Summary
Neuromuscular junctions (NMJs) ensure communication between motor neurons (MNs) and muscle; however, in MN disorders, such as amyotrophic lateral sclerosis (ALS), NMJs degenerate resulting in muscle atrophy. The aim of this study was to establish a versatile and reproducible in vitro model of a human motor unit to investigate the effects of ALS-causing mutations. Therefore, we generated a co-culture of human induced pluripotent stem cell (iPSC)-derived MNs and human primary mesoangioblast-derived myotubes in microfluidic devices. A chemotactic and volumetric gradient facilitated the growth of MN neurites through microgrooves resulting in the interaction with myotubes and the formation of NMJs. We observed that ALS-causing FUS mutations resulted in reduced neurite outgrowth as well as an impaired neurite regrowth upon axotomy. NMJ numbers were likewise reduced in the FUS-ALS model. Interestingly, the selective HDAC6 inhibitor, Tubastatin A, improved the neurite outgrowth, regrowth, and NMJ morphology, prompting HDAC6 inhibition as a potential therapeutic strategy for ALS.
Keywords: amyotrophic lateral sclerosis, FUS, microfluidic device, neurite outgrowth, neurite regrowth, neuromuscular junction, HDAC6, Tubastatin A
Highlights
-
•
Human motor units with functional NMJs can be generated using microfluidic devices
-
•
FUS-ALS motor units display impaired neurite regrowth, outgrowth and NMJ numbers
-
•
HDAC6 inhibition alleviate FUS-ALS motor unit pathology in vitro
This study provides a novel model of a motor unit in microfluidic devices. Van Den Bosch and colleagues report how mutations in FUS causing amyotrophic lateral sclerosis (ALS) compromise the motor neuron neurite outgrowth, regrowth, and number of neuromuscular junctions. These findings were alleviated by selective HDAC6 inhibition prompting this approach as a therapeutic strategy for ALS.
Introduction
Neuromuscular junctions (NMJs) are specialized synapses, which are crucial for the communication between spinal lower motor neurons (MNs) and skeletal muscle (Plomp 2018). NMJs become vulnerable in neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS), where the degeneration of NMJs results in muscle weakness and atrophy, ultimately causing respiratory insufficiency and death (Dadon-Nachum et al., 2010; Murray et al., 2010). There is ample evidence that the disconnection of the motor axon from the muscle is the first step in the disease process of ALS (Fischer et al., 2004; Martineau et al., 2018), making it an important mechanism to study. Initially, the MN will compensate for this retraction by axonal sprouting and collateral reinnervation. However, when the disease progresses, this compensation fails and the MN eventually dies, a phenomenon defined as the “dying-back” mechanism (Robberecht and Philips, 2013). Symptoms in patients start to occur when large populations of MNs are affected, resulting in weakness of muscle groups. MNs have very long axons, which makes them more susceptible to this dying-back mechanism compared with other neurons. This partially explains the selective vulnerability in ALS pathogenesis, and evidence of dying back and loss of NMJs has been reported in several ALS-mouse models and in patients (Fischer et al., 2004; Martineau et al., 2018; Nair et al., 2010; So et al., 2018; Tallon et al., 2016; Walker et al., 2015).
To further study the NMJs and their relation to ALS, it is beneficial to have a standardized, human cell-derived model of these NMJs in vitro, for which co-culturing of MNs and skeletal muscle is required. Co-cultures of these highly specialized cell types in single compartments have been developed using both human and animal cells and, in some studies, functional NMJs were established (Demestre et al., 2015; Guo et al., 2011; Lin et al., 2019; Puttonen et al., 2015; Umbach et al., 2012). However, such models are unable to account for the unique culture microenvironments occupied by cell-specific domains of MNs and muscle fibers (de Jongh et al., 2021; Millet and Gillette, 2012). The use of “Campenot” chambers (Campenot 1977) and microfluidic devices offers the opportunity to overcome this problem (Bellmann et al., 2019; Mills et al., 2018; Osaki et al., 2018a; Santhanam et al., 2018; Southam et al., 2013; Zahavi et al., 2015). The fluidically isolated compartments, between which only neurites can grow, not only allow for maintenance of cell-type-specific microenvironments, but also allow for isolation of subcellular compartments, such as the distal and the proximal part of the axon, for region-specific analyses (Naumann et al., 2018; Nijssen et al., 2018; Taylor et al., 2005).
While the development of customized microfluidic platforms (Bellmann et al., 2019; Osaki et al., 2018b) has significantly improved the potential for disease modeling, a standardized accessible method for the formation of human NMJs in vitro is lacking. Therefore, we aimed to develop a human-derived system, which combines commercially available microfluidic devices, induced pluripotent stem cell (iPSC)-derived MNs, and mesoangioblasts (MAB)-derived myotubes to generate and study functional human NMJs in a compartmentalized system. With the supplementation of agrin and laminin (A/L), we increased the clustering of nicotinic acetylcholine receptors (AChRs), the outgrowth of neurites and the formation of NMJs. Using this model, we discovered a compromised outgrowth and regrowth after axotomy of MN neurites, as well as an impairment in NMJ numbers in ALS-FUS mutant co-cultures in comparison with their CRISPR-Cas9 gene-edited isogenic controls. Interestingly, we could improve these phenotypes by treating with the selective histone deacetylase 6 (HDAC6) inhibitor, Tubastatin A (TubA). Our findings show that this in vitro model is a valuable tool to assess human NMJ physiology in health and disease.
Results
Generation of human iPSC-derived MNs and MAB-derived myotubes
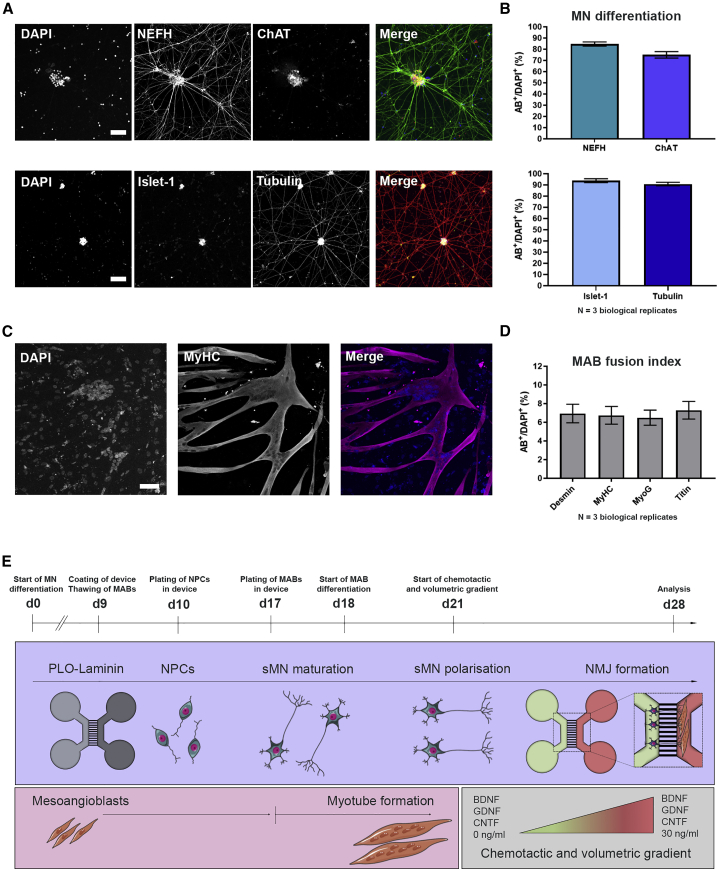
To generate human NMJs in vitro, we made use of human iPSCs (hiPSCs) and human primary MABs. MABs are vessel-associated mesenchymal stem cells, which are isolated from adult skeletal muscle tissue (Dellavalle et al., 2007; Rotini et al., 2018; Tonlorenzi et al., 2007). MABs were expanded and subsequently differentiated into myotubes over a 10-day period, while hiPSCs were differentiated into MNs via a 28-day protocol (Guo et al., 2017). MN differentiation efficiency and MAB fusion index were evaluated using immunocytochemistry (ICC). At day 28 of MN differentiation, the hiPSC-derived MNs stained positive for MN-specific markers neurofilament heavy chain (NEFH), choline acetyltransferase, and Islet-1, in addition to the pan-neuronal marker βIII-tubulin (Figures 1A and 1B), similar to previous studies utilizing the same MN differentiation protocol (Guo et al., 2017; Vandoorne et al., 2019). After 10 days of differentiation, the human MAB-derived multinucleated myotubes stained positive for specific muscle markers (Figures 1C, 1D, and S1).
