Abstract
DNA detection plays an important role in the rapid screening of cancers and early diagnosis of infectious diseases. Here, we developed a simple, versatile, electric field-enhanced (EFE), electrochemical CRISPR biosensor to detect DNA targets in a homogeneous solution phase. To improve the detection sensitivity, we applied a pulsed electric field to enrich nucleic acids on the electrode surface. The EFE electrochemical CRISPR biosensor takes advantage of the diffusivity difference between electrochemical oligonucleotide probes and CRISPR-cleaved probes toward a negatively charged working electrode, enabling simple and sensitive electrochemical detection of DNA without the need for complicated immobilization processing of electrochemical probes. Our developed CRISPR biosensor directly detects unamplified human papillomavirus-16 (HPV-16) DNA with a sensitivity of 1 pM. Further, the EFE electrochemical CRISPR biosensor coupled with recombinase polymerase amplification successfully detects HPV-16 DNA in clinical samples. Thus, the EFE electrochemical CRISPR biosensor provides a simple, robust, and sensitive detection method for nucleic acid-based molecular diagnostics.
Keywords: CRISPR-Cas12a, pulsed electric field, nucleic acid enrichment, electrochemical DNA biosensor, human papillomavirus (HPV)-associated cancer screening
Graphical Abstract

1. Introduction
Simple, rapid, and sensitive detection of nucleic acids plays a crucial role in early screening of cancer, infectious disease diagnostics, genotyping, and food safety monitoring. Polymerase chain reaction (PCR) methods have been widely used for nucleic acid detection due to their high sensitivity and specificity. However, PCR methods typically require bulky equipment, well-trained personnel, and long turnover times, limiting their utilization to only well-equipped laboratories. Recently, CRISPR-associated (Cas) proteins (e.g., Cas9, Cas12a, Cas13a) have emerged as tools for sequence-specific nucleic acid detection (Broughton et al., 2020; Gootenberg et al., 2017; Zhou et al., 2018). Due to their high sensitivity, specificity, and ability to be programmed, researchers are exploiting CRISPR-Cas proteins to develop various nucleic acid-based molecular diagnostic tools. For instance, several research groups have combined CRISPR-Cas12a programmed by CRISPR RNA (crRNA) with isothermal amplification to specifically detect nucleic acid targets with high sensitivity (Chen et al., 2018; Ding et al., 2020). By recognition of its target DNA, Cas12a protein can be specifically activated and can indiscriminately cleave single-stranded DNA (ssDNA) (trans-cleavage activity). However, most of these methods use ssDNA labeled with both fluorophore and quencher as the fluorescence probe for fluorescence detection, which relies on relatively expensive and complicated fluorescence detectors (Chen et al., 2018; Ding et al., 2020; Li et al., 2018; Wang et al., 2021).
Compared with fluorescence detection, electrochemical detection provides a simpler, lower cost, and more powerful strategy for nucleic acid detection. Recent efforts have established several electrochemical CRISPR-based biosensors for nucleic acid detection by taking advantage of the cleavage capabilities of CRISPR-Cas proteins (Bruch et al., 2019; Dai et al., 2019; Li et al., 2021; Zhang et al., 2020). However, most of these biosensors require the electrochemical probes to be immobilized on the electrode surface, which is a complicated and time-consuming process. In addition, due to the steric hindrance of the immobilized probes (Xiao et al., 2007), the immobilization approaches can suffer from reduced cleavage efficiency and selectivity on a heterogeneous surface compared to that in a homogeneous solution. Therefore, it is useful to develop a simple, sensitive, and versatile electrochemical CRISPR biosensing method for DNA detection without the need for a complex immobilization processing of electrochemical probes.
Various electrokinetic methods have been widely adapted to facilitate the concentration, transport, hybridization, and denaturation of DNA molecules (Bown and Meinhart, 2006; Fixe et al., 2004; Peterson et al., 2001; Radtkey et al., 2000; Sosnowski et al., 1997). For instance, Bown and Meinhart used a high-frequency electric field to concentrate DNA and minimize irreversible electrochemical reactions at the electrode surface (Bown and Meinhart, 2006). Sosnowski et al. proposed an electric field-assisted DNA immobilization approach to increase the DNA hybridization reaction rates (Sosnowski et al., 1997). By using an electric field, Fixe et al. demonstrated that DNA immobilization and hybridization rates were 109 times faster compared with passive control reactions without electric fields (Fixe et al., 2004). In addition, electrochemical DNA detection is compatible with electric field application on the electrode surface. Thus, electric fields can enrich nucleic acids and improve the detection sensitivity of electrochemical DNA biosensors.
