Abstract
Purpose
Deficiency of adenosine deaminase 2 (DADA2) is an inherited inborn error of immunity, characterized by autoinflammation (recurrent fever), vasculopathy (livedo racemosa, polyarteritis nodosa, lacunar ischemic strokes, and intracranial hemorrhages), immunodeficiency, lymphoproliferation, immune cytopenias, and bone marrow failure (BMF). Tumor necrosis factor (TNF-α) blockade is the treatment of choice for the vasculopathy, but often fails to reverse refractory cytopenia. We aimed to study the outcome of hematopoietic cell transplantation (HCT) in patients with DADA2.
Methods
We conducted a retrospective study on the outcome of HCT in patients with DADA2. The primary outcome was overall survival (OS).
Results
Thirty DADA2 patients from 12 countries received a total of 38 HCTs. The indications for HCT were BMF, immune cytopenia, malignancy, or immunodeficiency. Median age at HCT was 9 years (range: 2–28 years). The conditioning regimens for the final transplants were myeloablative (n = 20), reduced intensity (n = 8), or non-myeloablative (n = 2). Donors were HLA-matched related (n = 4), HLA-matched unrelated (n = 16), HLA-haploidentical (n = 2), or HLA-mismatched unrelated (n = 8). After a median follow-up of 2 years (range: 0.5–16 years), 2-year OS was 97%, and 2-year GvHD-free relapse-free survival was 73%. The hematological and immunological phenotypes resolved, and there were no new vascular events. Plasma ADA2 enzyme activity normalized in 16/17 patients tested. Six patients required more than one HCT.
Conclusion
HCT was an effective treatment for DADA2, successfully reversing the refractory cytopenia, as well as the vasculopathy and immunodeficiency.
Clinical Implications
HCT is a definitive cure for DADA2 with > 95% survival.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-021-01098-0.
Keywords: Hematopoietic cell transplantation, Deficiency of adenosine deaminase 2, DADA2, Inborn error of immunity, Bone marrow failure, Immunodeficiency, Autoinflammation
Introduction
In 2014, biallelic deleterious mutations in the cat eye chromosome region 1 gene (CECR1, subsequently renamed ADA2), encoding adenosine deaminase 2 (ADA2), were reported as the cause of a monogenic inborn error of immunity disease, deficiency of ADA2 (DADA2) (OMIM # 615,688) [1, 2]. The phenotype comprises recurrent fever and vasculopathy, ranging from livedo racemosa and polyarteritis nodosa (PAN) to intracranial vasculopathy with lacunar strokes and hemorrhages [1–3]. Cytopenias, either autoimmune or due to bone marrow failure (BMF), occur in 50% of patients and present as congenital pure red cell aplasia (PRCA), neutropenia, thrombocytopenia, or pancytopenia [4–6]. Immunodeficiency with hypogammaglobulinemia and recurrent viral and bacterial infections and malignant lymphoproliferation (T-large granular lymphocyte leukemia (T-LGL leukemia) and lymphoma) have also been described [5, 7–9]. DADA2 diagnosis is based on an absence or low levels of plasma ADA2 enzymatic activity and the demonstration of biallelic loss-of-function mutations of ADA2 [10]. The pathophysiology of DADA2 remains unclear. A picture emerges where ADA2 deficiency results in skewing of macrophage differentiation towards inflammatory M1 macrophages [11], leading to endothelial instability, as shown in a zebrafish model and in endothelial cell coculture systems [1]. Recent findings have revealed an even more complex interplay between endothelial cells and monocytes and macrophages with marked ADA2 secretion by endothelial cell lines [12].
Treatment of DADA2 is challenging and case mortality is estimated to be around 8%, mostly in childhood and related to vasculopathy-associated complications and infections [13–15]. None of the classical immunosuppressive drugs are an option for the long-term treatment of DADA2, because their efficacy is temporary, especially for the DADA2 related cytopenia, or because of the toxicity associated with long-term use. Anti-TNF agents, etanercept in particular, are the mainstay of treatment for the inflammatory and vasculopathy phenotypes [16]. However, anti-TNF agents do not cure the hematological phenotype and in a proportion of patients, vasculopathy persists despite anti-TNF treatment [17, 18]. Finally, the cost of life-long TNF-inhibition is a limitation for some patients. Hematopoietic cell transplantation (HCT) has been reported to result in a rapid and sustained resolution of the systemic inflammation and hematological phenotype, with all patients from a cohort of 14 patients surviving after HCT [5, 19–21]. We report here the results of a multinational study of a cohort of 30 patients with DADA2 undergoing HCT, including the previously reported cases.
Methods
Overview of the Study
We conducted an investigator-driven retrospective international non-interventional multicenter study on HCT for DADA2. Invitations to participate were sent to the physicians allied to the DADA2 Foundation, the European Group for Blood and Marrow Transplantation (EBMT), and the European Society for Immunodeficiencies (ESID). We also invited all authors of published single case reports on HCT in DADA2 to participate in the study. Data collection began after the Second Inaugural International Conference on DADA2 hosted by the DADA2 Foundation on November 9, 2018. The study was approved by the Ethics Committee of Leuven University Hospitals (study number S63982). The study was performed in accordance with the principles of the Declaration of Helsinki. The authors assume responsibility for the accuracy and completeness of the data and analyses and for fidelity to the study protocol.
Patients
The criteria for patient inclusion in the study were as follows: (1) genetic diagnosis of DADA2 and/or clinical findings consistent with DADA2 and plasma ADA2 activity level in the deficient range and (2) HCT performed with a follow-up time for survivors of at least 3 months after HCT. All participating physicians completed a questionnaire. All patients or their guardians gave written informed consent for data collection. Patients for whom incomplete data were obtained (indication for HCT, age at HCT, total nucleated cell dose or CD34 + stem cell dose, stem cell donor, conditioning regimen, graft-versus-host disease (GvHD) prophylaxis, time to engraftment, graft failure, conditioning for subsequent HCTs, chimerism) were excluded from the study.
HCT Data
Neutrophil engraftment was defined as the first of three consecutive days with a neutrophil count ≥ 0.5 × 109/L and platelet engraftment as the first of seven consecutive days with a platelet count ≥ 20 × 109/L without platelet transfusion in the prior 7 days. Full donor chimerism was defined as ≥ 95% donor cells in myeloid or whole-blood fractions. The type of test was at the discretion of the transplant center. Primary and secondary graft failures (GF) were defined according to EBMT guidelines. Second (or third) HCT was defined as the infusion of hematopoietic progenitor cell containing product, according to CIBMTR, regardless of conditioning regimen. The diagnosis and grading of acute and chronic GvHD were based on international standard criteria [22]. Transplant regimen, GvHD prophylaxis, antimicrobial prophylaxis, and pre-emptive treatment were chosen according to center preferences. Preparative regimens were classified as reduced intensity conditioning (RIC) if the dose of alkylating agents or TBI is reduced by at least 30% from a myeloablative conditioning (MAC) approach. A total dose of treosulfan > 30 g/m2 was considered MAC whether or not combined with another alkylator, whereas a total busulfan dose < 8 mg/kg and fludarabine-melphalan regimens were considered RIC.
