Abstract
Reactive oxygen species (ROS), such as the superoxide anion or hydrogen peroxide, have been established over decades of research as, on the one hand, important and versatile molecules involved in a plethora of homeostatic processes and, on the other hand, as inducers of damage, pathologies and diseases. Which effects ROS induce, strongly depends on the cell type and the source, amount, duration and location of ROS production. Similar to cellular pH and calcium levels, which are both strictly regulated and only altered by the cell when necessary, the redox balance of the cell is also tightly regulated, not only on the level of the whole cell but in every cellular compartment. However, a still widespread view present in the scientific community is that the location of ROS production is of no major importance and that ROS randomly diffuse from their cellular source of production throughout the whole cell and hit their redox-sensitive targets when passing by. Yet, evidence is growing that cells regulate ROS production and therefore their redox balance by strictly controlling ROS source activation as well as localization, amount and duration of ROS production. Hopefully, future studies in the field of redox biology will consider these factors and analyze cellular ROS more specifically in order to revise the view of ROS as freely flowing through the cell.
Keywords: mitochondria, NADPH oxidases, ROS sources, reactive oxygen species, redox balance, oxidative stress, ROS probes, ROS inhibitors
Reactive Oxygen Species: Handle With Care!
Reactive oxygen species (ROS) are molecules with higher reactivity than molecular oxygen (O2) (Fenton, 1894; Haber et al., 1997; D’Autreaux and Toledano, 2007; Hatz et al., 2007; Prousek, 2007; Das and Roychoudhury, 2014; Odobasic et al., 2016; Aratani, 2018; Juan et al., 2021) and include highly reactive free radicals, such as the superoxide anion (O2–) (Hayyan et al., 2016), and non-radical species, such as hydrogen peroxide (H2O2) (Chavez et al., 2009; Odobasic et al., 2016; Aratani, 2018).
A plethora of different ROS sources, such as NADPH oxidases (Nox) (Bedard and Krause, 2007; Nakano et al., 2008; Lambeth and Neish, 2014; Gluschko et al., 2018; Wolf et al., 2020), mitochondria (Afanas’ev et al., 1999; Han et al., 2003; Muller et al., 2004; Murphy, 2009; Brand, 2010; Shoshan-Barmatz et al., 2010; West et al., 2011a,b; Bezawork-Geleta et al., 2017; Zhao et al., 2019; Hadrava Vanova et al., 2020), xanthine oxidase (Nomura et al., 2014; Al-Shehri et al., 2020), peroxisomes (Lismont et al., 2015; Fransen et al., 2017; Shai et al., 2018) and cytochrome P450 oxidases (Omura and Sato, 1962; Zhang et al., 2020) can be responsible for cellular ROS production and it highly depends on the stimulus and the cell type whether a single or multiple ROS sources are activated, for how long this occurs and for what purpose (Banfi et al., 2004; Gavazzi et al., 2006; Bedard and Krause, 2007; Aguirre and Lambeth, 2010; Brand, 2010; Dikalova et al., 2010; Donko et al., 2010; Carnesecchi et al., 2011; Al-Mehdi et al., 2012; Lanciano et al., 2013; Kim et al., 2014; Lambeth and Neish, 2014; Ives et al., 2015; Xu et al., 2017; Herb et al., 2019b; Hernansanz-Agustin et al., 2020; Herb and Schramm, 2021).
O2– is the precursor of all cellular ROS (Chavez et al., 2009; Niethammer et al., 2009) and, under physiological pH, cannot diffuse over cell membranes due to its negative charge (Takahashi and Asada, 1983; Shoshan-Barmatz et al., 2010; Cordeiro, 2014). It has a half-life of a couple of seconds or even less (Marklund, 1976; Rapoport et al., 1994; D’Autreaux and Toledano, 2007; Taverne et al., 2013). Despite being not a strong oxidizing substance by itself, O2– readily oxidizes iron-sulfur structures of proteins (Liochev and Fridovich, 1999; Imlay, 2003), which can lead to protein malfunction and iron release from proteins. The released iron reacts with H2O2 to form the highly reactive and toxic OH radical (Liochev and Fridovich, 1999). Therefore, under healthy conditions cells keep O2– levels low (∼ 10–11-10–12 M) by compartmentalization and quick removal of O2–by superoxide dismutates (D’Autreaux and Toledano, 2007; Kehrer et al., 2010; Wang et al., 2018), which are expressed in all cellular compartments (Melov et al., 1999; Lambeth and Neish, 2014; Wang et al., 2018). A sustained increase in cellular O2– levels is associated with damage to cellular structures (Davies, 2016; Pizzino et al., 2017; Sies et al., 2017; Gutteridge and Halliwell, 2018; Su et al., 2019; Juan et al., 2021). However, O2– can also contribute to cellular signaling (Chen et al., 2009; Xu et al., 2017; Ren et al., 2021).
O2– quickly dismutates to H2O2, which is more, although not freely, diffusible for cellular membranes (Bienert et al., 2006; Wang et al., 2020; Chauvigne et al., 2021), which questions saturation of the cell with H2O2 to fulfill signaling functions in compartments, which are not in direct proximity to the ROS source (Beretta et al., 2020; Sies, 2021). Communication between cellular compartments can be achieved by aquaporins, which facilitate a controlled passage of H2O2 over membranes (Bienert and Chaumont, 2014; Wang et al., 2020). H2O2 has a longer cellular half-life (∼1 ms) with concentrations of ∼10–7 M under cellular homoeostatic conditions (D’Autreaux and Toledano, 2007). Because of these properties, it functions as an important signaling molecule involved in many different cellular processes (Kamata et al., 2005; Tonks, 2005; Rhee, 2006; Marinho et al., 2013; Holmstrom and Finkel, 2014; Romero et al., 2014; Jones et al., 2016; Short et al., 2016; Zhang et al., 2016; Herb et al., 2019b; Sies and Jones, 2020; Chauvigne et al., 2021). H2O2-mediated signaling is mainly based on the oxidation of cysteine residues of proteins (Chiarugi et al., 2001; Rhee, 2006; Herscovitch et al., 2008; Romero et al., 2014; Jones et al., 2016; Short et al., 2016; Herb et al., 2019b). These cysteine residues have a low pKa, are exposed to the cytosol and deprotonated to thiolate groups (Finkel, 2011; Poole, 2015). An increase to nanomolar concentrations (∼100 nM) of H2O2 is sufficient to induce reversible oxidation. This can lead to allosteric protein changes that alter the enzymatic function of the target proteins in many ways (Lee et al., 1998; Meng et al., 2002; Kamata et al., 2005; Tonks, 2005). ROS-mediated oxidation can also lead to covalent linkage of cysteine residues by disulfide bonds (Herscovitch et al., 2008; Zhou et al., 2014; Herb et al., 2019b). Since these H2O2-mediated protein oxidations can be reversed by the antioxidant defense system, they represent important redox switches involved in various cellular processes (Barford, 2004; Holmstrom and Finkel, 2014). Excessive H2O2 production, however, leads to further oxidation of the oxidized cysteines, which is an irreversible process and results in protein malfunction (Winterbourn and Hampton, 2008).
Growing evidence indicates that the redox status in different cellular compartments varies greatly (Fransen et al., 2017; Beretta et al., 2020; Chauvigne et al., 2021; Sies, 2021; Wang et al., 2021b), is tightly regulated (Nakamura, 2005; Kirkman and Gaetani, 2007; Kelley et al., 2010; Brigelius-Flohe and Maiorino, 2013; Poljsak et al., 2013; Couto et al., 2016; Jones et al., 2016; Chauvigne et al., 2021) and every elevation of ROS levels is controlled by the cell in various ways (Babior, 2002; Bulua et al., 2011; Hoeven et al., 2011; West et al., 2011a; Gluschko et al., 2018; Herb et al., 2019b; Chauvigne et al., 2021). The condition of ROS levels exceeding the capacity of cellular antioxidant defense systems is termed oxidative stress (Niki, 2016; Sies and Jones, 2020). Oxidative stress can be further divided into two subforms: (1) Oxidative distress, which represents excessive and prolonged oxidative stress, causes damage to cellular components and results in a number of different pathologies (Cross et al., 1987; Rangasamy et al., 2005; Thimmulappa et al., 2006). Notably, excessive oxidative distress is not always detrimental, if produced at the right place. Exceeded generation of oxidative stress in pathogen-containing phagosomes of phagocytes, for example, is an important factor of antimicrobial immunity (West et al., 2011a; Winterbourn and Kettle, 2013; Gluschko et al., 2018; Herb and Schramm, 2021). (2) Oxidative eustress represents a tightly controlled increase in cellular ROS levels (Niki, 2016; Sies and Jones, 2020), which are sufficient to fulfill important cellular processes, but do not induce critical damage to cellular structures (Tai et al., 2009; Finkel, 2011; Nathan and Cunningham-Bussel, 2013; Reczek and Chandel, 2014; Herb et al., 2019b).
Unfortunately, a lot of studies, which show the important role of ROS in various cellular processes, often suggest that ROS are produced in excess, saturate the cell and react randomly with redox-sensitive targets. This is mainly due to experimental setups that might lead to misinterpretation of the location of ROS production in cells.
Many studies use only one type of ROS probe, but do not provide an explanation for the choice, such as specificity for a cellular compartment or a defined type of ROS subspecies. Often probes are used that show neither a specificity for a ROS subspecies nor a defined cellular compartment, which leads to the frequently used terms “intracellular ROS” or “total cellular ROS,” which implicate that ROS once produced are equally distributed in the cell. Common examples for diffusible ROS probes are luminol (Caldefie-Chezet et al., 2002; Pavelkova and Kubala, 2004), 2′,7′-dichlordihydrofluorescein-diacetat (H2DCF-DA) (Ushijima et al., 1997; Hempel et al., 1999; Kim and Xue, 2020; Kim et al., 2021; Wang et al., 2021a) or dihydroethidium (DHE) (Gatliff et al., 2017; Wang and Zou, 2018; Zeller et al., 2021), which are regarded as compartment-specific but in fact they are not (Lundqvist and Dahlgren, 1996; Ushijima et al., 1997; Hempel et al., 1999; Wang and Zou, 2018). There are compartment-specific derivates available for these ROS probes, namely Isoluminol (Lundqvist and Dahlgren, 1996; Dahlgren and Karlsson, 1999; Caldefie-Chezet et al., 2002; Gluschko et al., 2018; Herb et al., 2019b; Wolf et al., 2020), 5-(and −6)-carboxy-2′,7′-dihydrochlorofluorescein-diacetat (5/6-Carboxy-DCF) (Hempel et al., 1999; Mak et al., 2017; Herb et al., 2019b; Wolf et al., 2020) and MitoSOX Red (MitoSOX) (Robinson et al., 2006; Mukhopadhyay et al., 2007) as alternatives, whose combined usage gives a much more confluent picture of the cellular ROS production. The preferable option for most precise ROS measurements concerning compatibility and specificity for ROS subspecies is represented by genetically modified cells, which express the ROS probe of choice in the cellular compartment of choice, like the HyPer family reporters and roGFP2-Orp1 (Belousov et al., 2006; Gutscher et al., 2009; Markvicheva et al., 2011; Bilan et al., 2013; Hernández-Barrera et al., 2013; Wang et al., 2021b). Another precise approach for compartment-specific ROS measurements is the coupling of ROS probes to cargo/particles, which can be engulfed by cells. This technique is especially useful in phagocytes like macrophages, to determine ROS levels in the phagosome (Geng et al., 2015; Ligeon et al., 2021a,b). For further reading on topics regarding ROS detection methods we want to point out to other reviews (Ermakova et al., 2014; Herb and Schramm, 2021).
