Abstract
Ferroptosis is a cell death modality triggered by excessive lipid peroxidation. Two recent studies (Zou et al., 2020; Cui et al., 2021) not only reveal critical roles of ether-linked phospholipids as an additional source for providing polyunsaturated-fatty-acid-containing phospholipids in driving ferroptosis, but also suggest a context-dependent role of TMEM189-mediated vinyl-ether phospholipid (plasmalogen) synthesis in ferroptosis.
Keywords: Ferroptosis, Lipid peroxidation, Ether phospholipids, Plasmalogen, TMEM189
Ether phospholipids (ePLs) are PLs with a fatty alcohol attached to the sn-1 position of the glycerol backbone via an ether bond (rather than a fatty acid linked to the glycerol backbone via an ester bond in typical diacyl PLs). There consist of two subclasses of ether PLs: alkyl-ether PLs and vinyl-ether PLs (also known as plasmalogens), depending on whether the ether-linked carbohydrate chain is saturated or contains a double bond between C1 and C2 (Fig. 1A). Ether PLs have important cellular functions in maintaining membrane structure, antioxidant defense, and cellular signaling, and its dysregulation can lead to diverse human diseases, such as peroxisomal disorders, neurodegenerative diseases, and cancer (Dean and Lodhi, 2018).
Fig. 1.
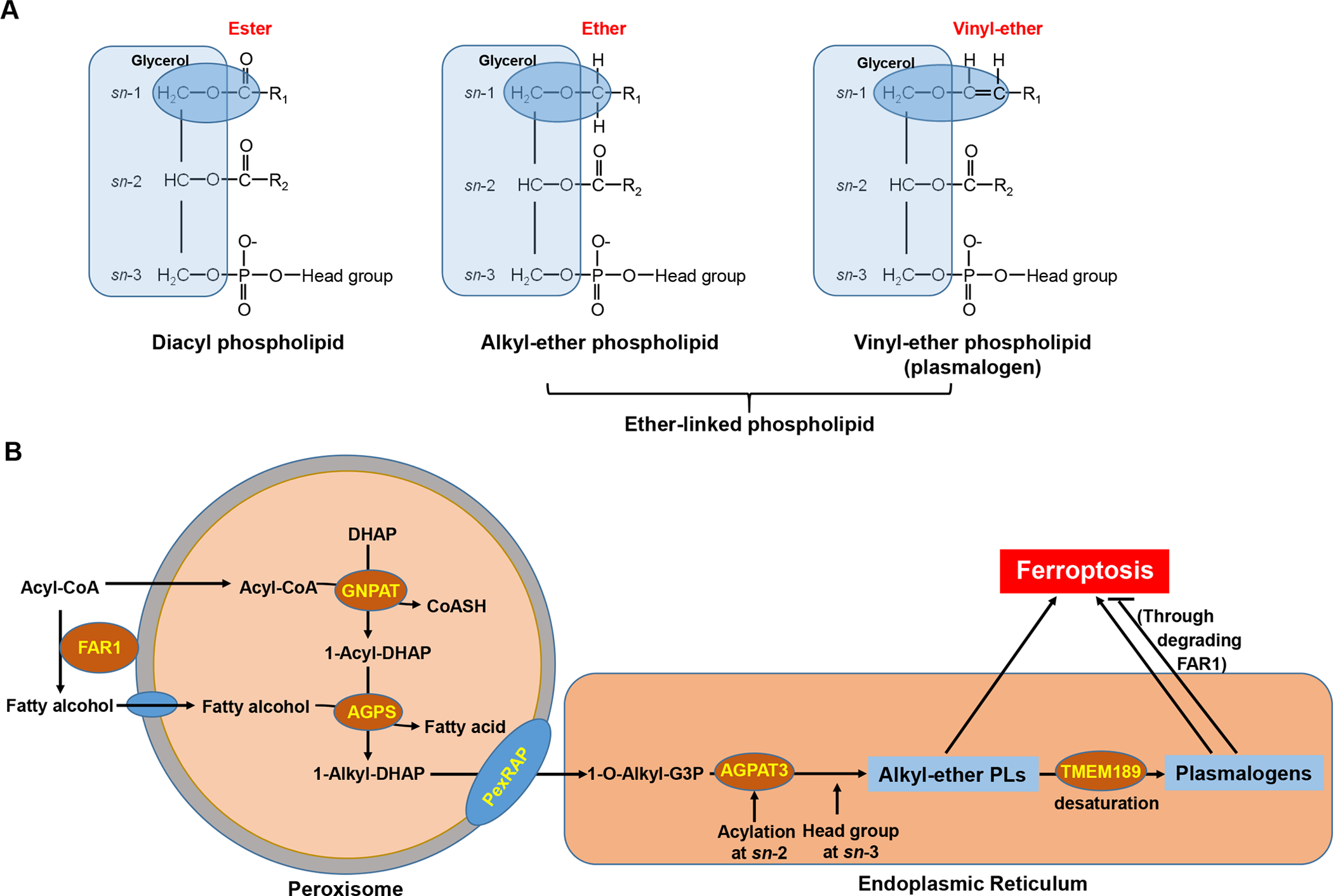
Chemical structures, biosynthesis pathways of ether-linked phospholipids and their roles in ferroptosis. A: Chemical structures of diacyl and ether-linked phospholipids. B: Schematic of ether-linked phospholipid biosynthesis pathways and their roles in regulating ferroptosis. See text for detailed discussion.
Ferroptosis is a unique cell death modality that is triggered by excessive lipid peroxidation on cellular membranes (Dixon et al., 2012; Stockwell et al., 2017). Lipid peroxides are produced during normal cellular activities, because cellular membranes constitute a large amount of polyunsaturated-fatty-acid-containing PLs (PUFA-PLs), which are