Abstract
Objective
To determine the risk factors for having diabetic retinopathy (DR) in children and young people (CYP) with type 1 diabetes (T1DM) at first screening.
Methods
Records from the Diabetes Eye Screening Wales (DESW) service for people in Wales, UK, with T1DM diagnosed under age 18 years were combined with other electronic health record (EHR) data in the Secure Anonymised Information Linkage (SAIL) Databank. Data close to the screening date were collected, and risk factors derived from multivariate, multinomial logistic regression modelling.
Results
Data from 4172 persons, with median (lower quartile, upper quartile) age 16.3 (13.0, 22.3) years and duration of diabetes 6.6 (2.3, 12.3) years were analysed. 62.6% (n = 2613) had no DR, 26.7% (n = 1112) background DR, and 10.7% (n = 447) had referable DR (RDR). No RDR was observed under 19 years of age. Factors associated with an increased risk of DR were diabetes duration, elevated HbA1c, and diastolic blood pressure. People diagnosed with T1DM at 12 years or older had an additional risk for each year they had diabetes compared to those diagnosed before age 12 controlling for the diabetes duration (odds ratios 1.23 and 1.34, respectively).
Conclusions
This study found that 37.4% of the study cohort had DR at first screening, the risk being greater the longer the duration of diabetes or higher the HbA1c and diastolic blood pressure. In addition, people diagnosed at 12 years of age or over were more likely to have DR with each additional year with diabetes.
Subject terms: Risk factors, Diabetes complications
Introduction
Visual impairment and blindness, as a consequence of diabetic retinopathy (DR), are amongst the most feared complications of diabetes. The incidence and prevalence of sight-threatening DR (STDR) has however been slowly decreasing over the last several decades despite the increase in the prevalence of diabetes [1–7]. It has been recently reported in England and Wales that DR is no longer the leading cause of blindness in the working age population [8]. Also, in a retrospective analysis of newly recorded certifications of visual impairment in Wales during 2007–2015 sight loss was reduced by 50% [9]. These observations may reflect the cumulative impact of better management of diabetes, the introduction of screening programmes, better management of risk factors and earlier and more effective ophthalmologic interventions.
Good glycaemic and blood pressure management are pivotal in both primary prevention and the prevention of progression of DR. The introduction of intensive insulin therapy to optimise glycaemic management in children has been observed to have a beneficial effect on DR in multiple studies [10–12]. In children aged 13–17 years with type 1 diabetes (T1DM) the risk of developing DR was reduced by 53% [10] while in children and young people (CYP) aged 12–20 years DR was also reduced by 12–52% [11]. The benefit of such intensive management in the adolescent years remains evident many years later (legacy effect) even when HbA1c values deteriorate, becoming similar to those undergoing conventional insulin therapy [12]. Currently, the treatment for STDR, which encompasses severe non-proliferative DR (pre-proliferative DR [PPDR] and proliferative DR (PDR), is primarily by laser photocoagulation and/or intravitreal injections of inhibitors of vascular endothelial growth factors (anti-VEGF). The relatively recent addition of anti-VEGF treatment has improved visual outcomes in those with PDR and/or clinically significant macular oedema [13]. Vitrectomy may also be required when these measures are considered inadequate. It is well accepted that DR remains asymptomatic until it reaches an advanced stage (STDR) and that the benefit from treatment is best achieved early. This is the basis for the introduction of screening for DR, which has been shown to be of clinical benefit but also cost-effective [14]. The detection of any DR should help to emphasise the need for improving glycaemic and blood pressure management, to prevent progression to STDR.
Previous studies have shown that ~0.3% of the Welsh population and 0.2% of CYP under 16 years have T1DM [15, 16]. The prevalence of DR in CYP with diabetes is low and extremely rare prior to puberty [17, 18]. The prevalence of DR has been found in CYP with diabetes to range between 10.5 and 57.6% depending on the age, duration of diabetes, methods of detecting DR, and the care setting [18–30]. The youngest ages at which DR and STDR have been recorded is 5 and 15 years, respectively, with the shortest duration of diabetes being 5 years and only five cases of STDR have been observed in children below the age of 18 years [29, 31]. However, these studies involved relatively small numbers and therefore there is a need to more clearly understand the epidemiology of DR and related risk factors in a population with T1DM diagnosed below the age of 18 years.
Systematic screening programmes for DR were introduced in the UK in 2003 with the recommendation to begin screening from the age of 12 years onwards [32]. However, the International Society for Paediatric and Adolescent Diabetes recommends annual screening to begin earlier from the age of 10 years or at the onset of puberty, if this is earlier [33]. In Wales there exists a single national community-based DR screening programme for all persons with diabetes aged 12 years and over using a standardised quality assured methodology for image capture and grading of DR, the guidelines for which originated from the Airlie House classification and its modified version used in the Early Treatment Diabetic Retinopathy Study [34, 35] which was simplified for the purpose of populations studies in the UK [36]. Grading involves a primary grader whose findings are checked by a secondary grader with differences resolved by a more senior tertiary grader to arrive at the final grading. Patients are referred to the hospital eye service if they have severe PPDR, PDR, and/or maculopathy for further assessment and treatment as required. This provided us with a unique opportunity to investigate the risk factors relating to DR in the population of children and young persons with T1DM diagnosed before the age of 18 years in Wales, at the time of their first screening event [37].