In this study, we developed a simple, sensitive, versatile, immobilization-free, electric field-enhanced (EFE), electrochemical CRISPR biosensor to detect DNA in a homogeneous solution phase. To improve the detection sensitivity, we applied a pulsed electric field to enrich nucleic acids on the working electrode surface. Unlike previously reported electrochemical CRISPR biosensors with immobilized electroactive probes (Bruch et al., 2019; Dai et al., 2019; Li et al., 2021; Zhang et al., 2020), our biosensor employs a methylene blue (MB)-labeled ssDNA (ssDNA-MB) as the electrochemical signaling probe in a homogeneous solution, eliminating the need for time-consuming immobilization procedures. By taking advantage of the trans-cleavage activity of CRISPR-Cas12a, the negatively charged ssDNA-MB probes are cleaved and release less negative MB-labeled probes, which can diffuse freely to the negatively charged working electrode and increase the electrochemical signal. To demonstrate the clinical utility of our EFE electrochemical CRISPR biosensor, we coupled it with recombinase polymerase amplification (RPA) to successfully detect HPV-16 DNA in clinical swab samples.
2. Experimental Section
2.1. Reagents and materials
All of the oligonucleotides (Supporting Information Table S1), crRNAs, and HPV-16 plasmid DNA were synthesized or purchased from Integrated DNA Technologies (IA, USA). LbCas12a, 10x NEBuffer 2.1 and nuclease-free water were purchased from New England BioLabs (MA, USA). The TwistAmp Liquid Basic Kit was purchased from TwistDx Ltd. (Maidenhead, UK) and the DNeasy Blood & Tissue Kit was purchased from Qiagen (Hilden, Germany). The electrochemical sensor and its detection platform were obtained from MicruX Technologies (Asturias, Spain). All other chemicals used were of analytical reagent grade.
2.2. CRISPR-based fluorescence detection
CRISPR-based fluorescence detection of HPV-16 DNA was carried out according to previous work (Chen et al., 2018). Briefly, the final CRISPR reaction system contained 200 nM LbCas12a, 250 nM crRNA, 1x buffer (50 mM NaCl, 10 mM Tris-HCl, 10 mM MgCl2, 100 μg/ml BSA, pH 7.9 at 25 °C), 1 μM single-stranded DNA fluorophore-quencher (ssDNA-FQ), and DNA target. For in-tube CRISPR detection, the CRISPR reaction tubes were incubated at 37 °C for 60 minutes and the fluorescence signals were monitored in real time using the Bio-Rad CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, CA).
For on-chip CRISPR detection, the CRISPR reaction occurred in the chamber of the electrochemical detection platform (MicruX Technologies, Asturias, Spain) (Supporting Information Figure S1). A digital block heater (VWR Mini Block Heater, PA, USA) was used to incubate the electrochemical detection platform at 37 °C. Mineral oil was added to the reaction chamber to minimize liquid evaporation during incubation. For fluorescence detection, a portable USB fluorescence microscope (AM4113T-GFBW, Dino-Lite Premier, AnMo Electronics, Taiwan, China) was used to record the fluorescence signals of the electrochemical chamber in real time (Kadimisetty et al., 2018) (Supporting Information Figure S2). Further, the fluorescence signals of the CRISPR reaction chamber were integrated and generated normalized average fluorescence intensities at every specified time interval (e.g., 1 min interval for 60 min). Normalized fluorescence intensities were plotted against time to obtain real-time fluorescence curves of CRISPR detection.
2.3. Pulsed electric field generation
During on-chip CRISPR-based detection, pulsed electric fields (Supporting Information Figure S3) were generated by Trueform Series Waveform Generators 33520B (Keysight Technologies, CA, USA). The pulsed electric field was applied to the electrochemical CRISPR biosensor, where the working electrode served as a positive electrode and the reference/counter electrodes served as ground electrodes.
