Abstract
The WAG/Rij strain of rats is commonly used as a preclinical model of genetic absence epilepsy. While widely utilized, the developmental trajectory of absence seizure expression has been only partially described. Moreover, sex differences in this strain have been under-explored. Here, we longitudinally monitored male and female WAG/Rij rats to quantify cortical spike-and-wave discharges (SWDs) monthly, from 4 to 10 months of age. In both male and female WAG/Rij rats, absence seizure susceptibility increased with age. In contrast to previous reports, we found a robust and consistent increase in absence epilepsy susceptibility in male WAG/Rij rats in comparison to females across months. The increased absence seizure susceptibility was characterized by increased number and duration of SWDs, and consequently increased total SWDs duration. These findings highlight a previously unrecognized sex difference in a model of absence epilepsy and narrow the knowledge gap of age-dependent expression of SWDs in the WAG/Rij strain.
Keywords: Absence epilepsy, WAG/Rij strain, EEG recordings, animal model, sex comparison, preclinical epilepsy
1. Introduction
Absence seizures are characterized by brief episodes (5-10 seconds) of generalized spike-wave discharges (SWDs) that start and end abruptly, are detected bilaterally on electroencephalography (EEG), and result in impaired consciousness (Crunelli and Leresche, 2002; Sarkisova and van Luijtelaar, 2011). These seizures are particularly common in the context of childhood and infant epilepsy syndromes, where psychological comorbidities associated with changes in brain connectivity have been reported (Killory et al., 2011; Masur et al., 2013; Tenney and Glauser, 2013). Absence epilepsy has been studied in a range of animal models, and the Wistar Albino Glaxo Rats from Rijswijk (WAG/Rij) strain is one of the most widely utilized (Coenen and Van Luijtelaar, 2003; Sarkisova and van Luijtelaar, 2011; Akman et al., 2010; Russo and Citraro, 2018). These animals can develop hundreds of SWDs (7–10 Hz) in a single day; the occurrence of absence seizures is consistent and the seizure susceptibility lasts into adulthood (Coenen and Van Luijtelaar, 2003). Moreover, WAG/Rijs display behavioral alterations associated with neuropsychiatric comorbidities commonly observed in persons with epilepsy, such as anxiety and depression (Sarkisova and Kulikov, 2006; Sarkisova and van Luijtelaar, 2011). SWDs in WAG/Rij rats can be suppressed by classical antiseizure medications (ASMs) that are effective against absence seizures in humans (Russo and Citraro, 2018).
Assessment of sex differences in seizure susceptibility and severity is of a growing interest because sex may play an important role in determining the efficacy of treatments and therapies. Sex differences have been described in human studies and preclinical models of epilepsy (for review see: (Christian et al., 2020). While some reports indicate that epilepsies are slightly more common in males than females, the incidence of generalized absence seizures is significantly higher in females (Savic, 2014; Reddy, 2017; Christian et al., 2020). Much of the preclinical epilepsy research on sex differences focused on models of temporal lobe epilepsy, including the pilocarpine and kainic-acid models (Mejías-Aponte et al., 2002; Velíšková and DeSantis, 2013; Scharfman and MacLusky, 2014; Twele et al., 2016), and relatively less has been done in models of absence epilepsy (Christian et al., 2020). That said, androgens, progestins and estrogens all influence seizure susceptibility in a variety of models (Velíšková and DeSantis, 2013; Christian et al., 2020), including the WAG/Rij strain (van Luijtelaar et al., 2001) and the gamma butyrolactone (GBL) pharmacological model of absence seizures (Santos et al., 2018). Nevertheless, despite the substantial number of studies conducted with the WAG/Rij strain, little has been done to investigate sex differences in the expression of absence seizures. One report found no difference in the incidence of SWDs across sex, but the developmental time course of absence epilepsy was evaluated using a small sample and at limited ages (Coenen and Van Luijtelaar, 1987). Since this study was conducted more than three decades ago (upwards of 90 generations), genetic drift may have occurred over time, as previously described in the Wistar Audiogenic Rats strain (Doretto et al., 2003). Similarly, differences in absence seizure expression have already been reported between four isolated colonies of Genetic Absence Epilepsy Rats from Strasbourg (GAERS) (Powell et al., 2014), suggesting that similar alterations may also occur in different colonies of WAG/Rij. The present study aimed to characterize and compare the longitudinal expression of absence seizures in both male and female WAG/Rij rats from 4 to 10 months old of age.
