Abstract
Background
Persistent cough and large amounts of purulent sputum affects many bronchiectasis patients. No studies have evaluated the efficacy and safety of bronchoscopic airway clearance therapy and bronchoalveolar lavage (B-ACT) for non-cystic fibrosis bronchiectasis patients with acute exacerbation.
Methods
A randomised controlled trial was conducted to explore the efficacy and safety of B-ACT among 189 bronchiectasis inpatients from February 1, 2018 to February 28, 2019. The primary outcome was the time to first acute exacerbation. Secondary outcomes included changes of health-related scores, length of hospital stay, hospitalization expenses and incidences of adverse events.
Findings
B-ACT therapy significantly prolonged the median days to first acute exacerbation when compared with control group (198 vs 168 days, HR 0·555 (0·322-0·958), p=0·012; effect size(r)= 0·94). Further analysis showed that B-ACT therapy was more beneficial for these patients with severe disease and greater symptoms. COPD Assessment Test (CAT) scores improved significantly on the third day (5·45 vs 4·85, 0·60 (0·09-1·11), p=0·023), and Leicester Cough Questionnaire (LCQ) scores improved obviously on the third and seventh days (1·53 vs 1·23, 0·30 (0·05-0·55), p=0·044; 1·66 vs 1·32, 0·34 (0·08-0·60), p=0·022; respectively) after B-ACT therapy. Adverse events associated with B-ACT were mostly transient and mild. Differences of the lengths of hospital stay and hospitalization expenses in both group was not significant.
Interpretation
B-ACT therapy significantly prolonged the time to first acute exacerbation after discharge, highlighting the importance of B-ACT therapy focused on symptom improvements in preventing exacerbation.
Funding
National Natural Science Foundation of China.
Trial registry
ClinicalTrials.gov; No.:NCT03643302; URL: www.clinicaltrials.gov.
Keywords: Acute exacerbation, B-ACT, Bronchiectasis, Efficacy
Research in context.
Evidence before this study
Current guidelines recommend ACT as stable state treatment for bronchiectasis, but the evidence on which to base guideline recommendations was limited. Furthermore, the efficacy of ACT on bronchiectasis with acute exacerbation is still unknown. British Thoracic Society Guideline for bronchiectasis highlights that randomised controlled trials are urgently needed to evaluate the effects of ACT in bronchiectasis patients who are undergoing an exacerbation. To our knowledge, this is the first randomised controlled trial that aims to explore the benefits and risks of B-ACT therapy in managing hospitalized bronchiectasis with acute exacerbation and identify special group of patients that benefit most from the newly promising treatment.
Added value of this study
B-ACT therapy significantly prolonged the time to first acute exacerbation after discharge and improved CAT and LCQ scores for bronchiectasis patients with acute exacerbation, especially for the subgroup of patients with a greater symptom burden at baseline, including those with BSI scores ≥8, the number of acute exacerbations in the past year ≥2, SGRQ scores ≥15, CAT scores ≥11, or LCQ scores <16.
Implications of all the available evidence
Our study highlighted the importance of B-ACT therapy focused on symptoms improvements in preventing exacerbation for highly symptomatic patients, thus, what we concluded here would be of great utility value and far-reaching instructive meaning to clinicians worldwide in developing individualized therapeutic choice for bronchiectasis patients.
Alt-text: Unlabelled box
1. Introduction
Bronchiectasis is a progressive respiratory disease characterised by permanent and irreversible bronchial wall dilatation and thickening. [1,2] With the annual increase of the prevalence of bronchiectasis worldwide, it has already caused serious economic burden on patients and the whole society [3], [4], [5]. A comprehensive scoring system, Bronchiectasis Severity Index (BSI), is an effective tool for identifying patients at high risk of exacerbations, hospitalization and future mortality [6]. Compared with mild patients, moderate to severe patients showed increased risk of mortality, hospitalisation rate and number of exacerbations within 4 years [7]. Therefore, additional consideration about the influence of disease severity on therapeutic efficacy should be taken into account by doctors when formulating treatment schemes. Persistent cough and sputum production are dominant symptoms of bronchiectasis. Bronchiectasis guidelines recommend that the clearance of excessive purulent sputum from the lung of bronchiectasis is important strategies to fight against recurrent acute exacerbation [8]. Airway clearance therapy (ACT) is a kind of airway physiotherapy and is indicated for patients with altered mucociliary escalator or compromised ability to expectorate airways secretions [9]. Common airway clearance techniques include active cycle of breathing techniques, autogenic drainage, postural drainage, clapping or vibration [9,10]. Guidelines recommend ACT as stable state treatment for bronchiectasis, but there was limited evidence on which to base guideline recommendations [11], [12], [13], [14]. Moreover, ACT recommendations for bronchiectasis largely rely on studies during a clinically stable stage, the effect of ACT on bronchiectasis with acute exacerbation is not clear.
British Thoracic Society Guideline for bronchiectasis highlights that randomised controlled trials are urgently required to evaluate the effects of ACT in bronchiectasis patients who are undergoing an exacerbation [11]. The aims of this study were to explore the benefits and risks of B-ACT therapy in managing hospitalized bronchiectasis with acute exacerbation and identify special group patients that benefit most from the newly promising treatment.