2.4. Electrochemical detection
For on-chip electrochemical detection, the ssDNA-FQ was replaced by electroactive ssDNA-MB probes and the electrochemical signals were detected at the end of the CRISPR reactions (e.g., 60 min). All electrochemical signal detections were performed on a CHI660D potentiostat (CH Instruments, TX, USA). Differential pulse voltammetry (DPV) was applied to determine the redox peak current.
2.5. Clinical sample preparation and HPV-16 DNA detection
De-identified clinical vaginal swab samples were obtained from the Hospital of the University of Pennsylvania and approved by its ethics committee (IRB protocol: #829760). HPV DNA was extracted from clinical samples by the DNeasy Blood & Tissue Kit according to the manufacturer’s protocol. Briefly, 200 μL clinical vaginal swab samples were centrifuged at 1,000 × g for 10 min to remove the liquid supernatant. The concentrated cells washed by PBS for 3 times were resuspended in 200 μL PBS, mixed with 20 μL proteinase K and 200 μL buffer AL (QIAamp DNeasy Blood and Tissue Kit), and then incubated at 56 °C for 10 min. The lysate was mixed with 200 μL ethanol and introduced into the DNeasy Mini spin column for nucleic acid extraction and purification. Subsequent to the sample introduction, 500 μL of Qiagen wash buffer 1 (AW1) and Qiagen wash buffer 2 (AW2) were, respectively, added into the spin column to remove any remaining amplification inhibitors. Then, the DNA was eluted by adding 200 μL Buffer AE.
For clinical sample detection, the HPV-16 DNA extracts were first pre-amplified by RPA amplification at 37 °C. The 25 μL RPA reaction solution contained 1x reaction buffer, 1x basic E-mix, 1x core reaction buffer, 14 mM MgOAc, 0.32 μM each of forward and reverse primers, 0.8 mM of each nucleotide (dATP, dTTP, dCTP, dGTP), and 2 μL DNA extracts. After RPA amplification, 2 μL amplicons were used for electrochemical CRISPR detection on the EFE electrochemical CRISPR biosensor.
3. Results and Discussion
3.1. EFE electrochemical CRISPR biosensor
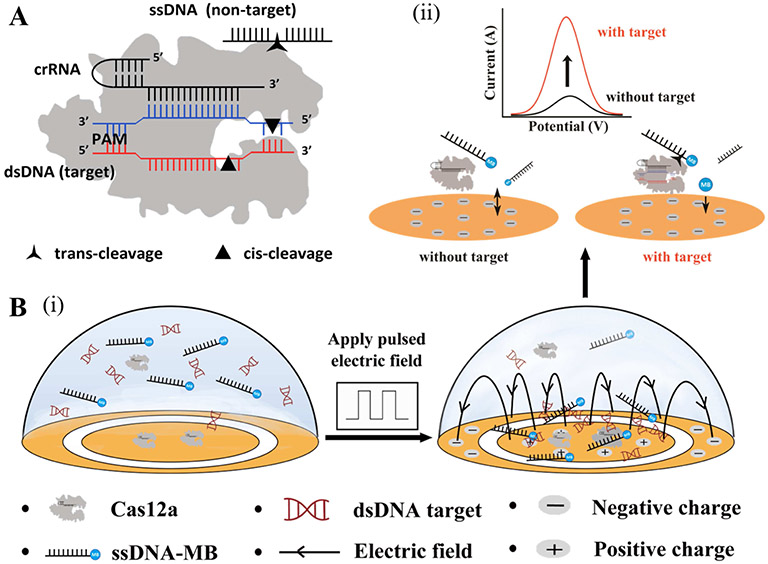
The working principle of the EFE, immobilization-free, electrochemical CRISPR biosensor is schematically illustrated in Figure 1. As shown in Figure 1A, the CRISPR-Cas12a protein has both cis-cleavage activity (target DNA cutting) and trans-cleavage activity (ssDNA cutting). To enhance the electrochemical detection sensitivity, our CRISPR biosensor uses a pulsed electric field to enrich DNAs (e.g., target DNA, ssDNA-MB) on the positively charged surface of the working electrode (Figure 1B (i)) due to the negative charge of the DNA molecules. In the absence of the target DNA, the ssDNA-MB probe is electrostatically repelled from the negatively charged electrode during differential pulse voltammetry detection (potential range of - 0.6 to 0 V) because the DNA itself is negatively charged, which leads to a low electrochemical current on the working electrode (Figure 1B (ii)). On the contrary, in the presence of the target DNA, the CRISPR-Cas12a protein is specifically activated and non-specifically cuts ssDNA-MB due to its trans-cleavage activity, releasing the CRISPR-cleaved, electroactive MB probes. Due to its less negative charge and smaller size, the cleaved MB probe has higher diffusivity toward and reduced electrostatic repulsion from the negatively charged electrode surface than that of the ssDNA-MB probes (Xuan et al., 2013), which results in an increased electrochemical signal (Figure 1B(ii)). Unlike previously reported electrochemical CRISPR biosensors (Bruch et al., 2019; Dai et al., 2019; Li et al., 2021; Zhang et al., 2020), our proposed CRISPR biosensor performs electrochemical detection of DNA targets in a homogeneous solution, which not only eliminates the need for complicated probe immobilization on the electrode surface, but also improves the reaction efficiency. Thus, our EFE electrochemical CRISPR biosensor provides a simple, highly sensitive, immobilization-free, electrochemical DNA detection strategy.
