Abstract
Objectives
To investigate the association of pericoronary adipose tissue mean attenuation (PCATMA) with coronary artery disease (CAD) characteristics on coronary computed tomography angiography (CCTA).
Methods
We retrospectively investigated 165 symptomatic patients who underwent third-generation dual-source CCTA at 70kVp: 93 with and 72 without CAD (204 arteries with plaque, 291 without plaque). CCTA was evaluated for presence and characteristics of CAD per artery. PCATMA was measured proximally and across the most severe stenosis. Patient-level, proximal PCATMA was defined as the mean of the proximal PCATMA of the three main coronary arteries. Analyses were performed on patient and vessel level.
Results
Mean proximal PCATMA was −96.2 ± 7.1 HU and −95.6 ± 7.8HU for patients with and without CAD (p = 0.644). In arteries with plaque, proximal and lesion-specific PCATMA was similar (−96.1 ± 9.6 HU, −95.9 ± 11.2 HU, p = 0.608). Lesion-specific PCATMA of arteries with plaque (−94.7 HU) differed from proximal PCATMA of arteries without plaque (−97.2 HU, p = 0.015). Minimal stenosis showed higher lesion-specific PCATMA (−94.0 HU) than severe stenosis (−98.5 HU, p = 0.030). Lesion-specific PCATMA of non-calcified, mixed, and calcified plaque was −96.5 HU, −94.6 HU, and −89.9 HU (p = 0.004). Vessel-based total plaque, lipid-rich necrotic core, and calcified plaque burden showed a very weak to moderate correlation with proximal PCATMA.
Conclusions
Lesion-specific PCATMA was higher in arteries with plaque than proximal PCATMA in arteries without plaque. Lesion-specific PCATMA was higher in non-calcified and mixed plaques compared to calcified plaques, and in minimal stenosis compared to severe; proximal PCATMA did not show these relationships. This suggests that lesion-specific PCATMA is related to plaque development and vulnerability.
Key Points
• In symptomatic patients undergoing CCTA at 70 kVp, PCATMA was higher in coronary arteries with plaque than those without plaque.
• PCATMA was higher for non-calcified and mixed plaques compared to calcified plaques, and for minimal stenosis compared to severe stenosis.
• In contrast to PCATMA measurement of the proximal vessels, lesion-specific PCATMA showed clear relationships with plaque presence and stenosis degree.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00330-021-07882-1.
Keywords: Computed tomography angiography, Atherosclerosis, Adipose tissue, Coronary arteries
Introduction
Coronary inflammation plays an important role in atherosclerosis development [1–3]. Detection and quantification of coronary inflammation could assist in early risk stratification of coronary artery disease (CAD) patients, possibly even before the development of coronary plaque [4]. Recently, a non-invasive biomarker for coronary inflammation was proposed: computed tomography angiography (CCTA) derived pericoronary adipose tissue mean attenuation (PCATMA) [5]. PCATMA has shown value as a predictor for cardiac mortality [6]. Few studies, predominantly using the proximal right coronary artery (RCA) as a representative location for patient-level analysis, have shown a relationship of PCATMA with CAD and atherosclerosis progression [5, 7–9].
CCTA-based plaque composition and stenosis severity give information about plaque vulnerability and hemodynamic significance, and can be used for prognostication [10–13]. A previous study showed a PCATMA difference of 3–4HU in the proximal RCA between CAD and non-CAD patients [5]. However, they found no significant difference of RCA-based PCATMA between non-calcified plaques (NCP) and mixed or calcified plaques (CP) in patients with high plaque burden. Another study demonstrated that increased NCP and total plaque burden were associated with higher PCATMA [8].
Most studies measured PCATMA at one proximal coronary location [5, 6, 8, 14]. Compared to proximal PCATMA, there may be a stronger relation of lesion-specific PCATMA with plaque considering a hypothesized local effect of coronary inflammation. Three PCATMA studies (35–199 patients) used a lesion-based measurement method considering all three main coronary arteries [9, 15, 16]. One study showed that lesion-specific PCATMA was higher around culprit lesions in acute coronary syndrome (ACS) patients compared to non-culprit lesions in ACS and CAD patients [15]. Another study revealed lesion-specific PCATMA was significantly increased in patients with abnormal FFR [9]. However, lesion-specific PCATMA failed to show a significant difference between patients with and without elevated high-sensitivity C-reactive protein [16]. Currently, there is a lack of knowledge on the relationship between PCATMA and plaque presence, plaque type, and stenosis severity. In addition, the majority of studies only investigated a single, proximally measured PCATMA value (mostly RCA) to represent overall pericoronary attenuation but did not investigate a potentially more relevant, focal PCATMA value across coronary plaque.
The aim of this study was to evaluate the relationship of proximal and lesion-specific PCATMA with coronary plaque presence, type, and severity.
Materials and methods
Study population
This single-center, cross-sectional study was performed at the University Medical Center Groningen. The study was compliant with the Declaration of Helsinki and approved by the institutional ethical review board, who waived the need for informed consent.
