDH expression (n = 3 experiments).
(E and F) Levels of secreted Aβ40 (E) and Aβ42 (F) from neurons detected by ELISA (n = 3 experiments for E, n = 5 experiments for F).
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant (Student's t test). Error bars represent SEM.
Extracellular cholesterol positively regulated the formation of lipid rafts and APP expression in hiPSC-derived neurons
Accumulation of intracellular cholesterol in hiPSC-derived ApoE4 astrocytes compared with isogenic ApoE3 astrocytes has been recently reported (Lin et al., 2018; Tcw et al., 2019), and Lin et al. further reported increased cholesterol secretion from ApoE4 astrocytes. Astrocytes supply cholesterol to neurons to support synapse formation and regulate membrane fluidity (Vance, 2012). Moreover, cholesterol, along with ganglioside and triglyceride, is a critical component of membrane lipid rafts, which provide a suitable platform for various membrane-bound proteins including glutamate receptors. APP and its processing secretases, β- and γ-secretase, are also known to be located in lipid rafts, while α-secretase is mainly expressed in non-lipid rafts (Cheng et al., 2007; Raffai and Weisgraber, 2003).
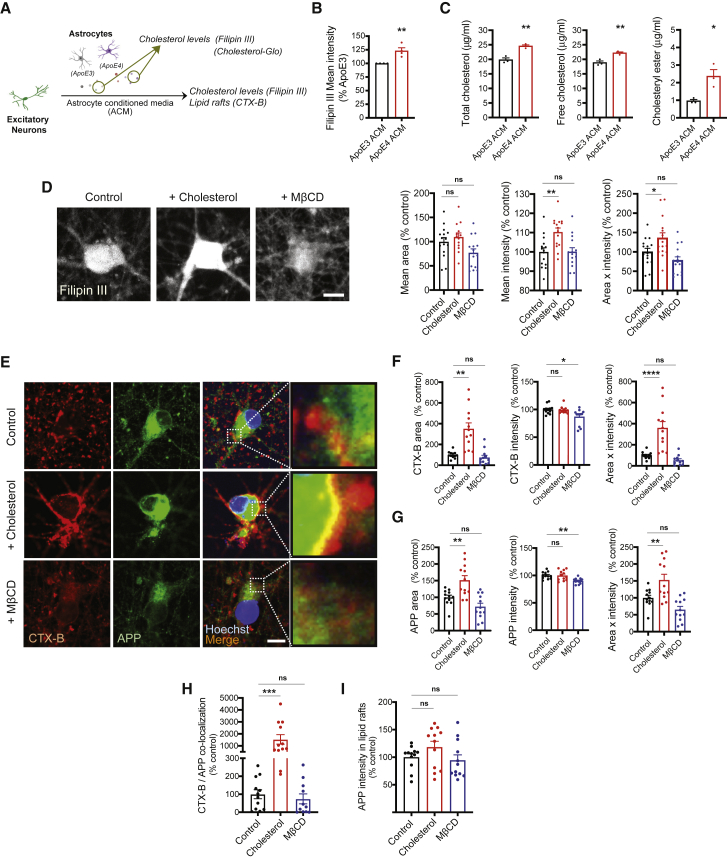
Previous studies have shown that increasing cholesterol in the membrane induced the formation of lipid rafts and increased Aβ production (Cossec et al., 2010; Marquer et al., 2014). Therefore, we hypothesized that increased cholesterol in ApoE4 ACM could be a key factor in upregulating APP and its processing by facilitating the formation of lipid rafts. First, we verified increased cholesterol secretion from ApoE4 astrocytes by measuring the signals of filipin III, which is naturally fluorescent upon cholesterol binding (Figures 2A and 2B) in ACM. We further measured exact levels of total cholesterol as well as its composition (free cholesterol and cholesteryl ester) in ApoE3 and ApoE4 ACM (Figure 2C). We repeatedly observed increased levels of total cholesterol (about 5 μg/mL higher) in ApoE4 ACM compared with ApoE3 ACM. Although free cholesterol is dominant in ACM, we also observed increased levels of cholesteryl ester in ApoE4 ACM, which could induce neuronal dysfunction as reported recently (van der Kant et al., 2019). To address whether such an increase of cholesterol is sufficient to promote APP expression and its processing in neurons, we next treated neurons with 20 μM cholesterol (about 7.6 5 μg/mL). We found that cholesterol treatment for 4 days was sufficient to increase neuronal cholesterol visualized by filipin III staining (Figure 2D). To directly deplete cholesterol in ACM, we applied methyl β-cyclodextrin (MβCD). We did not observe any alterations in cholesterol levels in neurons treated with MβCD-containing control