Abstract
Background
The pancreatic β cell, as the sole source of the vital hormone insulin, has been under intensive study for more than a century. Given the potential of newly created insulin-producing cells as a treatment or even cure of type 1 diabetes (T1D) and possibly in severe cases of type 2 diabetes (T2D), multiple academic and commercial laboratories are working to derive surrogate glucose-responsive, insulin-producing cells.
Scope of Review
The recent development of advanced phenotyping technologies, including molecular, epigenomic, histological, or functional, have greatly improved our understanding of the critical properties of human β cells. Using this information, here we summarize the salient features of normal, fully functional adult human β cells, and propose minimal criteria for what should rightfully be termed ‘β cells’ as opposed to insulin-producing but not fully-functional surrogates that we propose should be referred to as ‘β-like’ cells or insulin-producing cells.
Major Conclusions
Clear criteria can be established to differentiate fully functional, mature β cells from ‘β-like’ surrogates. In addition, we outline important knowledge gaps that must be addressed to enable a greater understanding of the β cell.
Keywords: Beta cell, Islet, Islet transplantation, Diabetes
Abbreviations: ESC, embryonic stem cell; HIRN, Human Islet Research Network; HIPP, Human Islet Phenotyping Program; IIDP, Integrated Islet Distribution Program; iPSC, inducible pluripotent stem cell; HPAP, Human Pancreas Analysis Program
1. Introduction
The β cell and its primary product, insulin, are essential for normal glucose homeostasis and organismal survival; β cell dysfunction or deficiency is central to the development of type 1 diabetes (T1D), type 2 diabetes (T2D), and almost all other forms of diabetes. Thus, considerable efforts are underway to amplify or functionally augment existing β cells or to generate new β cells for replacement therapy. For example, multiple groups of investigators have generated insulin-expressing cells from human embryonic stem cells (ESC) or from inducible pluripotent stem cells (iPSC), while others have tried to create “synthetic” β cells or insulin-producing cells from other cell types by transdifferentiation or reprogramming. Others have sought to identify regenerative therapeutic drugs to expand residual βcells in people with diabetes. Furthermore, some have proposed that β cell identity or function may be lost in diabetes, especially T2D, leading to discussion about how one defines a normal β cell. This Perspective seeks to integrate, summarize, and codify the hallmark features of a normal, adult human β cell, to outline critical knowledge gaps in the field, and to provide a framework for the appropriate use of the term “β cell.” That is, we assert that validating the identity and utility of any surrogate β cell source requires a clear definition of the native β cell state for comparison.
This attempt to define a β cell is reminiscent of efforts to precisely describe a topic or subject in art, religion, or philosophy, when there are many differing opinions and perspectives, all of which have validity. In that way, efforts of scientists to define a β cell could be described by the well-known “Parable of the Blind Men and the Elephant”:
“O how they cling and wrangle, some who claim
For preacher and monk the honored name!
For, quarreling, each to his view they cling.
Such folk see only one side of a thing.”
-Jainism and Buddhism. Udana 68–69: Parable of the Blind Men and the Elephant [1].
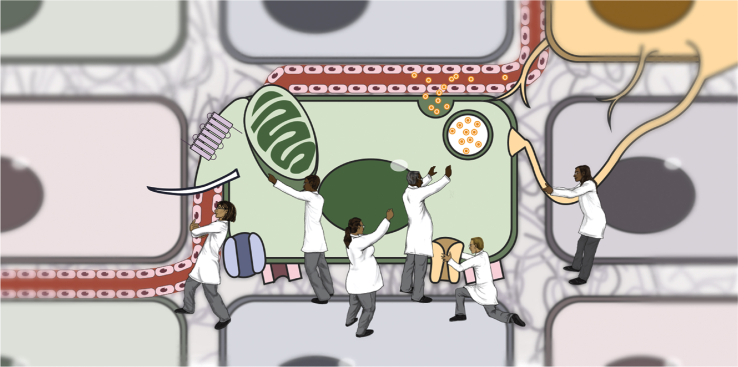
In this often used parable, six blind men, after feeling different parts of an elephant, are asked to describe what an elephant is like. Much like each blind person, islet biologists or scientists sometimes study or focus on one attribute in defining a β cell, for example, the transcriptional profile, the function of an ion channel, or the presence of a membrane receptor (Figure 1). While each blind person's report about a part of the elephant is technically correct, each perspective is limited and only when multiple features or processes are integrated does one “see” the whole elephant or in this case, is one able define a β cell. In our case, in addition to the philosophical challenge, there is an obvious translational challenge: we need a consensus operational definition of a human β cell to serve as a standard for efforts to generate clinically useful β cells from ESCs, iPSCs, or other cellular sources. Thus, this review focuses on isolated human islets as a comparator for such cells from new sources. Many other approaches, such as the pancreatic slice technique, are providing novel information about human islet cell function, physiology, and gene expression that will enable the field to refine the definition of a human β cell even further in the future.