Figure 1.
Characterization of monocultures and overview of NMJ protocol
(A) Confocal images of MNs stained with MN markers neurofilament heavy chain (NEFH), choline acetyltransferase (ChAT), and Islet-1, as well as the pan-neuronal marker βIII-tubulin (Tubulin) at day 28 of MN differentiation. Nuclei stained with DAPI. Scale bars, 75 μm.
(B) Number of cells positive for MN and pan-neuronal markers (AB+). Mean ± SEM of three biological replicates.
(C) Confocal images of myotube heavy chain (MyHC)-positive myotubes 10 days after initiation of differentiation. Scale bar, 75 μm.
(D) Quantification of mesoangioblast (MAB) fusion into multinucleated myotubes (fusion index) with myotube markers desmin, MyHC, myogenin (MyoG) or titin. Mean ± SEM of three biological replicates.
(E) Schematic overview of co-culture protocol and differentiation timeline (days 0–28). Day 0 (d0), differentiation of iPSCs into MN. Day 9 (d9), microfluidic devices are coated with poly-L-ornithine (PLO) and laminin, and MABs are thawed for expansion. Day 10 (d10), MN-NPCs are plated on one side (light gray) of the device. Day 17 (d17), MABs are seeded in the opposite side of the device (dark gray). Myotube differentiation is initiated (d18). Day 21 (d21), a volumetric and chemotactic gradient of neurotrophic factors (BDNF, GDNF and CNTF) is implemented to facilitate polarized growth of spinal MNs (sMN) through the microgrooves toward the myotubes to initiate the formation of NMJs (d28). Cell illustrations were modified from Smart Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
See also Figure S1.
NMJs spontaneously form in a MN-myotube microfluidic co-culture system
To generate NMJs through co-culturing of MNs and MABs, we utilized two types of Xona Microfluidics devices (SND75 and XC150). To ensure compartmentalization, MN-neural progenitor cells (NPCs) were plated at day 10 of MN differentiation on one side of the device (Figure 1E, light gray compartment) and allowed to differentiate for 1 week in the device, before MABs were plated at day 17 of MN differentiation on the other side of the fluidically isolated device (Figures 1E, dark gray compartment and S1). The difference in plating time was conducted to ensure an efficient co-culture between maturing MNs and fusing myotubes, to make full use of the culture window in which MAB-derived myotubes are viable.
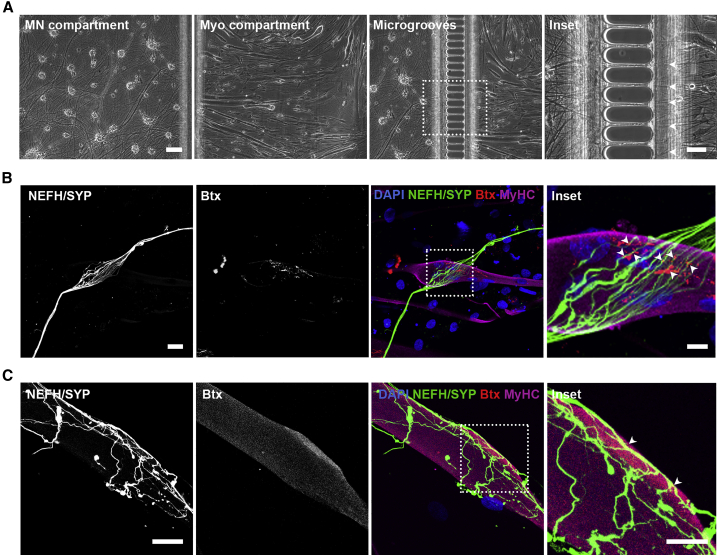
On day 21 of MN differentiation, a chemotactic growth factor and volumetric gradient was implemented to induce the migration of MN axons (Figure 1E, green compartment) through the microgrooves to the compartment containing myotubes (Figure 1E, red compartment). Due to these gradients, a larger proportion of axons migrated through the microgrooves from the MN compartment toward the myotube compartment (Figure 2A), where they were able to connect with clusters of α-bungarotoxin (Btx)-positive AChRs expressed on myotubes. AChRs represent an important guidance cue for MN axons and hence NMJ formation in vivo (Burden et al., 2018) and we could indeed observe interactions of MN neurites and AChR clusters on myotubes indicative of the formation of NMJ-like structures (referred to as NMJs from here on) (Figures 2B and 2C). However, only a limited number of AChR clusters and hence NMJs were identified in each culture.
Figure 2.
Co-culturing of MNs and myotubes in microfluidic devices leads to NMJ formation
(A) Bright-field micrographs of MN and myotube (Myo) compartments in the XC150 device on day 28. Insets: magnification of axonal migration through microgrooves (arrowheads). Scale bars, 100 μm and 50 μm (insets).
(B and C) Confocal micrograph of NMJ formation are shown as co-localization of presynaptic marker synaptophysin (SYP) and acetylcholine receptor marker α-bungarotoxin (Btx) on MyHC-labeled myotubes. Insets: magnification of co-localizations (arrowheads). Scale bars, 25 μm and 10 μm (insets).
Agrin and laminin stimulate neurite outgrowth and NMJ formation
To increase the NMJ numbers in our model, we tested whether supplementing the myotube culture medium with A/L had an effect, as these compounds have been shown to increase the AChR clustering at the sarcolemma as well as to enhance NMJ formation (Afshar Bakooshli et al., 2019; Burkin et al., 2000; Zhang et al., 2016). AChR clustering on cultured myotubes is independent of neuronal presence (Kummer et al., 2004; Osseni et al., 2020), so, to investigate the effect on human primary MABs-derived myotubes, we quantified AChR clustering with and without these compounds (Figure S2). Our data showed an increase in AChR clusters per myotube with A/L in comparison with untreated controls (Figure S2C). A/L supplementation did not affect the ability of MABs to fuse into myotubes (Figure S1).
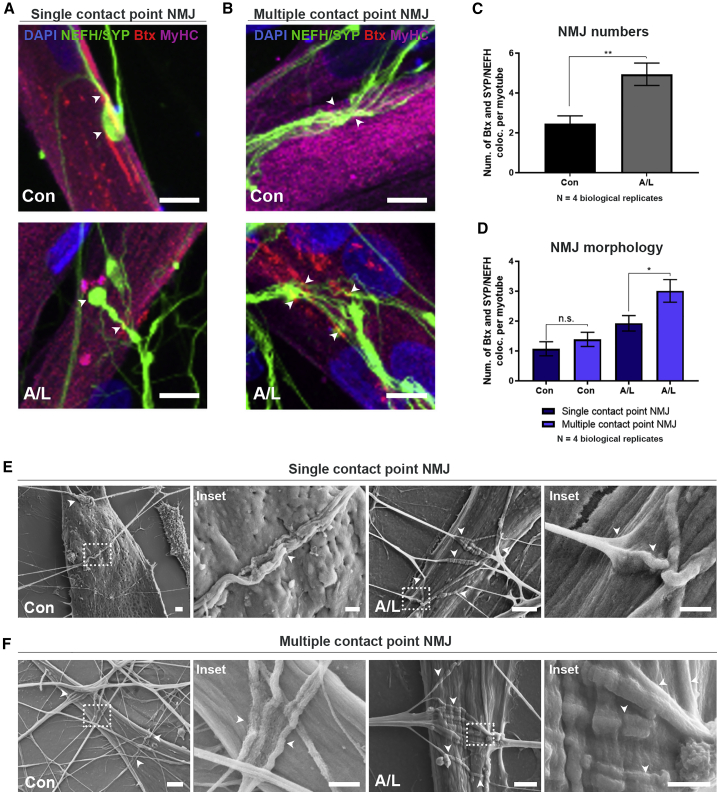
NMJs were identified through co-localization of distal neurites (NEFH and synaptophysin) with Btx-positive AChRs on MyHC-labeled myotubes and the number of interactions was normalized to the number of myotubes. The NMJs either appeared rudimentary and circular as single contact point NMJs (Figures 3A and S2D), or elongated with broad, flat, irregular multiple contact point NMJs (Figures 3B and S2E). In most cases, contacts between axons and myotubes did not produce distinct axon termination in endplate formation as seen in vivo but rather revealed a continuation of axonal growth after embedment into myotubes (Figure 3B). No obvious morphological differences in size, shape, or embedment form were observed immunocytochemically between controls and conditions with A/L (Figures 3 and S2). However, addition of A/L doubled the total number of NMJs per myotube in comparison with control devices (Figure 3C). A/L also increased the number of multiple contact point NMJs in comparison with single contact point NMJs (Figure 3D). Scanning electron microscopy (SEM) showed neurite embedment into the surface of myotubes (Figures 3E and 3F) in line with our ICC observations. In addition, we observed larger, elongated NMJs with A/L compared with untreated controls, further confirming the beneficial effect of these compounds.
