Abstract
Recent studies have brought to light the necessity to discern sex-specific differences in various pain states and different cell-types that mediate these differences. These studies have uncovered the role of neuroimmune interactions to mediate pain states in a sex-specific fashion. While investigating immune function in pain development, we discovered that females utilize immune components of sensory neurons to mediate neuropathic pain development. We utilized two novel transgenic mouse models that either restore expression of toll-like receptor (TLR) 4 in Nav1.8 nociceptors on a TLR4-null background (TLR4LoxTB) or remove TLR4 specifically from Nav1.8 nociceptors (TLR4fl/fl). After spared nerve injury (SNI), a model of neuropathic injury, we observed a robust female-specific onset of mechanical hypersensitivity in our transgenic animals. Female Nav1.8-TLR4fl/fl knockout animals were less mechanically sensitive than cre-negative TLR4fl/fl littermates. Conversely, female Nav1.8-TLR4LoxTB reactivated animals were as mechanically sensitive as their wild-type counterparts. These sex and cell-specific effects were not recapitulated in male animals of either strain. Additionally, we find the danger associated molecular pattern, high mobility group box-1 (HGMB1), a potent TLR4 agonist, localization and ATF3 expression in females is dependent on TLR4 expression in dorsal root ganglia (DRG) populations following SNI. These experiments provide novel evidence toward sensory neuron specific modulation of pain in a sex-dependent manner.
Keywords: Neuroimmune, Neuropathic Pain, Sex Differences, Mechanical Hypersensitivity, Nav1.8, TLR4, HMGB1
1. Introduction
The CDC lists chronic pain as a leading cause of long-term disability, with current treatments remaining ineffective (Dahlhamer et al., 2018). Grim side effects have tallied over 600,000 opioid-related deaths, with 64% of these cases linked to chronic pain patients since 2010 (Seth et al., 2018). These issues become more complex with females being more susceptible to certain forms of chronic pain (Joseph et al., 2003). Neuropathic pain results from disease or trauma to the nervous system, with injury-induced neuropathic pain estimated to occur in 20–50% of patients following routine operations (Kehlet et al., 2006). Sufferers often report a significantly reduced quality of life and the prevalence of chronic pain continues to rise as the number of effective therapeutics remain limited (McDermott et al., 2006). Recent literature highlighting sexual dimorphisms in the onset and chronicity of pain states have begun to alter the way researchers and clinicians approach mechanisms in pain and potential therapeutics. Therefore, a dire need exists to bolster research efforts to identify crucial therapeutic time windows as well as novel and effective alternatives for the treatment and abatement of chronic pain. Here we hope to Identify early time-points in the development of neuropathic pain that could represent a viable therapeutic window.
Toll-like receptors (TLRs) are pattern recognition receptors (PRRs) that initiate responses to infection and tissue injury that lead to inflammation and nocifensive behaviors (Hanamsagar et al., 2012; Nicotra et al., 2012; Xiang et al., 2015). TLR4 plays a pivotal role in chronic pain through the activation of pro-inflammatory cytokine production in non-neuronal cells. Pro-inflammatory mediators have been shown to directly sensitize neurons in the dorsal root ganglia (DRG) and exacerbate maladaptive plasticity (Ma et al., 2006). The dynamics of TLR4-mediated activation via microglia is well established where pharmacological inhibition of spinal TLR4 attenuates mechanical hypersensitivity in male, but not female mice (Sorge et al., 2011; Woller et al., 2016). Moreover, the role for T-cells regulating development of neuropathic pain in females has been alluded to, however, recent evidence leads us to believe that nociceptive sensory neurons are the primary facilitators of chronic pain etiology (Lopes et al., 2017). Differential expression of genes involved in nociceptive pathways have been identified with females expressing upregulated genetic markers that correlate with increased inflammation, synaptic transmission, and extracellular matrix (ECM) reorganization (Mecklenburg et al., 2020; Wangzhou et al., 2021). In the periphery, direct TLR4 stimulation is involved in dental pain via sensitization of trigeminal ganglion neurons (Diogenes et al., 2011), while TLR4 in dorsal root ganglia (DRG) cultures are implicated in chemotherapy-induced neuropathy (Li et al., 2015a). Recent studies suggest that TLR4 on peripheral monocytes may play a role in female-specific nociceptive states (Huck et al., 2021), while others refute this claim (Peng et al., 2016; Yu et al., 2020a). Due to its expression pattern in immune, mesenchymal, and neuronal cell populations, peripheral TLR4 signaling remains an elusive topic and important in both sexes. Importantly, the sex-specific role of TLR4 signaling in DRG neurons is unclear (Allette et al., 2014; Feldman et al., 2012; Hutchinson et al., 2008; Klawitter et al., 2014; Rudjito et al., 2021). In this study, we ask how endogenous TLR4 activation on peripheral nociceptors mediates the development of neuropathic pain.