Kaplan–Meier curves were plotted for overall survival (OS) and GvHD-free relapse-free survival (GRFS), and p values were obtained for Mantel-Cox log-rank tests performed with Graph-Pad Prism Software version 9. Values of p < 0.05 were considered statistically significant. GvHD relapse-free survival was calculated as the time from first HCT until the first occurrence of any of the following events: grades 3–4 aGvHD or moderate/severe cGvHD GF, disease relapse (poor graft function/graft failure with DADA2 disease relapse requiring repeat transplant), or death. HCTs inadvertently using affected donors were excluded. The cumulative incidence of GvHD and GF were also calculated using competing risk analysis, using R, for all HCT procedures, but excluding HCTs from affected donors.
Results
Patient Characteristics and Diagnosis of DADA2
We included 30 DADA2 patients undergoing HCT between 2000 and 2020 in this study. Four other patients were excluded due to incomplete data sets. Patients underwent HCT at 21 different centers from 12 countries in Europe and North America. Twenty of the patients have been reported before [4, 5, 7, 9, 19–21, 23–27]. Median age at disease onset was 2.25 years (range: birth to 16 years). Median age at genetic diagnosis was 12 years (range: 2–28 years) (Table 1). DADA2 diagnosis was confirmed at the molecular level in all patients, by demonstrating the presence of biallelic pathogenic ADA2 variants. Plasma ADA2 activity was assessed before HCT in 18 patients and was low in all cases. Twenty-six patients harbored known pathogenic ADA2 mutations. The R169Q variant was the most common mutation, found in 15 patients. Four patients harbored novel mutations, all with combined annotation-dependent depletion (CADD) scores above the mutation significance cutoff (MSC) for this gene; all these variants were private or had a MAF < 10–6 [28], strongly suggestive of a deleterious effect. Three of these patients were tested for ADA2 enzyme activity, which was found to be low or absent (Table 2). Patient and HCT characteristics are summarized in Table S1 in the Online Supplement.
Table 1.
Demographic and clinical features of the 30 DADA2 patients before HCT
| Patient ID | Sex/Ethnicity | Age at disease onset (y) | Age at genetic diagnosis (y) | DADA2 clinical manifestations | CD4, CD8, CD19, CD56 | IgG | IgA | IgM | Previous treatment | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| P1* | M, Caucasian | 0.5 | 5 | RCA, pancytopenia, splenomegaly, recurrent infections, LAP | 653, 93, 11, 0.5 | 997 | 2.4 | 7 | Prednisone, sirolimus, tacrolimus, IVIG | [5, 19, 21] |
| P2 | F, Tunisian | 7 | 28 | PRCA, stroke, EBV viremia, livedo, splenomegaly, aphthous ulcers | 370,84, 60, 48 | 1700 | 60 | 310 | Prednisone, everolimus, hydroxychloroquine, IVIG | [9] |
| P3 | F, Turkish | 1 | 3 | PRCA | 1460, 690, NA, 255 | 1200 | 77 | 77 | Prednisone | [24] |
| P4 | M, Turkish | 11 | 22 | PRCA, HSM, MDS-RCMD, recurrent infections, FTT | NA | 682 | 50 | 61 | Prednisone | [24] |
| P5 | M, Caucasian | 0.3 | 9 | PRCA, splenomegaly, IBD, recurrent fevers, aphthous ulcers | 607, 781, 685, 90 | 290 | 119 | 9 | Prednisone, anakinra | [21] |
| P6 | M, Caucasian | 0 | 15 | Neutropenia, HSM, LAP, livedo, strabismus, PAN, neuropathy | 1239, 714, 116, 339 | 1021 | 77 | 149 | Prednisone, etanercept, adalimumab, CsA, GCSF | |
| P7 | M, Caucasian | 2 | 22 | Anemia, lymphopenia, HSM, livedo, ICH, optic nerve atrophy, PAN | 50, 50, 1, 10 | 465 | 47 | 17 | Prednisone, azathioprine, infliximab, FFP, IVIG | [21] |
| P8 | M, Caucasian | 0 | 2 | PRCA, LAP, HSM, recurrent infections, liver fibrosis | 1763, 1037, 415, 104 | 426 | < 8 | < 6 | Prednisone, IVIG | [21] |
| P9 | F, Caucasian | 2.5 | 4 | RCA, pancytopenia, livedo, epilepsy, T-LGL, HSM, aphthous ulcer | 386, 257, NA, NA | 1000 | NA | 74 | Prednisone | [7, 21] |
| P10 | F, Caucasian | 7 | 11 | RCA, pancytopenia, AIHA, splenomegaly, ICH, livedo, arthritis | 528, 211, 26, 30 | 816 | < 26 | 25 | Prednisone, MTX, infliximab, IVIG | [21] |
| P11 | F, Caucasian | 1 | 26 | Pancytopenia, HSM, recurrent infections, PAN, bronchiectasis | 183, 108, 0, 2 | 700 | 42 | 33 | Prednisone, azathioprine, daratumumab, etanercept, CsA, rituximab, IVIG, GCSF | |
| P12* | M, Caucasian | 0.4 | 4 | Anemia, neutropenia, HSM, LAP, IBD, SAH, TIA, recurrent infections | 599, 278, 228, 147 | 436 | 17 | 54 | Prednisone, azathioprine, sirolimus, etanercept, IVIG | [21] |
| P13 | M, Caucasian | 4 | 4 | RCA, pancytopenia, FTT, fevers, arthralgia | 1926, 2005, 1, 74 | 1000 | 16 | 10 | Prednisone, etanercept, eltrombopag, infliximab, rituximab, IVIG | |
| P14 | F, Caucasian | 12 | 13 | Neutropenia, lymphopenia, recurrent infections, aphthous ulcers | 190, 172, 26, 35 | 730 | 81 | 27 | Etanercept | |
| P15 | M, Caucasian | 3 | 4 | PRCA, HSM, alopecia, recurrent fevers, strabismus, aphthous ulcers | 814, 459, 104, 60 | 605 | 40 | < 6 | Prednisone, MMF, CsA, sirolimus, IVIG, GCSF | [4, 21] |
| P16 | M, Caucasian | 0.1 | 13 | SAA, HSM, livedo, IDDM, GHD, hypothyroidism | 1610, 940, 1250, 1040 | 360 | 60 | 30 | None | [20, 21] |
| P17 | F, Caucasian | 0.3 | 6 | PRCA, splenomegaly, recurrent infections, livedo, arthritis, T-LGL | NA | 760 | 116 | 52 | Prednisone, etanercept | |
| P18 | M, Caucasian | 0.2 | 6 | RCA, neutropenia, HSM, LAP, portal HTN, hepatoportal sclerosis/fibrosis, recurrent infections | 154, 280, 225, 224 | 883 | 88 | 60 | Prednisone, etanercept, GCSF | |
| P19 | M, Hispanic | 14 | 15 | Neutropenia, HSM, NRH | 185, 310, 38, 26 | 1247 | 20 | 6 | Adalimumab, IVIG, GCSF | |
| P20 | F, Caucasian | 16 | 25 | Neutropenia, HSM, recurrent infections, lymphoproliferation | 1670, 1721, 106, 101 | NA | NA | NA | Prednisone, ATG, GCSF | [21] |
| P21 | F, Black | 12 | 12 | Anemia, neutropenia, LAP, HSM, recurrent infections, EBV, bronchiectasis, DLBCL | 170, 176, 127, 83 | 580 | 49 | 31 | Prednisone, rituximab, DLBCL-type chemotherapy | [25] |
| P22 | F, Hispanic | 7 | 21 | Neutropenia, recurrent infections | 361, 262, 0, 32 | NA | NA | NA | Prednisone, IVIG, GCSF | [21, 27] |
