Abstract
Intracranial vessel wall MR imaging (VWI) is increasingly being used as a valuable adjunct to conventional angiographic imaging techniques. This article will provide an updated review on intracranial VWI protocols and image interpretation. We review VWI technical considerations, describe common VWI imaging features of different intracranial vasculopathies and show illustrative cases. We review the role of VWI for differentiating among steno-occlusive vasculopathies, such as intracranial atherosclerotic plaque, dissections and Moyamoya disease. We also highlight how VWI may be used for the diagnostic work-up and surveillance of patients with vasculitis of the central nervous system and cerebral aneurysms.
Introduction
Over the last 2 decades, there has been an exponential increase in the number of vessel wall MR imaging (VWI) publications highlighting potential clinical applications for VWI. Therefore, an update incorporating this recent knowledge would be useful for review and clinical interpretation. Intracranial VWI has been applied to many different types of intracranial vasculopathies ranging from aneurysms to steno-occlusive vasculopathies. VWI has allowed us to investigate disease mechanisms by enabling us to directly visualize the site of pathology in the vessel walls. The diagnostic advantage of VWI to visualize vessel wall injury contrasts with conventional angiographic techniques, such as CT angiography (CTA), MR angiography (MRA), and Digital Subtraction Angiography (DSA), which provides luminal information only. Luminal changes such as stenosis and dilatation are often consequences of vessel wall pathology and thus may be less sensitive for detecting the etiology of a vasculopathy.
As reviewed by the American Society of Neuroradiology (ASNR) Vessel Wall Imaging study group, intracranial VWI may be useful diagnostically as an adjunct imaging tool to conventional luminal techniques.1 Intracranial VWI has utility in disease diagnosis or assessment of disease activity. Use of VWI is so far more firmly established for diagnostic purposes in particular for steno-occlusive diseases. Common clinical indications include evaluating patients with stroke of undetermined etiology, further characterizing angiographically detected stenosis, and, in some centers, evaluating cerebral aneurysms.1–4 In this review, we provide an overview of intracranial VWI techniques and highlight technical considerations for specific types of vasculopathies. We also share illustrative VWI cases for common clinical indications and review imaging features that may aid image interpretation.
Intracranial VWI Protocol Considerations
There are several key technical considerations for intracranial VWI. The recommended protocol from the ASNR VWI Study Group includes pre and postcontrast 3D T1-weighted (T1w) or proton density-weighted (PDw) VWI sequences.1 PD-weighted sequences provide higher signal-to-noise ratio of the vessel wall but contrast enhancement, if present, may be less conspicuous. 3D sequences based on a long-echo-train, variable-flip-angle refocusing pulse turbo spin-echo can reduce total scan time for the same area of coverage and permit multiplanar reformatting of torturous vessels. While certain limitations exist for 2D VWI, such as volume averaging at intracranial vascular obliquities,5,6 2D sequences may provide better imaging quality than 3D sequences when imaging is targeted at a specific vessel of interest.7 For these reasons, an optimal VWI protocol may depend on the vasculopathy of interest. A wide range of protocols have been reported for various clinical indications to date (Figure 1) likely reflecting this tailored approach to VWI.8
Figure 1: Common VWI Protocols Reported in the Literature.
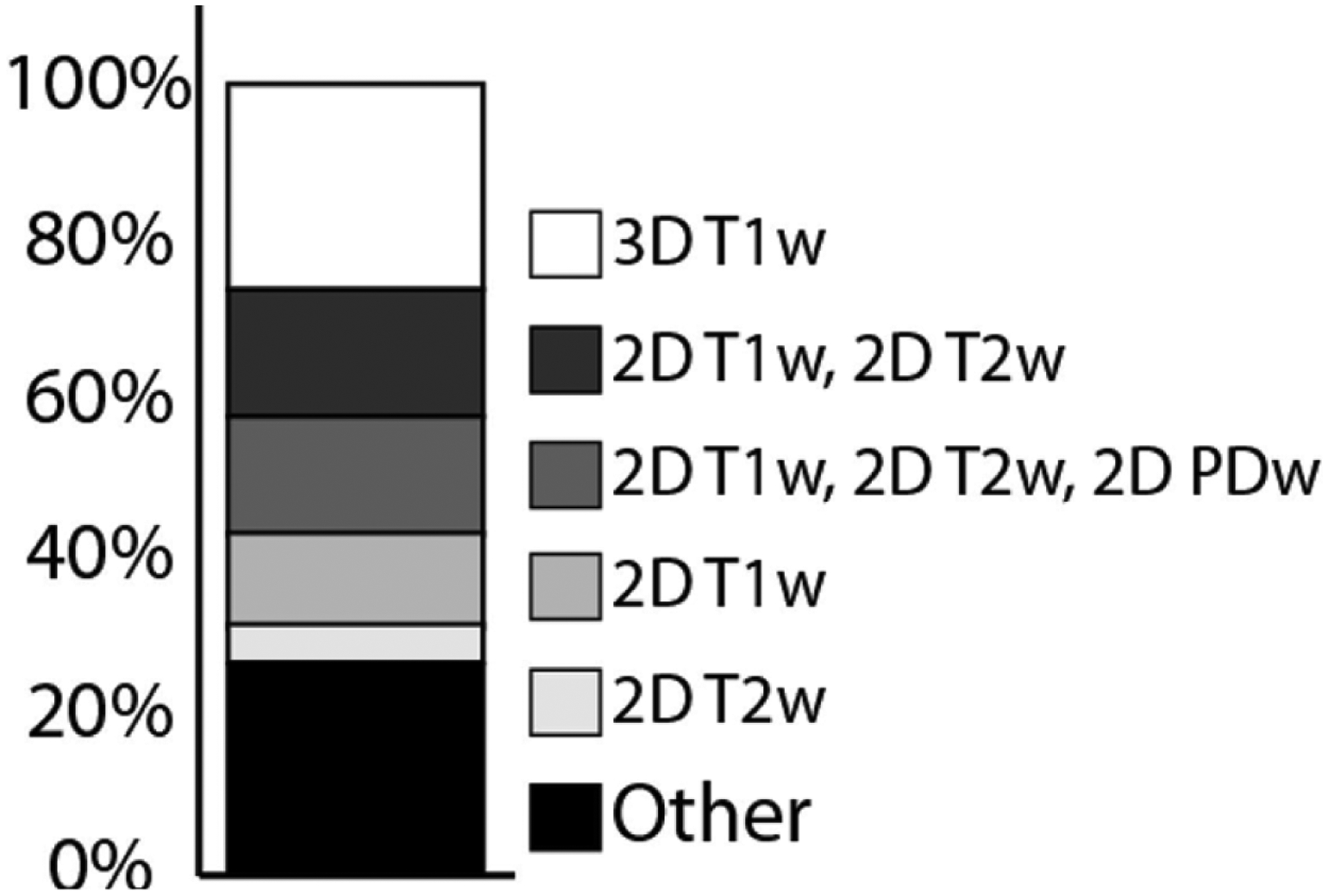
The most common VWI protocols reported in the literature are as follows: 3D T1-weighted (26%), 2D T1-weighted and 2D T2-weighted (16%); 2D T1-weighted, 2D T2-weighted, 2D PD-weighted (15%); 2D T1-weighted (12%); and 2D T2-weighted (5%). Other protocols include a combination of these pulse sequences and are further detailed in Song et al 2020.8
As part of the protocol, a 3D contrast-enhanced and/or time-of-flight (TOF) MRA is typically also included as it delineates the contour of the vessel lumen and confirms arterial flow.1 It is important to note that low velocity flow, often seen at sites of pronounced dilation or narrowing, can cause intravascular signal loss on TOF MRA as well as lead to a lack of blood flow suppression, artificial luminal narrowing and pseudo-enhancement on VWI. These considerations may lead to inclusion of a MRA sequence without and with gadolinium contrast as part of the VWI protocol.
VWI Technical Considerations
Important VWI technical considerations include ensuring adequate blood flow and cerebrospinal fluid (CSF) suppression, acquiring sufficiently high spatial resolutions to evaluate the vessel wall, and making the acquisition times clinically acceptable. Multiple different techniques can be used for the suppression of blood flow in VWI. For 2D acquisitions, spin echo sequences, spatial pre-saturation with a saturation band, and double inversion recovery preparation techniques can be used.9 For 3D acquisitions, blood flow suppression can be achieved by utilizing intravoxel dephasing. Laminar flow of luminal blood contains proton spins traveling at different velocities. These spins move through the magnetic field gradients and accumulate phases at different rates leading to intravoxel phase dispersion and subsequent signal loss.10 The intravoxel dephasing effect can be further enhanced by adding a motion-sensitizing magnetization preparation to 3D sequences.11,12
For 3D sequences, the most commonly used pulse sequence is the variable-flip-angle refocusing pulse turbo spin-echo sequence with vendor labels such as VISTA (volume isotropic turbo spin echo; Philips Healthcare), SPACE (sampling perfection with application-optimized contrasts by using different flip angle evolutions; Siemens), and CUBE (GE Healthcare). In addition to the inherent black-blood effect in these types of sequences, several flow suppression techniques are being investigated to achieve adequate suppression of both blood flow and CSF. For example, delay alternating with nutation for tailored excitation (DANTE) can effectively suppress the flow at a velocity as slow as 0.1 cm/s. However, flow suppression effect is also diminished when CSF flow is below 0.1 cm/s, which can be problematic when evaluating the middle cerebral arteries as they course in the Sylvian fissure.13 A trailing magnetization flip-down module14 (implemented on Siemens systems) or an anti-DRIVE preparation (implemented on Philips systems)15 can recycle and invert the magnetization of long-T2 spins, such as CSF, at the end of each long echo train to introduce nulling effects during the following inversion recovery period. This effect suppresses CSF signal independent of its velocity. However, these global flow suppression approaches can inevitably reduce the desired vessel wall T1 contrast weighting.16
Spatial resolution is another technical consideration for intracranial VWI. The ASNR VWI study group recommends an acquired resolution of 0.5 mm.1 Mean vessel wall thickness of ex vivo imaged circle of Willis specimens on 7 Tesla VWI reportedly ranges from 0.45 to 0.66 mm.17 The ability to image and precisely measure vessel wall features should be interpreted in light of the spatial resolution. While depicting vessel wall enhancement on lower-spatial resolutions may take advantage of partial volume effects, measuring wall remodeling index may be less precise if voxel sizes are larger than the true vessel wall thickness.
Motion degradation is also a common challenge in VWI due to long acquisition times. In VWI investigations, up to 17% of cases were excluded due to motion degradation.8 New motion-compensation approaches combining volumetric navigator and self-gating to compensate for bulk and localized movements in VWI have shown to improve overall image quality and vessel sharpness.18 Other strategies include the use of acceleration techniques such as extended echo train lengths with T1/T2 relaxation modulation,19 compressed sensing20,21 or limiting the imaging field of view to the area of interest, for example in aneurysm imaging.
Image Interpretation and Pitfalls
VWI image interpretation remains an active area of research. One key limitation of the intracranial VWI literature is the paucity of histopathologic correlation. The presence of vessel wall enhancement on intracranial VWI is thought to favor a pathologic process, particularly as most intracranial arteries lack vasa vasorum.22 However, the mechanism of vessel wall enhancement is incompletely understood and it is becoming increasingly apparent that multiple mechanisms causing vessel wall enhancement may occur among, and even within, disease and non-disease categories. Autopsy studies report vasa vasorum in segments of the circle of Willis in older individuals raising the possibility that mild vessel wall enhancement may be associated with aging.23 Artifactual wall thickening or enhancement can also be caused by incomplete blood flow suppression near the vessel wall or near curved large vessel segments. Slow flow in neighboring veins may also be misinterpreted as arterial wall enhancement. Inadequate spatial resolution may induce partial volume averaging and can lead to the overestimation of wall thickness. Awareness of these pitfalls is critical to interpreting VWI.