2. Methods
2.1. Study design
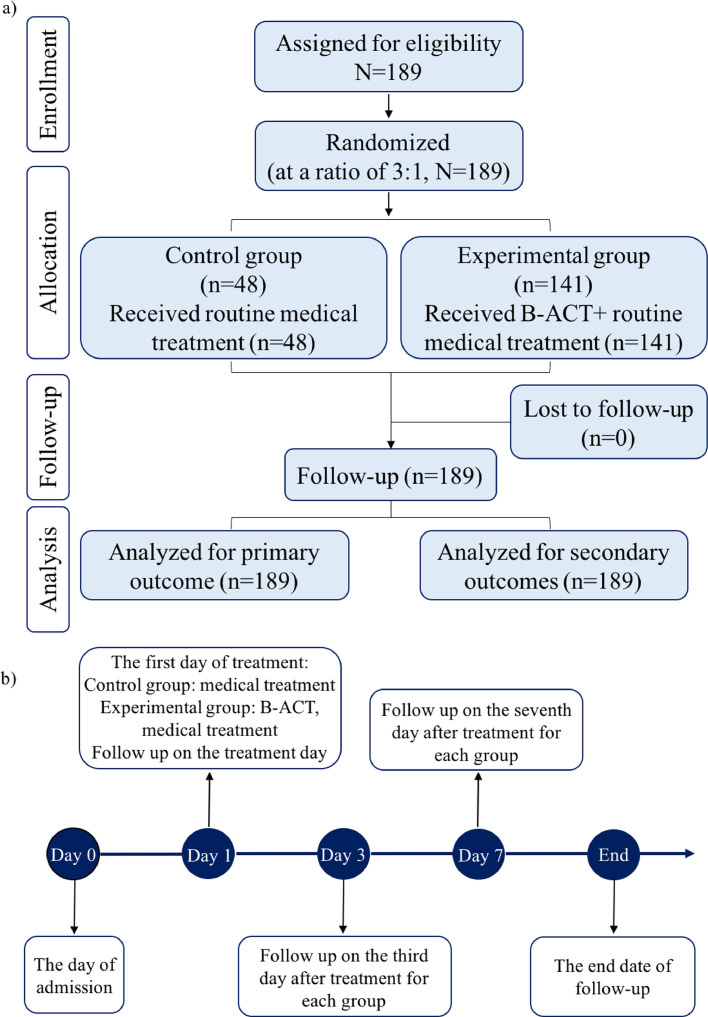
A randomised controlled trial had been conducted at Shanghai Pulmonary Hospital from February 1, 2018 to February 28, 2019. All patients enrolled in this study were moderate to severe bronchiectasis with acute exacerbation. Patients were screened and recommended through five hospitals in Bronchiectasis Treatment and Research Alliance of China (BEChina, http://www.chinabronchiectasis.com/) and then were uniformly introduced and admitted into Shanghai Pulmonary Hospital. All patients were followed up for healthy related scores at different time points (on the day right after the B-ACT therapy, and 3 days, 7 days after the treatment). In order to improve the accuracy of acute exacerbation information, patients were informed of the definition of acute exacerbation in advance, and were followed up by telephone every three months, which is relative to the individual's treatment date, regardless of whether an exacerbation occurred. And a uniform follow-up end point time of February 28, 2019 was set for both groups. Meanwhile, patients were given the phone number of the follow-up doctor and told to contact him within the first time when acute exacerbation occurred, so as to timely record acute exacerbation information. This study was approved by the ethical committee in Shanghai Pulmonary Hospital (approval number K17-112). Trial registration number: NCT03643302; URL: www.clinicaltrials.gov. All patients have signed the informed consent. Detailed procedures and timeline of the study design are shown in Figure 1a, 1b. An associated study protocol was shown in supplementary materials 1.
Fig. 1.
Flow diagram and timeline of the study design
The detailed procedure of the study is shown in panel a); The timeline of the study is shown in panel b).
2.2. Study participants and diagnosis of bronchiectasis and exacerbations
During the study period, we initially screened 229 bronchiectasis patients with acute exacerbation for feasiblility of bronchoscopy. Among them, 27 patients were not suitable for bronchoscopy, including those who are extremely weak, and cannot withstand any invasive procedures (16 patients), patients with uncontrolled hypertension (5 patients), active massive hemoptysis (4 patients), severe hypoxemia (2 patients). In addition, there were 13 patients who were suitable for bronchoscopy but are unwilling to do it. Finally, 189 patients who suitable for bronchiectasis were willing to participate this study and signed the informed consent. Eligible patients were ≥18 years of age, diagnosed with bronchiectasis according to guideline for bronchiectasis in adults [15]. In addition, all the enrolled patients had a history of acute exacerbation, were eligible for bronchoscopy and were willing to receive B-ACT therapy. Pulmonary exacerbation in patients with bronchiectasis was required to meet three or more of the following key symptoms for at least 48h: Cough; Sputum volume and/or consistency; Sputum purulence; Breathlessness and/or exercise tolerance; Fatigue and/or malaise; Haemoptysis, and a clinician determines that a change in bronchiectasis treatment is required [16]. Patients were excluded if (1) they did not receive a high-resolution CT (HRCT) chest scan in the past three months, (2) physicians determined that patient had other medical conditions that could affect the results of this study. Patients who were lost to follow-up or with major protocol violations need to withdraw after enrollment.
2.3. Sample size estimation, randomisation and blinding
Based on ten patients observed in preliminary clinical trial, which was independent of the current study, we found that the time to first acute exacerbation in the B-ACT treatment group was 17 days longer than that of the control group, with standard deviations of 87 and 75 days, respectively. Considering the small number of observed cases in the pre-experiment, we set the margin of non-inferiority (NIM) as 30 when calculating the sample size. Setting the significance level alpha (α) to 0.025, test power 1-β to 0.9, set the sample size ratio between the experiment group and the control group to 3:1, applying the software PASS 11, and calculate the sample size of control group was 40 cases and the experiment group was 120 cases. That is to say, when the final number of cases in the two groups is greater than or equal to the above value, the statistical difference test level can be reached. In this study, the random coding table with serial number 001-200 in proportion (3:1) was generated by the use of computer Excel software. Patients whose random number was less than or equal to 150 were assigned to the B-ACT group, and those whose random number was greater than 150 were assigned to the control group. The evaluator blind method was adopted in this study. That is, researchers who are responsible for assessing the improvement of patient's symptom score (CAT, LCQ, 6MWD) and for recording information of acute exacerbation were blinded. The investigators that responsible for giving B-ACT therapy and for recording subsequent adverse events remained unblinded. In order to avoid the subjective bias of the evaluator, the non-blind person should not disclose the blind information to the evaluator.
2.4. Study treatments
For patients in control group, they were given medical treatment according to the European Respiratory Society guideline [8], which including antibiotic and dispelling sputum therapies. During the hospitalization, all patients were given antibiotics as the physicians wish, the course of anti-infective treatment was 14 days. And for patients in B-ACT group, on the basis of the same treatment as the control group, they were also treated with B-ACT by attending physicians in respiratory department of our hospital. The operation steps of B-ACT are as follows:
Firstly, a comprehensive assessment of patients’ condition was conducted to determine whether the patient was able to tolerate bronchoscopy. Mainly through the following examinations: electrocardiogram, chest imaging data, blood test, infectious diseases related indicators detections and lung function examination if necessary. Preoperative analysis and discussion were conducted according to the requirements of bronchoscopy. Patients and their families were fully informed before bronchoscope, and the informed consents were signed.
Before the bronchoscopy, 2% lidocaine solution were used for laryngeal spray or atomized inhalation, and lidocaine gel were used for nasal cavity. Operators checked whether the patient had active denture and removed it in time to prevent aspiration. Oxygen was given to one side of the nasal tract and oxygen saturation and pulse were monitored. The sputum aspirator with negative pressure suction device was prepared before the operation and was connected to the bronchoscope. Continuous suction was performed from the trachea to the subsegmental during the entering of bronchoscope to remove the visible secretions from entire respiratory tract. After that, the bronchoscope entered the lavage segments, a total amount of 120 to 200ml normal saline was used for lavage (the volume various depending on the operator's judgement). Suction immediately after each lavage and a certain amount of lavage fluid was collected. Check for bleeding carefully before the withdrawal of the bronchoscope. A short video that showed the operation steps of B-ACT therapy was shown in supplementary materials 3.