| P23 | F, Caucasian | 3 | 5 | ALPS-like, recurrent infections, neutropenia, splenomegaly, livedo | 976, 738, 596, 263 | 2950 | 348 | 25 | Prednisone, sirolimus, MMF, CsA, GCSF | [23] |
| P24 | F, Algerian | 6 | NA | Neutropenia, AIHA, HSM, recurrent infections, livedo | 1208, 1779, 34, 134 | 225 | 2 | 37 | Prednisone, etanercept, sirolimus, rituximab, IVIG | |
| P25 | F, Turkish | 0.3 | 2 | PRCA, HSM, recurrent infections, livedo | 960, 980, 756, 142 | 1000 | 109 | 36 | Prednisone | [24] |
| P26 | F, Caucasian | 2 | 22 | Neutropenia, stroke, T-LGL, recurrent infections, splenomegaly, livedo | 590, 1450, 121, 6 | 487 | 55 | 394 | Prednisone, etanercept, hydroxychloroquine | |
| P27 | F, Caucasian | 1 | 9 | Pancytopenia, stroke, ICH, livedo, arthritis, AML, HSM, HTN, CMP | 273, 222, 23, 29 | 354 | 34 | 9 | Prednisone, etanercept, anakinra, azathioprine, IVIG | [21, 26] |
| P28 | F, Caucasian | 14 | 15 | RCA, neutropenia | 441, 276, 43, 35 | 499 | 11 | 15 | Prednisone, IVIG | [21] |
| P29* | M, Hispanic | 0 | 18 | PRCA, aphthous ulcers, moderate liver siderosis, hepatitis | NA | NA | NA | NA | Prednisone | [21] |
| P30* | F, Hispanic | 13 | 14 | RCA, neutropenia, liver fibrosis, recurrent warts, livedo | 588, 416, 176, 25 | 625 | 86 | 10 | Adalimumab, IVIG |
*Siblings (1 + 12 and 29 + 30); bold font indicates low values for age. Patients are arranged per donor then age. AIHA autoimmune hemolytic anemia, ATG antithymocyte globulin, CMP cardiomyopathy, CsA cyclosporine A, DLBCL diffuse large B cell lymphoma, F female, FFP fresh frozen plasma, GCSF granulocyte colony stimulating factor, GHD growth hormone deficiency, HSM hepatosplenomegaly, HTN hypertension, ICH intracranial hemorrhage, IBD inflammatory bowel disease, IDDM insulin-dependent diabetes mellitus, LAP lymphadenopathy, LGL large granular lymphocyte leukemia, M male, MDS-RCMD myelodysplastic syndrome-refractory cytopenia with multilineage dysplasia, MMF mycophenolate mofetil, NRH liver nodular regenerative hyperplasia, PAN polyarteritis nodosa, PRCA pure red cell aplasia, RCA red cell aplasia, SAH subarachnoid hemorrhage, TIA transient ischemic attack, URI upper respiratory infection, y year. Lymphocyte subsets (n x 106/L). IgG, IgA, IgM (mg/dL)
Table 2.
Genetics and ADA2 enzymatic activity for the 30 DADA2 patients
| Patient ID | ADA2 allele 1 | ADA2 allele 2 | ADA2 activity pre-HCT | ADA2 activity post-HCT |
|---|---|---|---|---|
| P1* | c.506G > A (p.R169Q) | c.506G > A (p.R169Q) | NA | 22.07a |
| P2 | c.(753 + 168_754-229)del | c.(1081 + 139_1082-92)del | 2b | 490b at 1y |
| P3 | c.680-681del (p.Y227fs*27) | c.680-681del (p.Y227fs*27) | NA | NA |
| P4 | c.1445 A > G (p.Y482C) | c.1445 A > G (p.Y482C) | NA | 44.38a |
| P5 | c.144del (p.R49fs) | c.47 + 2 T > C (splice site) | 0.2a | 11.7a |
| P6 | c.506G > A (p.R169Q) | c.139G > T (p.G47W) | 0.37a | 1.67 of normal |
| P7 | c.506C > T (p.R169Q) | c.2 T > C (p.M1T) | NA | NA |
| P8 | c.144delG (p.R49fs) | c.506G > A (p.R169Q) | NA | NA |
| P9 | c.506G > A (p.R169Q) | c.506G > A (p.R169Q) | 0.0a | NA |
| P10 | c.660C > A (p.Y220X) | c.660C > A (p.Y220X) | 2.5b | 403.6b at 2y |
| P11 | c.3936delG (p.R131Sfs) | c.3936delG (p.R131Sfs) | 1.2b | NA |
| P12* | c.506G > A (p.R169Q) | c.506G > A (p.R169Q) | 0.11a | 76.5b |
| P13 | c.506G > A (p.R169Q) | c.506G > A (p.R169Q) | 0b | 77.8b at 2.5 m |
| P14 | c.506G > A (p.R169Q) | c.506G > A (p.R169Q) | 0.09a | NA |
| P15 | c.1110C > A (p.N370K) | c.1072G > A (p.G358R) | 0.6a | 19.7a at 1y |
| P16 | c.506G > A (p.R169Q) | c.506G > A (p.R169Q) | NA | 8.3a at 10y |
| P17 | c.506G > A (p.R169Q) | c.932 T > G (p.L311R) | 0.3a | NA |
| P18 | c.336C > G (p.H112Q) | del exon 7 | 0.4a | 0.4 of normal |
| P19 | c.506G > A (p.R169Q) | c.336C > G (p.H112Q) | NA | NA |
| P20 | c.140G > T (p.G47V) | c.336C > G (p.H112Q) | NA | NA |
| P21 | c.934C > T (p.R312X) | c.709delC (p.Glu237fs) | 0.2a | 35.6a at 2 m |
| P22 | c.794C > G (p.S265X) | c.794C > G (p.S265X) | 0.0a | 10.8a at 1y |
| P23 | c.1367A > G (p.Y456C) | c.1196. G > A (p.W399X) | NA | 21.4a |
| P24 | c.140G > T (p.G47V) | c.140G > T (p.G47V) | NA | NA |
| P25 | c. 1072 G > A (p.G358R) | c. 1072 G > A (p.G358R) | 0.52a | NA |
| P26 | p.Lys188Pro | g.17188016_17188596del | 0a | 6a |
| P27 | c.506G > A (p.R169Q) | c.506G > A (p.R169Q) | 0.8a | 7.0a at 1y |
| P28 | c.144dupG (p.R49fs) | c.506G > A (p.R169Q) | NA | NA |
| P29* | c.506G > A (p.R169Q) | c.1072G > A (p.G358R) | NA | 22.3a |
| P30* | c.506G > A (p.R169Q) | c.1072G > A (p.G358R) | 0.3a | NA |
*Siblings (1 + 12 and 29 + 30)
aPlasma ADA2 (mU per mL): healthy controls (n = 27 + pooled normal plasma), 13.0 ± 5.1 (4.7–27.2). DADA2 patients (n = 55), 0.4 ± 0.5 (0–2.5)
bDried plasma spots ADA2 (mU/g protein): healthy controls (n = 106), 130.0 ± 53.2 (24.9–285). DADA2 patients (n = 78), 4.7 ± 4.8 (0–23.3)
Hematological Phenotype Pre-HCT
PRCA was documented in 8/30 patients, isolated neutropenia in 6/30, combined RCA and neutropenia in 5/30, severe aplastic anemia in 1/30, severe lymphopenia in 1/30, anemia and neutropenia in 2/30, autoimmune hemolytic anemia (AIHA) in 2/30, and pancytopenia in 5/30 patients, at presentation. Six patients had a hematological malignancy or myelodysplasia and received HCT as part of the therapeutic approach (P4, P9, P17, P21, P26, and P27). Twenty-nine patients had received at least one immunosuppressive treatment prior to HCT including 13 patients who received at least 3 lines of immunosuppressive medications. Fourteen patients had received anti-TNF agents before HCT, without effect on cytopenias. P6 received single agent etanercept, which failed to reverse neutropenia; a combination of adalimumab, cyclosporine, and low-dose prednisone resulted in normal neutrophil counts for 6 months prior to HCT. Two patients received pre-HCT Interleukin-1 receptor antagonist (anakinra), without amelioration of cytopenias or immune dysregulation. Seven patients received granulocyte colony stimulating factor (G-CSF) for neutropenia, with no response.
