Abstract
Background
Lower serum chloride is associated with a higher risk of mortality in the general population. However, the association has received little attention in peritoneal dialysis patients. The study aimed to examine the association between serum chloride and mortality in peritoneal dialysis patients.
Methods
In this multicenter retrospective cohort study, 2376 Chinese incident patients on peritoneal dialysis between January 1, 2005, and March 31, 2020, were included. Patients were grouped according to quartiles of serum chloride at baseline. The associations of baseline serum chloride and cardiovascular mortality and all-cause mortality were evaluated using cause-specific hazards models.
Findings
Of 2376 patients, the mean age was 45.9 (45.3,46.5) years, 50.1% of patients were men. The median serum chloride levels were 103.0 (99.0,106.9) mmol/L. During 9304.5 person-years of follow-up, 462 patients died, of which 235 deaths were caused by cardiovascular disease. The highest quartile group was associated with a higher risk of cardiovascular mortality (adjusted hazards ratio [HR], 2.95; 95% confidence interval [CI], 1.80 to 4.95) and all-cause mortality (adjusted HR, 2.03; 95% CI, 1.45 to 2.83) compared with the lowest quartile. The similar trend was also found when serum chloride levels were deal as continuous variable.
Interpretation
Higher serum chloride at the initial of peritoneal dialysis was associated with a higher risk of cardiovascular mortality and all-cause mortality in patients on peritoneal dialysis.
Funding
This work was supported by Shanghai Municipal Health Commission (2019SY018).
Keywords: Serum chloride, Cardio-vascular mortality, All-cause mortality, Peritoneal dialysis
Research in context.
Evidence before this study
Dyschloremia is common in patients with chronic kidney disease (CKD) when the estimated glomerular filtration rate declined to less than 20% to 25% of normal. We searched PubMed in May 2021 with the terms “serum chloride [Title/Abstract]” and “dialysis [Title/Abstract]”. There were 14 articles listed, one was describing the serum chloride and pre-dialysis CKD, others were irrelevant. Thus the association between serum chloride and mortality in patients undergoing CAPD need to be evaluated.
Added value of this study
We found that the highest quartile of serum chloride group was associated with a higher risk of cardiovascular mortality (adjusted hazards ratio [HR], 2.95; 95% confidence interval [CI], 1.80 to 4.95) and all-cause mortality (adjusted HR, 2.03; 95% CI, 1.45 to 2.83) compared with the lowest quartile. Each 1-mmol/L higher baseline serum chloride was associated with 4.0% and 7.0% higher risk of cardiovascular mortality (adjusted HR, 1.07; 95% CI, 1.04 to 1.10) and all-cause mortality (adjusted HR, 1.04; 95% CI, 1.02 to 1.07), respectively. The risk posed by higher serum chloride was independent of concomitant serum sodium, potassium, or calcium levels.
Implications of all the available evidence
Serum chloride measurement is part of the routine clinical screening of CAPD patients, but we usually pay attention to the serum sodium, potassium, calcium or phosphatase levels, and ignore the serum chloride. Our findings may be potentially remind medical workers to keeps serum chloride in focus simultaneously to identify high-risk patients on CAPD. Future studies in CAPD patients should consider serum chloride as a primary or secondary endpoint.
Alt-text: Unlabelled box
1. Introduction
Chloride is the principal anion in the extracellular fluid and has a significant role in maintaining acid-base balance, osmolality, and electro-neutrality of body fluids [[1], [2], [3]]. The principal dietary chloride intake is in the form of salt, and thus nutritional deficiencies of chloride are rare. Dyschloremia is a result of therapeutic interventions or represents a pathologic process. Renal losses of serum chloride ion can develop in the clinical settings of diuretic use and gastrointestinal losses, and hypochloremia can also occur with excess water gains such as congestive heart failure [1,4,5]. Iatrogenic mechanisms, including resuscitation with chloride-rich solutions, diuretic use, and excessive water loss, are the main causes of hyperchloremia [6]. Numerous studies showed that serum chloride concentration changes were associated with an increased risk of mortality in the different population settings [[7], [8], [9], [10], [11]]. In a Belgian general population cohort of 9106 patients free of symptomatic coronary heart disease with 10-year follow-up, serum chloride <100 mmol/L was associated with a 48% higher risk of all-cause mortality [11]. In a large retrospective study of non-cardiac surgery patients, preoperative hyperchloremia and hypochloremia were associated with an increase in 90-day mortality, and preoperative hypochloremia was associated with postoperative acute kidney disease [9].
The kidney is the primary regulator of homeostasis of chloride, and renal tubular reabsorption of chloride is essential for maintaining the volume of extracellular fluid [[12], [13], [14]]. Multiple electrolyte disorders and metabolic acidosis, which have diverse deleterious effects on organ function, are common in patients with chronic kidney disease (CKD) when the estimated glomerular filtration rate (eGFR) declines to less than 20% to 25% of normal [15,16]. A multicenter, prospective, observational study with 923 Japanese pre-dialysis CKD patients reported that low serum chloride was associated with an increased risk of 3-year cardiovascular disease (CVD) events and mortality [17]. After patients received long-term dialysis, electrolyte imbalance, including serum chloride, was significantly improved [18,19]. However, there to date is no data regarding the association of these patients. Therefore, the aim of this study was to examine the association between serum chloride and mortality in patients undergoing continuous ambulatory peritoneal dialysis (CAPD).