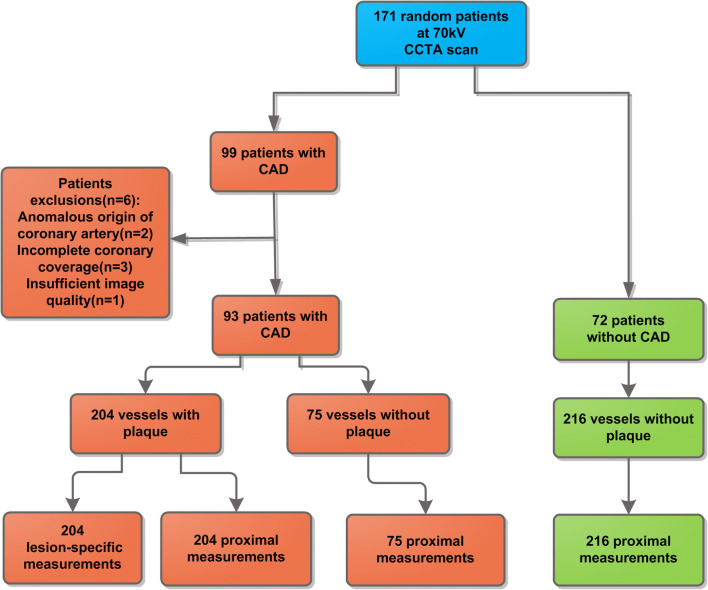
In total, 2621 patients underwent cardiac CTA for routine indications between January 2015 and November 2017. Of these patients, a random sample of 1280 patients was further characterized by gathering hospital record information on CT indication, demographics, and clinical risk factors, to be used in various CT analyses. In a previous analysis (Ma et al) [17], we studied a cohort of patients with a zero calcium score and no coronary plaque on CCTA (“normal patients”); from this population, we selected patients with CCTA at 70 kilovoltage peak (kVp) as a reference category for the current study (n = 72). From the 697 patients (out of 1280) who underwent CCTA because of angina, we randomly selected patients with CAD, defined as patients with plaque on their CCTA images, for the current analysis based on the following inclusion criteria: 1, age > 18 years; 2, CCTA performed at 70 kVp; 3, no coronary stents or coronary artery bypass grafts. Tube voltage was restricted to 70 kVp in view of known influence of kVp on PCATMA [17]. In total, 171 patients (72 + 99) were included. Six CAD patients were excluded for the following reasons: anomalous origin of coronary artery (n = 2), insufficient image quality (n = 1), incomplete coronary image coverage (n = 3) (Fig. 1). A radiologist with 10-year experience in cardiac radiology performed the CCTA evaluation (R.M.). In case of doubt, a radiologist with 14 years of experience was consulted and consensus was obtained (R.V.).
Fig. 1.
Flowchart of patient inclusion and PCATMA measurement analysis levels. kV is kilovoltage; CCTA is coronary computed tomography angiography; CAD coronary artery disease
CCTA scan protocol
CCTA imaging was performed according to the routine clinical protocol using third-generation dual-source CT (SOMATOM Force, Siemens Healthineers). First, a non-enhanced ECG-gated CT at a high pitch (tube voltage 120 kVp, reference tube current 64 mAs, reconstructed slice thickness 3.0mm) was performed for coronary calcium score (CACS) analysis. Subsequently, CCTA was performed using CarekV (kVp optimization assistance), depending on patient size; patients scanned at 70 kVp were included. ECG-gated high-pitch spiral scanning was performed in low, regular heart rate, otherwise ECG-triggered sequential scanning. Patients received sublingual nitroglycerin, unless contraindicated. If the heart rate was > 70–73 beats/min, the patient received intravenous beta-blocker, unless contraindicated. Contrast timing was determined using a test bolus. Iomeprol (Iomeron 350) was injected with dose- and flow-rate depending on patient characteristics and scan mode. A dual-injection technique was used followed by a saline flush. CCTA images were reconstructed at 0.6 mm thickness.
Patient characteristics
Baseline patient characteristics were collected from clinical records. Age, sex, and CAD risk factors were collected. The classification criteria of risk factors were as follows: (a) hypertension—systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg according to guidelines [18] and/or anti-hypertension medication use; (b) hyperlipidemia—patients with a low-density lipoprotein > 4.5 mmol/L or total cholesterol > 6.5 mmol/L based on guidelines [19] were considered as hyperlipidemic; lipid-lowering medications used at the time of CT scanning was considered as a separate factor indicating treated hyperlipidemia; (c) diabetes mellitus—anti-diabetic medication use; (d) smoking status was classified as non-smoker, current smoker, or former smoker. Depending on the risk factors, information was missing in 26 to 51 patients. If there was no mention of a risk factor, the risk factor was considered absent. Body mass index (BMI) information was collected as well.
Plaque analysis
Visual, qualitative analysis
For visual plaque evaluation only, the main coronary arteries, left anterior descending (LAD), left circumflex (LCx), and right coronary artery (RCA) were taken into account to optimize patient comparability. Plaque composition and diameter stenosis (DS) were assessed for the most severe plaque per coronary artery. Plaque components were classified into non-calcified plaque (NCP), mixed plaque, and calcified plaque (CP). Using visual analysis, CP was defined as plaque when it had > 75% volume with density higher than the luminal contrast; NCP was defined as plaque when it had > 75% volume with a density lower than the lumen contrast and higher than soft tissues around. Mixed plaque was defined as plaque comprising 25 to 75% volume with density higher than the luminal contrast [20, 21]. DS was classified into 4 stenosis categories: minimal, DS 1–24%; mild, DS 25–49%; moderate, DS 50–69%; and severe, DS 70–100% [22].
Quantitative analysis
Semi-automated software (Aquarius iNtuition, TeraRecon, Version 4.4.13) was used to measure the Agatston-based CACS on a per-patient level. The CACS was stratified into four categories: 0, 1–99, 100–399, and ≥ 400.