Also the combined use of only globally working ROS scavengers in combination with ROS probes that detect total cellular ROS can lead to results, which suggest that ROS are present in the whole cell after diffusion from the location of their production. With NAC as most prominent globally working ROS scavenger (Patriarca et al., 2005; Aldini et al., 2018; Ezerina et al., 2018) only the general involvement of ROS in the cellular process of interest can be investigated, but no compartment-specific ROS production can be analyzed. More examples of globally working ROS scavengers are Tempol (4-Hydroxy-Tempo) (dismutation of O2– into H2O2) (Bernardy et al., 2017; Herb et al., 2019b), Tiron (a global O2– scavenger) (Krishna et al., 1992; Hein and Kuo, 1998; Manzano et al., 2000), Trolox (globally scavenges OOH and OOR) (Davies et al., 1988; Dugas et al., 2000) and ebselen (effectively removes H2O2 and ONOO–) (Nakamura et al., 2002; Matsushita et al., 2004; Mugesh, 2013). All of the scavengers mentioned above are diffusible (Davies et al., 1988; Krishna et al., 1992; Hein and Kuo, 1998; Haj-Yehia et al., 1999; Dugas et al., 2000; Manzano et al., 2000; Rak et al., 2000; Nakamura et al., 2002; Matsushita et al., 2004; Mugesh, 2013; Herb and Schramm, 2021). Assessment of specific removal of ROS subspecies and therefore their involvement in cellular processes is possible with these substances, but they cannot be used to identify the specific compartment in which the ROS exert their function.
Not only the location of ROS production, but also their various sources and their activation, regulation and termination is of major importance for the understanding of the complex redox maintenance in cells. For the identification of ROS sources it is not always possible to provide genetic evidence with a knock-out system or by siRNA usage. ROS source inhibitors are in these cases an option to block ROS production and analyze possible ROS sources. There are a lot of specific ROS source inhibitors commercially available and the choice is continuously expanded (Murphy, 2009; Wind et al., 2010; Altenhofer et al., 2015; Herb and Schramm, 2021).
For Nox enzymes, as one of the most prominent ROS sources in many cell types, the well-validated general Nox inhibitors VAS2870 (Leusen et al., 1995; ten Freyhaus et al., 2006; Wind et al., 2010; Altenhofer et al., 2012, 2015) or GKT 137831 (Laleu et al., 2010; Sedeek et al., 2010; Gaggini et al., 2011; Aoyama et al., 2012; Strengert et al., 2014; Kim et al., 2021) can be used. Both inhibitors show no intrinsic antioxidant activity and do not inhibit other flavoproteins (Wind et al., 2010; Altenhofer et al., 2012; Teixeira et al., 2017). Both also inhibit Nox-derived ROS production in vitro and in vivo (Carnesecchi et al., 2009, 2011; Aoyama et al., 2012; Green et al., 2012; Bettaieb et al., 2015; Gorin et al., 2015). In sharp contrast to VAS2870 and GKT 137831, the substances apocynin and DPI are still used and falsely addressed as specific Nox inhibitors in many otherwise convincing and excellent studies (Barbieri et al., 2003; Kiritoshi et al., 2003; Dostert et al., 2008; Choi et al., 2011; Abuaita et al., 2015; Gatliff et al., 2017; Alonso et al., 2019; Fan et al., 2019; Damiano et al., 2020; Geng et al., 2020; Inomata et al., 2020; Prestes et al., 2020; Ahmad et al., 2021; Ligeon et al., 2021b; Martinez et al., 2021 #1039; Troia et al., 2021). Several studies have shown that apocynin directly scavenges ROS due to its antioxidant capacities (Aldieri et al., 2008; Heumuller et al., 2008; Mora-Pale et al., 2009; Wingler et al., 2011; Trevelin et al., 2016), while DPI inhibits flavoproteins in general (O’Donnell et al., 1993; Wind et al., 2010; Altenhofer et al., 2015) including Nox2 (Reis et al., 2020), but also various other targets, such as complex I of the mitochondrial electron transport chain (Bloxham, 1979; Lambeth et al., 2008; Bulua et al., 2011), iNOS (Stuehr et al., 1991; Geyer et al., 1997) or xanthine oxidase (O’Donnell et al., 1993; Wind et al., 2010) as well as calcium transporters (Tazzeo et al., 2009). Since genetic knock-out models with the CRISPR-Cas9 technology (Ledford, 2015; Anzalone et al., 2020; Carlson-Stevermer et al., 2020), either for cell lines, ex vivo cells or mice, as well as knock-down via siRNA (Han, 2018) are readily available tools for analyzing possible roles of Nox enzymes in cellular processes, the use of apocynin or DPI, especially in combination with diffusible ROS probes, should not be recommended, since it may lead to false interpretations of results regarding Nox enzyme involvement and the location of ROS production.
In mitochondria, the complexes of the ETC not only are essential for energy generation of the cell, but are also ROS production sites (Nohl et al., 2003; Lambeth and Neish, 2014). Inhibition of the complexes for analysis of ROS production might also result in energy deprivation and the energy status of the cell has to be checked every time these inhibitors are used. Typically used inhibitors are rotenone (Stowe and Camara, 2009; Heinz et al., 2017; Scialo et al., 2017), which inhibits complex I and increases ROS production inside the mitochondrial matrix (St-Pierre et al., 2002; Lambert and Brand, 2004; Panov et al., 2005; Stowe and Camara, 2009; Sena et al., 2013) and antimycin A (Murphy, 2009; Bleier and Drose, 2013), which inhibits complex III and increases ROS production into the intermembrane space (IMS) (Chen et al., 2003; Han et al., 2003; Al-Mehdi et al., 2012; Quinlan et al., 2012; Herb et al., 2019a). The most commonly used ROS probe for detection of mitochondrial ROS is MitoSOX, which measures O2– exclusively inside the mitochondrial matrix (Robinson et al., 2006; Mukhopadhyay et al., 2007; Ernst et al., 2021). However, since the ETC complexes show compartment-specific differences concerning ROS production (Fridovich, 1997; Murphy, 2009; Brand, 2010; West et al., 2011b; Herb and Schramm, 2021), this probe can only be used to measure ROS production inside mitochondria and, therefore, other cellular compartments should always be analyzed in addition. In healthy, undamaged mitochondria, ROS cannot escape the mitochondrial matrix because of the very effective antioxidative defense system (Roca and Ramakrishnan, 2013; Briston et al., 2017; Hos et al., 2017; Hernansanz-Agustin et al., 2020; Lin et al., 2020; Wang et al., 2020; Zhao et al., 2020). Only after prolonged overproduction or when the structure of the mitochondrial membranes is ruptured, either by opening of the mitochondrial permeability transition pore or direct damage, e.g., by pathogenic toxins, ROS can escape from the matrix into the cytosol (Koterski et al., 2005; Stavru et al., 2011; Roca and Ramakrishnan, 2013; Briston et al., 2017; Hos et al., 2017; Zhang Y. et al., 2019; Zhao et al., 2020). Nevertheless, the general term “mitochondrial ROS” is used in many studies, which often is synonymous for matrix-located mitochondrial ROS production measured by MitoSOX. ROS measurements in other cellular compartments as well as an explanation if and how the mitochondrial ROS escape from the matrix and fulfill their role in the cell, with a few exceptions (Koterski et al., 2005; Zhou et al., 2011; Roca and Ramakrishnan, 2013; Briston et al., 2017; Hos et al., 2017; Herb et al., 2019b; Zhao et al., 2020), are often not provided. Additionally, the usage of inhibitors of the ETC, like rotenone or antimycin A, which have compartment-specific effects on ROS production in combination with diffusible ROS probes can also lead to misinterpretations of the performed ROS measurements. For further reading concerning ROS scavengers and inhibitors, we like to point to other reviews (Wind et al., 2010; Altenhofer et al., 2015; Herb and Schramm, 2021).
ROS Production: The Dose Makes the Poison
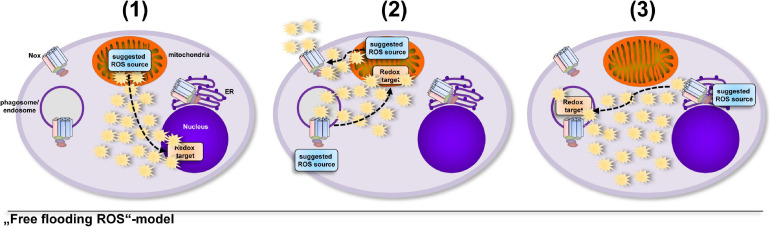
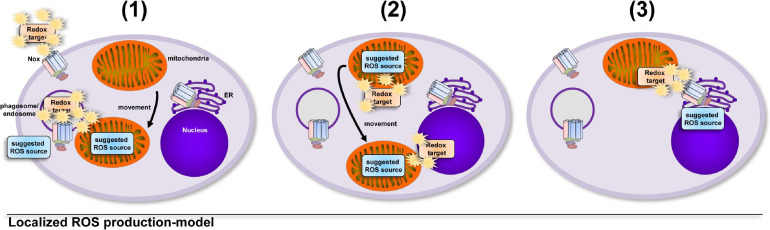
A model that involves an uncontrolled increase in total cellular ROS levels implies that cells take into account the collateral damage that ROS can inflict while enroute to their redox-sensitive target, that can be at a completely different cellular location (Bulua et al., 2011; Nazarewicz et al., 2013; Kelly et al., 2015; Garaude et al., 2016; Kim et al., 2017; Figure 1). But oxidative distress (Buczynski et al., 2013; Li et al., 2013; Bhattacharyya et al., 2014; Niki, 2016; Sweeney and McAuley, 2016; Yang et al., 2016; Sies and Jones, 2020) is a situation for the healthy cell that has to be avoided. Tightly controlled production of ROS in direct vicinity of a redox-sensitive target (Tai et al., 2009; Finkel, 2011; Nathan and Cunningham-Bussel, 2013; Reczek and Chandel, 2014; Herb et al., 2019b) requires much less ROS production and hence results in much less collateral damage, while fulfilling important cellular functions, i.e., oxidative eustress (Niki, 2016; Sies and Jones, 2020; Figure 2).
FIGURE 1.
Several studies suggested that ROS are produced in excess, saturate the cell and find their redox-sensitive targets at random. Usage of diffusible ROS probes, globally working ROS scavengers and unspecific inhibitors often place the suggested ROS source at a completely different location than the redox-sensitive target, which might lead to the interpretation that cells “take into account” the damage that ROS can inflict on their way to the target molecule. The importance of ROS in general for various cellular processes was shown by many excellent studies (Bulua et al., 2011; Nazarewicz et al., 2013; Kelly et al., 2015; Garaude et al., 2016; Kim et al., 2017), however, diffusible ROS probes or only one ROS probe are often used to determine ROS production in cells, which might lead to the suggestions, e.g., that (1) ROS escape from the mitochondrial matrix and regulate expression and secretion of cytokines (Bulua et al., 2011; Kelly et al., 2015), (2) extracellular Nox2-derived ROS modify enzyme activity in the mitochondrial matrix or matrix-derived ROS modulate Nox2 activity (Nazarewicz et al., 2013; Garaude et al., 2016) or (3) ROS produced by ER-located Nox4 reach the phagosome for inactivation of phagocytosed parasites (Kim et al., 2017).
