Abstract
Background:
Patients with medullary thyroid carcinoma (MTC) often receive lateral lymph node dissection with total thyroidectomy when calcitonin levels are elevated, even in the absence of structural disease, but the effect of this intervention on disease specific outcomes is not known.
Methods:
We retrospectively reviewed patients from 1986 to 2017 who underwent thyroidectomy with curative intent for MTC at our institution. The association of disease specific survival (DSS) and clinicopathologic features was examined using univariate and multivariate Cox regression.
Results:
We identified 316 patients who underwent curative resection for medullary thyroid carcinoma. Overall and disease specific survival were 76% and 86% at 10-years. To investigate the effect of prophylactic ipsilateral lateral lymph node dissection, we analyzed 89 patients without known structural disease in the neck lymph nodes at the time of resection and preoperative calcitonin > 200 pg/ml, of which 45 had an ipsilateral lateral lymph node dissection (LND) and 44 did not. There were no differences in tumor size or preoperative calcitonin levels. There was no difference at 10 years in cumulative incidence of recurrence in the neck (20.9% LND vs 30.4% no LND, p=0.46), cumulative incidence of distant recurrence (18.3% vs 18.4%, p=0.97), disease specific survival (86% vs 93%, p=0.53), or overall survival at (82% vs 90%, p=0.6).
Conclusion:
Lateral neck dissection in the absence of clinical or radiologic abnormal lymph nodes is not associated with improved survival in patients with MTC.
INTRODUCTION
Medullary thyroid carcinoma (MTC) is a neuroendocrine neoplasm arising from the parafollicular C cells of the thyroid. Cervical lymph node metastases are common and are a significant risk factor for recurrence and death1–3. There continues to be controversy regarding the extent of lymph node dissection at the time of thyroidectomy, particularly with regards to the lateral neck compartments1,4–6, and the most recent guidelines from the American Thyroid Association state that “dissection of the lymph nodes in the lateral compartments (levels II-V) may be considered based on serum calcitonin levels7.”
The debate regarding the extent of lymph node dissections for medullary thyroid carcinoma in the absence of structural disease has focused primarily on the likelihood of microscopic disease and on normalization of postoperative serum calcitonin levels3,8. Rates of lymph node metastasis in each compartment of the neck correlate well with basal serum calcitonin levels and tumor size9 which has led some recommendations for lymph node dissection in the absence of structural disease, including ipsilateral lateral dissection for calcitonin 20–200 pg/ml and bilateral dissection for calcitonin over 20010,11. However, this recommendation lacks correlation with long-term oncologic outcomes including recurrence in the neck, distant recurrence, and death from disease. Recurrences occur in the neck even after lymph node dissection12, and a better understanding of the effect on local recurrence, distant metastasis, and survival is needed.
We sought to characterize long term oncologic outcomes of recurrence and death for patients with MTC from a single institution, and describe factors associated with those outcomes. Further, we examine patients without structural disease using cutoff of basal serum calcitonin greater than 200 pg/ml to compare recurrence in the neck and reoperation, distant recurrence, and death from disease in patients that were selected for prophylactic lateral lymph node dissection and those in which it was not performed.
METHODS
Following institutional review board approval, we reviewed the cancer registry records at Memorial Sloan-Kettering Cancer Center (MSKCC) for patients with medullary thyroid cancer. We identified 340 patients that had a thyroidectomy for medullary thyroid cancer between 9/1986 and 1/2018. Twenty patients had distant metastatic disease at the time of surgery and four patients had gross residual disease in the neck leaving 316 patients who underwent thyroidectomy with curative intent as the patient cohort for this study. Clinical, pathologic, and follow-up data were obtained by review of the medical record.
Lymph node dissections were defined according to levels of the neck recorded in the operative and pathology reports. Only complete anatomic dissections were included and lymph node sampling was not recorded as a lymph node dissection. Levels II-V were classified as the lateral neck and designated as ipsilateral or contralateral to the primary site in the thyroid. The central neck was defined as levels VI and VII. Structural disease in the neck was defined as evidence for macroscopic disease in the lymph nodes of the neck detected by physical exam or cross-sectional imaging. Preoperative imaging was at the discretion of the treating team and radiology reports were analyzed for this study without rereview of the images. All patients with structural disease underwent lymphadenectomy of the involved compartment.
Persistent biochemical disease was defined as elevation of the first postoperative calcitonin or CEA over the upper limit of normal. Patient follow up was at the discretion of the treating physicians and most commonly included yearly neck ultrasound with calcitonin and CEA levels. Lymph node positivity was defined as at least one positive node. Locoregional recurrence was defined as structural recurrence in the neck and designated as lymph node or in the thyroid bed. Distant recurrence was defined as recurrence outside of the neck. Locoregional and distant recurrences were recorded independently and the time to recurrence or death was calculated from the date of surgery.