Quantification of the plaque composition was semi-automatically performed by the software (vascuCAP, Research Edition, Elucid Bioimaging) [23]. Automatic segmentation of the entire coronary lumen and wall was performed, allowing manual corrections if needed. Subsequently, the matrix burden, CP burden, and lipid-rich necrotic core (LRNC) burden were automatically calculated by the software on a per-vessel level [24]. The classification of the different plaque components, which was validated with plaque histology, was based on an adaptive threshold. The LRNC lower limit was defined as −300HU; LRNC-IPH boundary was defined as 25HU. The lower limit and upper limit of the CP were 250 and 3000HU. Matrix burden was calculated by dividing the total wall volume by the matrix volume, where the matrix is defined as normal organization tissues in the vessel wall [23]. Plaque burden was defined as 1-matrix burden [24].
PCATMA measurements
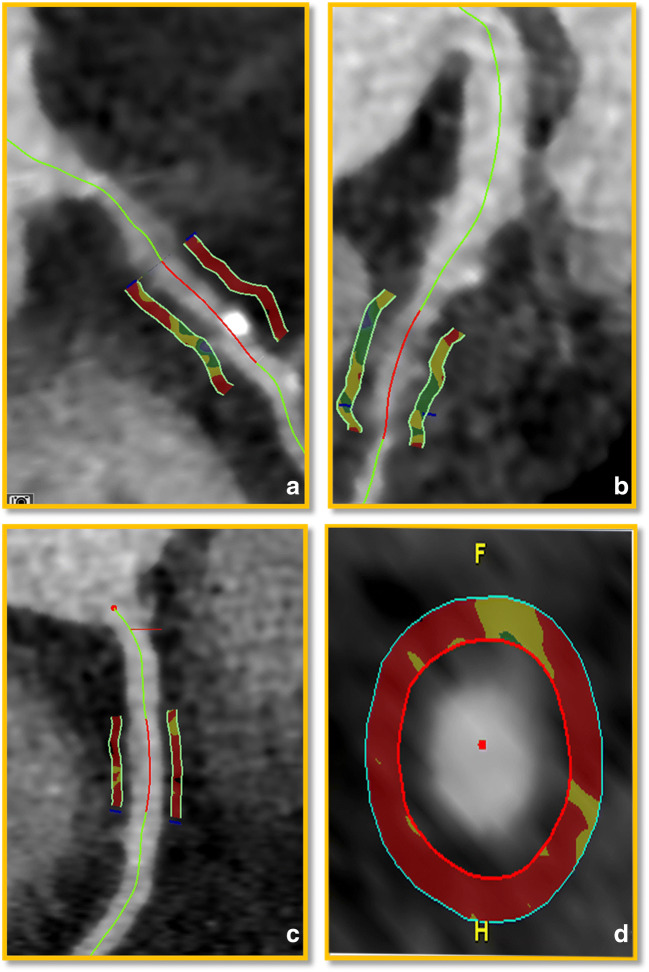
PCATMA was measured proximally in the RCA, LAD, and LCx, using dedicated software (Aquarius iNtuition, TeraRecon, Version 4.4.13). The starting point of the proximal PCATMA measurement was 10mm after the left main bifurcation for LAD, at the bifurcation point for LCx, and 10mm after the ostium for RCA [17]. In vessels with plaque, a lesion-specific PCATMA measurement was performed centered around the most severely stenotic plaque. The proximal and distal ends of the measurement were 5mm away from the lesion center. The measurement length and width for all measurements were 10mm and 1mm. A 1mm gap was left between the outer vessel wall, taking into account eccentric plaques, and the measured cylindrical volume to avoid artifacts. PCATMA was defined as the mean CT value in the measured area within the range of −190 to −30 HU (Fig. 2).
Fig. 2.
PCATMA measurements. a and b represent CCTA images from a 59-year-old male patient with CAD. a represents the lesion-specific PCATMA measurement in the RCA across a calcified plaque. b shows the lesion-specific PCATMA measurement across a non-calcified plaque in LAD. c and d represent CCTA images from a 56-year-old male patient without plaque. c shows the proximal PCATMA measurement of the RCA. d shows the cross-sectional view of the proximal PCATMA measurement in the RCA. The red zones indicate the areas used for PCATMA measurement
Data analysis
First, PCATMA was studied on per-patient level (Fig. 1). Patients with any coronary plaque were considered as CAD patients; patients without plaque were considered non-CAD patients. For the per-patient PCATMA, the mean of the proximal PCATMA values based on the three main coronary arteries was calculated to represent an overall, patient-based PCATMA value. Patient-based CACS and DS were analyzed in conjunction with the per-patient PCATMA. Patient-level categorization of DS degree was based on the most severe DS in all three coronary arteries. To allow comparison with prior studies that used only the proximal measurement of PCATMA of the RCA, we additionally performed analyses for RCA-based PCATMA. Additionally, a comparison of patients with and without at least 50% stenosis was performed. The total plaque burden of the main coronary arteries was considered as the patient-based plaque burden.
Second, vessel-based analysis was performed (Fig. 2). We discriminated arteries with any plaque, and arteries without plaque. CAD patients could contribute arteries without plaque. For arteries with multiple plaques, the lesion with the highest DS was used. The proximal PCATMA was used in arteries without plaque to compare with lesion-specific PCATMA in arteries with plaque. Lesion-specific PCATMA was analyzed based on plaque type and DS severity.
Statistical methods
Normality testing for continuous variables was performed with the Shapiro-Wilk test. Continuous variables are represented as mean± standard deviation (SD) or median (interquartile range [IQR]), according to distribution. The model estimated values are given in mean with 95% confidence interval (CI). Categorical variables were recorded as numbers (n) and percentages (%). Paired t-tests were used to evaluate differences between proximal and lesion-specific PCATMA. Independent t-tests were used to compare PCATMA measurements between patients. One-way analysis of variance (ANOVA) testing was used to compare PCATMA between categories of plaque type and DS severity. Spearman correlation testing was used to assess the correlation of PCATMA with plaque burden and plaque component burden.