FIGURE 2.
Growing evidence supports the hypothesis that cellular compartments show big differences and tight regulation of their redox status. The induction of ROS production is controlled by the cell in terms of location, source, duration and amount. The localized and timely controlled ROS production in the direct vicinity of the redox-sensitive target reduces the induced damage to cellular components and results in beneficial consequences for the cell, a condition termed as oxidative eustress (Sies, 2021). Examples of localized ROS production are (1) the production of antimicrobial ROS by Nox2 (Craig and Slauch, 2009; Gluschko et al., 2018) or mitochondria, which are recruited to pathogen-containing phagosomes (West et al., 2011a; Geng et al., 2015), (2) the recruitment of the redox-regulated target to ROS-producing mitochondria for NLRP3 inflammasome activation (Zhou et al., 2011) or the relocation of mitochondria to the nucleus for ROS-mediated nuclear signaling (Al-Mehdi et al., 2012) and (3) ROS production by ER-localized Nox4 during formation of mitochondria-associated membranes for regulation of calcium signaling (Beretta et al., 2020).
Of note, to reach levels at which the ROS can fulfill their important cellular roles, ROS production has to overcome the highly effective antioxidative defense systems of one or more cellular compartments (Nakamura, 2005; Kirkman and Gaetani, 2007; Kelley et al., 2010; Brigelius-Flohe and Maiorino, 2013; Poljsak et al., 2013; Lismont et al., 2015; Couto et al., 2016; Jones et al., 2016; Shai et al., 2018; Chauvigne et al., 2021; Herb and Schramm, 2021). Also H2O2-detoxifying enzymes, such as glutathione peroxidases or catalase, which can quickly decrease cellular H2O2 concentrations (Winterbourn and Hampton, 2008) as well as the inactivation of these scavenger enzymes by H2O2 itself broaden the regulatory potential of cells for selective and localized ROS signaling (Wood et al., 2003; Woo et al., 2010). This further places the model of ROS molecules, which “flood” the whole cell from a single location of ROS production in rather unrealistic light. Nevertheless, the model of ROS as “flooding” the cell is still popular in the scientific community, often referred to as “total cellular ROS levels” (Wang et al., 2013, 2019; Dinakar et al., 2016; Kim and Xue, 2020; Loth et al., 2020; Thorne et al., 2021), “intracellular ROS levels” (Tepel et al., 2000; Bensaad et al., 2009; Choi et al., 2011; Lee et al., 2017; Wang et al., 2019, 2021a; Wei et al., 2019; Zaidieh et al., 2019; Zhang W. et al., 2019; Mendiola et al., 2020; Winitchaikul et al., 2021; Zhong et al., 2021) or simply “ROS levels” (Chen et al., 2012, 2021; Wei et al., 2019; Agarwal and Ganesh, 2020; Kim et al., 2021; Knight et al., 2021; Zeller et al., 2021) in many studies, mainly because of the usage of diffusible ROS probes, which suggest free diffusion of ROS through the cell without regard of the location of ROS production. Mutations, e.g., in cancer cells (Schumacker, 2006; Liou and Storz, 2010; Reczek and Chandel, 2017; Zaidieh et al., 2019; Perillo et al., 2020), pathogenic invasion (West et al., 2011a; Abuaita et al., 2018; Gluschko et al., 2018; Roca et al., 2019) or metabolic disbalance (Li et al., 2016; Mak et al., 2017; Peng et al., 2021) are prominent examples, in which the ROS production of the cell can enter an uncontrolled stage and quickly overcome the antioxidative defense system leading to rapidly increased ROS levels in nearly every compartment of the cell with often detrimental consequences. In this context, cells can be regarded as “overflowing with ROS,” however, in healthy cells a redox balance between all producing and eliminating ROS sources, mediated by the antioxidant defense system is crucial for cellular functioning (Nathan and Cunningham-Bussel, 2013; Deshmukh et al., 2017; Suzuki et al., 2019; Wei et al., 2019; Baird and Yamamoto, 2020; Saito and Kimura, 2021). Importantly, in the extracellular milieu H2O2 can travel much further than inside the cell and fulfills important signaling (Levine et al., 1994; Sharma et al., 2012; Das and Roychoudhury, 2014; Hervera et al., 2018; Huang et al., 2019; Janku et al., 2019; Deng et al., 2020) and chemotactic functions (Niethammer et al., 2009; Rieger and Sagasti, 2011).
Concluding Remarks
In recent years, more and more studies supported the model–and highlighted the importance–of localized cellular ROS production in direct vicinity of the redox target (Meinhard and Grill, 2001; Veal et al., 2007; Go and Jones, 2008; Craig and Slauch, 2009; West et al., 2011a; Wink et al., 2011; Zhou et al., 2011; Al-Mehdi et al., 2012; Naviaux, 2012; Allan et al., 2014; Romero et al., 2014; Geng et al., 2015; To et al., 2017; Gluschko et al., 2018; Herb et al., 2019b; Acin-Perez et al., 2020; Beretta et al., 2020; Chanin et al., 2020; Miller et al., 2020; Sies and Jones, 2020; Herb and Schramm, 2021; Ligeon et al., 2021b; Sies, 2021; Wong et al., 2021). Therefore, the model of ROS molecules as “omnipresent and freely diffusing throughout the cell” should always be interpreted carefully in the context of research and highly depends on the proper use of ROS probes, scavengers and inhibitors. In healthy cells, ROS should be considered as molecules, whose production is tightly controlled in terms of stimulus, source, location, duration and amount.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MH and MS: conceptualization. MH: writing–original draft preparation and visualization. MH, AG, and MS: writing–review and editing and funding acquisition. MS: supervision and project administration. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This work was supported by the Deutsche Forschungsgemeins- chaft (DFG) grant SCHR 1627/2-1 to MS, by the Köln Fortune grant 278/2019 to AG, and by the Köln Fortune grant 302/2020 to MH.
References
- Abuaita B. H., Burkholder K. M., Boles B. R., O’Riordan M. X. (2015). The endoplasmic reticulum stress sensor inositol-requiring enzyme 1alpha augments bacterial killing through sustained oxidant production. mBio 6:e00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuaita B. H., Schultz T. L., O’Riordan M. X. (2018). Mitochondria-derived vesicles deliver antimicrobial reactive oxygen species to control phagosome-localized Staphylococcus aureus. Cell Host Microbe 24 625–636.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acin-Perez R., Iborra S., Marti-Mateos Y., Cook E. C. L., Conde-Garrosa R., Petcherski A., et al. (2020). Fgr kinase is required for proinflammatory macrophage activation during diet-induced obesity. Nat. Metab. 2 974–988. 10.1038/s42255-020-00273-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afanas’ev I. B., Ostrachovitch E. A., Korkina L. G. (1999). Lucigenin is a mediator of cytochrome C reduction but not of superoxide production. Arch. Biochem. Biophys. 366 267–274. 10.1006/abbi.1999.1215 [DOI] [PubMed] [Google Scholar]
- Agarwal S., Ganesh S. (2020). Perinuclear mitochondrial clustering, increased ROS levels, and HIF1 are required for the activation of HSF1 by heat stress. J. Cell Sci. 133:jcs245589. [DOI] [PubMed] [Google Scholar]
- Aguirre J., Lambeth J. D. (2010). Nox enzymes from fungus to fly to fish and what they tell us about Nox function in mammals. Free Radic. Biol. Med. 49 1342–1353. 10.1016/j.freeradbiomed.2010.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Nawaz M. I., Siddiquei M. M., Abu El-Asrar A. M. (2021). Apocynin ameliorates NADPH oxidase 4 (NOX4) induced oxidative damage in the hypoxic human retinal Muller cells and diabetic rat retina. Mol. Cell. Biochem. 476 2099–2109. 10.1007/s11010-021-04071-y [DOI] [PubMed] [Google Scholar]
- Aldieri E., Riganti C., Polimeni M., Gazzano E., Lussiana C., Campia I., et al. (2008). Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr. Drug Metab. 9 686–696. 10.2174/138920008786049285 [DOI] [PubMed] [Google Scholar]
- Aldini G., Altomare A., Baron G., Vistoli G., Carini M., Borsani L., et al. (2018). N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic. Res. 52 751–762. 10.1080/10715762.2018.1468564 [DOI] [PubMed] [Google Scholar]
- Allan E. R., Tailor P., Balce D. R., Pirzadeh P., Mckenna N. T., Renaux B., et al. (2014). NADPH oxidase modifies patterns of MHC class II-restricted epitopic repertoires through redox control of antigen processing. J. Immunol. 192 4989–5001. 10.4049/jimmunol.1302896 [DOI] [PubMed] [Google Scholar]
- Al-Mehdi A. B., Pastukh V. M., Swiger B. M., Reed D. J., Patel M. R., Bardwell G. C., et al. (2012). Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal. 5:ra47. 10.1126/scisignal.2002712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso D., Serrano E., Bermejo F. J., Corral R. S. (2019). HIF-1alpha-regulated MIF activation and Nox2-dependent ROS generation promote Leishmania amazonensis killing by macrophages under hypoxia. Cell. Immunol. 335 15–21. 10.1016/j.cellimm.2018.10.007 [DOI] [PubMed] [Google Scholar]
- Al-Shehri S. S., Duley J. A., Bansal N. (2020). Xanthine oxidase-lactoperoxidase system and innate immunity: biochemical actions and physiological roles. Redox Biol. 34:101524. 10.1016/j.redox.2020.101524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenhofer S., Kleikers P. W., Radermacher K. A., Scheurer P., Rob Hermans J. J., Schiffers P., et al. (2012). The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell. Mol. Life Sci. 69 2327–2343. 10.1007/s00018-012-1010-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenhofer S., Radermacher K. A., Kleikers P. W., Wingler K., Schmidt H. H. (2015). Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid. Redox Signal. 23 406–427. 10.1089/ars.2013.5814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone A. V., Koblan L. W., Liu D. R. (2020). Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38 824–844. 10.1038/s41587-020-0561-9 [DOI] [PubMed] [Google Scholar]
- Aoyama T., Paik Y. H., Watanabe S., Laleu B., Gaggini F., Fioraso-Cartier L., et al. (2012). Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56 2316–2327. 10.1002/hep.25938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratani Y. (2018). Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 640 47–52. 10.1016/j.abb.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Babior B. M. (2002). The activity of leukocyte NADPH oxidase: regulation by p47PHOX cysteine and serine residues. Antioxid. Redox Signal. 4 35–38. 10.1089/152308602753625834 [DOI] [PubMed] [Google Scholar]
- Baird L., Yamamoto M. (2020). The molecular mechanisms regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 40:e00099-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi B., Malgrange B., Knisz J., Steger K., Dubois-Dauphin M., Krause K. H. (2004). NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 279 46065–46072. 10.1074/jbc.m403046200 [DOI] [PubMed] [Google Scholar]
- Barbieri S. S., Eligini S., Brambilla M., Tremoli E., Colli S. (2003). Reactive oxygen species mediate cyclooxygenase-2 induction during monocyte to macrophage differentiation: critical role of NADPH oxidase. Cardiovasc. Res. 60 187–197. 10.1016/s0008-6363(03)00365-1 [DOI] [PubMed] [Google Scholar]
