Abstract
Purpose
To report the outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) for juvenile-onset primary open-angle glaucoma (JOAG).
Methods
A consecutive case series of JOAG patients who underwent GATT was reviewed with follow-up period of up to 18 months. Intraocular pressure (IOP), number of glaucoma medications and success rate were compared between eyes with and without prior glaucoma surgery, and between mild-to-moderate and severe cases defined based on Humphrey Visual Field mean deviation.
Results
In total, 59 eyes of 48 patients were included. Overall, IOP was reduced from 26.5 ± 9.0 mmHg on 3.7 ± 0.9 medications preoperatively to 14.7 ± 3.0 mmHg on 0.7 ± 1.2 medications at 12 months and to 14.1 ± 2.3 mmHg on 0.4 ± 0.8 medications at 18 months postoperatively (P < 0.001). The complete and qualified success rates were 70.8% and 81.2% at 12 months, and 58.6% and 81.2% at 18 months, respectively. Eyes with and without prior glaucoma surgery did not differ significantly in terms of postoperative IOP, glaucoma medication and success rate. In addition, GATT was effective for both mild-to-moderate and severe cases; the latter achieved a surgical success of 79.1%.
Conclusions
GATT is effective for JOAG. In particular, this case series suggests that GATT is promising in treating severe JOAG and those with prior glaucoma surgery.
Subject terms: Surgery, Outcomes research
Introduction
Juvenile-onset primary open-angle glaucoma (JOAG) is considered to be a subclass of primary open-angle glaucoma (POAG) occurring before the age of 35 years, thus affecting the most productive socioeconomic subgroup of the population [1]. Compared to adult-onset POAG, JOAG is usually more severe and rapidly progressive, often with a markedly high intraocular pressures (IOP) [2–5]. It has been shown that JOAG is often refractory to medical therapy alone, frequently requiring surgical interventions to achieve IOP control [4, 5]. Tabeculectomy [6–10], external trabeculotomy [11–13] and goniotomy [14, 15] are traditional surgical treatment of JOAG. Recently, the treatment paradigm has changed notably. Less-invasive, ab-interno canal-based procedures have been drawing much attention.
Gonioscopy-assisted transluminal trabeculotomy (GATT) is a novel ab-interno circumferential trabeculotomy first described by Grover et al. [16]. This procedure utilizes a blunted suture, microcatheter or TRAB360 device to circumferentially fracture the trabecular meshwork and inner wall of Schlemm’s canal to facilitate aqueous drainage through the conventional outflow pathway [16–18]. One of its major advantages is that GATT spares the conjunctiva, which is important to save ocular territory for future glaucoma surgeries [8, 9, 19, 20]. A couple of studies has shown that GATT alone or combined with cataract surgery is effective for patients with primary or secondary open-angle glaucoma [18, 21–24] and patients with previous filtering surgery [20, 25, 26]. However, to our knowledge, only one study has specifically addressed the efficacy of GATT in treating JOAG [27]. It has not been reported whether GATT was effective for JOAG patients with prior glaucoma surgery or for JOAG patients with severe glaucomatous damage.
The purpose of this study was to report the efficacy of microcatheter-assisted GATT for patients with different stages of JOAG and to compare the outcomes between eyes with and without prior glaucoma surgery.
Subjects and methods
Study design
This study was a retrospective, consecutive case series of patients with JOAG who underwent microcatheter-assisted GATT at Beijing Tongren Eye Centre between November 2017 and December 2018. This study was approved by the Institutional Ethics Committee of Beijing Tongren Hospital and conformed to the tenets of the Declaration of Helsinki. It was registered under the Chinese Clinical Trials Registry (ChiCTR1900028170). Written informed consent were obtained from all patients before surgery.
The inclusion criteria were JOAG patients (1) whose ages were between 16 and 35 years; (2) whose preoperative IOP ≥ 18 mmHg with progressive deterioration of visual field (VF) defects on maximal medications; or (3) who was intolerable to medical therapy. Patients with other types of glaucoma, a history of ocular trauma, a history of ocular surgery other than glaucoma surgery, with unidentifiable trabecular meshwork, with corneal abnormality that did not permit visualization of the angle or IOP measurement, and patients who were on anticoagulant therapy were excluded. Data were collected preoperatively and at the following postoperative visits: 1, 2, 3 days, 1, 2–3 weeks, 1, 3, 6 months, thereafter every 3–6 months.