Statistical Methods
Clinicopathologic characteristics were summarized using frequency/percentages for categorical variables, and median/ranges for continuous variables. Cumulative incidences of locoregional recurrence (LRR) and distant recurrence (DR) were estimated using competing risks method and compared between subgroups using Gray’s test. Fine and Grey regression method was used to examine association between potential risk factors and time to LRR and DR. Overall survival (OS) and disease specific survival (DSS) were calculated from the time of surgery until date of death or last follow up, estimated using Kaplan -Meier methods, and compared between subgroups using log-rank test. A Cox proportional hazards model was used to examine association between potential risk factors with DSS. Recurrence status was treated as time-dependent covariate in the Cox proportional hazards model. Final multivariable models included variables that were significantly associated with outcomes at p< 0.05 level. Preoperative and pos-operative CEA and calcitonin biomarkers were log-transformed.
Among the subgroup of patients without known structural disease in the neck lymph nodes at the time of resection and preoperative calcitonin > 200 pg/ml (n=89), Fisher’s exact test and Wilcoxon’s rank sum test were used to compare covariate distributions among subgroups. In addition, we evaluated the cumulative incidences of neck reoperation using the competing risks method and compared time to recurrence and survival between patients that had a prophylactic lateral neck lymph node dissection and those that did not.
All statistical analyses using SAS version 9.3 (SAS Institute, Inc, Cary, NC, USA) or R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). All p-values were two sided and statistical significance set at p <0.05.
RESULTS
Clinicopathologic Characteristics
In this study, the median age of 316 patients who underwent curative intent thyroidectomy for medullary thyroid carcinoma at our institution was 53.4 years (range 3.4–88) and 15 (5%) were younger than 18 years of age. Median follow up of survivors was 5.7 (range: 0.02–31.5) years. Clinicopathologic variables are listed in table 1. Lymph node dissection was performed at the discretion of the operating surgeon. Ninety-five (30%) patients underwent central with ipsilateral lateral lymph node dissection, 87 (28%) central only, 36 (11%) ipsilateral lateral only, 23 (7%) central and bilateral lateral, and 2 (1%) bilateral lateral without central. Seventy-three (23%) patients did not undergo any complete compartmental lymph node dissection. All 80 (26%) patients with evidence of structural lymph node disease underwent formal dissection of the involved compartment.
Table 1.
Clinicopathologic variables of 316 patients
| Variable** | Median (range) or N (%) |
|---|---|
| Age (years, IQR) | 53.4 (40–64) |
| Follow up (years) | 5.77 (3–31.5) |
| Gender (Female) | 163 (52%) |
| Bilateral | 51 (16%) |
| RET Mutant | 64 (21%) |
| Known Structural disease | 80 (26%) |
| Tumor size (cm) | 1.9 (1.1–3.2) |
| Node positive | 169 (54%) |
| Positive Central Node | 154 (49%) |
| Positive Lateral Node | 112 (35%) |
| Persistent biochemical disease* | 180 (62%) |
| Preop calcitonin (pg/ml), n=188 | 914 (258–4346) |
| Preop CEA (ng/ml), n=102 | 29 (6–92) |
| Post op calcitonin (pg/ml), n=294 | 32 (3–418) |
| Post op CEA (ng/ml), n=235 | 6 (2–17) |
| Neck Reoperation | 70 (22%) |
| Neck external beam radiation | 32 (10%) |
| Tyrosine Kinase Inhibitor | 39 (12%) |
| Cytotoxic Chemotherapy | 11 (4%) |
| Metastectomy | 11 (4%) |
| Year of Surgery | |
| 1986–1996 | 35 (11%) |
| 1997–2007 | 142(45%) |
| 2008–2018 | 139 (44%) |
Persistent biochemical disease was defined as an elevation above the upper limit of normal in the first postoperative calcitonin or CEA level; IQR=Inter-quartile ranges
For continuous variable, median and IQR were provided.
Preoperative imaging was obtained in 202 (64%) patients including 27 of 80 (34%) patients with evidence of structural disease and 175 of 236 (74%) of patients without evidence of structural disease, and 76 (85%) of the 89 patients later identified with no evidence of structural disease and a serum Ct > 200 pg/ml. In patients with structural disease the majority of lymph node metastasis were detected by physical exam (53/80, 66%); in patients that did have imaging, 13 were imaged with CT scan, 9 with ultrasound, and 5 with PET scan. In patients without structural disease the majority (137/236, 58%) had a preoperative ultrasound. Specifically, 82 patients underwent ultrasound only, 36 CT and ultrasound, 26 CT only, 11 PET and ultrasound, 8 MRI and ultrasound, 5 PET only, 5 MRI only, and 2 CT and MRI.