Methods
The study database was derived from both primary care (Welsh Longitudinal General Practice dataset, WLGP) and the Diabetic Eye Screening Wales (DESW) dataset and held in the Secure Anonymised Information Linkage (SAIL) Databank (Swansea University). SAIL is a repository of routinely collected electronic health record (EHR) data for people living in or receiving medical services in Wales [38, 39]. This study was reviewed by the independent Information Governance Review Panel of the SAIL Databank and approved under the ID: 0493. Ethical approval was not required since only anonymised data was used.
Data preparation
The study cohort consisted of people in Wales diagnosed with T1DM under the age of 18 years. The method used to identify persons with T1DM necessitated a recorded diagnosis of T1DM plus a prescription for insulin close to their earliest diagnosis date, or a hospital inpatient episode because of diabetic ketoacidosis, or a prescription for a medical device used in the management of T1DM (blood glucose and ketone monitoring equipment, for example, monitors and testing strips) on at least 5 occasions in the 12-months following diagnosis. In addition, the Brecon cohort, which is a national register of persons with T1DM diagnosed while living in Wales below the age of 15 years [40] was also used to ensure the cohort was as complete as possible.
DESW aims to conduct DR screening annually in all persons with diabetes registered with a GP located in Wales that meet the eligibility criteria (most notably, persons must be 12 years or older). When a person attends screening, after testing visual acuity, two 45° retinal fundus photographs (one centred on the fovea, and one nasal view) are captured for each eye following mydriasis. Trained graders then assess the images for the presence of DR, with images graded according to a standardised grading protocol [37]. The initial dataset consisted of the findings from the initial eye screening event which resulted in a successful assessment for at least one eye. In addition to the DR grading the current age, age at diagnosis of diabetes, duration of diabetes, gender, and whether the person was referred to a hospital eye department were recorded. The following data from primary care GP or reference sources obtained within 6 months of the date of initial DR screening were also included in the dataset: HbA1c, systolic and diastolic blood pressure, serum cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, creatinine, and Body Mass Index (BMI). Since this data was derived from the WLGP data, its availability depended on whether the test was performed by one of the 76% of general practices contributing data to the SAIL Databank [41]. A variable indicating whether persons were diagnosed with T1DM before the age of 12 years was also added to the data to enable modelling of interactions with duration of diabetes.
The DESW service commenced in 2003 attaining national coverage in 2007 with all data from both periods included in this study. The extract of the DESW data in the SAIL Databank used in this study ended at the end of January 2018.
Statistical methods
Median and quartiles are reported as measured values are typically not normally distributed. A univariate analysis was conducted to investigate differences between the two groups for each individual variable. For continuous variables the Mann–Whitney U test was employed while categorical variables were investigated using Pearson’s χ2 test. Secondly, multivariate models were constructed to compare a reference group consisting of people with no DR with two comparison groups: (i) people with evidence of any DR which was evaluated using binomial logistic regression and (ii) people with background diabetic retinopathy (BDR) or referable diabetic retinopathy (RDR, PPDR or worse) separately, which was evaluated using multinomial logistic regression.
Variables from the univariate analysis that were different between groups were used in the initial multivariate models and backwards stepwise logistic regression was performed until only those variables that differed significantly remained in the model. People diagnosed with T1DM before the age of 12 years are usually managed less intensively than those diagnosed after 12 years of age. Therefore, the model included a term that allowed for the interaction between the duration of diabetes and whether the person was diagnosed with T1DM under age 12 years or not. The logical variables indicating whether the person was diagnosed before the age of 12 years were retained regardless of whether they differed between groups, in order to evaluate their interaction with the duration of diabetes. In each of the logistic regression models, Nagelkere’s Pseudo R2 (denoted R2N) and the in-sample prediction accuracy, A, were used to evaluate the model’s goodness of fit.
Results
In Wales, during 2003–20018, 4495 people were diagnosed with T1DM under the age of 18 years and invited for DR screening from the age of 12 onwards. 305 (6.7%) did not attend screening and of the remaining 4190 people only 18 (0.4%) had ungradable images at their first screening event. The median age of the study cohort at the time of T1DM diagnosis was 10.6 years and at initial DR screening was 16.3 years with a median duration of diabetes of 6.6 years. The median HbA1c was 72.6 mmol/mol (8.8%) and blood pressure was 120/70 mmHg. (Table 1).
Table 1.