The outcome measures included IOP, number of glaucoma medications, and success rate and frequency of complications. Combination glaucoma medications were enumerated according to the number of active agents in the medication. An IOP of ≥30 mmHg within 1 month of surgery was defined as IOP spikes [28]. Microhyphema was defined as flared or layered blood ≤1 mm in the anterior chamber, and macrohyphema as layered blood >1 mm [29]. Surgical success was defined as: (1) an IOP of ≤18 mmHg and a reduction of IOP by 20% or more from baseline with (qualified success) or without (complete success) glaucoma medications; or for eyes with preoperative IOP of < 21 mmHg on 3 or 4 glaucoma medications but intolerant to the medications, postoperative IOP of ≤18 mmHg without any glaucoma medications; (2) no loss of light perception vision; and (3) no need for additional surgical procedure, which were also excluded from analysis subsequent to the intervention. Severity of glaucoma status were defined by mean deviation (MD) on Humphrey visual field 24-2 SITA-fast tests. Eyes with mild-to-moderate JOAG had MD ≥ −12 dB, while eyes with severe JOAG had MD < −12 dB.
Surgical procedure and postoperative care
GATT was performed as a stand-alone procedure by one of the two surgeons (NLW and HZW). As previously described [16], a clear corneal temporal incision was created, and the anterior chamber was filled with viscoelastics. A corneal paracentesis track, oriented tangentially, was then placed in the superonasal or the inferonasal quadrant. A small goniotomy was created in the nasal angle visualized with a Swan-Jacob goniolens. A microcatheter (iTrack 250A; Ellex iScience Inc., Fremont, CA) was used for catheterization, and a circumferential trabeculotomy was then performed by pulling both ends of the microcatheter. If the microcatheter stopped somewhere in Schlemm’s canal, an ab-interno cut down was performed to achieve a limited trabeculotomy, and then the microcatheter was placed in the opposite direction to enter the canal and to achieve as close to 360 degree as possible. The viscoelastic was roughly washed out, and the corneal wounds were hydrated to ensure a watertight closure.
After surgery, tobramycin-dexamethasone (TobraDex, Alcon, Rijksweg, Belgium) and Pranoprofen (Pranopulin, Senju Pharmaceutical, Osaka, Japan) eyedrops were administered for 2–4 weeks. The topical steroids were tapered per the surgeon’s discretion, with the main goal to control inflammation and prevent a steroid IOP response. Pilocarpine 2% (Bausch & Lomb, Rochester, NY, USA) was used 4 times daily for 3 months to hinder peripheral anterior synechia regardless of the IOP and was therefore not considered as an anti-glaucoma medication during this time.
Statistical analysis
Demographic and clinical characteristics between the groups were compared using Chi-square test, Mann–Whitney U or mixed-effects models, as appropriate. Repeated measures variables were analyzed using mixed-effects models with a Bonferroni-corrected significance level used for multiple comparison. The changes in IOP and the number of glaucoma medications between groups were compared using mixed-effect models to account for the clustering of eyes within a given patient. A Kaplan–Meier survival curve was constructed for the cumulative probability of success. Risk factors for surgical failure were identified using the Cox proportional hazards regression model. A P value of less than 0.05 (two-tailed) was considered statistically significant.
Results
Demographics and ocular characteristics
Table 1 summarizes demographic characteristics of 48 patients (59 eyes) in this study. The average age was 27.4 years (range, 17 to 35), and 38 patients were male (79.2%). The mean follow-up time was 14.9 months. Eyes were divided into two groups based on history of glaucoma surgeries. Twenty-six eyes (44.1%) had prior incisional glaucoma procedures, with filtering surgery being the most common. The time intervals between primary filtering surgery and GATT were less than 5 years in 17 eyes with median of 2 years. Eyes with prior glaucoma surgery (Group 2) did not significantly differ from those without prior surgeries (Group 1) in terms of the mean age, sex, preoperative IOP, preoperative medications, glaucoma severity and follow-up duration (all P > 0.05).
Table 1.
Demographics of study population included in this study.