2. Methods
2.1. Study design and participants
The manuscript adhered to STROBE guidelines. This was a retrospective cohort study of 3566 incident patients who used CAPD as the first renal replacement therapy in five tertiary hospitals in China from January 1, 2005 to March 31, 2020. The previous studies reported that patients with pre-existing CVD have a higher mortality than those without pre-existing CVD [[20], [21], [22]]. In addition, diuresis can affect chloride secretion [23]. Thus, we excluded those with pre-existing CVD or using diuretics. Based on these criteria, exclusion criteria included patients aged < 18 years, with < 3 months of follow-up, pre-existing CVD, using diuretics, missing of baseline serum chloride or other baseline covariates. The study protocol complied with the Declaration of Helsinki and had full approval from each Clinical Research Ethics Committee. The data were anonymous and the need for informed consent was therefore waived.
2.2. Data collection and measurements
Patients were advised to initiate dialysis with professional and clinical evaluation from nephrologists. In all patients, thorough medical records were reviewed by trained nurses in each dialysis center at study entry. Demographic data, comorbidities, and laboratory data at baseline were recorded. Baseline variables included age at study entry, sex, body mass index (BMI), diabetes mellitus (DM), hypertension, serum albumin, estimated glomerular filtration rate (eGFR), serum cholesterol, high-density cholesterol (HDL), low-density cholesterol (LDL), serum sodium, serum chloride, serum calcium, serum potassium, and hypersensitive C-reactive protein (hs-CRP). In China, the patient must receive the first dialysis in the hospital. Thus, most of the patient's data can be obtained within one week before the first dialysis. In order to minimize missing of data, we defined baseline as one month before the first CAPD (5.3 ± 1.2 days). All laboratory parameters from fasting blood samples were measured in the department of each tertiary hospital's laboratory. All patients received CAPD treatment. Conventional dialysis solutions (Dianeal 1.5%, 2.5%, or 4.25% dextrose; Baxter Healthcare, Guangzhou, China), Y sets, and twin bag systems were used in all CAPD patients. No patients received APD.
2.3. Follow-up and outcome measures
There was no exposure to all patients with any intervention. Patients needed to return to each center at least quarterly for an overall medical assessment. The trained nurses conducted monthly face-to-face interviews or monthly telephone interviews to assess their overall condition and related medications. The observation period for each patient lasted for ten years, or from the date of study entry to the date of death, transferring to hemodialysis, receiving renal transplantation, loss of follow-up, transferring to other dialysis centers, or the end of follow-up (31 May 2020). Patients who were lost to follow-up were censored at the date of the last examination.
The primary and secondary outcome measures were cardiovascular and all-cause mortality, respectively. We determined death causes based on medical files of admission. If patients died out of hospitals, we determined death causes according to interviewing with family members by telephone to acknowledge death's circumstances, combining with information from medical records of peritoneal dialysis centers. Cardiovascular mortality included death associated with an acute myocardial ischemic event, heart failure, hemorrhagic or thromboembolic stroke, malignant arrhythmia, and sudden cardiac death, based on the International Classification of Diseases Clinical Modification, 9th Revision. Sudden cardiac death is defined as unexpected, non-traumatic death occurring within 1 h of the onset of new or worsening symptoms (witnessed arrest) or, if un-witnessed, within 24 h of last being seen alive [24].
2.4. Statistical analysis
Means with 95% confidence interval (CI) were presented for normally distributed continuous variables and medians interquartile ranges (IQR) for skewed continuous variables. The Shapiro-Wilk test was used to assess the normality of the variables. Categorical variables were expressed as the number of patients. We use quartiles instead of others as we think this may better present the patients characteristics taking account into the sample size.
To examine the association between serum chlorine and mortality, we primarily constructed cause-specific hazard models (stcox). We then performed sub-distribution hazard (streg, distribution) models to confirm the association in the primary analysis. Non-cardiovascular mortality weas considered as a competing risk for cardiovascular mortality (stcrreg function). The main difference between the two hazard models is that patients experiencing a competing risk event remain in the risk settings in the sub-distribution hazard model, but they are removed in the cause-specific hazard models [[25], [26], [27]]. The results were presented as hazard ratios (HRs) and 95% confidence interval (CI). Cumulative observed outcomes were derived using the cumulative incidence function for a competing risk, and the difference between curves was examined using the Gray test. Restricted cubic splines (xblc) were used to examine the association between serum electrolyte as a continuous variable and the risk of mortality. In addition to the primary analysis, we further conducted multiple imputation analyses (mi) in 2512 patients who had data for serum chloride. Thus, chained equations were applied to fill in the 136 missing values of the co-variables at baseline.
