Abstract
Background
Oral sodium zirconium cyclosilicate (SZC) is a novel potassium binder capable of achieving a rapid reduction of serum potassium (sK+) and maintaining a long-term normokalemia. We undertook a meta-analysis to summarize and evaluate the effects surrounding SZC in patients with hyperkalemia.
Method
We searched data sources from MEDLINE (from 1950 to Sep 2020), EMBASE (from 1970 to Sep 2020), and the Cochrane Library database (from 1950 to Sep 2020) for eligible studies. All randomized controlled trials (RCTs) regarding comparison of therapeutic effects of SZC in hyperkalemia participants were included.
Results
Seven studies, including 1697 patients with hyperkalemia, were analyzed. SZC significantly reduced mean sK+ (−0.42 mmol/L; 95% CI: −0.63 to −0.20 mmol/L, p = 0.0001) compared with placebo, with a significantly greater proportion of patients with normokalemia (RR 3.48, 95% CI 1.49 to 8.11, p = 0.004). Subgroup analyses showed that the longer durations of SZC treatment, the greater magnitudes of potassium reduction when compared with those of placebo (p between subgroups = 0.01) at correction phase. Besides, it also demonstrated sK+ tended to decrease more in patients who got longer treatment or larger dosage of SZC at maintenance phase; however, the difference did not reach statistical significance. Additionally, the drug was equally effective in studies with larger than 50% of patients with chronic kidney disease (CKD) or diabetes or patients using renin-angiotensin aldosterone system inhibitor (RAAS) inhibitors (all p < 0.05). The risk of edema (4.30, 1.17 to 15.84; p = 0.03) in SZC group was higher than those of placebo group. No statistically significant differences in the risks of other adverse events were observed between the two groups.
Conclusions
SZC effectively decreased the sK+ level in patients with hyperkalemia within 48 h and had benefits in the long-term control of serum potassium in patients who continued to receive SZC with a favorable safety profile from available data.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10557-020-07134-2.
Keywords: Sodium zirconium cyclosilicate (SZC), Hyperkalemia, Serum potassium (sK+), Meta-analysis
Introduction
Hyperkalemia is a common electrolyte disorder that can cause potentially life-threatening arrhythmias [1]. An increased risk of hyperkalemia was observed in patients with chronic kidney disease (CKD, 22.8%) [2], heart failure (HF, 13.4%), diabetes (10.8%), and renin-angiotensin-aldosterone system inhibitor (RAASi, 14.2%) use [3–5]. However, the benefit of RAASi treatment in heart and kidney diseases is limited by some side effects, such as increased sK+, which is especially severe in patients with renal insufficiency [6]. It is worth noting that after the first hyperkalemia attack, 53.7 or 13.1% of patients experienced RAASi withdrawal or dose reduction, respectively [2]. Some observational cohorts also suggested that hyperkalemia was associated with reduction or cessation of RAAS inhibitors [7–9]. Furthermore, the occurrence of hyperkalemia in CKD patients is closely related to the poor prognosis [2]. Therefore, it is imperative to strengthen the management of hyperkalemia, especially for patients with CKD and hyperkalemia.
Over the years, the treatment for hyperkalemia mainly included low potassium diets, intravenous medications (intravenous insulin + glucose and diuretics), correction of acidosis, and oral medication (such as diuretics, sodium polystyrene sulfonate) [10]. Although intravenous insulin and glucose therapy can quickly reduce serum potassium levels by transferring potassium ions into cells, it does not change the total amount of potassium in the body, and the duration of the efficacy is short and hypoglycemia is a potential complication [10–12]. In addition, diuretics that promote the excretion of potassium ions by the kidneys are not selective for ions, and long-term use can cause renal impairment in patients [11]. Sodium polystyrene sulfonate (SPS) that excrete potassium ions through the intestine also has low selectivity for potassium ions with less compelling evidence from short-term studies (up to a week) for therapeutic effects [10, 13–16]. Furthermore, SPS is also associated with increased serious adverse gastrointestinal events [17].
Sodium zirconium cyclosilicate (SZC) is a stable inorganic crystal, and its structure highly matches the diameter of potassium ions [18, 19]. Its binding force with “potassium” is 25 times than those of other cations [18, 19]. SZC binds potassium ions in the entire digestive tract, which promotes the transfer of sK+ to the intestine and eliminates potassium from the body, thereby achieving rapid potassium reduction and long-term management of hyperkalemia [19, 20]. The data showed that the level of sK+ decreased significantly after 1 h receiving SZC treatment [21] and 98% of patients returned to normal range within 48 h; nearly 99% of patients had sK+ levels < 5.5 mmol/L in the following 1 year [22]. These clinical trials provided evidence on the effectiveness of SZC in reducing sK+. Although hundreds of patients were enrolled in each trial, the number of patients in each treatment group was small due to the stratification of drug dosages. Therefore, we conducted this systematic review and meta-analysis by pooling available interventional evidence to better identify the efficacy and safety of SZC in the treatment of hyperkalemia.
Method
Data Sources and Searches
Two investigators (Yaru Zhang and Ruiling Xu) independently searched and identified relevant studies from the following data sources: MEDLINE (from 1950 to Sep 2020), EMBASE (from 1970 to Sep 2020), and the Cochrane Library database (from 1950 to Sep 2020) using PubMed and Ovid search engines with the text words of “randomized controlled trial,” “hyperkalemia,” “sodium zirconium cyclosilicate,” or “SZC” or “ZS-9.” Trials were restricted to those published in English language. We also searched relevant reference lists from identified trials and review articles.