| Variables | Total | Group 1 | Group 2 | P |
|---|---|---|---|---|
| No. eyes (no. patients) | 59 (48) | 33 (26) | 26 (22) | – |
| Mean age (SD) (y) | 27.4 (5.4) | 28.0 (5.4) | 26.6 (5.6) | 0.366a |
| Range (y) | 17–35 | 17–35 | 18–35 | |
| Male, n (%) | 38 (79.2) | 20 (76.9) | 18 (81.8) | 0.953b |
| Prior glaucoma procedures, n (%) | 26 (44.1) | – | ||
| Incisional glaucoma surgery, n (%) | 26 (44.1) | |||
| Trabeculectomy, n | 20 | |||
| Ex-Press shunt, n | 3 | |||
| Ahmed valve, n | 0 | |||
| Trabectome, n | 2 | |||
| Canaloplasty, n | 1 | |||
| Selective laser trabeculoplasty, n (%) | 0 | |||
| The time interval between primary filtering surgery and GATT | ||||
| ≤2 y, n | 15 | |||
| ≤5 y, n | 17 | |||
| ≤10 y, n | 20 | |||
| >10 y, n | 3 | |||
| Number of prior surgeries, mean ± SD (range) | 0.5 ± 0.7 (0–3) | – | ||
| Preoperative IOP (mmHg), mean ± SD | 26.5 ± 9.0 | 25.4 ± 9.4 | 27.9 ± 8.5 | 0.285c |
| Preoperative medications, mean ± SD | 3.7 ± 0.9 | 3.8 ± 0.9 | 3.7 ± 0.9 | 0.654c |
| HVF MD values | ||||
| ≥−12 dB, n (%) | 16 (27.1) | 9 (27.3) | 7 (26.9) | 0.976b |
| < −12 dB, n (%) | 43 (72.9) | 24 (72.7) | 19 (73.1) | |
| Follow-up duration (months), mean ± SD | 14.5 ± 3.0 | 14.5 ± 3.0 | 14.6 ± 3.1 | 0.881a |
IOP intraocular pressure, y year, SD standard deviation, Group 1 Patients without prior glaucoma surgery, Group 2 Patients with prior glaucoma surgery.
aMann–Whitney U.
bChi-square test.
cMixed-effect model.
Surgical efficacy
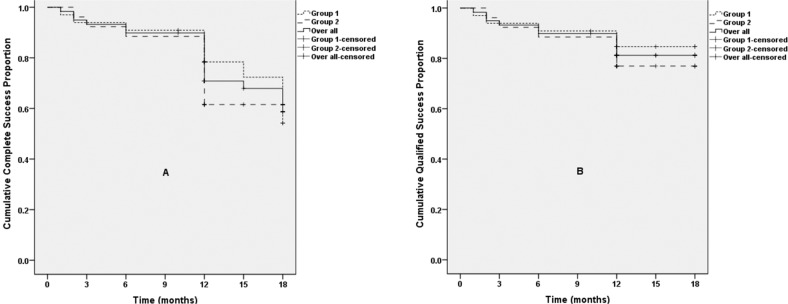
Overall, the mean IOP and number of glaucoma medications were significantly reduced at all postoperative visits compared to baseline (all P < 0.001). The mean IOP decreased from 26.5 ± 9.0 mmHg on 3.7 ± 0.9 medications preoperatively to 14.7 ± 3.0 mmHg on 0.7 ± 1.2 medications at 12 months and to 14.1 ± 2.3 mmHg on 0.4 ± 0.8 medications at 18 months postoperatively. The average reduction in IOP was 37.2% and 47.9% at 12 and 18 months, respectively. The number of glaucoma medications was reduced by an average of 3.0 and 3.3 at 12 and 18 months, respectively. While 55 eyes (93.2%) were treated with 3 or more glaucoma medications preoperatively, 35 eyes (59.3%) were off glaucoma medications entirely at 12 months, and 19 eyes (32.2%) at 18 months postoperatively. The overall complete success rate was 70.8% at 12 months and 58.6% at 18 months, respectively, and the qualified success rate was 81.2% at 12 and 18 months (Fig. 1).
Fig. 1. Kaplan–Meier analysis of the cumulative probabilities of surgical success.
Success was defined as: (1) an IOP of ≤ 18 mmHg and a reduction of IOP by 20% or more from baseline with (qualified success) or without (complete success) glaucoma medications; or for eyes with preoperative IOP of < 21 mmHg on 3 or 4 glaucoma medications but intolerant to the medications, postoperative IOP of ≤ 18 mmHg without any glaucoma medications; (2) no loss of light perception vision; and (3) no need for additional surgical procedure. Neither complete (A) nor qualified (B) success rate of GATT differed significantly between Group 1 and Group 2 (P > 0.05). The differences between Group 1 and Group 2 were analysed by the log-rank test. GATT: gonioscopy-assisted transluminal trabeculotomy; Group 1: eyes without prior glaucoma surgery; Group 2: eyes with prior glaucoma surgery.