particularly susceptible to peroxidation in an oxygen- and iron-rich cellular environment. Normally, lipid peroxides are kept in check and maintained at non-toxic levels by cellular antioxidant defense systems, prominent among which is glutathione peroxidase 4 (GPX4), an enzyme that constantly surveils cellular membranes and converts lipid peroxides to non-toxic lipid alcohols (Stockwell et al., 2017). Disruption of this critical defense system, such as by treatment with ferroptosis inducers (FINs) that inactivate GPX4, results in the accumulation of unchecked lipid peroxidation and subsequent ferroptotic cell death (Yang et al., 2014). While the role and mechanisms of GPX4 in defending against ferroptosis are well established, how lipid metabolism contributes to lipid peroxidation and ferroptosis remains less well understood.
Through conducting metabolite screenings, Cui et al. (2021) identified 1-hexadecanol (1-HE) as the metabolite with the potent ability to promote FIN-induced ferroptosis. 1-HE is a C-16 fatty alcohol that is reduced from palmitic acid (C16:0) through peroxisome-localized fatty acyl-CoA reductase (FAR1), which also mediates the reduction of stearic acid (C18:0) to its corresponding fatty alcohol 1-octadecanol (1-OE) (Dean and Lodhi, 2018) (Fig. 1B). Supplementation of saturated fatty acids (SFAs), such as palmitic acid or stearic acid, was previously shown to sensitize cells to FIN-induced ferroptosis (Lee et al., 2020). Cui et al. (2021) confirmed these observations and further showed that 1-HE or 1-OE supplementation exhibited even more potent ferroptosis-sensitizing effects than its corresponding SFA; importantly, the ferroptosis-sensitizing effect of SFAs, but not that of corresponding fatty alcohols, was abolished in FAR1 deficient cells, indicating that these SFAs promote ferroptosis through their conversion to fatty alcohols by FAR1. Further lipidomics analyses in 1-HE- or 1-OE- supplemented cells will help validate this model.