2. Methods
2.1. Animals
WAG/Rij rats (20 males and 20 females), were obtained from our colony at Georgetown University with breeders originally obtained from Charles River (Calco, Lecco, Italy). All rats were maintained in a temperature and humidity-controlled room in the Division of Comparative Medicine at Georgetown University with food and water ad libitum. All experimental procedures were performed during the light phase of the light-dark cycle (6:00 am. – 6:00 pm.). All procedures were approved by the Georgetown University Animal Care and Use committee (Protocol 2016-1184) and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Surgery and electroencephalography (EEG)
Three-months-old WAG/Rij rats were used. Animals were anesthetized with Ketamine (75 mg/kg; Zetamine; Vet Label) and Dexmedetomidine 0.5 mg/kg (Dexdormitor; Orion Pharma Pfizer) and placed into a Kopf stereotaxic frame (Tujunga, CA). For EEG recordings, six stainless-steel screw electrodes were bilaterally implanted over the cortex: two over the frontal cortex, two over posterior neocortex, and two over the cerebellum (ground and reference). Screws were placed ~2.5 mm anterior to bregma and 2 mm lateral to the midline for frontal electrodes, 5 mm posterior to bregma and 2.5 mm lateral to the midline for posterior cortical electrodes, and 3 mm posterior to lambda and 1 mm lateral to the midline for cerebellar electrodes. After surgery, animals were allowed to recover for two weeks.
Previous data reported that WAG/Rij rats do not present consistent SWDs during the first months of their life (Crunelli and Leresche, 2002; Coenen and Van Luijtelaar, 1987). Therefore, in the present study, we began the EEG monitoring the WAG/Rij rats at 4 months of age, on monthly basis until 10 months of age. WAG/Rij rats were placed in a clear acrylic cylindric chamber and were first habituated to handling and EEG tethering for two weeks before the first EEG recording. On the day of testing, rats were also habituated to experimental condition for 20-30 min before every recording session. EEG recording was performed using a preamplifier-amplifier system from Pinnacle Technologies and LabChart 8 (AD Instruments) for digitization and analysis, as previously described (Wicker et al., 2019). Each EEG recording session was done during the light phase (9:00 am. – 3:00 pm.) and lasted 120 minutes. Given prior reports showing circadian variability in the number of seizures per hour in WAG/Rij rats (Coenen et al., 1991), we compared the number of seizures, mean seizure duration, and total seizure duration in animals tested in the morning (9:00 am. – 12:00 pm.) to those of animals tested in the afternoon (12:00 pm. – 3:00 pm.) at various lifetime points (4-, 7-, and 10- month-old). Time of day was without effect on these measures (Supplemental Figure 1). The lack of difference between morning and afternoon testing is likely do to the fact that our testing started several hours after the onset of seizure episodes (seen at the very start of the light phase of the circadian cycle) and ended before the peak (at the start of the dark phase of the circadian cycle). Testing order was counterbalanced by sex and across sessions. Signal (1 kHz) was filtered (band pass 1-50 Hz) and SWDs were analyzed offline, using LabChart 8. Both generalized and focal SWDs have been reported in WAG/Rij (Midzianovskaia et al., 2001); because SWDs associated with absence seizures in humans are generalized (bilateral), we only considered generalized SWDs in the present study. The number of SWDs, mean SWDs duration, and maximum power frequency were calculated by two researchers both blinded to animal identity, using the criteria we have previously described (Wicker et al., 2019).
Recordings from 4- and 5-month-old rat were performed in a dedicated experimental room in our laboratory, while recordings from 6–10-month-old rats were performed in the Division of Comparative Medicine at Georgetown University, in the same room where rats were housed during the entire duration of the experiment, allowing reduction of potential stressors (e.g., transport) preceding the recording sessions. For these reasons, we did not perform direct comparisons between 4-5 months and 6-10 months of age rats.
We did not monitor estrous cycle phase in female rats to minimize stress; stress causes a biphasic effect in the number of SWDs in WAG/Rij rats, reducing SWDs during the first minutes after stressor and then, increasing the number SWDs. Furthermore, the repetitive exposure to stress causes additional impact in both the suppression and facilitation of absence seizure in WAG/Rij rats (Tolmacheva et al., 2012). Therefore, differential stress, as a result of estrous cycle monitoring, would have complicated and confounded our study of sex differences and absence seizures in WAG/Rij rats under basal condition. Moreover, a previous study assessed SWDs in WAG/Rij females during a complete 4-day estrous cycle and the only difference reported occurred during the first six hours of the dark period at proestrus, when frequency of SWDs increased; no difference was observed between days at any other hour (van Luijtelaar et al., 2001). Similarly, ovariectomized females WAG/Rij rats showed no alteration in SWDs expression under basal conditions (Tolmacheva and van Luijtelaar, 2007a).