The evolution of the disease pathology may also lead to misclassification. For example, although active vasculitis is associated with concentric vessel wall enhancement, eccentric involvement can also be seen, possibly due to superimposed atherosclerosis. Moreover, cases of treated vasculitis may show persistent wall enhancement. In fact, the presence of vessel wall enhancement does not necessarily indicate inflammation as non-inflammatory conditions such as moyamoya disease and non-inflammatory cerebral amyloid angiopathy have been associated with enhancement.24,25 An important step to avoid such pitfalls is the interpretation of VWI within the context of all available clinical data and in conjunction with other luminal and cross-sectional studies.
Intracranial Vasculopathies
Intracranial Atherosclerosis
Intracranial atherosclerosis (ICAS) is one of the most common causes of ischemic stroke and is especially prevalent in African, Asian, and Hispanic populations.26,27 In the evaluation of ICAS, VWI may be useful for the identification and characterization of a culprit (e.g., infarct causing) plaque (Figure 2), identification of plaque without substantial luminal narrowing (Figure 3), and assessment of branch occlusive disease where atherosclerotic plaque in the parent artery occludes perforator origins. Much has been learned about imaging plaque from cervical carotid artery atherosclerosis largely driven by the availability of carotid endarterectomy specimens. These carotid studies suggest the presence of an enhancing fibrous cap, an adjacent nonenhancing lipid core, and peripheral thin outer rim of enhancement representing vasa vasorum in the adventitia of the artery. Compared to carotid arteries, detailed imaging of intracranial plaque is more challenging, in part, due to plaque size.
Figure 2: VWI of Symptomatic ICAS with Vessel Wall Enhancement.
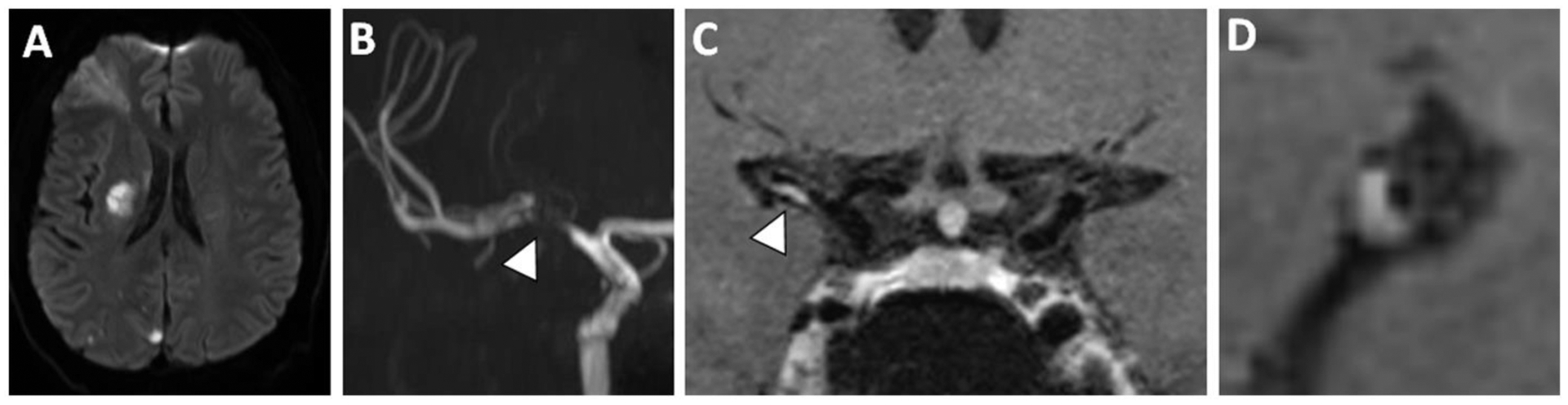
(A) A patient with a right middle cerebral artery (MCA) territory infarct showed (B) severe flow-limiting stenosis of the proximal right MCA on 3D TOF MRA. Postcontrast T1w VWI (C) coronal and (D) sagittal-oblique images show eccentric enhancement and is likely the causative culprit plaque.
Figure 3: VWI of Nonstenotic Basilar Artery Plaque.
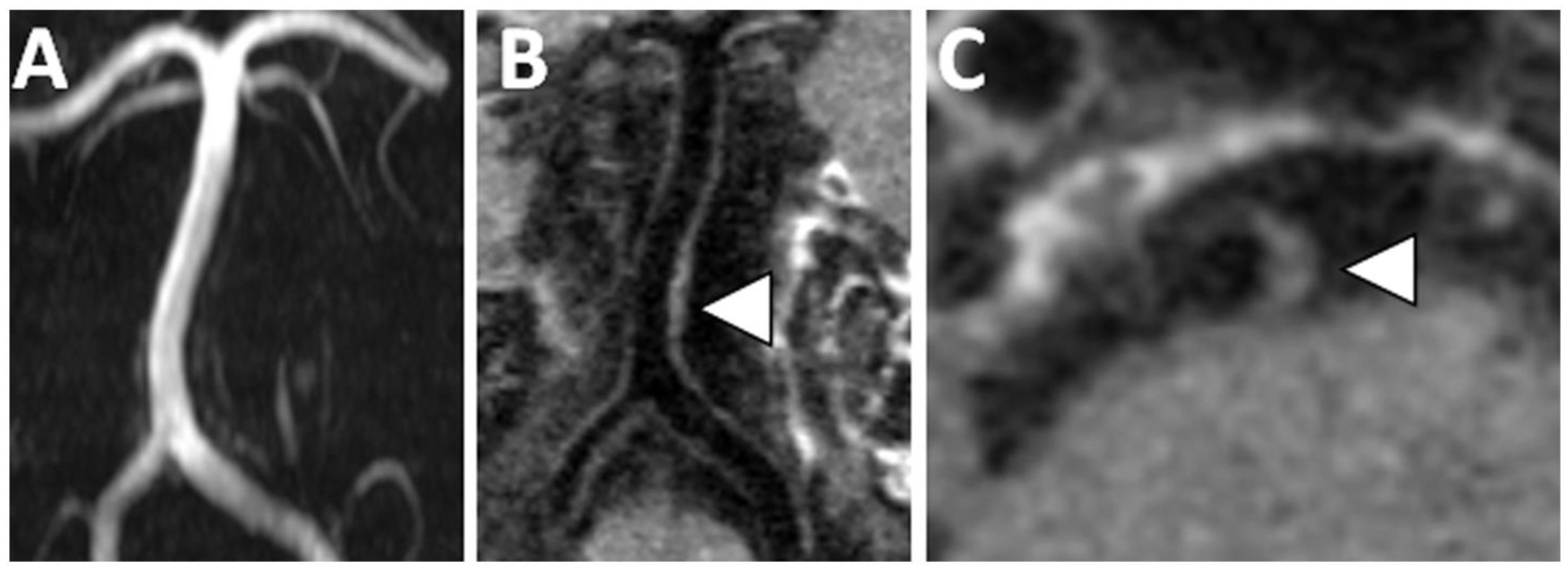
(A) A 3D TOF MRA shows no significant stenosis of the basilar artery. (B) However, on precontrast T1w VWI, there is eccentric vessel wall thickening along the left side-wall revealing plaque. (C) Axial VWI image through the basilar artery confirms eccentric vessel wall thickening.
The most common VWI feature used to identify culprit plaque is plaque enhancement. Numerous articles and recent meta-analyses show wall enhancement is significantly associated with culprit plaque with an odds ratio of 7.42 (95% CI 3.35–16.43) (Figure 4).28–31 Intracranial plaque enhancement has also been reported to be predictive of stroke recurrence.32 Additional VWI features such as intrinsic T1 hyperintensity (Figure 5), vessel wall thickening (e.g., eccentricity and outward (positive) wall remodeling), and plaque surface irregularity have also been described.33,34 Intrinsic T1 hyperintensity on precontrast VWI may be a surrogate imaging biomarker for intraplaque hemorrhage with few rare case reports histologically validating this finding.35,36 Studies show intrinsic T1 hyperintensity is more common in symptomatic than asymptomatic plaque37 and the degree of intrinsic T1 hyperintensity during medical therapy may be related to stroke recurrence.38 Notably, questions have been raised whether there is a differential composition of ICAS, including prevalence of intrinsic T1 hyperintensity, among races.39,40 However, larger studies are needed to understand environmental, genetic, and racial differences.
Figure 4: VWI of Symptomatic ICAS with T2 Hyperintensity and Enhancement.
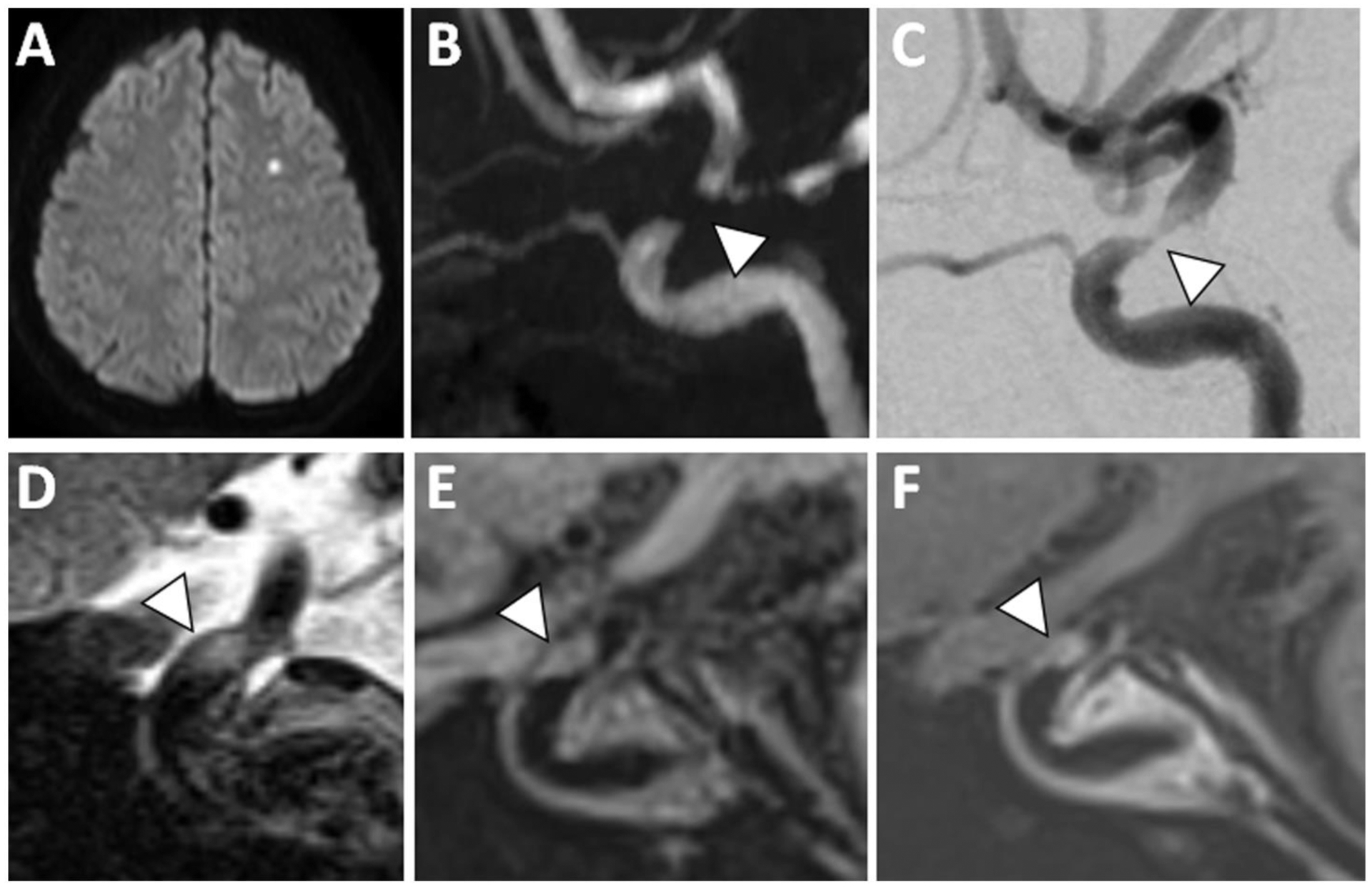
(A) A patient with acute infarction in the left centrum semiovale showed (B) severe flow-limiting stenosis in the left supraclinoid internal carotid artery on 3D TOF MRA (arrowhead). (C) Cerebral angiogram showed trace flow through this severely stenotic segment (arrowhead). (D) At the site of stenosis, sagittal T2w VWI showed eccentric T2 hyperintense signal (arrowhead). (E) Precontrast T1w VWI and (F) postcontrast T1w VWI reveals plaque enhancement (arrowhead).