Figure 3.
Agrin and laminin improve NMJ formation in microfluidic devices
(A and B) Confocal micrographs of NMJs in agrin (0.01 μg/mL)- and laminin (20 μg/mL)-supplemented (A/L) and untreated (Con) conditions on day 28 in XC150 devices. Single contact point NMJs (A). Multiple contact point NMJs (B). Scale bars, 10 μm. Arrowheads: Btx-SYP/NEFH co-localizations.
(C) Number of Btx and SYP/NEFH co-localizations per myotube.
(D) Quantifications of NMJ single and multiple contact point morphology.
(E and F) Representative scanning electron microscopy (SEM) images of NMJ formation in SND75 devices on day 28 in A/L-supplemented and control conditions. Single contact point NMJs (E). Multiple contact point NMJs (F). Insets: magnifications of NMJ (arrowheads). Scale bars, 2 μm and 1 μm (insets). (C) Unpaired t test. (D) One-way ANOVA with Tukey's multiple comparisons test. Mean ± SEM of four biological replicates. ∗p < 0.05, ∗∗p < 0.01.
See also Figures S2 and S3.
As A/L are signaling molecules important for neurite guidance, we investigated whether these supplements have an effect on neurite outgrowth in addition to NMJ formation. To quantify the difference, we acquired tile scan images of the myotube compartment and isolated the neurites (Figure S3). The images were converted to a mask and analyzed with a linear Scholl analysis script similar to a previously published method (Jocher et al., 2018) (Figures S3A and S3B). The Scholl analysis creates an intersection line every 50 μm, and the number of neurite pixels per intersection was quantified (Figure S3C). Due to the high density of neurites, which grow through the microgrooves in thick bundles, we omitted the first intersection at 50 μm distance from the microgroove exit. The initial exponential increase in pixel intersections correlated with the “debundling” and spreading of neurites post microgroove crossing (Figure S3C). Interestingly, we found an increase in neurite outgrowth due to A/L treatment.
Taken together, these results demonstrate the formation of human NMJs in our in vitro system. Supplementation of A/L promoted the outgrowth of neurites and increased the total number of NMJs per myotube as well as the contact areas between branching MN axons and myotubes further enhancing the embedment.
Human in vitro NMJs are functional through MN stimulation
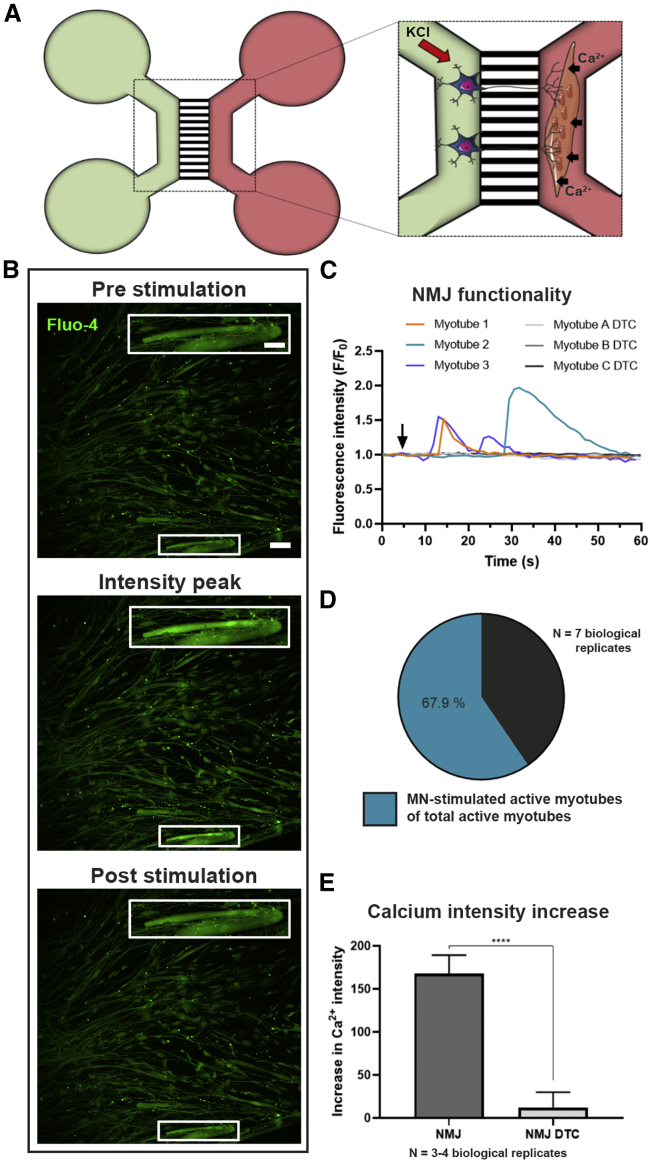
To evaluate whether the NMJs were functional, we performed live-cell Ca2+ imaging (Figure 4). MN somas and proximal axons were depolarized using 50 mM KCl and a subsequent increase in Ca2+ influx in the myotubes preloaded with the Ca2+-sensitive Fluo-4 dye was measured (Figure 4A). The fluidic isolation of the compartments in the microfluidic device ensured no direct contact between the high KCl solution and the myotubes (Figure S4A). Stimulation of MNs resulted in Ca2+ transient waves in the MN-innervated myotubes, indicating a functional connection through NMJ formation (Figures 4B and 4C).
Figure 4.
In vitro NMJs are functionally active
(A) Schematic overview of transient fluorescent Ca2+ imaging in XC150 devices on day 28. MN compartment (green) is stimulated with 50 mM KCl, which initiates an intracellular response through MN axons evoking an increase in Ca2+ influx in the Fluo-4-loaded myotubes (red compartment). Cell illustrations are modified from Smart Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
(B) Fluorescent micrograph examples of before, during, and after stimulation depicting a wave of increase in intracellular Ca2+ in myotubes upon MN stimulation. Inset shows a magnification of an innervated active myotube. Scale bars, 100 μm and 200 μm (insets).
(C) Representative Ca2+ influx curves in myotubes after KCl activation (arrow) of MNs before (myotube 1–3) and after 10 min treatment with NMJ blocker tubocurarine (myotube A-C DTC).
(D) Ratio of MN-stimulated active myotubes to directly KCl-activated myotubes.
(E) Effect of DTC on the intensity fluorescent increase due to Ca2+ influx induced by KCl increase in the MN compartment. Mean ± SEM of three to four biological replicates. Mann-Whitney test. ∗∗∗∗p < 0.0001.
See also Figure S4.
To differentiate between excitable and non-excitable myotubes, we stimulated the myotubes directly with KCl in the myotube compartment after MN stimulation (Figure S4B). Excitable myotubes were considered responsive to KCl through Ca2+ wave formation. Approximately 68% of myotubes of total directly KCl-stimulated active myotubes were responsive through MN stimulation (Figure 4D). Quantification of peak values of the Ca2+ influx was performed on myotubes demonstrating MN-dependent activity (Figure 4E). To confirm that the Ca2+ transients were due to NMJ transmission, NMJs were blocked using the nicotinic AChR competitive antagonist, tubocurarine (DTC) (Afshar Bakooshli et al., 2019), which successfully inhibited the Ca2+ transients in our system (Figures 4C and 4E). To assess whether the myotube excitability was dependent on MN presence, we cultured myotubes in 24-well plates and stimulated them with KCl (Figures S4C and S4D). Myotubes were active regardless of MN presence (Figure S4C), and they likewise emitted a Ca2+ response independent of DTC treatment, excluding a direct inhibitory effect of DTC on muscle depolarization (Figure S4D). In conclusion, these results confirm the functional MN innervation of myotubes through NMJ formation.