We conducted subgroup analyses to evaluate subgroups' modification effects on the relationship between serum chloride and mortality. Subgroups were stratified by age (<60 or ≥60 years old), sex (men or women), DM (with or without), hypertension (with or without), malnutrition (with, albumin <36.0 or without, albumin ≥36.0 g/L), and inflammation (with, hs-CRP ≥10.0 mg/L or without, hypersensitive C-reactive protein [hs-CRP] <10.0 mg/L). Both malnutrition and inflammation were defined according to the results of a large prospective study [28]. Statistical analyses were performed using Stata 15.1. statistical software (StataCorp, College Station, TX), and a pvalue < 0.05 was considered significant.
2.5. Role of the funding sources
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Baseline characteristics
We excluded 55 patients aged < 18 years, 62 patients with less than three months of follow-up, 368 patients with pre-existing CVD, and 446 patients taking diuretics, 123 patients missing baseline serum chloride, and 136 patients missing other baseline covariates. Therefore, 2376 patients were finally included in the final primary analysis (Fig. 1).
Fig. 1.
Flow-chart of eligible and ineligible patients.
The numbers of potential and eligible patients were shown on the left side, and the reasons for in ineligibility and the numbers of ineligible patients were shown on the right side. CAPD, continuous ambulatory peritoneal dialysis; CVD, cardio-vascular disease.
Table 1 presented the characteristic of patients according to quartiles of serum chloride. The mean age was 45.9 (45.3, 46.5) years old, 50.1% were men, and 14.4% were diabetic. The median serum chloride levels were 103.0 (99.0, 106.9) mmol/L. Age was older when serum chloride was higher. There were fewer patients with DM in the lowest quartile than in higher quartiles. Levels of serum albumin, eGFR, serum sodium, and potassium were higher, and serum cholesterol, HDL, LDL, calcium, and hs-CRP were lower when serum chloride was higher. However, there was no difference in sex, body mass index (BMI), and hypertension across the quartiles.
Table 1.
Baseline characteristics stratified by quartiles of serum chloride.
| Variables | Overall | Q1(≤99.0) | Q2(99.1 to 103.0) | Q3(103.1 to 106.9) | Q4(≥107.0) |
|---|---|---|---|---|---|
| N | 2376 | 551 | 627 | 600 | 598 |
| Age, years | 45.9 (45.3 46.5) | 44.1 (42.9 45.2) | 44.9 (43.7 46.0) | 45.2 (44.1 46.4) | 49.5 (48.4 50.6) |
| Men, n (%) | 1191 (50.1%) | 262 (47.5%) | 331 (52.8%) | 312 (52.0%) | 286 (47.8%) |
| BMI, kg/m2 | 21.9 (21.8 22.0) | 21.6 (21.3 21.9) | 22.0 (21.8 22.3) | 21.9 (21.6 22.1) | 22.1 (21.8 22.3) |
| DM, n (%) | 341 (14.4%) | 61 (11.1%) | 94 (15.0%) | 85 (14.2%) | 101 (16.9%) |
| Hypertension, n (%) | 1613 (67.9%) | 353 (64.1%) | 428 (68.3%) | 420 (70.0%) | 412 (68.9%) |
| Albumin, g/L | 34.6 (34.4 34.8) | 33.7 (33.3 34.2) | 34.7 (34.3 35.1) | 34.9 (34.5 35.3) | 35.1 (34.7 35.6) |
| eGFR, mL/min/1.73m2 | 7.7 (7.6 7.8) | 6.4 (6.2 6.7) | 7.8 (7.6 8.1) | 8.1 (7.9 8.4) | 8.5 (8.2 8.8) |
| Cholesterol, mmol/L | 4.1 (4.1 4.2) | 4.2 (4.1 4.3) | 4.2 (4.1 4.3) | 4.1 (4.0 4.2) | 4.0 (3.9 4.0) |
| HDL, mmol/L | 1.1 (1.1 1.2) | 1.2 (1.1 1.2) | 1.2 (1.1 1.2) | 1.1 (1.1 1.1) | 1.1 (1.1 1.1) |
| LDL, mmol/L | 2.3 (2.3 2.3) | 2.4 (2.3 2.4) | 2.4 (2.3 2.4) | 2.3 (2.2 2.4) | 2.2 (2.1 2.3) |
| Sodium, mmol/L | 139.9 (139.8 140.1) | 137.2 (136.8 137.6) | 140.0 (139.8 140.2) | 140.8 (140.6 141.1) | 141.6 (141.3 141.8) |
| Calcium, mmol/L | 2.0 (2.0 2.0) | 2.1 (2.0 2.1) | 2.0 (2.0 2.1) | 2.0 (2.0 2.0) | 1.9 (1.9 2.0) |
| Potassium, mmol/L | 4.2 (4.2 4.2) | 3.9 (3.8 4.0) | 4.1 (4.0 4.2) | 4.3 (4.2 4.3) | 4.6 (4.5 4.6) |
| hs-CRP, mg/L | 8.2 (7.7 8.7) | 11.3 (9.8 13.0) | 7.9 (6.9 8.9) | 7.7 (6.8 8.6) | 6.9 (6.2 7.7) |
| Centers | |||||
| 1 | 335 (14.1%) | 122 (22.1%) | 106 (16.9%) | 66 (11.0%) | 41 (6.9%) |
| 2 | 424 (17.8%) | 159 (28.9%) | 109 (17.4%) | 88 (14.7%) | 68 (11.4%) |
| 3 | 1203 (50.6%) | 153 (27.8%) | 274 (43.7%) | 343 (57.2%) | 433 (72.4%) |
| 4 | 66 (2.8%) | 5 (0.9%) | 8 (1.3%) | 20 (3.3%) | 33 (5.5%) |
| 5 | 348 (14.6%) | 112 (20.3%) | 130 (20.7%) | 83 (13.8%) | 23 (3.8%) |
Continuous variates were showed as mean (95% CI). Q, quartile; BMI, body mass index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL, high-density cholesterol; LDL, low-density cholesterol; hs-CRP, high-sensitivity C-reactive protein.