Study Selection
In this meta-analysis, RCTs regarding comparison of therapeutic effects of SZC in patients with hyperkalemia were collected. Inclusion criteria for studies were as follows: (i) study population comprised participants aged 18 years old or older with hyperkalemia (defined as sK+ 5.1 mmol/L); (ii) a comparison of SZC and placebo; (iii) reported study outcomes included the change in sK+, proportions of responders (defined as patients with sK+ < 6.0 mmol/L between 1 and 4 h, and < 5.0 mmol/L at 4 h, and not requiring additional therapy for hyperkalemia, or according to the definition criteria of the included studies), patient with normal sK+concentration (defined as patients with sK+ < 5.1 mmol/L, or according to the definition criteria of the included studies) between the SZC and placebo group at the end of study, and/or safety data of drug-related adverse events; and (iv) study design was RCT and sample size was larger than 20. We excluded studies without control group or lack of available data.
Data Extraction and Quality Assessment
We extracted information using standard data extraction forms, which included baseline patient characteristics, intervention, doses of drug, follow-up duration, outcome events, and adverse events. We used standard criteria (Cochrane risk of bias tool) to assess the inherent risk of bias of trials, as showed in Table S1. Two investigators (Yaru Zhang and Ruiling Xu) independently undertook data extraction and quality assessment using a standardized approach. Any disagreements between two investigators were resolved by consultation with a third reviewer (Youxia Liu). The study was conducted according to the PRISMA guidelines.
Data Synthesis and Statistical Analyses
We calculated relative risk (RR) and 95% confidence interval (CI) for outcome of proportions of responders with normokalemia using random-effects model. Weighted mean difference and standard deviation (SD) between groups were applied for continuous variable of the change in sK+. We analyzed heterogeneity by I2 statistic to describe the percentage of variability. Meta-regression was conducted to evaluate the impact of different dosages of SZC on the change of sK+. Begg Funnel plot was performed to assess potential publication bias. The results were considered significant with 2-sided p < 0.05. STATA, version 12.0 and Review Manager 5.1 software, were used to perform this meta-analysis. For data with high heterogeneity, we performed sensitivity analysis or subgroup analysis to check for instability and change in significance of the effect estimate.
Results
Study Characteristics and Quality Assessment
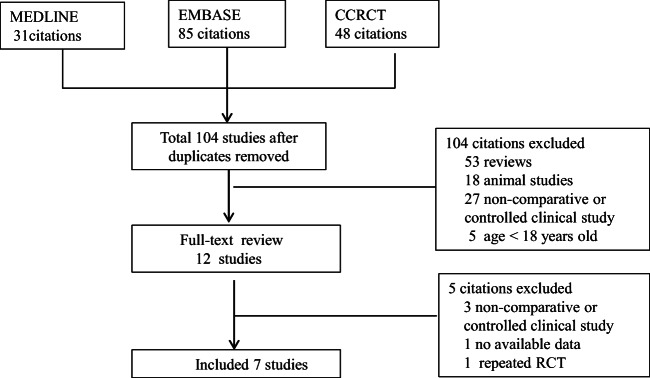
The literature search yielded 104 articles, and eventually, seven studies with 1697 patients were included in our meta-analysis according to the inclusion criteria (Fig. 1). Of the contained seven trials, all of them compared the efficacy of SZC with placebo. These studies were performed from 2014 to 2020, with sample sizes ranging from 70 to 754 and treatment duration ranging from 4 h to 28 days (Table 1). Baseline characteristics of patients in trials were estimated, and the results showed that the mean age was 65 ± 13 years, the mean serum potassium was 5.3 ± 0.8 mmol/L, and the mean weight was 83.7 ± 22.4 kg.
Fig. 1.
Process for identifying studies eligible for the meta-analysis
Table 1.
Characteristics of the randomized controlled studies included in meta-analysis for Sodium Zirconium Cyclosilicate (SZC)
| Study | Baseline sK+(mmol/L) | Number of patients | Treatment | Duration | Endpoint results | Medical history: n (%) | RAASi: n (%) | |
|---|---|---|---|---|---|---|---|---|
| SZC | control | |||||||
| David 2015 Phase 3 | 5.36 ± 0.66 | I-phasen =754 | I-phase: SZC 1.25-10 g tid | I-phase: placebo | I-phase: 48 h | Change in sK+ at 48 h | CKD: 463 (61.5%); HF: 300 (39.8%); DM: 451 (59.9%) | 502 (66.7%) |
| M-phase n = 543 | M-phase: SZC 1.25-10 g tid | M-phase: placebo | M-phase:14 days | |||||
| DIALIZE 2019 Phase 3b | NA | n = 196 | SZC 5 g qd or placebo on nondialysis days | placebo | 4 weeks | Proportion of responders | CKD: 196 (100%) | NA |
| ENERGIZE 2020 Phase 2 | 6.43 ± 0.7 | n = 70 | 10 g SZC tid + insulin + glucose during a 10-h period | placebo + insulin + glucose | 4 h | Change in sK+ at 4 h | CKD: 25 (41.7%), HF: NA; DM: 21(30%) | 25 (35.7%) |
|
HARMONIZE 2014 Phase 3 |
5.6 ± 0.4 | I-phase n = 258 | I-phase: SZC 10 g tid | I-phase: 48 h | Proportion of patient with normokalemia | CKD: 152 (64.1%); HF: 87 (36.7%); DM: 157 (66.2%) | 163 (68.8%) | |
| M-phase n = 237 | M-phase: SZC 5-15 g/day | M-phase: placebo | M-phase:14 days | |||||
|
HARMONIZE 2019 Phase 3 |
5.71 ± 0.5 | I-phase n = 267 | I-phase: SZC 10 g tid for 48 h | I-phase: 48 h | Proportion of patient with normokalemia | CKD: 209783%); HF: 50 (18.7%); DM: 172 (64.4%) | 204 (76.4%) | |
| M-phase n = 248 | M-phase: SZC 5 g and 10 g qd for 28 days | M-phase: placebo | M-phase:28 days | |||||
| Kashihara2020 Phase 2/3 | 5.6 ± 0.4 | 103 | SZC 5 g and10g tid for 48 h | placebo | 48 h | Proportion of patient with normokalemia | CKD: 78 (75.5%); HF: 14 (13.61%); DM: 62 (60.2%) | 80 (77.7%) |
| Stephen 2015 Phase 2 | 5.09 ± 0.37 | n = 90 | SZC 0.3 g, 3 g, and 10 g tid | placebo | 48 h | Change in sK+ at 48 h | CKD: 56(60.9%); HF: NA; DM: 50(55.5%) | NA |
SZC, Sodium zirconium cyclosilicate; I-phase, initial phase(or correction phase); M-phase, maintenance phase; tid, three times daily; qd, once daily; CKD, Chronic kidney disease; DM, Diabetic mellitus; HF, Heart failure; RAASi, renin-angiotensinaldosterone system inhibitor; NA, not available
The inherent risk of bias of trials was performed for all studies by Cochrane risk of bias tool (including sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other source of bias). As listed in Table S1, all studies had generally low risk of bias in all items.