Mutant FUS-ALS causes a reduction in NMJ numbers
To assess the effect of disease-causing ALS mutations in this human in vitro NMJ model, we used two iPSC lines with mutations in the FUS RNA binding protein (FUS) gene. One from a 17-year-old ALS patient with a de novo point mutation (P525L) and another one from a 71-year-old ALS patient with a R521H mutation. These patient lines were systematically compared with their corresponding CRISPR-Cas9 gene-edited isogenic P525P and R521R controls, respectively (Guo et al., 2017; Wang et al., 2018). MN differentiation verification with ICC showed no difference in the differentiation potential between the different lines with an approximate expression of 85%–95% of MN markers (Figures S4E–S4G).
NMJ quantifications revealed a higher number of NMJs per myotube in the P525P isogenic control system than in the P525L (Figures 5A, 5B, and S5), indicating a mutant FUS-dependent impairment of NMJs. A small difference was also observed between the R521R and R521H co-cultures. In addition, we observed a higher number of multiple contact point NMJs than single contact point NMJs (Figure 5C) in the isogenic control systems, while this difference was not observed in the mutant systems, suggesting that there is a difference in the maturation state between the ALS and control co-cultures.
Figure 5.
NMJs are impaired in ALS
(A) Confocal micrographs of NMJs with A/L from ALS-FUS (P525L, R521H) and isogenic control (P525P, R521R) MN/myotube co-cultures on day 28. Scale bars, 10 μm. Arrowheads: Btx-SYP/NEFH co-localizations.
(B) Quantification of NMJs as Btx and SYP/NEFH co-localizations and expressed per myotube.
(C) Quantification of NMJ single and multiple contact point morphology. Mean ± SEM of three to four biological replicates. (B) Unpaired t test or Mann-Whitney test. (C) Kruskal-Wallis test with Dunn's multiple comparisons test. ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
See also Figures S4, S5, and S7.
MN neurite outgrowth and regrowth is diminished in FUS-ALS
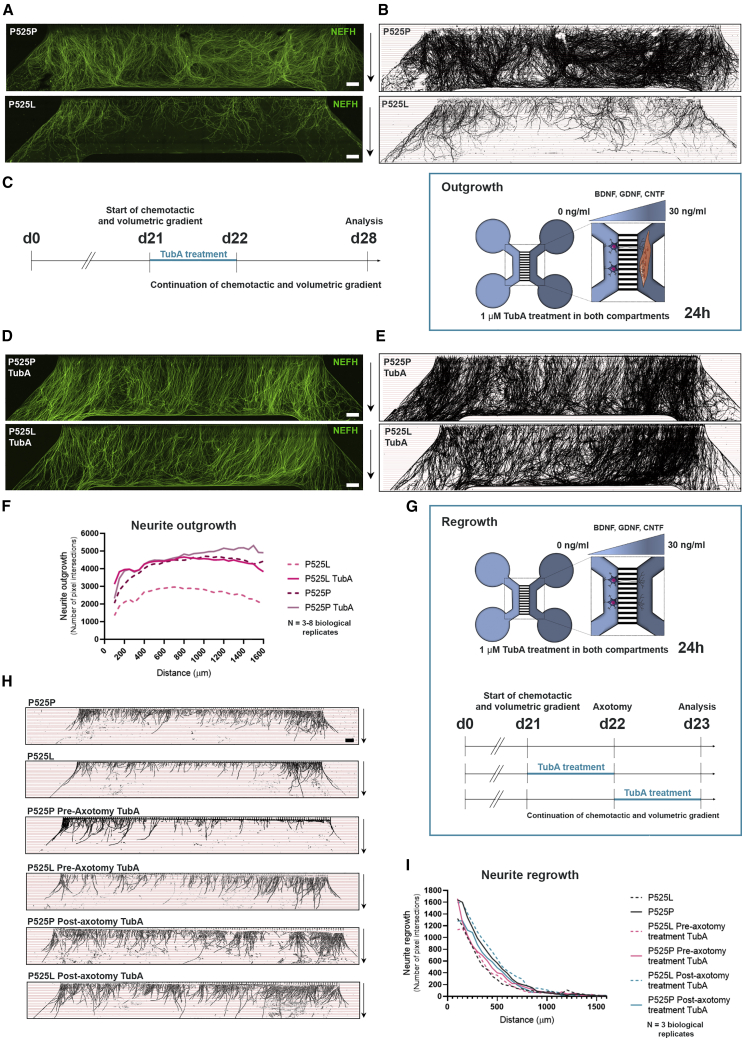
When culturing the motor unit systems for NMJ quantifications, we also observed a striking difference in neurite outgrowth between the mutant P525L and its isogenic control P525P (Figures 6A and 6B), which was confirmed with our linear Scholl analysis (Figure 6F). No obvious difference in outgrowth was observed in the other pair (R521H and R521R) (Figures S6A, S6B, and S6E). Next, we investigated whether this difference in neurite outgrowth was dependent on myotube presence (Figures S6F–S6K). Interestingly, we observed fewer neurites crossing in smaller bundles in the devices without myotubes, suggesting that myotubes play a role in the polarization of neurites in addition to the volumetric and chemotactic gradient. A similar decrease in neurite outgrowth in FUS-mutant P525L in comparison with isogenic control P525P systems was observed without myotubes present, although the difference was less pronounced. To test whether mutant and isogenic control MNs showed any variation in their initial neurite outgrowth, we cultured MN-NPCs from P525L and control P525P in 24-well plates and analyzed the neurite outgrowth using IncuCyte NeuroTrack software (Figures S7A and S7B). Images were taken every 2 h over the first 24 h after plating of NPCs and no difference in neurite outgrowth was observed between the two lines, suggesting that this phenotype only appears later upon MN maturation.
Figure 6.
ALS-dependent impairment in neurite outgrowth and regrowth is rescued by HDAC6 inhibition
(A) Tile scan confocal overviews of neurite outgrowth (NEFH) in the myotube compartment from P525L and P525P cultures at day 28. Arrows (right): growth direction from exit of microgrooves. Scale bars, 300 μm.
(B) Masks of tile scans with intersection lines at every 50 μm starting from microgroove exit.
(C) Schematic representation of outgrowth experimental time course implementing treatment with TubA. At day 21 (d21), both MN and myotube compartments were treated with 1 μM TubA for 24 h in addition to the start of the chemotactic and volumetric gradient.
(D) Tile scan confocal overviews of neurite outgrowth in myotube compartment at day 28 from P525L and P525P MN/myotube co-cultures with TubA treatment. Scale bars, 300 μm.
(E) Masks of tile scans with TubA treatment.
(F) Neurite outgrowth quantifications of the number of pixel intersections in P525L and P525P MN/myotube co-cultures with and without TubA treatment. Mean graph of three to eight biological replicates.
(G) Schematic representation of regrowth experimental time course implementing 24 h treatment with TubA before or after axotomy (day 22).
(H) Masks of tile scan overviews after 24 h neurite regrowth in empty myotube compartment from P525L and P525P cultures without, and with pre-axotomy and post-axotomy treatment with TubA. Arrows (right): growth direction from exit of microgrooves. Scale bar, 300 μm.
(I) Neurite regrowth quantifications of the number of pixel intersections. Mean graph of three biological replicates. Cell illustrations in (C and G) are modified from Smart Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
See also Figures S4, S6, and S7 and Tables S3 and S4.
We next investigated whether a similar difference could be found when evaluating MN neurite regrowth upon axotomy. Therefore, we cultured MNs from FUS-mutant P525L and its isogenic control P525P in devices without myotubes, and performed a combined mechanical and chemical axotomy at day 22 of MN maturation (Figure 6G). The neurites were allowed to regrow for 24 h before fixation, imaging, and linear Scholl analysis. Here, we also observed a reduction in the neurite regrowth in the P525L MNs in comparison with the control P525P MNs (Figures 6H and 6I).
Taken together, these results show that the number of NMJs and the MN neurite outgrowth/regrowth are affected by mutations in FUS in our motor unit system. These impairments can be successfully rescued by correction of the mutations, emphasizing that the effects are a direct cause of the point mutation in FUS.