3.2. Serum chloride and mortality
During 9304.5 person-years of follow-up (median 39.3 [3.0, 66.5] months), 462 (19.4%) patients died, 333 (14.0%) patients transferred to hemodialysis, 182 (7.7%) patients received renal transplantation, 28 (1.2%) patients transferred to other dialysis centers, and 53 (2.2%) patients had been the loss of follow-up. Of 462 deaths, 235 (50.9%) deaths were caused by CVD, 75 (16.2%) deaths were caused by infectious disease, 11 (2.3%) deaths were caused by malignancy, 82 (17.7%) deaths were caused by other reasons, and 59 (12.8%) deaths had unknown reasons. Deaths occurred in 79 (30.2/1000 person-years), 107 (40.4/1000 person-years), 127 (65.8/1000 person-years), and 149 (70.6/1000 person-years) patients from the lowest to the highest quartiles of serum chloride, respectively (Table 2).
Table 2.
Incidence rate of death according to quartiles of serum chloride.
| Outcomes | Overall (mmol/L) | Q1 (≤99.0) | Q2 (99.1 to 103.0) | Q3 (103.1 to 106.9) | Q4 (≥107.0) |
|---|---|---|---|---|---|
| No. of patients | 2376 | 551 | 627 | 600 | 598 |
| Person-years | 9304.5 | 2614.5 | 2650.1 | 1930.0 | 2109.9 |
| All-cause mortality | |||||
| Events | 462 | 79 | 107 | 127 | 149 |
| Events per 1000 person-years | 49.7 | 30.2 | 40.4 | 65.8 | 70.6 |
| Cardiovascular mortality | |||||
| Events | 235 | 30 | 46 | 75 | 84 |
| Events per 1000 person-years | 25.3 | 11.5 | 17.4 | 38.9 | 39.8 |
Q, quartile.
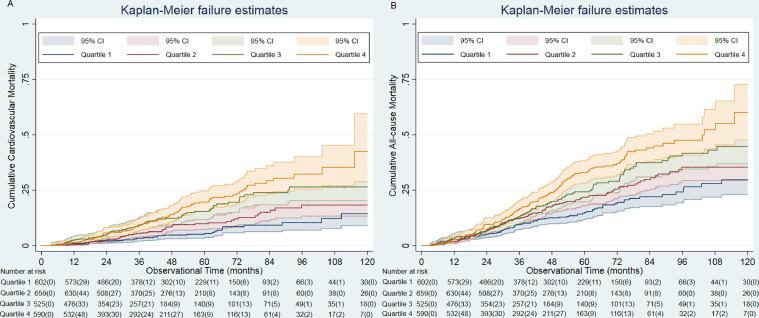
Mortality was significantly higher in the highest quartiles (p < 0.001) (Fig. 2). The cumulative incidence function of cardiovascular mortality showed a similar pattern (Supplemental Fig S1.). The unadjusted HRs (Model 1) for all-cause mortality in the cause-specific hazards model were 2.23 (95%CI, 1.69 to 2.93), 1.86 (95%CI, 1.40 to 2.46), and 1.35 (95%CI, 1.01 to 1.80) for the fourth, third, and second quartiles, respectively, compared with the first quartile (Table 3). The result was similar after adjustments for variables in Model 2. The final model (Model 3) revealed that the highest quartile of serum chloride had a 2.03-fold (95%CI, 1.45 to 2.83) higher risk of all-cause mortality than the lowest quartile. The association between serum chloride and cardiovascular mortality also showed a similar pattern (Table 3). In Model 3, the highest quartile had a 2.95-fold (95%CI, 1.80 to 4.95) higher risk of cardiovascular mortality than the lowest quartile.
Fig. 2.
Cumulative incidence of cardio-vascular and all-cause mortality according to baseline serum chloride.
Deaths occurred more as serum chloride was higher (p < 0.001, for the Gray test). Q, quartiles. Censured individual numbers in brackets.
Table 3.