Recurrence and Survival
Locoregional recurrence (LRR) was defined as histologically proven disease in the thyroid bed or in the central or lateral lymph node compartments of the neck. LRR was observed in 98 patients with a 5-year cumulative incidence of 28% (95% CI: 23%−34%) and 10-year cumulative incidence of 39% (95% CI: 32%−45%), Figure 1A. Seventy patients (72%) underwent reoperation with neck dissection following LRR for regional control. Surgery was offered after structural recurrence with or without biopsy for histologic confirmation. The most common site of recurrence was isolated recurrence in the ipsilateral lateral neck (n=54), followed by central neck alone (n=25), contralateral lateral (n=9), central and lateral (n=3 ipsilateral, n=2 contralateral), and bilateral lateral (n=4). Of the central neck recurrences, 5 occurred within the thyroid resection bed. Of the 61 patients that had a lymph node recurrence in the ipsilateral lateral neck, 33 (54%) previously underwent ipsilateral lateral lymph node dissection at initial operation. Factors associated with LRR on univariate analysis included tumor size (HR 1.15 [95% CI: 1.06–1.24], p=0.001), preoperative calcitonin (Ct) (HR 1.27 [95% CI: 1.09–1.48], p=0.003), postoperative Ct (HR 1.23 [95% CI: 1.14–1.32], p<0.001), postoperative CEA (HR 1.23 [95% CI: 1.07–1.41], p=0.003), positive lymph nodes (HR 3.43[95% CI: 2.18–5.39], p<0.001), and persistent biochemical disease defined as elevation in the first postoperative Ct or CEA (HR 7.8 [95% CI: 3.61–16.7], p<0.001). RET mutations (HR 1.03 [0.66–1.62], p=0.88) and surgical era (HR 0.81 [0.52–1.28] for 1997–2007 and HR 0.95 [0.54–1.65] for 1986–1996, p=0.64) were not associated with LRR. After controlling for tumor size, post-operative CEA, and structural disease, factors independently associated with LRR on multivariable analysis were postoperative Ct (HR 1.23 [95% CI: 1.08–1.39], p<0.001) and lymph node positivity (HR 2.35 [95% CI: 1.21–4.57], p=0.01).
Figure 1: Recurrence and survival in 316 patients with medullary thyroid carcinoma after thyroidectomy with curative intent.
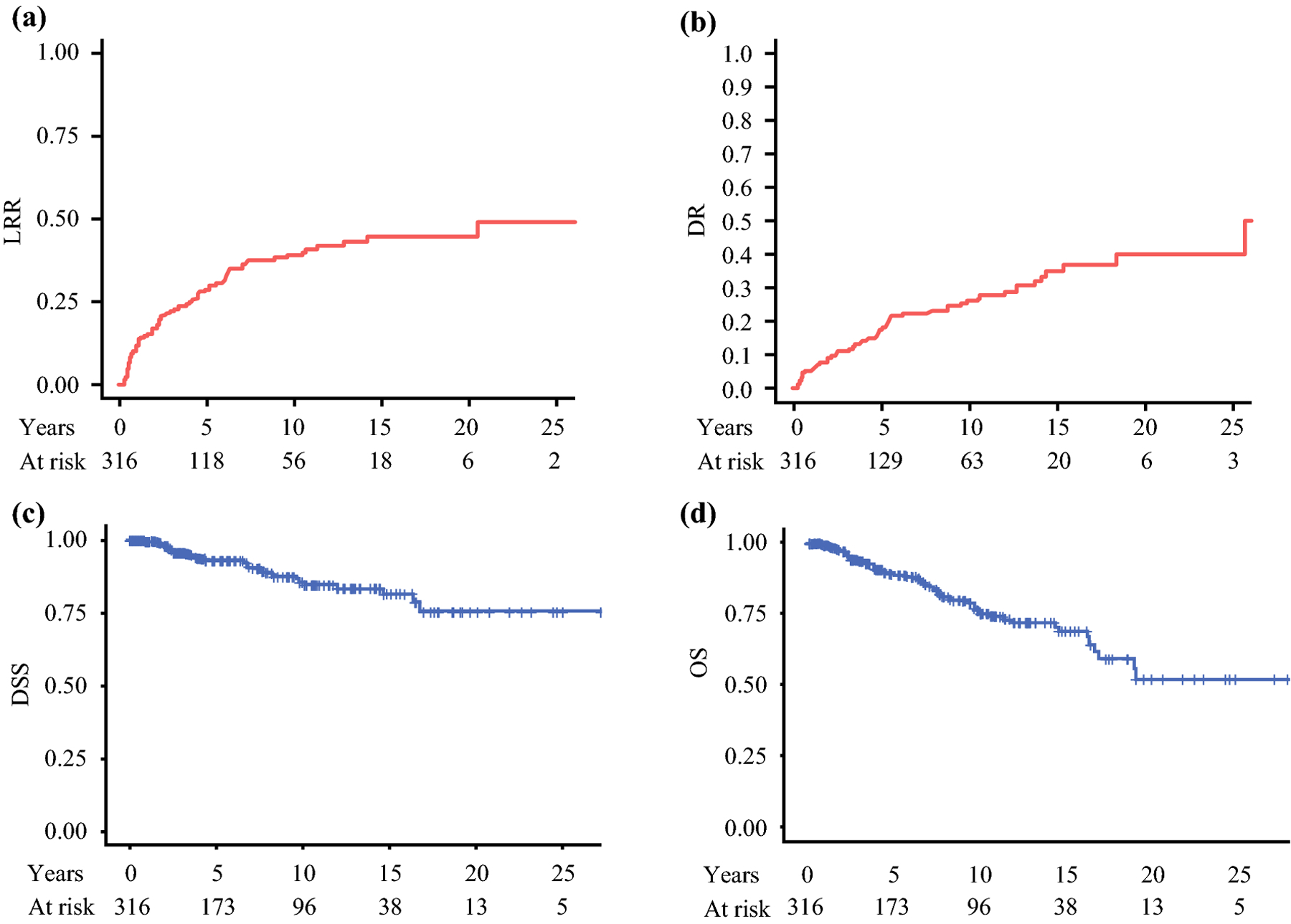