Fatty alcohols provide important precursors for ether PL biosynthesis, which is initiated in peroxisomes and subsequently completed in the endoplasmic reticulum (ER) (Dean and Lodhi, 2018) (Fig. 1B). Specifically, in peroxisomes, glyceronephosphate O-acyltransferase (GNPAT) generates 1-acyl-dihydroxyacetone phosphate (1-acyl-DHAP) from acyl-CoA and DHAP. Alkylglycerone phosphate synthase (AGPS) then exchanges the acyl chain in 1-acyl-DHAP with a fatty alcohol to form the ether bond, resulting in 1-alkyl-DHAP, which is subsequently converted to 1-O-alkyl-glycerol-3-phosphate (1-O-alkyl-G3P) by PexRAP (an acyl/alkyl DHAP reductase) (Fig. 1B). 1-O-alkyl-G3P then proceeds to the ER steps, including the addition of an acyl-CoA (often a PUFA-CoA) at the sn-2 position by 1-acyl-sn-glycerol-3-phosphate acyltransferase gamma (AGPAT3) and head group attachment at the sn-3 position, forming alkyl-ether PLs, which can further undergo desaturation by TMEM189 to generate plasmalogens (Gallego-Garcia et al., 2019; Werner et al., 2020) (Fig. 1B). Notably, through CRISPR-Cas9 screenings, another recent study by Zou et al. (2020) had uncovered genes involved in ether PL biosynthesis, including GNPAT, FAR1, AGPS, and AGPAT3 (but not TMEM189), as top pro-ferroptosis genes; importantly, this elegant study further revealed a dynamic regulation of ether lipid biosynthesis during cell state transitions, which enables cancer cells, neurons, and cardiomyocytes to change their sensitivity to ferroptosis. Both Zou et al. (2020) and Cui et al. (2021) confirmed that depletion of these ether PL biosynthesis genes markedly promoted ferroptosis resistance in diverse cancer cell lines. Zou et al. (2020) further showed that depletion of these genes resulted in decreased levels of PUFA-containing ePLs (PUFA-ePLs), and supplementation of PUFA-ePLs in cells with deficiency in ePL biosynthesis re-sensitized these cells to ferroptosis. Likewise, Cui et al. (2021) showed that ePL biosynthesis deficiency abolished the ferroptosis-promoting effect by 1-HE or 1-OE supplementation. Collectively, these data show that fatty alcohols act as the precursors for the synthesis of ePLs, which promote ferroptosis (Fig. 1B).
How do ePLs promote ferroptosis? Does the pro-ferroptosis effect of these ePLs relate to any specific chemical nature in them? Zou et al. (2020) showed that supplementing cells with either PUFA-ePLs or their non-ether-linked PL counterparts have similar sensitizing effects on ferroptosis, and importantly, deleting TMEM189 (the desaturase that introduces the double bond in the vinyl-ether to produce plasmalogens [Gallego-Garcia et al., 2019; Werner et al., 2020]) did not affect ferroptosis sensitivity. These data suggest that PUFA-ePLs are not intrinsically more sensitive to peroxidation than other PUFA-PLs, and the specific chemical nature of the ether linkage in these PUFA-ePLs does not appear to be important to their pro-ferroptosis function. Therefore, ePLs are important in driving ferroptosis perhaps because they represent a relatively abundant pool of PUFA-PLs that are available for peroxidation during ferroptotic stress.
However, this part also came with a major discrepancy between these two studies. While Zou et al. (2020) showed that TMEM189 deficiency did not affect ferroptosis sensitivity, Cui et al. (2021) revealed that TMEM189 deletion markedly sensitized cells to ferroptosis in different cancer cell lines and its overexpression had the opposite effect, which suggests that, opposite to other genes involved in ePL biosynthesis (such as GNPAT, FAR1, AGPS, and AGPAT3), TMEM189 has an anti-ferroptosis function. Mechanistically, Cui et al. (2021) showed that plasmalogens produced by TMEM189 degrade FAR1 through a negative feedback regulation, resulting in ferroptosis suppression. This discrepancy appears to lie in different cell lines used in these two studies, as Cui et al. (2021) showed that depleting TMEM189 in cell lines with high expression of TMEM189 resulted in much more dramatic effects on enhancing FAR1 levels and ferroptosis sensitivity than in cell lines with its low expression. Therefore, TMEM189-mediated plasmalogen synthesis might play a context dependent role in ferroptosis regulation. It remains to be determined whether other factors, such as tumor types, genetic background, or expression levels of other ferroptosis regulators, also contribute to the differential ferroptosis phenotypes in different cell lines with TMEM189 depletion. Additional lipidomics analyses in these TMEM189-depleted cell lines will also help clarify its exact role in regulating ferroptosis.