Tissue injury upregulates danger-associated molecular patterns (DAMPs) (Man et al., 2015; Wan et al., 2016). High-mobility group box 1 (HMGB1) is a DAMP capable of initiating inflammatory cascades via TLR4 (Yamasoba et al., 2016). HMGB1 is a nuclear-bound redox sensitive cytokine, that is released from the cell upon activation, during tissue damage and changes to oxidative states; disulfide HMGB1 (dsHMGB1), is the predominant species that has affinity for TLR4 (Agalave and Svensson, 2015; Yamasoba et al., 2016). Intrathecal administration of HMGB1 has been shown to produce robust mechanical allodynia (Agalave et al., 2021b; O’Connor et al., 2003). Elevated levels of circulating HMGB1 promote activation and priming states of immune cells; however, its action on neurons that express TLR4 to elicit pain behavior has not been assessed (Bestall et al., 2018). The notion of neuronal TLR signaling remains contentious as emerging evidence suggests DRG macrophages are involved in communication with sensory neurons following nerve injury in both sexes (Yu et al., 2020b). Interestingly, this does not relegate the fact that sensory neurons are activated and directly interact with immune cells (reverse communication). While the magnitude of neuropathic pain may not differ between sexes, the mechanisms which drive its development are clearly different. The timing and mode of communication between immune cells and neurons may help elucidate the mechanisms by which males and females differ.
Utilizing recently developed and validated transgenic models, we were able to specifically remove or reactivate TLR4 on Nav1.8+ nociceptors in a cre dependent fashion (Jia et al., 2021; Jia et al., 2014). We identified a distinct neuronal-mediated pathway in female mice which contributes to the early onset of peripheral nerve injury-induced mechanical hypersensitivity in addition to uncovering a direct mechanism of neuronal sensitization. We demonstrated that TLR4, expressed specifically on Nav1.8+ peripheral nociceptors, is responsible for mechanical hypersensitivity during the onset of peripheral nerve injury in female, but not male mice. Additionally, HMGB1 localization and autocrine signaling via these nociceptors, as well as paracrine action on large diameter sensory neurons in the DRG, contribute to pain sensitization in female mice. We believe TLR4 activation on Nav1.8+ nociceptors is one mechanism of pain sensitization unique to females.
2. Material and Methods
2.1. Animals
All animal experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Texas at Dallas. Mice were housed (4–5 per cage) in a temperature-controlled facility and maintained on a 12-hour light/dark cycle (lights on: 7am/ lights off 7pm). Mice had ad-libitum access to food and water and were eight to twelve-weeks-old during the experiments (male, 25–30 g: female 20–25 g). Transgenic mice expressing Cre recombinase under control of the Scn10a (Nav1.8) promoter were obtained initially from Professor John Wood (University College London), but are commercially available from Ifrafrontier (EMMA ID: 04582). Characterization of these mice showed that heterozygous cre animals have no pain phenotype and normal electrophysiological properties (Stirling et al., 2005). Genetically modified TLR4 floxed animals (has loxP sites flanking exon 2 and 3; TLR4fl/fl) (Jia et al., 2014) and TLR4 null-reactivatable animals (has a transcriptional blocker inserted into the TLR4 gene in between exon 2 and 3; TLR4LoxTB) (Jia et al., 2021)were a gift from Joel K. Elmquist, (UT Southwestern Medical Center) were bred with Nav1.8cre animals in-house (Nav1.8cre × TLR4fl/fl, TLR4fl/fl, Nav1.8cre × TLR4LoxTB, TLR4LoxTB) and used for all behavioral and biochemical assays (Jia et al., 2021; Jia et al., 2014). All cell-specific knockout (Nav1.8TLR4fl/fl) or cell-specific reactivated (Nav1.8TLR4LoxTB) mice are heterozygous for the Nav1.8-cre (Nav1.8cre) and are homozygous for the TLR4fl/fl or TLR4LoxTB gene, respectively. Phenotypically normal littermates lack Nav1.8cre and are homozygous for the TLR4fl/fl gene. Whole-body null or knockout mice (TLR4TB/TB) lack Nav1.8cre and are homozygous for the TLR4LoxTB gene. We purchased ROSA26LSLtdtomato (tdtomato) animals from Jackson lab (stock no. 007909) and crossed them with Nav1.8cre animals (Nav1.8tdT+) for reporter, IHC, and flow cytometry experiments. All strains were backcrossed at least eight generations to maintain C57BL/6J genetic background with animals from Jackson Lab (stock no. 000664). We used in-house bred C57BL/6J animals as wild-type (WT) controls.