ACM, suggesting minimal effects of cholesterol depletion in control ACM and/or the homeostatic mechanism of neurons to compensate for the possible loss of their cholesterol by MβCD. We then determined whether increased cholesterol induces lipid raft expansion in neurons. We accessed the levels of lipid rafts in neurons treated with cholesterol or MβCD by measuring the area or intensity of cholera toxin B (CTX-B), a previously reported well-known lipid raft marker (Lin et al., 2019), and found that cholesterol treatment increased the area of CTX-B signals without affecting intensity, suggesting the expansion of lipid rafts (Figures 2E and 2F). We also measured APP levels in neurons and found significant upregulation of APP by cholesterol treatment, due to increased area of APP signals rather than intensity (Figures 2E and 2G). We further found that the co-localization of APP and CTX-B was significantly increased by cholesterol treatment in neurons (Figures 2E and 2H). To determine whether APP upregulation is caused simply by the expansion of lipid rafts or whether more APP is recruited to the given area of lipid rafts, we measured the intensity of APP in the CTX-B/APP co-localized area. The data showed that there was no alteration in APP intensity in these regions (Figure 2I), suggesting that increased APP expression by extracellular cholesterol supply is mainly due to the increased area of lipid rafts. There was a slight reduction of lipid rafts and APP levels following MβCD treatment, a finding that also supports the regulatory role of cholesterol in neuronal lipid raft formation and APP expression.
Figure 2.
Extracellular cholesterol positively regulates the formation of lipid rafts and its association with APP in hiPSC-derived neurons
(A) Measurement of secretory levels of cholesterols in ApoE3 or ApoE4 ACM.
(B) Filipin III signals in ACM (n = 4 experiments).
(C) Levels of total cholesterol, free cholesterol, and cholesteryl ester in ACM (n = 3 experiments).
(D) Images of filipin III staining in neurons. Scale bar, 10 μm. Right: quantification of filipin III area, intensity, and area × intensity in neurons (n = 13–15 images from three experiments).
(E) Images of CTX-B and APP staining in neurons. Scale bar, 10 μm.
(F) CTX-B area, intensity, and area × intensity in neurons (n = 12 images from three experiments).
(G) APP area, intensity, and area × intensity in neurons (n = 12 images from three experiments).
(H) Co-localization of CTX-B and APP in neurons (n = 12 images from three experiments).
(I) APP intensity in CTX-B/APP co-localization area in neurons (n = 12 images from three experiments).
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant (Student's t test or ANOVA followed by Dunnett's post hoc test). Error bars represent SEM.