- Barford D. (2004). The role of cysteine residues as redox-sensitive regulatory switches. Curr. Opin. Struct. Biol. 14 679–686. 10.1016/j.sbi.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Bedard K., Krause K. H. (2007). The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87 245–313. 10.1152/physrev.00044.2005 [DOI] [PubMed] [Google Scholar]
- Belousov V. V., Fradkov A. F., Lukyanov K. A., Staroverov D. B., Shakhbazov K. S., Terskikh A. V., et al. (2006). Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 3 281–286. 10.1038/nmeth866 [DOI] [PubMed] [Google Scholar]
- Bensaad K., Cheung E. C., Vousden K. H. (2009). Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 28 3015–3026. 10.1038/emboj.2009.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta M., Santos C. X., Molenaar C., Hafstad A. D., Miller C. C., Revazian A., et al. (2020). Nox4 regulates InsP3 receptor-dependent Ca(2+) release into mitochondria to promote cell survival. EMBO J. 39:e103530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardy C. C. F., Zarpelon A. C., Pinho-Ribeiro F. A., Calixto-Campos C., Carvalho T. T., Fattori V., et al. (2017). Tempol, a superoxide dismutase mimetic agent, inhibits superoxide anion-induced inflammatory pain in mice. Biomed Res. Int. 2017:9584819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettaieb A., Jiang J. X., Sasaki Y., Chao T. I., Kiss Z., Chen X., et al. (2015). Hepatocyte Nicotinamide Adenine Dinucleotide Phosphate Reduced Oxidase 4 regulates stress signaling, fibrosis, and insulin sensitivity during development of steatohepatitis in mice. Gastroenterology 149 468–480.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezawork-Geleta A., Rohlena J., Dong L., Pacak K., Neuzil J. (2017). Mitochondrial complex II: at the crossroads. Trends Biochem. Sci. 42 312–325. 10.1016/j.tibs.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Sinha K., Sil P. C. (2014). Cytochrome P450s: mechanisms and biological implications in drug metabolism and its interaction with oxidative stress. Curr. Drug 15 719–742. 10.2174/1389200215666141125121659 [DOI] [PubMed] [Google Scholar]
- Bienert G. P., Chaumont F. (2014). Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 1840 1596–1604. 10.1016/j.bbagen.2013.09.017 [DOI] [PubMed] [Google Scholar]
- Bienert G. P., Schjoerring J. K., Jahn T. P. (2006). Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 1758 994–1003. [DOI] [PubMed] [Google Scholar]
- Bilan D. S., Pase L., Joosen L., Gorokhovatsky A. Y., Ermakova Y. G., Gadella T. W., et al. (2013). HyPer-3: a genetically encoded H(2)O(2) probe with improved performance for ratiometric and fluorescence lifetime imaging. ACS Chem. Biol. 8 535–542. 10.1021/cb300625g [DOI] [PubMed] [Google Scholar]
- Bleier L., Drose S. (2013). Superoxide generation by complex III: from mechanistic rationales to functional consequences. Biochim. Biophys. Acta 1827 1320–1331. 10.1016/j.bbabio.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Bloxham D. P. (1979). The relationship of diphenyleneiodonium-induced hypoglycaemia to the specific covalent modification of NADH-ubiquinone oxidoreductase. Biochem. Soc. Trans. 7 103–106. 10.1042/bst0070103 [DOI] [PubMed] [Google Scholar]
- Brand M. D. (2010). The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 45 466–472. 10.1016/j.exger.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohe R., Maiorino M. (2013). Glutathione peroxidases. Biochim. Biophys. Acta 1830 3289–3303. [DOI] [PubMed] [Google Scholar]
- Briston T., Roberts M., Lewis S., Powney B., Staddon J. M., Szabadkai G., et al. (2017). Mitochondrial permeability transition pore: sensitivity to opening and mechanistic dependence on substrate availability. Sci. Rep. 7:10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski B. W., Maduekwe E. T., O’reilly M. A. (2013). The role of hyperoxia in the pathogenesis of experimental BPD. Semin. Perinatol. 37 69–78. 10.1053/j.semperi.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulua A. C., Simon A., Maddipati R., Pelletier M., Park H., Kim K. Y., et al. (2011). Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 208 519–533. 10.1084/jem.20102049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldefie-Chezet F., Walrand S., Moinard C., Tridon A., Chassagne J., Vasson M. P. (2002). Is the neutrophil reactive oxygen species production measured by luminol and lucigenin chemiluminescence intra or extracellular? Comparison with DCFH-DA flow cytometry and cytochrome c reduction. Clin. Chim. Acta 319 9–17. 10.1016/s0009-8981(02)00015-3 [DOI] [PubMed] [Google Scholar]
- Carlson-Stevermer J., Kelso R., Kadina A., Joshi S., Rossi N., Walker J., et al. (2020). CRISPRoff enables spatio-temporal control of CRISPR editing. Nat. Commun. 11:5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnesecchi S., Deffert C., Donati Y., Basset O., Hinz B., Preynat-Seauve O., et al. (2011). A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid. Redox Signal. 15 607–619. 10.1089/ars.2010.3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnesecchi S., Deffert C., Pagano A., Garrido-Urbani S., Metrailler-Ruchonnet I., Schappi M., et al. (2009). NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am. J. Respir. Crit. Care Med. 180 972–981. 10.1164/rccm.200902-0296oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanin R. B., Winter M. G., Spiga L., Hughes E. R., Zhu W., Taylor S. J., et al. (2020). Epithelial-derived reactive Oxygen Species enable AppBCX-mediated aerobic respiration of Escherichia coli during intestinal inflammation. Cell Host Microbe 28 780–788.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvigne F., Ducat C., Ferre A., Hansen T., Carrascal M., Abian J., et al. (2021). A multiplier peroxiporin signal transduction pathway powers piscine spermatozoa. Proc. Natl. Acad. Sci. U.S.A. 118:e2019346118. 10.1073/pnas.2019346118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez V., Mohri-Shiomi A., Garsin D. A. (2009). Ce-Duox1/BLI-3 generates reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans. Infect. Immun. 77 4983–4989. 10.1128/iai.00627-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Mahar R., Merritt M. E., Denlinger D. L., Hahn D. A. (2021). ROS and hypoxia signaling regulate periodic metabolic arousal during insect dormancy to coordinate glucose, amino acid, and lipid metabolism. Proc. Natl. Acad. Sci. U.S.A. 118:e2017603118. 10.1073/pnas.2017603118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. C., Su L. T., Gonzalez-Pagan O., Overton J. D., Runnels L. W. (2012). A key role for Mg(2+) in TRPM7’s control of ROS levels during cell stress. Biochem. J. 445 441–448. 10.1042/bj20120248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Vazquez E. J., Moghaddas S., Hoppel C. L., Lesnefsky E. J. (2003). Production of reactive oxygen species by mitochondria: central role of complex III. J. Biol. Chem. 278 36027–36031. [DOI] [PubMed] [Google Scholar]
- Chen Y., Azad M. B., Gibson S. B. (2009). Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 16 1040–1052. 10.1038/cdd.2009.49 [DOI] [PubMed] [Google Scholar]
- Chiarugi P., Fiaschi T., Taddei M. L., Talini D., Giannoni E., Raugei G., et al. (2001). Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J. Biol. Chem. 276 33478–33487. 10.1074/jbc.m102302200 [DOI] [PubMed] [Google Scholar]
- Choi J., Ifuku M., Noda M., Guilarte T. R. (2011). Translocator protein (18 kDa)/peripheral benzodiazepine receptor specific ligands induce microglia functions consistent with an activated state. Glia 59 219–230. 10.1002/glia.21091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro R. M. (2014). Reactive oxygen species at phospholipid bilayers: distribution, mobility and permeation. Biochim. Biophys. Acta 1838 438–444. 10.1016/j.bbamem.2013.09.016 [DOI] [PubMed] [Google Scholar]
- Couto N., Wood J., Barber J. (2016). The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 95 27–42. 10.1016/j.freeradbiomed.2016.02.028 [DOI] [PubMed] [Google Scholar]
- Craig M., Slauch J. M. (2009). Phagocytic superoxide specifically damages an extracytoplasmic target to inhibit or kill Salmonella. PLoS One 4:e4975. 10.1371/journal.pone.0004975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., et al. (1987). Oxygen radicals and human disease. Ann. Intern. Med. 107 526–545. 10.7326/0003-4819-107-4-526 [DOI] [PubMed] [Google Scholar]
- Dahlgren C., Karlsson A. (1999). Respiratory burst in human neutrophils. J. Immunol. Methods 232 3–14. 10.1016/s0022-1759(99)00146-5 [DOI] [PubMed] [Google Scholar]
- Damiano S., Sozio C., La Rosa G., Santillo M. (2020). NOX-dependent signaling dysregulation in severe COVID-19: clues to effective treatments. Front. Cell. Infect. Microbiol. 10:608435. 10.3389/fcimb.2020.608435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K., Roychoudhury A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2:53. 10.3389/fenvs.2014.00053 [DOI] [Google Scholar]
- D’Autreaux B., Toledano M. B. (2007). ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8 813–824. 10.1038/nrm2256 [DOI] [PubMed] [Google Scholar]
- Davies K. J. (2016). Adaptive homeostasis. Mol. Aspects Med. 49 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. J., Forni L. G., Willson R. L. (1988). Vitamin E analogue Trolox C. E.s.r. and pulse-radiolysis studies of free-radical reactions. Biochem. J. 255 513–522. [PMC free article] [PubMed] [Google Scholar]
- Deng H., Yang W., Zhou Z., Tian R., Lin L., Ma Y., et al. (2020). Targeted scavenging of extracellular ROS relieves suppressive immunogenic cell death. Nat. Commun. 11:4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh P., Unni S., Krishnappa G., Padmanabhan B. (2017). The Keap1-Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 9 41–56. 10.1007/s12551-016-0244-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalova A. E., Gongora M. C., Harrison D. G., Lambeth J. D., Dikalov S., Griendling K. K. (2010). Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am. J. Physiol. Heart Circ. Physiol. 299 H673–H679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinakar C., Vishwakarma A., Raghavendra A. S., Padmasree K. (2016). Alternative oxidase pathway optimizes photosynthesis during osmotic and temperature stress by regulating cellular ROS, malate valve and antioxidative systems. Front. Plant Sci. 7:68. 10.3389/fpls.2016.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donko A., Ruisanchez E., Orient A., Enyedi B., Kapui R., Peterfi Z., et al. (2010). Urothelial cells produce hydrogen peroxide through the activation of Duox1. Free Radic. Biol. Med. 49 2040–2048. 10.1016/j.freeradbiomed.2010.09.027 [DOI] [PubMed] [Google Scholar]
- Dostert C., Petrilli V., Van Bruggen R., Steele C., Mossman B. T., Tschopp J. (2008). Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320 674–677. 10.1126/science.1156995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas A. J., Jr., Castaneda-Acosta J., Bonin G. C., Price K. L., Fischer N. H., Winston G. W. (2000). Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: structure-activity relationships. J. Nat. Prod. 63 327–331. 10.1021/np990352n [DOI] [PubMed] [Google Scholar]