In both subgroups, the mean IOP and number of glaucoma medications decreased significantly compared to baseline (all P < 0.001; Fig. 2). In the Group 1, the mean IOP decreased from 25.4 ± 9.4 mm Hg on 3.8 ± 0.9 medications preoperatively to 15.1 ± 1.9 mmHg on 0.6 ± 1.0 medications postoperatively at 12 months, and 14.9 ± 2.1 mmHg on 0.5 ± 1.1 medications at 18 months. In the Group 2, the mean IOP decreased from 27.9 ± 8.5 mmHg on 3.7 ± 0.9 medications preoperatively to 14.2 ± 4.0 mmHg on 0.8 ± 1.4 medications postoperatively at 12 months, and 13.1 ± 2.2 mmHg on 0.1 ± 0.3 medications at 18 months. The two groups did not differ significantly in terms of the mean IOP or number of glaucoma medications at any follow-up visit (all P > 0.05; Fig. 2). Neither complete (78.4% in Group 1 vs. 61.5% in Group 2, P = 0.759) nor qualified success rate (84.6% in Group 1 vs. 76.9% in Group 2, P = 0.483) at 12 months differed significantly between the two groups (Fig. 1). While GATT succeeded in 10 of 15 cases with interval of less than 2 years between primary filtering surgery and GATT, 4 of 8 eyes with interval of more than 2 years achieved success (P = 1.000).
Fig. 2. Preoperative versus postoperative IOP and glaucoma medications in eyes without prior glaucoma surgery (Group 1) and those with prior surgery (Group 2).
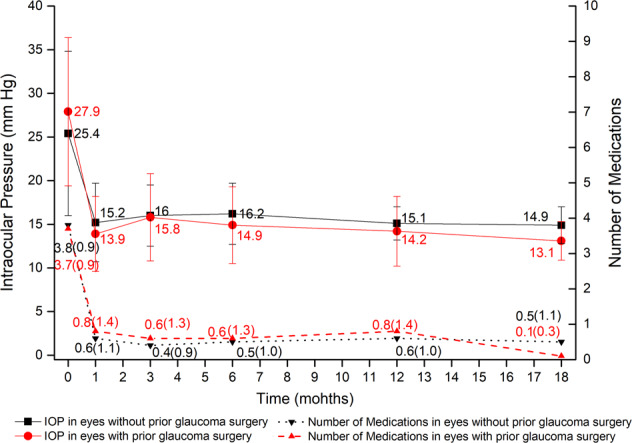
Compared to baseline, IOP and glaucoma medication burden decreased significantly at all postoperative visits (mixed-effect model, P < 0.001). The two groups did not differ significantly in terms of the mean IOP and number of glaucoma medications at each follow-up visit (mixed-effect model, P > 0.05).
The effect of GATT on eyes with different severities of JOAG were also studied. At any follow-up, there was no statistically significant difference in IOP reduction between mild-to-moderate and severe cases (all P > 0.05; Fig. 3). In addition, the success rate at postoperative 12 months for severe cases seemed to be lower than that for mild-to-moderate cases, but the difference was not statistically significant (P = 0.557; Fig. 4).
Fig. 3. The percent reduction of IOP in eyes with mild-to-moderate JOAG (MD ≥ −12 dB) and in eyes with severe JOAG (MD < −12 dB).
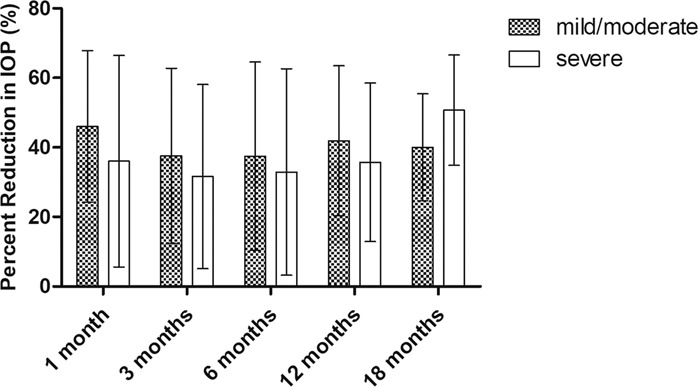
There was no statistically significant difference in IOP reduction between the two groups (mixed-effect model, P > 0.05). VF visual field, MD mean deviation.
Fig. 4. Kaplan–Meier analysis of the cumulative proportion of surgical success in mild-to-moderate (MD ≥ −12 dB) and severe (MD < −12 dB) JOAG.