Cholesterol in ApoE4 ACM is required to increase the formation of lipid rafts in hiPSC-derived neurons
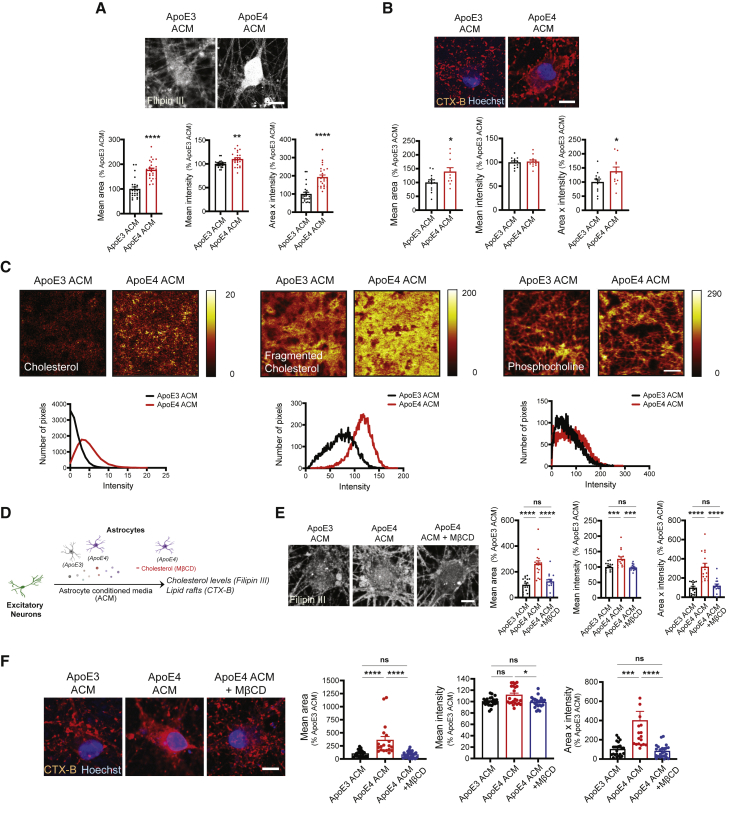
We next investigated whether the effects of ApoE4 ACM on neuronal cholesterol levels and lipid raft formation were due to cholesterol oversupply. We measured levels of filipin III in neurons cultured with ACM from either ApoE3 or ApoE4 astrocytes as described in Figure 1A, whereby ApoE4 ACM-treated neurons displayed increased filipin III signals (Figure 3A) as shown in neurons treated with cholesterol (Figures 2D and 2E). We also found that the area and total levels (area × intensity) of CTX-B were significantly increased in these neurons compared with those of ApoE3 ACM-treated neurons (Figure 3B). To directly measure cholesterol on the plasma membrane that is enriched in lipid rafts in a label-free way, we utilized time-of-flight secondary ion mass spectrometry (TOF-SIMS) imaging. We observed increased cholesterol and fragmented cholesterol levels in ApoE4 ACM-treated neurons compared with neurons with ApoE3 ACM, whereas phosphocholine, another metabolite in the plasma membrane, was not distinguishable between the two groups (Figure 3C). To determine whether the cholesterol in ApoE4 ACM is the major cause of the expansion of lipid rafts in neurons, we added MβCD to ApoE4 ACM during neuronal culture (Figure 3D) and found that the upregulation of neuronal cholesterol by ApoE4 ACM was significantly attenuated by MβCD, potentially due to its scavenging effect toward exogenous cholesterol (Figure 3E). The addition of MβCD to ApoE4 ACM also completely abolished the ApoE4 ACM-induced lipid raft expansion in neurons (Figure 3F).
Figure 3.
Cholesterol in ApoE4 ACM is required to increase the formation of lipid rafts in hiPSC-derived neurons
(A) Images of filipin III staining in neurons. Scale bar, 10 μm. Bottom: filipin III area, intensity, and area × intensity in neurons (n = 25 images from five experiments).
(B) Images of CTX-B staining in neurons. Scale bar, 10 μm. Bottom: CTX-B area, intensity, and area × intensity in neurons (n = 12 images from three experiments).
(C) TOF-SIMS imaging for membrane surface cholesterol, fragmented cholesterol, and phosphocholine in neurons. Scale bar, 50 μm. Bottom: relative intensity of cholesterol, fragmented cholesterol, and phosphocholine in each pixel of images.
(D) Experimental procedure to examine the effect of cholesterol or MβCD treatment on neurons.
(E) Images of filipin III staining in neurons. Scale bar, 10 μm. Right: filipin III area, intensity, and area × intensity in neurons (n = 15–16 images from four experiments).