Treatment Outcomes
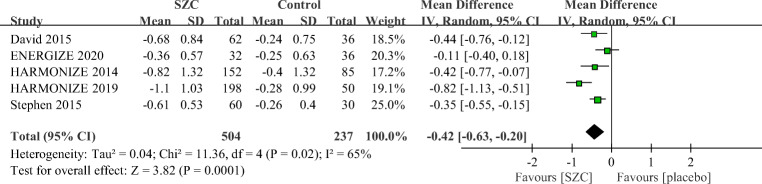
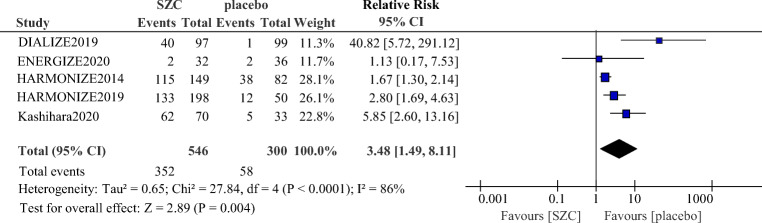
As showed in Fig. 2, patients who received SZC had a significant reduction of sK+ (−0.42 mmol/L; 95% CI: −0.63 to −0.20 mmol/L, p = 0.0001) compared with those who received placebo. SZC also significantly increased the proportion of responders with normokalemia (RR 3.48, 95% CI 1.49 to 8.11, p = 0.004, Fig. 3).
Fig. 2.
Comparison of change in serum potassium (sK+) between the SZC and placebo group. SZC, Sodium zirconium cyclosilicate
Fig. 3.
Comparison of proportions of responders with normokalemia between the SZC and placebo group. SZC, Sodium zirconium cyclosilicate
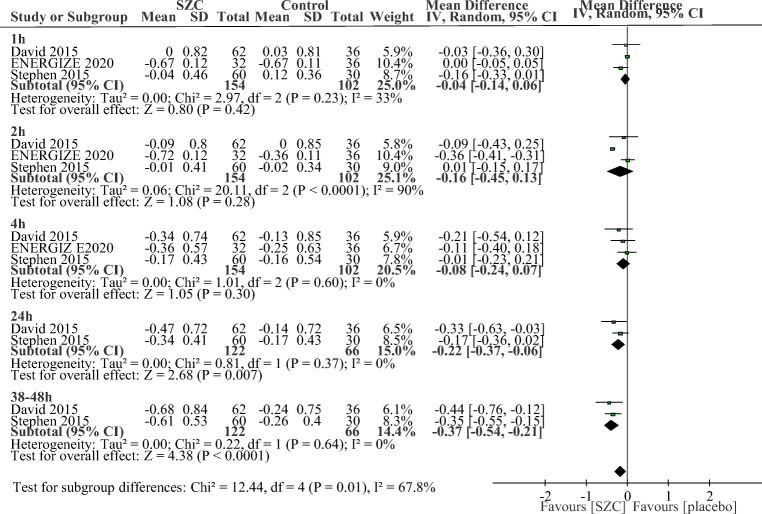
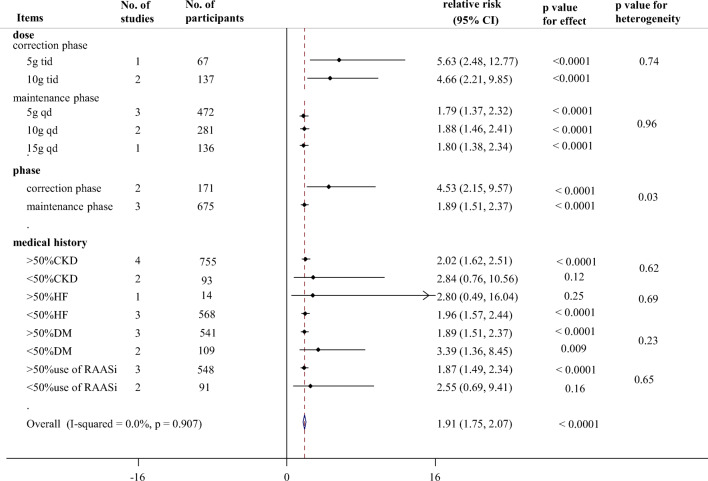
The drug was equally effective across various subgroups, including treatment duration, phase, dosage, and percentage of patients with CKD or diabetes or patients using RAAS inhibitor. We found that the longer durations of SZC treatment, the greater magnitudes of potassium reduction when compared with those of placebo at correction phase (p between subgroups = 0.01, Fig. 4). As shown in Fig. 4, greater reductions in sK+ were achieved by SZC at 24 h (−0.22 mmol/L; −0.37 to −0.06 mmol/L, p = 0.007) and 38–48 h (−0.37 mmol/L; −0.54 to −0.21 mmol/L, p < 0.0001) compared with placebo. However, SZC did not show significant advantages in the decrease of sK+ at 1 h (−0.04 mmol/L; −0.14 to 0.06 mmol/L), 2 h (−0.16 mmol/L; −0.45 to 0.13 mmol/L, p = 0.28) and 4 h (−0.08 mmol/L; −0.24 to 0.07 mmol/L, p = 0.30) when compared with placebo. At maintenance phase, SZC treatment were also associated with significant potassium reductions on day 15 (−0.47 mmol/L; −0.74 to −0.20 mmol/L, p = 0.0006, Supplement Fig. 1) and day 29 (−0.75 mmol/L; −0.98 to −0.51 mmol/L, p < 0.0001, Supplement Fig. 1). Besides, the proportions of responders with normokalemia were higher in patients with SZC, with RRs of 4.53 (95% CI 2.15 to 9.57, p < 0.0001, Fig. 5) at correction phase and 1.89 (95% CI 1.51 to 2.37, p < 0.0001, Fig. 5) at maintenance phase, and the RR at correction phase was significantly higher than that at maintenance phase (p between the two phase = 0.03, Fig. 5).