In all patients, A) cumulative incidence of locoregional recurrence is 28% (95% CI: 23%−34%) at 5 years and 39% (95% CI: 32%−45%) at 10 years, B) distant recurrence is 17% (95%CI: 13%−22%) at 5 years and 26% (95%CI: 20%−32%) at 10 years, C) disease specific survival is 93% (95% CI: 90%−96%) at 5 years and 86% (95% CI: 80%−91%) at 10 years, and D) overall survival is 89% (95% CI: 85%−93%) at 5 years and 76% (95% CI: 70%−83%) at 10 years.
Distant recurrence (DR) occurred in 70 patients. Five-year cumulative incidence of DR was 17% (95%CI: 13%−22%) and 10-year cumulative incidence was 26% (95%CI: 20%−32%), Figure 1B. DR were lung (n=20), liver (n=18), liver and lungs (n=10), bone (n=10), bone and liver (n=6), bone and lung (n=4), brain (n=1), and liver and brain (n=1). Factors associated with DR on univariate and multivariate analysis are listed in table 2. Seventy patients (22%) received additional treatment after curative intent thyroidectomy which in all cases was given after structural recurrence. Thirty-nine patients (12%) were treated with tyrosine kinase inhibitors, all after distant recurrence. Cytotoxic chemotherapy was given to 11 (3.5%) patients. Radiation was given to 43 (14%) patients, 11 directed at metastatic disease and 32 after recurrence in the neck. Metastectomy including liver resection/ablation and rib resection was performed in 11 (3.5%) patients.
Table 2.
Factors associated with Distant Recurrence in 316 patients.
| Factor | HR comparison | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age | Per 1-year increase | 0.99 (0.98–1.01) | 0.37 | n/a | n/a |
| Tumor size | Per 1-cm increase | 1.21 (1.11–1.31) | <0.001 | 1.07 (0.91–1.29) | 0.42 |
| LN positivity | Positive vs. negative | 2.43 (1.46–4.06) | 0.001 | 1.97 (1.00–3.89) | 0.051 |
| Ct pre-op* | Per 1-unit increase | 1.1 (0.9–1.34) | 0.34 | n/a | n/a |
| CEA pre-op* | Per 1-unit increase | 1.3 (0.9–1.86) | 0.16 | n/a | n/a |
| Ct post-op* | Per 1-unit increase | 1.22 (1.12–1.33) | <0.001 | 1.11 (0.98–1.25) | 0.11 |
| CEA post-op* | Per 1-unit increase | 1.43 (1.22–1.68) | <0.001 | 1.28 (1.02–1.60) | 0.033 |
| Structural disease | Yes vs. no | 1.76 (1.06–2.91) | 0.029 | 1.10 (0.55–2.23) | 0.78 |
| Persistent biochemical disease | Yes vs. no | 3.49 (1.67–7.31) | 0.001 | n/a | n/a |
| Year of Surgery | 0.324 | n/a | |||
| 1997–2007 | 1997–2007 vs. 2008–2018 | 1.38 (0.80–2.38) | n/a | ||
| 1986–1996 | 1986–1996 vs. 2008–2018 | 0.93 (0.45–1.89) | n/a | ||
| RET | Mut vs. WT | 1.20 (0.74–1.96) | 0.462 | n/a | n/a |
CEA (ng/ml) and Ct (pg/ml) levels were log-transformed.