Association between serum chloride and mortality using cause-specific hazard models.
| Model 1 (n = 2376) | Model 2 (n = 2376) | Model 3 (n = 2376) | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | P for trend | |||
| All-cause mortality | ||||||
| Chloride per 1-mmol/L increase | 1.05 (1.03, 1.06) | 1.03 (1.02, 1.05) | 1.04 (1.02, 1.07) | |||
| Quartiles of chloride, mmol/L | ||||||
| Q1, ≤99.0 | 1.00 | 1.00 | 1.00 | |||
| Q2, 99.1 to 103.0 | 1.35 (1.01, 1.80) | 1.33 (0.99, 1.78) | 1.41 (1.03, 1.91) | |||
| Q3, 103.1 to 106.9 | 1.86 (1.40, 2.46) | 1.80 (1.36, 2.39) | 1.71 (1.24, 2.36) | |||
| Q4, ≥107.0 | 2.23 (1.69, 2.93) | 1.82 (1.39, 2.40) | 2.03 (1.45, 2.83) | |||
| Cardiovascular mortality | ||||||
| Chloride, per 1-mmol/L increase | 1.07 (1.05, 1.10) | 1.05 (1.03, 1.08) | 1.07 (1.04, 1.10) | |||
| Quartiles of chloride, mmol/L | ||||||
| Q1, ≤99.0 | 1.00 | 1.00 | 1.00 | |||
| Q2, 99.1 to 103.0 | 1.52 (0.96, 2.41) | 1.51 (0.95, 2.39) | 1.71 (1.08, 2.71) | |||
| Q3, 103.1 to 106.9 | 2.90 (1.90, 4.43) | 2.82 (1.85, 4.31) | 2.83 (1.77, 4.52) | |||
| Q4, ≥107.0 | 3.30 (2.18, 5.01) | 2.71 (1.78, 4.11) | 2.95 (1.80, 4.95) |
Model 1: unadjusted crude HR. Model 2: adjusted for age, sex, BMI, DM, and hypertension. Model 3: model 2 plus albumin, eGFR, cholesterol, HDL, LDL, sodium, calcium, potassium, hs-CRP, and centers. Q, quartile; BMI, body mass index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; LDL, low density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; HR, hazards ratio; CI, confidence interval.
We also used the cause-specific hazard model to examine the association between serum sodium, potassium, and calcium and mortality, respectively (Supplemental Table S1, S2, and S3). We found that lower baseline serum sodium was associated with a higher risk of all-cause mortality (HR, 0.95; 95% CI, 0.93 to 0.98) and cardiovascular mortality (HR, 0.93; 95% CI, 0.90 to 0.97). Regression spline plots in Supplemental Figure S2 contrasted the risk of all-cause and cardiovascular mortality attributable to different serum electrolytes, which showed a relatively linear negative relationship between serum sodium and risk of all-cause and cardiovascular mortality.
3.3. Sensitivity analyses
To substantiate our findings, we conducted additional analyses using a multiple imputation method. After 136 missing values of baseline co-variables were imputed, there was no difference in distributions of baseline variables between the primary data and the imputed data (Supplemental Table S4). We further analyzed 2512 patients and found a robust association between higher serum chloride and increased risk of all-cause mortality and cardiovascular mortality (Supplemental Table S5). Distributions of baseline variables between the primary data and those patients without serum chloride (Supplemental Table S6) were not shown significance, which suggested that serum chloride were missed randomly.
3.4. Subgroup analyses
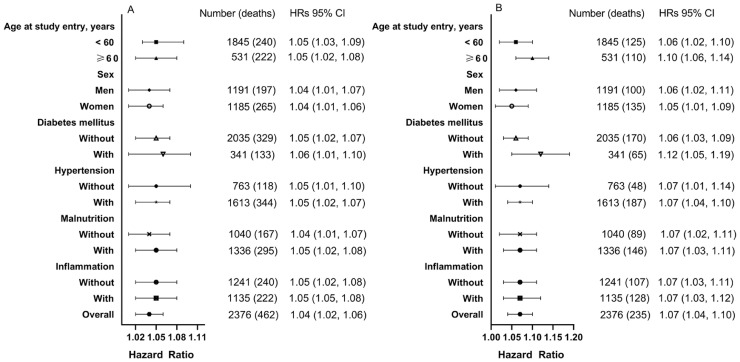
In overall patients, each 1-mmol/L higher baseline serum chloride was associated with 4.0% (95% CI, 1.02 to 1.07) higher risk of all-cause mortality and 7.0% (95% CI, 1.04 to 1.10) higher risk of cardiovascular mortality (Table 3). The associations of serum chloride and all-cause and cardiovascular mortality among subgroups had similar patterns (Fig. 3).
Fig. 3.
Subgroup association of baseline continuous serum chloride with all-cause and cardiovascular mortality.
The significant association between serum chloride and all-cause and cardiovascular mortality was seen in all subgroups. Cause-specific hazard ratios were adjusted for variables in Model 3, except for variable of subgroup. Malnutrition was defined as serum albumin <36 g/L; Inflammation was defined as hs-CPR ≥10 mg/L.
Since the study was a retrospective cohort design, we have collected as many patients’ data as possible during the study period and did not pre-estimate the sample size for this study. We made a post-hoc power analysis based on the available sample size using Power analysis based on cox regression method. An effect size of 2376 or greater was considered to be clinically significant. A post hoc statistical power calculation confirmed that the sample size available for this study provided in excess of 90% power to detect such clinically significant differences (Supplemental Materials: A post hoc statistical power calculation).