Figure 1.
Working principle of the EFE, immobilization-free electrochemical CRISPR biosensor for DNA detection. A) Trans-cleavage and cis-cleavage activities of CRISPR-Cas12a protein in the presence of crRNA, DNA target, and ssDNA (non-target). B) Operation procedures of the EFE, electrochemical CRISPR biosensor. (i) A pulsed electric field is applied to attract nucleic acids (e.g., electroactive ssDNA probe, dsDNA target) on the positively charged working electrode surface due to the static electric force. (ii) Electrochemical detection in the absence and presence of the target DNA. The trans-cleavage activity of activated Cas12a releases less negative MB-labeled probe, resulting in an increased electrochemical current during DPV detection.
3.2. Optimization of the ssDNA-MB probe concentration
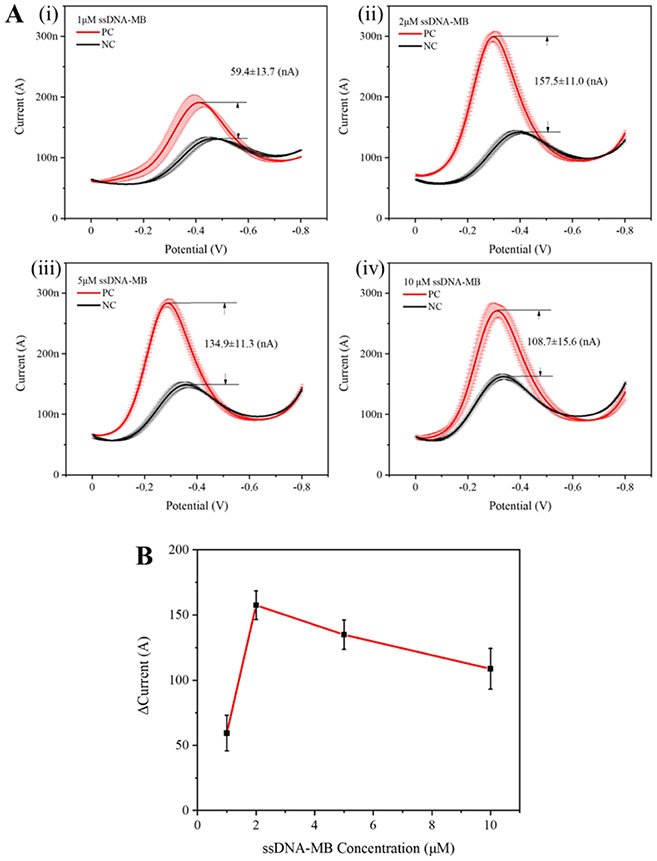
MB has been widely used either as an electrochemical hybridization indicator or as an electroactive probe for DNA strands in electrochemical DNA detection due to its high sensitivity and electrochemical activity (Erdem et al., 2001; Hsieh et al., 2012; Pänke et al., 2007; Yu et al., 2019). In our ssDNA-MB probe, the MB molecule is modified on the end of the ssDNA oligonucleotides, which is crucial for providing the electrostatic force due to its negative charge in our EFE electrochemical CRISPR biosensor. The electroactive ssDNA-MB probe consists of a five-mer single-strand DNA and an MB tag labeled at the 3′ terminus (Table S1). We optimized the amount of ssDNA-MB probe by evaluating different ssDNA-MB probe concentrations ranging from 1 to 10 μM. As shown in Figure 2, the concentration of 2 μM ssDNA-MB probes showed the best signal-to-background ratio (157.5±11.0 nA). Interestingly, further increasing the ssDNA-MB probe concentration reduced the electrochemical current difference. We attributed this result to the increased background signals because more ssDNA-MB probes would diffuse to the electrode surface by overcoming the electrostatic repulsion at high probe concentrations. Therefore, we used the 2 μM concentration of the ssDNA-MB probe in all subsequent experiments due to its optimal signal-to-background ratio.