Disease specific survival (DSS) was 93% (95% CI: 90%−96%) at 5 years and 86% (95% CI: 80%−91%) at 10 years, figure 1C. Overall survival for the cohort was 89% (95% CI: 85%−93%) at 5 years and 76% (95% CI: 70%−83%) at 10 years, figure 1D. At 10 years 25 patients were alive and had follow up. Of the 32 patients that died from disease, 30 were from metastatic disease and 2 patients died from invasive disease in the neck. Factors associated with DSS are listed in table 3.
Table 3.
Factors associated with Disease-Specific Mortality in 316 patients.
| Factor | HR comparison | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age | Per 1-year increase | 1.02 (1.0–1.04) | 0.082 | n/a | n/a |
| Tumor size | Per 1-cm increase | 1.35 (1.19–1.53) | <0.001 | 1.26 (1.10–1.44) | 0.001 |
| LN positivity | Positive vs. negative | 2.22 (1.02–4.79) | 0.38 | 0.99 (0.37–2.69) | 0.99 |
| Ct pre-op* | Per 1-unit increase | 1.06 (0.86–1.31) | 0.57 | n/a | n/a |
| CEA pre-op* | Per 1-unit increase | 1.14 (0.71–1.84) | 0.59 | n/a | n/a |
| Ct post-op* | Per 1-unit increase | 1.14 (1.01–1.29) | 0.054 | n/a | n/a |
| CEA post-op* | Per 1-unit increase | 1.4 (1.13–1.72) | 0.001 | n/a | n/a |
| Structural disease | Yes vs. no | 2.4 (1.17–4.93) | 0.014 | 1.23 (0.52–2.95) | 0.638 |
| Persistent biochemical disease | Yes vs. no | 2.14 (0.81–5.65) | 0.12 | n/a | n/a |
| LRR | Time-dependent covariate | 4.93 (2.30–10.55) | <0.001 | 3.64 (1.52–8.71) | 0.004 |
| Year of Surgery | 0.511 | n/a | |||
| 1997–2007 | 1997–2007 vs. 2008–2018 | 1.16 (0.47–2.85) | n/a | ||
| 1986–1996 | 1986–1996 vs. 2008–2018 | 0.61 (0.16–2.29) | n/a | ||
| RET | Mut vs. WT | 0.29 (0.09–0.94) | 0.029 | 0.34 (0.10–1.15) | 0.083 |
CEA and Ct levels were log-transformed.
There were 92 patients for which there was no preoperative calcitonin level and no preoperative evidence of structural disease. Of those patients, 39 (42%) underwent central lymph node dissection and 19 (21%) underwent ipsilateral lateral lymph node dissection. At 10 years, cumulative incidence of LRR was 41% % (95% CI: 29%−53%), cumulative incidence of DR was 31% (95% CI: 20%−42%), DSS was 89% (95% CI: 82%−98%), and OS was 93% (95% CI: 87%−99%), all of which did not show a statistical difference from the remaining 213 patients.
Impact of Elective Lymph Node Dissection
To examine the impact of elective lymph node dissection we examined the subset of the study cohort that had no evidence of structural disease in the neck and had a preoperative basal serum calcitonin level of greater than 200 pg/ml. Eighty-nine patients met these criteria, of which 45 underwent an elective ipsilateral lateral lymph node dissection (LND) and 44 did not (No LND). Comparison of clinicopathologic features showed no significant differences between the two groups in tumor size or preoperative Ct and CEA, although patients in the no LND group were older (median age 58 vs 50, p=0.04), table 4. There was also no difference in postoperative Ct (median 16 pg/ml vs 16, p=0.9) or CEA (median 6 ng/ml vs 5, p=0.5). Both groups had a similar rate of persistent biochemical disease on first postoperative labs (59% vs 49%, p=0.50) and similar percentages of each group were treated during each decade of the study (p=0.94).
Table 4.