Demographic and laboratory test information on the cohort of people with T1DM at the time of first DR screening event.
| Age range | 0–6 | 6–12 | 12–18 | Whole cohort | ||||
|---|---|---|---|---|---|---|---|---|
| Description | Count | Median (LQ, UQ) | Count | Median (LQ, UQ) | Count | Median (LQ, UQ) | Count | Median (LQ, UQ) |
| Total n | 851 (100%) | 1747 (100%) | 1592 (100%) | 4190 (100%) | ||||
| Female gender | 425 (50%) | 914 (52%) | 631 (40%) | 1971 (47.0%) | ||||
| Age at diagnosis (years) | 851 (100%) | 3.47 (2.2, 4.8) | 1747 (100%) | 9.5 (8.0, 10.8) | 1592 (100%) | 14.2 (13.0, 15.9) | 4190 (100%) | 10.6 (7.0, 13.4) |
| Age at screening (years) | 851 (100%) | 14.2 (12.3, 21.4) | 1747 (100%) | 14.1 (12.4, 20.6) | 1592 (100%) | 17.9 (15.2, 24.0) | 4190 (100%) | 16.3 (13.0, 22.3) |
| Diabetes duration (years) | 851 (100%) | 11.3 (8.9, 17.9) | 1747 (100%) | 5.6 (3.1, 11.4) | 1586 (100%) | 2.7 (0.8, 9.8) | 4186 (99.9%) | 6.6 (2.3, 12.3) |
| HbA1c (mmol/mol) | 390 (46%) | 74.0 (65.0, 86.8) | 822 (47%) | 73.7 (63.9, 87.7) | 743 (47%) | 70.4 (56.2, 84.6) | 1957 (46.7%) | 72.6 (61.7, 86.0) |
| HbA1c (%) | 390 (46%) | 8.9 (8.1, 10.1) | 822 (47%) | 8.9 (8.0, 10.2) | 743 (47%) | 8.6 (7.3, 9.9) | 1957 (46.7%) | 8.8 (7.8, 10.0) |
| Systolic pressure (mmHg) | 383 (45%) | 120.0 (110.0, 130.0) | 814 (47%) | 119.0 (110.0, 128.0) | 931 (59%) | 120.0 (110.0, 130.0) | 2129 (50.8%) | 120.0 (110.0, 130.0) |
| Diastolic pressure (mmHg) | 383 (45%) | 70.0 (62.0, 78.0) | 814 (47%) | 70.0 (63.0, 78.0) | 931 (59%) | 70.0 (65.0, 80.0) | 2129 (50.8%) | 70.0 (61.0, 79.0) |
| Cholesterol (mmol/l) | 245 (29%) | 4.5 (3.9, 5.1) | 507 (29%) | 4.4 (3.8, 5.1) | 639 (40%) | 4.3 (3.7, 5.0) | 1392 (33.2%) | 4.4 (3.8, 5.1) |
| LDL (mmol/l) | 144 (17%) | 2.5 (2.0, 3.0) | 302 (17%) | 2.3 (1.8, 2.9) | 406 (26%) | 2.3 (1.8, 2.9) | 853 (20.4%) | 2.3 (1.8, 2.9) |
| HDL (mmol/l) | 162 (19%) | 1.5 (1.3, 1.7) | 334 (19%) | 1.4 (1.2, 1.7) | 438 (28%) | 1.3 (1.1, 1.6) | 935 (22.3%) | 1.4 (1.2, 1.7) |
| Creatinine (µmol/l) | 203 (24%) | 76.0 (64.5, 89.0) | 469 (27%) | 73.0 (63.0, 85.0) | 656 (41%) | 74.0 (63.0, 86.0) | 1330 (31.7%) | 74 (63, 86) |
| BMI (kg/m2) | 294 (35%) | 23.8 (21.12, 27.2) | 611 (35%) | 23.4 (21.1, 26.7) | 784 (49%) | 24.0 (21.3, 27.0) | 1691 (40.4%) | 23.8 (21.1, 26.9) |
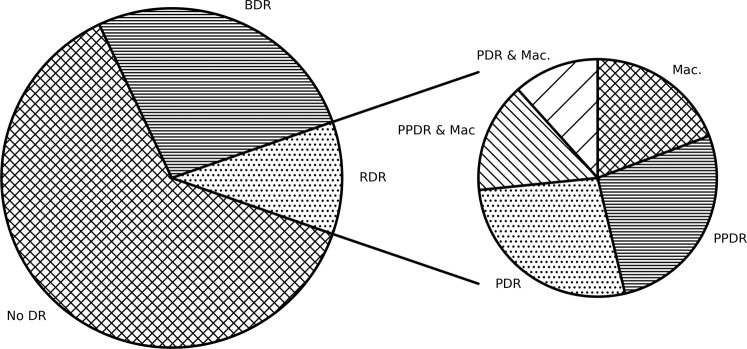
Of the 4172 people with gradable images at their first screening event 62.6% (2613) did not have any evidence of DR, 26.7% (1112) had BDR and 10.7% (447) had RDR with 4.1% (173) having PDR in one or both eyes (Fig. 1). Those who presented with any DR at their first screening event had higher HbA1c, blood pressure, LDL, cholesterol, creatinine and a longer duration of diabetes and these differences were even greater in those who presented with a referable level of DR (Supplementary Table 1).