2.2. Surgical Procedures
The spared nerve injury (SNI) model of neuropathic pain was used. Mice were anesthetized under isoflurane anesthesia (1.0–2.5 %). The ipsilateral thigh was shaved and cleaned with betadine (Dynarex, 1425) and 70% ethanol (Decon Labs, 2701). The skin and muscle of the left thigh were incised with a #11 scalpel blade (Thermo, 22–079-691) and the sciatic nerve along with its three branches (common peroneal, tibial, and sural) was exposed. A tight ligature using a 5–0 silk suture (VWR, MV-682) was placed around the proximal tibial and common peroneal branches, after which the nerve distal to the ligature was transected, taking care to not stretch or damage the sural nerve (Decosterd and Woolf, 2000). Sham surgeries were done identically to the SNI surgery; however, no portion of the sciatic nerve was ligated or transected. The skin was closed using an auto clip (Fine Science Tools, 12022–09) and mice were then given a subcutaneous injection of 5 mg/mL Gentamicin (Sigma, G1272) as a preventative antibiotic and returned to their home cages to recover (Decosterd and Woolf, 2000). Mice were monitored daily for the duration of the experiment. Mechanical hypersensitivity and cold allodynia were then assessed on postoperative days 1, 3, 5 and 7. Baseline values were taken 24 hours prior to surgery.
2.3. Behavioral Testing
To measure mechanical hypersensitivity, mice were individually placed on an elevated wire grid inside acrylic behavior racks and allowed to habituate for approximately 2 hours. The ipsilateral hind paw was then stimulated with von Frey filaments (Stoelting, 58011) using the up-down experimental paradigm (Chaplan et al., 1994). To assess cold allodynia in our SNI model, mice were individually placed on the same elevated wire grid and behavior racks and allowed to habituate for approximately 2 hours before testing. Approximately 100 μL of biology grade acetone (Fisher, AI6P-4) was then applied to the ipsilateral hind paw using a 1 mL syringe (VWR, 309659) attached to a 25 G needle (VWR, 305125). Latency of behavioral response over a 60 second period was measured (Choi et al., 1994). Behavior racks were cleaned with a 1:3 ratio of a plant-based deodorant-free cleaner (Seventh Generation™, 22719BK-5) to eliminate odor cues between each reading, baseline, and experiment. Baseline values were taken 24 hours prior to performing surgery. Mechanical hypersensitivity and cold allodynia were then measured on postoperative days 1, 3, 5, and 7. Mechanical measures were always taken before thermal. All behavioral testing was done between 10 a.m. and 2:00 p.m. All behavior data sets are additionally represented as effect sizes. It is determined by calculating the cumulative difference between the value for each time point and the baseline value (Agalave et al., 2021a; Hassler et al., 2019). Behavioral experiments were performed by T.A.S., L.R.B., and M.D.B. Experimenters were blinded to genotype, surgical condition, or both.