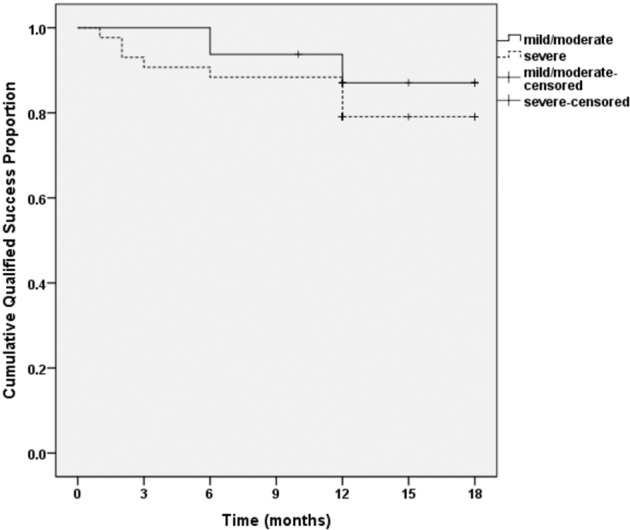
The difference in success rate between mild-to-moderate and severe cases was not statistically significant (the log-rank test, P > 0.05). VF visual field, MD mean deviation.
Complications and secondary procedures
No patient experienced any vision-threatening complications over the whole follow-up period. Microhyphema occurred in 29 eyes, and macrohyphema in other 30 eyes on the first day postoperatively. In 55 (93.2%) eyes, hyphema resolved by the first 2 weeks postoperatively. Twenty-nine (48.3%) eyes experienced IOP spike, 90% of which occurred in the first week after surgery. Mostly, IOP spike was controlled by glaucoma medications or by releasing aqueous humor from the paracentesis site created during GATT. Three eyes with massive hyphema and IOP spike underwent anterior chamber washout. Eighteen patients (30.0%) demonstrated varying levels of ciliochoroidal detachment, which resolved simultaneously within the first month after surgery in most of the cases. Topical pilocarpine was suspended in eyes presenting wide and extensive ciliochoroidal detachment until ciliochoroidal detachment resolved. One eye experienced a sustained cyclodialysis cleft without hypotony and was prescribed topical anti-inflammatory. In total, 5 eyes underwent secondary surgery for IOP controlling (2 goniosynechialysis, 2 trabeculectomy and 1 transscleral cyclophotocoagulation). Group 1 and Group 2 did not differ in terms of the frequency of macrohyphema (P = 0.091), IOP spike (P = 0.522) or ciliochoroidal detachment (P = 0.595).
Risk factors related to surgical failure
We examined the risk factors associated with GATT failure in this study. Surgical failure was not significantly associated with age, sex, prior glaucoma surgery, preoperative IOP, preoperative medications, severity of glaucomatous HVF defects, postoperative hyphema or ciliochoroidal detachment in the multivariate cox regression model. IOP spike was a risk factor for surgical failure of GATT in this study (hazard ratio (95% CI): 3.189 (1.204, 8.448)).
Discussion
Surgical treatment of JOAG has been challenging. This study demonstrated the safety and efficacy of stand-alone GATT in treating JOAG patients. This procedure was effective for approximately 80% of JOAG cases in this series. More importantly, our results indicated that for the first time, the surgical success rate for cases with prior glaucoma surgery was comparable to that for eyes without prior surgery. In addition, GATT was also effective for patients with severe JOAG.
Previously, 360-degree trabeculotomy was performed via an ab-externo approach. IOP-lowering effect of GATT in this study was similar to prior studies of ab-externo circumferential trabeculotomy for JOAG [30, 31]. Considering that ab-interno procedure spares the conjunctiva, GATT may be preferred for patients with JOAG over ab-externo trabeculotomy. Grover et al. were the first to report that postoperative IOP and number of glaucoma medications decreased significantly in patients with primary congenital glaucoma and JOAG [27]. However, there were only 14 patients in that study. With larger sample size, our current study not only achieved consistent results with theirs, but also enable to explore the effect of priory surgery and severity of glaucoma status on the surgical outcome and to identify the risk factor associated with surgical failure.
Previous study demonstrated that GATT alone was safe and effective for 60–70% of older patients with open-angle glaucoma and prior incisional glaucoma surgery (mean age of 67.7 years) [21]. The current study focused on much younger patients with mean age of 27.4 years, and nearly half of the eyes had prior filtration surgeries. Our results showed that the IOP-lowering effect of GATT for eyes with prior surgery was comparable to that for eyes without prior surgery, which may be explained as follows. Firstly, the primary outflow resistance in JOAG is mainly located at the trabecular meshwork and inner wall of Schlemm’s canal rather than distal pathway [4, 32]. Therefore, eyes with JOAG may have preferred outcomes. Secondly, prior filtering surgery may only affect very short segment of Schlemm’s canal with most of Schlemm’s canal still intact. Unroof the majority of intact Schlemm’s canal is able to achieve significant IOP reduction in these eyes.