Fig. 1. The proportion of CYP with T1DM that have DR at first screening.
Proportion of the population with no DR (62.6%), BDR (26.7%), or RDR (10.7%) at first screening and the proportion of people that have PPDR (2.9%), PDR (2.9%), maculopathy (2.1%), PPDR with maculopathy (1.6%), and PDR with maculopathy (1.2%) at first screening.
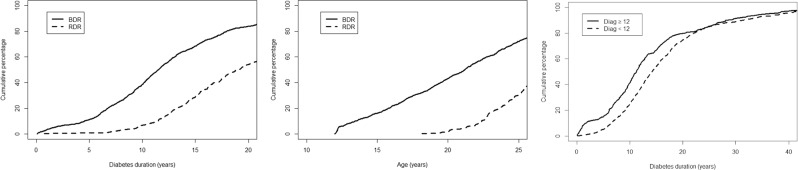
People who had had diabetes for a longer time were more likely to have DR at first screening, with the proportion of the population with DR increasing with increasing duration of diabetes almost linearly up to ~17 years duration (Fig. 2a). After 5, 10, 15, and 20 years of diabetes duration 11.0%, 38.6%, 68.4%, and 83.9%, respectively had evidence of BDR. RDR was only observed in those people having had diabetes for at least 8 years, thereafter the proportion of people with RDR increased linearly (Fig. 2a). We found, after 10, 15, and 20 years duration 4.6%, 27.9%, and 53.6% of people had RDR, respectively. None of the CYP had evidence of RDR before the age of 18 years (Fig. 2b). We observed that 11.4% of 12-year-old at first screening had evidence of early DR, increasing to 31.9% for 18-year-old (Fig. 2b). A smaller proportion of people aged under 12 years at diagnosis of T1DM had DR at first screening than people diagnosed at age 12 years or older when controlling for duration of diabetes (Fig. 2c). Those people diagnosed with T1DM at or over the age of 12 years acquired an additional risk of developing DR for each year they had T1DM than people diagnosed under the age of 12 years (Table 2). This difference in the proportion of people with DR persisted until ~20 years duration of diabetes, when the proportion of people with DR in both groups became comparable (Fig. 2c).
Fig. 2. How proportion of people with DR at first screening varies by demographic factors.
Fraction of persons diagnosed with BDR or RDR at first screening as a function of diabetes duration (left), age at screening (middle) and the fraction of people diagnosed aged less than 12 and aged 12 or older with any DR as a function of diabetes duration (right).
Table 2.
Results from the (a) multivariate binomial logistic regression model, (b) multivariate multinomial logistic regression model.
| (a) | ||
|---|---|---|
| Variable | OR (95% CI), No DR vs. Any DR | |
| Diabetes duration (diagnosed < 12) | 1.23 (1.20, 1.26) | |
| Diabetes duration (diagnosed ≥ 12) | 1.34 (1.30, 1.37) | |
| HbA1c (per 10 mmol/mol) | 1.09 (1.04, 1.15) | |
| (b) | ||
|---|---|---|
| Variable | OR (95% CI), No DR vs. BDR | OR (95% CI), No DR vs. RDR |
| Diabetes duration (diagnosed < 12) | 1.22 (1.19, 1.24) | 1.29 (1.26, 1.33) |
| Diabetes duration (diagnosed ≥ 12) | 1.32 (1.29, 1.36) | 1.40 (1.36, 1.44) |
| HbA1c (per 10 mmol/mol) | 1.07 (1.02, 1.14) | 1.19 (1.10, 1.29) |
| Diastolic pressure | 1.02 (1.01, 1.04) | 1.04 (1.02, 1.06) |
(a) RN2 = 0.764, A = 0.827.
(b) RN2 = 0.782, A = 0.721.
In a multivariate binomial logistic regression analysis, presenting at first screening with an elevated HbA1c (odds ratio [OR] 1.09) and duration of diabetes (OR 1.23 for people diagnosed under age 12 and 1.34 for people diagnosed at age 12 or older) carried an increased risk of having DR (Table 2a). In the multivariate, multinomial model increased HbA1c, diastolic blood pressure, and duration of diabetes were observed to increase the risk of BDR and RDR, with duration of diabetes having the greatest effect (OR 1.22 for BDR and 1.29 for RDR in people diagnosed under 12 years, and 1.32 and 1.40 for BDR and RDR respectively in people diagnosed at 12 years or over, Table 2b). The accuracy of the bivariate model was slightly better than the multivariate model which is to be expected as classifying people into three groups is a more difficult problem than classifying them into two groups. The Nagelkerke R2N indicates the multivariate model was a slightly better fit than the bivariate model, but both models fit the data well, having R2N > 0.75.