2.4. Immunohistochemistry
Three days post SNI, mice were anesthetized using 100% isoflurane and were subsequently euthanized via decapitation. Dorsal root ganglia (L3–5) were extracted and post-fixed in 4% paraformaldehyde made in 1 × phosphate buffered saline (PBS) (Sigma, 158127) for 4 hours and then cryoprotected for 48 hours in a 30% sucrose (Sigma, S0389) solution made in 1 × (PBS). Frozen tissues (DRGs: 16 μm) were then transversally sectioned on a cryostat and mounted onto SuperFrost Plus (Fisher, 12–544-7) charged microscope slides. Slides were then allowed to dry for 1 hour before being placed in the −80° C freezer overnight. Slides were then rehydrated in 1 × PBS for 5 minutes before being incubated in a blocking/permeabilization solution consisting of: 1 × PBS, 2% heat-inactivated normal goat serum (Sigma, G9023), 1% bovine serum albumin (Sigma, A9576–50ML) 0.1% Triton (Sigma, X100), 0.05% Tween-20 (Sigma, P1379) and 0.05% sodium azide (Ricca Chemical, R7144800). Slides were then incubated overnight at 4° C in a primary antibody cocktail diluted in blocking/permeabilization solution. The following day slides were washed three times for 5 minutes each in 1 × PBS + 0.05% Tween-20 and incubated for 2 hours at room temperature in their respective secondary antibodies diluted in blocking/permeabilization solution. Slides were then washed three times for 5 minutes each in 1 × PBS + 0.05% Tween-20 and mounted using Prolong gold (Thermo, P36974) mounting media and a 1.5 mm glass coverslip (Thermo, 08–774-384). Slides were stored at 4° C until imaging. Images used for analysis and quantification were taken on a Zeiss Axiobserver 7 epifluorescent Microscope at 20x. Representative images were taken at 20x (ATF3 and tdTomato images) or 40x (HMGB1 images) using an Olympus FluoView 3000 RS confocal microscope. Analysis of images was done using ImageJ Version 1.48 (National Institutes of Health, Bethesda, MD) for Mac OS X (Apple). Immunohistochemistry (IHC) experiments and analyses were performed by T.A.S., and L.R.B. Experimenters were blinded to sex, genotype, and surgical condition. For a complete list of antibodies used refer to Table 1.
Table 1.
Antibodies used in IHC and flow experiments.
| Antibody | Company | Catalog number | Working dilution |
|---|---|---|---|
|
| |||
| Antibodies used for IHC | |||
| Anti-Nav1.8 sodium channel | Neuromab | 75-166 | 1:500 |
| Anti-Neurofilament 200 | Millipore Sigma | MAB5266 | 1:1000 |
| Anti-HMGB1 | Abcam | AB79823 | 1:500 |
| Anti-ATF3 | Abcam | AB207434 | 1:500 |
| Goat anti-mouse Alexa Fluor 488 | Invitrogen | A21131 | 1:1000 |
| Goat anti-mouse Alexa Fluor 647 | Invitrogen | A21240 | 1:1000 |
| Goat anti-rabbit Alexa Fluor 568 | Invitrogen | A11011 | 1:1000 |
| Antibodies used for flow | |||
| Anti-CD45 Super Bright 645 conjugate | eBioscience | 64-0451-82 | 1:200 |
| Anti-TLR4/MD-2 Complex APC conjugate | eBioscience | 17-9924-82 | 1:200 |
| Anti-CD16/32 | eBioscience | 16016185 | 1:1000 |
| Anti-NeuN Alexa Fluor 488 conjugate | Millipore Sigma | MAB377X | 1:200 |
2.5. Flow Cytometry