HDAC6 inhibition rescues mutant FUS-mediated impairments of neurite outgrowth and regrowth
Many studies demonstrated beneficial effects of HDAC6 inhibition in various models of neurodegenerative diseases Benoy et al., 2017, Benoy et al., 2018; d’Ydewalle et al., 2011; Fazal et al., 2021; Guo et al., 2017; Krukowski et al., 2017; Mao et al., 2017; Van Helleputte et al., 2018). HDAC6 is primarily found in the cytosol where it can deacetylate α-tubulin (Prior et al., 2018; Rossaert and Van Den Bosch, 2020), and we showed previously that HDAC6 inhibition can rescue axonal transport defects in mutant FUS MNs (Guo et al., 2017). As the impairment in neurite outgrowth and regrowth could correlate with axonal transport deficits, we investigated whether HDAC6 inhibition could also improve these phenotypes.
Therefore, we first treated our MN-myotube co-cultures with the specific HDAC6 inhibitor, TubA (1 μM), in both the MN and the myotube compartment for 24 h, at day 21 when the chemotactic and volumetric gradient was imposed (Figure 6C). Interestingly, TubA treatment had a striking effect on the neurite outgrowth in the mutant P525L co-cultures, improving the outgrowth of neurites to a similar level as the isogenic control P525P (Figures 6D–6F). TubA treatment also increased the outgrowth of neurites for the other FUS mutation (R521H) to a level above the control (Figures S6C and S6E), but had no effect on the initial outgrowth of P525P and P525L MN neurites during the first 24 h of culture (Figures S7A and S7B). TubA treatment had no effect on the previously described cytoplasmic mislocalization of FUS in the P525L MNs (Figures S7C and S7D) (Guo et al., 2017).
We next treated our P525L and P525P MNs in the devices for the regrowth experiment with TubA in both compartments. MNs were treated for 24 h either from days 21 to 22 (pre-axotomy) or from days 22 to 23 (post-axotomy) (Figure 6G). Remarkably, post-axotomy TubA treatment could rescue the FUS-mediated impairment in regrowth in the P525L MNs, while pre-axotomy TubA treatment had no effect (Figures 6H and 6I). These results suggest that HDAC6 inhibition could be useful as a treatment after injury rather than a prophylactic therapy.
HDAC6 inhibition improves NMJ formation in FUS-ALS
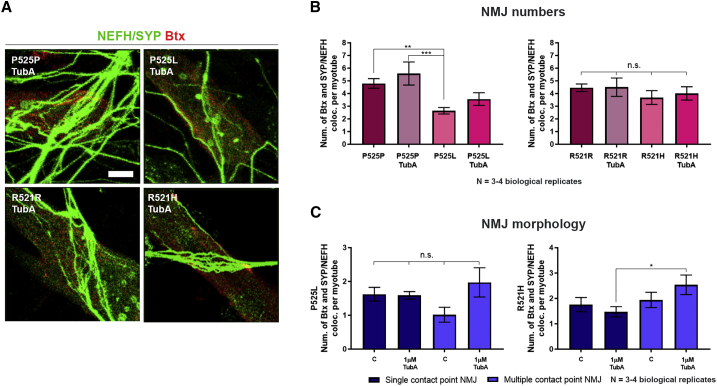
Since TubA treatment proved to have a beneficial effect on neurite outgrowth and regrowth, and HDAC6 recently has been demonstrated to regulate AChR receptor clustering (Osseni et al., 2020), we next investigated whether this would have an effect on the formation of NMJs. Indeed, compared with our control NMJ data, we observed that 24 h TubA treatment resulted in a tendency toward an increase in total and multiple contact point NMJ numbers in FUS-ALS P525L co-culture systems, although this did not reach statistical significance (Figures 7A and 7B). However, we could demonstrate a significant increase in multiple contact point NMJs in the FUS-ALS R521H co-cultures reaching similar levels as its corresponding control (R521R, Figure 7C).
Figure 7.
HDAC6 inhibition improves ALS-dependent impairments in NMJ morphology
(A) Confocal micrographs of NMJs with TubA treatment. Scale bar, 10 μm.
(B) Quantification of NMJs as Btx-SYP/NEFH co-localization per myotube with and without TubA treatment. Control data without TubA treatment are identical to Figure 5B.
(C) Morphological analysis of NMJs without (C) and with TubA treatment. Control data (C) are identical to Figure 5C. Mean ± SEM from three to four biological replicates. One-way ANOVA with Tukey's multiple comparisons test or Kruskal-Wallis test with Dunn's multiple comparisons test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
See also Figures S4 and S7.
In conclusion, we observe a beneficial effect of HDAC6 inhibition on neurite outgrowth, regrowth, and NMJ formation in mutant FUS co-cultures illustrating that our in vitro system can be used to test new therapeutic strategies for MN diseases.
Discussion
In this study, we developed a standardized co-culture model of hiPSC-derived MN and human primary MAB-derived myotubes in commercial microfluidic devices resulting in the formation of functional human NMJs. We observed that supplementation of A/L promoted polarized MN outgrowth as well as NMJ formation, further enhancing the output of this in vitro model. In contrast, mutations in FUS had a striking negative effect on neurite outgrowth, neurite regrowth, and NMJ numbers. We could successfully normalize these phenotypes by correcting the point mutations, emphasizing that the impairments are a direct cause of the mutations in FUS. Finally, we discovered that HDAC6 inhibition can improve the neurite outgrowth/regrowth impairments and partially restore the formation of NMJs, further prompting HDAC6 inhibition as a potential therapeutic strategy in ALS.
A human NMJ model eliminates the challenge of interspecies variability and reduces the need for overexpression of mutated genes in animal models, which might not fully recapitulate human disease etiology or pathology (Greek and Hansen, 2013; Morrice et al., 2018). In terms of NMJ morphology, it is evident that rodent NMJs are larger, more complex, and less fragmented than human NMJs, and that human NMJs are more stable across the human adult lifespan (Jones et al., 2017). These differences highlight the importance of a human model of NMJs for mechanistic insights in human NMJ physiology and disease. This model generates single contact point NMJs, which resemble “nummular” morphology in human NMJs from amputates (Jones et al., 2017). In addition, large, irregular multiple contact point NMJs are generated, which present a more complex morphology compared with the nummular NMJs and might therefore be a sign of further maturation.
An advantage of our system is the use of hiPSC-derived MNs, which allows for full adaptability to other MN disorders, including sporadic forms of ALS. In addition, the use of MABs in an NMJ co-culture model has, to our knowledge, not been reported before. MABs present a valid alternative to human satellite cells or myoblasts, since they age less, can undergo more passages, and are generally easier to keep in culture (Dellavalle et al., 2007). Prolonging myotube viability in vitro and hence increased NMJ maturity could likely be accomplished by the incorporation of a dynamic adherence system allowing for myotube contractility (Osaki et al., 2018b). However, this requires a redesign of the commercial microfluidic device. An ideal model would contain both iPSC-derived MNs and myotubes from the same donor; however, current protocols display large variabilities in myotube fusion index (Jiwlawat et al., 2018), which lowers the compatibility and compromises the output of our system.
Using our model, we investigated the effects of mutant FUS on the formation of human NMJs. Mutations in FUS cause the mislocalization of FUS from the nucleus to the cytoplasm, which has recently been reported in other familial as well as sporadic cases of ALS (Tyzack et al., 2019). Interestingly, we saw a reduction in total NMJs with an almost 50% decrease in the FUS-P525L system in comparison with the control P525P. The FUS mutations did not affect the amount of single contact point NMJs, but had a large impact on the number of multiple contact point NMJs. This is in line with the dying-back mechanism where mature NMJs are lost in disease, while newly formed immature NMJs are made simultaneously to compensate for the lack of muscle innervation. However, we did not observe a significant increase in single contact point NMJs in the mutated system, which could explain such a compensatory mechanism, although a tendency could be observed. As a consequence, we cannot exclude that the P525L findings are a result of a delayed maturation of the system influenced by the impairment in neurite outgrowth. Importantly, as we do not report a clear neurite outgrowth deficiency in the R521H motor unit, this indicates that NMJ numbers are affected independently of maturation of the system but are rather due to the ALS-causing mutation itself. The smaller difference in NMJ numbers between FUS-ALS R521H and control R521R correlates with a general observation of mild phenotypes in this late-onset R521H patient in comparison with the rather aggressive phenotypes found in the juvenile-onset P525L-ALS patient in this system.