Immunological and Vasculitis Phenotype Pre-HCT
IgG levels were low in 12/27 tested patients, IgA levels were low in 13/26, and IgM levels were low in 15/27 tested patients. Recurrent infections were reported in 17 of the 30 patients, mostly viral infections (in 14/17). Herpesvirus infections predominated, with three patients suffering from recurrent herpes zoster, one having protracted CMV infection, one with severe chicken pox, one with recurrent cutaneous HSV-1, two with HHV6 viremia, and four with EBV viremia (2 transient, 1 chronic, and 1 in the context of lymphoproliferative disease). Warts (n = 4), and mollusca contagiosum (n = 4) were also reported. Immunoglobulin substitution treatment was administered to 15 of the 30 patients before HCT, and splenomegaly was reported in 23/30 patients. Fifteen of 30 patients had reported vasculitis prior to HCT (Table 1): 9 had livedo racemosa, 3 had polyarteritis nodosa (PAN) (P6 with livedo, P7 with ICH and livedo, P11 isolated), three patients had ischemic stroke (P2, 26, 27), and four had intracranial hemorrhage (P7,10, 27, P12).
Transplant Characteristics
The indications for HCT were cytopenia with or without immunodeficiency and/or lymphoproliferation or malignancy (Table 3). The median age at HCT was 9 years (range: 2–28). Six of the 30 patients had received HCT before the description of DADA2 in 2014. Two patients (P16, P29) were inadvertently transplanted using affected siblings as donors and received salvage second HCT from unrelated donors. A total of 38 HCTs were performed for 30 patients. Twenty patients received MAC (P12 with two subsequent HCTs), eight received RIC, and two received NMA conditioning for the final curative transplant (Tables 3 and S1). The most commonly used regimen (in 11 patients) was treosulfan/fludarabine ± thiotepa with antithymocyte globulin (ATG) or alemtuzumab. Serotherapy was used in 25/30 patients: ATG in 10 (rabbit ATG in all except horse ATG in P6 and P18) and alemtuzumab in 15 patients. The source of the stem cells for the final transplant was peripheral blood (PB) for 10 and bone marrow (BM) for 20 patients. Donors were HLA-matched related (n = 4), HLA-haploidentical sibling (n = 2), 10/10 HLA-matched unrelated (n = 16), and 9/10 HLA-mismatched unrelated (n = 8) for the final transplant (Tables 3 and S1). Two of the six related donors carried heterozygous ADA2 mutations (P3, P22), three were healthy (P1, P2, P21), and one donor was of unknown status (P4).
Table 3.
HCT data and post-HCT complications for the 30 DADA2 patients
| Patient ID | Year of HCT | Age at HCT (y)/sex | Indication for HCT | HLA match/graft source | Conditioning | GvHD prophylaxis | CD34+ dose (× 106/kg) | aGvHD/grade | cGvHD | Other comp | Last donor chimerism | Last follow-up (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1* | 2009 | 3/M | RCA, neutropenia | MSD BM | Bu/Cy (MAC) | MMF/CsA | 7.5 |
Gut 3 |
No | ITP, SOS on D + 60 | 3 y > 95% | 114 |
| P2 | 2018 | 28/F | PRCA | MSD BM | Bu/Flu (MAC) | CsA | 4.3 | No | No | None | 2 y 91% (myeloid 100%) | 26 |
| P3 | 2016 | 3/F | PRCA | MRD BM (ADA2 carrier) | Bu/Flu/TT (MAC) | MTX/CsA | 5.4 | No | No | None | 2 y 100% | 48 |
| P4 | 2016 | 23/M | PRCA | MRD PB (unknown ADA2 status) | Bu/Flu (MAC) | MMF/PTCy | 10.5 | Skin 1 | Skin + mouth, mild | None | 6 m 100% | 41 |
| P5 | 2015 | 7/M | PRCA | 10/10 MUD BM | Bu/Cy/ATG (MAC) | MTX/CsA | TNC = 7.5 | No | No | None | 1 y 100% | 60 |
| P6 | 2019 | 17/M | Neutropenia | 10/10 MUD PB | Bu/Cy/pentostatin/hATG (RIC) | MMF/Tacro/PTCy | 7.7 | Skin 1 | No | None | 1 y 100% | 20 |
| P7 | 2016 | 23/M | Severe lymphopenia | 10/10 MUD BM | Bu/Flu/Alem (MAC) | MTX/CsA | 6.4 | No | No | None | 6 m > 95% | 50 |
| P8 | 2016 | 2/M | PRCA, recurrent CMV | 10/10 MUD BM | Flu/Treo/TT/ATG (MAC) | MTX/CsA | 8.3 | Skin 2 | No | None | 6 m 100% | 50 |
| P9 | 2016 | 5/F | RCA, neutropenia | 10/10 MUD BM | Flu/Treo/TT/Alem (MAC) | MMF/CsA | 9 | Skin 1 | No | None | 1 y 98% | 48 |
| P10 | 2016 | 11/F | Pancytopenia, autoimmunity | 10/10 MUD PB | Flu/Treo/TT/ATG (MAC) | MTX/CsA | 3.2 | Skin 1 | No | Steatosis hepatis | 1 y 100% | 50 |
| P11 | 2019 | 28/F | Pancytopenia | 10/10 MUD BM | Flu/Treo/TT/Alem (MAC) | MMF/CsA | 4.8 | No | No | None | 6 m 100% | 12 |
| P12* | 2016 | 5/M | Recurrent TIA, immunodeficiency | 10/10 MUD PB (2 boosts for declining MC)** | Flu/Treo/Alem (MAC) | MMF/CsA | 8.1 | Skin 1 | No | None | 3 y > 95% | 54 |
| P13 | 2020 | 6/M | PRCA | 10/10 MUD BM | Flu/Treo/Alem (MAC) | MMF/Tacro | 5.7 | No | No | None | 6 m 100% | 6 |
| P14 | 2018 | 14/F | Neutropenia, severe lymphopenia | 10/10 MUD PB | Flu/Treo/Alem (MAC) | MMF/CsA | 15 | Skin 1 | No | Mild bronchiectasis | 2 y 100% | 22 |
| P15 | 2016 | 4/M | PRCA, neutropenia | 10/10 MUD BM | Flu/Mel/Alem (RIC) | MTX/Tacro | 3.2 | No | No | ITP D + 42 | 1 y 98% | 52 |
| P16 | 2003 | 4/M | Refractory SAA | 10/10 MUD BM (1st HCT from affected MSD) | Flu/TBI/Alem (RIC) | MTX/CsA | 1.4 | No | No | None | 3 y 100% | 200 |
| P17 | 2019 | 6/F | Immune dysregulation | 10/10 MUD BM | Flu/Treo/TT/Alem (MAC) | CsA | 10.8 | Skin 1 | Skin, moderate | None | 3 m 96% | 11 |
| P18.1 | 2018 | 7/M | RCA, neutropenia | 10/10 MUD PB | Bu/Cy/pentostatin/hATG (RIC) | MMF/Tacro/PTCy | 9 | No | No | Secondary GF | ||