(F) Images of CTX-B staining in neurons. Scale bar, 10 μm. Right: CTX-B area, intensity, and area × intensity in neurons (n = 15–16 images from four experiments).
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant (Student's t test or ANOVA followed by Tukey's post hoc test). Error bars represent SEM.
Cholesterol in ApoE4 ACM is required to induce APP upregulation and Aβ42 secretion in hiPSC-derived neurons
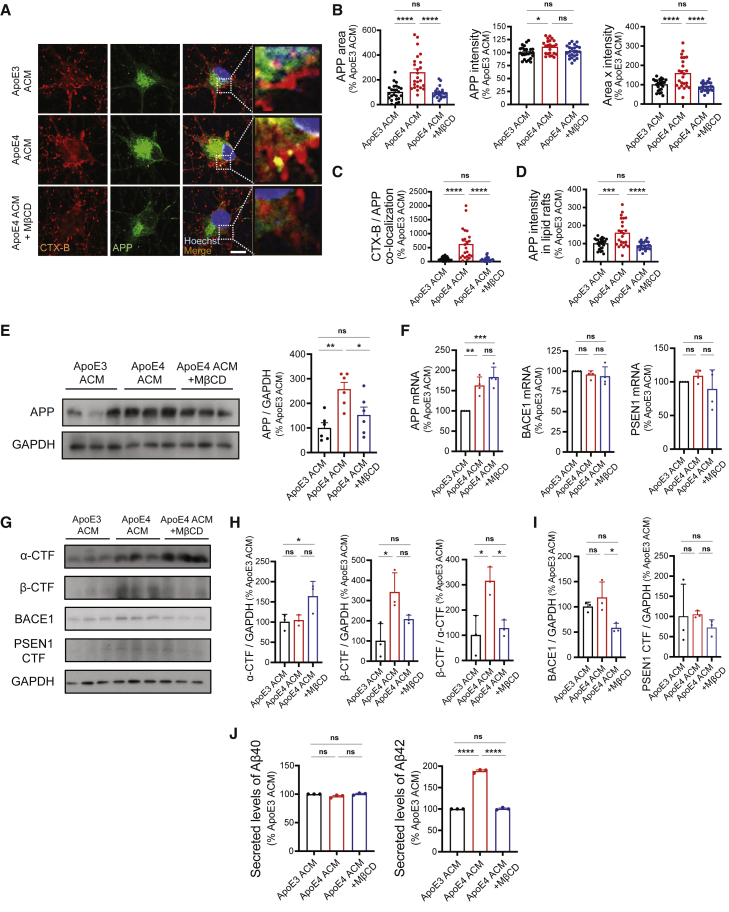
To determine whether cholesterol in ApoE4 ACM is necessary for the upregulation of APP and its metabolism to produce Aβ in neurons, we examined APP expression in neurons cultured with ApoE3 ACM, ApoE4 ACM, or MβCD-included ApoE4 ACM (Figure 3D). As shown in Figure 1C, neurons cultured with ApoE4 ACM showed increased expression of APP compared with those cultured with ApoE3 ACM (Figures 4A and 4B). However, in the presence of MβCD, ApoE4 ACM was not able to induce significant upregulation of APP (Figures 4A, 4B, and 4E). Increased co-localization of lipid rafts and APP by ApoE4 ACM was also abolished in neurons treated with MβCD (Figures 4A, 4C, and 4D). We examined whether APP processing was altered by ApoE4 ACM preferentially to Aβ generation as shown in Figure 1F. First, we found that ApoE4 ACM increases APP transcription without affecting the levels of BACE1 and PSEN1 transcripts (Figure 4F). Unlike the protein levels of APP, this was not inhibited by MβCD treatment. Although inhibition of cholesterol was sufficient to prevent APP protein upregulation (Figure 4E), these data suggest that secretory factors other than cholesterol from ApoE4 ACM or ApoE4 itself as shown by Huang et al. (2017) could increase APP transcription. We then examined the levels of APP C-terminal fragments (CTFs) and observed increased generation of β-CTF over α-CTF by ApoE4 ACM, which was completely abolished by MβCD (Figures 4G and 4H). The levels of BACE1 and PSEN1 CTF were not altered by ApoE4 ACM (Figures 4G and 4I). Finally, we observed that MβCD treatment inhibited the ApoE4 ACM-induced increase in Aβ42 secretion in neurons (Figure 4F). Taken together, these data suggest that an excess supply of cholesterol is responsible for ApoE4 ACM-mediated amyloidogenic processing of APP.