2.3. Audiogenic seizure screening
A subset of WAG/Rij rats also display susceptibility to audiogenic seizures (AGS), characterized by wild running, and tonic-clonic motor seizures evoked in response to intense sound stimulation (Midzyanovskaya et al., 2004; Vinogradova, 2008), however, these rats present low susceptibility to AGS in comparison to other “pure” rodent audiogenic strains (Vinogradova, 2017). Therefore, we screened WAG/Rij rats to identify AGS-sensitive and AGS-resistant rats. Previous reports demonstrated that AGS-resistant WAG/Rij rats developed longer SWDs in comparison to AGS-sensitive WAG/Rij rats (Midzyanovskaya et al., 2004). Furthermore, AGS episodes can suppress absence seizures in WAG/Rij for about 1 hour; this suppression effect was likely due to AGS-induced cortical spreading depression (Vinogradova et al., 2005). Therefore, we sought to eliminate AGS-sensitive WAG/Rij rats from this study.
Animals were screened every month on the day following each EEG recording session. Each screening consisted of three acoustic stimulations, every two days, and if animals expressed at least one AGS episode they were considered seizure susceptible. In each session, rats were placed on an acrylic chamber (height: 60 cm, diameter: 30 cm) located at a soundproof chamber. Animals were allowed to explore the chamber for one minute, then the acoustic stimulation, which consisted of 120 dB mixed sounds delivered by an electrical bell, was applied until the onset of wild running, or for 60 seconds, if no seizures occurred. Convulsive behaviors of AGS were analyzed and scored as previously described (Wicker et al., 2019), where: 0 = no seizure; 1 = one wild running; 2 = two or more bouts of wild running; 3 = wild running followed by generalized tonic-clonic seizures.
Only 2 out of 20 animals (10%), both female, developed AGS during the 7 months observation period (4-10 months old). In one animal, AGS were observed at 7-10 months old; of the 21 acoustic stimulations, 12 AGS episodes were observed (median severity = 2). In the second animal, AGS were observed at 8-10 months old; of the 21 acoustic stimulations, 7 AGS episodes were observed (median severity = 1). Only AGS-resistant WAG/Rij rats were included in data analysis as the low (n=2) number of AGS-sensitive animals precluded a systematic analysis of that group. This frequency of AGS susceptibility is in line with the 10-30% susceptibility to AGS that has been previously reported (Midzyanovskaya et al., 2004).
2.4. Spectral Analysis
To calculate the maximum power frequency of peaks within the SWD, we applied a Fast Fourier transform (FFT) with a frequency resolution of 0.1 Hz and a Hann window with 93.75% window overlap. In all animals the maximum power frequency corresponded to the fundamental frequency with lower power first and second harmonics evident in the spectrogram (see representative Spectra in Supplemental Fig 2). Maximum power was calculated for each discharge in each animal and averaged on a within-subject basis for each recording session.
2.5. Statistical analysis
Statistical analyses were performed using the GraphPad Prism (GraphPad Software, Inc, La Jolla, CA). Number and duration of SWDs were expressed as mean ± SEM. Data were analyzed using a Mixed Effects analysis (with age as a within subject factor and sex as a between subject factor). Degrees of freedom were corrected using the Greenhaus-Geisser correction. Pairwise comparisons were adjusted for multiplicity using the Holm-Sidak method. p values <0.05 were considered statistically significant.
During the study, one female and two males prematurely lost their EEG implants and were thus excluded from the analysis. Moreover, one male was a statistical outlier (as determined by the ROUT test in GraphPad prism) and was also excluded from the analysis. Therefore, 17 males and 17 females remained to the final statistical analysis. Due to technical error with one preamplifier, we had to exclude a single individual session from 1 male at 6 months, and from another at 7 months, 2 females at 6 months, 2 different females at 8 months, and 3 different females at 10 months. No more than one data point was excluded for any given animal. The statistical approach we selected (Mixed Effects Model) is tolerant to data points missing at random, and thus the remainder of the data for these animals were retained.