Minimally invasive glaucoma surgeries are usually recommended for early or moderate glaucoma. Grover et al. [22] reported that eyes with advanced POAG had a very large proportion of failure at 6 months after GATT. In contrast, our results showed that GATT was effective in treating advanced JOAG and that glaucoma severity was not a risk factor for surgical failure, which was consistent with the study by Aktas et al. [23]. The efficacy of GATT is related to the function of the distal outflow system, which may differ across studies. Recently, Aktas et al. [33] have found a significant correlation between postoperative IOP and the extent of the episcleral venous fluid wave in patients with advanced glaucoma, while the latter was not correlated to HVF MD values. Moreover, postoperative scarring response in the remaining trabecular leaflets may also differ across studies. Future studies are needed to determine how postoperative scarring affects surgical efficacy in patients with JOAG. In this study, the IOP-lowering effect achieved by GATT for severe JOAG seemed to be comparable to that for mild-to-moderate cases. However, limited by our small sample size, this needs further investigation.
Consistent with previous literature, we found that GATT is safe for eyes with JOAG. In addition, prior glaucoma surgery did not increase the risk of hyphema, IOP spike or ciliochoroidal detachment after GATT in our series. The frequency of IOP spike seems higher in this study than what were reported in previous publications [16, 22, 24, 28, 34, 35]. It may be related to high frequency of follow ups with close monitoring of IOP during the first month after GATT in our study. Fortunately, the IOP spike in most of cases only appeared briefly. The cause of IOP spike may be multifactorial. Postoperative hyphema increases risk of IOP spike. If a patient’s drainage function cannot compensate for the increased flow resistance caused by bleeding, IOP spike may occur. IOP spike may be also associated with resolution of ciliochoroidal detachment, which may lead to transient IOP spike. Consistent with Rahmatnejad et al. [34], we found that eyes with IOP spike were more likely to experience surgical failure. Although the specific mechanism of IOP spike has not been fully understood, close monitoring and timely management is important to treat postoperative IOP spike.
The current study had limitations. First, it was a retrospective study. Some cases did not undergo anterior-segment optic coherence tomography within the first week after surgery, and thus we may underestimate the frequency of transient ciliochoroidal detachment. There was also no grading for macrohyphema, so we cannot determine the association between surgical failure and macrohyphema. Second, this study was limited by the short follow-up duration and relatively small sample size.
In summary, our study showed that GATT significantly lowered IOP and the burden of glaucoma medications for eyes with JOAG. In particular, this procedure was safe and effective for cases with severe glaucoma and those with prior incisional glaucoma surgery. Further study is needed to determine the long-term success of GATT in treating JOAG patients.
Summary
What was known before
The surgical treatment of JOAG has been typically challenging. In particular, JOAG cases with prior glaucoma surgery are at high-risk for failure of traditional glaucoma filtering surgeries.
GATT alone or combined with cataract surgery is effective for patients with primary or secondary open-angle glaucoma. However, to our knowledge, only one small series addressing the efficacy of GATT in treating JOAG has been reported so far.
What this study adds
The success rate of GATT for JOAG with prior glaucoma surgery was comparable to that for eyes without prior surgery. GATT represents a reasonable choice of repeat surgical treatment for JOAG with prior glaucoma surgery.
GATT was effective to treat eyes with severe JOAG.