More and more studies show impaired neurite outgrowth in ALS. These include differences in axonal branching (Akiyama et al., 2019), impairments in neurite elongation speed (Osaki et al., 2018b), and in neurite length (Fujimori et al., 2018). However, the underlying mechanism has yet to be established. We observed that the mutant FUS-dependent reduction in neurite outgrowth and regrowth could be rescued by selective inhibition of HDAC6, which suggests a link to the widespread defects of the MN axonal transport machinery (Guo et al., 2019). Our studies in peripheral neuropathies demonstrated a correlation between an improvement of axonal transport due to HDAC6 inhibition and the reinnervation of NMJs in vivo (Benoy et al., 2018, 2017; d’Ydewalle et al., 2011; Van Helleputte et al., 2018). Further studies are crucial to investigate the potential of HDAC6 inhibition in ALS, although our in vivo results in a FUS mouse model already indicate that also other HDACs might play an important role (Rossaert et al., 2019).
Overall, we established a versatile and relatively easy to use motor unit system to study the functionality of human NMJs in culture. This co-culture model has broad application potential and is suited to test therapeutic strategies focusing on reinnervation and/or the stabilization of NMJs.
Experimental procedures
See supplemental information for detailed descriptions of the methods.
Cell lines and reagents
Healthy human control iPSCs (SBAD2 line) generated from a 51-year-old white male donor were provided by the Stem Cell Institute Leuven (SCIL, Leuven, Belgium), and, in addition, two previously characterized FUS-mutant iPSC lines from a 17-year-old male ALS patient carrying a de novo mutation (P525L) and a 71-year-old female ALS patient (R521H). The FUS-ALS lines were systematically compared with their corresponding CRISPR-Cas9 gene-edited isogenic control (P525P and R521R) generated by CellSystems (Troisdorf, Germany) (Guo et al., 2017; Vandoorne et al., 2019; Wang et al., 2018). Human control primary MABs were obtained from a healthy 58-year-old donor (SCIL).
Chemicals and cell culture reagents were purchased from Thermo Fisher Scientific (Waltham, MA, USA) unless specified otherwise.
Co-culturing myotubes and MNs in a microfluidic device
Human MABs were isolated and cultured as described previously (Dellavalle et al., 2007; Tonlorenzi et al., 2007). The iPSC-derived MN differentiation protocol utilized is based on a modified version of the Maury et al. protocol (Maury et al., 2015) and has previously been described (Guo et al., 2017; Wang et al., 2018).
Day 10 MN-NPCs were seeded in two wells and a channel on one side of the microfluidic device at 125,000 cells per well (total of 250,000 NPCs/device). One week later, MABs were seeded in the two wells and channel opposite to the MNs in the microfluidic device at 100,000 cells per well (total of 200,000 MABs/device). At day 21, a chemotactic and volumetric gradient was established. MN compartments received 100 μL/well neuronal medium without neurotrophic factor, while myotube compartments received 200 μL/well neuronal medium supplemented with 30 ng/mL BDNF (PeproTech, Rocky Hill, NJ, USA, cat. no. 450-02B), GDNF (PeproTech, cat. no. 450-10B) and CNTF (PeproTech, cat. no. 450-13B) in addition to 20 μg/mL laminin (Sigma, cat. no. L2020-1MG) and 0.01 μg/mL recombinant human agrin protein (R&D Systems, cat. no. 6624-AG-050) (A/L). The growth factor and volume gradients, including A/L, were kept at each medium change, which was performed every other day for a week.
TubA treatment
Treatments were made for 24 h with 1–3 μM TubA (Selleckchem, Houston, TX, USA, cat. no. S8049).
Immunocytochemistry and SEM
Immunocytochemistry and SEM are described in the supplemental information. See also Tables S1 and S2.
Neurite axotomy and regrowth
The axotomy was performed using a similar method as described previously (Nijssen et al., 2018).
Neurite outgrowth and regrowth quantifications
Tile scan images of NEFH fluorescence were taken using an inverted Leica SP8 DMI8 confocal microscope and neurites were isolated using ilastik 1.3.3post1 Pixel Classification software. Total number of pixel intersections was quantified per intersection line utilizing a custom ImageJ 1.52p software linear School analysis script.
For MN-NPC neurite outgrowth quantifications, NPCs were imaged for 24 h using an IncuCyte ZOOM device with the IncuCyte ZOOM 2016A software (Essen BioScience) and analyzed with the IncuCyte NeuroTrack Phase Neurites software.
Calcium fluorescent imaging
On day 28 of MN differentiation, myotube compartments were incubated for 25 min with 5 μM Fluo-4 AM (cat. no. F14201). MNs were stimulated with 50 mM KCl and Fluo-4 fluorescence was recorded in the myotube compartment. In addition, myotube compartments were treated with 19 μM of the AChR competitive antagonist tubocurarine hydrochloride pentahydrate (DTC) (Sigma, cat. no. T2379-100G) for 10 min. Myotubes were cultured without MNs in μ-plates (ibidi, Planegg, Germany, cat. no. 82,406) and tested for Ca2+ functionality, as well as in devices without MNs to confirm fluidic isolation upon KCl stimulation in the MN compartment. All recordings were acquired and analyzed using a Nikon A1R confocal microscope and NIS-Elements AR 4.30.02 software.
Statistics
Data are presented as mean ± SEM, unless indicated otherwise. Statistical analyses were made in GraphPad Prism 7.04. Data were tested for normal Gaussian distribution using the Anderson-Darling test, D'Agostino-Pearson omnibus normality test, and Shapiro-Wilk normality test. Statistical analyses were determined using unpaired t test or Mann-Whitney test for differences of mean between two groups and one-way ANOVA with Tukey's multiple comparisons test and Kruskal-Wallis test with Dunn's multiple comparisons test for difference of means between multiple groups. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Data and code availability
The datasets used and analyzed during this study are available in the source data file. ImageJ and Nikon software scripts are available upon request.
Author contributions
K.S.D., L.F., and T.V. planned and designed the experiments. E.N.K. and K.S.D. optimized the system. K.S.D. performed most of the experiments and did the data analysis. L.F. and K.S.D. performed ICC. K.S.D. and P.B. performed SEM. A.K. assisted in neurite outgrowth/regrowth quantifications and wrote the Nikon script. B.P. wrote the ImageJ script. E.R. and J.B. helped with the axotomy experiments. M.S. provided mesoangioblasts and valuable ideas for the project. G.G. provided advice for mesoangioblast culturing and differentiation. P.V.D. provided ideas for the project. L.V.D.B. planned and supervised the project. K.S.D. wrote the paper. L.F., T.V., and L.V.D.B. edited the manuscript. All authors read and approved the final version of the paper.
Declaration of interests
L.V.D.B. has a patent on the use of HDAC inhibitors in Charcot-Marie-Tooth disease (US-2013227717-A1), is scientific co-founder of Augustine Therapeutics and a member of its scientific advisory board. The other authors declare no competing interests.
Acknowledgments
The authors thank Sebastian Munck and Nikky Corthout from LiMoNe, Research Group Molecular Neurobiology (VIB-KU Leuven) for discussions concerning Ca2+ live-cell imaging. This research was supported by the Fulbright Commission to Belgium and Luxembourg, the VIB, the KU Leuven (C1 and “Opening the Future” Fund), the “Fund for Scientific Research Flanders” (FWO-Vlaanderen), the Agency for Innovation by Science and Technology (IWT; SBO-iPSCAF), the Belgian Government (Interuniversity Attraction Poles Program P7/16 initiated by the Belgian Federal Science Policy Office), the Thierry Latran Foundation, the “Association Belge contre les Maladies neuro-Musculaires” (ABMM), Target ALS, and the ALS Liga België (A Cure for ALS). T.V., E.R., and J.B. are supported by strategic basic research PhD fellowships awarded by FWO-Vlaanderen.
Published: April 22, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2021.03.029.