Figure 2.
Optimization of the ssDNA-MB probe concentration for the EFE electrochemical CRISPR biosensor. A) Electrochemical current of the positive and negative samples at different ssDNA-MB probe concentrations (1, 2, 5, and 10 μM) (i-iv). PC is the positive control with 500 pM HPV-16 DNA target. NC is the negative control without HPV-16 DNA target. B) Comparison of the electrochemical current difference at ssDNA-MB concentrations ranging from 1 μM to 10 μM. Error bars represent the means ± s.d. from three replicates (n = 3).
3.3. Optimization of the pulsed electric field
Previous research indicates that the electric field provides an ideal strategy for DNA manipulation and concentration with different electric waveforms (Bown and Meinhart, 2006; Fixe et al., 2005, 2004; Peterson et al., 2001; Radtkey et al., 2000; Sosnowski et al., 1997; Swami et al., 2009). Here, we applied a pulsed electric field (Figure S3) to the electrochemical electrodes during the CRISPR-based detection. The pulsed electric field consisted of an AC electric field and a DC offset. We set the offset potential as half of the peak-to-peak potential (i.e., the amplitude of the electric field) and the pulse width as 50% of T (i.e., the period). To facilitate real-time monitoring of the CRISPR detection signal during pulsed electric field optimization, we used a portable Dino-Lite digital fluorescence microscope to record the fluorescence images of the CRISPR reaction solution in the electrochemical chamber (Figure 3A and Supporting Information Video S1). First, we determined the effects of different peak-to-peak potential values ranging from 0 to 100 mV on the CRISPR biosensor. As shown in Figures 3B and S4, we found that the higher the peak-to-peak amplitude, the stronger the fluorescence signals of the CRISPR detection, thus demonstrating that the pulsed electric field can enrich the DNA and accelerate CRISPR detection. However, when the peak-to-peak amplitude reached 100 mV, the fluorescence signal decreased, which may be attributed to the redox reaction on the electrode surface when relatively high voltage is applied. In our experiment, a peak-to-peak amplitude of 10 mV resulted in the strongest fluorescence signals. Next, we investigated the effects of the frequency of the pulsed electric field. As shown in Figures 3C and S5, we observed no significant difference when we applied various frequencies (from 1 Hz to 100 Hz). Therefore, we utilized the optimized pulsed electric field with a peak-to-peak amplitude of 10 mV and a frequency of 1 Hz for all subsequent experiments.
Figure 3.
Optimization of the pulsed electric field for CRISPR-based DNA detection. A) Fluorescence images of the reaction chambers of the electrochemical CRISPR biosensor at different incubation times (0, 20, 40, and 60 min). B) Effect of the peak-to-peak amplitude (0, 1, 10, and 100 mV) on the CRISPR-based DNA detection. C) Effect of the frequency (1, 10, and 100 Hz) on the CRISPR-based DNA detection. The relative fluorescence intensities were recorded at the endpoint of the CRISPR reaction. PC is the positive control with 500 pM HPV-16 DNA target. NC is the negative control without HPV-16 DNA target. Error bars represent the means ± s.d. from three replicates (n = 3).