2.5. Data collection and outcomes
All data collections were performed according to standardised protocol by clinical physicians involved in this research. Data on general conditions, clinical symptoms, radiographic manifestations, lung function indexes and healthy related scores such as St George's Respiratory Questionnaire (SGRQ) [17], CAT [18], modified Medical Research Council (mMRC) [19], LCQ [20] and the 6 Minute Walking Distance (6-MWD) [21] of all patients were recorded at different time points. Lung function parameters included forced expiratory volume in 1s (FEV1), percentage of predicted FEV1 value (FEV1%), forced vital capacity (FVC) and FEV1/FVC ratio. The primary outcome was the time to first acute exacerbation after discharge. Secondary outcomes included the frequency of exacerbations, the change of health-related scores, lengths of hospital stay, hospitalization expenses and incidences of adverse events.
2.6. Statistical analysis
Categorical variables were presented as frequencies and percentages, while continuous variables were tabulated as mean (standard deviation) for normally distribute variables or median (IQR) for non-normally distribute variables. The Kolmogorov-Smirnov test and Levene test were applied for analysing the normality and homogeneity of variables. Independent group t-test was used for normally distributed variables, and Mann-Whitney U test was applied for non-normally distributed variables. Categorical variables were compared by the Chi-square test or Fisher's exact test. The multivariable analysis (Cox proportional hazards model) was applied to assess independent risk factors for the time to first acute exacerbation after discharge. The difference of primary outcome between two groups were compared by the method of Kaplan-Meier and the log-rank test. The repeated measures ANOVA was adopt to compare the scores changes at different time points. Statistical significance was considered when the two-tailed p < 0·05. All statistical analyses and diagramming were performed by SPSS (version 23·0), GraphPad Prism (version 8·0), Origin Pro (version 26·0) and Ziostation2 (version 2·4·0·2) softwares.
2.7. Role of the funding source
The funders had no role in the design and conduct of the study; collection, management, analysis, interpretation of the data, review, or approval of the manuscript; and decision to submit the manuscript for publication. No authors have been paid to write this article by any pharmaceutical companies or agencies.
3. Results
3.1. Study population description
The data of 189 bronchiectasis inpatients were included in the study. Of all patients, 141(74·6%) patients were randomly assigned to the B-ACT group, and 48 (25·4%) to the control group. The baseline characteristics of all patients in both groups were similar in terms of demographics, disease severity, comorbidities, health-related scores, lung function and pulmonary segments involved in CT scans (Table 1).
Table 1.
Differences of baseline characteristics of bronchiectasis patients with acute exacerbation in both groups were not significant.
| Control group | B-ACT group | |
|---|---|---|
| N | 48 | 141 |
| Demographics | ||
| Age, yrs | 60·5 (49·3 to 71·5) | 58·0 (48·5 to 65·0) |
| Female, n (%) | 26 (54·2%) | 93 (66·0%) |
| BSI score | ||
| Moderate (5-8) | 13 (27·1%) | 48 (34·0%) |
| Severe (≥ 9) | 35 (72·9%) | 93 (66·0%) |
| Age of onset,yrs | 47·5 (34·0 to 56·8) | 46·0 (30·0 to 58·0) |
| Duration of disease,yrs | 9·0 (2·0 to 20·0) | 7·0 (2·0 to 20·0) |
| AE numbers/year | 2·0 (2·0 to 3·0) | 2·0 (1·0 to 3·0) |
| Comorbidities, n (%) | ||
| Hypertension | 8 (16·7%) | 17 (12·1%) |
| Diabetes | 4 (8·3%) | 9 (6·4%) |
| Coronary heart disease | 1 (2·1%) | 5 (3·5%) |
| Arrhythmia | 4 (8·3%) | 10 (7·1%) |
| Chronic nasosinusitis | 2 (4·2%) | 8 (5·7%) |
| Healthy-related scores | ||
| SGRQ score | 18·0 (14·0 to 24·0) | 17·0 (14·0 to 21·0) |
| mMRC grading | 1·0 (0·0 to 1·0) | 1·0 (0·0 to 1·0) |
| CAT score | 16·5 (11·0 to 20·0) | 14·0 (11·0 to 19·0) |
| LCQ score | 15·3 (13·5 to 18·0) | 15·0 (13·5 to 16·7) |
| 6-MWD, m | 399·0 (340·0 to 469·0) | 421·0 (376·0 to 470·0) |
| Lung function | ||
| FEV1 | 1·4 (0·8 to 2·3) | 1·8 (1·4 to 2·2) |
| FEV1% | 60·8 (26·7) | 71·3 (21·0) |
| FEV1/FVC | 72·1 (58·6 to 83·3) | 77·6 (66·5 to 83·9) |
| Radiography involved lung segments | 7·0 (5·0 to 10·0) | 6·0 (3·0 to 9·0) |
| Hemoglobin (g/L) | 124·4 (15·2) | 124·9 (15·1) |
Quantitative data are summarized as mean (SD) for normally distribute variables or median (IQR) for non-normally distribute variables and qualitative data are presented as n (percentage). Normal distribution variables, non-normal distribution variables and categorical variables were compared by student's t test, Mann-Whitney U test and chi-square test respectively. BMI, Body Mass Index; BSI, Bronchiectasis Severity Index; AE, Acute Exacerbation; SGRQ, St. George's Respiratory questionnaire; mMRC, modified British Medical Research Council; CAT, COPD Assessment Test; LCQ, Leicester Cough Questionnaire; 6MWD, 6 Minute Walking Distance; FEV1, Forced Expiratory Volume in 1s; FVC, Forced Vital Capacity.