Fig. 4.
Time-related subgroup analysis of change in serum potassium (sK+) between SZC and placebo group at correction phase (48 h) after start of treatment. SZC, Sodium zirconium cyclosilicate
Fig. 5.
Summary of subgroup analyses for proportions of responders with normokalemia between the SZC and placebo group. >50% CKD: the proportion of patients with CKD was larger than 50% in the included studies; SZC, Sodium zirconium cyclosilicate; CKD, chronic kidney disease; DM, diabetic mellitus; RAASi, renin-angiotensin aldosterone system inhibitor; tid: three times daily; qd: once daily; HF, heart failure
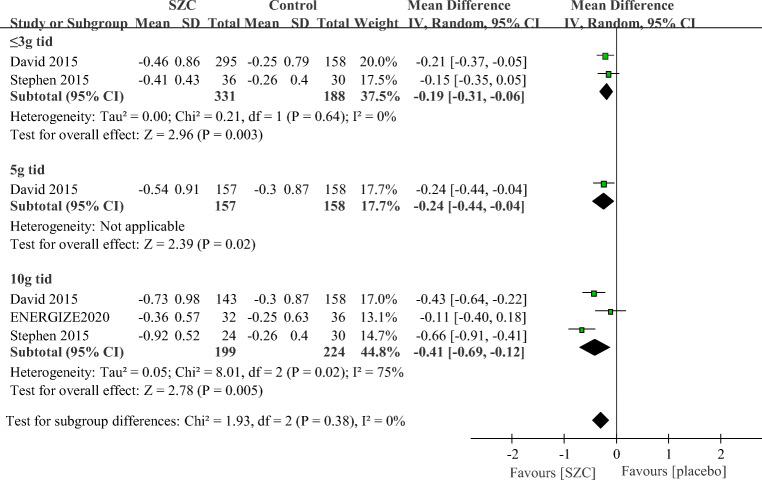
Subgroup analyses showed that patients taking SZC 10 g orally three times a day (tid) significantly decreased sK+ level to the greatest extent (−0.41 mmol/L; −0.69 to −0.12 mmol/L, p = 0.005, Fig. 6), followed by 5 g tid (−0.24 mmol/L; −0.44 to −0.04 mmol/L, p = 0.02) and 3 g tid (−0.19 mmol/L; −0.31 to −0.06 mmol/L, p = 0.003) compared with those who received placebo at correction phase. At maintenance phase, three different doses (5 g qd, 10 g qd and 15 g qd) of SZC could significantly decrease the levels of sK+ (−0.53 mmol/L; −0.79 to −0.27 mmol/L, p < 0.0001; −0.92 mmol/L; −1.25 to −0.59 mmol/L, p < 0.0001; −0.80 mmol/L; −1.27 to −0.33 mmol/L, p = 0.0009, Supplement Fig. 2), respectively. Meta-regression analyses showed that the different dosages of SZC had no significant effect on the change of sK+ at correction phase (p = 0.61) and maintenance phase (p = 0.17). For the proportion of responders with normokalemia (Fig. 5), the results also showed the RRs were higher in patients who received SZC (5.63, 2.48–12.77; 4.66, 2.21–9.85 for 5 g tid and 10 g tid doses at correction phase; 1.79, 1.37–2.32; 1.88, 1.46–2.41; 1.80, 1.38–2.34 for 5 g qd, 10 g qd, and 15 g doses, respectively, at maintenance phase).
Fig. 6.
Subgroup analysis of different doses in the change of serum potassium (sK+) between the ZS-9 and placebo group at correction phase (48 h) after start of treatment. SZC, Sodium zirconium cyclosilicate; tid: three times daily
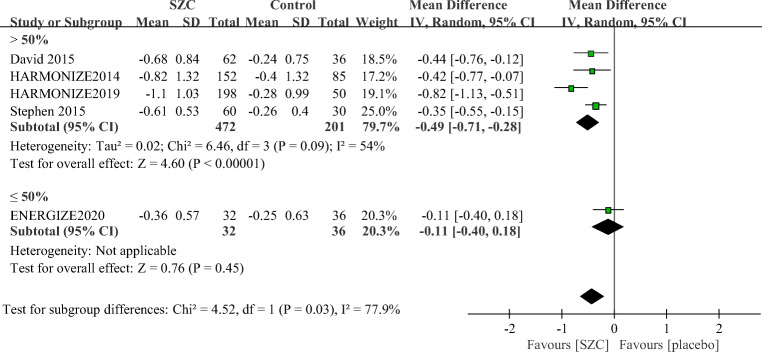
Additionally, as shown in Fig. 5, a higher proportion of patients in SZC group attained normokalemia at the end of their treatment compared with those in the placebo group in studies with more than 50% of patients with CKD (2.08, 1.71–2.53), diabetes (1.96, 1.60–2.40), or RAASi use (1.94, 1.58–2.38). There was no significant difference in proportions of responders with normokalemia between the SZC and placebo group in studies with more than 50% of patients with HF (2.80, 0.49–16.04, p = 25). Besides, there were no significant differences between subgroups based on the proportion of those patients above and below 50%. Similarly, a greater reduction in sK+ was achieved with SZC compared with placebo in studies with more than 50% of patients with CKD, HF, diabetes, or patients using RAAS inhibitors (−0.49 mmol/L; −0.71 to −0.28 mmol/L, p < 0.0001, Fig. 7).