- Ermakova Y. G., Bilan D. S., Matlashov M. E., Mishina N. M., Markvicheva K. N., Subach O. M., et al. (2014). Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat. Commun. 5:5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst O., Sun J., Lin B., Banoth B., Dorrington M. G., Liang J., et al. (2021). A genome-wide screen uncovers multiple roles for mitochondrial nucleoside diphosphate kinase D in inflammasome activation. Sci. Signal. 14:eabe0387. 10.1126/scisignal.abe0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezerina D., Takano Y., Hanaoka K., Urano Y., Dick T. P. (2018). N-Acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and Sulfane Sulfur Production. Cell Chem. Biol. 25 447–459.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L. M., Geng L., Cahill-Smith S., Liu F., Douglas G., Mckenzie C. A., et al. (2019). Nox2 contributes to age-related oxidative damage to neurons and the cerebral vasculature. J. Clin. Invest. 129 3374–3386. 10.1172/jci125173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton H. J. H. (1894). LXXIII.—Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 65 899–910. 10.1039/ct8946500899 [DOI] [Google Scholar]
- Finkel T. (2011). Signal transduction by reactive oxygen species. J. Cell Biol. 194 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen M., Lismont C., Walton P. (2017). The peroxisome-mitochondria connection: How and Why? Int. J. Mol. Sci. 18:1126. 10.3390/ijms18061126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. (1997). Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J. Biol. Chem. 272 18515–18517. 10.1074/jbc.272.30.18515 [DOI] [PubMed] [Google Scholar]
- Gaggini F., Laleu B., Orchard M., Fioraso-Cartier L., Cagnon L., Houngninou-Molango S., et al. (2011). Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg. Med. Chem. 19 6989–6999. 10.1016/j.bmc.2011.10.016 [DOI] [PubMed] [Google Scholar]
- Garaude J., Acin-Perez R., Martinez-Cano S., Enamorado M., Ugolini M., Nistal-Villan E., et al. (2016). Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat. Immunol. 17 1037–1045. 10.1038/ni.3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatliff J., East D. A., Singh A., Alvarez M. S., Frison M., Matic I., et al. (2017). A role for TSPO in mitochondrial Ca(2+) homeostasis and redox stress signaling. Cell Death Dis. 8:e2896. 10.1038/cddis.2017.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi G., Banfi B., Deffert C., Fiette L., Schappi M., Herrmann F., et al. (2006). Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 580 497–504. 10.1016/j.febslet.2005.12.049 [DOI] [PubMed] [Google Scholar]
- Geng J., Sun X., Wang P., Zhang S., Wang X., Wu H., et al. (2015). Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat. Immunol. 16 1142–1152. 10.1038/ni.3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L., Fan L. M., Liu F., Smith C., Li J. (2020). Nox2 dependent redox-regulation of microglial response to amyloid-beta stimulation and microgliosis in aging. Sci. Rep. 10:1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer O., Podos S. M., Mittag T. (1997). Nitric oxide synthase activity in tissues of the bovine eye. Graefes Arch. Clin. Exp. Ophthalmol. 235 786–793. 10.1007/bf02332864 [DOI] [PubMed] [Google Scholar]
- Gluschko A., Herb M., Wiegmann K., Krut O., Neiss W. F., Utermohlen O., et al. (2018). The beta2 Integrin Mac-1 induces protective LC3-associated phagocytosis of Listeria monocytogenes. Cell Host Microbe 23 324–337.e5. [DOI] [PubMed] [Google Scholar]
- Go Y. M., Jones D. P. (2008). Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta 1780 1273–1290. 10.1016/j.bbagen.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin Y., Cavaglieri R. C., Khazim K., Lee D. Y., Bruno F., Thakur S., et al. (2015). Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Renal Physiol. 308 F1276–F1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E., Murphy T. C., Kang B. Y., Kleinhenz J. M., Szyndralewiez C., Page P., et al. (2012). The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am. J. Respir. Cell Mol. Biol. 47 718–726. 10.1165/rcmb.2011-0418oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutscher M., Sobotta M. C., Wabnitz G. H., Ballikaya S., Meyer A. J., Samstag Y., et al. (2009). Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J. Biol. Chem. 284 31532–31540. 10.1074/jbc.m109.059246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. C., Halliwell B. (2018). Mini-Review: Oxidative stress, redox stress or redox success? Biochem. Biophys. Res. Commun. 502 183–186. 10.1016/j.bbrc.2018.05.045 [DOI] [PubMed] [Google Scholar]
- Haber F., Weiss J., Pope W. J. (1997). The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 147 332–351. 10.1098/rspa.1934.0221 [DOI] [Google Scholar]
- Hadrava Vanova K., Kraus M., Neuzil J., Rohlena J. (2020). Mitochondrial complex II and reactive oxygen species in disease and therapy. Redox Rep. 25 26–32. 10.1080/13510002.2020.1752002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Yehia A. I., Nassar T., Assaf P., Nassar H., Anggard E. E. (1999). Effects of the superoxide dismutase-mimic compound TEMPOL on oxidant stress-mediated endothelial dysfunction. Antioxid. Redox Signal. 1 221–232. 10.1089/ars.1999.1.2-221 [DOI] [PubMed] [Google Scholar]
- Han D., Antunes F., Canali R., Rettori D., Cadenas E. (2003). Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J. Biol. Chem. 278 5557–5563. 10.1074/jbc.m210269200 [DOI] [PubMed] [Google Scholar]
- Han H. (2018). RNA interference to knock down gene expression. Methods Mol. Biol. 1706 293–302. 10.1007/978-1-4939-7471-9_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatz S., Lambert J. D., Ogilby P. R. (2007). Measuring the lifetime of singlet oxygen in a single cell: addressing the issue of cell viability. Photochem. Photobiol. Sci. 6 1106–1116. 10.1039/b707313e [DOI] [PubMed] [Google Scholar]
- Hayyan M., Hashim M. A., Alnashef I. M. (2016). Superoxide ion: generation and chemical implications. Chem. Rev. 116 3029–3085. 10.1021/acs.chemrev.5b00407 [DOI] [PubMed] [Google Scholar]
- Hein T. W., Kuo L. (1998). LDLs impair vasomotor function of the coronary microcirculation: role of superoxide anions. Circ. Res. 83 404–414. 10.1161/01.res.83.4.404 [DOI] [PubMed] [Google Scholar]
- Heinz S., Freyberger A., Lawrenz B., Schladt L., Schmuck G., Ellinger-Ziegelbauer H. (2017). Mechanistic investigations of the mitochondrial complex I inhibitor rotenone in the context of pharmacological and safety evaluation. Sci. Rep. 7:45465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel S. L., Buettner G. R., O’malley Y. Q., Wessels D. A., Flaherty D. M. (1999). Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic. Biol. Med. 27 146–159. [DOI] [PubMed] [Google Scholar]
- Herb M., Schramm M. (2021). Functions of ROS in macrophages and antimicrobial immunity. Antioxidants 10:313. 10.3390/antiox10020313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herb M., Farid A., Gluschko A., Kronke M., Schramm M. (2019a). Highly efficient transfection of primary macrophages with in vitro transcribed mRNA. J. Vis. Exp. 153. [DOI] [PubMed] [Google Scholar]
- Herb M., Gluschko A., Wiegmann K., Farid A., Wolf A., Utermohlen O., et al. (2019b). Mitochondrial reactive oxygen species enable proinflammatory signaling through disulfide linkage of NEMO. Sci. Signal. 12:eaar5926. 10.1126/scisignal.aar5926 [DOI] [PubMed] [Google Scholar]
- Hernández-Barrera A., Quinto C., Johnson E. A., Wu H.-M., Cheung A. Y., Cárdenas L. (2013). Using hyper as a molecular probe to visualize hydrogen peroxide in living plant cells: a method with virtually unlimited potential in plant biology. Methods Enzymol. 527 275–290. 10.1016/b978-0-12-405882-8.00015-5 [DOI] [PubMed] [Google Scholar]
- Hernansanz-Agustin P., Choya-Foces C., Carregal-Romero S., Ramos E., Oliva T., Villa-Pina T., et al. (2020). Na(+) controls hypoxic signalling by the mitochondrial respiratory chain. Nature 586 287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscovitch M., Comb W., Ennis T., Coleman K., Yong S., Armstead B., et al. (2008). Intermolecular disulfide bond formation in the NEMO dimer requires Cys54 and Cys347. Biochem. Biophys. Res. Commun. 367 103–108. 10.1016/j.bbrc.2007.12.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervera A., De Virgiliis F., Palmisano I., Zhou L., Tantardini E., Kong G., et al. (2018). Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 20 307–319. 10.1038/s41556-018-0039-x [DOI] [PubMed] [Google Scholar]
- Heumuller S., Wind S., Barbosa-Sicard E., Schmidt H. H., Busse R., Schroder K., et al. (2008). Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51 211–217. 10.1161/hypertensionaha.107.100214 [DOI] [PubMed] [Google Scholar]
- Hoeven R., Mccallum K. C., Cruz M. R., Garsin D. A. (2011). Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS Pathog. 7:e1002453. 10.1371/journal.ppat.1002453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom K. M., Finkel T. (2014). Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 15 411–421. 10.1038/nrm3801 [DOI] [PubMed] [Google Scholar]
- Hos N. J., Ganesan R., Gutierrez S., Hos D., Klimek J., Abdullah Z., et al. (2017). Type I interferon enhances necroptosis of Salmonella Typhimurium-infected macrophages by impairing antioxidative stress responses. J. Cell Biol. 216 4107–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Ullah F., Zhou D. X., Yi M., Zhao Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 10:800. 10.3389/fpls.2019.00800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J. A. (2003). Pathways of oxidative damage. Annu. Rev. Microbiol. 57 395–418. 10.1146/annurev.micro.57.030502.090938 [DOI] [PubMed] [Google Scholar]
- Inomata M., Xu S., Chandra P., Meydani S. N., Takemura G., Philips J. A., et al. (2020). Macrophage LC3-associated phagocytosis is an immune defense against Streptococcus pneumoniae that diminishes with host aging. Proc. Natl. Acad. Sci. U.S.A. 117 33561–33569. 10.1073/pnas.2015368117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives A., Nomura J., Martinon F., Roger T., Leroy D., Miner J. N., et al. (2015). Xanthine oxidoreductase regulates macrophage IL1beta secretion upon NLRP3 inflammasome activation. Nat. Commun. 6:6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku M., Luhova L., Petrivalsky M. (2019). On the Origin and Fate of Reactive Oxygen Species in Plant Cell Compartments. Antioxidants 8:105. 10.3390/antiox8040105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. I., Meshulam T., Oliveira M. F., Burritt N., Corkey B. E. (2016). Extracellular redox regulation of intracellular reactive oxygen generation, mitochondrial function and lipid turnover in cultured human Adipocytes. PLoS One 11:e0164011. 10.1371/journal.pone.0164011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan C. A., De La Lastra J. M. P., Plou F. J., Perez-Lebena E. (2021). The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, Lipids and Proteins) and induced pathologies. Int. J. Mol. Sci. 22, 4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata H., Honda S., Maeda S., Chang L., Hirata H., Karin M. (2005). Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120 649–661. 10.1016/j.cell.2004.12.041 [DOI] [PubMed] [Google Scholar]
- Kehrer J. P., Robertson J. D., Smith C. V. (2010). “1.14 - Free radicals and reactive oxygen species,” in Comprehensive Toxicology, 2nd Edn, ed. Mcqueen C. A. (Oxford: Elsevier; ), 277–307. [Google Scholar]