3.2. Primary outcome
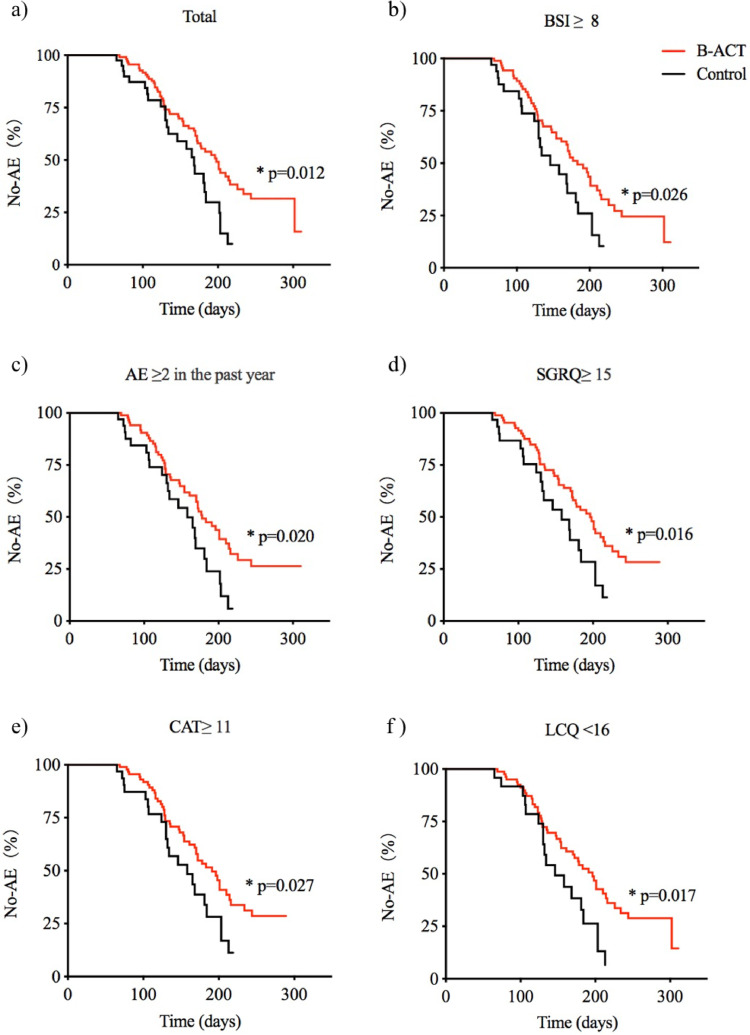
The multivariable analysis of relevant factors associated with the time to first acute exacerbation after discharge were shown in table 2. Factors including the B-ACT therapy (OR 0·57, 0·35-0·93; p=0·024), numbers of AE in the past year (OR 1·76, 1·35-2·31; p<0·0001), and haemoglobin (g/L) (OR 0·99, 0·97-1·00; p=0·042) were independently associated with the time to first acute exacerbation. The time to first acute exacerbation after discharge was followed up by telephone in both groups, and the observation endpoint was set on February 28, 2019. The difference in the follow-up time between the two groups was not significant (127(74·3-168·8) days vs 129(80·5-190·0) days, p=0·193) and the Kaplan-Meier survival analysis was used for the primary outcome. The median time to first acute exacerbation after discharge was longer in B-ACT group than that in control group (198 days vs 168 days, HR 0·555 (0·322-0·958), p= 0·012; effect size(r)= 0·94) (Figure 2a). Further analysis showed that B-ACT therapy was more effective in prolonging the median time to first acute exacerbation after discharge for patients with BSI scores more than 8 (183 days vs 146 days, HR 0·574 (0·323-1·022), p= 0·026), with two or more acute exacerbations in the past year (178 days vs 158 days, HR 0·558 (0·312-0·999), p= 0·020), with SQRQ scores more than 15 (196 days vs 158 days, HR 0·536 (0·289-0·992), p= 0·016), with CAT scores more than 11 (191 days vs 158 days, HR 0·567 (0·314-1·025), p= 0·027), or with LCQ scores less than 16 (196 days vs 146 days, HR 0·527 (0·275-1·010), p= 0·017) (Figure 2b-2f, Supplementary Figure S1).
Table 2.
Multivariate analysis of the independent risk factors associated with the time to fist acute exacerbation after discharge.
| Variables | Odds ratio (95%CI) | p value |
|---|---|---|
| B-ACT | 0·57 (0·35-0·93) | 0·024 |
| Numbers of AE in the past year | 1·76 (1·35-2·31) | <0·0001 |
| Hemoglobin (g/L) | 0·99 (0·97-1·00) | 0·042 |
ACT, Airway Clearance Therapy; AE, Acute Exacerbation; B, Bronchoscopic; CI, confidence interval.
Fig. 2.
Subgroups analysis of the target populations who benefit more from the B-ACT therapy according to comparison of the time to first acute exacerbation after discharge
Differences of the time to first acute exacerbation after discharge for total population, patients with BSI scores ≥8, the number of acute exacerbations in the past year ≥2, SGRQ scores ≥15, CAT scores ≥11, or LCQ scores <16 between the two different treatment groups are shown in panel a), b), c), d), e) and f) respectively. *: p<0.05.
3.3. Secondary outcomes
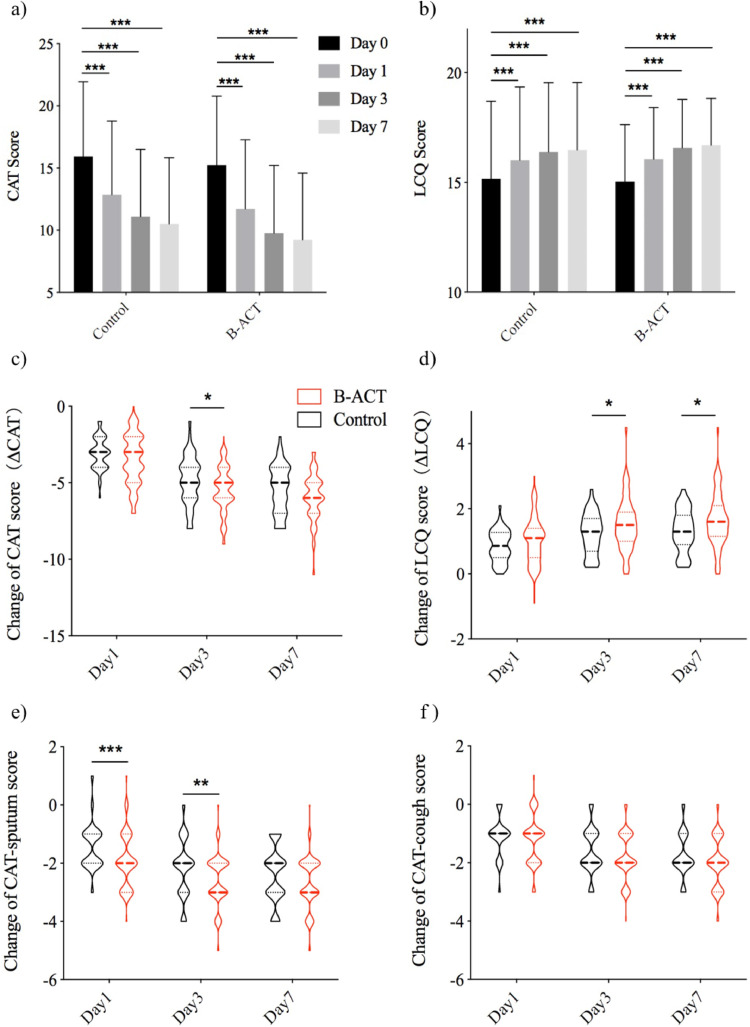
During the follow up period, the frequency of exacerbations in the B-ACT group was lower than that in the control group (0·43 (0·55) vs 0·69 (0·75), p=0·038). The CAT and LCQ scores in both group were improved significantly at different time points after treatment (all p<0·05) (Figure 3a and b). Moreover, changes of CAT and LCQ scores between admission day and different time points after the treatment were compared in both groups and in different subgroups of B-ACT group as well.The change of the CAT score in B-ACT group on the day3 after B-ACT therapy was more pronounced than that in control group (5·45 vs 4·85, 0·60 (0·09-1·11), p=0·023) (Figure 3c). And the improvement of LCQ score was larger in B-ACT group than that in control group on the day3 and day7 after B-ACT treatment (1·53 vs 1·23, 0·30 (0·05-0·55), p=0·044; 1·66 vs 1·32, 0·34 (0·08-0·60), p=0·022; respectively) (Figure 3d). Further analysis showed that the change of CAT-sputum score in the B-ACT group was greater than that in control group on the day1 (1·92 vs 1·42, 0·51 (0·20-0·81), p=0·001) and day3 after B-ACT therapy (2·59 vs 2·17, 0·42 (0·11-0·73), p=0.009), but the difference of the change of CAT-cough score between both groups was not significant (Figure 3e and f). The improvement of 6MWD in B-ACT group was larger than that in control group, although the difference was not significant (7·97 vs 6·69, 1·28 (-0·89-3·45), p=0·335) (Supplementary Figure S2). Differences of the lengths of hospital stay and hospitalization expenses in both group was not significant (Supplementary Figure S3).