Figure 1.
Efforts by islet scientists to define a β cell. The schematic shows individual scientists interacting with one of the key cellular process of the β cell such as the nucleus, an ion channel, or a secretory granule. None of these individual processes provide a full understanding of what defines the β cell.
While much has been learned about the β cell, islet, and pancreas from rodents and model organisms, this discussion focuses on the human β cell, reflecting the increasing evidence that human islets and β cells differ in important ways from their mammalian counterparts, especially from rodents. This is not to minimize fundamental discoveries about β cell development, differentiation, and function from studies of model organisms and cell-based systems, but to highlight the importance and challenges of translating information to the human arena.
The authors of this publication are or have been part of the Human Islet Research Network (HIRN; https://hirnetwork.org/), which is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to advance understanding of the human islet and β cell by supporting and facilitating collaborative research related to the loss of functional β cell mass in T1D. HIRN has scores of investigators working in five interdisciplinary research consortia, supported by Coordinating and Bioinformatics Centers:
-
•
The Consortium on Beta Cell Death and Survival (CBDS) is working with human tissues and samples to search for highly specific biomarkers of β cell injury and to develop strategies to halt β cell destruction.
-
•
The Consortium on Human Islet Biomimetics (CHIB) is integrating advances in β cell biology, stem cell biology, and tissue engineering technologies to develop microfluidic devices that support functional human islets as well as to provide means to study islet–immune interactions ex vivo.
-
•
The Consortium on Modeling Autoimmune Interactions (CMAI) is developing innovative approaches to model basic aspects of human T1D immunobiology by using in vivo and in vitro platforms.
-
•
The Consortium on Targeting and Regeneration (CTAR) is working to increase, regenerate, or maintain functional β cell mass in T1D through targeted manipulation of islet cell plasticity or engineered protection of β cells from immune-mediated destruction.
-
•
The Human Pancreas Analysis Consortium (HPAC) is investigating the physical and functional organization of the human islet tissue environment, the cell–cell relationships within the pancreatic tissue ecosystem, and the contributions of non-endocrine components (acinar, ductal, vascular, perivascular, neuronal, lymphatic, immune) to islet cell function and dysfunction.
This manuscript reflects extensive discussion by this group of HIRN investigators from these consortia and strives to address the following critical questions:
-
1.
What is a “normal” β cell?
-
2.
How does one determine if an insulin-producing cell is a β cell?
-
3.
What are critical gaps in our understanding of human β cell biology?
-
4.
What are practical suggestions to fill these knowledge gaps?