3. Results
3.1. Time-course evolution of absence seizure expression in male and female WAG/Rij rats
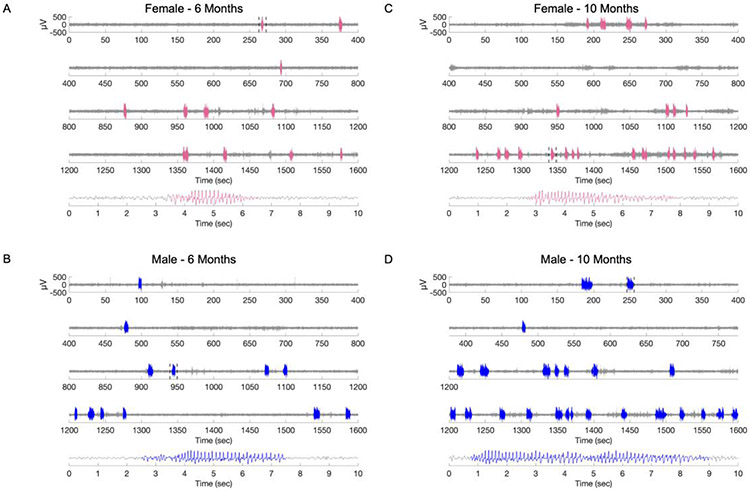
The two observers displayed a high degree of inter-rater reliability (R2=0.89 for mean SWD duration, R2=0.82 for the number of SWDs). Representative EEG traces from a female and a male rat at 6 months of age are shown in Figure 1 panel A and B, respectively. EEG from the same animals at 10 months of age are shown in Figure 1 panel C and D, respectively. Results from 4- and 5-months old WAG/Rijs are shown in Supplementary Figure 3.
Figure 1. Electrographic characterization of spike-wave discharges (SWDs) in male and female WAG/Rij rats.
Representative electrographic recordings from A: a 6-month-old female and B: a 6-month-old male WAG/Rij rat. C-D: Representative electrographic recordings of the same animals as in A and B at 10 months of age. The areas highlighted in blue (male) and pink (female) indicate SWDs. The area bracketed by dotted lines is shown in an expanded time scale at the bottom of each panel.
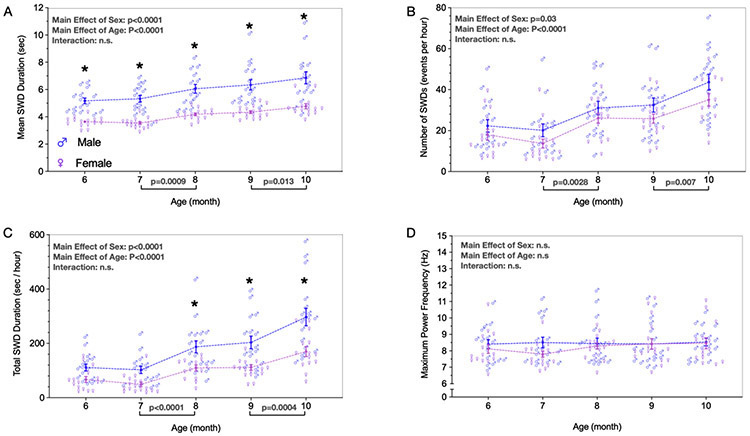
Mean SWD duration (Fig. 2A) differed as a function of both sex (F1,32 = 63.59; p<0.0001) and age (F1.989, 59.17 = 18.29; p<0.0001), but no notable interaction was detected (F4, 119 = 0.3682; p=0.8309). In every month analyzed, males showed longer SWDs than females (ps≤0.0002). When we analyzed the mean SWD duration across consecutive months, we only detected a significant increase between 7 to 8 months (p=0.0009) and between 9 to 10 months (p=0.0128).
Figure 2. Spike-wave discharges (SWDs) characteristics in males and females WAG/Rij rats from 6 to 10 months old.
A: Mean SWD duration (seconds). B: Number of SWDs (events per hour). C: Total SWD duration (seconds per hour). D: Maximum power frequency (Hz). Data are expressed as mean ± standard error mean (SEM). n=17 males, n=17 females.
The number of SWDs observed per hour (Fig. 2B) also differed as a function of sex (F1,32 = 5.151; p=0.0301) and age (F2.953, 88.60 = 18,30; p<0.0001), with a no notable interaction (F4, 120 = 0.244, p=0.912). However, pairwise comparisons revealed no difference between males and females at any time point (ps≥0.29). Similar as observed for mean SWD duration, when the number of SWDs was analyzed across consecutive months, an increase was observed between 7 to 8 months (p=0.0028) and between 9 to 10 months (p=0.007).
As a third measure which incorporates both mean SWD duration and number of SWDs, we examined total SWD duration (seconds per hour) (Fig. 2C). We found a significant main effect of sex (F1, 32 = 24.37; p<0.0001) and age (F4, 119 = 31.15; p<0.0001). No notable interaction between factors was observed (F4, 119 = 2,044; p=0.0926). Differences between consecutive months were observed between 7 to 8 months (p<0.0001) and between 9 to 10 months (p=0.0004). No notable differences were detected in the total SWD duration when comparing males and females at 6 (p=0.0886) and 7 (p=0.0518) months of age. However, the total SWD duration was significantly increased in males when compared to females in 8, 9, and 10 months old (ps≤0.0073), these differences became more evident at 10 months old (p<0.0001).