Fig. 3.
The improvement of CAT and LCQ scores were more significant in the B-ACT group at different time points after treatment
The overall trends of the change of CAT and LCQ scores at different time points in two groups were shown in panel a) and b). The change of CAT score, LCQ score, CAT-sputum score and CAT-cough score were shown in panel c), d), e) and f). *: p<0.05, **: p<0.01, ***: p<0.001.
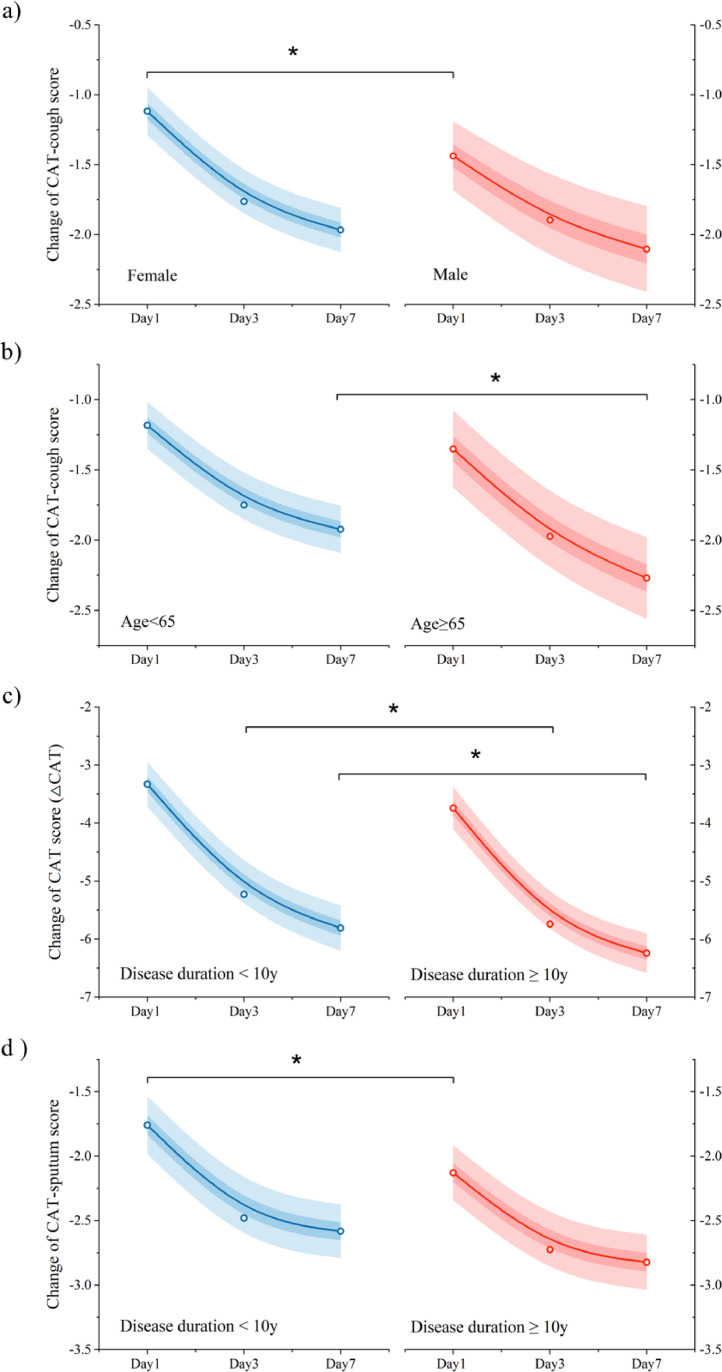
Further analysis have showed that the change of CAT-cough score after B-ACT therapy was larger in male patients than that in female on the B-ACT day (1·4 vs 1·1, p=0·049) (Figure 4a). And patients who were younger than 65 showed less decline in CAT-cough scores than those who were 65 or older on the seventh day after the treatment (1·9 vs 2·3, p=0·048) (Figure 4b). Moreover, patients with a disease duration less than 10 years had a less CAT score decline on the third and seventh days after B-ACT therapy (5·23 vs 5·74, 0·51 (0·04-0·99), p=0·018; 5·81 vs 6·24, 0·43 (0·10-0·96), p=0·029; respectively), and a less CAT-sputum score decline on the B-ACT day (1·76 vs 2·13, 0·37 (0·06-0·68), p=0·013) than that of patients with a disease duration of 10 years or more (Figure 4c, 4d).
Fig. 4.
B-ACT therapy was more suitable for improving CAT scores in patients with long disease course
Differences of the changes of CAT-cough score at different time points after B-ACT therapy in different subgroup related to sex and age were shown in panel a) and b); Comparisons of the change of CAT-score and CAT-sputum score at different time points after B-ACT therapy between patients with disease duration less than 10 years and those with more than 10 years were shown in panel c) and d). *: p<0.05. The empty circles represent the actual mean value of the score change, the lines represent the fitting line of the mean value of the score change, the light shading represents the 95% confidence interval of the score change, and the dark shading represents the 50% confidence interval of the score change.
3.4. Adverse events
During the follow up period, we conducted detailed consultation in the experimental group to evaluate the occurrence of adverse events related to B-ACT therapy. There were no serious complications such as severe arrhythmia, airway spasm, and massive hemoptysis occurred. Incidences of different adverse events among 141 patients in the experimental group were showed in Table 3. Five (3·6%) patients developed low fever (<38°C) after B-ACT therapy, and the temperature turned normal within a few hours after physical cooling treatment. Besides, incidences of adverse events related to B-ACT therapy were compared in different subgroups related to age and sex. Pharyngeal discomfort was more common in patients younger than 65 than that in patients over 65 (39(37·5%, 28·0%-47·0%) vs 7(18·9%, 5·7%-32·2%); OR 2·57, 1·03-6·41; p=0·038). Incidences of other adverse events such as nausea, chest discomfort, fever and blood in sputum were not significantly different between different subgroups (Table 3).