Figure 5: VWI of Symptomatic ICAS with T1 Hyperintensity.
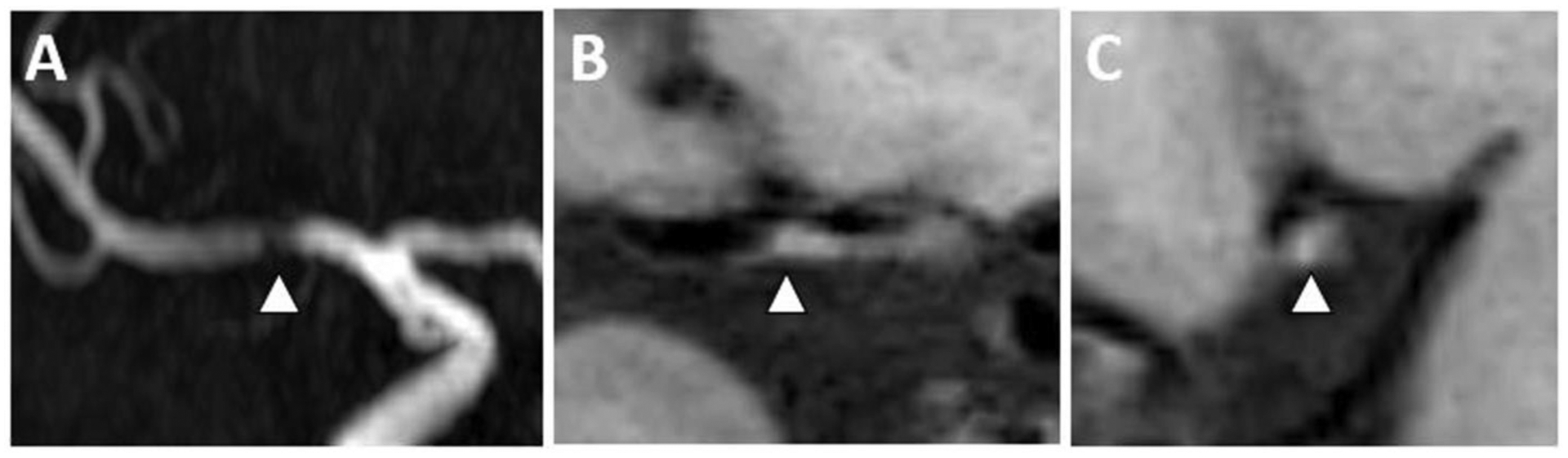
(A) A 3D TOF MRA shows flow-limiting right M1 MCA stenosis (arrowhead). (B) Precontrast T1w VWI coronal and (C) sagittal planes through the MCA stenosis shows eccentric wall thickening (arrowhead) with intrinsic T1 hyperintense signal (arrowhead) of a symptomatic plaque.
Vessel wall thickening has also been of great interest as eccentricity was reported in earlier studies to help distinguish ICAS from other vasculopathies. However, VWI and autopsy studies report an overlap of eccentric and concentric vessel wall thickening in plaque morphology.41,42 Furthermore, more recent meta-analyses have not shown eccentricity to be strongly associated with plaque.33 Other vessel wall adaptions include the ability of the vessel wall to remodel inwardly or outwardly. Outward (positive) wall remodeling has been strongly associated with symptomatic ICAS with an odds ratio of 5.60 (95% CI 2.23–14.03) in a meta-analysis.34 VWI has a particularly important role in detecting plaque with outward remodeling as the lumen is typically preserved (Figure 3). Studies show improved detection of abnormal vessel segments, including ICAS, with VWI compared to luminal imaging alone.2,43 Additionally, VWI can help further characterize the location and quadrant of ICAS along the artery wall in perforator infarcts in branch occlusive disease.44,45 Finally, presence of T2 hyperintense signal may be helpful in identifying ICAS.42 T2 signal may reflect the fibrous cap, evolving intraplaque hemorrhage or thrombus.46,47
Moyamoya Disease
VWI may be useful for the diagnosis of moyamoya disease (MMD) and assessment of disease activity. MMD is an idiopathic steno-occlusive vasculopathy that can involve the distal internal carotid arteries and proximal middle and anterior cerebral arteries. MMD without associated small vessel collateralization can be indistinguishable from other steno-occlusive diseases, such as atherosclerosis on luminal imaging. VWI features of MMD appear to be inward (negative) remodeling, as the tunica media decreases in thickness and the vessel shrinks usually with concentric vessel wall involvement (Figure 6).48–50 In later stages, the involved arteries may no longer be identifiable. Histopathologic studies also report reduced outer vessel wall diameters, thinning of the media, thicker intima and an overall lack of inflammatory cells in the vessel wall.51 Vessel wall enhancement is reportedly variable ranging from absent to marked. Since MMD is generally considered to be a non-inflammatory condition, it has been speculated that when present, vessel wall enhancement may represent intimal proliferation.52 Recent studies have also indicated the presence of enhancement may be predictive of stenosis progression or ischemic infarcts.53,54 Additional prospective confirmatory studies would be of value.
Figure 6: VWI of Moyamoya Disease.
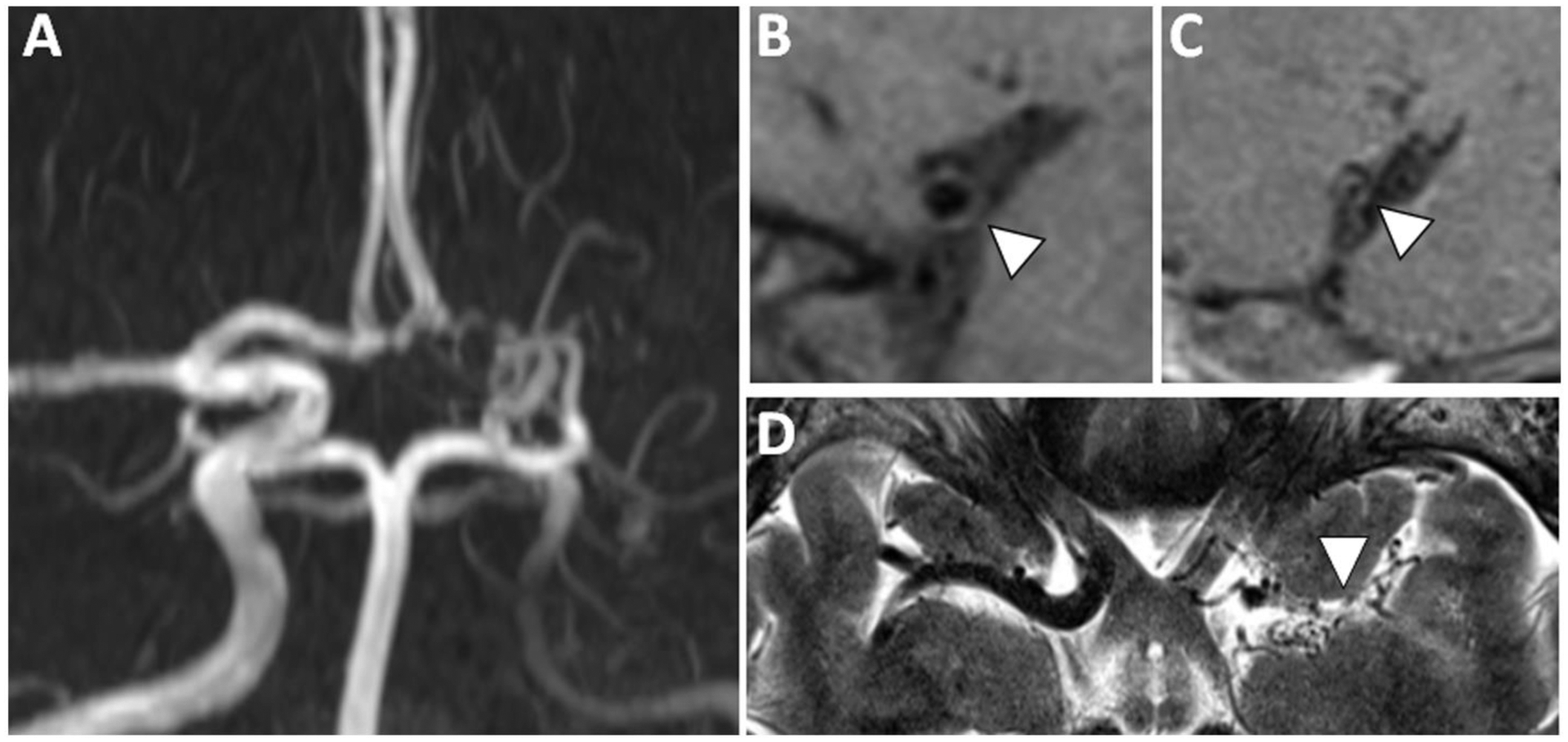
(A) 3D TOF MRA shows severe stenosis of the left internal carotid artery terminus, severe irregularity of the left A1 anterior cerebral artery and nonvisualization of the left M1 middle cerebral artery (MCA) with lenticulostriate collaterals in a patient with moyamoya disease (MMD). (B) Sagittal-oblique precontrast T1w VWI of the right M1 MCA shows a thin vessel wall with preservation of the lumen diameter and appears normal. (C) The affected left M1 MCA shows small outer and inner diameters consistent with inward (negative) wall remodeling, a feature reported in MMD. (D) Axial T2w VWI shows lenticulostriate collaterals at the site of the left M1 MCA.