Patients with no structural disease and Ct > 200
| Variable | No LND | LND | p-value |
|---|---|---|---|
| N = 44 | N = 45 | ||
| Age at Procedure, year median (range) | 58 (52–63) | 50 (39–60) | 0.04 |
| Gender (Female) | 23 (52%) | 29 (64%) | 0.3 |
| Size (cm), median (range) | 1.80 (1.50–2.58) | 2.35 (1.50–3.05) | 0.2 |
| Preoperative Imaging (any) | 38 (86%) | 38 (84%) | 1.0 |
| Preoperative neck ultrasound | 33 (75%) | 30 (67%) | 0.49 |
| Number of Lymph Nodes Removed, median (range) | 4 (0–10) | 24 (8–64) | <0.001 |
| Number of Positive Lymph Nodes, median (range) | 0 (0–5) | 2 (0–41) | 0.004 |
| Ct pre-op | 1142 (581–3472) | 1058 (567–5018) | >0.9 |
| CEA pre-op | 25 (15–103) | 35 (14–93) | 0.8 |
| Ct post-op | 16 (2–176) | 16 (3–75) | 0.9 |
| CEA post-op | 6 (2–9) | 5 (2–17) | 0.5 |
| Persistent Biochemical Disease | 26 (59%) | 22 (49%) | 0.5 |
| Surgical Era | 0.94 | ||
| 1986–1996 | 3 (7%) | 2 (4%) | |
| 1997–2007 | 16 (36%) | 16 (36%) | |
| 2008–2018 | 25 (57%) | 27 (60%) |
At the time of analysis, 17 patients developed LRR of whom: 7 had LND. At 10 years, cumulative incidence of LRR in patients that had LND was 21% (95%CI: 9%−37%) and 30% (95%CI: 13%−50%) in patients that did not, p=0.46, figure 2A. In patients that had LND, 5 patients had LRR; 2 (40%) in the central neck, 2 (40%) ipsilateral lateral only, and 1 (20%) ipsilateral lateral and contralateral lateral and in the no LND group there were 6 LRR, 2 (33%) in the central neck and 4 (67%) in the ipsilateral lateral neck. Cumulative incidence of reoperation in the neck was 16% (95%CI: 5%−31%) at 10 years in the LND group and 23% (95%CI: 8%−42%) in the no LND group, p=0.43. Among this subgroup, 10 patients developed DR and ten-year cumulative incidence of DR was 18% (95%CI: 6%−36%) in the LND group and 18% (95%CI: 5%−38%) in the no LND group, p=0.97, figure 2B. We did not detect a significant difference in OS or DSS according to whether a lymph node dissection was performed. DSS (Figure 2C) was 86% (95% CI: 71%−100%) at 10 years in the LND group and 93% (95% CI: 80%−100%) in the no LND group, p=0.53, and overall survival (Figure 2D) was 82% (95% CI: 67%−100%) and 90% (95% CI: 76%−100%) at 10-years respectively, p=0.6.
Figure 2: Recurrence and survival in patients without structural lymph node disease and preoperative calcitonin >200 pg/ml.
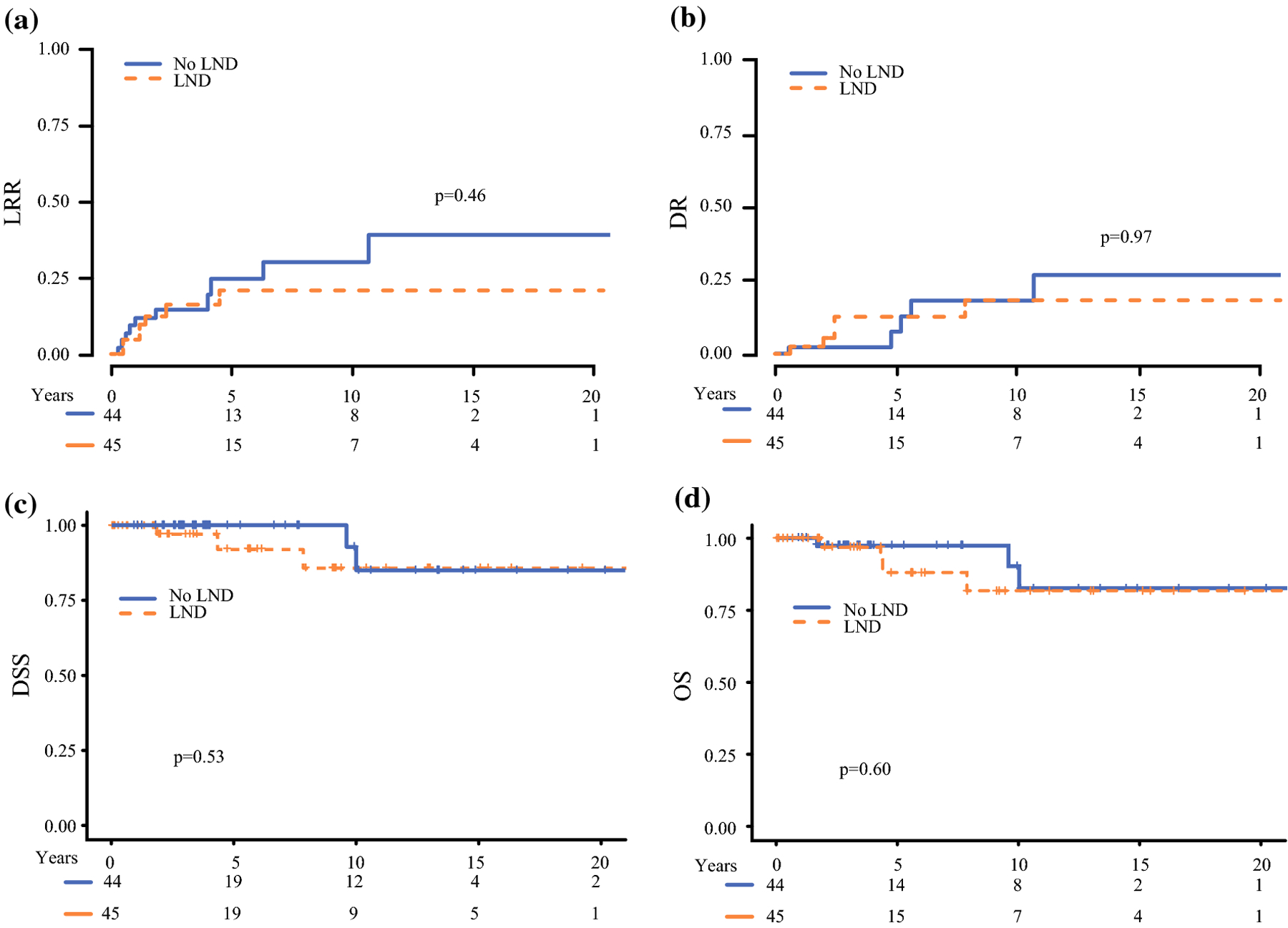
In patients with high preoperative calcitonin and no evidence of structural disease in the neck, ipsilateral lateral lymph node dissection (LND) was not associated with a difference in A) locoregional recurrence B) distant recurrence, C) disease specific survival, or D) overall survival
DISCUSSION
In this study we define the long-term oncologic outcomes for patients with medullary thyroid carcinoma in the largest series reported from a single institution. We describe factors associated with recurrence and mortality and examine the effect of elective lymph node dissection in patients without structural disease.