2. What is a “normal” β cell?
Despite decades of studies, there is a need for consensus on what constitutes an authentic, mature β cell. This question is not simply a matter of semantics. Insulin synthesis and release by a cell is not sufficient for that cell to be labeled a normal, fully competent β cell. Unfortunately, the scientific literature is replete with frequent references to insulin-producing cells as “β cells” when the cells clearly do not, or have not been demonstrated to, exhibit physiologically regulated insulin secretion. This type of misnomer is further exacerbated when cells from outside pancreatic ontogeny are called “pancreatic β cells.” While it is not possible to comprehensively summarize all properties of mature β cells in a schematic, some key features are summarized in Figure 2. We argue that β cells should have a minimum of six key properties (Table 1): (1) synthesis of physiological quantities of insulin and proper processing of insulin precursors to mature insulin to maintain not just the kinetics of insulin secretion but also its sustained production, which in the native β cell means that 10–30% of total cellular protein is insulin or an insulin precursor; (2) storage of insulin in abundant (>5,000 per cell) secretory granules so that for a single secretory stimulus, typically <0.1% of the total insulin content is released; (3) rapid (seconds to minutes) secretion of insulin in response to glucose which is enhanced by incretins; (4) rapid (minutes) cessation of insulin secretion in response to low glucose; (5) a robust insulin stimulatory quotient (high versus low glucose) (see Figure 2B for an example of stimulated insulin secretory profile in isolated islets); and (6) absence of a secretory response to pyruvate or lactate. In this way, an organism, by secreting or not secreting insulin, maintains its blood glucose in a very narrow physiologic range, integrating an influx of a variety of signals and nutrients during feeding, periods of fasting, or physical activity. Certainly, an array of other hormones, metabolites, and processes in many organs contribute to blood glucose regulation, but insulin is the most critical, evidenced by the fact that untreated completed insulin deficiency leads to a severe wasting syndrome and death. Note that here we discuss the features of the healthy adult human β cell; very young human β cells have quite different molecular and physiological properties that are appropriate for the fetal or neonatal time period and are not discussed herein. Likewise, this review does not discuss the many alterations that occur in β cells in diabetes.
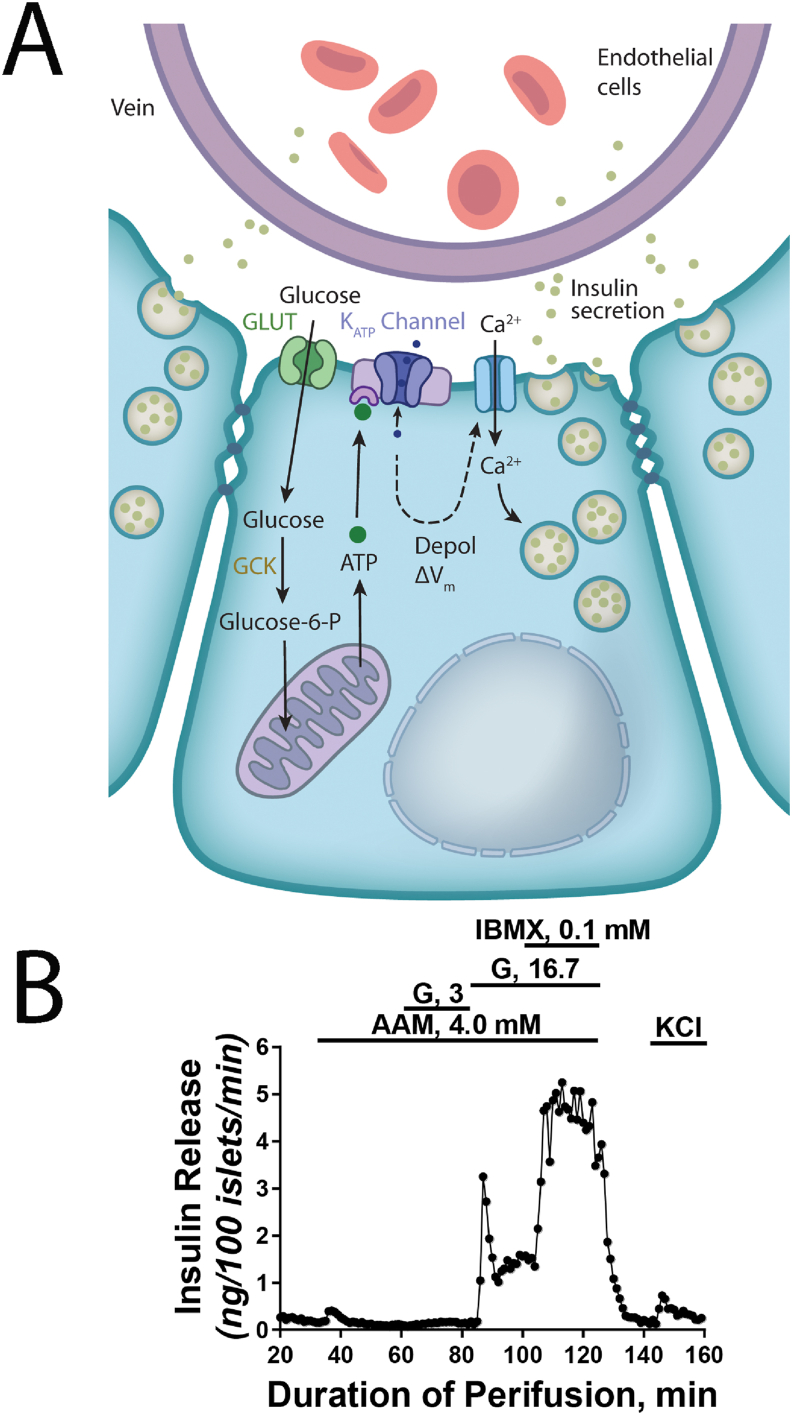
Figure 2.