Discussion
This study involved a large cohort (4172) of CYP diagnosed with T1DM under the age of 18 years and investigated the proportion with DR and associated risk factors at their first DR screening event. In this cohort the presence of any DR was seen in 37.4 and 10.7% had RDR although no one was found with RDR under the age of 18 years. The fraction of people with BDR at first screening increased almost linearly with age, with ~31.8% having BDR at first screening at age 18. Although none of the cohort had RDR at their first screening before the age of 19 years there was a linear increase thereafter increasing to 30.1% at the age of 25 years at first screening. Increased diabetes duration, elevated HbA1c, and diastolic blood pressure conferred a higher risk of having any DR, BDR or RDR at first screening.
To our surprise our retinal graders recorded the presence of BDR in ~10% of our cohort within the first 2 years after diagnosis at variance with previous studies [35, 42–44]. This is difficult to explain but may in part reflect the high quality of retinal images acquired and the rigorous grading procedure at DESW and/or a prolonged asymptomatic period prior to the diagnosis of diabetes. Another contributing factor may be that many of the diagnoses of DR at this stage is acknowledged to rely on a small number of microaneurysms, or even a solitary one. Similarly, the DCCT study observed that 9.9% of people with T1DM had evidence of DR within the first 2 years since diagnosis, based on 7-field stereoscopic colour retinal photographs, increasing to 15% with the addition of fluorescein angiography [45]. Consistent with many other studies [46–48], we demonstrated in our study that the longer the duration of diabetes the greater the risk of developing DR. The proportion with BDR at 5 and 10 years was ~11.0% and 38.6%, respectively, and whereas there was no RDR seen up to 8 years after diagnosis, at 10 and 20 years duration ~4.6 and 53.6% had developed RDR.
We also observed that a greater proportion of those diagnosed with T1DM after the age of 12 years had DR when compared to those diagnosed prior to 12 years for the same diabetes duration. The median time to DR in those diagnosed after the age of 12 years was 10 years in comparison to a median time of 12 years in those diagnosed before the age of 12 years. The adverse impact of puberty on the risk of progression of DR has been observed in many other populations [42, 49, 50] although not in others [51].
Our study also found that a higher HbA1c was a risk factor for DR at first screening which is in agreement with many previous studies performed in the UK [5, 46], Europe [47, 52], and the US [48, 53]. The finding that increased diastolic blood pressure specifically increases the risk of DR at first screening is also in agreement with previous work [48]. In CYP hypertension is relatively uncommon and the median blood pressures in groups that had no DR, BDR, and RDR at first screening were all in the normal range for adults, in particular the median diastolic blood pressure was in the ideal range for all groups. We note defining hypertension in CYP is usually done with reference to percentiles taking age into account rather than using absolute cut-offs, but often people with blood pressure under 120/80 mmHg are classified as having normal blood pressure regardless of age. We observed that a modest increase in diastolic blood pressure causes a relatively large increase in risk of DR at first screening, even when the diastolic blood pressure is within the normal range.
Other risk factors for DR found in some previous studies were HbA1c variability, total cholesterol, HDL, age at diabetes diagnosis [5, 52], and male gender [47]. However, in our study cohort we found total cholesterol, LDL, HDL, triglycerides, and gender not to be associated with the occurrence of DR. It is difficult to compare our results with previous longitudinal studies due to differences in study population and design.
A limitation of this study was that persons having undertaken screening but did not have additional EHR data which included the putative risk factors of interest within 6 months of the screening which was required by the model and therefore were excluded from the cohort and subsequent analysis. Only if the measurement of HbA1c is available within 6 months of the screening date is the person included in the model. Adding more variables to the model compounds this difficulty, leading to quite small cohorts due to the relatively high levels of missing data. The key factor that influences whether the data is missing or not is when the measurements were performed, and since these data are gathered at all times through the year the data can be considered to be missing at random and consequently will not affect the results of statistical modelling. This limitation would be common to all study designs that incorporate routine data. Furthermore, this study did not have access to data from the hospital based ophthalmological services to confirm the diagnoses of RDR. However, a great advantage of our study is that the cohort of persons with T1DM is much larger than has been reported in previous work and that the DESW adopts standardised practices and data collection methods, and has the ability to link to other EHR data via the SAIL Databank, which also covers all of Wales.
Summary
What was known before
Longitudinal studies have investigated risk factors for diabetic retinopathy in various populations.
Screening services have improved outcomes and reduced incidence of blindness in people with diabetes.
People with type 1 diabetes tend to experience poorer outcomes than those with type 2 diabetes because they often have more difficulty with glycaemic management.
What this study adds
In our cohort of people with type 1 diabetes 37.4% had diabetic retinopathy and 10.7% had referable diabetic retinopathy at the first screening.