Vasculitis
Uses of VWI in the evaluation of vasculitis include diagnosis, localization for biopsy, and disease activity assessment.55 Vasculitis of the central nervous system (CNS) can be due to an infectious or immune mediated etiology, which can be further divided into primary angiitis of the CNS (PACNS) or secondary involvement of systemic vasculitides. VWI features of vasculitis include vessel wall enhancement and thickening that are usually concentric and can also show peri-adventitial (or perivascular) enhancement of adjacent brain parenchyma.56 There is substantial overlap of the VWI features of infectious or immune-mediated vasculitis. Thus, clinical factors are crucial to add diagnostic specificity and a complete neurologic and rheumatologic work-up may be required. Technical considerations for CNS vasculitis include whole brain VWI coverage to assess the proximal and distal arteries.57 Pulse sequences to assess for pachymeningeal and leptomeningeal enhancement as well as for microhemorrhages may be helpful in image interpretation.
Inflammatory Vasculitis
VWI imaging features of PACNS have been associated with concentric, homogeneous, and smooth vessel wall enhancement (Figure 7).58,59 It is hypothesized that the marked arterial wall enhancement in CNS vasculitis is due to contrast leakage from the arterial lumen directly into an inflamed wall or from engorged and enlarged vasa vasorum.60,61 Angiographic imaging may show multifocal irregular luminal narrowing concurrent with acute and chronic infarcts. Due to high false negative detection rates with DSA and MRA,62 other noninvasive imaging modalities, such as intracranial VWI, to detect vessel wall inflammation would be prudent prior to pursuing invasive brain biopsies, which can lead to meaningful complications.63 It is worth noting that PACNS is a heterogeneous disease and can affect small caliber pial and leptomeningeal vessels, which may be below the resolution of achievable VWI spatial resolutions.64 Thus inflammatory vasculitis may still be a possibility in the absence of vessel wall enhancement.
Figure 7: VWI of Primary Angiitis of the Central Nervous System.
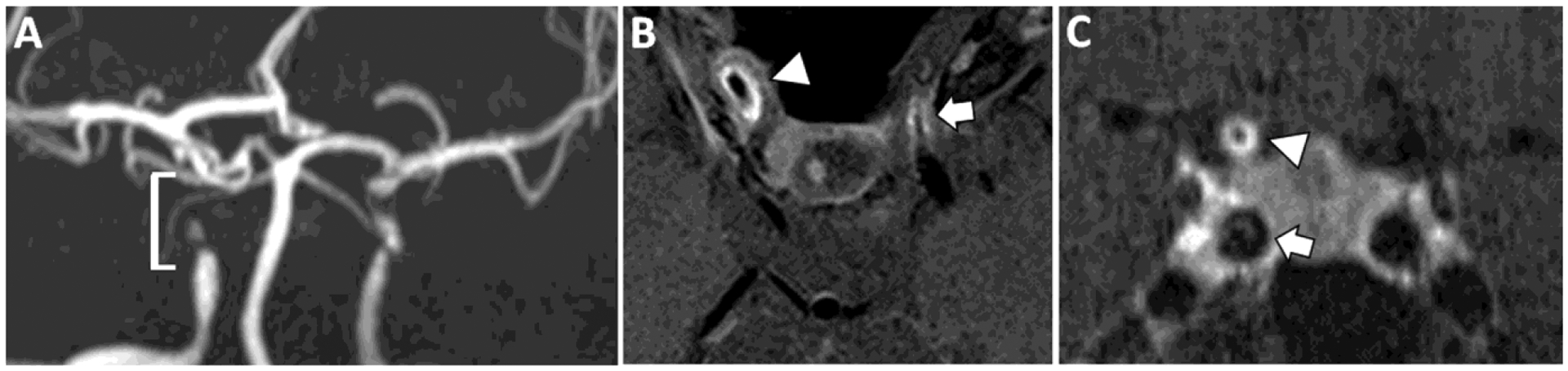
(A) A patient with a diagnosis of primary angiitis of the CNS shows flow-limiting stenosis of the right cavernous and supraclinoid internal carotid artery (ICA, bracket). Similar findings are seen in the contralateral left ICA. (B) Postcontrast T1w VWI shows avid concentric vessel wall thickening and enhancement of the stenotic ICA segments (right, arrowhead and left, arrow). (C) Coronal postcontrast T1w VWI confirms concentric vessel wall thickening and enhancement of the right supraclinoid ICA (arrowhead). By contrast, the right cavernous ICA (arrowhead) shows features of atherosclerotic plaque with eccentric wall thickening and a linear overlying enhancing rim favored to be a fibrous cap (arrow).
Infectious Vasculitis
Infectious CNS vasculitis can be caused by many different types of organisms including bacteria, fungi, and viruses. Infectious vasculitides can be categorized as a primary cerebrovascular infection or a secondary infection of the vessel wall due to a primary brain infection (e.g., meningitis). For the latter, the vasculitic component is a complication and targeted infectious therapy in addition to adjunctive treatment with steroids or anti-inflammatory treatment may be needed.65,66 Serial VWI may be able to help ensure resolution of the vasculitic complication.67
One of the more common causes of infectious cerebrovascular CNS vasculitis is varicella zoster vasculitis (VZV). Following a primary infection, the virus remains latent in the neurons of the cranial nerves, dorsal root, and autonomic ganglia of the neuroaxis. With aging and in immune-compromised states, VZV reactivation can occur leading to herpes zoster. After reactivation, the virus can spread transaxonally from cranial or cervical ganglia to infect the arterial adventitia and extend transmurally causing a vasculitis.68 On VWI, vessel wall inflammation manifests as concentric wall thickening and enhancement and can also demonstrate perivascular enhancement (Figure 8).69
Figure 8: VWI of VZV vasculitis.

(A) A patient with a diagnosis of varicella zoster vasculitis shows multiple segments of avid wall thickening and enhancement along the course of the bilateral anterior cerebral arteries (arrows) including the pericallosal artery (arrowhead) on postcontrast T1w VWI. (B) Coronal plane of the enhancing pericallosal artery segment shows both concentric wall enhancement as well as enhancement extending beyond the vessel wall borders, characteristic of perivascular enhancement (arrowhead). (C–D) After 2 months of treatment with acyclovir, there was improvement in the degree of vessel wall and perivascular enhancement.
Arterial Dissection
Arterial dissections are challenging diagnoses. The dissection can occur at the intimal or adventitial layer of the vessel wall and result in different complications and imaging manifestations.70 When a subintimal dissection occurs, an intramural hematoma between the media and internal elastic lamina forms and can lead to luminal narrowing or occlusion. Sequela of subintimal dissections include ischemia by hypoperfusion, ostial occlusion, or thromboemboli. A subadventitial dissection results in the hematoma extending through the media and adventitia, which can lead to subarachnoid hemorrhage. In some cases, a pseudoaneurysm forms. However, in other cases, the luminal contours are preserved, and these lesions can be angiographically occult.33
Conventional T1-weighted turbo spin echo imaging with fat suppression is often used to image intramural hematoma within the false lumen. However, the higher isotropic spatial resolution and black-blood imaging technique of VWI can improve characterization and detection. 3D VWI has been shown to better characterize and detect vertebrobasilar dissections than both MRA and 2D spin echo techniques.71 The inclusion of a precontrast T1-weighted VWI sequence with fat suppression is a requisite for the detection of dissections. Postcontrast VWI may be helpful for characterizing chronic dissections by showing vessel wall thickening and enhancement, particularly if the intramural hematoma has evolved and no longer shows intrinsic T1-hyperintense blood products.72 Furthermore, postcontrast imaging may also be helpful in the detection of both intramural hematoma and intraluminal thrombus, as the thin intimal flap can be seen separating the true and false lumens (Figure 9).73
Figure 9: Intracranial Vertebral Artery Dissection on VWI.
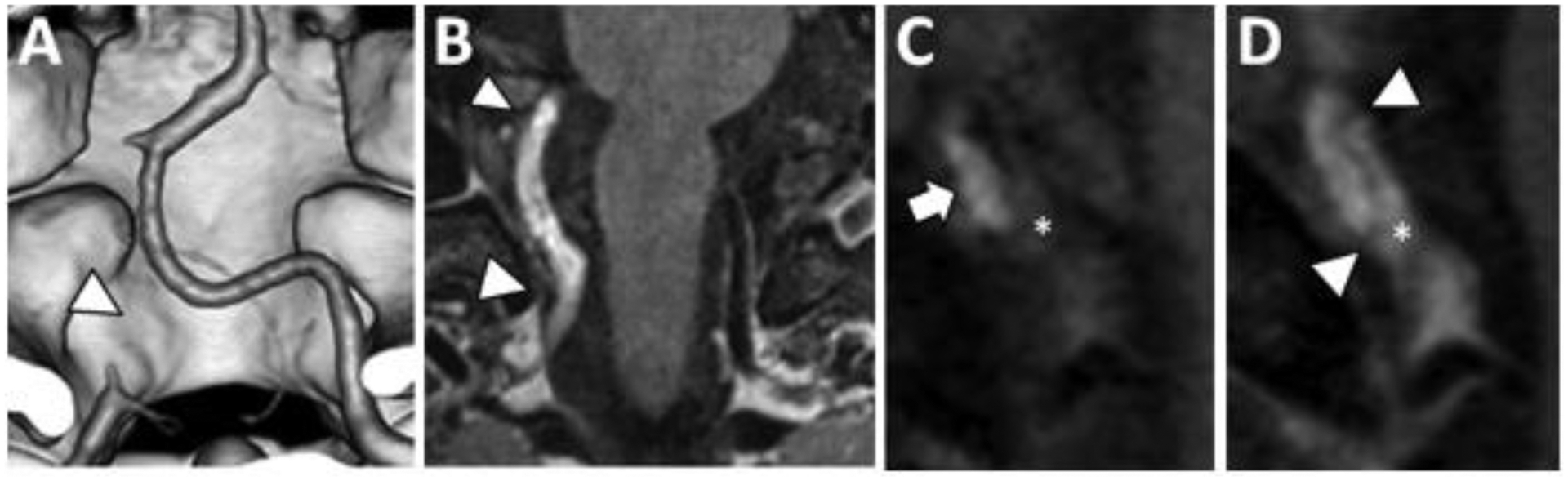
(A) Coronal 3D surface rendered CTA image shows occlusion of the right intradural vertebral artery (arrowhead). (B) Coronal postcontrast T1w VWI shows abnormal signal in a long segment of the right intradural vertebral artery (arrowheads). (C) Coronal oblique precontrast T1w VWI shows mural hematoma (arrow) and intraluminal thrombus (asterisk). (D) The intraluminal thrombus enhances on coronal oblique postcontrast T1w VWI (asterisk). A thin intimal flap is detected (arrowheads) spiraling through the lumen and separating the intraluminal thrombus from the intramural hematoma.
Intracerebral Aneurysm
The two primary categories of VWI for aneurysms include the identification of a ruptured aneurysm and risk-stratification of an unruptured aneurysm. Aneurysm wall enhancement (AWE) may reflect rupture-induced inflammation or inflammatory changes related to aneurysm formation or growth. Studies have shown, however, that AWE is nonspecific and has been associated with macrophage infiltration, neovascularization, atherosclerotic changes, and wall-adherent thrombus.74–78
The unique geometric morphology of aneurysms requires several technical VWI considerations. Complex flow dynamics can influence the aneurysm wall signal intensity and lead to wall-adjacent hyperintensities, sometimes described as “pseudo-enhancement.” The application of MSDE or DANTE preparation pulses improves performance of slow-flow suppression and mitigates this pitfall.79 Voxel size also influences the efficiency of blood signal suppression though the effect depends on intra-aneurysmal flow velocity. For aneurysms with higher flow rates, a smaller voxel size achieves better signal suppression likely due to faster outflow within the voxel. With slower intra-aneurysmal flow rates, a larger voxel size is more efficient for signal suppression due to intravoxel dephasing.80 For clinical applications, a smaller voxel size is recommended to accurately depict morphological features.1 Postmortem and intraoperatively acquired intracranial aneurysms report aneurysm walls measure between 0.02 to 0.50 mm.81 Aneurysms that are partially thrombosed will require additional attention as enhancing thrombus near the wall may mimic wall inflammation.74 Additional technical considerations include limiting the field-of-view to the aneurysm only thereby achieving higher spatial resolutions over shorter acquisition times. However, this may require a radiologist to monitor the examination and identify the area of interest.