| P18.2 | 2019 | 10/10 MUD PB | Flu/Cy/Alem (NMA) | CsA/PTCy | 3.7 | No | No | Unstable graft | ||||
| P18.3 | 2019 | 10/10 MUD PB CD34 selected + 2 week post-HCT DLI | Flu/rATG (NMA) | None | 7.4 | Skin, liver, gut 2 | No | NRH, siderosis | 8 m 100% | 24 | ||
| P19.1 | 2017 | 19/M | Neutropenia | 10/10 MUD BM | Bu/Cy/pentostatin (RIC) | MMF/Siro/PTCy | 4.5 | No | No | SOS on D + 21, secondary GF | ||
| P19.2 | 2018 | 10/10 MUD PB | Flu/Alem (NMA) | CsA | 8.5 | No | No | Stable NRH | 1 y 100% | 36 | ||
| P20 | 2014 | 23/F | Neutropenia | 10/10 MUD BM | Flu/Mel/Alem (RIC) | Prednisone/CsA | TNC = 2.1 | No | No | None | 2 y 98% | 76 |
| P21 | 2018 | 13/F | Diffuse large B-cell lymphoma | Haplo brother PB | Flu/Mel/TT/ATG/rituximab (RIC) | alpha–beta TCD | 7 | No | No | None | 1 y 100% | 30 |
| P22 | 2013 | 20/F | Neutropenia | Haplo sister BM (ADA2 carrier) | Flu/Bu/Cy/TBI200 (MAC) | Tacro/MMF/PTCy | 0.5 | No | No | None | 3 y 100% | 78 |
| P23 | 2015 | 5/F | Neutropenia | 9/10 MMUD BM | Bu/Flu/TT/ATG/ rituximab (MAC) | MTX/CsA | 4.2 | Skin/gut 2 | Gut, mild | None | 1 y 100% | 45 |
| P24 | 2018 | 9/F | Neutropenia, AIHA | 9/10 MMUD BM | Bu/Flu/Alem/Rituximab (MAC) | MMF/CsA/PTCy | 5.6 | No | No | SOS on D + 12, ARDS D + 54 | 1 m 100% | 2 (dead) |
| P25 | 2020 | 4/F | PRCA | 9/10 MMUD PB | Flu/Treo/TT/ATG (MAC) | MTX/CsA | 4.5 | Skin 2 | No | None | 1 m 100% to 11 m 55% | 12 |
| P26 | 2018 | 24/F | Neutropenia | 9/10 MMUD BM | Flu/Treo/TT/ATG/ rituximab (MAC) | MTX/CsA | 3 | No | No | None | 2 y 100% | 51 |
| P27 | 2012 | 8/F | Pancytopenia | 9/10 MMUD BM | Flu/Treo/Alem (MAC) | MTX/CsA | 8.6 | No | No | None | 5 y 100% | 98 |
| P28.1 | 2016 | 16/F | RCA, neutropenia | 9/10 MMUD BM | Flu/Treo/TT/ATG (MAC) | MTX/CsA | 1.4 | No | No | Primary GF | ||
| P28.2 | 9/10 MMUD PB (different donor) | Flu/TT/ATG (RIC) (alpha–beta TCD) | MTX/CsA | 4.9 | No | No | None | 1 m 100% | 42 | |||
| P29* | 2007 | 9/M | PRCA | 9/10 MMUD PB (1st HCT from affected MSD) | Flu/TBI450/Alem (RIC) | MTX/CsA | 7.9 | Skin 2 | Skin + liver moderate | AIHA D + 70 bridging liver fibrosis | 3 y 95% | 155 |
| P30* | 2017 | 16/F | RCA, neutropenia | 9/10 MMUD BM | Flu/Mel/TT/Alem (RIC) | prednisone/CsA | TNC = 2.5 | No | Skin mild | Hepatitis | 1 y 100% | 36 |
*Siblings (1 + 12 and 29 + 30); **unconditioned boosts 1 month apart; AIHA autoimmune hemolytic anemia, Alem alemtuzumab, ARDS acute respiratory distress syndrome, hATG horse antithymocyte globulin, BM bone marrow, Bu busulfan, comp complications, CsA cyclosporine A, DLI donor lymphocyte infusion, Flu fludarabine, GF graft failure, GvHD graft versus host disease, HCT hematopoietic cell transplant, m month, MC mixed chimerism, Mel melphalan, MTX methotrexate, MMF mycophenolate mofetil, MSD HLA-matched sibling donor, MUD HLA-matched unrelated donor, MMUD HLA-mismatched unrelated donor, PB peripheral blood, NRH nodular regenerative hyperplasia, PRCA pure red cell aplasia, RCA red cell aplasia, Siro sirolimus, SOS sinusoidal obstruction syndrome, TBI total body irradiation, TNC total nucleated cell dose, Treo treosulfan, TT thiotepa, PTCy post-transplant cyclophosphamide, y year
Engraftment, Graft Failure, Transplant-Related Morbidity, Survival
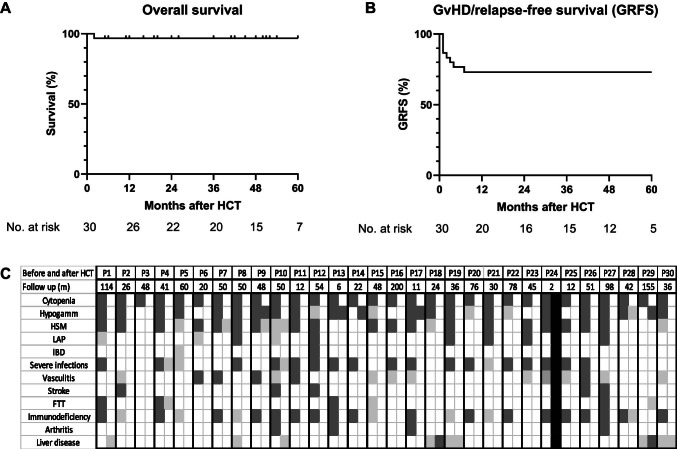
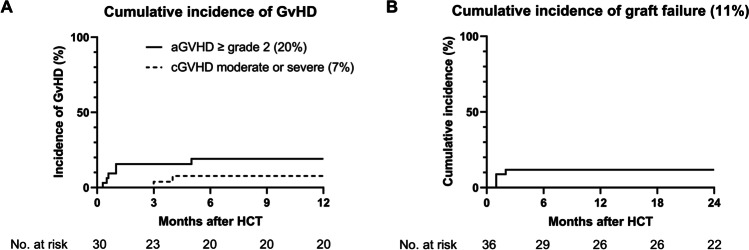
Median engraftment was d + 20 for neutrophils and d + 23 for platelets. In 25/28 patients receiving grafts from unaffected donors, full donor chimerism was achieved by d + 30. Overall survival at 2 years was 97%, with a median follow-up of survivors of 2 years (range: 0.5–16), accounting for 1545 patient-months post-HCT (Fig. 1A). P24 passed away 2 months post-HCT due to respiratory failure secondary to parainfluenza pneumonia despite full donor chimerism and treatment with steroids and etanercept for the suspicion of immune reconstitution inflammatory syndrome. Viral reactivation occurred in 17/30 patients (56%). Adenovirus, CMV, and BK were most frequent involved, each observed in six patients. GRFS was 73% at 2 years, with all events occurring within the first year post-HCT. Of those transplanted with affected donors (n = 2), P16 had primary graft failure requiring salvage HCT, and P29 had obtained near-full donor chimerism but failed erythroid line engraftment and remained with PRCA. Three patients experienced graft failure. P18 required two and P19 one subsequent HCT for secondary GF. The latter two patients were found to have aggregates of CD8 + T cells in their BM with low donor T cell chimerism (9% and 0%) (Fig. 1B, Fig. 2B). P28 required a second HCT likely due to low stem cell dose (1.4 × 106/kg). P12 originally received MAC, but subsequently required two unconditioned HCTs due to drop in whole blood donor chimerism to 30% and new-onset RCA and agranulocytosis unresponsive to G-CSF. Cumulative incidence of aGvHD grades 2–4 was 20% at 1 year. Moderate-severe chronic GvHD developed in 2/30 patients (7%) at 1 year (Fig. 2A). P1, P19, and P24 developed sinusoidal obstruction syndrome (SOS), which responded to fluid restriction and diuresis in P1 and P19 and to defibrotide in P24. All three patients with SOS received either high-dose cyclophosphamide or myeloablative busulfan.