A generalized linear model was used to evaluate the influencing factors for patient-based PCATMA. Using mixed models with random intercepts, the model estimated marginal means and 95% CI of the corrected PCATMA were calculated. The basic model included age, sex, and vessel, while the advanced models included CAD risk factors. The models did not include BMI because of 43 missing values. PCATMA was taken as a dependent variable in order to study the relationship between PCATMA and plaque features. A p value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 25 (IBM).
Results
Patient demographics
In total, 93 patients with CAD and 72 patients without CAD were included. Figure 2 shows an overview of the inclusion process. Patient characteristics are given in Table 1. Patients with CAD were significantly older (60.9 ± 8.7 vs. 51.2 ± 12.6 years, p < 0.001) and had significantly more hypertension (39 [41.9%] vs. 16 [22.2%], p = 0.008) and hyperlipidemia (39 [41.9%] vs. 12 [16.7%], p < 0.001) compared to patients without CAD.
Table 1.
Patient characteristics
| Variables | CAD patients | Non-CAD patients | p value |
|---|---|---|---|
| n | 93 | 72 | |
| Male, n (%) | 43 (46.2%) | 23 (31.9%) | 0.063 |
| Age (years) (SD) | 60.9 ± 8.7 | 51.2 ± 12.6 | < 0.001 |
| BMI (kg/m2) (SD)* | 24.2 ± 2.9 | 23.2 ± 3.1 | 0.092 |
| Hypertension, n (%) | 39 (41.9%) | 16 (22.2%) | 0.008 |
| Diabetes mellitus, n (%) | 10 (10.8%) | 3 (4.2%) | 0.119 |
| Hyperlipidemia, n (%) | 39 (41.9%) | 12 (16.7%) | < 0 .001 |
| Statin use, n (%) | 23 (24.7%) | 6 (8.3%) | 0.005 |
| Smoking, n (%) | 0.144 | ||
| Former smoker | 22 (23.7%) | 8 (11.1%) | |
| Current smoker | 26 (28.0%) | 18 (25.0%) | |
| Family history of CAD, n (%) | 41 (44.1%) | 22 (30.6%) | 0.076 |
| Indication for CCTA, n (%) | 0.517 | ||
| Typical angina | 12 (12.9%) | 8 (11.1%) | |
| Atypical angina | 50 (53.8%) | 36 (50%) | |
| Non-anginal chest pain | 2 (2.2%) | 7 (9.7%) | |
| Dyspnea/dyspnea d’ effort | 7 (7.5%) | 5 (6.9%) | |
| Others* | 22 (23.7%) | 16 (22.2) |
BMI body mass index; SD standard deviation; CCTA coronary computed tomography angiography. BMI information was available for 122 patients. *Others included arrhythmias or high-risk profile
Patient-based PCATMA analysis
An overview of PCATMA values for CAD and non-CAD patients, CACS, and DS category is provided in Table 2. There was no correlation between PCATMA and CACS (r = −0.006, p = 0.939). Correlation of PCATMA with DS category and plaque burden was very weak (r = 0.073, p = 0.486 and r = −0.092, p = 0.383). When corrected for age and sex, PCATMA showed no difference between patients with and without CAD (−95.7 HU vs −95.6 HU, p = 0.933). PCATMA was significantly different between sexes (men: −94.0 HU vs. women: −97.3 HU, p = 0.007). Results for proximal RCA-based PCATMA are provided in Table S1 and Table S2.
Table 2.
PCATMA by CAC score and degree of stenosis, per-patient analysis
| Patient-level evaluation | Mean proximal PCATMA | p value |
|---|---|---|
| 0.325 | ||
| CAC score 0 | −95.4 ± 7.9 HU (n = 78) | |
| CAC score 1–99 | −96.9 ± 7.1 HU (n = 35) | |
| CAC score 100–399 | −97.3 ± 5. 7HU (n = 34) | |
| CAC score > 400 | −94.0 ± 8.5 HU (n = 18) | |
| 0.644 | ||
| no CAD | −95.6 ± 7.8 HU (n = 72) | |
| With CAD | −96.2 ± 7.1 HU (n = 93) | |
| 0.825 | ||
| DS < 50% | −96.0 ± 7. 3HU (n = 121) | |
| DS ≥ 50% | −95.7 ± 7.6 HU (n = 44) | |
| 0.580 | ||
| DS 1–24% | −98.3 ± 6.5 HU (n = 16) | |
| DS 25–49% | −95.8 ± 6.7 HU (n = 33) | |
| DS 50–69% | −94.9 ± 6.8 HU (n = 16) | |
| DS 70–100% | −96.2 ± 8.2 HU (n = 28) |
DS diameter stenosis; CAC coronary artery calcium; PCATMA pericoronary adipose tissues mean attenuation
Vessel-based proximal PCATMA analysis
There were 204 arteries with plaque and 291 without plaque (216 from patients without CAD and 75 from patients with CAD). The mean proximal PCATMA of vessels without plaque was −95.6 ± 9.6 HU and −96.3 ± 8.3 HU for patients with and without CAD, respectively (p = 0.567). The different plaque components or degrees of stenosis groups did not show a difference in proximal PCATMA.