3.4. Analytical performance of the EFE electrochemical CRISPR biosensor
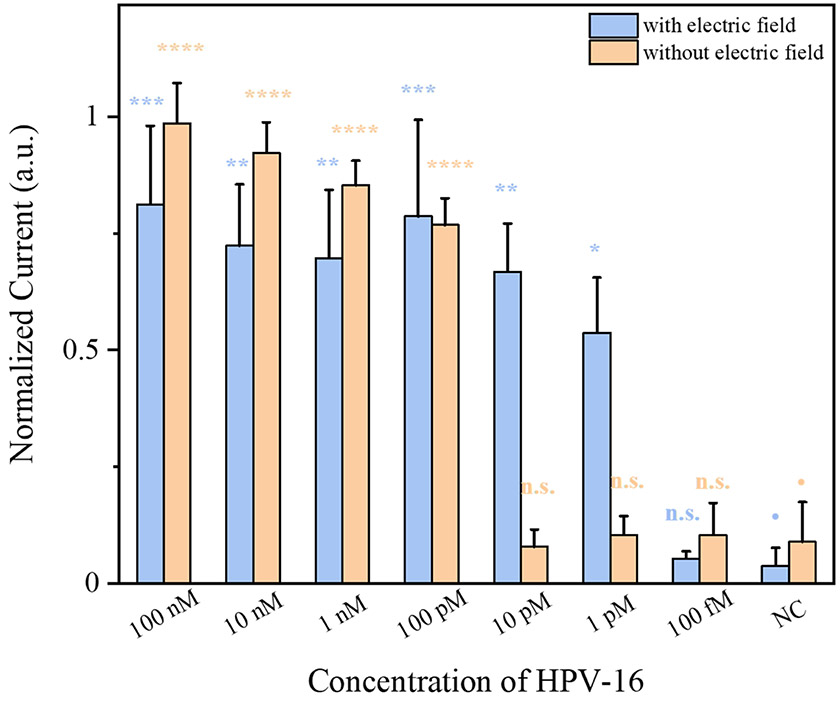
Under the optimized experimental conditions, we determined the analytical sensitivity of our EFE electrochemical CRISPR biosensor by directly detecting a tenfold serial dilution of unamplified HPV-16 DNA. For comparison, we evaluated the analytical performance of the electrochemical CRISPR biosensor with and without the pulsed electric field applied. As shown in Figure 4, without applying a pulsed electric field, the electrochemical CRISPR biosensor could detect 100 pM of HPV-16 DNA target, which is comparable to that of the conventional CRIPSR-based fluorescence detection in the reaction tubes (Supporting Information Figure S6). On the contrary, when we applied the pulsed electric field, the electrochemical CRISPR biosensor consistently detected 1 pM of HPV-16 DNA target, which is 100 times higher than when the pulsed electric field was not applied. In addition, compared with previous electrochemical CRISPR biosensors with immobilized probes (Dai et al., 2019), our EFE electrochemical CRISPR biosensor showed 50 times higher sensitivity in the detection of unamplified HPV-16 DNA. The improved sensitivity of our CRISPR biosensor can be attributed to electric field-assisted DNA enrichment induced by the pulsed electric field. Therefore, applying the pulsed electric field significantly improves the detection sensitivity of the electrochemical CRISPR biosensor for DNA detection.
Figure 4.
Comparison of HPV-16 DNA detection by the electrochemical CRISPR biosensor with and without applying a pulsed electric field. Statistical analysis was performed using a one-way ANOVA test with Tukey’s comparison test, where n.s. = not significant with p > 0.05 and the asterisks (*, **, ***, ****) denote significant differences with p values (* = 0.001 <P ≤0.05, ** = 0.0001 <P ≤ 0.001, *** = 0.00001 < P ≤ 0.0001, **** = P ≤ 0.00001). Error bars represent the means ± s.d. from three replicates (n = 3).
3.5. Clinical validation of the EFE electrochemical CRISPR biosensor
The high-risk HPV-16 is the most prevalent genotype in HPV-associated cancers, including cervical cancer (Ogilvie et al., 2018; Schiffman et al., 2007; Yin et al., 2020). To validate the clinical utility of our EFE electrochemical CRISPR biosensor, we applied it to clinical swab samples, including two negative samples and four positive samples, for HPV-16 DNA detection. To meet clinical requirements, we first pre-amplified the HPV-16 DNA samples extracted from clinical swab samples by RPA amplification before performing electrochemical detection. As shown in Figure 5 and Supporting Information Figure S7, all four positive clinical samples consistently showed a higher electrochemical peak in their DPV curves, which was not the case for the two negative samples. For comparison, we also tested the HPV-16 DNA samples using conventional CRISPR-based fluorescence detection in the reaction tubes after RPA pre-amplification (Supporting Information Figure S8), which showed results consistent with those of our EFE electrochemical CRISPR biosensor (Figure 5). Therefore, our EFE electrochemical CRISPR biosensor is suitable for clinical diagnostic applications in the detection of HPV-associated cancer and other infectious diseases.