Figure 4.
Cholesterol in ApoE4 ACM is required to induce APP upregulation and Aβ42 secretion in hiPSC-derived neurons
(A) Images of CTX-B and APP staining in neurons. Scale bar, 10 μm.
(B) APP area, intensity, and area × intensity in neurons (n = 23–24 images from six experiments).
(C) Co-localization of CTX-B and APP in neurons (n = 23–24 images from six experiments).
(D) APP intensity in CTX-B/APP co-localization area in neurons (n = 23–24 images from six experiments).
(E) Western blotting for APP in neurons. Right: levels of APP were normalized to GAPDH expression in neurons (n = 6 experiments).
(F) Levels of APP, BACE1, and PSEN1 mRNA in neurons (n = 3 experiments).
(G) Western blotting for α-CTF and β-CTF of APP, BACE1, and PSEN1 in neurons.
(H) Levels of α-CTF and β-CTF of APP (normalized to GAPDH expression), and the ratio of β-CTF/α-CTF in neurons (n = 3 experiments).
(I) Levels of BACE1 and PSEN1 were normalized to GAPDH expression in neurons (n = 3 experiments).
(J) Levels of secreted Aβ40 and Aβ42 from neurons detected by ELISA (n = 3 experiments).
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant (ANOVA followed by Tukey's post hoc test). Error bars represent SEM.
Discussion
Abnormal neuronal cholesterol levels have been linked to AD-related pathology both in vitro and in vivo. For example, increased levels of cholesterol were observed in AD brain samples, and the severity of pathology was correlated with cholesterol levels (Cutler et al., 2004; Lazar et al., 2013). Inhibition of cholesterol efflux by reducing the expression of CYP46A1, a cholesterol 24S-hydroxylase, in a neuron-specific manner was shown to result in cognitive deficits and neuronal death in wild-type mice. The study further showed the recruitment of APP to lipid rafts and Aβ upregulation before neuronal death in both wild-type and APP23 mice (Djelti et al., 2015). Here, we revealed the contribution of secretory cholesterol from ApoE4 astrocytes to lipid raft expansion and APP expression, which promotes Aβ42 secretion in neurons.
A recent study showed that Chinese hamster ovary cell lines expressing familial AD-associated PSEN1 ΔE9 display increased levels of cholesterol, which leads to the enrichment of APP in lipid rafts (Cho et al., 2019). Similarly, a previous study suggested binding between cholesterol and β-CTF of APP (Beel et al., 2008). These data propose the active role of cholesterol in recruiting APP to lipid rafts. Here, we found that cholesterol oversupply by ApoE4 astrocytes increases APP levels in lipid rafts. Although we did not find a difference in APP intensity (local clustering density) in lipid rafts by extracellular cholesterol supply (Figure 2I), neurons cultured in ApoE4 ACM displayed increased APP intensity in lipid rafts, which was abolished by MβCD treatment (Figure 4D). Cholesterol was shown to increase the proximity between APP and BACE1 in the membrane (Marquer et al., 2011). Although we did not observe change in BACE1 and PSEN1 expression, further studies are required to determine the expression pattern of β- and γ-secretases on lipid rafts in neurons when they are cultured with ApoE3 or ApoE4 ACM.