Flow cytometric analysis of isolated DRGs was performed based on previous protocols with some modifications (Chiu et al., 2014). In brief, ipsilateral and contralateral DRGs (L3–4) were collected in ice cold sterile 1 × DPBS (Hyclone, SH30028). Samples were centrifuged at 400 × g for 3 minutes. Supernatants were removed and samples were treated with Collagenase A (Sigma, 10103586001) and incubated in a 37°C water bath for 20 minutes. Samples were then centrifuged at 400 × g for 3 minutes. Supernatants were removed and the samples were treated with Collagenase D (Sigma, 1188866001) and a 10% v/v of papain (Roche, 10108014001) in HBSS for another 20 minutes. Cells were centrifuged at 400 × g and the pellet were resuspended in Enzyme T (Sigma, 10109886001) (soybean trypsin inhibitor made in 1:1 bovine serum albumin and DMEM/F12 media (Thermo, 10565161) supplemented with 10% fetal bovine serum (FBS) (Hyclone, SH30088.03) and 1% pen/strep (Sigma, P4333)) to stop the enzymatic reaction. Digested tissues were triturated using a 1 mL fire-polished Pasteur pipette tip and passed through a 70-micron nylon mesh cell strainer (Sigma, CLS431751–50EA), with a subsequent wash using flow buffer (0.5% bovine serum albumin with 0.02% glucose (Sigma, G7528) made in 1 × DPBS). Resultant suspension was centrifuged at 400 × g for 3 minutes and resuspended in flow blocking buffer (anti-CD16/32 purified antibody diluted in flow buffer) for 20 minutes to block fc receptors. Samples were incubated with pre-conjugated extracellular flow antibodies (CD45 & TLR4) for 45 minutes. Samples were then washed with flow buffer, then centrifuged at 400 × g for 3 minutes and resuspended in a fixation/permeabilization buffer (BD Biosciences, 555028) for 45 minutes. Samples were then centrifuged at 400 × g for 3 minutes and were washed twice using the manufacturer provided wash buffer (1×). Samples were incubated with pre-conjugated intracellular flow antibodies (NeuN) for 60 minutes and washed with DAPI for 5 minutes. Samples were then centrifuged at 400 × g for 3 minutes and were washed twice using wash buffer and were then resuspended in flow buffer. Appropriate compensation controls and isotypes were used for determination and gating. After gating DAPI positive cells (to determine debris), immune cells were identified with gating for CD45 and were further gated to identify expression of TLR4. Neurons were initially identified with gating for NeuN and were further gated to identify expression of TLR4. Stained samples were analyzed using a Special Order (4-laser) Becton-Dickinson Fortessa analyzer (Red Oaks, CA) and data were analyzed using FlowJo and FCS Express software (De Novo Software, Los Angeles, CA). Flow cytometry experiments and analyses were performed by T.A.S., M.E.L., and M.D.B. Experimenters were blinded to genotype and surgical condition. For a complete list of antibodies used refer to Table 1.
2.6. Statistical Analysis
Prism 8.01 software (GraphPad, San Diego, CA, USA) was utilized to generate all graphs and statistical analysis. Behavioral data was analyzed using repeated measures Two-Way ANOVA with Tukey’s post hoc tests. Effect size, immunohistochemical, and flow cytometric data were analyzed using Ordinary Two-Way ANOVA with Tukey’s post hoc tests. All data are represented as the standard error of the mean (SEM). A p-value of <0.05 was used to determine statistical significance. All statistical values and corresponding tests can be found in Tables 2-7. All behavioral, immunohistochemical, and flow cytometric experiments and analyses were performed by blinded experimenters.
Table 2.
Statistical values for analyses performed within Figure 1. Paw withdrawal threshold and cold allodynia behavioral data were analyzed using repeated measures Two-Way ANOVA with Tukey’s post hoc. Effect size data were analyzed using Ordinary Two-Way ANOVA with Tukey’s post hoc. ATF3 and HMGB1 localization datasets were analyzed using Ordinary Two-Way ANOVA with Tukey’s post hoc. Significance was set at p<0.05 for all datasets. Bolded values are statistically significant.