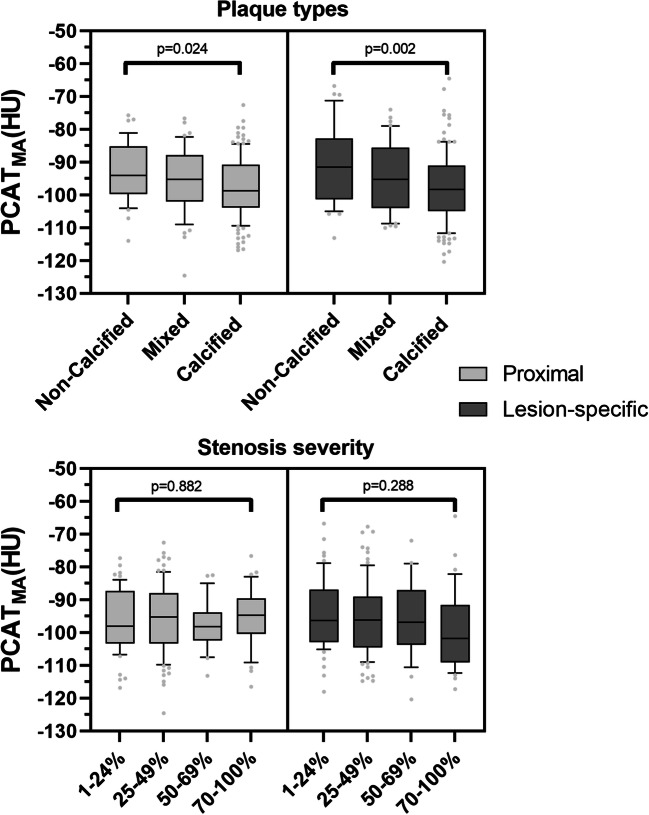
Vessel-based lesion-specific PCATMA analysis
Lesion-specific PCATMA showed a significant difference (p = 0.002) for the coronary lesions with different plaque components. However, there was no significant difference in degrees of stenosis (p = 0.288). In arteries with plaque (n = 204), the median [IQR] plaque burden was 32.9% [29.6–37.5%], showing a weak correlation with PCATMA (r = −0.260, p < 0.001). The median LRNC plaque burden was 9.9% [5.9–13.7%], showing a moderate correlation with PCATMA (r = −0.325, p < 0.001). Median CP burden was 4.1% [1.9–7.9%], with a weak correlation between PCATMA and CP burden (r = −0.097, p = 0.167).
Figure 3 gives an overview of proximal and lesion-specific PCATMA measurements for different plaque components and degrees of stenosis.
Fig. 3.
Proximal and lesion-specific PCATMA by plaque type and stenosis severity. PCATMA pericoronary adipose tissue mean attenuation
Model-based analysis of PCATMA
In the basic model, the corrected mean (95% CI) PCATMA was −94.1 HU (−95.7; −92.5 HU) in vessels with plaque (lesion-specific) and −96.3 HU −97.8; −94.9 HU) in vessels without plaque in non-CAD patients (proximal) (p = 0.026) (Table 3). Sex (p = 0.032), age (p = 0.018), and vessel (LAD, LCx, RCA) had significant effects on PCATMA (p < 0.001). The mean (95% CI) lesion-specific PCATMA of NCP, mixed, and CP was −90.2 HU (−93.8; −86.7 HU), −94.8 HU (−98.0; −91.6 HU), and −96.6 (−98.6; −94.5 HU), respectively (p = 0.006). For DS categories, the overall group effect did not reach statistical significance (p = 0.073), but PCATMA of severe DS was significantly different from minimal DS (p = 0.037). For the advanced models, including CAD risk factors, the differences remained significant (Table 3). For the model with all healthy and diseased vessels, there was a significant difference of PCATMA between patients with and without statin use (−97.6 HU vs −94.3 HU, p = 0.039). Table S3 shows results comparing proximal PCATMA between plaque types and DS using all arteries with and without plaque combined.
Table 3.
Mixed linear models for PCATMA and plaque characteristics
| Categories | Basic models | Advanced models | ||||
|---|---|---|---|---|---|---|
| Estimated fixed effect (95% CI) | Estimated mean (95% CI) (HU) |
p value | Estimated fixed effect (95% CI) |
Estimated mean (95% CI) (HU) |
p value | |
| Models of vessels with and without plaque | ||||||
| Vessels without plaque | 0 (Ref) | −96.3 (−97.8; −94.9) | 0.026* | 0 (0) | −97.2 (−100.0; −94.3) | 0.015* |
| Vessels with plaque | 3.7 (1.0; 6.4) | −94.1 (−95.7; −92.5) | 0.026 | 3.9 (1.2;6.7) | −94.7 (−97.5; −92.0) | 0.015 |
| Models of vessels with plaque | ||||||
| Type of plaque | ||||||
| Non-calcified (n = 38) | 4.5 (−0.6; 9.7) | −90.2 (−93.8; −86.7) | 0.001 | 4.7 (−0.5;9.8) | −89.9 (−94.3; −85.4) | 0.001 |
| Mixed (n = 45) | 1.3 (−4.3; 6.8) | −94.8 (−98.0; −91.6) | 0.329 | 0.9 (−4.6; 6.5) | −94.6 (−98.6; −90.5) | 0.301 |
| Calcified (n = 121) | 0 (Ref) | −96.6 (−98.6; −94.5) | 0.006* | 0(Ref) | −96.5 (−99.8; −93.2) | 0.004* |
| Degree of stenosis | ||||||
| 1–24% (n = 59) | 0 (Ref) | −94.4 (−97.2; −91.6) | 0.073* | 0 (Ref) | −94.0 (−97.9; −90.1) | 0.079* |
| 25–49% (n = 85) | 0.5 (−4.5; 5.5) | −94.1 (−96.5; −91.7) | 0.856 | 0.2 (−4.8; 5.2) | −93.8 (−97.6; −90.1) | 0.927 |
| 50–69% (n = 26) | 5.0 (−5.1; 15.1) | −93.2 (−97.5; −88.8) | 0.622 | 3.7 (−6.5; 13.8) | −93.3 (−98.4; −88.3) | 0.798 |
| 70–100% (n = 34) | −3.5 (−10.2; 3.2) | −98.8 (−102.2; −95.3) | 0.037 | −3.6 (−10.3; 3.1) | −98.5 (−102.9; −94.1) | 0.030 |
| Plaque component burden | ||||||
| LRNC burden | −0.8 (−1.2; 0.4) | 0.009 | −0.7 (−1.1; −0.3) | 0.014 | ||
| Calcified plaque burden | −0.3 (−0.9; 0.3) | 0.326 | −0.3 (−0.9; 0.3) | 0.336 | ||
| Plaque burden | −0.6 (−1.0; −0.2) | 0.003 | −0.6 (−1.0; −0.2) | 0.007 | ||
CAD coronary artery disease; CI confidence interval; HU Hounsfield unit; PCATMA pericoronary adipose tissues mean attenuation; LRNC lipid-rich necrosis core. Values are lesion-specific PCATMA values, apart from vessels without plaque (proximal PCATMA). * is the fixed effect p value of the factor
After correction for CAD risk factors, LRNC burden and plaque burden had significant effects (estimate: −0.8 vs. −0.6) on proximal PCATMA, while the CP burden had no significant effects on proximal PCATMA (Table 3).