- Kelley E. E., Khoo N. K., Hundley N. J., Malik U. Z., Freeman B. A., Tarpey M. M. (2010). Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 48 493–498. 10.1016/j.freeradbiomed.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B., Tannahill G. M., Murphy M. P., O’neill L. A. (2015). Metformin Inhibits the Production of Reactive Oxygen Species from NADH:Ubiquinone Oxidoreductase to Limit Induction of Interleukin-1beta (IL-1beta) and Boosts Interleukin-10 (IL-10) in Lipopolysaccharide (LPS)-activated Macrophages. J. Biol. Chem. 290 20348–20359. 10.1074/jbc.m115.662114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. K., Lee H. Y., Riaz T. A., Bhattarai K. R., Chaudhary M., Ahn J. H., et al. (2021). Chalcone suppresses tumor growth through NOX4-IRE1alpha sulfonation-RIDD-miR-23b axis. Redox Biol. 40:101853. 10.1016/j.redox.2021.101853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Xue X. (2020). Detection of total reactive Oxygen Species in Adherent Cells by 2′,7′-Dichlorodihydrofluorescein Diacetate Staining. J. Vis. Exp. 160:10.3791/60682. 10.3791/60682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Lee J., Bae S. J., Kim Y., Park B. J., Choi J. W., et al. (2017). NADPH oxidase 4 is required for the generation of macrophage migration inhibitory factor and host defense against Toxoplasma gondii infection. Sci. Rep. 7:6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. H., Choi S., Han E. J., Hong B. K., Choi S. Y., Kwon H. M., et al. (2014). The xanthine oxidase-NFAT5 pathway regulates macrophage activation and TLR-induced inflammatory arthritis. Eur. J. Immunol. 44 2721–2736. 10.1002/eji.201343669 [DOI] [PubMed] [Google Scholar]
- Kiritoshi S., Nishikawa T., Sonoda K., Kukidome D., Senokuchi T., Matsuo T., et al. (2003). Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes Metab. Res. Rev. 52 2570–2577. 10.2337/diabetes.52.10.2570 [DOI] [PubMed] [Google Scholar]
- Kirkman H. N., Gaetani G. F. (2007). Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem. Sci. 32 44–50. 10.1016/j.tibs.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Knight L. J., Martis R. M., Acosta M. L., Donaldson P. J., Lim J. C. (2021). Detection of reduced mitochondrial ROS production but increased ROS levels and oxidative damage in the young xCT knockout mouse retina. Invest. Ophthalmol. Vis. Sci. 62 2230–2230. [Google Scholar]
- Koterski J. F., Nahvi M., Venkatesan M. M., Haimovich B. (2005). Virulent Shigella flexneri causes damage to mitochondria and triggers necrosis in infected human monocyte-derived macrophages. Infect. Immun. 73 504–513. 10.1128/iai.73.1.504-513.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna C. M., Liebmann J. E., Kaufman D., Degraff W., Hahn S. M., Mcmurry T., et al. (1992). The catecholic metal sequestering agent 1,2-dihydroxybenzene-3,5-disulfonate confers protection against oxidative cell damage. Arch. Biochem. Biophys. 294 98–106. 10.1016/0003-9861(92)90142-j [DOI] [PubMed] [Google Scholar]
- Laleu B., Gaggini F., Orchard M., Fioraso-Cartier L., Cagnon L., Houngninou-Molango S., et al. (2010). First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J. Med. Chem. 53 7715–7730. 10.1021/jm100773e [DOI] [PubMed] [Google Scholar]
- Lambert A. J., Brand M. D. (2004). Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J. Biol. Chem. 279 39414–39420. 10.1074/jbc.m406576200 [DOI] [PubMed] [Google Scholar]
- Lambeth J. D., Neish A. S. (2014). Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu. Rev. Pathol. 9 119–145. 10.1146/annurev-pathol-012513-104651 [DOI] [PubMed] [Google Scholar]
- Lambeth J. D., Krause K. H., Clark R. A. (2008). NOX enzymes as novel targets for drug development. Semin. Immunopathol. 30 339–363. 10.1007/s00281-008-0123-6 [DOI] [PubMed] [Google Scholar]
- Lanciano P., Khalfaoui-Hassani B., Selamoglu N., Ghelli A., Rugolo M., Daldal F. (2013). Molecular mechanisms of superoxide production by complex III: a bacterial versus human mitochondrial comparative case study. Biochim. Biophys. Acta 1827 1332–1339. 10.1016/j.bbabio.2013.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford H. (2015). CRISPR, the disruptor. Nature 522 20–24. 10.1038/522020a [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Bae H. C., Lee H., Jang Y., Park Y. H., Kim J. H., et al. (2017). Intracellular ROS levels determine the apoptotic potential of keratinocyte by Quantum Dot via blockade of AKT Phosphorylation. Exp. Dermatol. 26 1046–1052. 10.1111/exd.13365 [DOI] [PubMed] [Google Scholar]
- Lee S. R., Kwon K. S., Kim S. R., Rhee S. G. (1998). Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 273 15366–15372. 10.1074/jbc.273.25.15366 [DOI] [PubMed] [Google Scholar]
- Leusen J. H., Fluiter K., Hilarius P. M., Roos D., Verhoeven A. J., Bolscher B. G. (1995). Interactions between the cytosolic components p47phox and p67phox of the human neutrophil NADPH oxidase that are not required for activation in the cell-free system. J. Biol. Chem. 270 11216–11221. [DOI] [PubMed] [Google Scholar]
- Levine A., Tenhaken R., Dixon R., Lamb C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79 583–593. 10.1016/0092-8674(94)90544-4 [DOI] [PubMed] [Google Scholar]
- Li T. Y., Sun Y., Liang Y., Liu Q., Shi Y., Zhang C. S., et al. (2016). ULK1/2 constitute a bifurcate node controlling glucose metabolic fluxes in addition to autophagy. Mol. Cell 62 359–370. 10.1016/j.molcel.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Li X., Fang P., Mai J., Choi E. T., Wang H., Yang X. F. (2013). Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 6:19. 10.1186/1756-8722-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligeon L. A., Pena-Francesch M., Munz C. (2021a). Measuring oxidation within LC3-associated phagosomes that optimizes MHC class II restricted antigen presentation. Methods Cell Biol. 164 187–200. 10.1016/bs.mcb.2021.02.003 [DOI] [PubMed] [Google Scholar]
- Ligeon L. A., Pena-Francesch M., Vanoaica L. D., Nunez N. G., Talwar D., Dick T. P., et al. (2021b). Oxidation inhibits autophagy protein deconjugation from phagosomes to sustain MHC class II restricted antigen presentation. Nat. Commun. 12:1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. K., Lin K. J., Lin K. L., Liou C. W., Chen S. D., Chuang Y. C., et al. (2020). When friendship turns sour: effective communication between mitochondria and intracellular organelles in Parkinson’s disease. Front. Cell. Dev. Biol. 8:607392. 10.3389/fcell.2020.607392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liochev S. I., Fridovich I. (1999). Superoxide and iron: partners in crime. IUBMB Life 48 157–161. 10.1080/713803492 [DOI] [PubMed] [Google Scholar]
- Liou G. Y., Storz P. (2010). Reactive oxygen species in cancer. Free Radic. Res. 44 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lismont C., Nordgren M., Van Veldhoven P. P., Fransen M. (2015). Redox interplay between mitochondria and peroxisomes. Front. Cell Dev. Biol. 3:35. 10.3389/fcell.2015.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loth M. K., Guariglia S. R., Re D. B., Perez J., De Paiva V. N., Dziedzic J. L., et al. (2020). A novel interaction of translocator protein 18 kDa (TSPO) with NADPH Oxidase in Microglia. Mol. Neurobiol. 57 4467–4487. 10.1007/s12035-020-02042-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist H., Dahlgren C. (1996). Isoluminol-enhanced chemiluminescence: a sensitive method to study the release of superoxide anion from human neutrophils. Free Radic. Biol. Med. 20 785–792. 10.1016/0891-5849(95)02189-2 [DOI] [PubMed] [Google Scholar]
- Mak T. W., Grusdat M., Duncan G. S., Dostert C., Nonnenmacher Y., Cox M., et al. (2017). Glutathione primes T cell metabolism for inflammation. Immunity 46 675–689. 10.1016/j.immuni.2017.03.019 [DOI] [PubMed] [Google Scholar]
- Manzano V. M., Munoz J. C., Jimenez J. R., Puyol M. R., Puyol D. R., Kitamura M., et al. (2000). Human renal mesangial cells are a target for the anti-inflammatory action of 9-cis retinoic acid. Br. J. Pharmacol. 131 1673–1683. 10.1038/sj.bjp.0703728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho H. S., Cyrne L., Cadenas E., Antunes F. (2013). The cellular steady-state of H2O2: latency concepts and gradients. Methods Enzymol. 527 3–19. 10.1016/b978-0-12-405882-8.00001-5 [DOI] [PubMed] [Google Scholar]
- Marklund S. (1976). Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J. Biol. Chem. 251 7504–7507. 10.1016/s0021-9258(17)32878-8 [DOI] [PubMed] [Google Scholar]
- Markvicheva K. N., Bilan D. S., Mishina N. M., Gorokhovatsky A. Y., Vinokurov L. M., Lukyanov S., et al. (2011). A genetically encoded sensor for H2O2 with expanded dynamic range. Bioorg. Med. Chem. 19 1079–1084. 10.1016/j.bmc.2010.07.014 [DOI] [PubMed] [Google Scholar]
- Martinez M. A., Ubeda A., Trillo M. A. (2021). Role of NADPH oxidase in MAPK signaling activation by a 50 Hz magnetic field in human neuroblastoma cells. Electromagn. Biol. Med. 40 103–116. 10.1080/15368378.2020.1851250 [DOI] [PubMed] [Google Scholar]
- Matsushita T., Fukuda K., Yamamoto H., Yamazaki K., Tomiyama T., Oh M., et al. (2004). Effect of ebselen, a scavenger of reactive oxygen species, on chondrocyte metabolism. Mod. Rheumatol. 14 25–30. 10.3109/s10165-003-0261-6 [DOI] [PubMed] [Google Scholar]
- Meinhard M., Grill E. (2001). Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. FEBS Lett. 508 443–446. 10.1016/s0014-5793(01)03106-4 [DOI] [PubMed] [Google Scholar]
- Melov S., Coskun P., Patel M., Tuinstra R., Cottrell B., Jun A. S., et al. (1999). Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. U.S.A. 96 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola A. S., Ryu J. K., Bardehle S., Meyer-Franke A., Ang K. K., Wilson C., et al. (2020). Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat. Immunol. 21 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng T. C., Fukada T., Tonks N. K. (2002). Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9 387–399. 10.1016/s1097-2765(02)00445-8 [DOI] [PubMed] [Google Scholar]
- Miller B. M., Liou M. J., Zhang L. F., Nguyen H., Litvak Y., Schorr E. M., et al. (2020). Anaerobic respiration of NOX1-derived hydrogen peroxide licenses bacterial growth at the colonic surface. Cell Host Microbe 28 789–797.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Pale M., Weiwer M., Yu J., Linhardt R. J., Dordick J. S. (2009). Inhibition of human vascular NADPH oxidase by apocynin derived oligophenols. Bioorg. Med. Chem. 17 5146–5152. 10.1016/j.bmc.2009.05.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugesh G. (2013). Glutathione peroxidase activity of ebselen and its analogues: some insights into the complex chemical mechanisms underlying the antioxidant activity. Curr. Chem. Biol. 7 47–56. 10.2174/2212796811307010005 [DOI] [Google Scholar]
- Mukhopadhyay P., Rajesh M., Hasko G., Hawkins B. J., Madesh M., Pacher P. (2007). Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protoc. 2 2295–2301. 10.1038/nprot.2007.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F. L., Liu Y., Van Remmen H. (2004). Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 279 49064–49073. 10.1074/jbc.m407715200 [DOI] [PubMed] [Google Scholar]