Patients with high preoperative calcitonin and without structural disease selected for ipsilateral lateral compartment lymph node dissection at the time of initial thyroidectomy had equivalent distant recurrence and survival to patients who did not undergo initial lymph node dissection. Patients in this study were selected by surgeon preference for treatment rather than randomization. Previously age, post-operative calcitonin, sex, tumor size and lymph node positive disease have been identified as factors associated with survival13,14. The no LND patients were older than the LND patients, which would bias that group to worse survival, which was not observed and further supports the conclusion that omitting lymph node dissection does not negatively impact survival. The other factors associated with recurrence and survival were well matched between patients undergoing LND and those that did not.
Omission of prophylactic LND was not associated with worse survival, but there was a non-statistically significant increase in the rate of lateral neck recurrence and reoperation. Further, there may exist a high risk group, such as young patients with familial disease15, who are more likely to benefit. Interestingly the rate of recurrence in the neck was very similar over the first 5 years from initial operation and only started to increase in the no LND group after that point. These findings support a disease model where prophylactic LND removes microscopic, slow growing disease that takes many years to manifest if it becomes clinically apparent at all. The finding of similar distant recurrence rates indicates that the potential for residual microscopic lymph node disease does not increase the risk of metastasis and that locoregional recurrence is salvageable with neck dissection. It was shown four decades ago that patients with lymph node positive disease have low rates of biochemical cure16, further indicating that the mechanism of long term survival in patients with lymph node positive disease is not aggressive surgical control in the neck. Similarly, randomized controlled trials in breast cancer (ACOSOG Z0011)17 and melanoma (MSLT-2)18 have demonstrated the safety of nodal observation strategies and shown no survival advantage with complete lymph node dissection in select patients with nodal disease. These findings are consistent with a model of disease progression where systemic disease does not result as a product of stepwise progression through the lymph nodes and aggressive attempts to control microscopic disease in the lymph nodes does not affect metastasis or survival.
One population at high risk for local recurrence is young patients with familial disease15, which could represent a group that could benefit from more aggressive upfront surgery. Further study is needed to determine the biologic determinants of metastasis and the role of surgery to affect that outcome.
In this study we chose a preoperative calcitonin cutoff above 200 pg/ml for our comparison of elective lymph node dissection because it is a cutoff at which some advocate for bilateral lateral lymph node dissection in the absence of known structural disease10. Based on Machens’ study, the probability of positive lymph nodes in the ipsilateral lateral compartment with a basal serum calcitonin > 200 pg/ml is 59%. It would follow that if no survival advantage is observed with ipsilateral lateral lymph node dissection with a probability of microscopic disease of 59%, that there would also be no survival advantage with contralateral lateral lymph node dissection with a positive lymph node rate of 16%.