Key properties of β cells. (A) The schematic shows several β cells arranged in a rosette around a small capillary within the islet. Islet β cells are electrically coupled, allowing for partial synchronization of the secretory response in vivo. Glucose, the major secretagoue, enters the β cell via a high capacity facilitative glucose transporter (primarily GLUT1 with low level of GLUT2 in human β cells) and is metabolized by the high Km enzyme Glucokinase (GCK) to Glucose-6-phosphate which is further metabolized through glycolysis and mitochondrial oxidative phosphorylation for the production of ATP. Increase in the ATP/ADP ratio is sensed by the ATP-dependent potassium channel (KATP channel) in the apical membrane, causing membrane depolarization and the opening of voltage-gated Ca2+ channels. Increase in cytoplasmic calcium triggers the fusion of insulin-containing secretory vesicles with the plasma membrane and release of insulin into the bloodstream. (B) Insulin secretion by isolated human pancreatic islets. In the presence of a physiological amino acid mixture, insulin release is extremely low at 3 mM glucose. Changing the medium to high glucose (16.7 mM) stimulates very rapid insulin secretion, with peak levels (‘first phase’) reached within minutes. Note that both peak and sustained (‘second phase’) levels of insulin release are more than 10-fold higher than basal levels. Insulin secretion can be stimulated further through the addition of 3-isobutyl-1-methylxanthine (IBMX), a competitive non-selective phosphodiesterase inhibitor which raises intracellular cAMP. Depolarization via the addition of KCl stimulates the release of remaining readily releasable insulin granules. We thank Drs. Franz Matschinsky, Doris Stoffers, and Nicolai Doliba from the University of Pennsylvania for providing the figure.
Table 1.
Critical properties of human β cells.
|
|
|
|
|
|
A normal, mature β cell establishes and couples the six core properties listed above through an intricate, coordinated array of intracellular molecular processes and within a complex and supporting microenvironment. For instance, β cells are integrated with other cell types within the pancreatic islet that is a vascularized, innervated mini-organ. Thus, β cells are in close contact with each other and communicate with other islet endocrine cells, the intraislet extracellular matrix, nerve fibers, and endothelial cells. There is evidence that the β cell microenvironment is crucial for finely regulated insulin secretion in vivo, but it is clear that islets and β cells isolated from previously healthy organ donors display regulated insulin secretion also in vitro, indicating that core β cell properties can be maintained autonomously (Figure 2B). It should be noted that while the non-diabetic donors of the isolated islets analyzed by dozens of labs have not undergone dynamic glucose tolerance testing prior to islet isolation, the donors did have a non-diabetic A1C (<5.7%), reflecting their overall normal glucose tolerance over the prior 3 months. An alternative approach for investigating human islet function is the study of pancreatic slices obtained from individuals undergoing pancreatic surgery for other reasons (non-malignant pancreatic tumor) who have had pre-operative glucose tolerance testing [2,3]. In ex vivo studies of pancreatic slices, glucose- and nutrient-stimulated insulin secretion is robust and are overall in line what has been observed in isolated islets; such studies of these surgical specimens will continue to provide important information to the field. We note that the important question of whether transplantation of only β cells, rather than whole islet replacement, is sufficient to restore normal glucose homeostasis when endogenous β cell mass is insufficient is beyond the scope of this article.