We found that diabetes duration, elevated HbA1c, and diastolic blood pressure increase the risk of having any grade of retinopathy at the first screening.
People diagnosed with type 1 diabetes at or over the age of 12 years acquired a slightly larger additional risk of DR for each year of diabetes than people diagnosed under the age of 12 years.
Supplementary information
Acknowledgements
AA acknowledges financial support from Health Data Research UK (NIWA1), which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, National Institute for Health Research (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation and Wellcome.
This study makes use of anonymised data held in the SAIL Databank, which is part of the national e-health records research infrastructure for Wales. We would like to acknowledge all the data providers who make anonymised data available for research.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41433-020-01326-8) contains supplementary material, which is available to authorized users.
References
- 1.Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26:1258–64. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Lee KE, Knudtson MD, Gangnon RE, Klein BE. Changes in visual impairment prevalence by period of diagnosis of diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2009;116:1937–42. doi: 10.1016/j.ophtha.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong TY, Mwamburi M, Klein R, Larsen M, Flynn H, Hernandez-Medina M, et al. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care. 2009;32:2307–13. doi: 10.2337/dc09-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero-Aroca P, Fernández-Balart J, Baget-Bernaldiz M, Martinez-Salcedo I, Méndez-Marín I, Salvat-Serra M, et al. Changes in the diabetic retinopathy epidemiology after 14 years in a population of Type 1 and 2 diabetic patients after the new diabetes mellitus diagnosis criteria and a more strict control of the patients. J Diabetes Complicat. 2009;23:229–38. doi: 10.1016/j.jdiacomp.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Ng SM, Ayoola OO, McGuigan M, Chandrasekaran S. A multicentre study evaluating the risk and prevalence of diabetic retinopathy in children and young people with type 1 diabetes mellitus. Diabetes Metab Syndr. 2019;13:744–6. doi: 10.1016/j.dsx.2018.11.063. [DOI] [PubMed] [Google Scholar]
- 6.Klein BE. Reduction in risk of progression of diabetic retinopathy. N. Engl J Med. 2010;363:287–8. doi: 10.1056/NEJMe1005667. [DOI] [PubMed] [Google Scholar]
- 7.Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7:140–9. doi: 10.1016/S2213-8587(18)30128-1. [DOI] [PubMed] [Google Scholar]
- 8.Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open. 2014;4:e004015. doi: 10.1136/bmjopen-2013-004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas RL, Luzio SD, North RV, Banerjee S, Zekite A, Bunce C, et al. Retrospective analysis of newly recorded certifications of visual impairment due to diabetic retinopathy in Wales during 2007-2015. BMJ Open. 2017;7:e015024. doi: 10.1136/bmjopen-2016-015024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 11.Downie E, Craig ME, Hing S, Cusumano J, Chan AK, Donaghue KC. Continued reduction in the prevalence of retinopathy in adolescents with type 1 diabetes: role of insulin therapy and glycemic control. Diabetes Care. 2011;34:2368–73. doi: 10.2337/dc11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleary PA, Dahms W, Goldstein D, Malone J, Tamborlane WV. Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT) J Pediatr. 2001;139:804–12. doi: 10.1067/mpd.2001.118887. [DOI] [PubMed] [Google Scholar]
- 13.Cho WB, Oh SB, Moon JW, Kim HC. Panretinal photocoagulation combined with intravitreal bevacizumab in high-risk proliferative diabetic retinopathy. Retina. 2009;29:516–22. doi: 10.1097/IAE.0b013e31819a5fc2. [DOI] [PubMed] [Google Scholar]
- 14.Javitt JC, Aiello LP. Cost-effectiveness of detecting and treating diabetic retinopathy. Ann Intern Med. 1996;125:939-. doi: 10.7326/0003-4819-125-11-199612010-00020. [DOI] [PubMed] [Google Scholar]
- 15.Holman N, Young B, Gadsby R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabet Med. 2015;32:1119–20. doi: 10.1111/dme.12791. [DOI] [PubMed] [Google Scholar]
- 16.Maahs DM, Hermann JM, Holman N, Foster NC, Kapellen TM, Allgrove J, et al. Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the US, Austria, and Germany. Diabetes Care. 2015;38:1876–82. doi: 10.2337/dc15-0780. [DOI] [PubMed] [Google Scholar]