Acknowledgments
Funding
This work was supported by National Natural Science Foundation of China (81730027). The funding organization had no role in the design or conduct of this research.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yiwei Wang, Huaizhou Wang
References
- 1.Gupta V, Dutta P, Mary OV, Kapoor KS, Sihota R, Kumar G. Effect of glaucoma on the quality of life of young patients. Investig Ophthalmol Vis Sci. 2011;52:8433–7. doi: 10.1167/iovs.11-7551. [DOI] [PubMed] [Google Scholar]
- 2.Wiggs JL, Del Bono EA, Schuman JS, Hutchinson BT, Walton DS. Clinical features of five pedigrees genetically linked to the juvenile glaucoma locus on chromosome 1q21-q31. Ophthalmology. 1995;102:1782–9. doi: 10.1016/S0161-6420(95)30793-2. [DOI] [PubMed] [Google Scholar]
- 3.Johnson AT, Richards JE, Boehnke M, Stringham HM, Herman SB, Wong DJ, et al. Clinical phenotype of juvenile-onset primary open-angle glaucoma linked to chromosome 1q. Ophthalmology. 1996;103:808–14. doi: 10.1016/S0161-6420(96)30611-8. [DOI] [PubMed] [Google Scholar]
- 4.Turalba AV, Chen TC. Clinical and genetic characteristics of primary juvenile-onset open-angle glaucoma (JOAG) Semin Ophthalmol. 2008;23:19–25. doi: 10.1080/08820530701745199. [DOI] [PubMed] [Google Scholar]
- 5.Kwun Y, Lee EJ, Han JC, Kee C. Clinical characteristics of juvenile-onset open angle glaucoma. Korean J Ophthalmol. 2016;30:127–33. doi: 10.3341/kjo.2016.30.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai JC, Chang HW, Kao CN, Lai IC, Teng MC. Trabeculectomy with mitomycin C versus trabeculectomy alone for juvenile primary open-angle glaucoma. Ophthalmologica. 2003;217:24–30. doi: 10.1159/000068250. [DOI] [PubMed] [Google Scholar]
- 7.Nash DL, Crouch ER, Crouch ER., Jr Comparison of EX-PRESS shunt and trabeculectomy with mitomycin-C in congenital and juvenile glaucoma. J Glaucoma. 2017;26:e58–e63. doi: 10.1097/IJG.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 8.Pathania D, Senthil S, Rao HL, Mandal AK, Garudadari CS. Outcomes of trabeculectomy in juvenile open angle glaucoma. Indian J Ophthalmol. 2014;62:224–8. doi: 10.4103/0301-4738.101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gressel MG, Heuer DK, Parrish RK., 2nd Trabeculectomy in young patients. Ophthalmology. 1984;91:1242–6. doi: 10.1016/S0161-6420(84)34179-3. [DOI] [PubMed] [Google Scholar]
- 10.Jacobi PC, Dietlein TS, Krieglstein GK. Primary trabeculectomy in young adults: long-term clinical results and factors influencing the outcome. Ophthalmic Surg Lasers. 1999;30:637–46. doi: 10.3928/1542-8877-19990901-07. [DOI] [PubMed] [Google Scholar]
- 11.Kjer B, Kessing SV. Trabeculotomy in juvenile primary open-angle glaucoma. Ophthalmic Surg. 1993;24:663–8. [PubMed] [Google Scholar]
- 12.Kubota T, Takada Y, Inomata H. Surgical outcomes of trabeculotomy combined with sinusotomy for juvenile glaucoma. Jpn J Ophthalmol. 2001;45:499–502. doi: 10.1016/S0021-5155(01)00375-6. [DOI] [PubMed] [Google Scholar]
- 13.Gupta V, Ov M, Rao A, Sharma A, Sihota R. Long-term structural and functional outcomes of therapy in juvenile-onset primary open-angle glaucoma: a five-year follow-up. Ophthalmologica. 2012;228:19–25. doi: 10.1159/000334033. [DOI] [PubMed] [Google Scholar]
- 14.McPherson SD, Jr., Berry DP. Goniotomy vs external trabeculotomy for developmental glaucoma. Am J Ophthalmol. 1983;95:427–31. doi: 10.1016/0002-9394(83)90260-X. [DOI] [PubMed] [Google Scholar]
- 15.Yeung HH, Walton DS. Goniotomy for juvenile open-angle glaucoma. J Glaucoma. 2010;19:1–4. doi: 10.1097/IJG.0b013e3181a2fa31. [DOI] [PubMed] [Google Scholar]
- 16.Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes de Oca I, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121:855–61. doi: 10.1016/j.ophtha.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Grover DS, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy (GATT): thermal suture modification with a dye-stained rounded tip. J Glaucoma. 2016;25:501–4. doi: 10.1097/IJG.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 18.Sarkisian SR, Mathews B, Ding K, Patel A, Nicek Z. 360 degrees ab-interno trabeculotomy in refractory primary open-angle glaucoma. Clin Ophthalmol. 2019;13:161–8. doi: 10.2147/OPTH.S189260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AGIS Investigators The Advanced Glaucoma Intervention Study (AGIS): 11. risk factors for failure of trabeculectomy and argon laser trabeculoplasty. Am J Ophthalmol. 2002;134:481–98. doi: 10.1016/S0002-9394(02)01658-6. [DOI] [PubMed] [Google Scholar]