The authors of this Perspective argue that insulin-producing cells, whether studied in vitro, ex vivo, or in vivo, should not be referred to as “β cells” without clear evidence of having met the criteria or properties outlined here. We suggest that insulin-containing cells that do not meet all six criteria be referred to with more conservative terms, such as “insulin-producing cells”, “insulin+ cells”, or ‘β-like cells”. In this way, cells derived from ESC, iPSC, or non-islet cell types would be described as insulin-producing cells (unless the six criteria above are met), and thus be distinguished from insulin+ β cells in a pancreatic islet.
3. How does one determine whether an insulin-producing cell is a normal β cell?
Through multidisciplinary efforts, we have learned a great deal about the molecular machinery comprising and regulating a normal β cell [[2], [3], [4], [5]]. For example, there is general agreement about the number and properties of insulin secretory granules in β cells, the insulin mRNA and protein content, and the molecules and activities responsible for the metabolic and electrical events of glucose sensing and insulin secretion. More recently, the transcriptional profile of human β cells, including from individual islet cells, are being defined with the likely conclusion that the mRNA repertoire and epigenetic features in human β cells is heterogeneous from cell to cell [[6], [7], [8], [9]]. We note that while many of the discussed features are defined as present or absent, many exist along a spectrum.
We suggest that multiple, operational features be used to establish that a β cell is normal (Table 1). Physiologically regulated insulin production and secretion require a series of complex intracellular events involving glucose transport and metabolism and electrical activity. Thus, we propose that a combination of function (glucose regulation of insulin secretion and cessation of insulin secretion) and gene expression be the standards that insulin-producing cells should meet before the term β cell can be applied. Assessment of insulin secretion should use physiologically relevant conditions. For example, we assert that “low” glucose concentrations should not be < 2.8 mM (50 mg/dl), as this is close to the lower bound of circulating glucose in humans, and stimulatory concentrations should not exceed 16.7 mM (300 mg/dl). Many insulin+ cell preparations that exhibit a 20% increase in insulin secretion have been termed “glucose responsive”, though the dynamic range of insulin secretion in vivo must be much larger in order to be maintain euglycemia. Importantly, isolated islets obtained from non-diabetic organ donors frequently have stimulatory quotients in excess of 10-fold (Figure 2B) and islets studied in pancreatic slides respond to glucose stimulation. Moreover, in vivo studies of transplanted insulin-producing cells in immunocompromised mice should assess regulated insulin secretion, not simply reversal of hyperglycemia. Ideally, these studies would include a series of physiological challenges, like glucose tolerance testing.
In addition, a single feature of normal β cells, such as the expression of a β cell-enriched transcription factor like PDX1 or MAFA by immunostaining, is not sufficient to establish a cell as a normal β cell. Furthermore, cells that lack a specific feature such as the characteristic insulin secretory granule number and composition are not likely to process proinsulin or preproinsulin appropriately, or secrete insulin with normal kinetics and in sufficient quantity. Likewise, cells that do not express the canonical β cell transcription factors are not likely to express the receptors, enzymes, and ion channels required to appropriately secrete insulin in response to physiologic stimuli. Ample evidence for this notion comes from monogenic forms of diabetes or hyperinsulinism resulting from mutations in key transcription factors or ion channels that result in impaired β cell function and diabetes [10]. In addition, suppression of ‘disallowed’ genes such as those encoding lactate dehydrogenase, hexokinase other than glucokinase, or monocarboxylate transporter 1 (MCT1) is essential for proper β cell function, as, for instance, expression of MCT1 in β cells causes exercise-induced hypoglycemia [11]. Thus, we suggest that multiple assays are needed to establish operationally that the insulin-producing cell is a “normal” β cell. Importantly, the approaches used to interrogate an insulin-producing cell should be specifically stated in publications. Insulin-producing cells lacking one or more of the six key properties should not be referred to as β cells.