- Murphy M. P. (2009). How mitochondria produce reactive oxygen species. Biochem. J. 417 1–13. 10.1042/bj20081386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. (2005). Thioredoxin and its related molecules: update 2005. Antioxid. Redox Signal. 7 823–828. 10.1089/ars.2005.7.823 [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Feng Q., Kumagai T., Torikai K., Ohigashi H., Osawa T., et al. (2002). Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis. J. Biol. Chem. 277 2687–2694. 10.1074/jbc.m109641200 [DOI] [PubMed] [Google Scholar]
- Nakano Y., Longo-Guess C. M., Bergstrom D. E., Nauseef W. M., Jones S. M., Banfi B. (2008). Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J. Clin. Invest. 118 1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Cunningham-Bussel A. (2013). Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 13 349–361. 10.1038/nri3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naviaux R. K. (2012). Oxidative shielding or oxidative stress? J. Pharmacol. Exp. Ther. 342 608–618. 10.1124/jpet.112.192120 [DOI] [PubMed] [Google Scholar]
- Nazarewicz R. R., Dikalova A. E., Bikineyeva A., Dikalov S. I. (2013). Nox2 as a potential target of mitochondrial superoxide and its role in endothelial oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 305 H1131–H1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer P., Grabher C., Look A. T., Mitchison T. J. (2009). A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in Zebrafish. Nature 459 996–999. 10.1038/nature08119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki E. (2016). Oxidative stress and antioxidants: Distress or eustress? Arch. Biochem. Biophys. 595 19–24. 10.1016/j.abb.2015.11.017 [DOI] [PubMed] [Google Scholar]
- Nohl H., Kozlov A. V., Gille L., Staniek K. (2003). Cell respiration and formation of reactive oxygen species: facts and artefacts. Biochem. Soc. Trans. 31 1308–1311. 10.1042/bst0311308 [DOI] [PubMed] [Google Scholar]
- Nomura J., Busso N., Ives A., Matsui C., Tsujimoto S., Shirakura T., et al. (2014). Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Sci. Rep. 4:4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odobasic D., Kitching A. R., Holdsworth S. R. (2016). Neutrophil-mediated regulation of innate and adaptive immunity: the role of Myeloperoxidase. J. Immunol. Res. 2016:2349817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell B. V., Tew D. G., Jones O. T., England P. J. (1993). Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 290(Pt 1), 41–49. 10.1042/bj2900041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T., Sato R. (1962). A new cytochrome in liver microsomes. J. Biol. Chem. 237 1375–1376. [PubMed] [Google Scholar]
- Panov A., Dikalov S., Shalbuyeva N., Taylor G., Sherer T., Greenamyre J. T. (2005). Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J. Biol. Chem. 280 42026–42035. [DOI] [PubMed] [Google Scholar]
- Patriarca S., Furfaro A. L., Domenicotti C., Odetti P., Cottalasso D., Marinari U. M., et al. (2005). Supplementation with N-acetylcysteine and taurine failed to restore glutathione content in liver of streptozotocin-induced diabetics rats but protected from oxidative stress. Biochim. Biophys. Acta 1741 48–54. 10.1016/j.bbadis.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Pavelkova M., Kubala L. (2004). Luminol-, isoluminol- and lucigenin-enhanced chemiluminescence of rat blood phagocytes stimulated with different activators. Luminescence 19 37–42. 10.1002/bio.754 [DOI] [PubMed] [Google Scholar]
- Peng H. Y., Lucavs J., Ballard D., Das J. K., Kumar A., Wang L., et al. (2021). Metabolic reprogramming and reactive Oxygen Species in T cell immunity. Front. Immunol. 12:652687. 10.3389/fimmu.2021.652687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., et al. (2020). ROS in cancer therapy: the bright side of the moon. Exp. Mol. Med. 52 192–203. 10.1038/s12276-020-0384-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., et al. (2017). Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017:8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljsak B., Suput D., Milisav I. (2013). Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013:956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole L. B. (2015). The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 80 148–157. 10.1016/j.freeradbiomed.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestes E. B., Alves L. S., Rodrigues D. A. S., Dutra F. F., Fernandez P. L., Paiva C. N., et al. (2020). Mitochondrial reactive oxygen species participate in signaling triggered by Heme in macrophages and upon Hemolysis. J. Immunol. 205 2795–2805. 10.4049/jimmunol.1900886 [DOI] [PubMed] [Google Scholar]
- Prousek J. (2007). Fenton chemistry in biology and medicine. Pure Appl. Chem. 79 2325–2338. 10.1351/pac200779122325 [DOI] [Google Scholar]
- Quinlan C. L., Orr A. L., Perevoshchikova I. V., Treberg J. R., Ackrell B. A., Brand M. D. (2012). Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 287 27255–27264. 10.1074/jbc.m112.374629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak R., Chao D. L., Pluta R. M., Mitchell J. B., Oldfield E. H., Watson J. C. (2000). Neuroprotection by the stable nitroxide Tempol during reperfusion in a rat model of transient focal ischemia. J. Neurosurg. 92 646–651. 10.3171/jns.2000.92.4.0646 [DOI] [PubMed] [Google Scholar]
- Rangasamy T., Guo J., Mitzner W. A., Roman J., Singh A., Fryer A. D., et al. (2005). Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 202 47–59. 10.1084/jem.20050538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R., Hanukoglu I., Sklan D. (1994). A fluorimetric assay for hydrogen peroxide, suitable for NAD(P)H-dependent superoxide generating redox systems. Anal. Biochem. 218 309–313. 10.1006/abio.1994.1183 [DOI] [PubMed] [Google Scholar]
- Reczek C. R., Chandel N. S. (2014). ROS-dependent signal transduction. Curr. Opin. Cell Biol. 33C 8–13. 10.1016/j.ceb.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek C. R., Chandel N. S. (2017). The two faces of reactive oxygen species in cancer. Annu. Rev. Cancer Biol. 1 79–98. 10.1146/annurev-cancerbio-041916-065808 [DOI] [Google Scholar]
- Reis J., Massari M., Marchese S., Ceccon M., Aalbers F. S., Corana F., et al. (2020). A closer look into NADPH oxidase inhibitors: validation and insight into their mechanism of action. Redox Biol. 32:101466. 10.1016/j.redox.2020.101466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Wang M., Wang Y., Huang A. (2021). Superoxide anion generation response to wound in Arabidopsis hypocotyl cutting. Plant Signal. Behav. 16:1848086. 10.1080/15592324.2020.1848086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G. (2006). Cell signaling. H2O2, a necessary evil for cell signaling. Science 312 1882–1883. 10.1126/science.1130481 [DOI] [PubMed] [Google Scholar]
- Rieger S., Sagasti A. (2011). Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the Zebrafish skin. PLoS Biol. 9:e1000621. 10.1371/journal.pbio.1000621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K. M., Janes M. S., Pehar M., Monette J. S., Ross M. F., Hagen T. M., et al. (2006). Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. U.S.A. 103 15038–15043. 10.1073/pnas.0601945103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca F. J., Ramakrishnan L. (2013). TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153 521–534. 10.1016/j.cell.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca F. J., Whitworth L. J., Redmond S., Jones A. A., Ramakrishnan L. (2019). TNF induces pathogenic programmed macrophage necrosis in tuberculosis through a mitochondrial-lysosomal-endoplasmic reticulum circuit. Cell 178 1344–1361.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero L. C., Aroca M. A., Laureano-Marin A. M., Moreno I., Garcia I., Gotor C. (2014). Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana. Mol. Plant 7 264–276. 10.1093/mp/sst168 [DOI] [PubMed] [Google Scholar]
- Saito Y., Kimura W. (2021). Roles of phase separation for cellular redox maintenance. Front. Genet. 12:691946. 10.3389/fgene.2021.691946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacker P. T. (2006). Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10 175–176. 10.1016/j.ccr.2006.08.015 [DOI] [PubMed] [Google Scholar]
- Scialo F., Fernandez-Ayala D. J., Sanz A. (2017). Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front. Physiol. 8:428. 10.3389/fphys.2017.00428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeek M., Callera G., Montezano A., Gutsol A., Heitz F., Szyndralewiez C., et al. (2010). Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am. J. Physiol. Renal Physiol. 299 F1348–F1358. [DOI] [PubMed] [Google Scholar]
- Sena L. A., Li S., Jairaman A., Prakriya M., Ezponda T., Hildeman D. A., et al. (2013). Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38 225–236. 10.1016/j.immuni.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai N., Yifrach E., Van Roermund C. W. T., Cohen N., Bibi C., IJlst L., et al. (2018). Systematic mapping of contact sites reveals tethers and a function for the peroxisome-mitochondria contact. Nat. Commun. 9:1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Jha A. B., Dubey R. S., Pessarakli M. (2012). Reactive oxygen Species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012:217037. [Google Scholar]
- Short J. D., Downs K., Tavakoli S., Asmis R. (2016). Protein Thiol Redox signaling in monocytes and macrophages. Antioxid. Redox Signal. 25 816–835. 10.1089/ars.2016.6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz V., De Pinto V., Zweckstetter M., Raviv Z., Keinan N., Arbel N. (2010). VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Aspects Med. 31 227–285. 10.1016/j.mam.2010.03.002 [DOI] [PubMed] [Google Scholar]
- Sies H. (2021). Oxidative eustress: on constant alert for redox homeostasis. Redox Biol. 41:101867. 10.1016/j.redox.2021.101867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H., Jones D. P. (2020). Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21 363–383. 10.1038/s41580-020-0230-3 [DOI] [PubMed] [Google Scholar]
- Sies H., Berndt C., Jones D. P. (2017). Oxidative stress. Annu. Rev. Biochem. 86 715–748. [DOI] [PubMed] [Google Scholar]
- Stavru F., Bouillaud F., Sartori A., Ricquier D., Cossart P. (2011). Listeria monocytogenes transiently alters mitochondrial dynamics during infection. Proc. Natl. Acad. Sci. U.S.A. 108 3612–3617. 10.1073/pnas.1100126108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe D. F., Camara A. K. (2009). Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid. Redox Signal. 11 1373–1414. 10.1089/ars.2008.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J., Buckingham J. A., Roebuck S. J., Brand M. D. (2002). Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 277 44784–44790. 10.1074/jbc.m207217200 [DOI] [PubMed] [Google Scholar]
- Strengert M., Jennings R., Davanture S., Hayes P., Gabriel G., Knaus U. G. (2014). Mucosal reactive oxygen species are required for antiviral response: role of Duox in influenza a virus infection. Antioxid. Redox Signal. 20 2695–2709. 10.1089/ars.2013.5353 [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Fasehun O. A., Kwon N. S., Gross S. S., Gonzalez J. A., Levi R., et al. (1991). Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J. 5 98–103. 10.1096/fasebj.5.1.1703974 [DOI] [PubMed] [Google Scholar]