Cholesterol was also shown to increase Aβ production through the facilitation of APP endocytosis to endosomes (Cossec et al., 2010). Moreover, cholesterol loading to the neuronal plasma membrane was shown to result in enlarged endosomes (Marquer et al., 2014). In the current study, we revealed for the first time the impact of ApoE4 astrocytes on neuronal cholesterol and lipid rafts that affect APP processing toward Aβ production. Additionally, its effect was not attenuated by inhibition of clathrin-mediated endocytosis. Further studies are required to determine whether astrocytic cholesterol promotes APP processing on lipid rafts or by facilitating raft-dependent APP endocytosis.
Lipid rafts provide a platform for not only APP but also multiple neuronal membrane proteins that are important for synaptic functions (Hering et al., 2003). Previously, cholesterol was shown to promote synapse maturation, whereas depletion of cholesterol significantly reduced the lipid raft domain and synapses (Hering et al., 2003; Mauch et al., 2001). The positive correlation between neuronal activity and Aβ production has been supported by multiple studies (Bero et al., 2011; Das et al., 2013), so it is possible that the expansion of lipid rafts by ApoE4 ACM could also contribute to the upregulation of Aβ by increasing neuronal activity.
APOE4, the strongest genetic risk factor for AD, has recently been shown to have detrimental effects on astrocytes, including endocytic defects and impaired homeostatic functions (Fernandez et al., 2019; Narayan et al., 2020). It is not clear, however, whether and how altered astrocytic properties could affect neighboring neurons and induce AD-associated pathology. Here, we revealed that ApoE4 astrocytes could regulate neuronal APP metabolism to induce amyloidosis through cholesterol oversupply. This study provides new insight into the contribution of ApoE4 and astrocytes to amyloidosis in AD, as well as the importance of regulating astrocytic APOE isotypes and its cholesterol oversupply for disease intervention.
Experimental procedures
A detailed description of all materials and methods is presented in supplemental experimental procedures.
iPSC culture
The use of human iPSCs was approved by the Institutional Review Board of DGIST (Permit Number: DGIST-190829-BR-071-01). The ApoE3 iPSC line was generated from the Coriell Institute's fibroblast line derived from a healthy individual (age 75 years, female; #AG09173) by Dr. Yankner’s Laboratory at Harvard Medical School (Meyer et al., 2019). The ApoE4 isogenic line was generated by CRISPR/Cas9 genome editing as previously described (Lin et al., 2018). For ApoE3/E4 heterozygous ACM, an iPSC line derived from a healthy individual (age 22 years, female; #GM23720) was obtained from the Coriell Institute. iPSCs were maintained on Matrigel (Corning #354277)-coated plate in mTeSR1 medium (STEMCELL Technologies) at 37°C with 5% CO2-conditioned incubator. A detailed description of differentiation into neural progenitor cells, astrocytes, or neurons is presented in supplemental experimental procedures.
Statistical analysis
Prism 8 (GraphPad) was used for statistical analysis. Unpaired Student's t test or one-way ANOVA test with Tukey's or Dunnett's post hoc analysis was used.
Author contributions
S.-I.L. performed most of the biochemical experiments and analyzed data. W.J. set up the protocols for biochemical experiments and performed pilot experiments. S.-I.L. and H. Lim performed TOF-SIMS imaging and analyzed data. S.C. and H. Lee generated and cultured iPSC-derived astrocytes. Y.J. generated and cultured iPSC-derived neurons. J.C. performed immunocytochemistry. Y.-T.L., L-H.T., and D.W.M. provided resources. S.-I.L., W.J., and J.S. conceptualized the project. S.-I.L., W.J., S.C., S.B., and J.S. wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
We thank all members of the Seo lab for fruitful advice and discussions. This work was supported by the NIH grant (NIA RF1AG062377) to L.-H.T., the National Research Foundation of Korea grants funded by the Ministry of Science, ICT (2021R1C1C2010928 to H.L., 2021R1A2C1008704 to D.W.M., 2018M3C7A1056275, 2019R1C1C1008591 to J.S.), and the POSCO Science Fellowship of POSCO TJ Park Foundation to J.S.