It is critical to acknowledge challenges in defining the features of the normal β cell in that manipulation of the tissue/cells of interest during isolation/procurement or how the tissue/cells are collected may alter aspects of the cellular phenotype or impact data interpretation. For example, one study concluded that isolation of islets and subsequent culture affected gene expression profile by microarray [12]. However, it is clear that isolated human islets in general have robust insulin secretion in vitro and that islets isolated from individuals with type 1 or type 2 diabetes mimic the in vivo insulin secretion phenotype. Because of concerns of the impact of islet isolation, some have used laser capture microdissection of islets from surgical specimens to obtain transcriptome profiles [3] or the pancreatic slide technique mentioned above. A challenge for laser capture microdissection of islets is that it collects a mixture of cells including alpha, beta, delta, endothelial cells, etc., and the contribution of various cell types varies greatly among humans [13].
4. What are critical gaps in our understanding of human β cell biology?
Understanding how β cells are lost or become dysfunctional in T1D or T2D and developing effective and safe cell protective, regenerative, or replacement strategies will require additional assessment criteria that are the subject of active research (Table 2). For example, how many human β cell subtypes are there, and does their distribution change in response to physiologic or pathophysiologic stimuli or stressors? Furthermore, are β cell subtypes permanent, or do they represent transient and interchangeable states? How does the human β cell change during the progression from infancy to adulthood to old age? Answering these questions will require new experimental approaches and the rigorous application of existing assessments of human islets in vitro and in vivo.
Table 2.
Incompletely defined molecules, processes, or characteristics of normal human β cells.
|
|
|
|
A major challenge historically has been the absence of a human β cell “gold standard” that is widely accepted. In large part, this is because the primary source of “normal” human islets and β cells for research from cadaveric organ donors or pancreatic tissue following partial or total pancreatectomy have been limited in supply. Recent progress in reliable procurement, distribution, and assessment of primary human islets has begun to address this need, but there are remaining challenges. In addition to obvious biologic/demographic variables such as age, BMI, and ethnic background and careful pre-morbid phenotyping to exclude diabetes, there are complex pre-death variables (e.g., time in the intensive care unit, pre-terminal drug treatments, cause of death, etc.) that are impossible to control and may influence gene expression or hormone secretion, leading to appropriate concerns about the variability of human islet preparations. Thus, in spite of recent improvements, the challenge of procuring “normal” human islets and β cells is far from solved. Beyond providing an ample number of human islet preparations to minimize the consequences of inter-individual variability, investigations of primary human islets need rigorous standardization [14]. As an important initial step for providing minimum islet standard evaluations, the major supplier of human islets for research in the United States, the Integrated Islet Distribution Program (IIDP; https://iidp.coh.org/), created a central islet quality core facility (Human Islet Phenotyping Program, HIPP), which is systemically evaluating every human islet preparation distributed for research in the U.S [15]. Similar rigorous evaluations of human islet preparations is also routine by investigators of the European Consortium for Islet Transplantation (ECIT) and other European centers [16], as well as the Alberta Diabetes Institute in Edmonton (www.bcell.org/isletcore.html). These efforts are allowing investigators using human islets to link their experimental data from a specific islet batch to assessments of the health and function of that islet preparation using Research Resource Identifiers (RRIDs). Additional efforts to standardize and integrate data from a range of investigators using IIDP-distributed islets, and adoption of this model by other islet distribution programs, should be prioritized and will foster development of more comprehensive, useful profiles of normal human islets. Finally, comprehensive evaluations that go far beyond functional assays and integrate transcriptional and epigenomic profiling of individual islet preparations are now available via HIRN and PANC-DB (https://hpap.pmacs.upenn.edu), where data on individual human islet preparations, including those of T2D, T2D pre-diabetic, T1D, and non-diabetic autoantibody-positive and autoantibody-negative donors, are continuously being added and are available to any investigator [17], and by collaborative efforts such as the Innovative Medicines Initiative (https://www.imi.europa.eu/news-events/newsroom/imi-impact-diabetes) and T2DSystems (https://www.t2dsystems.eu/).
