Abstract
Purpose
To report a case of bilateral anterior uveitis undergoing atezolizumab treatment for advanced non-small cell lung cancer (NSCLC).
Observations
A 64-year-old man was receiving atezolizumab for metastatic programmed cell death ligand 1 (PD-L1) positive NSCLC as first line therapy. Three weeks after first atezolizumab administration, he complained of blurry vision in both eyes and was referred to our clinic. At initial presentation, slit lamp examination revealed bilateral Descemet membrane folds, fine keratic precipitates, and anterior chamber cells 2+. Dilated fundus examination showed no abnormal findings. A complete laboratory evaluation ruled out infectious or autoimmune causes of the uveitis and he was diagnosed with uveitis caused by atezolizumab. Atezolizumab was suspended, administration of topical corticosteroid was initiated, and the anterior uveitis was resolved within one month.
Conclusion and importance
This is the first case report of bilateral anterior uveitis associated with atezolizumab and that PD-1 and PD-L1 inhibitors cause uveitis.
Keywords: Atezolizumab, PD-L1 inhibitor, Anterior uveitis, Immune-related adverse events (irAEs)
1. Introduction
Cell surface checkpoint ligands, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) or its ligand, programmed cell death ligand 1 (PD-L1), are inhibitory signaling pathways in immune cells that maintain the peripheral tolerance of self-antigens.1 Recently, immune checkpoint inhibitors (ICIs) have proven useful in antitumor immunotherapy and been approved to treat various cancers. However, these agents are associated with distinctive adverse events collectively known as immune-related adverse events (irAEs).2 Although irAEs may affect any organ, various types of ocular adverse events including dry eye, uveitis, and myasthenia gravis with ocular involvement, are well-recognized.3,4 We report a case of bilateral anterior uveitis in a patient with metastatic non-small cell lung cancer (NSCLC) treated by atezolizumab immunotherapy, PD-L1 inhibitor.
2. Case report
A 64-year-old male presented to our ophthalmology clinic with sudden onset bilateral blurred vision three weeks after starting intravenous treatment with atezolizumab (1200mg) as first line therapy for metastatic PD-L1 positive NSCLC. He had noticed ocular redness in both eyes one week before his sudden bilateral visual acuity impairment. At presentation, he showed significantly decreased visual acuity, 20/50 in the right eye (OD) and 20/40 in the left (OS). Intraocular pressure was normal. Slit lamp examination revealed bilateral Descemet membrane folds, fine pigmented keratic precipitates on the posterior surface of the cornea, and anterior chamber cells 2+ (using SUN Working Group criterion5), but no iris nodules, synechiae, or corneal superficial punctate keratopathy (Fig. 1A–D). Fundus examination findings were normal (Fig. 2A and B). Optical coherence tomography (OCT) revealed no sign of macular edema or another abnormality (Fig. 2C and D). Fundus fluorescein angiography showed no abnormal findings. A complete laboratory evaluation ruled out infectious or autoimmune causes of uveitis. The final diagnosis was bilateral anterior uveitis presumed secondary to atezolizumab. A decision to postpone the second cycle of intravenous atezolizumab infusion and treat him topically with 0.1% sodium phosphate betamethasone eye drops every 3-h and tropicamide eye drops twice a day was initiated. An examination conducted on day 14 following initial presentation revealed improved visual acuity (20/20 for both eyes), reduction of keratic precipitates, disappearance of anterior chamber cells and Descemet membrane folds. At one month after the onset of eye inflammation, his anterior uveitis had resolved completely and topical steroid was tapered off. The second cycle of atezolizumab was started during which uveitis did not recur, infusion related reaction occurred and the third cycle of atezolizumab was cancelled. The cancer seemed to be in partial remission and two months after atezolizumab was interrupted, treatment was restarted with PD-1 inhibitor pembrolizumab.
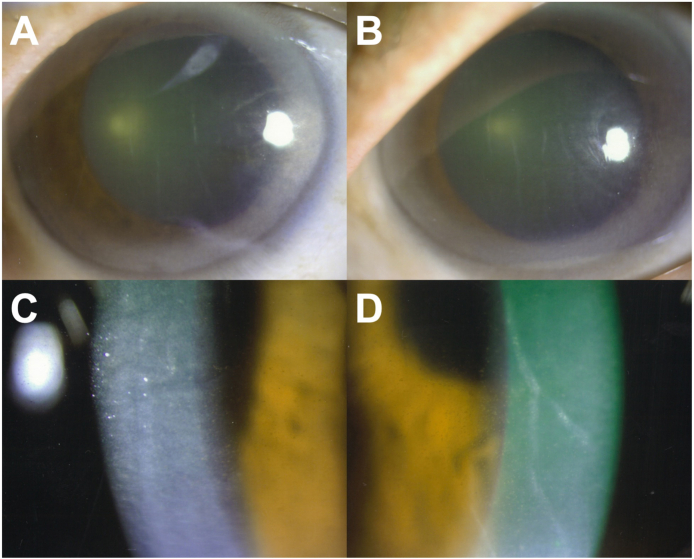
Fig. 1.