We also evaluated the spectral characteristics of SWDs over age (Fig. 2D). We found no notable effect of sex (F1, 32 = 0,7851; p=0.3822), and neither a main effect of age (F2.823, 83.99 = 0.9953; p= 0.3956), nor an interaction between both age and sex (F4, 119 = 1.258; p=0.2905). Given the absence of main effects or interactions, we did not perform any pairwise comparisons.
Finally, the average coefficient of variability across timepoints in females did not differ from that of males on any measure: mean SWD duration (t=0.615, df=32, p=0.54), number of SWDs (t=0.57, df=32, p=0.57), total SWDs duration (t=1.29, df=32, p=0.20), or maximal power frequency (t=0.616, df=32, p=0.54).
4. Discussion
In the present study, we performed a longitudinal assessment of absence seizures in WAG/Rij rats and compared seizure expression between males and females from 4 to 10 months of age. We found that male WAG/Rij rats are more susceptible to absence seizures than females, presenting longer and increased number of SWDs than females, as well as increased total SWDs duration. These differences were consistently observed from 4 to 10 months of age. Furthermore, the susceptibility to absence epilepsy progressively increased with age in both sexes, with older WAG/Rij rats demonstrating greater mean SWDs duration, number of SWDs, and consequently, total SWD duration.
We choose the WAG/Rij model because it is one of the most widely utilized for the study of absence seizures, and it offers clear advantages as a genetic model, because seizures occur spontaneously with no need for drug treatment (e.g., gamma butyrolactone) or other procedures, like inhibition of cholesterol biosynthesis to trigger seizures (Coenen and Van Luijtelaar, 2003; Persad et al., 2002; Vanluijtelaar and Sitnikova, 2006; Russo and Citraro, 2018). This makes the model particularly well-suited for long-term and/or repeated measure designs. Our longitudinal monitoring data showed relatively stable seizure burden in both males and females from 4 to 6 months of age, with increases in seizure susceptibility most evident in 8-10 months old. These findings are generally consistent with a prior report of generalized SWDs emerging in WAG/Rij rats between 2 and 4 months of age and increasing SWDs burden by 8 months of age (Coenen and Van Luijtelaar, 1987); but here, we reported an additional increase in absence epilepsy susceptibility and severity in older animals. In the present study, SWDs were 100% penetrant and were evident in every recording session, supporting the WAG/Rij strain as a valuable tool for epilepsy research.
We did not monitor estrous cycle in the present study because stressful procedures can induce biphasic effects on SWDs in WAG/Rijs, with an initial suppression of SWDs in the first 15 minutes, followed by an increase in the number SWDS for at least 60 minutes (Tolmacheva et al., 2012). Moreover, repetitive exposure to stressors presented an additional impact on both seizure suppression and seizure severity (Tolmacheva et al., 2012). In line with the impact of stress on SWDs expression in WAG/Rijs, it has been reported that systemic injections of corticosterone increased SWDs expression in WAG/Rij rats (Schridde and van Luijtelaar, 2004). A prior study in the WAG/Rij rat model, did, however, examine how estrous stage impacted SWDs; aside from circadian variation, similar to that observed in male WAG/Rij rats (Van Luijtelaar and Coenen, 1988), the only difference in females across the cycle was an increase in the number of SWDs during the first six hours of the dark period at proestrus. No difference was observed between days at any other hour (Luijtelaar et al., 2001). Conversely, SWDs were suppressed in pregnant female WAG/Rij rats; this suppression was associated with increased progesterone levels (Tolmacheva et al., 2004). A similar profile was found after intracerebral injection of progesterone in male WAG/Rij (Tolmacheva and van Luijtelaar, 2007b). By contrast, systemic acute injections of progesterone increased SWDs in non-pregnant female WAG/Rij rats (van Luijtelaar et al., 2001).
Effects of gonadectomy on SWDs in WAG/Rijs has likewise produced varying results. Yildiz et al. (2011) observed increased SWDs in ovariectomized WAG/Rijs between 7 to 11 days after ovariectomy. However, van Luijtelaar et al., (1996) showed that ovariectomized females did not show differences in SWDs in comparison to intact females 3 months after ovariectomy, while testosterone was shown to suppress SWDs in male WAG/Rij rats (van Luijtelaar et al., 1996). Similarly, Tolmacheva and van Luijtelaar, (2007a) showed no difference in basal SWDs expression in female WAG/Rijs compared to sham operated females for more than 1 month after surgery. However, ovariectomized females were shown to be more susceptible to stress-induced alterations in SWDs, suggesting that ovarian hormones may not control the basal expression of SWDs, but, conversely, they can modulate the impact of stressors in absence seizures (Tolmacheva and van Luijtelaar, 2007a). Therefore, given these prior reports in the WAG/Rij strain, we opted not to assess estrous cycle variation in the present study. Since all EEG recordings in the current study occurred during the light phase, under basal conditions, we think it unlikely that estrous cycle impacted our results. Moreover, we observed that the average coefficient of variability for females was equivalent to that for males across all measures. While this certainly does not rule out an estrous cycle effect in females, it does highlight that contrary to common concerns about increased variability in females, as compared to males, this may not be a concern in the WAG/Rij strain.