Fig. 1.
Kaplan–Meier curves representing A overall survival, B GvHD-free, relapse-free survival (GRFS). GvHD relapse-free survival was calculated as the time from first HCT until the first occurrence of any of the following events: grades 3–4 aGvHD or moderate/severe cGvHD GF, disease relapse (poor graft function/graft failure with DADA2 disease relapse requiring repeat transplant), or death. Overall survival is calculated on total number of patients (n = 30); GRFS is calculated on total number of HCT procedures, excluding the two procedures performed with stem cells from an affected sibling (n = 34). C Effect of HCT on clinical features resolution. Black squares indicate death post-HCT. Dark gray squares represent the presence of a clinical feature/phenotype. Light gray squares represent major improvement in clinical features. White squares represent complete resolution of clinical features. Each patient is presented by 2 attached columns (before and after HCT) for comparison. Follow-up time post-HCT for each patient is shown in months (second row). Severe infections represent any viral, bacterial, or fungal infection that required antiviral or antifungal treatment or led to sepsis. FTT, failure to thrive; HSM, hepatosplenomegaly; LAP, lymphadenopathy
Fig. 2.
Kaplan–Meier curves representing A cumulative incidence of GvHD (a, acute grade 2 or higher; c, chronic moderate or severe); B cumulative incidence of graft failure. Cumulative incidence of GvHD and cumulative incidence of graft failure are calculated on total number of HCT procedures, excluding the two procedures performed with stem cells from an affected sibling (n = 34)
Cure of DADA2
HCT cured the hematological phenotype in all patients, as confirmed at the most recent follow-up visit (Fig. 1C). No central vascular events were reported after engraftment (Fig. 1C). ADA2 plasma enzyme activity normalized in 16/17 patients tested post-HCT (Table 2). This normalization occurred as early as d + 12, coinciding with the reappearance of monocytes in the PB, as demonstrated by the prospective monitoring of plasma ADA2 enzyme activity in one patient [29]. At last follow-up, 29 patients were still alive, and 28 of these patients displayed full donor chimerism. Donor chimerism fell to 55% in P25 but remained stable over several months, with no evidence of disease. Transient hematological autoimmunity post-HCT was reported in three patients: ITP in two and AIHA in one. This autoimmunity responded to various treatment regimens (intravenous immunoglobulins (IVIG)/steroids/sirolimus/rituximab/bortezomib/romiplostim). Five patients are still on IVIG (one less than a year after HCT, one for mild bronchiectasis, and three post-rituximab treatment).
Discussion
We show here that HCT for DADA2 cures the hematological and immunological phenotypes of DADA2, with no new vascular events, with excellent survival, after a median follow-up of 2 years. Outside this study, two additional patients with DADA2 have received HCT and are reported to be alive and well [30, 31]. Another two patients have also undergone HCT, one of whom died after receiving a graft from a donor heterozygous for the pathogenic mutation in ADA2 (P. Stepensky, personal communication). Adding these patients to the current report, 32 of the 34 DADA2 patients who have undergone HCT were cured. Despite the temporary resolution of neutropenia by a multidrug approach (cyclosporine, steroids, adalimumab) in one patient prior to HCT, this option is not feasible in the long term, and HCT is, therefore, a valuable alternative. HCT also resolved the vascular phenotype in all 15 patients with vasculitis. No additional central nervous system vascular events were reported post-engraftment. Overall, the available data show an absence of new vascular events after engraftment post-HCT, demonstrating that hematopoietic cell-derived ADA2 plays a non-redundant role in restoring monocyte-endothelium interactions [12].
In the presence of this vascular phenotype, despite the impossibility of formal comparisons with other transplant indications, it is advisable to avoid high dose or untargeted busulfan and/or cyclophosphamide or high-dose radiation and to consider preventive measures for SOS. Liver disease was prominent and had a multifactorial etiology, directly related to DADA2 in some cases, but not in others (vasculitis, SOS, inflammation (DADA2/GvHD), iron overload, drug toxicity, GvHD). We therefore advise involving hepatologists in the care of DADA2 patients’ right from diagnosis, with imaging, functional assessment, liver biopsy, and iron chelation if indicated, and opting for the least hepatotoxic conditioning regimen. The evaluation of pre/post-HCT renal disease, although less well described, requires a similarly high level of attention.
Three patients who were grafted with a non-diseased donor, suffered GF. Low CD34+ stem cell dose probably contributed to GF of P28. The choice of conditioning may have contributed to secondary GF in P18 and P19, both of whom had low donor T cell chimerism preceding GF. Our data thus suggest that robust host lymphodepletion, more than myeloablation, is essential in preventing GF. It remains a matter of debate whether a related donor with a single deleterious allele of ADA2 can be considered a suitable donor. Two of the six related donors carried heterozygous ADA2 mutations (P3, P22: both HCT were successful), three were healthy (P1, P2, P21: all HCTs were successful), and one donor was of unknown status (P4). P25 has suffered no disease relapse, with a whole blood donor chimerism of 55%. In P12, whole blood donor chimerism fell to 30%, resulting in disease relapse, suggesting that there is a minimum required level of donor chimerism in DADA2. Indeed, patients carrying only a single pathogenic allele, with intermediate levels of ADA2 enzyme activity, have been reported to manifest DADA2 symptoms [10, 32]. In contrast, 9/10 MMUD and MUD may be considered suitable options for donors: There were 16 MUD and 8 MMUD transplants in this cohort.