5. What are practical suggestions to fill these knowledge gaps?
While striving for specificity and focus, this perspective is intended as a first step, a working document, and is certainly not the definitive word. We trust this commentary will stimulate additional discussion about important questions like “What is a β cell?” or “What parameters should be used to evaluate insulin-producing cells?” We anticipate growth in knowledge about the questions and unknowns posed in Table 2 will lead to improvement and refinement of criteria for ‘normal’ β cells listed in Table 1. We envision that perhaps this working document, available to all, would be updated and revised as new attributes of the normal β cell are discovered.
Since our understanding of human β cells is rapidly advancing and evolving, we suggest several immediate useful steps for those interested in human β cells and diabetes, including:
-
•
Careful and deliberate use of the phrases “β cell” and “insulin-producing cells” in publications and scientific presentations. We encourage that authors, manuscripts, presentations, and communications (including press releases) refrain from using the term β cell unless the ‘core six’ features mentioned above are demonstrated. In the absence of such proof, we advocate for the phrase “insulin-producing cells” or “β-like cell” for cells derived from ESC, iPSC, or non-islet cell types.
-
•
Authors and publications should clearly state the criteria for how insulin-producing cells were evaluated and how the cells under investigation compared to normal β cells. We suggest that multiple assessments from Table 1 be used in such evaluations.
-
•
Continued discussions in the field about the minimal standards for defining a cell as a β cell. We hope this perspective will stimulate ongoing discussion and dialogue.
-
•
Continued efforts to standardize human islet preparations used for research and to systemically evaluate those preparations distributed to investigators.
-
•
Efforts to integrate the rapidly expanding interdisciplinary information relevant to human β cells. There is a great need for a careful alignment and integration of the large amount of transcriptional profiling of human β cells that is now in the public arena. Such approaches to define “normal” will be crucial for the field to understand normal age-related changes and derangements in islets in T1D, T2D, and other forms of diabetes.
-
•
Continued worldwide support for research on human islets and β cells with integration of the new data across investigators, scientific disciplines, countries, and funding agencies.
-
•
In accord with the considerations detailed above, we also foresee a similar set of ‘working’ criteria to be applied in naming other islet cell types generated from renewable sources, like glucagon-producing α cells, and somatostatin-producing δ cells.
We hope that these recommendations from this HIRN working group will provide practical standards for the diabetes research community, enhance the reproducibility of studies in the field, and provide suggestions for future research directions.
Acknowledgements
We apologize to our colleagues whose relevant and important work could not be cited due to space limitations or whose discussions and ideas we mention but do not attribute. Related work in the authors' laboratories has been supported by the Human Islet Research Network (HIRN; RRID:SCR_014393; https://hirnetwork.org) and by NIDDK grants UC4DK112217 (KHK), U01DK123594 (KHK), UC4DK112232 (ACP), U01DK123716 (ACP and SKK), UC4DK116271 (KHK and YD), U01DK123716 (ACP), DK106755 (ACP), U01DK120429 (MS), UG3DK122639 (MS), UC4DK104202 (MS), U01DK123743 (SKK and ACP), UC4DK104211 (ACP, AFS, SKK), DK108120 (ACP), DK116873 (AFS), DK116904 (AFS), DK125285 (AFS), DK105015 (AFS), UC4DK104155 (MC-T), DK123329 (MC-T), DK122160 (MC-T), DK122638 (CS), and DK020541, DK20593, DK19525, DK116074, and by the Department of Veterans Affairs (BX000666; ACP). All authors reviewed and edited the final manuscript.
Contributor Information
Klaus H. Kaestner, Email: kaestner@pennmedicine.upenn.edu.
Alvin C. Powers, Email: al.powers@vumc.org.
Conflict of interest
AFS is an inventor on patents filed by the Icahn School of Medicine at Mount Sinai. The other authors declare no conflicts of interests.