- 20.Law SK, Shih K, Tran DH, Coleman AL, Caprioli J. Long-term outcomes of repeat vs initial trabeculectomy in open-angle glaucoma. Am J Ophthalmol. 2009;148:685–.e681. doi: 10.1016/j.ajo.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 21.Grover DS, Godfrey DG, Smith O, Shi W, Feuer WJ, Fellman RL. Outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in eyes with prior incisional glaucoma surgery. J Glaucoma. 2017;26:41–5. doi: 10.1097/IJG.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 22.Grover DS, Smith O, Fellman RL, Godfrey DG, Gupta A, Montes de Oca I, et al. Gonioscopy assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy - 24-month follow-up. J Glaucoma. 2018;27:393–401. doi: 10.1097/IJG.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 23.Aktas Z, Ucgul AY, Bektas C, Sahin, Karamert S. Surgical outcomes of prolene gonioscopy assisted transluminal trabeculotomy in patients with moderate to advanced open angle glaucoma. J Glaucoma. 2019;28:884–8. doi: 10.1097/IJG.0000000000001331. [DOI] [PubMed] [Google Scholar]
- 24.Boese EA, Shah M. Gonioscopy-assisted transluminal trabeculotomy (GATT) is an effective procedure for steroid-induced glaucoma. J Glaucoma. 2019;28:803–7. doi: 10.1097/IJG.0000000000001317. [DOI] [PubMed] [Google Scholar]
- 25.Broadway DC, Chang LP. Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J Glaucoma. 2001;10:237–49. doi: 10.1097/00061198-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Sugimoto Y, Mochizuki H, Ohkubo S, Higashide T, Sugiyama K, Kiuchi Y. Intraocular pressure outcomes and risk factors for failure in the collaborative bleb-related infection incidence and treatment study. Ophthalmology. 2015;122:2223–33. doi: 10.1016/j.ophtha.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 27.Grover DS, Smith O, Fellman RL, Godfrey DG, Butler MR, Montes de Oca I, et al. Gonioscopy assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy for the treatment of primary congenital glaucoma and juvenile open angle glaucoma. Br J Ophthalmol. 2015;99:1092–6. doi: 10.1136/bjophthalmol-2014-306269. [DOI] [PubMed] [Google Scholar]
- 28.Sato T, Hirata A, Mizoguchi T. Prospective, noncomparative, nonrandomized case study of short-term outcomes of 360 degrees suture trabeculotomy ab interno in patients with open-angle glaucoma. Clin Ophthalmol. 2015;9:63–8. doi: 10.2147/OPTH.S75739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alnahrawy O, Blumenstock G, Ziemssen F, Szurman P, Leitritz MA, Dimopoulos S, et al. Exit strategies in canaloplasty: intraoperative conversion into 180-degree trabeculotomy or 360-degree trabeculotomy in cases of unsuccessful catheterisation of Schlemm’s canal: influence of degree of canal cleavage. Graefes Arch Clin Exp Ophthalmol. 2015;253:779–84. doi: 10.1007/s00417-015-2955-9. [DOI] [PubMed] [Google Scholar]
- 30.Dao JB, Sarkisian SR, Jr., Freedman SF. Illuminated microcatheter-facilitated 360-degree trabeculotomy for refractory aphakic and juvenile open-angle glaucoma. J Glaucoma. 2014;23:449–54. doi: 10.1097/IJG.0b013e31829484df. [DOI] [PubMed] [Google Scholar]
- 31.Lim ME, Dao JB, Freedman SF. 360-degree trabeculotomy for medically refractory glaucoma following cataract surgery and juvenile open-angle glaucoma. Am J Ophthalmol. 2017;175:1–7. doi: 10.1016/j.ajo.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Furuyoshi N, Furuyoshi M, Futa R, Gottanka J, Lutjen-Drecoll E. Ultrastructural changes in the trabecular meshwork of juvenile glaucoma. Ophthalmologica. 1997;211:140–6. doi: 10.1159/000310781. [DOI] [PubMed] [Google Scholar]
- 33.Aktas Z, Ozmen MC, Atalay HT, Ucgul AY. Evaluation of episcleral venous fluid wave during gonioscopy assisted transluminal trabeculotomy in patients with advanced glaucoma. Eye. 2019;33:668–73. doi: 10.1038/s41433-018-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahmatnejad K, Pruzan NL, Amanullah S, Shaukat BA, Resende AF, Waisbourd M, et al. Surgical outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in patients with open-angle glaucoma. J Glaucoma. 2017;26:1137–43. doi: 10.1097/IJG.0000000000000802. [DOI] [PubMed] [Google Scholar]
- 35.Sato T, Kawaji T, Hirata A, Mizoguchi T. 360-degree suture trabeculotomy ab interno to treat open-angle glaucoma: 2-year outcomes. Clin Ophthalmol. 2018;12:915–23. doi: 10.2147/OPTH.S161238. [DOI] [PMC free article] [PubMed] [Google Scholar]