Table 3.
The occurrence of adverse events in B-ACT group was low and pharyngeal discomfort was more common in younger patients.
| B-ACT related adverse events | All | Age |
Sex |
||||
|---|---|---|---|---|---|---|---|
| <65 years | ≥65 years | p | Female | Male | p | ||
| N | 141 | 104 | 37 | 93 | 48 | ||
| Nausea | 8 (5·7%) | 6 (5·8%) | 2 (5·4%) | 0·94 | 6 (5·8%) | 2 (5·4%) | 0·94 |
| Chest discomfort | 15 (10·6%) | 12 (11·5%) | 3 (8·1%) | 0·56 | 7 (7·5%) | 8 (16·7%) | 0·095 |
| Pharyngeal discomfort | 46 (32·6%) | 39 (37·5%) | 7 (18·9%) | 0·038 | 29 (31·2%) | 17 (35·4%) | 0·61 |
| Fever | 5 (3·6%) | 4 (3·9%) | 1 (2·7%) | 0·75 | 2 (2·2%) | 3 (6·3%) | 0·21 |
| Blood in sputum | 15 (10·6%) | 12 (11·5%) | 3 (8·1%) | 0·56 | 10 (10·8%) | 5 (10·4%) | 0·95 |
Data are summarized as n (percentage) for categorical data. Categorical variables were compared by chi-square test. Statistical significance was considered when the two-tailed P < 0·05. ACT, Airway Clearance Therapy; B, Bronchoscopic.
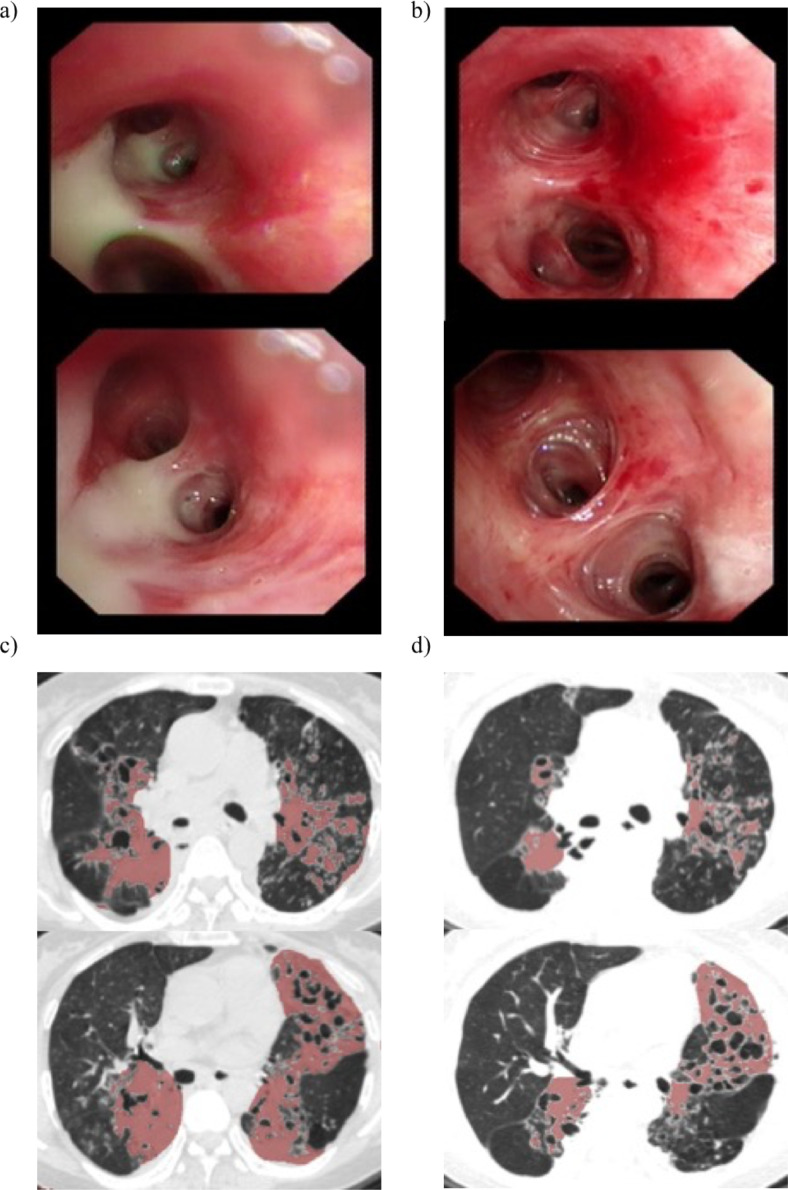
Through the bronchoscopy image, we could clearly see that the patient had a large amount of purulent sputum retained in the airway before the B-ACT therapy (Figure 5a), but the sputum disappeared after the treatment and the therapeutic effect of airway clearance was obvious (Figure 5b). From CT scans of bronchiectasis patient, there were obvious inflammatory exudative lesions on both sides before the B-ACT therapy (Figure 5c), and the exudative lesions in the same layer of CT scans were significantly reduced three months later after the treatment (Figure 5d).
Fig. 5.
The massive purulent sputum retention in patients’ airway was reduced significantly after B-ACT therapy
Large amounts of purulent sputum retained in patient's airway before the B-ACT therapy are shown in panel a), and the disappeared sputum in the same place after the treatment are shown in panel b). Panel c) showed obvious inflammatory exudative lesions on both sides before the B-ACT therapy, and improvement of inflammatory exudation in the same CT scanning plane for the same patient three months later after B-ACT therapy were shown in panel d); Red shading on the CT scans represent the focal area of the lung in a patient with bronchiectasis.
4. Discussion
This is the first perspective study to show a longer time to first exacerbation following B-ACT therapy in bronchiectasis with acute exacerbation. Repeated acute exacerbations are one of key targets for therapy in international bronchiectasis guidelines because of that they are major driver of disease progression and associated with high healthcare costs. Some studies had showed that ACT was associated with improvement in sputum expectoration, HRQOL, lung function, and reduced symptoms of coughing for bronchiectasis [22], [23], [24], [25], [26], [27], [28], [29]. Lee AL et al [30] found that 8 weeks of supervised exercise training and review of ACTs were associated with decreased frequency of exacerbations over 12-month follow-up and longer time to first exacerbation, which was consistent with ours. More importantly, we found that B-ACT therapy was more beneficial in prolonging the time to first acute exacerbation for special patients with severe disease and greater symptoms, including those with BSI scores ≥8, the number of acute exacerbations in the past year ≥2, SQRQ scores ≥15, CAT scores ≥11, or LCQ scores <16. Thus, what we concluded here would be of great utility value to clinicians worldwide in developing individualized therapies for bronchiectasis patients. Also, our results showed that B-ACT therapy could reduce the production of sputum and improve CAT and LCQ scores of bronchiectasis patients with acute exacerbation significantly. The possible explanation was that bronchoscopic ACT treatment could reduce sputum retention in deep airways significantly, which was beneficial for reducing further airway damage by halting the vicious cycle of bacterial colonization and subsequent inflammation. Therefore, the time to first acute exacerbation is prolonged and the quality of life is improved.