Slit lamp examination at initial presentation.
Photographs of the anterior segment showing considerable Descemet membrane folds resulting from anterior uveitis (A and B). Slit lamp images of extensive fine pigmented keratic precipitates with anterior chamber cells in both eyes. Right eye (OD) in left column and left eye (OS) in right column.
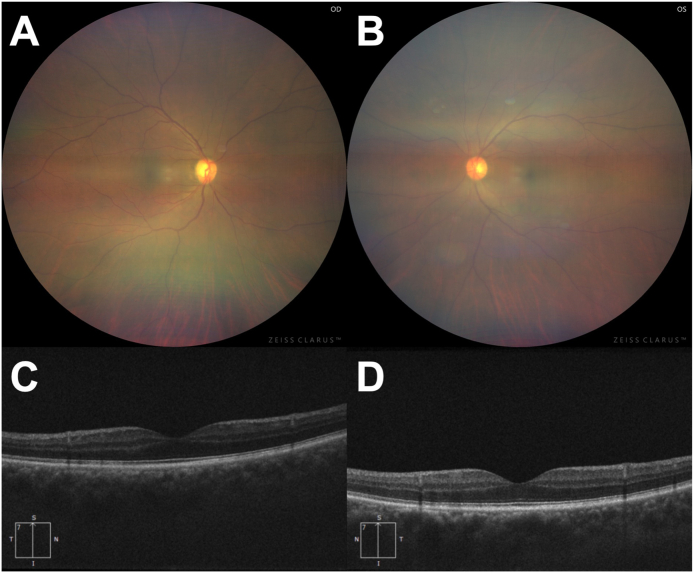
Fig. 2.
Fundus photographs and vertical sections of optical coherence tomographic (OCT) scans.
Although this widefield fundus photo is unclear due to anterior inflammation of the eye, fundi of both eyes appear normal (A and B). OCT showed no abnormality in either eye (C and D). Right eye (OD) in left column and left eye (OS) in right column.
3. Discussion
Only one case study in the literature report the association of PD-L1 inhibitors with anterior uveitis.6 To the best of our knowledge, this is the first case report of bilateral anterior uveitis associated with administration of atezolizumab.
Usually, T cells that recognize self-antigens are selected and deleted in the thymus through a central tolerance mechanism. However, some autoreactive T cells escape thymic deletion, and mechanisms which induce immune tolerance in the peripheral tissues are required for these escaped autoreactive T cells. Binding of cell surface checkpoint ligands leads to inhibition of an excessive autoimmune responses. Recent studies clarified that binding of ligands (PD-L1 expressed by cancer cells) to PD-1 on the surface of tumor-reactive T cells helps cancer cells evade immune attack via inhibitory signals.7,8
Atezolizumab is a humanized IgG1 monoclonal antibody which recognizes PD-L1, and exerts antitumor effects by removing inhibition of effector T cells through blocking PD-1/PD-L1 signaling.9 But the problem is that this PD-L1 inhibitor induces irAEs, specific to ICIs, in various organs and tissues. IrAEs occur at relatively high frequency, and the incidence rates of irAEs are reportedly 72% in CTLA-4 inhibitors and 66% in PD-1 inhibitors or PD-L1 inhibitors.10,11
The pathogenic mechanisms of irAEs have not been fully elucidated, but two possible mechanisms have been suggested. Firstly, action of ICIs blocks inhibitory signaling, thereby triggering the activation of effector T cells which recognize antigens present in both tumors and healthy tissues. This event presumably causes direct damage to tissues.12 Actually, blocking the interaction of PD-L1 receptors on the surface of retinal pigment epithelium (RPE) cells with PD-1 receptors on T cells results in a continued Th1 response targeted at RPE cells and ocular inflammation within the uvea. Secondly, PD-L1, which expresses in non-immune organs and immune cells (such as dendritic cells, monocytes, and macrophages), plays an important role in maintenance of immune tolerance.13,14 When this function is disturbed, failure of immunological homeostasis occurs potentially inducing irAEs. Yang et al. reported that PD-L1 protein was expressed in human ocular cell lines, and suggested that PD-L1 has a presumptive role in controlling ocular inflammation by inhibiting proinflammation cytokines (such as IFN-γ, TNF-α) and a Th2 cytokine produced by activated T cells.15
According to Brahmer et al., the most commonly occurring ICIs-induced ocular irAEs were dry eye at incidence rates of approximately 1% and intraocular inflammation is much less frequent.16 The review by Dalvin et al. also revealed that the majority of patients who developed ICIs-induced uveitis presented with anterior segment inflammation, although only three of 24 patients reportedly experienced inflammation localized to the posterior segment of the eye.3
Ocular irAEs induced by atezolizumab have rarely been reported. Bitton et al. reported a woman who developed bilateral conjunctivitis with marked superficial punctate keratitis, inferior fornix shortening, and tarsal conjunctival fibrosis.17 Another study by Venkat et al. reported a woman who had inflammatory superior limbic keratitis and subsequent bilateral posterior uveitis.18 Fang et al. also reported 3 cases of unspecified “eye inflammation” and 1 case of “uveitis”.4 In our case, however, anterior segment inflammation was found in both eyes of the patient. Likewise, other studies have reported that many patients who developed ICIs-related uveitis presented with anterior uveitis; this is the second case report of anterior uveitis caused by PD-L1 inhibition.6