| Dataset | Main Effect | Interactions | Multiple Comparisons | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F (DFn, DFd) | p-value | F (DFn, DFd) | p-value | Effect | Groups | POD | p-value | ||
| Mechanical Sensitivity | Paw Withdrawal | Surgery: F (3, 24) = 140.6 Time: F (2.218, 53.24) = 66.65 |
p<0.0001
p<0.0001 |
F (12, 96) = 19.05 | p<0.0001 | Surgery | Male Female |
BL 1 3 5 7 BL 1 3 5 7 |
p=0.7619 p=0.0093 p<0.0001 p<0.0001 p<0.0001 p=0.4393 p=0.0030 p<0.0001 p<0.0001 p<0.0001 |
| Effect Size | Surgery: F (1, 24) = 81.93 Sex: F (1, 24) = 1.397 |
p<0.0001 p=0.2487 |
F (1, 24) = 0.0411 | p=0.8411 | Surgery | Male Female |
p<0.0001
p<0.0001 |
||
| Cold Allodynia | Response | Surgery: F (3, 24) = 58.11 Time: F (2.891, 69.39) = 31.56 |
p<0.0001
p<0.0001 |
F (12, 96) = 9.429 | p<0.0001 | Surgery | Male Female |
BL 1 3 5 7 BL 1 3 5 7 |
p=0.4227 p=0.0293 p=0.0027 p=0.0001 p=0.0028 p=0.9964 p=0.2647 p=0.0020 p=0.0056 p=0.0009 |
| Effect Size | Surgery: F (1, 24) = 129.9 Sex: F (1, 24) = 1.013 |
p<0.0001 p=0.3242 |
F (1, 24) = 0.0726 | p=0.7899 | Surgery | Male Female |
p<0.0001
p<0.0001 |
||
| ATF3 Localization | NF200+ Neurons | Surgery: F (1, 12) = 120.4 Sex: F (1, 12) = 4.019 |
p<0.0001 p=0.0681 |
F (1, 12) = 1.347 | p=0.2684 | Surgery | Male Female |
p<0.0001
p<0.0001 |
|
| Nav1.8+ Neurons | Surgery: F (1, 12) = 646.2 Sex: F (1, 12) = 48.07 |
p<0.0001
p<0.0001 |
F (1, 12) = 34.88 | p<0.0001 | Surgery Sex |
Male Female Sham SNI |
p<0.0001 p<0.0001 p>0.9999 p<0.0001 |
||
| HMGB1 Localization | NF200+ Neurons | Surgery: F (1, 12) = 11.47 Sex: F (1, 12) = 0.2560 |
p=0.0054 p=0.0622 |
F (1, 12) = 0.2705 | p=0.6124 | Surgery | Male Female |
p=0.1267 p=0.0341 |
|
| Nav1.8+ Neurons | Surgery: F (1, 12) = 16.92 Sex: F (1, 12) = 1.880 |
p=0.0014 p=0.1954 |
F (1, 12) = 5.222 | p=0.0413 | Surgery Sex |
Male Female Sham SNI |
p=0.9148 p=0.0034 p=0.5843 p=0.0959 |
||
3. Results
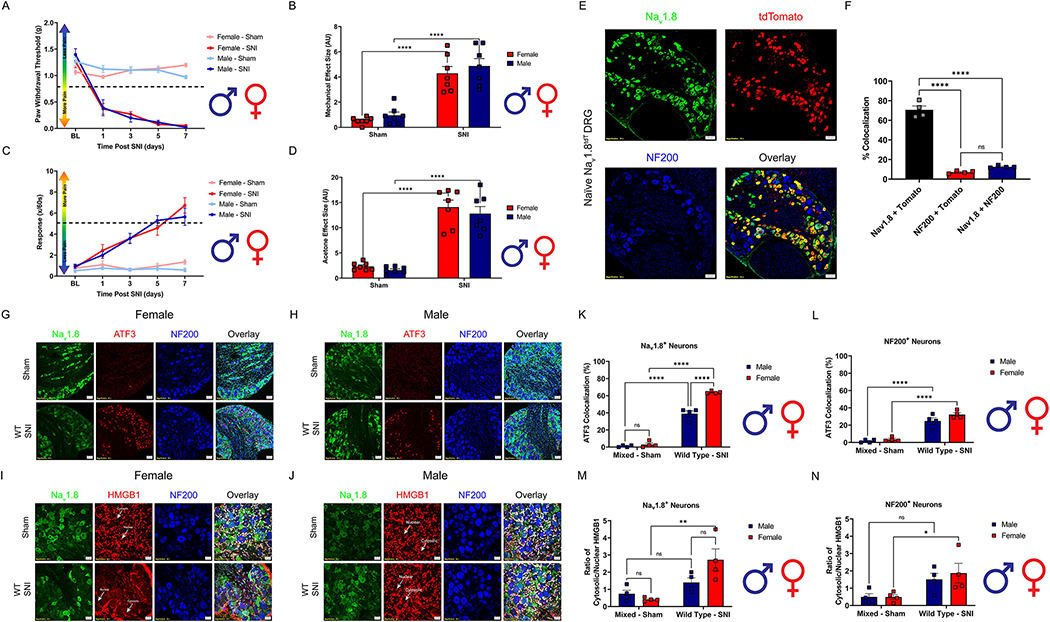
3.1. The severity of mechanical hypersensitivity and cold allodynia in a neuropathic pain model is similar between males and females, but protein expression levels in the lumbar DRG are different.