Interestingly, a previous study found no sex differences in WAG/Rij rats at 2.5, 4 or 6 months of age (Coenen and Van Luijtelaar, 1987). In the present study, by contrast, we found a robust main effect of sex on the number of SWDs, the mean SWD duration, and total duration of absence seizures, mainly in older rats (8-10 months of age). There are several possible explanations for this difference. First, previous study by Coenen and Van Luijtelaar (1987) was conducted three decades ago and genetic drift (upwards of 90 generations) may occur over time in genetic models of epilepsy (Doretto et al., 2003). Additionally, founder effects in sub-colonies may also lead to divergence over time, as reported in the GAERS, a genetic model of absence epilepsy (Powell et al., 2014). In the GAERS, differences in absence seizure expression were reported in four different colonies, which included differences in seizure duration, seizures per minute, and percentage of time in seizure (Powell et al., 2014). The WAG/Rij rats in our colony were derived from founders purchased from Charles River (Calco, Lecco, Italy) ~6 generations ago and it is likely that a genetic drift may have occurred. Moreover, the study by Coenen and Van Luijtelaar (1987) was less powered using only 6 rats per sex. In contrast, we sampled a larger number (n=17 per group) of males and females resulting in better powered study to detect sex differences.
Our finding of enhanced susceptibility to develop absence seizures in males is consistent with previous study using the GBL model (Santos et al., 2018); similar results were also found in the Brown Norway rat strain with males displaying longer and more frequent SWDs than females (Jandó et al., 1995). However, these differences are not consistent across all absence models, since the number and duration of absence seizures do not differ across sex in the GAERS strain (van Luijtelaar et al., 2014), and SWDs duration is higher in females than males in the AY-9944 model of atypical absence seizures (Persad et al., 2002).
In conclusion, we observed that male WAG/Rij rats are more susceptible to develop absence seizures than females during adulthood, presenting longer SWDs, an increased number of SWDs, and a greater total SWD duration. Additionally, the susceptibility to develop absence seizures increased with age regardless of sex. Our findings reinforce the importance to assess sex differences in seizure susceptibility for better management and treatment of epilepsy.
Supplementary Material
Highlights.
Male WAG/Rij rats showed greater mean SWDs duration than female WAG/Rij rats.
Male WAG/Rij rats showed increased number of SWDs compared to female WAG/Rij rats.
Total SWDs duration was increased in male WAG/Rij rats in comparison to females.
SWDs increased with age in both male and female WAG/Rij rats.
Acknowledgments
WLL holds Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil) grants: CAPES, (Finance code 001); CAPES-Print (Process no. 88887.370299/2019-00). Research was supported by R01NS097762 from the National Institute of Neurological Disorders and Stroke to PAF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
None of the authors has any conflict of interest to disclosure.
References
- Akman O, Demiralp T, Ates N, Onat FY, 2010. Electroencephalographic differences between WAG/Rij and GAERS rat models of absence epilepsy. Epilepsy Research 89, 185–193. 10.1016/j.eplepsyres.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Christian CA, Reddy DS, Maguire J, Forcelli PA, 2020. Sex Differences in the Epilepsies and Associated Comorbidities: Implications for Use and Development of Pharmacotherapies. Pharmacol Rev 72, 767–800. 10.1124/pr.119.017392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen AML, Drinkenburg WHIM, Peeters BWMM, Vossen JMH, vanLuijtelaar ELJM, 1991. Absence epilepsy and the level of vigilance in rats of the WAG/Rij strain. Neuroscience & Biobehavioral Reviews 15, 259–263. 10.1016/S0149-7634(05)80005-3 [DOI] [PubMed] [Google Scholar]
- Coenen AML, Van Luijtelaar ELJM, 2003. Genetic Animal Models for Absence Epilepsy: A Review of the WAG/Rij Strain of Rats. Behavior Genetics 33, 635–655. [DOI] [PubMed] [Google Scholar]