It should be noted that the double bond in plasmalogens is believed to provide plasmalogens with some antioxidant activities against reactive oxygen species (Dean and Lodhi, 2018), and it has been speculated that plasmalogens might have an anti-ferroptosis role. In support of this, another recent study showed that deficiency of AGPS orthologue ads-1 in C. elegans or treatment with an AGPS inhibitor in mammalian cells decreased plasmalogen levels and promoted ferroptosis, which supports an anti-ferroptosis role of plasmalogens (Perez et al., 2020). However, the data from pharmacologic inhibition of AGPS in this study was different from the results from Zou et al. (2020) showing that genetic ablation of AGPS by CRISPR suppressed ferroptosis.
Overall, these recent studies reveal a somewhat complex picture on the role of ePLs, particularly plasmalogens, in regulating lipid peroxidation and ferroptosis. The studies by Zou et al. (2020) and Cui et al. (2021) convincingly showed that alkyl-ether PLs promote ferroptosis (Fig. 1B); consequently, genetic ablation of genes involved in alkyl-ether PL biosynthesis (FAR1, GNPAT, AGPS, and AGPAT3) drives ferroptosis resistance. However, it remains to be addressed why treatment with an AGPS inhibitor promoted ferroptosis as shown in another study (Perez et al., 2020). A direct comparison of AGPS genetic deletion and AGPS inhibitor treatment in the same cell lines should clarify this question. These studies also raised several outstanding questions, including: Does the ratio of PUFA ester-linked PLs to PUFA ether-linked PLs influence cellular sensitivity to ferroptosis? What is the exact role of TMEM189-mediated plasmalogen synthesis in ferroptosis (anti-ferroptosis or no effect on ferroptosis) (Fig. 1B)? Does its role in ferroptosis regulation depend on cellular contexts such as FAR1 expression levels and/or PUFA composition in cells? How does TMEM189 downregulation lead to FAR1 upregulation and the subsequent ether lipid biosynthesis changes? Is the TMEM189-FAR1 crosstalk conserved in other cellular settings such as non-neoplastic cells? Does the anti-ferroptosis function of plasmalogens simply result from their feed-back regulation on FAR1 or relate to their antioxidant activities? Like any other important scientific discovery, these recent studies present fascinating questions for further understanding this intriguing cell death mechanism in future studies.
Acknowledgements
We apologize to the colleagues whose relevant work cannot be cited here due to space limitations. Research in the authors’ lab has been supported by The University of Texas MD Anderson Cancer Center, National Institutes of Health grants R01CA181196, R01CA244144, and R01CA247992 (to B. Gan) and by Cancer Center Support (Core) Grant P30 CA016672 from the National Cancer Institute (to The University of Texas MD Anderson Cancer Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Boyi Gan is an inventor on patent applications involving targeting ferroptosis in cancer therapy. The other authors declare no competing interests.
References
- Cui W, Liu D, Gu W, and Chu B, 2021. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JM, and Lodhi IJ, 2018. Structural and functional roles of ether lipids. Protein Cell 9, 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. , 2012. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Garcia A, Monera-Girona AJ, Pajares-Martinez E, Bastida-Martinez E, Perez-Castano R, Iniesta AA, Fontes M, Padmanabhan S, and Elias-Arnanz M, 2019. A bacterial light response reveals an orphan desaturase for human plasmalogen synthesis. Science 366, 128–132. [DOI] [PubMed] [Google Scholar]
- Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L, Tyagi S, Ma L, Westbrook TF, Steinberg GR, et al. , 2020. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol 22, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Magtanong L, Dixon SJ, Watts JL, 2020. Dietary Lipids Induce Ferroptosis in Caenorhabditiselegans and Human Cancer Cells. Dev. Cell 54, 447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, et al. , 2017. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171, 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ER, Keller MA, Sailer S, Lackner K, Koch J, Hermann M, Coassin S, Golderer G, Werner-Felmayer G, Zoeller RA, et al. , 2020. The TMEM189 gene encodes plasmanylethanolamine desaturase which introduces the characteristic vinyl ether double bond into plasmalogens. Proc. Natl. Acad. Sci. U. S. A 117, 7792–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, et al. , 2014. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P, Paradkar S, Boehnke N, Deik AA, Reinhardt F, et al. , 2020. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 585, 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]