Ruptured cerebral aneurysms
Nontraumatic subarachnoid hemorrhage is a devastating disease with a mortality rate up to 45% within 1 month after the event. A ruptured saccular aneurysm is the most common cause.82 Obtaining a CTA and DSA to identify the source of hemorrhage is typically first-line of evaluation. However, when there are multiple aneurysms identified, VWI may be useful for identifying the culprit aneurysm. Up to 30% of patients with nontraumatic subarachnoid hemorrhage have multiple aneurysms.83 Several studies have shown that in patients with multiple aneurysms, the ruptured aneurysm shows AWE.84,85 In studies that correlate in vivo VWI with subsequent histology of clipped aneurysms, the imaged vessel wall enhancement corresponds to an area of wall thinning and inflammation, confirming rupture-induced inflammation.74
VWI may also guide treatment in settings of angiogram-negative subarachnoid hemorrhage. After a comprehensive angiographic assessment, up to 20% of these cases show no identifiable lesion.86,87 For these negative cases, 10% have recurrent hemorrhage and 20% have poor clinical outcomes.88 The ability to detect a culprit lesion in these cases is critical to the patient’s prognosis. Jung et al showed that VWI was able to detect the culprit source of subarachnoid hemorrhage in 53% of cases that revealed no source on cerebral catheter angiogram.89 Culprit lesions identified by VWI included arterial dissections (n=5), blister aneurysms (n=3), and fusiform aneurysm (n=1). Cox et al reported detection of a basilar perforator aneurysm by VWI that was not seen on initial CTA and DSA imaging.90 This may be due to a small thrombus filling the aneurysm sac in the acute setting. These cases highlight a potential invaluable diagnostic role for patients with nontraumatic subarachnoid hemorrhage.
Unruptured cerebral aneurysms
VWI assessment for aneurysm stability is potentially very useful. Primary treatment considerations remain largely based on results from the International Study of Unruptured Intracranial Aneurysms Study.91 In addition to aneurysm size and location, numerous other patient and morphologic features of aneurysms have been assessed and incorporated into prediction scores. However, additional markers to predict aneurysm rupture would be useful. This is an active area of investigation with several multicenter prospective studies underway.
Edjlali et al performed a prospective VWI study of 87 patients with 108 aneurysms and showed that among features of size, location, multiplicity of aneurysms, daily aspirin intake, and AWE, only AWE was significantly associated with unstable (ruptured, symptomatic, or evolving) status (OR 9.20, 95% CI 2.92–29).92 A meta-analysis of longitudinal studies further supported this result identifying aneurysms with AWE on initial VWI had a significantly higher risk of being unstable at follow-up with a risk ratio of 3.6 (95% CI 1.7–7.5). The study also highlighted a high negative predictive value (96%, 95% CI 93.2–97.7%) indicating the absence of wall enhancement may be a predictor of aneurysm stability (Figure 10).93
Figure 10: Stable basilar tip aneurysm without enhancement.
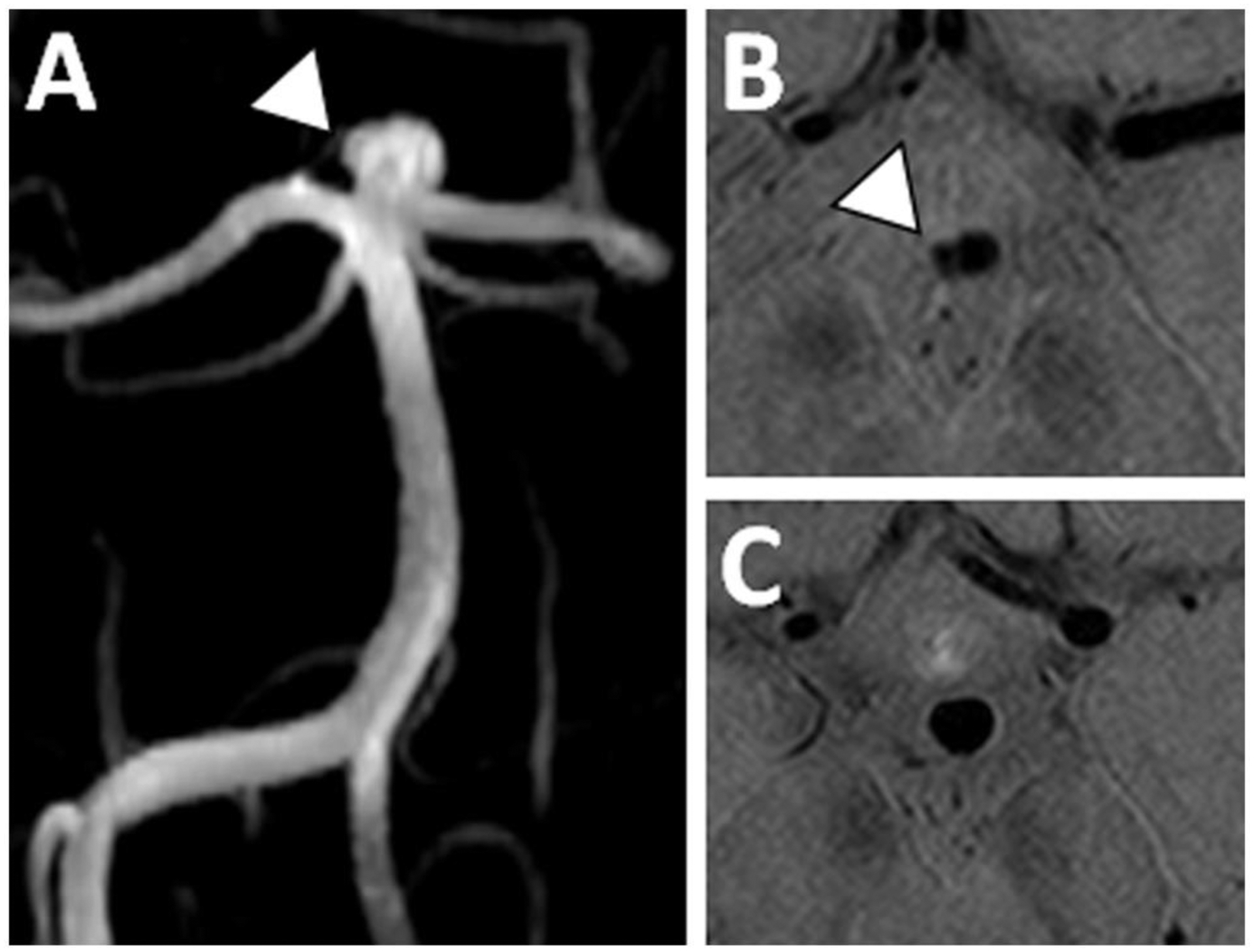
(A) A 3D TOF MRA shows a basilar tip aneurysm with a small daughter sac along the superolateral wall (arrowhead). (B–C) Axial postcontrast PDw VWI shows no aneurysm wall enhancement near the aneurysm daughter sac (B) nor near the aneurysm neck (C).
These results are promising but AWE should be clinically interpreted with caution. Reports of ruptured aneurysms without AWE suggest some aneurysms with thin thrombosis-lined hypocellular walls may not show enhancement.94 Conversely, Edjlali et al also reported that nearly 1/3 of the stable aneurysms also showed AWE,92 which may be explained by a vessel wall remodeling and repair mechanism (Figure 11). These mechanisms involve the secretion of matrix metalloproteinases which allow for the migration and proliferation of vascular smooth muscle cells to form a thickened fibroid layer on the luminal surface.95–97 Finally, while slow-flow artifacts adjacent to the inner wall often mimic AWE, slow-flow artifacts are more prone in areas of low wall sheer stress, which is associated with aneurysm rupture.98,99 Thus, the presence of slow flow artifacts and pseudo-enhancement near the aneurysm wall may also be an indirect marker of wall inflammation. The literature to date largely consists of retrospective, cross-sectional studies with varied definitions of AWE leading to some conflicting conclusions. More rigorous prospective longitudinal studies, accounting for VWI technique, aneurysm geometry, and flow dynamics, are needed to understand the role of VWI for aneurysm surveillance.
Figure 11: Stable fusiform pericallosal aneurysm with enhancement.
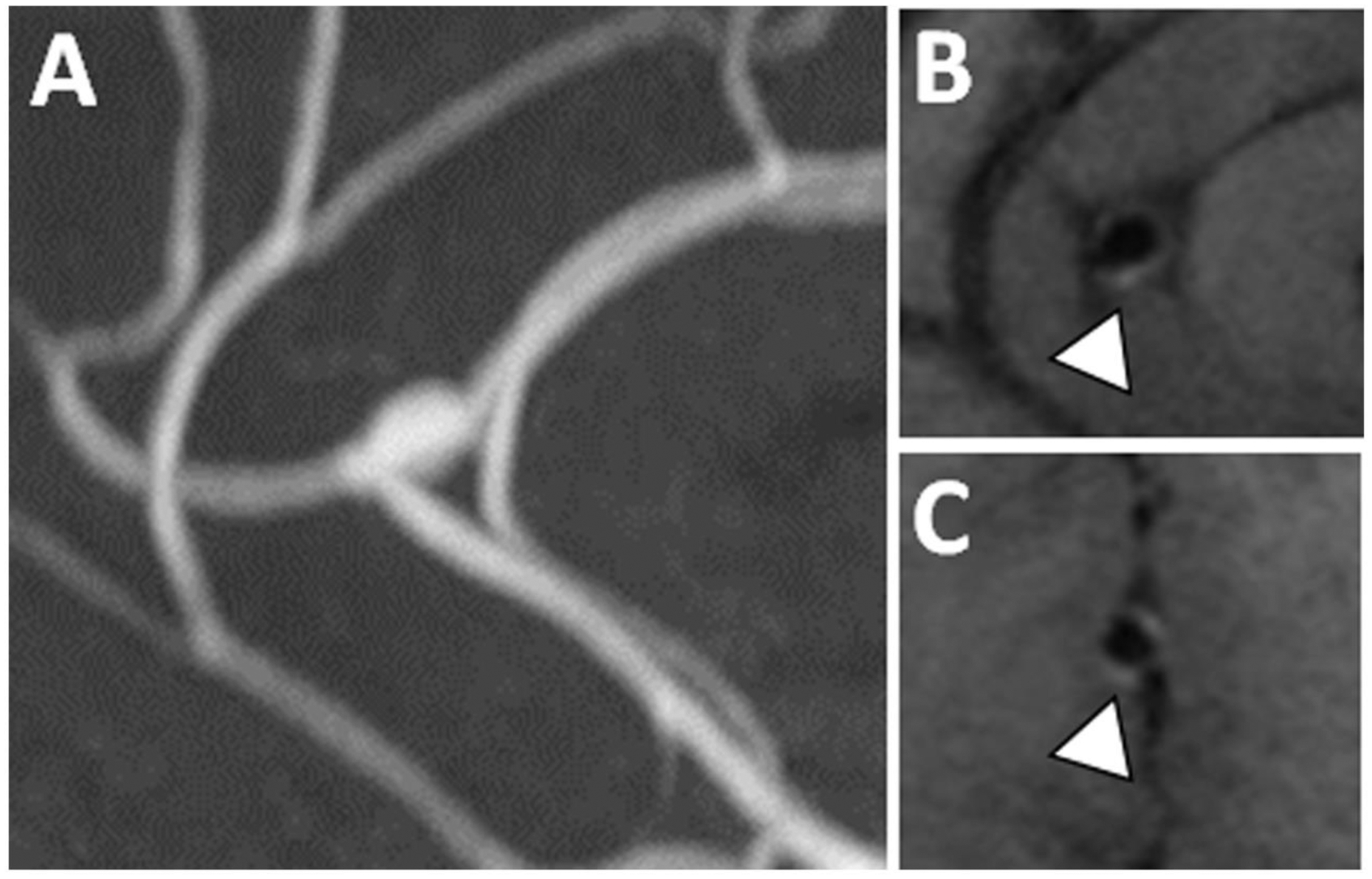
(A) A fusiform pericallosal artery aneurysm seen on 3D TOF MRA was imaged with postcontrast T1w VWI. (B–C) A thin rim of aneurysm wall enhancement was present on postcontrast T1w VWI in this asymptomatic patient.