Discussion
This study investigated the relationship between PCATMA and plaque presence, plaque type, and stenosis severity in the main coronary arteries in symptomatic patients undergoing CCTA at 70 kVp. PCATMA was higher in vessels with plaque than in vessels without plaque, taking into account patients’ risk factors. Lesion-specific PCATMA was higher for non-calcified and mixed plaques compared to calcified plaques, and for minimal stenosis compared to severe stenosis. In contrast to proximal PCATMA, lesion-specific PCATMA showed clear relationships with plaque presence and stenosis degree.
The proof-of-concept paper by Antonopoulos et al [5] demonstrated that RCA-based PCATMA differed by approximately 3HU between CAD and non-CAD patients, where CAD was defined as the presence of a stenosis of more than 50%. As PCATMA values vary between coronary arteries and plaque distribution among the coronary arteries, with the LAD most often affected, taking only the RCA as a PCATMA reference location may not accurately represent the patient’s PCATMA status. Oikonomou et al [6] reported that increased PCATMA in the RCA and LAD rather than LCx was related to increased cardiac mortality risk. Gaibazzi et al [25] reported significant differences between the LAD/RCA and the LCX in vessels with a stenosis < 50%, with a HU difference of approximately 1.5 HU on 120kVp scans. In our previous study, comparing PCATMA at different kVp levels in patients without plaque, there were significant differences between the PCATMA of LAD, LCX, and RCA with a HU difference around 2~4 HU [17].
Besides the coronary artery, the measurement location may also have a significant effect on PCATMA. Goeller et al [8] showed that, although there was a correlation between PCATMA and epicardial adipose tissue (EAT), there was no correlation between changes in EAT and plaque burden progression. Dai et al [16] found no relationship between lesion-specific PCATMA and high-sensitive C-reactive protein, suggesting that PCATMA may be associated with local coronary inflammation rather than global inflammation. Previously mentioned studies used lesion-specific PCATMA only; few investigated the relationship with coronary plaque. Kwiecinski et al [26] found that increased lesion-specific PCATMA in patients with high-risk plaque was related to focal 18F-NaF PET uptake. Lin et al [27] reported on the relationship of PCAT radiomic features and PCATMA in the proximal RCA and around (non-) culprit lesions at presentation and 6 months post-MI, in comparison to stable CAD and non-CAD cases. They report that the most significant radiomic parameters distinguishing patients with and without MI were based on texture and geometry, yielding information not included in PCAT attenuation. They found that radiomic features were not different between culprit and non-culprit lesions, where the PCATMA showed a significant difference. The authors mention that PCATMA may have utility as a lesion-specific imaging biomarker, while radiomics features may have more value as a patient-specific biomarker of systemic inflammation. Our study, using both proximal and lesion-based PCATMA, confirms that lesion-specific PCATMA is a better representation of focal inflammation and plaque development. Only lesion-specific PCATMA measurements showed a difference between vessels with and without plaque. Using an adjusted model, the PCATMA of vessels with plaque was around 2HU higher than those without plaque. This result is similar to the HU difference in the study by Antonopoulos et al [5].
Lesion-specific PCATMA differed by DS categories, taking into account age, sex, and coronary artery. Our results suggest that there may be more inflammation in mild and moderate DS than in severe DS. This fits with the hypothesis that as the plaque becomes more stabilized and more calcified in severe DS, inflammation could be relatively decreased [28]. Inflammatory cytokines play a critical role in the development and progression of coronary atherosclerosis [29, 30]. The theory behind PCATMA is that vessel wall atherosclerosis inhibits adipocyte maturation and lipid accumulation in the pericoronary fat tissue, increasing the attenuation. Additionally, corresponding increases in edema and amount of inflammatory cells possibly result in an additional increase in PCATMA in patients at risk of or with CAD [31, 32]. Results from previous studies suggest that the relationship between coronary inflammation and PCATMA may be more evident in NCP than CP, since CPs are relatively stable and have only a minimal inflammatory component [31, 32]. Goeller et al [8] investigated the relationship between PCATMA and progression of plaque burden on CCTA. Measuring patient-based plaque burden/composition and RCA-based PCATMA, they found that PCATMA is related to progression of total plaque burden and NCP burden. PCATMA > −75 HU of the proximal RCA was independently associated with increased NCP burden at 120kVp CCTA [8]. However, similar to our results, they found that there was no relationship with CP burden. In our study, the model-adjusted, lesion-specific PCATMA values for NCP were 5–7 HU higher compared to CP and mixed plaques at 70kVp CCTA, measured in the three main coronary arteries. Our study showed only a weak correlation between vessel-based plaque burden and per-vessel PCATMA, and no significant correlation between patient-based total plaque burden and patient-based PCATMA. The per-vessel LRNC burden had a moderate correlation with PCATMA whereas the CP burden showed a very poor correlation. Recent research revealed that LRNC burden is capable of predicting myocardial infarction better than CAC scoring, cardiovascular risk scores, and coronary artery stenosis [33].