Published: August 26, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2021.07.017.
Supplemental information
References
- 2021 Alzheimer's Disease Facts and Figures 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- Beel A.J., Mobley C.K., Kim H.J., Tian F., Hadziselimovic A., Jap B., Prestegard J.H., Sanders C.R. Structural studies of the transmembrane C-terminal domain of the amyloid precursor protein (APP): does APP function as a cholesterol sensor? Biochemistry. 2008;47:9428–9446. doi: 10.1021/bi800993c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero A.W., Yan P., Roh J.H., Cirrito J.R., Stewart F.R., Raichle M.E., Lee J.-M., Holtzman D.M. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canter R.G., Penney J., Tsai L.-H. The road to restoring neural circuits for the treatment of Alzheimer's disease. Nature. 2016;539:187–196. doi: 10.1038/nature20412. [DOI] [PubMed] [Google Scholar]
- Cheng H., Vetrivel K.S., Gong P., Meckler X., Parent A., Thinakaran G. Mechanisms of disease: new therapeutic strategies for Alzheimer's disease--targeting APP processing in lipid rafts. Nat. Clin. Pract. Neurol. 2007;3:374–382. doi: 10.1038/ncpneuro0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.Y., Kwon O.-H., Park M.K., Kim T.-W., Chung S. Elevated cellular cholesterol in Familial Alzheimer's presenilin 1 mutation is associated with lipid raft localization of β-amyloid precursor protein. PLoS One. 2019;14:e0210535. doi: 10.1371/journal.pone.0210535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossec J.-C., Simon A., Marquer C., Moldrich R.X., Leterrier C., Rossier J., Duyckaerts C., Lenkei Z., Potier M.-C. Clathrin-dependent APP endocytosis and Aβ secretion are highly sensitive to the level of plasma membrane cholesterol. Biochim. Biophys. Acta. 2010;1801:846–852. doi: 10.1016/j.bbalip.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Cutler R.G., Kelly J., Storie K., Pedersen W.A., Tammara A., Hatanpaa K., Troncoso J.C., Mattson M.P. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc. Natl. Acad. Sci. U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U., Scott D.A., Ganguly A., Koo E.H., Tang Y., Roy S. Activity-induced convergenceof APP and BACE-1 in acidic microdomains via an endocytosis-dependent pathway. Neuron. 2013;79:447–460. doi: 10.1016/j.neuron.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djelti F., Braudeau J., Hudry E., Dhenain M., Varin J., Bièche I., Marquer C., Chali F., Ayciriex S., Auzeil N., et al. CYP46A1 inhibition, brain cholesterol accumulation and neurodegeneration pave the way for Alzheimer's disease. Brain. 2015;138:2383–2398. doi: 10.1093/brain/awv166. [DOI] [PubMed] [Google Scholar]
- Fernandez C.G., Hamby M.E., McReynolds M.L., Ray W.J. The role of APOE4 in disrupting the homeostatic functions of astrocytes and microglia in aging and Alzheimer's disease. Front. Aging Neurosci. 2019;11:14. doi: 10.3389/fnagi.2019.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H., Lin C.-C., Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J. Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.W.A., Zhou B., Wernig W., Südhof T. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell. 2017;168:427–441.e21. doi: 10.1016/j.cell.2016.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar A.N., Bich C., Panchal M., Desbenoit N., Petit V.W., Touboul D., Dauphinot L., Marquer C., Laprevote O., Brunelle A., Duyckaerts C. Time-of-flight secondary ion mass spectrometry (TOF-SIMS) imaging reveals cholesterol overload in the cerebral cortex of Alzheimer disease patients. Acta Neuropathol. 2013;125:133–144. doi: 10.1007/s00401-012-1041-1. [DOI] [PubMed] [Google Scholar]