- Coenen AML, Van Luijtelaar ELJM, 1987. The WAG/Rij rat model for absence epilepsy: age and sex factors. Epilepsy Research 1, 297–301. 10.1016/0920-1211(87)90005-2 [DOI] [PubMed] [Google Scholar]
- Crunelli V, Leresche N, 2002. Childhood absence epilepsy: Genes, channels, neurons and networks. Nat Rev Neurosci 3, 371–382. 10.1038/nrn811 [DOI] [PubMed] [Google Scholar]
- Doretto MC, Fonseca CG, Lôbo RB, Terra VC, Oliveira JAC, Garcia-Cairasco N, 2003. Quantitative Study of the Response to Genetic Selection of the Wistar Audiogenic Rat Strain (WAR). Behavior Genetics 33, 33–42. [DOI] [PubMed] [Google Scholar]
- Jando’ G, Carpi D, Kandel A, Urioste R, Horvath Z, Pierre E, Vadi D, Vadasz C, Buzsa’ki G, 1995. Spike-and-wave epilepsy in rats: Sex differences and inheritance of physiological traits. Neuroscience 64, 301–317 10.1016/0306-4522(94)00329-4 [DOI] [PubMed] [Google Scholar]
- Killory BD, Bai X, Negishi M, Vega C, Spann MN, Vestal M, Guo J, Berman R, Danielson N, Trejo J, Shisler D, Novotny EJ, Constable RT, Blumenfeld H, 2011. Impaired attention and network connectivity in childhood absence epilepsy. Neuroimage 56, 2209–2217. 10.1016/j.neuroimage.2011.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur D, Shinnar S, Cnaan A, Shinnar RC, Clark P, Wang J, Weiss EF, Hirtz DG, Glauser TA, Group F the C.A.E.S., 2013. Pretreatment cognitive deficits and treatment effects on attention in childhood absence epilepsy. Neurology 81, 1572–1580. 10.1212/WNL.0b013e3182a9f3ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzianovskaia IS, Kuznetsova GD, Coenen AML, Spiridonov AM, van Luijtelaar ELJM, 2001. Electrophysiological and pharmacological characteristics of two types of spike-wave discharges in WAG/Rij rats. Brain Research 911, 62–70. 10.1016/S0006-8993(01)02705-6 [DOI] [PubMed] [Google Scholar]
- Midzyanovskaya IS, Kuznetsova GD, Vinogradova LV, Shatskova AB, Coenen AML, Luijtelaar G. van, 2004. Mixed forms of epilepsy in a subpopulation of WAG/Rij rats. Epilepsy & Behavior 5, 655–661. 10.1016/j.yebeh.2004.06.021 [DOI] [PubMed] [Google Scholar]
- Persad V, Cortez MA, Carter Snead O, 2002. A chronic model of atypical absence seizures: studies of developmental and gender sensitivity. Epilepsy Research 48, 111–119. 10.1016/S0920-1211(01)00319-9 [DOI] [PubMed] [Google Scholar]
- Powell KL, Tang H, Ng C, Guillemain I, Dieuset G, Dezsi G, Çarçak N, Onat F, Martin B, O’Brien TJ, Depaulis, Jones NC, 2014. Seizure expression, behavior, and brain morphology differences in colonies of Genetic Absence Epilepsy Rats from Strasbourg. Epilepsia 55, 1959–1968. 10.1111/epi.12840 [DOI] [PubMed] [Google Scholar]
- Reddy DS, 2017. The neuroendocrine basis of sex differences in epilepsy. Pharmacology Biochemistry and Behavior 152, 97–104. 10.1016/j.pbb.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, 2018. Pharmacology of epileptogenesis and related comorbidities in the WAG/Rij rat model of genetic absence epilepsy. Journal of Neuroscience Methods 310, 54–62. 10.1016/j.jneumeth.2018.05.020 [DOI] [PubMed] [Google Scholar]
- Santos VR, Kobayashi I, Hammack R, Danko G, Forcelli PA, 2018. Impact of strain, sex, and estrous cycle on gamma butyrolactone-evoked absence seizures in rats. Epilepsy Research 147, 62–70. 10.1016/j.eplepsyres.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisova K, van Luijtelaar G, 2011. The WAG/Rij strain: A genetic animal model of absence epilepsy with comorbidity of depressiony. Progress in Neuro-Psychopharmacology and Biological Psychiatry 35, 854–876. 10.1016/j.pnpbp.2010.11.010 [DOI] [PubMed] [Google Scholar]
- Sarkisova K. Yu., Kulikov MA, 2006. Behavioral characteristics of WAG/Rij rats susceptible and non-susceptible to audiogenic seizures. Behavioural Brain Research 166, 9–18. 10.1016/j.bbr.2005.07.024 [DOI] [PubMed] [Google Scholar]
- Savic I, 2014. Sex differences in human epilepsy. Experimental Neurology 259, 38–43. 10.1016/j.expneurol.2014.04.009 [DOI] [PubMed] [Google Scholar]