Figure 5.
HPV-16 DNA detection in clinical samples by using the EFE electrochemical CRISPR biosensor after RPA pre-amplification. A) Electrochemical response curves of the EFE electrochemical CRISPR biosensor for HPV-16 DNA detection in clinical samples. B) Normalized electrochemical current of the EFE electrochemical CRISPR biosensor for HPV-16 DNA detection in clinical samples. S1, S2, S4, and S5 are positive clinical samples. S3 and S6 are negative samples. PC and NC are, respectively, the positive and negative controls. Error bars represent the means ± s.d. from three replicates (n = 3).
4. Conclusion
In this study, we developed a simple, sensitive, and versatile electrochemical CRISPR biosensor for DNA detection in homogeneous solutions by combining electric field-assisted DNA enrichment with a CRISPR-based assay. Compared to previous electrochemical CRISPR biosensors, our EFE electrochemical CRISPR biosensor offers several advantages. i) By leveraging the pulsed electric field, the developed electrochemical CRISPR biosensor can detect 1 pM HPV-16 DNA target without amplification, which is 100 times higher than conventional electrochemical CRISPR detection. To the best of our knowledge, this is the first demonstration of applying a pulsed electric field to improve CRISPR-based DNA biosensing. ii) Unlike existing electrochemical CRISPR biosensors (Bruch et al., 2019; Dai et al., 2019; Li et al., 2021; Zhang et al., 2020), our biosensor enables CRISPR-Cas12a cleavage in a homogeneous solution phase rather than the heterogeneous electrode/solution interface, which improves the CRISPR detection efficiency by minimizing steric hindrance of the immobilized probes and eliminates the need for the tedious probe immobilization process. iii) By coupling with isothermal amplification, our EFE electrochemical CRISPR biosensor can detect HPV-16 DNA in clinical samples, enabling simple and sensitive point-of-care molecular diagnostics. To further simplify the operation of the CRISPR biosensor, we will adapt the developed CRISPR biosensing methodology to “one-pot” RPA/CRISPR assay (Ding et al., 2020) in the future. Therefore, the developed EFE electrochemical biosensor represents a promising path toward realizing simple, portable, and affordable electrochemical DNA detection for clinical applications at the point of care.
Supplementary Material
Highlights.
A simple, electric field-enhanced (EFE), electrochemical CRISPR biosensor was developed for DNA detection in a homogeneous solution phase
A pulsed electric field was applied to enrich nucleic acids on the electrode surface, enabling highly sensitive CRISPR-based DNA biosensing
The EFE electrochemical CRISPR biosensor was applied to detect unamplified HPV-16 DNA with a sensitivity of 1 pM
Clinical application of the developed CRISPR biosensor was successfully demonstrated by detecting HPV-16 DNA in clinical samples
Acknowledgments
The work was supported, in part, by National Institutes of Health (NIH) grants R01 CA214072, R01 EB023607, and R61 AI154642.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bown MR, Meinhart CD, 2006. Microfluid. Nanofluidics 2, 513–523. [Google Scholar]
- Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, Singh J, Miao X, Streithorst JA, Granados A, Sotomayor-Gonzalez A, Zorn K, Gopez A, Hsu E, Gu W, Miller S, Pan CY, Guevara H, Wadford DA, Chen JS, Chiu CY, 2020. Nat. Biotechnol 38, 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruch R, Baaske J, Chatelle C, Meirich M, Madlener S, Weber W, Dincer C, Urban GA, 2019. Adv. Mater 31, 1905311. [DOI] [PubMed] [Google Scholar]
