To the Editor:
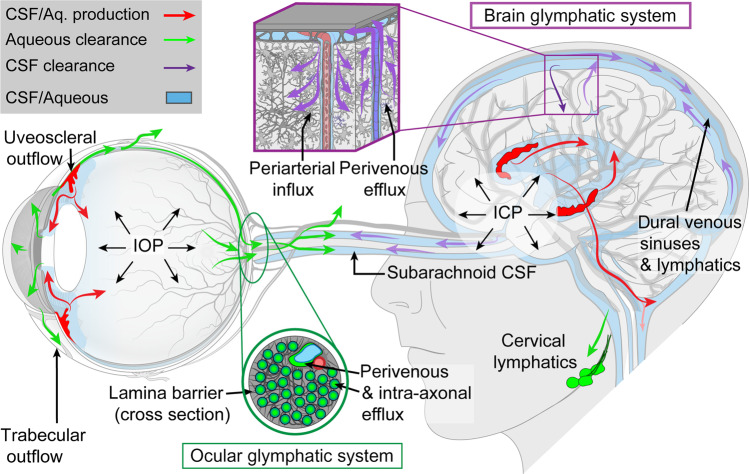
Previous work of Nedergaard’s team has led to the discovery of the glymphatic system in the brain (Fig. 1), a cerebrospinal fluid (CSF) transport system that facilitates the clearance of neurotoxic molecules, including amyloid-β (Aβ), through a brain-wide network of perivascular spaces [1]. CSF enters the brain along periarterial spaces to exchange with interstitial fluid, which is in turn cleared from the brain along perivenous spaces. Glymphatic transport is facilitated by aquaporin-4 (AQP4) water channels on astrocytic endfeet ensheathing the cerebral vasculature. Now, Nedergaard’s team identified a novel “ocular glymphatic clearance” route (Fig. 1) for fluid and wastes from the intraocular space via the proximal optic nerve in rodents [2, 3]. Aβ was cleared from the retina and vitreous via a pathway dependent on glial water channel AQP4 and driven by the trans-lamina cribrosa pressure difference and light-induced pupil constriction [2, 3]. In addition, the authors demonstrated that this clearance pathway was impaired in two distinct murine models of high intraocular pressure glaucoma, due to defects in the lamina barrier [2, 3].
Fig. 1. Macroscopic overview of the brain and ocular glymphatic systems, emphasizing the role played by pressure gradients, hydrostatic barriers and lymphatic drainage, shown in the context of known pathways for aqueous humour and cerebrospinal fluid (CSF) efflux.
ICP intracranial pressure, IOP intraocular pressure. Figure reproduced from [3].
The discovery of this entirely new ocular glymphatic clearance system may lead to a paradigm shift in our understanding of fluid dynamics in the eye, and has the potential to become a game-changer in our understanding of the pathogenesis of a wide range of ophthalmic conditions, including glaucoma. Moreover, this landmark study may lead to new opportunities for the development of novel diagnostic and therapeutic strategies for many ocular diseases.
Here, I raise the question of whether the normal-tension and high-tension forms of primary open-angle glaucoma may at least partially result from brain and ocular glymphatic system disturbances, respectively. The optic nerve, a white matter tract of the CNS, is surrounded by CSF within the subarachnoid space (SAS) [4]. Recently, Mathieu et al. [4] provided the first evidence to support the existence of a glymphatic pathway in the optic nerve following tracer injection into the CSF of live mice. Their findings indicated that CSF enters the optic nerve via spaces surrounding blood vessels, bordered by AQP4-positive astrocytic endfeet, up to and including the glia lamina, the mouse equivalent of the human lamina cribrosa. The authors suggested that CSF flow through the optic nerve may play a role in neurotoxin clearance in the laminar and retrolaminar optic nerve, with potential implications for the pathogenesis of glaucoma [4]. Their findings also point to the importance of intact CSF flow in the SAS of the optic nerve, without which CSF entry into the optic nerve via a glymphatic pathway may be impaired. Interestingly, impaired CSF dynamics within the optic nerve SAS have been demonstrated in normal-tension glaucoma (NTG) [5]. Given that there exists a perivascular transport system within the optic nerve, similar and likely continuous with the glymphatic system in the rest of the CNS, it is conceivable that some cases of NTG are actually the expression of glymphatic dysfunction in natural brain aging and CNS diseases, including Alzheimer’s disease [6]. High-tension glaucoma, on the other hand, might then primarily result from pathological changes in ocular glymphatic solute transport, as reported by Nedergaard and colleagues.
Compliance with ethical standards
Conflict of interest
The author declares that he has no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Lou N, Eberhardt A, Yang Y, Kusk P, Xu Q, et al. An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci Transl Med. 2020;12:eaaw3210. doi: 10.1126/scitranslmed.aaw3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangroo Thrane V, Hynnekleiv L, Wang X, Thrane AS, Krohn J, Nedergaard M. Twists and turns of ocular glymphatic clearance—new study reveals surprising findings in glaucoma. Acta Ophthalmol. 2020. 10.1111/aos.14524. [DOI] [PMC free article] [PubMed]
- 4.Mathieu E, Gupta N, Ahari A, Zhou X, Hanna J, Yücel YH. Evidence for cerebrospinal fluid entry into the optic nerve via a glymphatic pathway. Investig Ophthalmol Vis Sci. 2017;58:4784–91. doi: 10.1167/iovs.17-22290. [DOI] [PubMed] [Google Scholar]
- 5.Pircher A, Montali M, Wostyn P, Pircher J, Berberat J, Remonda L, et al. Impaired cerebrospinal fluid dynamics along the entire optic nerve in normal-tension glaucoma. Acta Ophthalmol. 2018;96:e562–569. doi: 10.1111/aos.13647. [DOI] [PubMed] [Google Scholar]
- 6.Wostyn P. The “ocular glymphatic clearance system”: a key missing piece of the Alzheimer’s disease-glaucoma puzzle found? Eye. 2020 doi: 10.1038/s41433-020-1008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]