Fig. 7.
Subgroup analysis according to the proportion of patients with CKD or DM or patients using RAAS inhibitors in the included studies of change in serum potassium (sK+) between the ZS-9 group and placebo group. SZC, Sodium zirconium cyclosilicate; CKD, chronic kidney disease; DM, diabetic mellitus; RAASi, renin-angiotensin aldosterone system inhibitor
Adverse Effects
Table 2 displayed the pooled clinical trial results of adverse events. Among adverse events of SZC, the risk of edema in SZC group was 4.30 times higher than that of placebo group (4.30, 1.17 to 15.84; p = 0.03). No statistically significant differences were observed in the risks of other adverse events including any adverse event, serious adverse event, gastrointestinal disorders (including nausea, vomiting, diarrhea, and constipation), cardiac disorders, urinary tract infection, hypokalemia between SZC, and placebo therapy (Table 2, all p > 0.05).
Table 2.
Comparison of adverse events between the SZC and placebo group
| Adverse events | Reporting Study (n) | SZC group (n/n) | Placebo group (n/n) | RR (95%CI) |
p value | |
|---|---|---|---|---|---|---|
| Any Adverse Event | 7 | 289/1205 | 116/492 | 1.25(0.98,1.60) | 0.07 | |
| Serious Adverse Event | 4 | 27/480 | 14/271 | 1.42 (0.71, 2.84) | 0.33 | |
| Gastrointestinal disorders | Nausea | 4 | 12/749 | 4/251 | 1.49(0.51, 4.42) | 0.47 |
| Diarrhea | 4 | 20/950 | 11/337 | 0.77(0.36, 1.65) | 0.5 | |
| vomiting | 2 | 4/655 | 3/188 | 0.47(0.04, 5.04) | 0.53 | |
| Constipation | 4 | 17/517 | 9/267 | 0.91(0.22, 3.82) | 0.9 | |
| Cardiac disorders | 3 | 18/945 | 2/293 | 1.72 (0.51, 576) | 0.38 | |
| Urinary tract infection | 3 | 8/853 | 0/238 | 2.19 (0.39, 12.30) | 0.37 | |
| Edema | 2 | 32/350 | 2/135 | 4.30 (1.17, 15.84) | 0.03 | |
| Hypokalemia | 3 | 6/383 | 3/172 | 1.42 (0.39, 5.24) | 0.6 | |
RR: Relative Risk; SZC: Sodium zirconium cyclosilicate
Sensitivity Analysis and Publication Bias
Sensitivity analysis was performed for change in sK+ and the proportion of responders with normokalemia. The results revealed that no studies had a significant effect on the results as showed in Supplement Fig. 3. Statistical testing showed no evidence of publication bias for the change of the proportion of responders with normokalemia, which was displayed in Supplement Fig. 4.
Discussion
This systematic review and meta-analysis was conducted to estimate the efficacy and safety of SZC for hyperkalemia, and eventually, 8 RCTs met our inclusion criteria [21, 23–29]. Our results showed that SZC effectively decreased the sK+ in patients with hyperkalemia within 48 h and had benefits for the long-term control of sK+ in patients who continued to receive SZC with a favorable safety profile from available data. Compared with placebo, the advantages in decreasing sK+ of SZC at 1, 2, and 4 h were not found according to our results. Besides, SZC seemed to safely and effectively normalize and maintain potassium levels in patients with CKD, HF, diabetes, or RAASi use.
Consistent with our findings, available data showed that SZC was associated with a significant decrease in sK+ within 48 h and had benefits for the long-term control of sK+. A previous meta-analyses and systematic review published in 2017 evaluated the effects of patiromer and SZC on hyperkalemia, and the results showed that SZC reduced sK+ 0.67 mEq/L at 48 h [30]. Kosiborod et al. [24] found that 84 and 98% of patients had normal sK+ concentration by 24 h and 48 h with SZC treatment, respectively. Several published studies evaluated the long-term effects of potassium control. Bruce et al. [22] found most participants (82%) achieved normokalemia after administration of SZC 30 g (three 10-g doses) over 24 h and the treatment with SZC resulted in a reduction in potassium from ≥ 5.1 mmol/L at baseline to 4.7 mmol/L within 3–12 months. In HARMONIZE study with an 11-month open-label extension, 88.3% of patients maintained mean sK+ within the normokalemic range for ≤ 11 months during ongoing SZC treatment [31]. In our study, SZC was associated with a decrease in sK+ levels and a higher proportion of patients with normokalemia with 48 h and maintained normokalemia during 2–4 weeks. The present study provided additional evidence to support the viewpoint that SZC contributed to effectively reducing sK+ concentration and maintaining long-term normokalemia. However, compared with placebo, the effects for decreasing sK+ of SZC at 1, 2, and 4 h were not found according to our study. A study conducted by Mikhail showed that in population with mean sK+ level at baseline larger than 6.0 mmol per liter, the mean sK+ level declined by 0.4 mmol per liter (95% CI, 0.2 to 0.5) at 1 h, by 0.6 mmol per liter (95% CI, 0.4 to 0.8) at 2 h, and by 0.7 mmol per liter (95% CI, 0.6–0.9) at 4 h (p < 0.001 for the comparison of each time point with baseline) after one 10-g dose of SZC [32]. A study published in The New England journal of medicine showed that the SZC significantly decreased sK+ from baseline by 0.11 mEq/l at 1 h after the first 10-g dose, as compared with an increase of 0.01 mmol per liter in the placebo group (p = 0.009). This disparity may be contributed by that the dose of SZC in the above studies were 10 g, and the dose of SZC in our study included 0.3 g, 3 g, etc., which may have weakened the effect of SZC in reducing sK+. We did not conduct a subgroup analysis of the potassium-lowering effect of different doses of SZC within 4 h because of poorly available data. The study conducted by Naoki [29] showed that median time to normalization of sK+ concentration was shorter with SZC 10 g versus placebo (1.8 h vs. 3.9 h) and was similar for the SZC 5 g and placebo groups (3.9 and 3.9 h, respectively), suggesting that the time to normalization of sK+ concentration was shorter with larger dose of SZC. Thus the absence of positive effects at 1, 2, and 4 h in our meta-analysis should not be considered as conflictive with the general literature. We believe that when more and more large-scale studies are included in the future, the advantages of SZC in rapidly reducing the sK+ of patients with hyperkalemia, especially those with severe hyperkalemia, will be more prominent.