The main advantage of B-ACT therapy is to clear phlegm retention in patients’ airway and reduce daily symptoms of cough and sputum production, thus it can be found in our study that patients with severe disease and greater symptoms benefited more from the treatment. B-ACT therapy achieved a longer time to first exacerbation in the subgroup of patients with a greater symptom burden at baseline and highlighted the importance of B-ACT therapy focused on symptoms improvements in preventing exacerbation. Recent study had shown that patients with greater symptoms would be at higher risk of exacerbations and therefore a therapy aimed at improving daily symptoms would also reduce exacerbations in highly symptomatic patients [31]. Our study verified the conclusion from this study in different way, which showed symptom driven treatment may be more important for bronchiectasis patients.
Furthermore, our results showed that elderly patients with long course of disease had more significant improvement in CAT scores after B-ACT therapy, which suggesting that B-ACT therapy may be more suitable for improving daily symptoms in this group of patients. Previous studies have shown that there was an age-related decrease in muscle mass and quality in the trunk and limbs [32]. Muscle weakness and inactivity may affect disease progression, health-related quality of life, frequency of infectious exacerbations and ability to mobilize secretions [33]. We speculate from the results of above researches that elderly patients with reduced muscle function cough less effectively than younger patients. After B-ACT therapy, the improvement of cough score was more significant in elderly patients, and there was no difference in sputum score improvement. We think the reason is that the treatment could help patient clear airway secretions, and reduce ineffective cough in elderly patients, which improve cough score more apparent.
B-ACT therapy was a safe and effective method of diagnosis and treatment technique for patients, as adverse events associated with it were mostly transient and mild. Other studies had reached similar conclusions. Pervin et al. [34] found that ARDS patients were well tolerated throughout the treatment process under electronic bronchoscope. Besides, previous study had shown that people of different ages response differently to all stimuli [35], younger participants are more sensitive to hot, cold, touch, sour and salty stimuli than older participants which was consistent with our findings that the pharyngeal tolerance of elderly patients to bronchoscope stimulation was higher than that of young patients, and gender did not affect the incidence of adverse events.
Although the study was designed in accordance with the non-inferiority trial initially, after the end of the trial, the therapeutic effect of the trial group is higher than that of the control group, and the primary outcome of the two groups are directly subjected to statistical testing, reached the level of statistical difference. Thus it can be considered that the treatment effect of the trial group is higher than that of the control group, and the conclusion of superiority can be drawn. However, our study has its limitations. This study is a preliminary exploration to provide ideas for further higher-quality studies and should be confirmed by confirmatory studies. And a unified observation endpoint was set in this study and the length of follow-up time for different individuals was inconsistent, thus the primary endpoint will not be as robust as for a study with a fixed and longer follow-up period. In addition, in the subgroup analysis of the primary endpoint, we reached a preliminary conclusion that patients with severe disease and greater symptoms benefit more from treatment. It should be noted that these are only preliminary results after simple subgroup analysis, and there may be confounding factors. But it still has important significance for the clinical treatment of the disease, and provides early exploration ideas for more clinical trials in the near future. Finally, it is important to note that conclusions of our study can only be extended to bronchiectasis patients with acute exacerbation who are suitable and able to tolerate the bronchoscopy.
In conclusion, B-ACT therapy significantly prolonged the time to first acute exacerbation after discharge and improved CAT and LCQ scores for bronchiectasis patients with acute exacerbation, especially for those with a greater symptom burden at baseline, highlighting the importance of B-ACT therapy in preventing exacerbation for highly symptomatic patients. Hopefully, this will provide a novel evidence-based, individualized therapeutic choice for bronchiectasis patients.
Contributors
Y Liu, HW Lu, SY Gu, WW Wang contributed equally to the work. Concept and design: JF Xu. Acquisition, analysis, or interpretation of data: HW Lu, SY Gu, WW Wang, J Ge, ZJ Jie, JG Jia, ZT Gao, J Li, JY Shi, S Liang, KB Cheng, JW Bai. Drafting of the manuscript: Y Liu, JF Xu. Critical revision of the manuscript for important intellectual content: Y Liu, HW Lu, SY Gu, JF Xu. Statistical analysis: Y Liu, WW Wang, J Li. Administrative, technical, or material support: JF Xu. Supervision: JF Xu, JM Qu. The corresponding author JF Xu had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of Competing Interest
All authors declare that they have no competing interests.
Acknowledgments
Acknowledgement
The authors would like to express our sincere thanks to all the hospital staffs and the patients for their contributions to the study.
Data sharing statement
The researchers will share de-identified individual data and study protocol following completion of a data use agreement. Data are available from jfxu@tongji.edu.cn.
Sources of Funding
This work had been funded by the National Natural Science Fund for Distinguished Young Scholars (No. 81925001, JFX); Key Scientific Inovation Project of Shanghai Municipal Education Commission (No. 202101070007-E00097, JFX); the Project of the Shanghai Hospital Development Center (No. 16CR3036A, JFX); Shanghai Municipal Commission of Health and Family Panning (No. ZY2018-2020 FWTX 3022, HWL) and Innovation Project of Shanghai Pulmonary Hospital.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103587.
Contributor Information
Jie-Ming Qu, Email: jmqu0906@163.com.
Jin-Fu Xu, Email: jfxu@tongji.edu.cn.