Although treatment for irAEs differs by type of adverse event, the main treatments for irAEs include discontinuation of treatment with ICIs and immunosuppressive therapy with corticosteroids or with other immunosuppressive drugs including biologics. Findings of uveitis are classified and graded using the latest version of the Common Terminology Criteria for Adverse Events (CTCAE).19 In general, when a patient has grade 2 uveitis, clinicians should suspend ICIs treatment and administer topical corticosteroids, cycloplegic agents, or systemic corticosteroids. Clinicians may resume ICIs treatment when uveitis returns to grade 1 or less. Continued topical/ocular corticosteroids are permitted when resuming therapy to manage and minimize local toxicity. When a patient has grade 3 uveitis, treatment with ICIs is permanently discontinued in favor of treatment with corticosteroids.20 In our case, uveitis was classified in CTCAE grade 2, the symptoms immediately improved after atezolizumab treatment was suspended and the patient received only topical betamethasone for approximately four weeks because the inflammation had been confined to the anterior segment of his eye.
4. Conclusions
We encountered a patient with metastatic NSCLC who developed bilateral anterior uveitis after atezolizumab administration. In our case, the uveitis was resolved with topical application of steroid eyedrops and treatment of atezolizumab was continued. We believe this report will be informative to ophthalmologists involved in consultations regarding patients receiving atezolizumab and emphasize the utmost importance of communication between oncologists and organ specialists such as ophthalmologists to manage irAEs.
Patient consent
Written informed consent was obtained from the patient to publish and report this data.
Funding
No funding was received by any of the authors for writing this manuscript.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Declaration of competing interest
All authors declare they have no financial disclosures.
Acknowledgments
The authors are indebted to Mr. David Price for English proofreading.
References
- 1.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Canc. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 3.Dalvin L.A., Shields C.L., Orloff M. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063–1078. doi: 10.1097/IAE.0000000000002181. [DOI] [PubMed] [Google Scholar]
- 4.Fang T., Maberley D.A., Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319–322. doi: 10.1016/j.joco.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabs D.A., Nussenblatt R.B., Rosenbaum J.T. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh R.A., Chaon B.C., Berkenstock M.K. Ocular complications of checkpoint inhibitors and immunotherapeutic agents: a case series. Ocul Immunol Inflamm. 2020;9:1–6. doi: 10.1080/09273948.2020.1766082. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 8.Chen L., Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumeister S.H., Freeman G.J., Dranoff G., Sharpe A.H. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–573. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand A., Kostine M., Barnetche T., Truchetet M.E., Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Zhou S., Yang F. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008–1019. doi: 10.1001/jamaoncol.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson D.B., Balko J.M., Compton M.L. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okazaki T., Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 14.Keir M.E., Liang S.C., Guleria I. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W., Li H., Chen P.W. PD-L1 expression on human ocular cells and its possible role in regulating immune-mediated ocular inflammation. Invest Ophthalmol Vis Sci. 2009;50(1):273–280. doi: 10.1167/iovs.08-2397. [DOI] [PubMed] [Google Scholar]
- 16.Brahmer J.R., Tykodi S.S., Chow L.Q. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bitton K., Michot J.M., Barreau E. Prevalence and clinical patterns of ocular complications associated with anti-PD-1/PD-L1 anticancer immunotherapy. Am J Ophthalmol. 2019;202:109–117. doi: 10.1016/j.ajo.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Venkat A.G., Arepalli S., Sharma S. Local therapy for cancer therapy-associated uveitis: a case series and review of the literature. Br J Ophthalmol. 2020;104(5):703–711. doi: 10.1136/bjophthalmol-2019-314403. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE V.5.0) U.S. Department of Health and Human Services; 2019. [Google Scholar]
- 20.Brahmer J.R., Lacchetti C., Schneider B.J. Management of immuno-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]