Our further subgroup analysis revealed that compared with placebo, three doses of SZC at correction phase (10 g tid, 5 g tid, or ≤ 3 g tid) and at maintenance phase (15 g qd, 10 g qd, or 5 g qd) resulted in the decrease of sK+ levels and a higher proportion of patients with normokalemia. It demonstrated patients with hyperkalemia got larger dosage of SZC their sK+ tended to decrease more; however, the difference did not reach statistical significance. The study conducted by Naoki [29] showed that the exponential rates of sK+ change from 0 to 48 h versus placebo were greater with larger doses of SZC, and they also found the time for sK+ to return to normal level was shorter with larger dose of SZC, suggesting that SZC lowed sK+ with dose dependency.
As mentioned above, CKD, HF, diabetes, or the use of RAASi were high risks for hyperkalemia [2]. Kosiborod et al. found SZC reduced and maintained normal potassium for up to 4 weeks in patients with CKD, heart failure, and diabetes [24]. A study of hyperkalemia patients with renal insufficiency found that during SZC treatment, the average sK+ levels decreased from 5.7 to 4.8 mmol/L in patients with eGFR < 30 mL/min/1.73 m2 and from 5.6 to 4.7 mmol/L in those with eGFR ≥30 mL/min/1.73 m2 at day 365 [33]. They also pointed the proportions of patients who reached normal potassium during the maintenance period were 82 and 90% for the eGFR < 30 and ≥ 30 mL/min/1.73 m2 group at day 365, respectively. In a recent Dialize study, the use of SZC in patients undergoing hemodialysis was able to maintain sK+ levels between 4.0 and 5.0 mmol/L during dialysis with a few records of adverse events [27]. Hyperkalemia usually limits the use of RAASi in patients who were expected to derive the most benefit from these classes of drugs. Bruce et al. [22] found that 87% of patients continued to take RAASi or increased their dose among 483 RAASi users with hyperkalemia at baseline and 14% started RAASi treatment among 263 RAASi-naïve participants after initiating SZC. A study conducted by Jared et al. [34] demonstrated that SZC safely and effectively normalized and maintained potassium levels (71–85% vs 48% for placebo, p < 0.01) and prevented hyperkalemia from redeveloping in patients receiving RAASi for up to 4 and 8 weeks. These results were generally in accordance with our results, suggesting that SZC was a potentially effective treatment for patients with CKD or diabetes or those receiving RAASi treatment. However, there was no significant difference in proportions of responders with normokalemia between the SZC and placebo group in studies with more than 50% of patients with HF according to our meta-analysis. We further analyzed the reasons and found that this result was driven by only one study with a small sample size. Therefore, the identification of sub-groups of patients who may benefit is important to help guide treatment and should be explored in future studies.
The safety of SZC is the main issues of concern. The current research reported the side effects of SZC mainly included gastrointestinal disorders (including nausea, vomiting, diarrhea and constipation), cardiac disorders, urinary tract infection, edema and hypokalemia [20–30, 33]. In our meta-analysis, of all adverse events mentioned above, SZC only increased the risk of edema compared with placebo. No statistically significant differences in the risks of other adverse events including any adverse event, serious adverse event, gastrointestinal disorders (including nausea, vomiting, diarrhea, and constipation), cardiac disorders, urinary tract infection and hypokalemia were found between SZC and placebo therapy. These results indicated that SZC had a favorable safety.
A larger sample size and rigorous statistic methodology were the strengths of this meta-analysis. The overall low risk of bias of included studies was an additional strength of this review. However, our study had some limitations. Firstly, scant primary data prevented us from undertaking subgroup analysis of effect of SZC in patients with different degrees of hyperkalemia, especially in patients with severe hyperkalemia. Secondly, the duration of drug treatment and follow-up of the included studies in our study did not exceed 2 months. The safety is still uncertain when SZC is used for more than 1 year. Thirdly, we did not register this analysis with PROSPERO. More and larger RCTs with longer maintenance-period are needed in the future. Fourthly, in the meta-analysis, studies with open-label extension were included, which may introduce a bias where patients who do not tolerate the drug are unlikely to take part in the extension study.
Conclusion
SZC effectively decreased the sK+ in patients with hyperkalemia within 48 h and had benefits in the long-term control of sK+ in patients who continued to receive SZC with a favorable safety profile from available data. Besides, SZC seemed to safely and effectively normalize and maintain potassium levels in patients with CKD or diabetes or those receiving RAASi treatment. We believe that when more and more large-scale studies are included in the future, the advantages of SZC in rapidly reducing the sK+ of patients with hyperkalemia, especially those with severe hyperkalemia, will be more prominent.