- 17.Kernell A, Dedorsson I, Johansson B, Wickström C, Ludvigsson J, Tuvemo T, et al. Prevalence of diabetic retinopathy in children and adolescents with IDDM A population-based multicentre study. Diabetologia. 1997;40:307–10. doi: 10.1007/s001250050679. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Retinopathy in young-onset diabetic patients. Diabetes Care. 1985;8:311–5. doi: 10.2337/diacare.8.4.311. [DOI] [PubMed] [Google Scholar]
- 19.Kubin M, Tossavainen P, Hannula V, Lahti S, Hautala N, Falck A. Prevalence of retinopathy in Finnish children and adolescents with type 1 diabetes: a cross-sectional population-based retrospective study. Arch Dis Child. 2011;96:963–8. doi: 10.1136/adc.2011.210807. [DOI] [PubMed] [Google Scholar]
- 20.Kullberg C, Abrahamsson M, Arnqvist H, Finnström K, Ludvigsson J. Prevalence of retinopathy differs with age at onset of diabetes in a population of patients with Type 1 diabetes. Diabet Med. 2002;19:924–31. doi: 10.1046/j.1464-5491.2002.00839.x. [DOI] [PubMed] [Google Scholar]
- 21.Maguire A, Chan A, Cusumano J, Hing S, Craig M, Silink M, et al. The case for biennial retinopathy screening in children and adolescents. J Am Assoc Pediatr Ophthalmol Strabismus. 2006;10:189. doi: 10.1016/j.jaapos.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 22.LeCaire T, Palta M, Zhang H, Allen C, Klein R, D’Alessio D. Lower-than-expected prevalence and severity of retinopathy in an incident cohort followed during the first 4–14 years of type 1 diabetes: the Wisconsin Diabetes Registry Study. Am J Epidemiol. 2006;164:143–50. doi: 10.1093/aje/kwj166. [DOI] [PubMed] [Google Scholar]
- 23.Falck A, Käär ML, Laatikainen L. A prospective, longitudinal study examining the development of retinopathy in children with diabetes. Acta Paediatrica. 1996;85:313–9. doi: 10.1111/j.1651-2227.1996.tb14023.x. [DOI] [PubMed] [Google Scholar]
- 24.Geloneck MM, Forbes BJ, Shaffer J, Ying G-S, Binenbaum G. Ocular complications in children with diabetes mellitus. Ophthalmology. 2015;122:2457–64. doi: 10.1016/j.ophtha.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahill M, Wallace D, Travers S, Lipinski H, Aldington S, Costigan C, et al. Detection and prevalence of early diabetic retinopathy in juvenile diabetics with diabetes for 10 years or more. Eye. 2000;14:847–50. doi: 10.1038/eye.2000.234. [DOI] [PubMed] [Google Scholar]
- 26.Demirel F, Tepe D, Kara Ö, Esen İ. Microvascular complications in adolescents with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. 2013;5:145. doi: 10.4274/Jcrpe.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dujić MP, Ignjatović Z. Juvenile diabetes eye complications and treatment. Vojnosanitetski Pregl. 2009;66:729–32. doi: 10.2298/VSP0909729D. [DOI] [PubMed] [Google Scholar]
- 28.Florkowski CM, Scott RS, Coope PA, Graham PJ, Moir CL. Age at diagnosis, glycaemic control and the development of retinopathy in a population-based cohort of Type 1 diabetic subjects in Canterbury, New Zealand. Diabetes Res Clin Pract. 2001;52:125–31. doi: 10.1016/S0168-8227(00)00248-5. [DOI] [PubMed] [Google Scholar]
- 29.Holl R, Lang GE, Grabert M, Heinze E, Lang G, Debatin K-M. Diabetic retinopathy in pediatric patients with type-1 diabetes: effect of diabetes duration, prepubertal and pubertal onset of diabetes, and metabolic control. J Pediatrics. 1998;132:790–4. doi: 10.1016/S0022-3476(98)70305-1. [DOI] [PubMed] [Google Scholar]
- 30.Olsen BS, Sjølie AK, Hougaard P, Johannesen J, Marinelli K, Jacobsen BB, et al. The significance of the prepubertal diabetes duration for the development of retinopathy and nephropathy in patients with type 1 diabetes. J Diabetes Complicat. 2004;18:160–4. doi: 10.1016/S1056-8727(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520–6. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 32.Ghanchi F. The Royal College of Ophthalmologists’ clinical guidelines for diabetic retinopathy: a summary. Eye. 2013;27:285–7. doi: 10.1038/eye.2012.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donaghue K, Wadwa R, Dimeglio L, Wong T, Chiarelli F, Marcovecchio M, et al. Microvascular and macrovascular complications in children and adolescents: Microvascular and macrovascular complications. Pediatr Diabetes. 2014;15:257–69. doi: 10.1111/pedi.12180. [DOI] [PubMed] [Google Scholar]
- 34.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. doi: 10.1016/S0161-6420(13)38012-9. [DOI] [PubMed] [Google Scholar]
- 35.The Diabetic Retinopathy Study Research Group Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Investig Ophthalmol Vis Sci. 1981;21:210–26. [PubMed] [Google Scholar]