The indication for HCT in this cohort was cytopenia and/or malignancy and immunodeficiency, not responding to treatment with TNF inhibitors. DADA2 patients with refractory BMF or immune cytopenia should be referred early on for HCT evaluation, given the morbidity and mortality due to hemorrhage, iron overload, infection, and long-term treatment with multiple immunosuppressive agents [18]. HCT is also a treatment option for patients who do not have access to anti-TNF agents even in the absence of immune cytopenia, BMF, or immunodeficiency. Theoretically, HCT could be a treatment option for patients on long-term treatment with anti-TNF inhibitors who develop neutralizing anti-drug antibodies [33]. Gene therapy (GT) is a promising option for the future, but is unlikely to be available to all DADA2 patients worldwide. In addition, GT requires conditioning and the presence of a sufficient number of autologous hematopoietic stem cells and may fail to reverse immune cytopenias if residual host T cells remain. Thus, GT is only feasible in patients without refractory cytopenia/BMF/malignancy, without prohibitive organ dysfunction, and without host T cell-mediated cytopenias. In the latter, HCT is likely the only potential curative treatment. For both HCT and GT, it emerges that full replacement of the host T cell compartment, and at least partial replacement of the myeloid compartment with ADA2 sufficient cells, is required.
In conclusion, we report here experience with the treatment of 30 DADA2 patients with cytopenia, BMF and immunodeficiency by HCT. All but one of the patients are alive and well and are cured at a median follow-up of 2 years. This successful treatment of an auto-inflammatory condition paves the way for application of HCT in other auto-inflammatory conditions refractory to classic immunosuppressive approaches.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the affected children and their parents for their participation and their confidence. We thank the DADA2 Foundation (www.dada2.org) for organizing the Inaugural International Conference on the Deficiency of ADA2, with special thanks to Chip Chambers, MD for forming the foundation. We thank Ms. Jo Vencken for technical assistance and all colleagues who contributed to the care for these patients. We thank Nancy J. Ganson, PhD and Susan J. Kelly, PhD at Duke University Center, Durham, for performing numerous ADA2 enzyme activity measurements. We also thank Amy Cruickshank, DO at the University of New Mexico, for her contributions. This study was made possible by collaboration with the European Society of Blood and Bone Marrow Transplantation (EBMT), Inborn Errors Working Party (IEWP), and the DADA2 Foundation and European Society for Immunodeficiencies. SU and NA would like to thank ERARE for supporting DBA molecular work-up as a part of the EuroDBA Project. This work is supported by ERN-RITA.
Abbreviations
- HCT
Hematopoietic cell transplantation
- DADA2
Deficiency of adenosine deaminase type 2
- TNF-α
Tumor necrosis factor alpha
- BMF
Bone marrow failure
- PAN
Polyarteritis nodosa
- GvHD
Graft-versus-host disease
- GF
Graft failure
- GRFS
GvHD-free relapse-free survival
- MAC
Myeloablative conditioning
- RIC
Reduced intensity conditioning
- NMA
Non-myeloablative conditioning
- PRCA
Pure red cell aplasia
- MSD
HLA-matched sibling donor
- MUD
HLA-matched unrelated donor
- ATG
Anti-thymocyte globulin
Author Contribution
HH, GB, and I. Meyts collected, analyzed, and interpreted data and wrote the manuscript. HH, SO, SU, IOB, NA, MT, MK, JS, DD, DDH, APH, SMH, RK, GS, ARK, I. Müller, MdS, SD, F. Babor, F. Barzaghi, RB, JVM, VB, SC, MPC, PS, NB, DM, GLG, DB, J. Dalal, JB, ED, J. Dara, CLL, SH, SJ, YK, TG, LM, CS, FC, SS, AS, KW, CC, MH, AO, JK, and I. Meyts provided clinical information from patients and edited the manuscript. MH provided the ADA2 enzyme assay activity data for most of the patients and edited the manuscript. GB helped with data analysis. All the authors contributed to clinical care, data gathering and analysis and edited and approved the final manuscript.
Funding
This project was funded in whole or in part by federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. This research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and (in part) by the National Institute of Allergy and Infectious Diseases, the Sir Jules Thorn Charitable Trust (12/JTA), and the Wellcome Trust (207556_Z_17_Z). Specific ERARE grant funding was attributed to the EuroDBA researchers of Turkey by the Scientific and Technological Research Council of Turkey (TÜBITAK, 315S192).SD is supported by the Personal Research Foundation – Flanders grant 11F4421N. CLL received support from the Immune Deficiency Foundation and Yale University School of Medicine. IM is a Senior Clinical Investigator at the Research Foundation – Flanders and is supported by the CSL Behring Chair of Primary Immunodeficiencies paid to KU Leuven; by the KU Leuven C1 Grant C16/18/007; by a VIB GC PID Grant, by the FWO Grants G0C8517N, G0B5120N, and G0E8420N; and by the Jeffrey Modell Foundation. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No. 948959, MORE2ADA2).
Availability of Data and Materials
Available upon request to the corresponding authors.
Declarations
Ethics Approval
This retrospective study was approved by the Ethics Committee of Leuven University Hospitals (study number S63982 and S63077) and by individual local Ethics Committees.
Consent to Participate
Written informed consent was obtained from participants or their guardians.
Consent for Publication
Patients signed informed consent regarding publishing their data.
Conflict of Interest
The authors have no competing financial interests to declare with respect to this work. IM is holder of CSL-Behring Chair paid to KU Leuven. ARK is a speaker for SOBI. JVM served on an advisory Board for Takeda.
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/30/2022
A Correction to this paper has been published: 10.1007/s10875-022-01280-y
Contributor Information
Hasan Hashem, Email: hh.08847@khcc.jo.
Isabelle Meyts, Email: isabelle.meyts@uzleuven.be.