4. Discussion
In this study, we first reported that higher serum chloride at baseline was associated with a significantly increased risk of 10-year all-cause and cardiovascular mortality in patients undergoing CAPD. The risk posed by higher serum chloride was independent of concomitant serum sodium, potassium, or calcium levels, suggesting that this was a chloride-specific finding and did not reflect risks associated with other electrolyte disorders.
Based on existing evidence, the association between serum chloride and mortality is inconsistent in different research participants. In critically ill patients, numerous studies had reported that dyschloremia was associated with an increase in short-term mortality [[29], [30], [31]]. Another retrospective cohort study reported that a U-shaped relationship between serum chloride and short-term mortality among 106,505 patients >20 years of age who were performed noncardiac surgery [9]. In the general population settings, the large BIRNH cohort of 9106 participants without coronary heart disease at baseline with 10-year mortality follow-up observed a strong, independent, graded, and inverse relationship between baseline serum chloride levels and all-cause and cardiovascular mortality [11]. Another study of 12,968 hypertensive patients with follow up for 35 years also reported that low serum chloride (<100 mmol/L) is associated with more significant cardiovascular mortality and all-cause mortality risk independent of obvious confounders [32]. Consistent findings were also observed in heart failure participants [33]. In patients with pre-dialysis CKD, a similar association of serum chloride and mortality also was observed. A prospective cohort study with a median follow-up of 33 months from Japan reported that lower baseline serum chloride was associated with higher mortality and cardiovascular events in 923 pre-dialysis CKD patients [17]. The authors of this study reported that when patients were stratified into quartiles of serum chloride, the analysis showed the J-shaped association between serum chloride and mortality. However, these findings above were inconsistent with those in patients on CAPD in the present study. We found that there was an increasing linear dose-response relationship between baseline serum chloride levels and long-term all-cause and cardiovascular mortalities. A previous study showed that CKD patients were more prone to fluid and electrolyte imbalance with metabolic acidosis, compared to those with normal kidney function [15]. In more advanced CKD with high serum chloride, long-lasting therapy with sodium bicarbonate is extensively used [23]. Thus, taking sodium bicarbonate may avoid the harm of high chloride to PD patients. In addition, as all we have known, patients undergoing CAPD are more likely to be older age, more comorbidities, and disorders of laboratory variables. Therefore, inconsistent findings may be that there was the significant difference in the demographic and clinical characteristics and laboratory variables in the different population settings. Notably, the association between baseline serum sodium and mortality in our study was consistent with the findings of a large observational study, which also reported that in 4687 incident peritoneal dialysis patients, lower baseline serum sodium was independently associated with higher death risk [34].
The findings of subgroup analyses highlight the consistency in the association between serum chloride levels and risk of death from any cause and cardiovascular disease across selected cardiovascular risk factors. The strength in the association between serum chloride and the risk of death from any cause and cardiovascular disease may slightly vary by age, sex, diabetes mellitus, malnutrition, and inflammation, with the range of HRs from 1.04 to 1.07 per 1-mmol/L increase of serum chloride at the start of CAPD. These subgroup analyses further supported the association of serum chloride and mortality, suggesting that the findings were robust in our study. Nonetheless, there remained a question of whether serum chloride directly affected the prognosis or just was a marker of adverse events in CAPD patients. In addition, regression spline analyses suggested linear relationships between mortality risk and serum chloride, sodium, potassium and calcium but that this requires further investigation and analysis. Notedly, the relationships between mortality risk and both serum potassium and serum calcium cease to be significant when serum chloride is added to the hazard models - but for serum sodium. The opposite occurs whereby the previously non-significant association with mortality risk becomes statistically significant when sodium chloride is added to the hazard model. Thus, the relationship between serum chloride and serum sodium merits further investigation.
The potential mechanisms underlying the association between higher serum chloride and mortality remain to be determined. The movement of chloride ions on the cell plasma membrane involves regulating cell volume, smooth muscle cell contraction, transepithelial fluid transport, and synaptic transmission [35]. In vitro cell models had demonstrated an increased pro-inflammatory response to hyper-chloremic metabolic acidosis mediated by nitric oxide and higher interleukin-6 (IL-6) to interleukin-10 (IL-10) ratio, compared with lactic acidosis [36]. Another study also reported that hyper-chloremic metabolic acidosis in a murine sepsis model increased circulating interleukin-6, interleukin-10, and tumor necrosis factor [37].
Our study's strengths were its sizeable multicenter sample size, the restricted inclusion and exclusion criteria of the sample, and rigorous multivariate regression analyses. Nonetheless, our study had some limitations. First, as a retrospective observational study, this study cannot necessarily prove causation between serum chloride and mortality, and the exclusion of individuals (n = 123) without baseline serum chloride from our analysis may have resulted in bias. Second, although confounding variables were adjusted using rigorous multivariate regression analyses, residual confounding by unmeasured covariates cannot have been eliminated completely. Third, because only a single measurement of serum chloride was reported, bias from regression toward the mean may underestimate the associations’ strength and whether changes in serum chloride levels over time strength predictive value will need further study. Fourth, all eligible patients were from China, suggesting our findings may lack generalization to other ethnic population settings. Fifth, serum bicarbonate or sodium bicarbonate administration can influence both serum chloride and outcomes but were not collected in the present study. Changes in serum chloride occur with concurrent changes in serum sodium, potassium, and bicarbonate [32], and it is difficult to disentangle the independent effects of serum chloride on mortality. Sixth, the "young" mean age of 45.9 years and the median follow-up of 39 months may affect the precise of the association. Finally, determination of death causes based on medical files or family interviews without biopsy may lead to incorrect death classification.