This study has several limitations. Although our treatment groups are well matched, patients were not randomized to treatment and these findings would ideally be validated with a randomized-controlled trial which would provide data without the potential for selection bias. Additionally, we included patients over a 30-year period in which effective systemic therapy has been developed. The majority of patients in that analysis were from the last decade and patients in the LND and No LND had similar percentages per decade of the study but combining data from different treatment periods could affect conclusions. There was variation in preoperative labs and imaging which could have affected preoperative assessment of burden of disease. Finally, associations of LND status with OS, DSS, time to LRR, and time to DR were based on a subset of 89 patients and should be interpreted cautiously.
This is the first report in the literature of recurrence and survival related to the extent of lymph node dissection for medullary thyroid carcinoma. Acknowledging the limitations above, our data show that prophylactic lymph node dissection in patients with MTC, no structural disease outside the thyroid, and a preoperative calcitonin level of > 200 pg/ml is not associated with improved survival. These results may be considered when deciding if the potential for oncologic benefit justifies the morbidity of prophylactic lateral neck dissection.
Synopsis.
Medullary thyroid cancer is a rare neuroendocrine neoplasm. Rates of lymph node involvement based on preoperative calcitonin levels have been described, but the oncologic effect of lateral lymph node dissection at the time of thyroidectomy in these patients is not known. Performance of lateral neck dissection in patients with high preoperative calcitonin levels (> 200 pg/ml) was not associated with improved recurrence or survival.
ACKNOWLEDGEMENTS
The work was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Scollo C, Baudin E, Travagli JP, et al. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab. 2003;88(5):2070–2075. [DOI] [PubMed] [Google Scholar]
- 2.Machens A, Holzhausen H, Dralle H. Contralateral cervical and mediastinal lymph node metastasis in medullary thyroid cancer: Systemic disease? Surgery. 2006;139(1):28–32. [DOI] [PubMed] [Google Scholar]
- 3.Weber T, Schilling T, Frank-Raue K, et al. Impact of modified radical neck dissection on biochemical cure in medullary thyroid carcinomas. Surgery. 2001;130(6):1044–1049. [DOI] [PubMed] [Google Scholar]
- 4.Ellenhorn JD, Shah JP, Brennan MF. Impact of therapeutic regional lymph node dissection for medullary carcinoma of the thyroid gland. Surgery. 1993;114(6):1078–1081; discussion 1081–1072. [PubMed] [Google Scholar]
- 5.Fleming JB, Lee JE, Bouvet M, et al. Surgical strategy for the treatment of medullary thyroid carcinoma. Ann Surg. 1999;230(5):697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machens A, Holzhausen HJ, Dralle H. Contralateral cervical and mediastinal lymph node metastasis in medullary thyroid cancer: systemic disease? Surgery. 2006;139(1):28–32. [DOI] [PubMed] [Google Scholar]
- 7.Wells SA Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machens A, Hauptmann S, Dralle H. Prediction of lateral lymph node metastases in medullary thyroid cancer. Br J Surg. 2008;95(5):586–591. [DOI] [PubMed] [Google Scholar]
- 9.Moley JF, DeBenedetti MK. Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg. 1999;229(6):880–887; discussion 887–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab. 2010;95(6):2655–2663. [DOI] [PubMed] [Google Scholar]
- 11.Dralle H, Machens A. Surgical management of the lateral neck compartment for metastatic thyroid cancer. Curr Opin Oncol. 2013;25(1):20–26. [DOI] [PubMed] [Google Scholar]
- 12.Franc S, Niccoli-Sire P, Cohen R, et al. Complete surgical lymph node resection does not prevent authentic recurrences of medullary thyroid carcinoma. Clin Endocrinol (Oxf). 2001;55(3):403–409. [DOI] [PubMed] [Google Scholar]
- 13.Ho AS, Wang L, Palmer FL, et al. Postoperative Nomogram for Predicting Cancer-Specific Mortality in Medullary Thyroid Cancer. Ann Surg Oncol. 2015;22(8):2700–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo EJ, Sho S, Li N, Zanocco KA, Yeh MW, Livhits MJ. Risk Factors Associated With Reoperation and Disease-Specific Mortality in Patients With Medullary Thyroid Carcinoma. JAMA Surg. 2018;153(1):52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spanheimer PM, Ganly I, Chou J, et al. Long-Term Oncologic Outcomes After Curative Resection of Familial Medullary Thyroid Carcinoma. Annals of Surgical Oncology. 2019;26(13):4423–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norton JA, Doppman JL, Brennan MF. Localization and resection of clinically inapparent medullary carcinoma of the thyroid. Surgery. 1980;87(6):616–622. [PubMed] [Google Scholar]
- 17.Giuliano AE, Ballman KV, McCall L, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017;318(10):918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faries MB, Thompson JF, Cochran AJ, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med. 2017;376(23):2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]