Supplemental information
References
- Afshar Bakooshli M., Lippmann E.S., Mulcahy B., Iyer N., Nguyen C.T., Tung K., Stewart B.A., van den Dorpel H., Fuehrmann T., Shoichet M., et al. A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. eLife. 2019;8:e44530. doi: 10.7554/eLife.44530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T., Suzuki N., Ishikawa M., Fujimori K., Sone T., Kawada J., Funayama R., Fujishima F., Mitsuzawa S., Ikeda K., et al. Aberrant axon branching via Fos-B dysregulation in FUS-ALS motor neurons. EBioMedicine. 2019;45:362–378. doi: 10.1016/j.ebiom.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmann J., Goswami R.Y., Girardo S., Rein N., Hosseinzadeh Z., Hicks M.R., Busskamp V., Pyle A.D., Werner C., Sterneckert J. A customizable microfluidic platform for medium-throughput modeling of neuromuscular circuits. Biomaterials. 2019;225:119537. doi: 10.1016/j.biomaterials.2019.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoy V., Van Helleputte L., Prior R., d’Ydewalle C., Haeck W., Geens N., Scheveneels W., Schevenels B., Cader M.Z., Talbot K., et al. HDAC6 is a therapeutic target in mutant GARS-induced Charcot-Marie-Tooth disease. Brain. 2018;141:673–687. doi: 10.1093/brain/awx375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoy V., Vanden Berghe P., Jarpe M., Van Damme P., Robberecht W., Van Den Bosch L. Development of improved HDAC6 inhibitors as pharmacological therapy for axonal Charcot-Marie-Tooth disease. Neurotherapeutics. 2017;14:417–428. doi: 10.1007/s13311-016-0501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden S.J., Huijbers M.G., Remedio L. Fundamental molecules and mechanisms for forming and maintaining neuromuscular synapses. Int. J. Mol. Sci. 2018;19:490. doi: 10.3390/ijms19020490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkin D.J., Kim J.E., Gu M., Kaufman S.J. Laminin and alpha 7 beta 1 integrin regulate agrin-induced clustering of acetylcholine receptors. J. Cell Sci. 2000;113:2877–2886. doi: 10.1242/jcs.113.16.2877. [DOI] [PubMed] [Google Scholar]
- Campenot R.B. Local control of neurite development by nerve growth factor. Proc. Natl. Acad. Sci. U S A. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Ydewalle C., Krishnan J., Chiheb D.M., Van Damme P., Irobi J., Kozikowski A.P., Vanden Berghe P., Timmerman V., Robberecht W., Van Den Bosch L. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat. Med. 2011;17:968–974. doi: 10.1038/nm.2396. [DOI] [PubMed] [Google Scholar]
- Dadon-Nachum M., Melamed E., Offen D. The “dying-back” phenomenon of motor neurons in ALS. J. Mol. Neurosci. 2010;43:470–477. doi: 10.1007/s12031-010-9467-1. [DOI] [PubMed] [Google Scholar]
- de Jongh R., Spijkers X.M., Pasteuning-Vuhman S., Vulto P., Pasterkamp R.J. Neuromuscular junction-on-a-chip: ALS disease modeling and read-out development in microfluidic devices. J. Neurochem. 2021 doi: 10.1111/jnc.15289. [DOI] [PubMed] [Google Scholar]
- Dellavalle A., Sampaolesi M., Tonlorenzi R., Tagliafico E., Sacchetti B., Perani L., Innocenzi A., Galvez B.G., Messina G., Morosetti R., et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Demestre M., Orth M., Föhr K.J., Achberger K., Ludolph A.C., Liebau S., Boeckers T.M. Formation and characterisation of neuromuscular junctions between hiPSC derived motoneurons and myotubes. Stem Cell Res. 2015;15:328–336. doi: 10.1016/j.scr.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Fazal R., Boeynaems S., Swijsen A., De Decker M., Fumagalli L., Moisse M., Vanneste J., Guo W., Boon R., Vercruysse T., et al. HDAC6 inhibition restores TDP-43 pathology and axonal transport defects in human motor neurons with TARDBP mutations. EMBO J. 2021;40:e106177. doi: 10.15252/embj.2020106177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L.R., Culver D.G., Tennant P., Davis A.A., Wang M., Castellano-Sanchez A., Khan J., Polak M.A., Glass J.D. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp. Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Fujimori K., Ishikawa M., Otomo A., Atsuta N., Nakamura R., Akiyama T., Hadano S., Aoki M., Saya H., Sobue G., et al. Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat. Med. 2018;24:1579–1589. doi: 10.1038/s41591-018-0140-5. [DOI] [PubMed] [Google Scholar]
- Greek R., Hansen L.A. Questions regarding the predictive value of one evolved complex adaptive system for a second: exemplified by the SOD1 mouse. Prog. Biophys. Mol. Biol. 2013;113:231–253. doi: 10.1016/j.pbiomolbio.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Guo W., Naujock M., Fumagalli L., Vandoorne T., Baatsen P., Boon R., Ordovás L., Patel A., Welters M., Vanwelden T., et al. HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat. Commun. 2017;8:861. doi: 10.1038/s41467-017-00911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Stoklund Dittlau K., Van Den Bosch L. Axonal transport defects and neurodegeneration: molecular mechanisms and therapeutic implications. Semin. Cell Dev. Biol. 2019;99:133–150. doi: 10.1016/j.semcdb.2019.07.010. [DOI] [PubMed] [Google Scholar]
- Guo X., Gonzalez M., Stancescu M., Vandenburgh H.H., Hickman J.J. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials. 2011;32:9602–9611. doi: 10.1016/j.biomaterials.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiwlawat N., Lynch E., Jeffrey J., Van Dyke J.M., Suzuki M. Current progress and challenges for skeletal muscle differentiation from human pluripotent stem cells using transgene-free approaches. Stem Cells Int. 2018;11:6241681. doi: 10.1155/2018/6241681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocher G., Mannschatz S.H., Offterdinger M., Schweigreiter R. Microfluidics of small-population neurons allows for a precise quantification of the peripheral axonal growth state. Front. Cell. Neurosci. 2018;12:166. doi: 10.3389/fncel.2018.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.A., Harrison C., Eaton S.L., Llavero Hurtado M., Graham L.C., Alkhammash L., Oladiran O.A., Gale A., Lamont D.J., Simpson H., et al. Cellular and molecular anatomy of the human neuromuscular junction. Cell Rep. 2017;21:2348–2356. doi: 10.1016/j.celrep.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K., Ma J., Golonzhka O., Laumet G.O., Gutti T., Van Duzer J.H., Mazitschek R., Jarpe M.B., Heijnen C.J., Kavelaars A. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain. 2017;158:1126–1137. doi: 10.1097/j.pain.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer T.T., Misgeld T., Lichtman J.W., Sanes J.R. Nerve-independent formation of a topologically complex postsynaptic apparatus. J. Cell Biol. 2004;164:1077–1087. doi: 10.1083/jcb.200401115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.Y., Yoshida M., Li L.T., Ikenaka A., Oshima S., Nakagawa K., Sakurai H., Matsui E., Nakahata T., Saito M.K. iPSC-derived functional human neuromuscular junctions model the pathophysiology of neuromuscular diseases. JCI Insight. 2019;4:e124299. doi: 10.1172/jci.insight.124299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C.X., Wen X., Jin S., Zhang Y.Q. Increased acetylation of microtubules rescues human tau-induced microtubule defects and neuromuscular junction abnormalities in Drosophila. Dis. Model. Mech. 2017;10:1245–1252. doi: 10.1242/dmm.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau É., Di Polo A., Vande Velde C., Robitaille R. Dynamic neuromuscular remodeling precedes motor-unit loss in a mouse model of ALS. eLife. 2018;7:e41973. doi: 10.7554/eLife.41973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury Y., Côme J., Piskorowski R.A., Salah-Mohellibi N., Chevaleyre V., Peschanski M., Martinat C., Nedelec S. Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nat. Biotechnol. 2015;33:89–96. doi: 10.1038/nbt.3049. [DOI] [PubMed] [Google Scholar]