Reversible Cerebral Vasoconstriction Syndrome
Reversible cerebral vasoconstriction syndrome (RCVS) is an autonomic disease that often presents with a severe onset thunderclap headache. On imaging, it is associated with multifocal vasoconstriction of intracranial arteries that is reversible within 3 months. RCVS typically affects young or middle aged females. RCVS is associated with specific triggers, such as vasoactive drugs post-partum status, blood transfusions, among others.100,101 Recently, the RCVS2 score was developed incorporating imaging and clinical features to distinguish RCVS from mimics.102 Positive points in the score include presence of thunderclap headaches, female sex, an identified vasoactive trigger, subarachnoid hemorrhage, and lack of (large vessel) carotid artery involvement. Although RCVS is typically clinically benign, complications such as ischemia and hemorrhage can lead to substantial morbidity.
Identifying RCVS from other intracranial steno-occlusive disease is important as treatment strategies differ. RCVS is managed conservatively and the triggering agent, once identified, is stopped. Misdiagnosis and administering an immunosuppressive or anti-inflammatory agent can worsen the vasoconstriction in RCVS.103 Angiographic imaging will typically show a beaded pattern of multifocal alternating vasoconstriction interspersed with normal caliber or slightly dilated vessel diameter. In vasospastic arteries, smooth muscle cells not only shorten in length but also increasingly overlap resulting in a drastic increase in wall thickness and concurrent luminal narrowing.104 In RCVS, limited histopathologic studies have also shown an absence of arterial wall inflammation.105 In keeping with this, most VWI studies suggest a relative paucity of vessel wall enhancement in RCVS given the non-inflammatory pathophysiology.58,106 However, diffuse, smooth vessel wall enhancement has also been reported in RCVS,42 raising the possibility of a non-inflammatory vessel wall mechanism of contrast leakage (Figure 12). The varied VWI appearances of RCVS are not yet well understood. More studies are warranted to gain insight into the pathophysiology of RCVS and mechanisms of contrast enhancement on VWI.
Figure 12: VWI of Reversible Cerebral Vasoconstriction Syndrome.
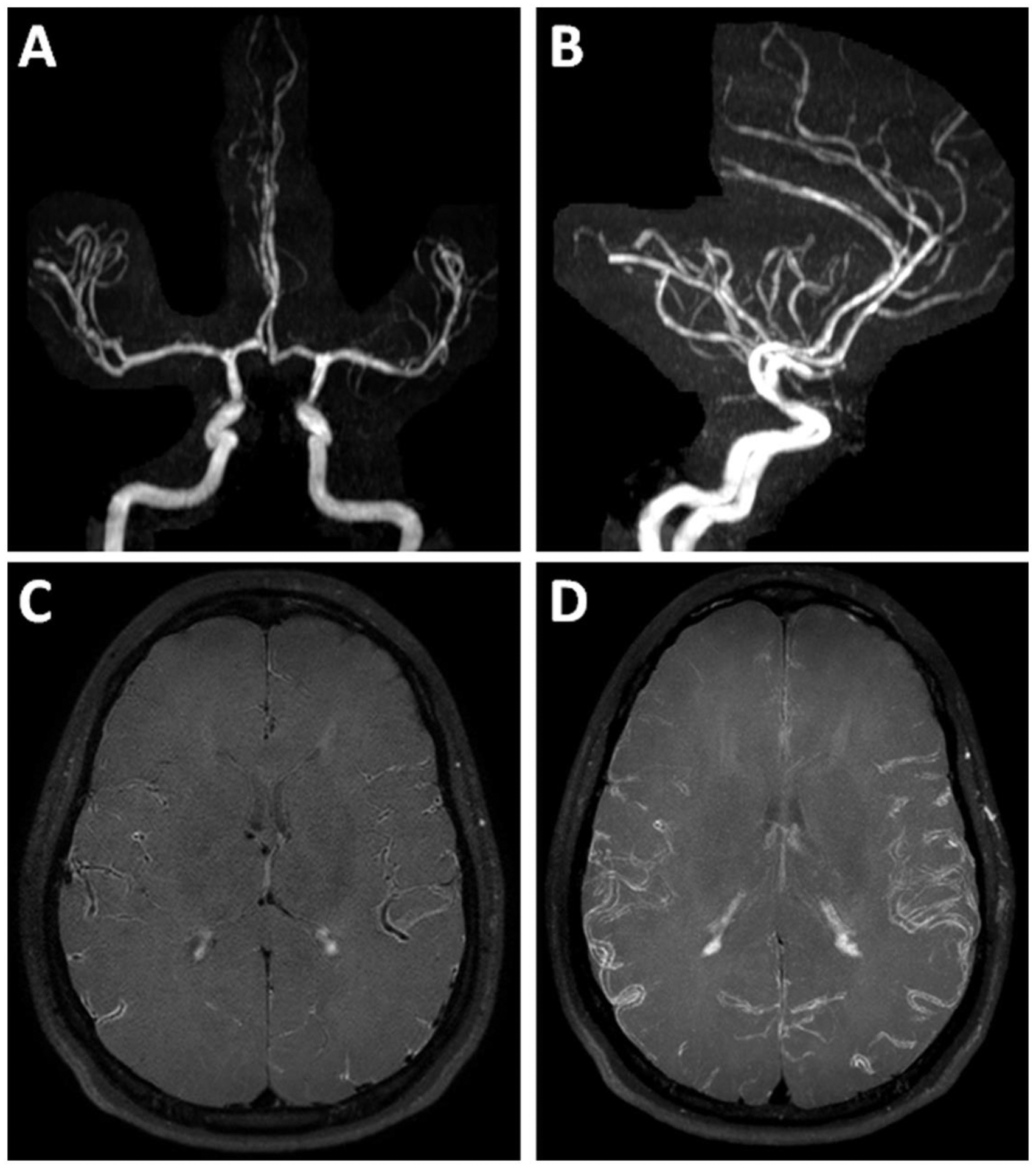
(A–B) 3D TOF MRA maximum image projection images show diffuse irregularity of the cerebral vasculature. (C) Axial postcontrast T1w VWI and (D) a maximum image projection shows diffuse vessel wall enhancement. Clinical symptoms of severe headache resolved after starting a calcium channel blocker. Patient was diagnosed with RCVS after a negative laboratory work-up. A 1-month follow-up MRI showed complete resolution of the imaging findings (not shown).
Cerebral Amyloid Angiopathy
Cerebral amyloid angiopathy (CAA) is characterized by amyloid beta-peptide deposition within the vessel walls of small- to medium-sized blood vessels in the brains and leptomeninges. While lobar hemorrhage is one of the most common acute presentations in older patients with CAA, microinfarcts are also present in up to 30%–60% of patients107 and contribute to cortical thinning and cognitive decline.108,109 Few studies have reported the presence of vessel wall enhancement with both inflammatory and non-inflammatory CAA. A case series showed presence of vessel wall enhancement in 2 of 5 cases of biopsy-proven non-inflammatory CAA.25 They hypothesized that the disruption of the blood-brain barrier and increased permeability in CAA may explain vessel wall enhancement. Notably, numerous microhemorrhages were also present in the 2 cases with vessel wall enhancement. In a larger retrospective study, 58% of cases among 50 patients diagnosed with CAA showed vessel wall enhancement. In this study, acute and future ischemic strokes in patients with CAA were significantly associated with vessel wall enhancement.110 More studies are warranted to understand the utility of VWI for CAA.
Future Directions
As intracranial VWI techniques continue to evolve, comparisons of protocols, techniques, and imaging features to study will likely differ by vasculopathy type. There is increasing awareness that different CNS vasculopathies may have different imaging features that can be interpreted qualitatively or quantitatively. Quantification of VWI imaging characteristics have recently become popular because of its precision. Inter-, intra-observer, and scan-rescan reproducibility in vessel wall morphology quantification are excellent,111,112 which makes the modality suitable for treatment response tracking in, for example, ICAS.38 Automated vessel wall image analysis will make the interpretation of intracranial VWI more reproducible and time efficient and less expertise dependent, thus potentially facilitating widespread applications of VWI in clinical practice.113 VWI radiomics will likely help study population-based trends for intracranial vasculopathies.114 Prospective, longitudinal studies are needed to understand the evolution of pathologic VWI findings for CNS vasculopathies and assess the impact of VWI on patient outcomes.
Summary
Intracranial VWI has a wide array of potential clinical applications. As technical innovations improve our ability to image small intracranial vessel walls and our scientific understanding of imaging appearances of intracranial vasculopathies expand, VWI will likely take an important role as part of the diagnostic work-up of patients with cerebrovascular diseases.