In conclusion, higher serum chloride at the initial of CAPD was associated with a higher risk of all-cause and cardiovascular mortality in patients undergoing CAPD. As serum chloride measurement is part of the routine clinical screening, our findings may be potentially translatable into clinical settings to identify high-risk patients on CAPD. Future studies in CAPD patients should consider serum chloride as a primary or secondary endpoint.
Supplementary Material
Revised Supplemental Materials.docx
Contributors
The authors’ responsibilities were as follows: Lei Zhou: formal analysis, writing- original draft; Xiaoyang wang: resources, methodology; Xiaojing Zhan and Xiaoran Feng: resources, data curation; Niansong Wang: data verification; Fenen Peng and Yueqiang Wen: resources, data verification; Xianfeng Wu: conceptualization, investigation and writing-review & editing. Xinafeng Wu accessed and was responsible for the raw data associated with the study.
Data sharing statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
Funding
This work was supported by Shanghai Municipal Health Commission (2019SY018).
Declaration of Competing Interest
None.
Acknowledgements
We express our gratitude to all patients who participated in the study.
Reference
- 1.Berend K., van Hulsteijn L.H., Gans R.O. Chloride: the queen of electrolytes? Eur J Intern Med. 2012;23(3):203–211. doi: 10.1016/j.ejim.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Yunos N.M., Bellomo R., Story D., Kellum J. Bench-to-bedside review: chloride in critical illness. Crit Care. 2010;14(4):226. doi: 10.1186/cc9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langer T., Ferrari M., Zazzeron L., Gattinoni L., Caironi P. Effects of intravenous solutions on acid-base equilibrium: from crystalloids to colloids and blood components. Anaesthesiol Intensive Ther. 2014;46(5):350–360. doi: 10.5603/AIT.2014.0059. [DOI] [PubMed] [Google Scholar]
- 4.Zazzeron L., Ottolina D., Scotti E. Real-time urinary electrolyte monitoring after furosemide administration in surgical ICU patients with normal renal function. Ann Intensive Care. 2016;6(1):72. doi: 10.1186/s13613-016-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yunos N.M., Bellomo R., Hegarty C., Story D., Ho L., Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 6.Bandak G., Kashani K.B. Chloride in intensive care units: a key electrolyte. F1000Res. 2017;6:1930. doi: 10.12688/f1000research.11401.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neyra J.A., Canepa-Escaro F., Li X. Association of Hyperchloremia With Hospital Mortality in Critically Ill Septic Patients. Crit Care Med. 2015;43(9):1938–1944. doi: 10.1097/CCM.0000000000001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tani M., Morimatsu H., Takatsu F., Morita K. The incidence and prognostic value of hypochloremia in critically ill patients. Sci World J. 2012;2012 doi: 10.1100/2012/474185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh T.K., Do S.H., Jeon Y.T., Kim J., Na H.S., Hwang J.W. Association of Preoperative Serum Chloride Levels With Mortality and Morbidity After Noncardiac Surgery: a Retrospective Cohort Study. Anesth Analg. 2019;129(6):1494–1501. doi: 10.1213/ANE.0000000000003958. [DOI] [PubMed] [Google Scholar]
- 10.Oh H.J., Kim S.J., Kim Y.C. An increased chloride level in hypochloremia is associated with decreased mortality in patients with severe sepsis or septic shock. Sci Rep. 2017;7(1):15883. doi: 10.1038/s41598-017-16238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bacquer D., De Backer G., De Buyzere M., Kornitzer M. Is low serum chloride level a risk factor for cardiovascular mortality? J Cardiovasc Risk. 1998;5(3):177–184. [PubMed] [Google Scholar]
- 12.Grill A., Schiessl I.M., Gess B., Fremter K., Hammer A., Castrop H. Salt-losing nephropathy in mice with a null mutation of the Clcnk2 gene. Acta Physiol (Oxf) 2016;218(3):198–211. doi: 10.1111/apha.12755. [DOI] [PubMed] [Google Scholar]
- 13.Hennings J.C., Andrini O., Picard N. The ClC-K2 Chloride Channel Is Critical for Salt Handling in the Distal Nephron. J Am Soc Nephrol. 2017;28(1):209–217. doi: 10.1681/ASN.2016010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wall S.M., Kim Y.H., Stanley L. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl- conservation. Hypertension. 2004;44(6):982–987. doi: 10.1161/01.HYP.0000145863.96091.89. [DOI] [PubMed] [Google Scholar]
- 15.Eustace J.A., Astor B., Muntner P.M., Ikizler T.A., Coresh J. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int. 2004;65(3):1031–1040. doi: 10.1111/j.1523-1755.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- 16.Kraut J.A., Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005;45(6):978–993. doi: 10.1053/j.ajkd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Mandai S., Kanda E., Iimori S. Association of serum chloride level with mortality and cardiovascular events in chronic kidney disease: the CKD-ROUTE study. Clin Exp Nephrol. 2017;21(1):104–111. doi: 10.1007/s10157-016-1261-0. [DOI] [PubMed] [Google Scholar]