References
- 1.Zhou Q, Yang D, Ombrello AK, Zavialov AV, Toro C, Zavialov AV, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370(10):911–920. doi: 10.1056/NEJMoa1307361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NavonElkan P, Pierce SB, Segel R, Walsh T, Barash J, Padeh S, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med. 2014;370(10):921–931. doi: 10.1056/NEJMoa1307362. [DOI] [PubMed] [Google Scholar]
- 3.Caorsi R, Penco F, Schena F, Gattorno M. Monogenic polyarteritis: the lesson of ADA2 deficiency. Pediatr Rheumatol Online J. 2016;14(1):51. doi: 10.1186/s12969-016-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashem H, Egler R, Dalal J. Refractory pure red cell aplasia manifesting as deficiency of adenosine deaminase 2. J Pediatr Hematol Oncol. 2017;39(5):e293–e296. doi: 10.1097/MPH.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 5.Van Eyck Jr L, Hershfield MS, Pombal D, Kelly SJ, Ganson NJ, Moens L, et al. Hematopoietic stem cell transplantation rescues the immunologic phenotype and prevents vasculopathy in patients with adenosine deaminase 2 deficiency. J Allergy Clin Immunol. 2015;135(1):283–7 e5. doi: 10.1016/j.jaci.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Ami T, Revel-Vilk S, Brooks R, Shaag A, Hershfield MS, Kelly SJ, et al. Extending the clinical phenotype of adenosine deaminase 2 deficiency. J Pediatr. 2016;177:316–320. doi: 10.1016/j.jpeds.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 7.Trotta L, Martelius T, Siitonen T, Hautala T, Hamalainen S, Juntti H, et al. ADA2 deficiency: clonal lymphoproliferation in a subset of patients. J Allergy Clin Immunol. 2018;141(4):1534–7. [DOI] [PubMed]
- 8.Alabbas F, Elyamany G, Alsharif O, Hershfield M, Meyts I. Childhood Hodgkin lymphoma: think DADA2. J Clin Immunol. 2019;39(1):26–29. doi: 10.1007/s10875-019-0590-7. [DOI] [PubMed] [Google Scholar]
- 9.Le Voyer T, Boutboul D, Ledoux-Pilon A, de Fontbrune FS, Boursier G, Latour S, et al. Late-onset EBV susceptibility and refractory pure red cell aplasia revealing DADA2. J Clin Immunol. 2020;40(6):948–953. doi: 10.1007/s10875-020-00812-8. [DOI] [PubMed] [Google Scholar]
- 10.Schnappauf O, Zhou Q, Moura NS, Ombrello AK, Michael DG, Deuitch N, et al. Deficiency of adenosine deaminase 2 (DADA2): hidden variants, reduced penetrance, and unusual inheritance. J Clin Immunol. 2020;40(6):917–926. doi: 10.1007/s10875-020-00817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinon F, Aksentijevich I. New players driving inflammation in monogenic autoinflammatory diseases. Nat Rev Rheumatol. 2015;11(1):11–20. doi: 10.1038/nrrheum.2014.158. [DOI] [PubMed] [Google Scholar]
- 12.Dhanwani R, Takahashi M, Mathews IT, Lenzi C, Romanov A, Watrous JD, et al. Cellular sensing of extracellular purine nucleosides triggers an innate IFN-beta response. Sci Adv. 2020;6(30):eaba3688. doi: 10.1126/sciadv.aba3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyts I, Aksentijevich I. Deficiency of adenosine deaminase 2 (DADA2): updates on the phenotype, genetics, pathogenesis, and treatment. J Clin Immunol. 2018;38(5):569–578. doi: 10.1007/s10875-018-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahin S, Adrovic A, Kasapcopur O. A monogenic autoinflammatory disease with fatal vasculitis: deficiency of adenosine deaminase 2. Curr Opin Rheumatol. 2020;32(1):3–14. doi: 10.1097/BOR.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 15.Sharma A, Naidu G, Sharma V, Jha S, Dhooria A, Dhir V, et al. Deficiency of adenosine deaminase 2 (DADA2) in adults and children: experience from India. Arthritis Rheumatol. 2020;73(2):276–285 [DOI] [PMC free article] [PubMed]
- 16.Ombrello AK, Qin J, Hoffmann PM, Kumar P, Stone D, Jones A, et al. Treatment strategies for deficiency of adenosine deaminase 2 N Engl J Med. 2019;380(16):1582–4. [DOI] [PMC free article] [PubMed]
- 17.Cooray S, Omyinmi E, Hong Y, Papadopoulou C, Harper L, Al-Abadi E, et al. Anti-tumour necrosis factor treatment for the prevention of ischaemic events in patients with deficiency of adenosine deaminase 2 (DADA2). Rheumatology (Oxford). 2021. 10.1093/rheumatology/keaa837 [DOI] [PubMed]
- 18.Lee PY, Kellner ES, Huang Y, Furutani E, Huang Z, Bainter W, et al. Genotype and functional correlates of disease phenotype in deficiency of adenosine deaminase 2 (DADA2) J Allergy Clin Immunol. 2020;145(6):1664–72 e10. doi: 10.1016/j.jaci.2019.12.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Eyck L, Liston A, Wouters C. Mutant ADA2 in vasculopathies. N Engl J Med. 2014;371(5):480. doi: 10.1056/NEJMc1405506. [DOI] [PubMed] [Google Scholar]
- 20.van Montfrans J, Zavialov A, Zhou Q. Mutant ADA2 in vasculopathies. N Engl J Med. 2014;371(5):478. doi: 10.1056/NEJMc1405506. [DOI] [PubMed] [Google Scholar]
- 21.Hashem H, Kumar AR, Muller I, Babor F, Bredius R, Dalal J, et al. Hematopoietic stem cell transplantation rescues the hematological, immunological, and vascular phenotype in DADA2. Blood. 2017;130(24):2682–2688. doi: 10.1182/blood-2017-07-798660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129(1):30–37. doi: 10.1182/blood-2016-07-686642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barzaghi F, Minniti F, Mauro M, Bortoli M, Balter R, Bonetti E, et al. ALPS-Like phenotype caused by ADA2 deficiency rescued by allogeneic hematopoietic stem cell transplantation. Front Immunol. 2018;9:2767. doi: 10.3389/fimmu.2018.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozen S, Batu ED, Taskiran EZ, Ozkara HA, Unal S, Guleray N, et al. A Monogenic disease with a variety of phenotypes: deficiency of adenosine deaminase 2. J Rheumatol. 2020;47(1):117–125. doi: 10.3899/jrheum.181384. [DOI] [PubMed] [Google Scholar]
- 25.Brooks JP, Rice AJ, Ji W, Lanahan SM, Konstantino M, Dara J, et al. Uncontrolled Epstein-Barr virus as an atypical presentation of deficiency in ADA2 (DADA2). J Clin Immunol. 2021;41(3):680–3. [DOI] [PubMed]
- 26.Van Montfrans JM, Hartman EA, Braun KP, Hennekam EA, Hak EA, Nederkoorn PJ, et al. Phenotypic variability in patients with ADA2 deficiency due to identical homozygous R169Q mutations. Rheumatology (Oxford) 2016;55(5):902–910. doi: 10.1093/rheumatology/kev439. [DOI] [PubMed] [Google Scholar]
- 27.Hsu AP, West RR, Calvo KR, Cuellar-Rodriguez J, Parta M, Kelly SJ, et al. Adenosine deaminase type 2 deficiency masquerading as GATA2 deficiency: successful hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2016;138(2):628–30 e2. doi: 10.1016/j.jaci.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itan Y, Bryson K, Thomas MG. Detecting gene duplications in the human lineage. Ann Hum Genet. 2010;74(6):555–565. doi: 10.1111/j.1469-1809.2010.00609.x. [DOI] [PubMed] [Google Scholar]
- 29.Bucciol G, Delafontaine S, Segers H, Bossuyt X, Hershfield MS, Moens L, et al. Hematopoietic stem cell transplantation in ADA2 deficiency: early restoration of ADA2 enzyme activity and disease relapse upon drop of donor chimerism. J Clin Immunol. 2017;37(8):746–750. doi: 10.1007/s10875-017-0449-8. [DOI] [PubMed] [Google Scholar]
- 30.Gibson PG. Allergic bronchopulmonary aspergillosis. Semin Respir Crit Care Med. 2006;27(2):185–191. doi: 10.1055/s-2006-939521. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Wang W, Wang Y, Hou J, Ying W, Hui X, et al. A Chinese DADA2 patient: report of two novel mutations and successful HSCT. Immunogenetics. 2019;71(4):299–305. doi: 10.1007/s00251-018-01101-w. [DOI] [PubMed] [Google Scholar]
- 32.Carmona-Rivera C, Khaznadar SS, Shwin KW, Irizarry-Caro JA, O'Neil LJ, Liu Y, et al. Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2. Blood. 2019;134(4):395–406. doi: 10.1182/blood.2018892752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klareskog L, Gaubitz M, Rodriguez-Valverde V, Malaise M, Dougados M, Wajdula J, et al. Assessment of long-term safety and efficacy of etanercept in a 5-year extension study in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2011;29(2):238–247. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available upon request to the corresponding authors.