- Schridde U, van Luijtelaar G, 2004. Corticosterone increases spike-wave discharges in a dose- and time-dependent manner in WAG/Rij rats. Pharmacology Biochemistry and Behavior 78, 369–375. 10.1016/j.pbb.2004.04.012 [DOI] [PubMed] [Google Scholar]
- Tenney JR, Glauser TA, 2013. The Current State of Absence Epilepsy: Can We Have Your Attention?: The Current State of Absence Epilepsy. Epilepsy Curr 13, 135–140. 10.5698/1535-7511-13.3.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmacheva EA, Chepurnov SA, Chepumova NE, Kochetkov YA, van Luijtelaar G, 2004. Absence seizures during pregnancy in WAG/Rij rats. Physiology & Behavior 81, 623–627. 10.1016/j.physbeh.2004.02.028 [DOI] [PubMed] [Google Scholar]
- Tolmacheva EA, Oitzl MS, van Luijtelaar G, 2012. Stress, glucocorticoids and absences in a genetic epilepsy model. Hormones and Behavior 61, 706–710. 10.1016/j.yhbeh.2012.03.004 [DOI] [PubMed] [Google Scholar]
- Tolmacheva EA, van Luijtelaar G, 2007a. The role of ovarian steroid hormones in the regulation of basal and stress induced absence seizures. The Journal of Steroid Biochemistry and Molecular Biology 104, 281–288. 10.1016/j.jsbmb.2007.03.017 [DOI] [PubMed] [Google Scholar]
- Tolmacheva EA, van Luijtelaar G, 2007b. Absence seizures are reduced by the enhancement of GABA-ergic inhibition in the hippocampus in WAG/Rij rats. Neuroscience Letters 416, 17–21 10.1016/j.neulet.2007.01.038 [DOI] [PubMed] [Google Scholar]
- Van Luijtelaar ELJM, Coenen AML, 1988. Circadian rhythmicity in absence epilepsy in rats. Epilepsy Research 2, 331–336. 10.1016/0920-1211(88)90042-3 [DOI] [PubMed] [Google Scholar]
- van Luijtelaar ELJM, Dirksen R, Vree TB, van Haaren F, 1996. Effects of acute and chronic cocaine administration on EEG and behaviour in intact and castrated male and intact and ovariectomized female rats. Brain Research Bulletin 40, 43–50. 10.1016/0361-9230(96)00005-6 [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Budziszewska B, Jaworska-Feil L, Ellis J, Coenen A, Lasoń W, 2001. The ovarian hormones and absence epilepsy: a long-term EEG study and pharmacological effects in a genetic absence epilepsy model. Epilepsy Research 46, 225–239. 10.1016/S0920-1211(01)00277-7 [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Onat FY, Gallagher MJ, 2014. Animal models of absence epilepsies: What do they model and do sex and sex hormones matter? Neurobiology of Disease 72, 167–179. 10.1016/j.nbd.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanluijtelaar G, Sitnikova E, 2006. Global and focal aspects of absence epilepsy: The contribution of genetic models. Neuroscience & Biobehavioral Reviews 30, 983–1003. 10.1016/j.neubiorev.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Velíšková J, DeSantis KA, 2013. Sex and hormonal influences on seizures and epilepsy. Hormones and Behavior 63, 267–277. 10.1016/j.yhbeh.2012.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova L, Kuznetsova G, Coenen A, 2005. Audiogenic seizures associated with a cortical spreading depression wave suppress spike-wave discharges in rats. Physiology & Behavior 86, 554–558. 10.1016/j.physbeh.2005.08.017 [DOI] [PubMed] [Google Scholar]
- Vinogradova LV, 2017. Audiogenic kindling and secondary subcortico-cortical epileptogenesis: Behavioral correlates and electrographic features. Epilepsy & Behavior 71, 142–153. 10.1016/j.yebeh.2015.06.014 [DOI] [PubMed] [Google Scholar]
- Vinogradova LV, 2008. Audiogenic kindling in Wistar and WAG/Rij rats: Kindling-prone and kindling-resistant subpopulations. Epilepsia 49, 1665–1674. 10.1111/j.1528-1167.2008.01617.x [DOI] [PubMed] [Google Scholar]
- Wicker E, Beck VC, Kulick-Soper C, Kulick-Soper CV, Hyder SK, Campos-Rodriguez C, Khan T, N’Gouemo P, Forcelli PA, 2019. Descending projections from the substantia nigra pars reticulata differentially control seizures. Proc Natl Acad Sci USA 116, 27084–27094. 10.1073/pnas.1908176117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz OK, Yildiz C, Durmus N, Gulturk S, Benek S, Cetin A, 2011. Ovariectomy enhances spike-wave discharges in WAG/Rij rats. Neurology, Psychiatry and Brain Research 17, 67–70. 10.1016/j.npbr.2011.06.005 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.