- 18.Lefebvre A., de Vernejoul M.C., Gueris J., Goldfarb B., Graulet A.M., Morieux C. Optimal correction of acidosis changes progression of dialysis osteodystrophy. Kidney Int. 1989;36(6):1112–1118. doi: 10.1038/ki.1989.309. [DOI] [PubMed] [Google Scholar]
- 19.Movilli E., Zani R., Carli O. Correction of metabolic acidosis increases serum albumin concentrations and decreases kinetically evaluated protein intake in haemodialysis patients: a prospective study. Nephrol Dial Transplant. 1998;13(7):1719–1722. doi: 10.1093/ndt/13.7.1719. [DOI] [PubMed] [Google Scholar]
- 20.Wu X., Yang X., Liu X. Patient Survival and Technique Failure in Continuous Ambulatory Peritoneal Dialysis Patients with Prior Stroke. Perit Dial Int. 2016;36(3):308–314. doi: 10.3747/pdi.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stack A.G., Molony D.A., Rahman N.S., Dosekun A., Murthy B. Impact of dialysis modality on survival of new ESRD patients with congestive heart failure in the United States. Kidney Int. 2003;64(3):1071–1079. doi: 10.1046/j.1523-1755.2003.00165.x. [DOI] [PubMed] [Google Scholar]
- 22.Ganesh S.K., Hulbert-Shearon T., Port F.K., Eagle K., Stack A.G. Mortality differences by dialysis modality among incident ESRD patients with and without coronary artery disease. J Am Soc Nephrol. 2003;14(2):415–424. doi: 10.1097/01.asn.0000043140.23422.4f. [DOI] [PubMed] [Google Scholar]
- 23.Adeva-Andany M.M., Fernandez-Fernandez C., Mourino-Bayolo D., Castro-Quintela E., Dominguez-Montero A. Sodium bicarbonate therapy in patients with metabolic acidosis. ScientificWorldJournal. 2014;2014 doi: 10.1155/2014/627673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European Heart Rhythm A. Heart Rhythm S., Zipes D.P. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) J Am Coll Cardiol. 2006;48(5):e247–e346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Noordzij M., Leffondre K., van Stralen K.J., Zoccali C., Dekker F.W., Jager K.J. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28(11):2670–2677. doi: 10.1093/ndt/gft355. [DOI] [PubMed] [Google Scholar]
- 26.Lau B., Cole S.R., Gange S.J. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z. Survival analysis in the presence of competing risks. Ann Transl Med. 2017;5(3):47. doi: 10.21037/atm.2016.08.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Coresh J., Eustace J.A. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004;291(4):451–459. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 29.McCluskey S.A., Karkouti K., Wijeysundera D., Minkovich L., Tait G., Beattie W.S. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg. 2013;117(2):412–421. doi: 10.1213/ANE.0b013e318293d81e. [DOI] [PubMed] [Google Scholar]
- 30.Van Regenmortel N., Verbrugghe W., Van den Wyngaert T., Jorens P.G. Impact of chloride and strong ion difference on ICU and hospital mortality in a mixed intensive care population. Ann Intensive Care. 2016;6(1):91. doi: 10.1186/s13613-016-0193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ditch K.L., Flahive J.M., West A.M., Osgood M.L., Hyperchloremia uehlschlegel S. Not Concomitant Hypernatremia, independently predicts early mortality in critically Ill moderate-severe traumatic brain injury patients. Neurocrit Care. 2020 doi: 10.1007/s12028-020-00928-0. [DOI] [PubMed] [Google Scholar]
- 32.McCallum L., Jeemon P., Hastie C.E. Serum chloride is an independent predictor of mortality in hypertensive patients. Hypertension. 2013;62(5):836–843. doi: 10.1161/HYPERTENSIONAHA.113.01793. [DOI] [PubMed] [Google Scholar]
- 33.Felker G.M., Allen L.A., Pocock S.J. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50(1):40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 34.Ravel V.A., Streja E., Mehrotra R. Serum sodium and mortality in a national peritoneal dialysis cohort. Nephrol Dial Transplant. 2017;32(7):1224–1233. doi: 10.1093/ndt/gfw254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duran C., Thompson C.H., Xiao Q., Hartzell H.C. Chloride channels: often enigmatic, rarely predictable. Annu Rev Physiol. 2010;72:95–121. doi: 10.1146/annurev-physiol-021909-135811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kellum J.A., Song M., Li J. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264.7 cells. Am J Physiol Regul Integr Comp Physiol. 2004;286(4):R686–R692. doi: 10.1152/ajpregu.00564.2003. [DOI] [PubMed] [Google Scholar]
- 37.Kellum J.A., Song M., Almasri E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest. 2006;130(4):962–967. doi: 10.1378/chest.130.4.962. [DOI] [PubMed] [Google Scholar]