There are reports that show that lipid-lowering medication could decrease the EAT attenuation independent of decreasing lipid values [34]. Our study also shows a significant effect of lipid-lowering medication on PCATMA values, supporting the idea that statins have an effect on cardiac fat attenuation and, potentially, adipose tissue activity [35]. Additionally, we found that vessel, sex, and age had significant effects on PCATMA. The relationship between age, sex, and CAD has been reported frequently [36–38]. Men showed generally higher PCATMA values than women (−94.0 vs −97.3 HU). Gender-specific hormones may be the reason for the different effects on coronary inflammation.
Limitations
This is a single-center, cross-sectional study of patients with clinically indicated CCTA. No follow-up information is available; hence, CCTA results cannot be related to cardiovascular prognosis. Although our study demonstrates a relationship between plaque presence, type, and stenosis degree with PCATMA, it was not designed to show direct causality between inflammatory status, plaque characterization, and PCATMA. Plaque burden quantification was performed by automatic software, allowing manual corrections. In general, automatic analysis might be sensitive to errors due to image artifacts or decreased image quality and errors in segmentation. To avoid these errors in this study, scans were selected on image quality (2 scans were excluded), and at each segmentation step, the segmentation was visually assessed and manually corrected when necessary by an experienced radiologist to avoid errors. Window levels could be adjusted manually to reduce, for example, blooming effects from calcifications in order to optimize the segmentation and automated analysis.
Conclusion
PCATMA was higher in coronary arteries with plaque, compared to vessels without plaque. Lesion-specific PCATMA was higher in NCP and mixed plaque compared to CP, and in minimal stenosis compared to severe stenosis. Proximally measured PCATMA only showed differences by plaque composition, and only when corrected for clinical parameters. This suggests that in particular lesion-specific PCATMA is related to plaque development and vulnerability.
Supplementary Information
(DOCX 23 kb)
Acknowledgements
Financial support provided by the China Scholarship Council (CSC) to the first author is gratefully acknowledged.
Abbreviations
- BMI
Body mass index
- CAD
Coronary artery disease
- CCTA
Coronary computed tomography angiography
- CP
Calcified plaque
- DS
Diameter stenosis
- ICC
Intra-class correlation coefficient
- IQR
Interquartile range
- kVp
Kilovoltage peak
- LAD
Left anterior descending coronary artery
- LCx
Left circumflex coronary artery
- LRNC
Lipid-rich necrotic core
- NCP
Non-calcified plaque
- PCATMA
Pericoronary adipose tissue mean attenuation
- RCA
Right coronary artery
- SD
Standard deviation
Funding
The first author of this study has received funding from the China Scholarship Council (CSC).
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Rozemarijn Vliegenthart.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent for this retrospective study was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Please note that the PCATMA values of patients without CAD, used as controls for the extensive analyses of patients with CAD in the current study, were reported in our previous study in European Radiology (doi: 10.1007/s00330-020-07069-0. PMID: 32700017). The former study focused on the influence of kVp and coronary artery on PCATMA values in a normal population.
Methodology
• retrospective
• ross-sectional study/diagnostic study/observational
• performed at one institution
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114:1867–1879. doi: 10.1161/CIRCRESAHA.114.302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrington RA. Targeting Inflammation in Coronary Artery Disease. N Engl J Med. 2017;377:1197–1198. doi: 10.1056/NEJMe1709904. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 4.Steg PG, Ducrocq G. Future of the prevention and treatment of coronary artery disease. Circ J. 2016;80:1067–1072. doi: 10.1253/circj.CJ-16-0266. [DOI] [PubMed] [Google Scholar]
- 5.Antonopoulos AS, Sanna F, Sabharwal N, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9:eaal2658. doi: 10.1126/scitranslmed.aal2658. [DOI] [PubMed] [Google Scholar]
- 6.Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392:929–939. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elnabawi YA, Oikonomou EK, Dey AK, et al. Association of biologic therapy with coronary inflammation in patients with psoriasis as assessed by perivascular fat attenuation index. JAMA Cardiol. 2019;4:885–891. doi: 10.1001/jamacardio.2019.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goeller M, Tamarappoo BK, Kwan AC, et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2019;20:636–643. doi: 10.1093/ehjci/jez013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu M, Dai X, Deng J, Lu Z, Shen C, Zhang J. Diagnostic performance of perivascular fat attenuation index to predict hemodynamic significance of coronary stenosis: a preliminary coronary computed tomography angiography study. Eur Radiol. 2020;30:673–681. doi: 10.1007/s00330-019-06400-8. [DOI] [PubMed] [Google Scholar]
- 10.Nadjiri J, Hausleiter J, Jahnichen C, et al. Incremental prognostic value of quantitative plaque assessment in coronary CT angiography during 5 years of follow up. J Cardiovasc Comput Tomogr. 2016;10:97–104. doi: 10.1016/j.jcct.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Nance JW, Jr, Schlett CL, Schoepf UJ, et al. Incremental prognostic value of different components of coronary atherosclerotic plaque at cardiac CT angiography beyond coronary calcification in patients with acute chest pain. Radiology. 2012;264:679–690. doi: 10.1148/radiol.12112350. [DOI] [PubMed] [Google Scholar]