Supplementary Information
(PDF 229 kb)
(DOCX 30 kb)
Authors’ Contributions
Youxia Liu designed the study. Yaru Zhang, Ruiling Xu, and Junying Xu collected the data. Na Zhao and Fajuan Cheng analyzed the results. Lihong Long, Fanghao Wang, and Junya Jia confirmed information. Yaru Zhang and Ruiling Xu drafted the manuscript. Youxia Liu and Shan Lin revised the manuscript.
Funding
This study was funded by the National Natural Science Foundation (81600553, 81600554, 81601421) and the Shandong Provincial Natural Science Foundation (Grant No. ZR2016HB09).
Data Availability
Not applicable.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaru Zhang and Ruiling Xu contributed equally to this work.
Contributor Information
Yaru Zhang, Email: 15122459564@163.com.
Ruiling Xu, Email: 13895043418@163.com.com.
Fanghao Wang, Email: wfh-92-05-09@163.com.
Youxia Liu, Email: 5liuyouxia@163.com.
Junying Xu, Email: 3380929601@qq.com.
Na Zhao, Email: orange_3420@163.com.
Fajuan Cheng, Email: chengfajuan2009@163.com.
Lihong Long, Email: 1356348980@qq.com.
Junya Jia, Email: jiajunya@126.com.
Shan Lin, Email: linshan1012@163.com.
References
- 1.Khanagavi J, Gupta T, Aronow WS, Shah T, Garg J, Ahn C, Sule S, Peterson S. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251–257. doi: 10.5114/aoms.2014.42577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashihara N, Kohsaka S, Kanda E, Okami S, Yajima T. Hyperkalemia in real-world patients under continuous medical Care in Japan. Kidney Int Rep. 2019;4(9):1248–1260. doi: 10.1016/j.ekir.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, He D, Zhang W, Xing Y, Guo Y, Wang F, Jia J, Yan T, Liu Y, Lin S. ACE inhibitor benefit to kidney and cardiovascular outcomes for patients with non-Dialysis chronic kidney disease stages 3-5: a network meta-analysis of randomised clinical trials. Drugs. 2020;80:797–811. doi: 10.1007/s40265-020-01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao G, Wang Z, Guo R, Chen Z, Wang X, Zhang L, Li W. Effects of ACEI/ARB in hypertensive patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled studies. BMC Cardiovasc Disord. 2014;14:148. doi: 10.1186/1471-2261-14-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savarese G, Vasko P, Jonsson Å, Edner M, Dahlström U, Lund LH. The Swedish heart failure registry: a living, ongoing quality assurance and research in heart failure. Ups J Med Sci. 2019;124(1):65–69. doi: 10.1080/03009734.2018.1490831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoro A, Mandreoli M. [Hyperkalemia as a limiting factor in the use of drugs that block the Renin Angiotensin Aldosterone System (RAAS)]. G Ital Nefrol. 2018. [PubMed]
- 7.Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia After Initiating Renin-Angiotensin System Blockade: The Stockholm Creatinine Measurements (SCREAM) Project. J Am Heart Assoc. 2017;6(7). [DOI] [PMC free article] [PubMed]
- 8.Jun M, Jardine MJ, Perkovic V, Pilard Q, Billot L, Rodgers A, Rogers K, Gallagher M. Hyperkalemia and renin-angiotensin aldosterone system inhibitor therapy in chronic kidney disease: a general practice-based, observational study. PLoS One. 2019;14(3):e0213192. doi: 10.1371/journal.pone.0213192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazich I, Bakris GL. Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. Semin Nephrol. 2014;34(3):333–339. doi: 10.1016/j.semnephrol.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Palmer BF, Clegg DJ. Diagnosis and treatment of hyperkalemia. Cleve Clin J Med. 2017;84(12):934–942. doi: 10.3949/ccjm.84a.17056. [DOI] [PubMed] [Google Scholar]
- 11.Cowan AC, Gharib EG, Weir MA. Advances in the management of hyperkalemia in chronic kidney disease. Curr Opin Nephrol Hypertens. 2017;26(3):235–239. doi: 10.1097/MNH.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 12.McNicholas BA, Pham MH, Carli K, et al. Treatment of hyperkalemia with a low-dose insulin protocol is effective and results in reduced hypoglycemia. Kidney Int Rep. 2018;3(2):328–336. doi: 10.1016/j.ekir.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clase CM, Carrero JJ, Ellison DH, Grams ME, Hemmelgarn BR, Jardine MJ, Kovesdy CP, Kline GA, Lindner G, Obrador GT, Palmer BF, Cheung M, Wheeler DC, Winkelmayer WC, Pecoits-Filho R, Ashuntantang GE, Bakker SJL, Bakris GL, Bhandari S, Burdmann EA, Campbell KL, Charytan DM, Clegg DJ, Cuppari L, Goldsmith D, Hallan SI, He J, Herzog CA, Hoenig MP, Hoorn EJ, Leipziger JG, Leonberg-Yoo AK, Lerma EV, Lopez-Almaraz JE, Małyszko J, Mann JFE, Marklund M, McDonough AA, Nagahama M, Navaneethan SD, Pitt B, Pochynyuk OM, Proença de Moraes T, Rafique Z, Robinson BM, Roger SD, Rossignol P, Singer AJ, Smyth A, Sood MM, Walsh M, Weir MR, Wingo CS. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2020;97(1):42–61. doi: 10.1016/j.kint.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Hagan AE, Farrington CA, Wall GC, et al. Sodium polystyrene sulfonate for the treatment of acute hyperkalemia: a retrospective study. Clin Nephrol. 2016;85(1):38–43. doi: 10.5414/CN108628. [DOI] [PubMed] [Google Scholar]
- 15.Hunt TV, DeMott JM, Ackerbauer KA, et al. Single-dose sodium polystyrene sulfonate for hyperkalemia in chronic kidney disease or end-stage renal disease. Clin Kidney J. 2019;12(3):408–413. doi: 10.1093/ckj/sfy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepage L, Dufour AC, Doiron J, Handfield K, Desforges K, Bell R, Vallée M, Savoie M, Perreault S, Laurin LP, Pichette V, Lafrance JP. Randomized clinical trial of sodium polystyrene Sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10(12):2136–2142. doi: 10.2215/CJN.03640415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noel JA, Bota SE, Petrcich W, Garg AX, Carrero JJ, Harel Z, Tangri N, Clark EG, Komenda P, Sood MM. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene Sulfonate use in patients of advanced age. JAMA Intern Med. 2019;179(8):1025–1033. doi: 10.1001/jamainternmed.2019.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.周庆飞. Sodium zirconium cyclosilicate(Lokelma). 中国药物化学杂志. 028(006):515.