- Millet L.J., Gillette M.U. New perspectives on neuronal development via microfluidic environments. Trends Neurosci. 2012;35:752–761. doi: 10.1016/j.tins.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills R., Taylor-Weiner H., Correia J.C., Agudelo L.Z., Allodi I., Kolonelou C., Martinez-Redondo V., Ferreira D.M.S., Nichterwitz S., Comley L.H., et al. Neurturin is a PGC-1α1-controlled myokine that promotes motor neuron recruitment and neuromuscular junction formation. Mol. Metab. 2018;7:12–22. doi: 10.1016/j.molmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrice J.R., Gregory-Evans C.Y., Shaw C.A. Animal models of amyotrophic lateral sclerosis: a comparison of model validity. Neural Regen. Res. 2018;13:2050–2054. doi: 10.4103/1673-5374.241445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L.M., Talbot K., Gillingwater T.H. Neuromuscular synaptic vulnerability in motor neuron disease: amyotrophic lateral sclerosis and spinal muscular atrophy. Neuropathol. Appl. Neurobiol. 2010;36:133–156. doi: 10.1111/j.1365-2990.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Nair G., Carew J.D., Usher S., Lu D., Hu X.P., Benatar M. Diffusion tensor imaging reveals regional differences in the cervical spinal cord in amyotrophic lateral sclerosis. Neuroimage. 2010;53:576–583. doi: 10.1016/j.neuroimage.2010.06.060. [DOI] [PubMed] [Google Scholar]
- Naumann M., Pal A., Goswami A., Lojewski X., Japtok J., Vehlow A., Naujock M., Günther R., Jin M., Stanslowsky N., et al. Impaired DNA damage response signaling by FUS-NLS mutations leads to neurodegeneration and FUS aggregate formation. Nat. Commun. 2018;9:335. doi: 10.1038/s41467-017-02299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijssen J., Aguila J., Hoogstraaten R., Kee N., Hedlund E. Axon-seq decodes the motor axon transcriptome and its modulation in response to ALS. Stem Cell Reports. 2018;11:1565–1578. doi: 10.1016/j.stemcr.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki T., Sivathanu V., Kamm R.D. Engineered 3D vascular and neuronal networks in a microfluidic platform. Sci. Rep. 2018;8:5168. doi: 10.1038/s41598-018-23512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki T., Uzel S.G.M., Kamm R.D. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci. Adv. 2018;4:eaat5847. doi: 10.1126/sciadv.aat5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osseni A., Ravel-chapuis A., Thomas J., Gache V., Schaeffer L., Jasmin B.J. HDAC6 regulates microtubule stability and clustering of AChRs at neuromuscular junctions. JCB. 2020;219:e201901099. doi: 10.1083/jcb.201901099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp J.J. In: Myasthenia Gravis and Related Disorders. Kaminski H.J., Kusner L.L., editors. Springer International Publishing; 2018. Neuromuscular junction physiology and pathophysiology; pp. 1–12. [Google Scholar]
- Prior R., Van Helleputte L., Klingl Y.E., Van Den Bosch L. HDAC6 as a potential therapeutic Target for peripheral nerve disorders. Expert Opin. Ther. Targets. 2018;22:993–1007. doi: 10.1080/14728222.2018.1541235. [DOI] [PubMed] [Google Scholar]
- Puttonen K.A., Ruponen M., Naumenko N., Hovatta O.H., Tavi P., Koistinaho J. Generation of functional neuromuscular junctions from human pluripotent stem cell lines. Front. Cell. Neurosci. 2015;9:473. doi: 10.3389/fncel.2015.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht W., Philips T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013;14:248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- Rossaert E., Van Den Bosch L. HDAC6 inhibitors: translating genetic and molecular insights into a therapy for axonal CMT. Brain Res. 2020;1733:146692. doi: 10.1016/j.brainres.2020.146692. [DOI] [PubMed] [Google Scholar]
- Rossaert E., Pollari E., Jaspers T., Van Helleputte L., Jarpe M., Van Damme P., De Bock K., Moisse M., Van Den Bosch L. Restoration of histone acetylation ameliorates disease and metabolic abnormalities in a FUS mouse model. Acta Neuropathol. Commun. 2019;7:107. doi: 10.1186/s40478-019-0750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotini A., Martínez-Sarrà E., Duelen R., Costamagna D., Di Filippo E.S., Giacomazzi G., Grosemans H., Fulle S., Sampaolesi M. Aging affects the in vivo regenerative potential of human mesoangioblasts. Aging Cell. 2018;17:e12714. doi: 10.1111/acel.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam N., Kumanchik L., Guo X., Sommerhage F., Cai Y., Jackson M., Martin C., Saad G., McAleer C.W., Wang Y., et al. Stem cell derived phenotypic human neuromuscular junction model for dose response evaluation of therapeutics. Biomaterials. 2018;166:64–78. doi: 10.1016/j.biomaterials.2018.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So E., Mitchell J.C., Memmi C., Chennell G., Vizcay-Barrena G., Allison L., Shaw C.E., Vance C. Mitochondrial abnormalities and disruption of the neuromuscular junction precede the clinical phenotype and motor neuron loss in hFUSWT transgenic mice. Hum. Mol. Genet. 2018;27:463–474. doi: 10.1093/hmg/ddx415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southam K.A., King A.E., Blizzard C.A., McCormack G.H., Dickson T.C. Microfluidic primary culture model of the lower motor neuron-neuromuscular junction circuit. J. Neurosci. Methods. 2013;218:164–169. doi: 10.1016/j.jneumeth.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Tallon C., Russell K.A., Sakhalkar S., Andrapallayal N., Farah M.H. Length-dependent axo-terminal degeneration at the neuromuscular synapses of type II muscle in SOD1 mice. Neuroscience. 2016;312:179–189. doi: 10.1016/j.neuroscience.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A.M., Blurton-Jones M., Rhee S.W., Cribbs D.H., Cotman C.W., Jeon N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonlorenzi R., Dellavalle A., Schnapp E., Cossu G., Sampaolesi M. Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr. Protoc. Stem Cell Biol. 2007;2:2B.1. doi: 10.1002/9780470151808.sc02b01s3. [DOI] [PubMed] [Google Scholar]
- Tyzack G.E., Luisier R., Taha D.M., Neeves J., Modic M., Mitchell J.S., Meyer I., Greensmith L., Newcombe J., Ule J., et al. Widespread FUS mislocalization is a molecular hallmark of amyotrophic lateral sclerosis. Brain. 2019;142:2572–2580. doi: 10.1093/brain/awz217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach J.A., Adams K.L., Gundersen C.B., Novitch B.G. Functional neuromuscular junctions formed by embryonic stem cell-derived motor neurons. PLoS One. 2012;7:e36049. doi: 10.1371/journal.pone.0036049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Helleputte L., Kater M., Cook D.P., Eykens C., Rossaert E., Haeck W., Jaspers T., Geens N., Vanden Berghe P., Gysemans C., et al. Inhibition of histone deacetylase 6 (HDAC6) protects against vincristine-induced peripheral neuropathies and inhibits tumor growth. Neurobiol. Dis. 2018;111:59–69. doi: 10.1016/j.nbd.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Vandoorne T., Veys K., Guo W., Sicart A., Vints K., Swijsen A., Moisse M., Eelen G., Gounko N.V., Fumagalli L., et al. Differentiation but not ALS mutations in FUS rewires motor neuron metabolism. Nat. Commun. 2019;10:4147. doi: 10.1038/s41467-019-12099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A.K., Spiller K.J., Ge G., Zheng A., Xu Y., Zhou M., Tripathy K., Kwong L.K., Trojanowski J.Q., Lee V.M.Y. Functional recovery in new mouse models of ALS/FTLD after clearance of pathological cytoplasmic TDP-43. Acta Neuropathol. 2015;130:643–660. doi: 10.1007/s00401-015-1460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Guo W., Mitra J., Hegde P.M., Vandoorne T., Eckelmann B.J., Mitra S., Tomkinson A.E., Van Den Bosch L., Hegde M.L. Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in amyotrophic lateral sclerosis. Nat. Commun. 2018;9:3683. doi: 10.1038/s41467-018-06111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahavi E.E., Ionescu A., Gluska S., Gradus T., Ben-Yaakov K., Perlson E. A compartmentalized microfluidic neuromuscular co-culture system reveals spatial aspects of GDNF functions. J. Cell Sci. 2015;128:1241–1252. doi: 10.1242/jcs.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.G.X., Quigley A.F., Bourke J.L., Nowell C.J., Myers D.E., Choong P.F.M., Kapsa R.M.I. Combination of agrin and laminin increase acetylcholine receptor clustering and enhance functional neuromuscular junction formation in vitro. Dev. Neurobiol. 2016;76:551–565. doi: 10.1002/dneu.22331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during this study are available in the source data file. ImageJ and Nikon software scripts are available upon request.