- Su L. J., Zhang J. H., Gomez H., Murugan R., Hong X., Xu D., et al. (2019). Reactive Oxygen Species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019:5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Muramatsu A., Saito R., Iso T., Shibata T., Kuwata K., et al. (2019). Molecular mechanism of cellular oxidative stress sensing by Keap1. Cell Rep. 28 746–758.e4. [DOI] [PubMed] [Google Scholar]
- Sweeney R. M., McAuley D. F. (2016). Acute respiratory distress syndrome. Lancet 388 2416–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T. C., Wong-Faull D. C., Claycomb R., Wong D. L. (2009). Hypoxic stress-induced changes in adrenergic function: role of HIF1 alpha. J. Neurochem. 109 513–524. 10.1111/j.1471-4159.2009.05978.x [DOI] [PubMed] [Google Scholar]
- Takahashi M. A., Asada K. (1983). Superoxide anion permeability of phospholipid membranes and chloroplast thylakoids. Arch. Biochem. Biophys. 226 558–566. 10.1016/0003-9861(83)90325-9 [DOI] [PubMed] [Google Scholar]
- Taverne Y. J., Bogers A. J., Duncker D. J., Merkus D. (2013). Reactive oxygen species and the cardiovascular system. Oxid. Med. Cell. Longev. 2013: 862423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazzeo T., Worek F., Janssen L. (2009). The NADPH oxidase inhibitor diphenyleneiodonium is also a potent inhibitor of cholinesterases and the internal Ca(2+) pump. Br. J. Pharmacol. 158 790–796. 10.1111/j.1476-5381.2009.00394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira G., Szyndralewiez C., Molango S., Carnesecchi S., Heitz F., Wiesel P., et al. (2017). Therapeutic potential of NADPH oxidase 1/4 inhibitors. Br. J. Pharmacol. 174 1647–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Freyhaus H., Huntgeburth M., Wingler K., Schnitker J., Baumer A. T., Vantler M., et al. (2006). Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc. Res. 71 331–341. 10.1016/j.cardiores.2006.01.022 [DOI] [PubMed] [Google Scholar]
- Tepel M., Echelmeyer M., Orie N. N., Zidek W. (2000). Increased intracellular reactive oxygen species in patients with end-stage renal failure: effect of hemodialysis. Kidney Int. 58 867–872. 10.1046/j.1523-1755.2000.00236.x [DOI] [PubMed] [Google Scholar]
- Thimmulappa R. K., Lee H., Rangasamy T., Reddy S. P., Yamamoto M., Kensler T. W., et al. (2006). Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 116 984–995. 10.1172/jci25790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne L. S., Rochford G., Williams T. D., Southam A. D., Rodriguez-Blanco G., Dunn W. B., et al. (2021). Cytoglobin protects cancer cells from apoptosis by regulation of mitochondrial cardiolipin. Sci. Rep. 11:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To E. E., Vlahos R., Luong R., Halls M. L., Reading P. C., King P. T., et al. (2017). Endosomal NOX2 oxidase exacerbates virus pathogenicity and is a target for antiviral therapy. Nat. Commun. 8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K. (2005). Redox redux: revisiting PTPs and the control of cell signaling. Cell 121 667–670. 10.1016/j.cell.2005.05.016 [DOI] [PubMed] [Google Scholar]
- Trevelin S. C., Dos Santos C. X., Ferreira R. G., De Sa Lima L., Silva R. L., Scavone C., et al. (2016). Apocynin and Nox2 regulate NF-kappaB by modifying thioredoxin-1 redox-state. Sci. Rep. 6:34581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troia A., Knutsen R. H., Halabi C. M., Malide D., Yu Z. X., Wardlaw-Pickett A., et al. (2021). Inhibition of NOX1 Mitigates Blood Pressure Increases in Elastin Insufficiency. Function (Oxf) 2:zqab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima Y., Totsune H., Nishida A., Nakano M. (1997). Chemiluminescence from human polymorphonuclear leukocytes activated with opsonized zymosan. Free Radic. Biol. Med. 22 401–409. 10.1016/s0891-5849(96)00329-2 [DOI] [PubMed] [Google Scholar]
- Veal E. A., Day A. M., Morgan B. A. (2007). Hydrogen peroxide sensing and signaling. Mol. Cell 26 1–14. 10.1016/j.molcel.2007.03.016 [DOI] [PubMed] [Google Scholar]
- Wang H., Schoebel S., Schmitz F., Dong H., Hedfalk K. (2020). Characterization of aquaporin-driven hydrogen peroxide transport. Biochim. Biophys. Acta Biomembr. 1862:183065. 10.1016/j.bbamem.2019.183065 [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang W., Wang S., Wang Y., Chu X., Ji H., et al. (2021a). Lactobacillus plantarum exhibits antioxidant and cytoprotective activities in porcine intestinal epithelial cells exposed to hydrogen peroxide. Oxid. Med. Cell. Longev. 2021:8936907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Jiang Z., Yue N., Jin X., Zhang X., Li Z., et al. (2021b). Barley stripe mosaic virus gammab protein disrupts chloroplast antioxidant defenses to optimize viral replication. EMBO J 40:e107660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Geng J., Gao J., Zhao H., Li J., Shi Y., et al. (2019). Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat. Commun. 10:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zou M. H. (2018). Measurement of reactive oxygen Species (ROS) and Mitochondrial ROS in AMPK Knockout Mice Blood Vessels. Methods Mol. Biol. 1732 507–517. 10.1007/978-1-4939-7598-3_32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Branicky R., Noe A., Hekimi S. (2018). Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 217 1915–1928. 10.1083/jcb.201708007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Cai F., Chen X., Luo M., Hu L., Lu Y. (2013). The role of mitochondria-derived reactive oxygen species in hyperthermia-induced platelet apoptosis. PLoS One 8:e75044. 10.1371/journal.pone.0075044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R., Enaka M., Muragaki Y. (2019). Activation of KEAP1/NRF2/P62 signaling alleviates high phosphate-induced calcification of vascular smooth muscle cells by suppressing reactive oxygen species production. Sci. Rep. 9:10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A. P., Brodsky I. E., Rahner C., Woo D. K., Erdjument-Bromage H., Tempst P., et al. (2011a). TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472 476–480. 10.1038/nature09973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A. P., Shadel G. S., Ghosh S. (2011b). Mitochondria in innate immune responses. Nat. Rev. Immunol. 11 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind S., Beuerlein K., Eucker T., Muller H., Scheurer P., Armitage M. E., et al. (2010). Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br. J. Pharmacol. 161 885–898. 10.1111/j.1476-5381.2010.00920.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler K., Hermans J. J., Schiffers P., Moens A., Paul M., Schmidt H. H. (2011). NOX1, 2, 4, 5: counting out oxidative stress. Br. J. Pharmacol. 164 866–883. 10.1111/j.1476-5381.2011.01249.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winitchaikul T., Sawong S., Surangkul D., Srikummool M., Somran J., Pekthong D., et al. (2021). Calotropis gigantea stem bark extract induced apoptosis related to ROS and ATP production in colon cancer cells. PLoS One 16:e0254392. 10.1371/journal.pone.0254392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D. A., Hines H. B., Cheng R. Y., Switzer C. H., Flores-Santana W., Vitek M. P., et al. (2011). Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 89 873–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Hampton M. B. (2008). Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 45 549–561. 10.1016/j.freeradbiomed.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Kettle A. J. (2013). Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 18 642–660. 10.1089/ars.2012.4827 [DOI] [PubMed] [Google Scholar]
- Wolf A., Herb M., Schramm M., Langmann T. (2020). The TSPO-NOX1 axis controls phagocyte-triggered pathological angiogenesis in the eye. Nat. Commun. 11:2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. S., Mezera V., Dighe P., Melov S., Gerencser A. A., Sweis R. F., et al. (2021). Superoxide produced by mitochondrial site IQ inactivates cardiac succinate dehydrogenase and induces hepatic steatosis in Sod2 knockout mice. Free Radic. Biol. Med. 164 223–232. 10.1016/j.freeradbiomed.2020.12.447 [DOI] [PubMed] [Google Scholar]
- Woo H. A., Yim S. H., Shin D. H., Kang D., Yu D. Y., Rhee S. G. (2010). Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell 140 517–528. 10.1016/j.cell.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Wood Z. A., Poole L. B., Karplus P. A. (2003). Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300 650–653. 10.1126/science.1080405 [DOI] [PubMed] [Google Scholar]
- Xu J., Tran T., Padilla Marcia C. S., Braun D. M., Goggin F. L. (2017). Superoxide-responsive gene expression in Arabidopsis thaliana and Zea mays. Plant Physiol. Biochem. 117 51–60. 10.1016/j.plaphy.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Yang Y., Karakhanova S., Hartwig W., D’haese J. G., Philippov P. P., Werner J., et al. (2016). Mitochondria and mitochondrial ROS in cancer: novel targets for anticancer therapy. J. Cell. Physiol. 231 2570–2581. 10.1002/jcp.25349 [DOI] [PubMed] [Google Scholar]
- Zaidieh T., Smith J. R., Ball K. E., An Q. (2019). ROS as a novel indicator to predict anticancer drug efficacy. BMC Cancer 19:1224. 10.1186/s12885-019-6438-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller J., Bogner B., Kiefer J., Braig D., Winninger O., Fricke M., et al. (2021). CRP Enhances the Innate Killing Mechanisms Phagocytosis and ROS formation in a conformation and complement-dependent manner. Front. Immunol. 12:754. 10.3389/fimmu.2018.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y., et al. (2016). ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016:4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Hu X., Shen Q., Xing D. (2019). Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat. Commun. 10:1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. D., Peng Y. Q., Zhao J., Li Q., Yu X. J., Acevedo-Rocha C. G., et al. (2020). Bacterial cytochrome P450-catalyzed regio- and stereoselective steroid hydroxylation enabled by directed evolution and rational design. Bioresour. Bioprocess. 7:2. [Google Scholar]
- Zhang Y., Yao Y., Qiu X., Wang G., Hu Z., Chen S., et al. (2019). Listeria hijacks host mitophagy through a novel mitophagy receptor to evade killing. Nat. Immunol. 20 433–446. 10.1038/s41590-019-0324-2 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Liu J., Deng H., Ma R., Liao J. Y., Liang H., et al. (2020). Targeting mitochondria-located circRNA SCAR Alleviates NASH via Reducing mROS Output. Cell 183 76–93.e22. [DOI] [PubMed] [Google Scholar]
- Zhao R. Z., Jiang S., Zhang L., Yu Z. B. (2019). Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 44 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Li Q., Luo H., Holmdahl R. (2021). Neutrophil-derived reactive oxygen species promote tumor colonization. Commun. Biol. 4:865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Yeo A. T., Ballarano C., Weber U., Allen K. N., Gilmore T. D., et al. (2014). Disulfide-mediated stabilization of the IkappaB kinase binding domain of NF-kappaB essential modulator (NEMO). Biochemistry 53 7929–7944. 10.1021/bi500920n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Yazdi A. S., Menu P., Tschopp J. (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469 221–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.