- 36.Harding S, Greenwood R, Aldington S, Gibson J, Owens D, Taylor R, et al. Grading and disease management in national screening for diabetic retinopathy in England and Wales. Diabet Med. 2003;20:965–71. doi: 10.1111/j.1464-5491.2003.01077.x. [DOI] [PubMed] [Google Scholar]
- 37.Thomas RL, Dunstan FD, Luzio SD, Chowdhury SR, North RV, Hale SL, et al. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br J Ophthalmol. 2015;99:64–8. doi: 10.1136/bjophthalmol-2013-304017. [DOI] [PubMed] [Google Scholar]
- 38.Ford DV, Jones KH, Verplancke J-P, Lyons RA, John G, Brown G, et al. The SAIL Databank: building a national architecture for e-health research and evaluation. BMC Health Serv Res. 2009;9:157. doi: 10.1186/1472-6963-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyons RA, Jones KH, John G, Brooks CJ, Verplancke J-P, Ford DV, et al. The SAIL databank: linking multiple health and social care datasets. BMC Med Inform Decis Mak. 2009;9:3. doi: 10.1186/1472-6947-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sayers A, Thayer D, Harvey JN, Luzio S, Atkinson MD, French R, et al. Evidence for a persistent, major excess in all cause admissions to hospital in children with type-1 diabetes: results from a large Welsh national matched community cohort study. BMJ Open. 2015;5:e005644. doi: 10.1136/bmjopen-2014-005644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thayer D, Rees A, Kennedy J, Collins H, Harris D, Halcox J, et al. Measuring follow-up time in routinely-collected health datasets: Challenges and solutions. PLoS One. 2020;15:e0228545. doi: 10.1371/journal.pone.0228545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol. 1989;107:244–9. doi: 10.1001/archopht.1989.01070010250031. [DOI] [PubMed] [Google Scholar]
- 43.Dwyer MS, Melton LJ, Ballard DJ, Palumbo PJ, Trautmann JC, Chu C-P. Incidence of diabetic retinopathy and blindness: a population-based study in Rochester, Minnesota. Diabetes Care. 1985;8:316–22. doi: 10.2337/diacare.8.4.316. [DOI] [PubMed] [Google Scholar]
- 44.Burger W, Hovener G, Dusterhus R, Hartmann R, Weber B. Prevalence and development of retinopathy in children and adolescents with type 1 (insulin-dependent) diabetes mellitus. A longitudinal study. Diabetologia. 1986;29:17–22. doi: 10.1007/BF02427275. [DOI] [PubMed] [Google Scholar]
- 45.Malone JI, Morrison AD, Pavan PR, Cuthbertson DD. Prevalence and significance of retinopathy in subjects with type 1 diabetes of less than 5 years’ duration screened for the diabetes control and complications trial. Diabetes Care. 2001;24:522–6. doi: 10.2337/diacare.24.3.522. [DOI] [PubMed] [Google Scholar]
- 46.Dhillon N, Karthikeyan A, Castle A, Dodson P, Högler W, Kirk J, et al. Natural history of retinopathy in children and young people with type 1 diabetes. Eye. 2016;30:987–91. doi: 10.1038/eye.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jansson RW, Hufthammer KO, Krohn J. Diabetic retinopathy in type 1 diabetes patients in Western Norway. Acta Ophthalmol. 2018;96:465–74. doi: 10.1111/aos.13654. [DOI] [PubMed] [Google Scholar]
- 48.Hainsworth DP, Bebu I, Aiello LP, Sivitz W, Gubitosi-Klug R, Malone J, et al. Risk factors for retinopathy in type 1 diabetes: the DCCT/EDIC Study. Diabetes Care. 2019;42:875–82. doi: 10.2337/dc18-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sterky G, Wall S. Determinants of microangiopathy in growth‐onset diabetes: with special reference to retinupathy and glycaemic control. Acta Pædiatrica. 1986;75:1–45. doi: 10.1111/j.1651-2227.1986.tb14937.x. [DOI] [PubMed] [Google Scholar]
- 50.Klein BE, Moss SE, Klein R. Is menarche associated with diabetic retinopathy? Diabetes Care. 1990;13:1034–8. doi: 10.2337/diacare.13.10.1034. [DOI] [PubMed] [Google Scholar]
- 51.Kokkonen J, Laatikainen L, Dickhoff K, Miettinen R, Tuominen M, Lautala P, et al. Ocular complications in young adults with insulin‐dependent diabetes mellitus since childhood. Acta Paediatrica. 1994;83:273–8. doi: 10.1111/j.1651-2227.1994.tb18093.x. [DOI] [PubMed] [Google Scholar]
- 52.Schreur V, van Asten F, Ng H, Weeda J, Groenewoud JM, Tack CJ, et al. Risk factors for development and progression of diabetic retinopathy in Dutch patients with type 1 diabetes mellitus. Acta Ophthalmol. 2018;96:459–64. doi: 10.1111/aos.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein R, Lee KE, Gangnon RE, Klein BE. The 25-year incidence of visual impairment in type 1 diabetes mellitus the wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology. 2010;117:63–70. doi: 10.1016/j.ophtha.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.