- 19.Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One. 2014;9(12):e114686. doi: 10.1371/journal.pone.0114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang A, Leon A, Nuttall M, et al. In vitro ion exchange capacity and selectivity of ZS-9, a novel, selective CATION trap for the treatment of hyperkalemia. Am J Kidney Dis. 2014;63(5):B115. [Google Scholar]
- 21.Packham DK, Rasmussen HS, Lavin PT, el-Shahawy MA, Roger SD, Block G, Qunibi W, Pergola P, Singh B. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372(3):222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 22.Spinowitz BS, Fishbane S, Pergola PE, Roger SD, Lerma EV, Butler J, von Haehling S, Adler SH, Zhao J, Singh B, Lavin PT, McCullough PA, Kosiborod M, Packham DK, on behalf of the ZS-005 Study Investigators Sodium zirconium Cyclosilicate among individuals with hyperkalemia: a 12-month phase 3 study. Clin J Am Soc Nephrol. 2019;14(6):798–809. doi: 10.2215/CJN.12651018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peacock WF, Rafique Z, Vishnevskiy K, Michelson E, Vishneva E, Zvereva T, Nahra R, Li D, Miller J. Emergency potassium normalization treatment including sodium zirconium Cyclosilicate: a phase II, randomized, double-blind, placebo-controlled study (ENERGIZE) Acad Emerg Med. 2020;27:475–486. doi: 10.1111/acem.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, Roger SD, Yang A, Lerma E, Singh B. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312(21):2223–2233. doi: 10.1001/jama.2014.15688. [DOI] [PubMed] [Google Scholar]
- 25.Anker SD, Kosiborod M, Zannad F, Piña IL, McCullough PA, Filippatos G, van der Meer P, Ponikowski P, Rasmussen HS, Lavin PT, Singh B, Yang A, Deedwania P. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail. 2015;17(10):1050–1056. doi: 10.1002/ejhf.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zannad F, Hsu BG, Maeda Y, Shin SK, Vishneva EM, Rensfeldt M, Eklund S, Zhao J. Efficacy and safety of sodium zirconium cyclosilicate for hyperkalaemia: the randomized, placebo-controlled HARMONIZE-global study. ESC Heart Fail. 2020;7(1):54–64. doi: 10.1002/ehf2.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fishbane S, Ford M, Fukagawa M, McCafferty K, Rastogi A, Spinowitz B, Staroselskiy K, Vishnevskiy K, Lisovskaja V, al-Shurbaji A, Guzman N, Bhandari S. A phase 3b, randomized, double-blind, placebo-controlled study of sodium zirconium Cyclosilicate for reducing the incidence of Predialysis hyperkalemia. J Am Soc Nephrol. 2019;30(9):1723–1733. doi: 10.1681/ASN.2019050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015;88(2):404–411. doi: 10.1038/ki.2014.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashihara N, Nishio T, Osonoi T, Saka Y, Imasawa T, Ohtake T, Mizuno H, Shibagaki Y, Kim H, Yajima T, Sarai N. Correction of serum potassium with sodium zirconium cyclosilicate in Japanese patients with hyperkalemia: a randomized, dose-response, phase 2/3 study. Clin Exp Nephrol. 2020;24:1144–1153. doi: 10.1007/s10157-020-01937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of Patiromer and sodium zirconium Cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy. 2017;37(4):401–411. doi: 10.1002/phar.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roger SD, Spinowitz BS, Lerma EV, Singh B, Packham DK, al-Shurbaji A, Kosiborod M. Efficacy and safety of sodium zirconium Cyclosilicate for treatment of hyperkalemia: an 11-month open-label extension of HARMONIZE. Am J Nephrol. 2019;50(6):473–480. doi: 10.1159/000504078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosiborod M, Peacock WF, Packham DK. Sodium zirconium cyclosilicate for urgent therapy of severe hyperkalemia. N Engl J Med. 2015;372(16):1577–1578. doi: 10.1056/NEJMc1500353. [DOI] [PubMed] [Google Scholar]
- 33.Roger SD, Lavin PT, Lerma EV, McCullough PA, Butler J, Spinowitz BS, et al. Long-term safety and efficacy of sodium zirconium cyclosilicate for hyperkalaemia in patients with mild/moderate versus severe/end-stage chronic kidney disease: comparative results from an open-label, phase 3 study. Nephrol Dial Transplant. 2020. [DOI] [PMC free article] [PubMed]
- 34.Schaefer JA, Gales MA. Potassium-binding agents to facilitate renin-angiotensin-aldosterone system inhibitor therapy. Ann Pharmacother. 2016;50(6):502–510. doi: 10.1177/1060028016640794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 229 kb)
(DOCX 30 kb)
Data Availability Statement
Not applicable.