Acknowledgement:
Supported in part by the Institute for Translational Medicine and Therapeutics/Thomas B. McCabe and Jeannette E. Laws McCabe Fund (JWS) and NIH/NHLBI R01 HL147355 (ZF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 38:218–29, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song JW, Obusez EC, Raymond SB, et al. Vessel Wall MRI Added to MR Angiography in the Evaluation of Suspected Vasculopathies. J Neuroimaging 29:454–7, 2019. [DOI] [PubMed] [Google Scholar]
- 3.Song JW, Guiry SC, Shou H, et al. Qualitative Assessment and Reporting Quality of Intracranial Vessel Wall MR Imaging Studies: A Systematic Review. AJNR Am J Neuroradiol 40:2025–32, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaafsma JD, Rawal S, Coutinho JM, et al. Diagnostic Impact of Intracranial Vessel Wall MRI in 205 Patients with Ischemic Stroke or TIA. AJNR Am J Neuroradiol 40:1701–6, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antiga L, Wasserman BA, Steinman DA On the overestimation of early wall thickening at the carotid bulb by black blood MRI, with implications for coronary and vulnerable plaque imaging. Magn Reson Med 60:1020–8, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Qiao Y, Steinman DA, Qin Q, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. Journal of magnetic resonance imaging: JMRI 34:22–30, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Lindenholz A, van der Kolk AG, Zwanenburg JJM, et al. The Use and Pitfalls of Intracranial Vessel Wall Imaging: How We Do It. Radiology 286:12–28, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Song JW, Moon BF, Burke MP,et al. MRI of Intracranial Vessel Wall MR: A Systematic Review. Journal of Neuroimaging 30:428–442, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelman RR, Mattle HP, Wallner B, et al. Extracranial carotid arteries: evaluation with “black blood” MR angiography. Radiology 177:45–50, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Alexander AL, Buswell HR, Sun Y, et al. Intracranial black-blood MR angiography with high-resolution 3D fast spin echo. Magn Reson Med 40:298–310, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Nagahata S, Nagahata M, Obara M, et al. Wall Enhancement of the Intracranial Aneurysms Revealed by Magnetic Resonance Vessel Wall Imaging Using Three-Dimensional Turbo Spin-Echo Sequence with Motion-Sensitized Driven-Equilibrium: A Sign of Ruptured Aneurysm? Clin Neuroradiol 26:277–83, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Fan Z, Zhang Z, Chung YC, et al. Carotid arterial wall MRI at 3T using 3D variable-flip-angle turbo spin-echo (TSE) with flow-sensitive dephasing (FSD). J Magn Reson Imaging 31:645–54, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Helle M, Zhou Z, et al. Joint blood and cerebrospinal fluid suppression for intracranial vessel wall MRI. Magnetic Resonance in Medicine 75:831–8, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Fan Z, Yang Q, Deng Z, et al. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid-attenuated T1-weighted 3D turbo spin echo. Magnetic Resonance in Medicine 77:1142–50, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Zhang X, Qin Q, et al. Improved cerebrospinal fluid suppression for intracranial vessel wall MRI. Journal of magnetic resonance imaging: JMRI 44:665–72, 2016. [DOI] [PubMed] [Google Scholar]
- 16.Cogswell PM, Siero JCW, Lants SK, et al. Variable impact of CSF flow suppression on quantitative 3.0T intracranial vessel wall measurements. J Magn Reson Imaging 48:1120–8, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harteveld AA, Denswil NP, Van HW, et al. Ex vivo vessel wall thickness measurements of the human circle of Willis using 7T MRI. Atherosclerosis 273:106–14, 2018. [DOI] [PubMed] [Google Scholar]
- 18.Hu Z, van der Kouwe A, Han F, et al. Motion-compensated 3D turbo spin-echo for more robust MR intracranial vessel wall imaging. Magn Reson Med 86:637–47, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Q, Deng Z, Bi X, et al. Whole-brain vessel wall MRI: A parameter tune-up solution to improve the scan efficiency of three-dimensional variable flip-angle turbo spin-echo. Journal of Magnetic Resonance Imaging 46:751–7, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guggenberger K, Krafft AJ, Ludwig U, et al. High-resolution Compressed-sensing T1 Black-blood MRI: A New Multipurpose Sequence in Vascular Neuroimaging? Clin Neuroradiol 31:207–216, 2019. [DOI] [PubMed] [Google Scholar]
- 21.Zhu C, Tian B, Chen L, et al. Accelerated whole brain intracranial vessel wall imaging using black blood fast spin echo with compressed sensing (CS-SPACE). MAGMA 31:457–67, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portanova A, Hakakian N, Mikulis DJ, et al. Intracranial vasa vasorum: insights and implications for imaging. Radiology 267:667–79, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Harteveld AA, van der Kolk AG, van der Worp HB, et al. High-resolution intracranial vessel wall MRI in an elderly asymptomatic population: comparison of 3T and 7T. Eur Radiol 27:1585–95, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muraoka S, Taoka T, Kawai H, et al. Changes in Vessel Wall Enhancement Related to the Recent Neurological Symptoms in Patients with Moyamoya Disease. Neurol Med Chir (Tokyo) 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao Q, Tsankova NM, Shoirah H, et al. Vessel Wall MRI Enhancement in Noninflammatory Cerebral Amyloid Angiopathy. AJNR Am J Neuroradiol 41:446–8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardener H, Sacco RL, Rundek T, et al. Race and Ethnic Disparities in Stroke Incidence in the Northern Manhattan Study. Stroke 51:1064–9, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorelick PB, Wong KS, Bae HJ, et al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 39:2396–9, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Swartz RH, Bhuta SS, Farb RI, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 72:627–34, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Vergouwen MD, Silver FL, Mandell DM, et al. Eccentric narrowing and enhancement of symptomatic middle cerebral artery stenoses in patients with recent ischemic stroke. Arch Neurol 68:338–42, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 271:534–42, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu F, Ma Q, Song H, et al. Differential Features of Culprit Intracranial Atherosclerotic Lesions: A Whole-Brain Vessel Wall Imaging Study in Patients With Acute Ischemic Stroke. J Am Heart Assoc 7:10.1161/JAHA.118.009705, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JM, Jung KH, Sohn CH, et al. Intracranial plaque enhancement from high resolution vessel wall magnetic resonance imaging predicts stroke recurrence. Int J Stroke 11:171–9, 2016. [DOI] [PubMed] [Google Scholar]
- 33.Lee HN, Ryu CW, Yun SJ Vessel-Wall Magnetic Resonance Imaging of Intracranial Atherosclerotic Plaque and Ischemic Stroke: A Systematic Review and Meta-Analysis. Front Neurol 9:1032, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song JW, Pavlou A, Xiao J, et al. Vessel Wall Magnetic Resonance Imaging Biomarkers of Symptomatic Intracranial Atherosclerosis: A Meta-Analysis. Stroke 52:193–202, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen XY, Wong KS, Lam WW, et al. High signal on T1 sequence of magnetic resonance imaging confirmed to be intraplaque haemorrhage by histology in middle cerebral artery. Int J Stroke 9:E19, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Turan TN, Rumboldt Z, Granholm AC, et al. Intracranial atherosclerosis: correlation between in-vivo 3T high resolution MRI and pathology. Atherosclerosis 237:460–3, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol 71:195–8, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Xiao J, Song SS, Schlick KH, et al. Disparate trends of atherosclerotic plaque evolution in stroke patients under 18-month follow-up: a 3D whole-brain magnetic resonance vessel wall imaging study. Neuroradiol J:19714009211026920, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moossy J Pathology of cerebral atherosclerosis. Influence of age, race, and gender. Stroke 24:I22–2, 1993. [PubMed] [Google Scholar]
- 40.Watase H, Shen M, Sui B, et al. Differences in atheroma between Caucasian and Asian subjects with anterior stroke: A vessel wall MRI study. Stroke Vasc Neurol 6:25–32, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang WJ, Chen XY, Zhao HL, et al. In Vitro Assessment of Histology Verified Intracranial Atherosclerotic Disease by 1.5T Magnetic Resonance Imaging: Concentric or Eccentric? Stroke 47:527–30, 2016. [DOI] [PubMed] [Google Scholar]
- 42.Mossa-Basha M, Shibata DK, Hallam DK, et al. Added Value of Vessel Wall Magnetic Resonance Imaging for Differentiation of Nonocclusive Intracranial Vasculopathies. Stroke 48:3026–33, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim DK, Verdoorn JT, Gunderson TM, et al. Comparison of non-contrast vessel wall imaging and 3-D time-of-flight MRA for atherosclerotic stenosis and plaque characterization within intracranial arteries. J Neuroradiol 47:266–271, 2019. [DOI] [PubMed] [Google Scholar]
- 44.Ryoo S, Lee MJ, Cha J, et al. Differential Vascular Pathophysiologic Types of Intracranial Atherosclerotic Stroke: A High-Resolution Wall Magnetic Resonance Imaging Study. Stroke 46:2815–21, 2015. [DOI] [PubMed] [Google Scholar]
- 45.Song JW, Reyes-Esteves S, Cucchiara BL Vessel Wall Imaging of Basilar Artery Perforator Disease. Ann Neurol 89:617–8, 2021. [DOI] [PubMed] [Google Scholar]
- 46.Chu B, Ferguson MS, Underhill H, et al. Images in cardiovascular medicine. Detection of carotid atherosclerotic plaque ulceration, calcification, and thrombosis by multicontrast weighted magnetic resonance imaging. Circulation 112:3, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Kampschulte A, Ferguson MS, Kerwin WS, et al. Differentiation of intraplaque versus juxtaluminal hemorrhage/thrombus in advanced human carotid atherosclerotic lesions by in vivo magnetic resonance imaging. Circulation 110:3239–44, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Yuan M, Liu ZQ, Wang ZQ, et al. High-resolution MR imaging of the arterial wall in moyamoya disease. Neurosci Lett 584:77–82, 2015. [DOI] [PubMed] [Google Scholar]
- 49.Ya J, Zhou D, Ding J, et al. High-resolution combined arterial spin labeling MR for identifying cerebral arterial stenosis induced by moyamoya disease or atherosclerosis. Ann Transl Med 8:87, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryoo S, Cha J, Kim SJ, et al. High-resolution magnetic resonance wall imaging findings of Moyamoya disease. Stroke 45:2457–60, 2014. [DOI] [PubMed] [Google Scholar]
- 51.Takagi Y, Kikuta K, Nozaki K, et al. Histological features of middle cerebral arteries from patients treated for Moyamoya disease. Neurol Med Chir (Tokyo) 47:1–4, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Takagi Y, Kikuta K, Nozaki K, et al. Expression of hypoxia-inducing factor-1 alpha and endoglin in intimal hyperplasia of the middle cerebral artery of patients with Moyamoya disease. Neurosurgery 60:338,45; discussion 345, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Kathuveetil A, Sylaja PN, Senthilvelan S, et al. Vessel Wall Thickening and Enhancement in High-Resolution Intracranial Vessel Wall Imaging: A Predictor of Future Ischemic Events in Moyamoya Disease. AJNR Am J Neuroradiol 41:100–5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muraoka S, Araki Y, Taoka T, et al. Prediction of Intracranial Arterial Stenosis Progression in Patients with Moyamoya Vasculopathy: Contrast-Enhanced High-Resolution Magnetic Resonance Vessel Wall Imaging. World Neurosurg 116:e1114–21, 2018. [DOI] [PubMed] [Google Scholar]
- 55.Zeiler SR, Qiao Y, Pardo CA, et al. Vessel Wall MRI for Targeting Biopsies of Intracranial Vasculitis. AJNR Am J Neuroradiol 39:2034–6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arnett N, Pavlou A, Burke MP, et al. Vessel wall MR imaging of central nervous system vasculitis: a systematic review. Neuroradiology 2021. [DOI] [PubMed] [Google Scholar]