Appendix. Supplementary materials
Reference
- 1.Tino G. Bronchiectasis: Phenotyping an Orphan Disease. Am J Respir Crit Care Med. 2018;197(11):1371–1373. doi: 10.1164/rccm.201802-0211ED. [DOI] [PubMed] [Google Scholar]
- 2.Boyton RJ, Altmann DM. Bronchiectasis: current concepts in pathogenesis, immunology, and microbiology. Ann Rev Pathol. 2016;11:523–554. doi: 10.1146/annurev-pathol-012615-044344. [DOI] [PubMed] [Google Scholar]
- 3.Ringshausen FC, Rademacher J, Pink I. Increasing bronchiectasis prevalence in Germany, 2009-2017: a population-based cohort study. Eur Respir J. 2019;54(6) doi: 10.1183/13993003.00499-2019. [DOI] [PubMed] [Google Scholar]
- 4.Choi H, Yang B, Nam H. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J. 2019;54(2) doi: 10.1183/13993003.00194-2019. [DOI] [PubMed] [Google Scholar]
- 5.Snell N, Gibson J, Jarrold I, Quint JK. Epidemiology of bronchiectasis in the UK: Findings from the British lung foundation's 'Respiratory health of the nation' project. Respir Med. 2019;158:21–23. doi: 10.1016/j.rmed.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Chalmers JD, Goeminne P, Aliberti S. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189(5):576–585. doi: 10.1164/rccm.201309-1575OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonnell MJ, Aliberti S, Goeminne PC. Multidimensional severity assessment in bronchiectasis: an analysis of seven European cohorts. Thorax. 2016;71(12):1110–1118. doi: 10.1136/thoraxjnl-2016-208481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polverino E, Goeminne PC, McDonnell MJ. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3) doi: 10.1183/13993003.00629-2017. [DOI] [PubMed] [Google Scholar]
- 9.Volsko TA. Airway clearance therapy: finding the evidence. Respir Care. 2013;58(10):1669–1678. doi: 10.4187/respcare.02590. [DOI] [PubMed] [Google Scholar]
- 10.Hoo ZH, Daniels T, Wildman MJ, Teare MD, Bradley JM. Airway clearance techniques used by people with cystic fibrosis in the UK. Physiotherapy. 2015;101(4):340–348. doi: 10.1016/j.physio.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Hill AT, Sullivan AL, Chalmers JD. British Thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019;74(Suppl 1):1–69. doi: 10.1136/thoraxjnl-2018-212463. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Garcia MA, Maiz L, Olveira C. Spanish Guidelines on Treatment of Bronchiectasis in Adults. Arch Bronconeumol. 2018;54(2):88–98. doi: 10.1016/j.arbres.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Spinou A, Chalmers JD. Respiratory physiotherapy in the bronchiectasis guidelines: is there a loud voice we are yet to hear? Eur Respir J. 2019;54(3) doi: 10.1183/13993003.01610-2019. [DOI] [PubMed] [Google Scholar]
- 14.Wong C, Sullivan C, Jayaram L. ELTGOL airway clearance in bronchiectasis: laying the bricks of evidence. Eur Respir J. 2018;51(1) doi: 10.1183/13993003.02232-2017. [DOI] [PubMed] [Google Scholar]
- 15.Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65(Suppl 1):i1–58. doi: 10.1136/thx.2010.136119. [DOI] [PubMed] [Google Scholar]
- 16.Hill AT, Haworth CS, Aliberti S. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J. 2017;49(6) doi: 10.1183/13993003.00051-2017. [DOI] [PubMed] [Google Scholar]
- 17.St Jones PW. George's Respiratory Questionnaire: MCID. Copd. 2005;2(1):75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 18.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 19.Stenton C. The MRC breathlessness scale. Occup Med (Lond) 2008;58(3):226–227. doi: 10.1093/occmed/kqm162. [DOI] [PubMed] [Google Scholar]
- 20.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zugck C, Kruger C, Durr S. Is the 6-minute walk test a reliable substitute for peak oxygen uptake in patients with dilated cardiomyopathy? Eur Heart J. 2000;21(7):540–549. doi: 10.1053/euhj.1999.1861. [DOI] [PubMed] [Google Scholar]
- 22.Muñoz G, de Gracia J, Buxó M, Alvarez A, Vendrell M. Long-term benefits of airway clearance in bronchiectasis: a randomised placebo-controlled trial. Eur Respir J. 2018;51(1) doi: 10.1183/13993003.01926-2017. [DOI] [PubMed] [Google Scholar]
- 23.Lee AL, Burge AT, Holland AE. Airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev. 2015;(11) doi: 10.1002/14651858.CD008351.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutalithas K, Watkin G, Willig B, Wardlaw A, Pavord ID, Birring SS. Improvement in health status following bronchopulmonary hygiene physical therapy in patients with bronchiectasis. Respir Med. 2008;102(8):1140–1144. doi: 10.1016/j.rmed.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Lee AL, Williamson HC, Lorensini S, Spencer LM. The effects of oscillating positive expiratory pressure therapy in adults with stable non-cystic fibrosis bronchiectasis: A systematic review. Chron Respir Dis. 2015;12(1):36–46. doi: 10.1177/1479972314562407. [DOI] [PubMed] [Google Scholar]
- 26.Nicolini A, Cardini F, Landucci N, Lanata S, Ferrari-Bravo M, Barlascini C. Effectiveness of treatment with high-frequency chest wall oscillation in patients with bronchiectasis. BMC Pulmonary Med. 2013;13:21. doi: 10.1186/1471-2466-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naraparaju S, Vaishali K, Venkatesan P, Acharya V. A comparison of the Acapella and a threshold inspiratory muscle trainer for sputum clearance in bronchiectasis-A pilot study. Physiother Theory Pract. 2010;26(6):353–357. doi: 10.3109/09593981003596616. [DOI] [PubMed] [Google Scholar]
- 28.Figueiredo PH, Zin WA, Guimaraes FS. Flutter valve improves respiratory mechanics and sputum production in patients with bronchiectasis. Physiother Res Int. 2012;17(1):12–20. doi: 10.1002/pri.507. [DOI] [PubMed] [Google Scholar]
- 29.Patterson JE, Bradley JM, Hewitt O, Bradbury I, Elborn JS. Airway clearance in bronchiectasis: a randomized crossover trial of active cycle of breathing techniques versus Acapella. Respiration. 2005;72(3):239–242. doi: 10.1159/000085363. [DOI] [PubMed] [Google Scholar]
- 30.Lee AL, Hill CJ, Cecins N. The short and long term effects of exercise training in non-cystic fibrosis bronchiectasis–a randomised controlled trial. Respir Res. 2014;15:44. doi: 10.1186/1465-9921-15-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao YH, Abo Leyah H, Finch S. Relationship between Symptoms, Exacerbations, and Treatment Response in Bronchiectasis. Am J Respir Crit Care Med. 2020;201(12):1499–1507. doi: 10.1164/rccm.201910-1972OC. [DOI] [PubMed] [Google Scholar]
- 32.Fukumoto Y, Ikezoe T, Yamada Y. Age-Related Ultrasound Changes in Muscle Quantity and Quality in Women. Ultrasound Med Biol. 2015;41(11):3013–3017. doi: 10.1016/j.ultrasmedbio.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Burtin C, Hebestreit H. Rehabilitation in patients with chronic respiratory disease other than chronic obstructive pulmonary disease: exercise and physical activity interventions in cystic fibrosis and non-cystic fibrosis bronchiectasis. Respiration. 2015;89(3):181–189. doi: 10.1159/000375170. [DOI] [PubMed] [Google Scholar]
- 34.Korkmaz Ekren P, Basarik Aydogan B, Gurgun A, Tasbakan MS, Bacakoglu F, Nava S. Can fiberoptic bronchoscopy be applied to critically ill patients treated with noninvasive ventilation for acute respiratory distress syndrome? Prospective observational study. BMC Pulmonary Med. 2016;16(1):89. doi: 10.1186/s12890-016-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heft MW, Robinson ME. Age differences in suprathreshold sensory function. Age (Dordr) 2014;36(1):1–8. doi: 10.1007/s11357-013-9536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.