- 12.Park HB, Heo R, Hartaigh BO, et al. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging. 2015;8:1–10. doi: 10.1016/j.jcmg.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dey D, Gaur S, Ovrehus KA, et al. Integrated prediction of lesion-specific ischaemia from quantitative coronary CT angiography using machine learning: a multicentre study. Eur Radiol. 2018;28:2655–2664. doi: 10.1007/s00330-017-5223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomura CH, Assuncao-Jr AN, Guimaraes PO, et al. Association between perivascular inflammation and downstream myocardial perfusion in patients with suspected coronary artery disease. Eur Heart J Cardiovasc Imaging. 2020;21:599–605. doi: 10.1093/ehjci/jeaa023. [DOI] [PubMed] [Google Scholar]
- 15.Goeller M, Achenbach S, Cadet S, et al. Pericoronary adipose tissue computed tomography attenuation and high-risk plaque characteristics in acute coronary syndrome compared with stable coronary artery disease. JAMA Cardiol. 2018;3:858–863. doi: 10.1001/jamacardio.2018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai X, Deng J, Yu M, Lu Z, Shen C, Zhang J. Perivascular fat attenuation index and high-risk plaque features evaluated by coronary CT angiography: relationship with serum inflammatory marker level. Int J Cardiovasc Imaging. 2020;36:723–730. doi: 10.1007/s10554-019-01758-8. [DOI] [PubMed] [Google Scholar]
- 17.Ma R, Ties D, van Assen M, et al. Towards reference values of pericoronary adipose tissue attenuation: impact of coronary artery and tube voltage in coronary computed tomography angiography. Eur Radiol. 2020;30:6838–6846. doi: 10.1007/s00330-020-07069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 19.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 20.Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010;210:150–154. doi: 10.1016/j.atherosclerosis.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Cheng VY, Wolak A, Gutstein A, et al. Low-density lipoprotein and noncalcified coronary plaque composition in patients with newly diagnosed coronary artery disease on computed tomographic angiography. Am J Cardiol. 2010;105:761–766. doi: 10.1016/j.amjcard.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) Coronary artery Disease - reporting and data system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2016;10:269–281. doi: 10.1016/j.jcct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Sheahan M, Ma X, Paik D, et al. Atherosclerotic plaque tissue: noninvasive quantitative assessment of characteristics with software-aided measurements from conventional CT angiography. Radiology. 2018;286:622–631. doi: 10.1148/radiol.2017170127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Assen M, Varga-Szemes A, Schoepf UJ, et al. Automated plaque analysis for the prognostication of major adverse cardiac events. Eur J Radiol. 2019;116:76–83. doi: 10.1016/j.ejrad.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Gaibazzi N, Martini C, Botti A, Pinazzi A, Bottazzi B, Palumbo AA. Coronary inflammation by computed tomography pericoronary fat attenuation in MINOCA and Tako-Tsubo syndrome. J Am Heart Assoc. 2019;8:e013235. doi: 10.1161/JAHA.119.013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwiecinski J, Dey D, Cadet S, et al. Peri-coronary adipose tissue density is associated with (18)F-sodium fluoride coronary uptake in stable patients with high-risk plaques. JACC Cardiovasc Imaging. 2019;12:2000–2010. doi: 10.1016/j.jcmg.2018.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin A, Kolossvary M, Yuvaraj J, et al. Myocardial infarction associates with a distinct pericoronary adipose tissue radiomic phenotype: a prospective case-control study. JACC Cardiovasc Imaging. 2020;13:2371–2383. doi: 10.1016/j.jcmg.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shioi A, Ikari Y. Plaque calcification during atherosclerosis progression and regression. J Atheroscler Thromb. 2018;25:294–303. doi: 10.5551/jat.RV17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 30.Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on Atherothrombosis (2009) Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 54:2129–2138 [DOI] [PMC free article] [PubMed]
- 31.Margaritis M, Antonopoulos AS, Digby J, et al. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127:2209–2221. doi: 10.1161/CIRCULATIONAHA.112.001133. [DOI] [PubMed] [Google Scholar]
- 32.Antonopoulos AS, Margaritis M, Coutinho P, et al. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes. 2015;64:2207–2219. doi: 10.2337/db14-1011. [DOI] [PubMed] [Google Scholar]
- 33.Williams MC, Kwiecinski J, Doris M, et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial (Scottish Computed Tomography of the HEART) Circulation. 2020;141:1452–1462. doi: 10.1161/CIRCULATIONAHA.119.044720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raggi P, Gadiyaram V, Zhang C, Chen Z, Lopaschuk G, Stillman AE. Statins reduce epicardial adipose tissue attenuation independent of lipid lowering: a potential pleiotropic effect. J Am Heart Assoc. 2019;8:e013104. doi: 10.1161/JAHA.119.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JH, Park YS, Kim YJ, et al. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimibe. J Cardiovasc Ultrasound. 2010;18:121–126. doi: 10.4250/jcu.2010.18.4.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz-Garcia J, Lerman A, Weisz G, et al. Age- and gender-related changes in plaque composition in patients with acute coronary syndrome: the PROSPECT study. EuroIntervention. 2012;8:929–938. doi: 10.4244/EIJV8I8A142. [DOI] [PubMed] [Google Scholar]
- 37.Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5:S62–S72. doi: 10.1016/j.jcmg.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds HR, Shaw LJ, Min JK, et al. Association of sex with severity of coronary artery disease, ischemia, and symptom burden in patients with moderate or severe ischemia: secondary analysis of the ISCHEMIA randomized clinical trial. JAMA Cardiol. 2020;5:773–786. doi: 10.1001/jamacardio.2020.0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 23 kb)