To establish if any sexual dimorphisms exist in the magnitude of pain following neuropathic injury, SNI was performed on both male and female WT mice. No significant differences were found between male and female SNI groups in either mechanical hypersensitivity or cold allodynia (Fig. 1A–D, Table 2). Emerging evidence suggests that the mechanisms that mediate neuronal sensitization during the early onset of neuropathic pain differ between sexes (Ross et al., 2018; Tajerian et al., 2015). Myeloid-derived immune cells are strongly implicated in driving the early onset of pain following injury, however; differences between sexes in direct neuronal sensitization in the lumbar DRG are poorly understood (Peng et al., 2016). To test the hypothesis that neuronal injury after SNI is different between males and females we first utilized our Nav1.8tdT reporter line to verify colocalization between our Nav1.8 antibody and cre-mediated tdTomato expression in Nav1.8+ nociceptors. Naïve male and female Nav1.8tdT lumbar DRGs (L3–5) were immunoassayed with Nav1.8 and NF200 antibody (Fig. 1E, Table 2). They were then assessed for colocalization with tdTomato. We found there to be a high degree of colocalization (75%) between Nav1.8 antibody and tdTomato expression (Fig. 1F, Table 2). This indicates robust specificity of the Nav1.8 antibody with endogenous expression of tdTomato. Next, we used histochemical markers to identify small (Nav1.8) and large (NF200) diameter nociceptors in the DRG (L3–5) of male and female WT mice. We then examined HMGB1 cytosolic localization and ATF3 expression in these distinct neuronal populations 3 days post SNI (Fig. 1G–J, Table 2). We discovered a sexual dimorphism where both male and female small diameter nociceptors (Nav1.8+) exhibit significantly elevated expression of ATF3 3 days post SNI, but females have significantly more ATF3 expressed as compared to males. (Fig. 1K, Table 2). Both males and females have significantly increased ATF3 expression in their large diameter sensory neurons (NF200+) 3 days post SNI as compared to sham controls (Fig. 1L, Table 2). It is evident that these neuronal subpopulations exhibit signs of cellular injury in both males and females after SNI. We then measured cellular localization of HMGB1 in these neuronal subpopulations. Only female WT mice exhibit significantly elevated cytosolic localization of HMGB1 3 days post SNI in their Nav1.8+ nociceptors as compared to sham controls (Fig. 1M, Table 2). Additionally, only female WT mice exhibit significantly elevated cytosolic localization of HMGB1 3 days post SNI in their NF200+ neurons as compared to sham controls (Fig. 1N, Table 2). Taken together, our data reveals an inherent sexual dimorphism in both inflammatory signaling and neuronal injury in the context of neuropathic pain. This simple, yet enlightening finding pushed us to further investigate the dichotomy of sex and cell-specific sensitization in the peripheral nervous system.
Figure 1.
The magnitude of pain after SNI does not differ between sexes following SNI, however; the mechanisms during its onset at the level of the lumbar (L3–5) DRGs are. Spared-nerve injury was performed on the left hindlimb of male and female mice. A, Hind paw mechanical withdrawal thresholds were measured prior to surgery and on days: 1, 3, 5, and 7 post-surgery in both male and female sham (n=7) and SNI (n=7) wild type mice. B, Data for males and females combined shown as mechanical effect size. C, Hind paw response to application of acetone were measured prior to surgery and on days: 1, 3, 5, and 7 post-surgery in both male and female sham (n=7) and SNI (n=7) mice. D, Data for males and females combined shown as acetone effect size. E, Naïve Nav1.8tdT female and male lumbar (L3–5) DRGs were immunostained with Nav1.8 (green), tdTomato (red) and NF200 (Blue); representative images from n=4 mice). Scale bar: 50 μm. Magnification: 20x. F, Quantification of colocalization between Nav1.8, tdTomato and NF200 (n=4). G-H Female and male WT lumbar DRGs (L3–5) were immunostained 3D post SNI with DAPI (teal), Nav1.8 (green), ATF3 (red) and NF200 (blue; representative images from n=4 mice). Scale bar: 50 μm. Magnification: 20x. K, Quantification of ATF3 colocalization with Nav1.8+ neurons of both sexes 3 days after SNI (n=4 per group). L, Quantification of ATF3 colocalization with NF200+ neurons of both sexes 3 days after SNI (n=4 per group). I-J, Female, and male WT lumbar DRGs (L3–5) were immunostained 3D post SNI with DAPI (teal), Nav1.8 (green), HMGB1 (red) and NF200 (blue; representative images from n=4 mice). White arrows point to an example of nuclear or cytosolic localization of HMGB1. Scale bar: 20 μm. Magnification: 40x. M, Quantification of HMGB1 cytosolic localization in Nav1.8+ neurons of both sexes 3 days after SNI (n=4 per group). N, Quantification of HMGB1 cytosolic localization in NF200+ neurons of both sexes 3 days after SNI (n=4 per group). *p < 0.05; **p < 0.01; ****p < 0.0001. BL = Baseline.