References
- 1.Wikipedia, https://en.wikipedia.org/wiki/Blind_men_and_an_elephant.
- 2.Solimena M., Schulte A.M., Marselli L., Ehehalt F., Richter D., Kleeberg M. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia. 2018;61(3):641–657. doi: 10.1007/s00125-017-4500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wigger L., Barovic M., Brunner A.D., Marzetta F., Schoniger E., Mehl F. Multi-omics profiling of living human pancreatic islet donors reveals heterogeneous beta cell trajectories towards type 2 diabetes. Nat Metab. 2021;3(7):1017–1031. doi: 10.1038/s42255-021-00420-9. [DOI] [PubMed] [Google Scholar]
- 4.Marshall S.M. The pancreas in health and in diabetes. Diabetologia. 2020;63(10):1962–1965. doi: 10.1007/s00125-020-05235-z. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson M.A., Campbell-Thompson M., Kusmartseva I., Kaestner K.H. Organisation of the human pancreas in health and in diabetes. Diabetologia. 2020;63(10):1966–1973. doi: 10.1007/s00125-020-05203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y.J., Schug J., Won K.J., Liu C., Naji A., Avrahami D. Single-cell transcriptomics of the human endocrine pancreas. Diabetes. 2016;65(10):3028–3038. doi: 10.2337/db16-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y.J., Kaestner K.H. Single-cell RNA-seq of the pancreatic islets--a promise not yet fulfilled? Cell Metabolism. 2019;29(3):539–544. doi: 10.1016/j.cmet.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mawla A.M., Huising M.O. Navigating the depths and avoiding the shallows of pancreatic islet cell transcriptomes. Diabetes. 2019;68(7):1380–1393. doi: 10.2337/dbi18-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiou J., Zeng C., Cheng Z., Han J.Y., Schlichting M., Huang S. Single cell chromatin accessibility reveals pancreatic islet cell type- and state-specific regulatory programs of diabetes risk. Nature Genetics. 2021 doi: 10.1038/s41588-021-00823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hattersley A.T., Patel K.A. Precision diabetes: learning from monogenic diabetes. Diabetologia. 2017;60(5):769–777. doi: 10.1007/s00125-017-4226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otonkoski T., Jiao H., Kaminen-Ahola N., Tapia-Paez I., Ullah M.S., Parton L.E. Physical exercise-induced hypoglycemia caused by failed silencing of monocarboxylate transporter 1 in pancreatic beta cells. The American Journal of Human Genetics. 2007;81(3):467–474. doi: 10.1086/520960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negi S., Jetha A., Aikin R., Hasilo C., Sladek R., Paraskevas S. Analysis of beta-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. PloS One. 2012;7(1) doi: 10.1371/journal.pone.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y.J., Golson M.L., Schug J., Traum D., Liu C., Vivek K. Single-cell mass cytometry Analysis of the human endocrine pancreas. Cell Metabolism. 2016;24(4):616–626. doi: 10.1016/j.cmet.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart N.J., Powers A.C. Use of human islets to understand islet biology and diabetes: progress, challenges and suggestions. Diabetologia. 2019;62(2):212–222. doi: 10.1007/s00125-018-4772-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brissova M., Niland J.C., Cravens J., Olack B., Sowinski J., Evans-Molina C. The integrated islet distribution program answers the call for improved human islet phenotyping and reporting of human islet characteristics in research articles. Diabetes. 2019;68(7):1363–1365. doi: 10.2337/dbi19-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchetti P., Schulte A.M., Marselli L., Schoniger E., Bugliani M., Kramer W. Fostering improved human islet research: a European perspective. Diabetologia. 2019;62(8):1514–1516. doi: 10.1007/s00125-019-4911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaestner K.H., Powers A.C., Naji A., Atkinson M.A., Consortium H. NIH initiative to improve understanding of the pancreas, islet, and autoimmunity in type 1 diabetes: the human pancreas Analysis program (HPAP) Diabetes. 2019;68(7):1394–1402. doi: 10.2337/db19-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]