- Chen JS, Ma E, Harrington LB, Da Costa M, Tian X, Palefsky JM, Doudna JA, 2018. Science. 360, 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Somoza RA, Wang L, Welter JF, Li Y, Caplan AI, Liu CC, 2019. Angew. Chemie - Int. Ed 58, 17399–17405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Yin K, Li Z, Lalla RV, Ballesteros E, Sfeir MM, Liu C, 2020. Nat. Commun 11, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem A, Kerman K, Meric B, Ozsoz M, 2001. Electroanalysis 13, 219–223. [Google Scholar]
- Fixe F, Branz HM, Louro N, Chu V, Prazeres DMF, Conde JP, 2005. Nanotechnology 16, 2061–2071. [DOI] [PubMed] [Google Scholar]
- Fixe F, Branz HM, Louro N, Chu V, Prazeres DMF, Conde JP, 2004. Biosens. Bioelectron 19, 1591–1597. [DOI] [PubMed] [Google Scholar]
- Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, Verdine V, Donghia N, Daringer NM, Freije CA, Myhrvold C, Bhattacharyya RP, Livny J, Regev A, Koonin EV, Hung DT, Sabeti PC, Collins JJ, Zhang F, 2017. Science (80-. ) 356, 438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh K, Patterson AS, Ferguson BS, Plaxco KW, Soh HT, 2012. Angew. Chemie - Int. Ed 51, 4896–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadimisetty K, Song J, Doto AM, Hwang Y, Peng J, Mauk MG, Bushman FD, Gross R, Jarvis JN, Liu C, 2018. Biosens. Bioelectron 109, 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Ye Q, Chen M, Zhou B, Zhang J, Pang R, Xue L, Wang J, Zeng H, Wu S, Zhang Y, Ding Y, Wu Q, 2021. Biosens. Bioelectron 179, 113073. [DOI] [PubMed] [Google Scholar]
- Li SY, Cheng QX, Li XY, Zhang ZL, Gao S, Cao RB, Zhao GP, Wang J, Wang JM, 2018. Cell Discov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie GS, Van Niekerk D, Krajden M, Smith LW, Cook D, Gondara L, Ceballos K, Quinlan D, Lee M, Martin RE, Gentile L, Peacock S, Stuart GCE, Franco EL, Coldman AJ, 2018. JAMA - J. Am. Med. Assoc 320, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pänke O, Kirbs A, Lisdat F, 2007. Biosens. Bioelectron 22, 2656–2662. [DOI] [PubMed] [Google Scholar]
- Peterson AW, Heaton RJ, Georgiadis RM, 2001. Nucleic Acids Res. 29, 5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtkey R, Feng L, Muralhidar M, Duhon M, Canter D, DiPierro D, Fallon S, Tu E, McElfresh K, Nerenberg M, Sosnowski R, 2000. Nucleic Acids Res. 28, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S, 2007. Lancet. [DOI] [PubMed] [Google Scholar]
- Sosnowski RG, Tu E, Butler WF, O’Connell JP, Heller MJ, 1997. Proc. Natl. Acad. Sci. U. S. A 94, 1119–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swami N, Chou CF, Ramamurthy V, Chaurey V, 2009. Lab Chip 9, 3212–3220. [DOI] [PubMed] [Google Scholar]
- Wang R, Chen R, Qian C, Pang Y, Wu J, Li F, 2021. Sensors Actuators, B Chem. 326, 128618. [Google Scholar]
- Xiao Y, Qu X, Plaxco KW, Heeger AJ, 2007. J. Am. Chem. Soc 129, 11896–11897. [DOI] [PubMed] [Google Scholar]
- Xuan F, Luo X, Hsing IM, 2013. Anal. Chem 85, 4586–4593. [DOI] [PubMed] [Google Scholar]
- Yin K, Ding X, Li Z, Zhao H, Cooper K, Liu C, 2020. Anal. Chem 92, 8561–8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Luan Y, Li H, Wang W, Wang X, Zhang Q, 2019. Sensors Actuators, B Chem. 284, 73–80. [Google Scholar]
- Zhang D, Yan Y, Que H, Yang T, Cheng X, Ding S, Zhang X, Cheng W, 2020. ACS Sensors 5, 557–562. [DOI] [PubMed] [Google Scholar]
- Zhou W, Hu L, Ying L, Zhao Z, Chu PK, Yu XF, 2018. Nat. Commun 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.