- Lin H.J., Jiang Z.P., Lo H.R., Feng C.L., Chen C.J., Yang C.Y., Huang M.Z., Wu H.Y., Chen Y.A., Chen Y., et al. Coalescence of RAGE in lipid rafts in response to cytolethal distending toxin-induced inflammation. Front. Immunol. 2019;10:109. doi: 10.3389/fimmu.2019.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-T., Seo J., Gao F., Feldman H.M., Wen H.-L., Penney J., Cam H.P., Gjoneska E., Raja W.K., Cheng J., et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer's disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98:1141–1154.e7. doi: 10.1016/j.neuron.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-C., Liu C.-C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-C., Zhao N., Fu Y., Wang N., Linares C., Tsai C.-W., Bu G. ApoE4 accelerates early seeding of amyloid pathology. Neuron. 2017;96:1024–1032.e3. doi: 10.1016/j.neuron.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquer C., Devauges V., Cossec J.-C., Liot G., Lécart S., Saudou F., Duyckaerts C., Lévêque-Fort S., Potier M.-C. Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. FASEB J. 2011;25:1295–1305. doi: 10.1096/fj.10-168633. [DOI] [PubMed] [Google Scholar]
- Marquer C., Laine J., Dauphinot L., Hanbouch L., Lemercier-Neuillet C., Pierrot N., Bossers K., Le M., Corlier F., Benstaali C., et al. Increasing membrane cholesterol of neurons in culture recapitulates Alzheimer’s disease early phenotypes. Mol. Neurodegener. 2014;9:60. doi: 10.1186/1750-1326-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch D.H., Nägler K., Schumacher S., Göritz C., Müller E.C., Otto A., Pfrieger F.W. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Meyer K., Feldman H.M., Lu T., Drake D., Lim E.T., Ling K.-H., Bishop N.A., Pan Y., Seo J., Lin Y.-T., et al. REST and neural gene network dysregulation in iPSC models of Alzheimer's disease. Cell Rep. 2019;26:1112–1127.e9. doi: 10.1016/j.celrep.2019.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P., Sienski G., Bonner J.M., Lin Y.-T., Seo J., Baru V., Haque A., Milo B., Akay L.A., Graziosi A., et al. PICALM rescues endocytic defects caused by the Alzheimer's disease risk factor APOE4. Cell Rep. 2020;33:108224. doi: 10.1016/j.celrep.2020.108224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R.J., Wong P.C. Amyloid precursor protein processing and Alzheimer's disease. Annu. Rev. Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffai R.L., Weisgraber K.H. Cholesterol: from heart attacks to Alzheimer's disease. J. Lipid Res. 2003;44:1423–1430. doi: 10.1194/jlr.R300007-JLR200. [DOI] [PubMed] [Google Scholar]
- Tcw J., Liang S.A., Qian L., Pipalia N.H., Chao M.J., Shi Y., Bertelsen S.E., Kapoor M., Marcora E., Sikora E., et al. Cholesterol and matrisome pathways dysregulated in human APOE ε4 glia. bioRxiv. 2019 doi: 10.1101/713362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance J.E. Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis. Model. Mech. 2012;5:746–755. doi: 10.1242/dmm.010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kant R., Langness V.F., Herrera C.M., Williams D.A., Fong L.K., Leestemaker Y., Steenvoorden E., Rynearson K.D., Brouwers J.F., Helms J.B., et al. Cholesterol metabolism is a druggable axis that independently regulates tau and amyloid-β in iPSC-derived Alzheimer’s disease neurons. Cell Stem Cell. 2019;24:363–375. doi: 10.1016/j.stem.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Najm R., Xu Q., Jeong D.-E., Walker D., Balestra M.E., Yoon S.Y., Yuan H., Li G., Miller Z.A., et al. Gain of toxic apolipoprotein E4 effects in human iPSC-derived neurons is ameliorated by a small-molecule structure corrector. Nat. Med. 2018;24:647–657. doi: 10.1038/s41591-018-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.