- 57.Song JW, Shou H, Obusez EC, et al. Spatial Distribution of Intracranial Vessel Wall Enhancement in Hypertension and Primary Angiitis of the CNS. Sci Rep 9:19270–5, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obusez EC, Hui F, Hajj-Ali R, et al. High-resolution MRI vessel wall imaging: spatial and temporal patterns of reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. AJNR Am J Neuroradiol 35:1527–32, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lehman VT, Brinjikji W, Kallmes DF, et al. Clinical interpretation of high-resolution vessel wall MRI of intracranial arterial diseases. Br J Radiol 89:20160496, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choe YH, Kim DK, Koh EM, et al. Takayasu arteritis: diagnosis with MR imaging and MR angiography in acute and chronic active stages. J Magn Reson Imaging 10:751–7, 1999. [DOI] [PubMed] [Google Scholar]
- 61.Choe YH, Han BK, Koh EM, et al. Takayasu’s arteritis: assessment of disease activity with contrast-enhanced MR imaging. AJR Am J Roentgenol 175:505–11, 2000. [DOI] [PubMed] [Google Scholar]
- 62.de Boysson H, Boulouis G, Parienti JJ, et al. Concordance of Time-of-Flight MRA and Digital Subtraction Angiography in Adult Primary Central Nervous System Vasculitis. AJNR Am J Neuroradiol 38:1917–22, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torres J, Loomis C, Cucchiara B, et al. Diagnostic Yield and Safety of Brain Biopsy for Suspected Primary Central Nervous System Angiitis. Stroke 47:2127–9, 2016. [DOI] [PubMed] [Google Scholar]
- 64.de Boysson H, Zuber M, Naggara O, et al. Primary angiitis of the central nervous system: description of the first fifty-two adults enrolled in the French cohort of patients with primary vasculitis of the central nervous system. Arthritis Rheumatol 66:1315–26, 2014. [DOI] [PubMed] [Google Scholar]
- 65.Nagel MA, Gilden D Neurological complications of varicella zoster virus reactivation. Curr Opin Neurol 27:356–60, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brouwer MC, Heckenberg SG, de Gans J, et al. Nationwide implementation of adjunctive dexamethasone therapy for pneumococcal meningitis. Neurology 75:1533–9, 2010. [DOI] [PubMed] [Google Scholar]
- 67.Song JW, Lehman L, Rivkin M, et al. Serial vessel wall MR imaging of pediatric tuberculous vasculitis. Neurol Clin Pract 9:459–61, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagel MA, Cohrs RJ, Mahalingam R, et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging, and virologic features. Neurology 70:853–60, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song JW, Ojeda S, Romero JM High resolution vessel wall MRI and vasculopathy related to herpes zoster ophthalmicus. Clin Imaging 50:336–9, 2018. [DOI] [PubMed] [Google Scholar]
- 70.Krings T, Choi IS The many faces of intracranial arterial dissections. Interv Neuroradiol 16:151–60, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takano K, Yamashita S, Takemoto K, et al. MRI of intracranial vertebral artery dissection: evaluation of intramural haematoma using a black blood, variable-flip-angle 3D turbo spin-echo sequence. Neuroradiology 55:845–51, 2013. [DOI] [PubMed] [Google Scholar]
- 72.Wu Y, Wu F, Liu Y, et al. High-Resolution Magnetic Resonance Imaging of Cervicocranial Artery Dissection: Imaging Features Associated With Stroke. Stroke 50:3101–7, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atsina KB, Rothstein A, Messe SR, et al. Intracranial vessel wall MR imaging of an intradural vertebral artery dissection. Clin Imaging 68:108–10, 2020. [DOI] [PubMed] [Google Scholar]
- 74.Matsushige T, Shimonaga K, Mizoue T, et al. Focal Aneurysm Wall Enhancement on Magnetic Resonance Imaging Indicates Intraluminal Thrombus and the Rupture Point. World Neurosurg 127:e578–84, 2019. [DOI] [PubMed] [Google Scholar]
- 75.Sato T, Matsushige T, Chen B, et al. Wall Contrast Enhancement of Thrombosed Intracranial Aneurysms at 7T MRI. AJNR Am J Neuroradiol 40:1106–11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimonaga K, Matsushige T, Ishii D, et al. Clinicopathological Insights From Vessel Wall Imaging of Unruptured Intracranial Aneurysms. Stroke 49:2516–9, 2018. [DOI] [PubMed] [Google Scholar]
- 77.Larsen N, von der Brelie C, Trick D, et al. Vessel Wall Enhancement in Unruptured Intracranial Aneurysms: An Indicator for Higher Risk of Rupture? High-Resolution MR Imaging and Correlated Histologic Findings. AJNR Am J Neuroradiol 39:1617–21, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Larsen N, Fluh C, Saalfeld S, et al. Multimodal validation of focal enhancement in intracranial aneurysms as a surrogate marker for aneurysm instability. Neuroradiology 62:1627–35, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cornelissen BMW, Leemans EL, Coolen BF, et al. Insufficient slow-flow suppression mimicking aneurysm wall enhancement in magnetic resonance vessel wall imaging: a phantom study. Neurosurg Focus 47:E19, 2019. [DOI] [PubMed] [Google Scholar]
- 80.Pravdivtseva MS, Gaidzik F, Berg P, et al. Pseudo-Enhancement in Intracranial Aneurysms on Black-Blood MRI: Effects of Flow Rate, Spatial Resolution, and Additional Flow Suppression. J Magn Reson Imaging 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suzuki J, Ohara H Clinicopathological study of cerebral aneurysms. Origin, rupture, repair, and growth. J Neurosurg 48:505–14, 1978. [DOI] [PubMed] [Google Scholar]
- 82.van Gijn J, Rinkel GJ Subarachnoid haemorrhage: diagnosis, causes and management. Brain 124:249–78, 2001. [DOI] [PubMed] [Google Scholar]
- 83.Juvela S Risk factors for multiple intracranial aneurysms. Stroke 31:392–7, 2000. [DOI] [PubMed] [Google Scholar]
- 84.Matouk CC, Mandell DM, Gunel M, et al. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: proof of principle. Neurosurgery 72:492,6; discussion 496, 2013. [DOI] [PubMed] [Google Scholar]
- 85.Omodaka S, Endo H, Niizuma K, et al. Circumferential Wall Enhancement on Magnetic Resonance Imaging is Useful to Identify Rupture Site in Patients with Multiple Cerebral Aneurysms. Neurosurgery 82:638–44, 2018. [DOI] [PubMed] [Google Scholar]
- 86.Duong H, Melancon D, Tampieri D, et al. The negative angiogram in subarachnoid haemorrhage. Neuroradiology 38:15–9, 1996. [DOI] [PubMed] [Google Scholar]
- 87.Rinkel GJ, Wijdicks EF, Hasan D, et al. Outcome in patients with subarachnoid haemorrhage and negative angiography according to pattern of haemorrhage on computed tomography. Lancet 338:964–8, 1991. [DOI] [PubMed] [Google Scholar]
- 88.Bederson JB, Connolly E, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 40:994–1025, 2009. [DOI] [PubMed] [Google Scholar]
- 89.Jung HN, Suh SI, Ryoo I, et al. Usefulness of 3D High-resolution Vessel Wall MRI in Diffuse Nonaneurysmal SAH Patients. Clin Neuroradiol 2021. [DOI] [PubMed] [Google Scholar]
- 90.Cox M, Song JW, Nabavizadeh SA, et al. Detection of Angiographically Occult Ruptured Basilar Sidewall Perforator Aneurysm by Vessel Wall MR Imaging. Neurohospitalist 11:156–9, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiebers DO, Whisnant JP, Huston J, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–10, 2003. [DOI] [PubMed] [Google Scholar]
- 92.Edjlali M, Gentric JC, Regent-Rodriguez C, et al. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms? Stroke 45:3704–6, 2014. [DOI] [PubMed] [Google Scholar]
- 93.Larson AS, Lehman VT, Lanzino G, et al. Lack of Baseline Intracranial Aneurysm Wall Enhancement Predicts Future Stability: A Systematic Review and Meta-Analysis of Longitudinal Studies. AJNR Am J Neuroradiol 41:1606–10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chyatte D, Bruno G, Desai S, et al. Inflammation and intracranial aneurysms. Neurosurgery 45:1137–7, 1999. [DOI] [PubMed] [Google Scholar]
- 95.Frosen J, Piippo A, Paetau A, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke 35:2287–93, 2004. [DOI] [PubMed] [Google Scholar]
- 96.Intengan HD, Schiffrin EL Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38:581–7, 2001. [DOI] [PubMed] [Google Scholar]
- 97.Zempo N, Kenagy RD, Au YP, et al. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J Vasc Surg 20:209–17, 1994. [DOI] [PubMed] [Google Scholar]
- 98.Hadad S, Mut F, Chung BJ, et al. Regional Aneurysm Wall Enhancement is Affected by Local Hemodynamics: A 7T MRI Study. AJNR Am J Neuroradiol 42:464–70, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao W, Qi T, He S, et al. Low Wall Shear Stress Is Associated with Local Aneurysm Wall Enhancement on High-Resolution MR Vessel Wall Imaging. AJNR Am J Neuroradiol 39:2082–7, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miller TR, Shivashankar R, Mossa-Basha M, et al. Reversible Cerebral Vasoconstriction Syndrome, Part 1: Epidemiology, Pathogenesis, and Clinical Course. AJNR Am J Neuroradiol 36:1392–9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miller TR, Shivashankar R, Mossa-Basha M, et al. Reversible Cerebral Vasoconstriction Syndrome, Part 2: Diagnostic Work-Up, Imaging Evaluation, and Differential Diagnosis. AJNR Am J Neuroradiol 36:1580–8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rocha EA, Topcuoglu MA, Silva GS, et al. RCVS2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology 92:e639–47, 2019. [DOI] [PubMed] [Google Scholar]
- 103.Singhal AB, Topcuoglu MA, Fok JW, et al. Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Ann Neurol 79:882–94, 2016. [DOI] [PubMed] [Google Scholar]
- 104.Findlay JM, Weir BK, Kanamaru K, et al. Arterial wall changes in cerebral vasospasm. Neurosurgery 25:736–6, 1989. [DOI] [PubMed] [Google Scholar]
- 105.Singhal AB, erly WT, Schaefer PW, et al. Case records of the Massachusetts General Hospital. Case 8–2009. A 36-year-old woman with headache, hypertension, and seizure 2 weeks post partum. N Engl J Med 360:1126–37, 2009. [DOI] [PubMed] [Google Scholar]
- 106.Mandell DM, Matouk CC, Farb RI, et al. Vessel wall MRI to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: preliminary results. Stroke 43:860–2, 2012. [DOI] [PubMed] [Google Scholar]
- 107.van Veluw SJ, Charidimou A, van der Kouwe AJ, et al. Microbleed and microinfarct detection in amyloid angiopathy: a high-resolution MRI-histopathology study. Brain 139:3151–62, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fotiadis P, van Rooden S, van der Grond J, et al. Cortical atrophy in patients with cerebral amyloid angiopathy: a case-control study. Lancet Neurol 15:811–9, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Castellani RJ, Smith MA, Perry G, et al. Cerebral amyloid angiopathy: major contributor or decorative response to Alzheimer’s disease pathogenesis. Neurobiol Aging 25:599–4, 2004. [DOI] [PubMed] [Google Scholar]
- 110.McNally JS, Sakata A, Alexander MD, et al. Vessel Wall Enhancement on Black-Blood MRI Predicts Acute and Future Stroke in Cerebral Amyloid Angiopathy. AJNR Am J Neuroradiol 42:1038–1045, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang N, Zhang F, Deng Z, et al. 3D whole-brain vessel wall cardiovascular magnetic resonance imaging: a study on the reliability in the quantification of intracranial vessel dimensions. J Cardiovasc Magn Reson 20:39-z, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang N, Liu X, Xiao J, et al. Plaque Morphologic Quantification Reliability of 3D Whole-Brain Vessel Wall Imaging in Patients With Intracranial Atherosclerotic Disease: A Comparison With Conventional 3D Targeted Vessel Wall Imaging. J Magn Reson Imaging 54:166–74, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shi F, Yang Q, Guo X, et al. Vessel Wall Segmentation Using Convolutional Neural Networks. IEEE Trans Biomed Eng 66:2840–2847, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi Z, Zhu C, Degnan AJ, et al. Identification of high-risk plaque features in intracranial atherosclerosis: initial experience using a radiomic approach. Eur Radiol 28:3912–21, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