3.2. Sensory neuron and immune cell expression of TLR4
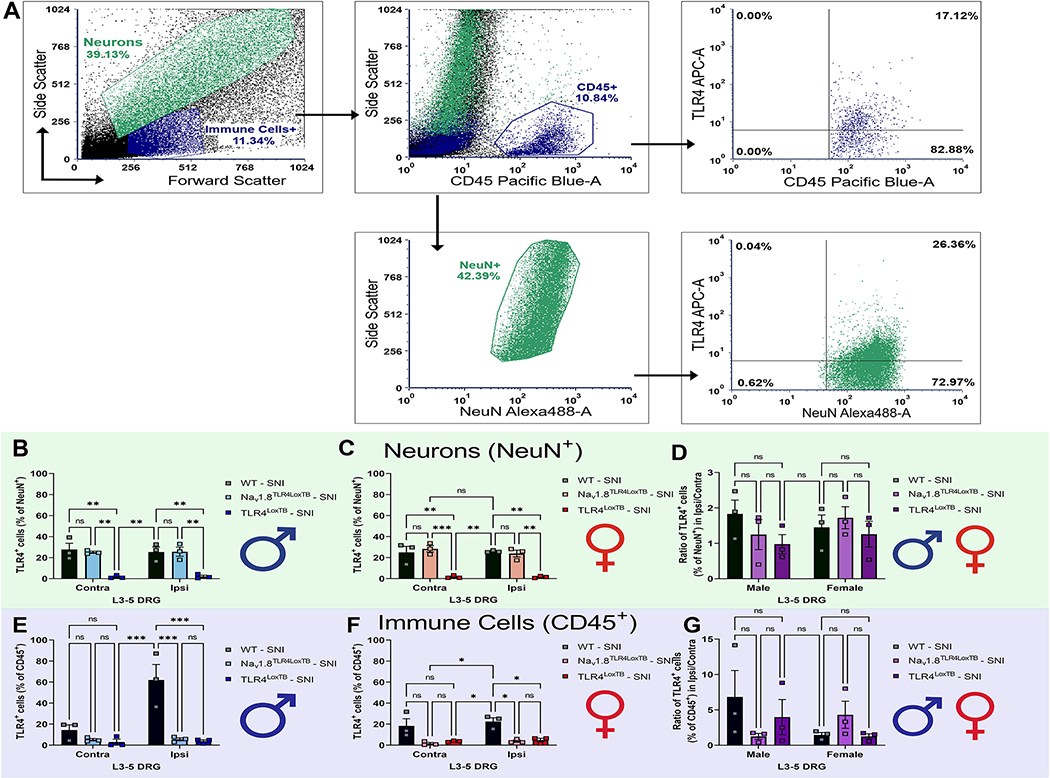
To investigate baseline and surgery-induced levels of TLR4 on DRG cell populations, ipsilateral and contralateral DRGs (L3-L5) were isolated from males and females 3 days post SNI (Chiu et al., 2014). Moreover, we verified protein expression of our null-re-expression animals when we added whole-body null (TLR4LoxTB) and cell-specific re-expression (Nav1.8TLR4LoxTB) groups to the study. Neuronal (NeuN+) and immune cell (CD45+) populations were identified and assessed for TLR4 at various stages and genotypes (Fig. 2A). We determined that there were no significant sex or surgery-induced differences in TLR4 protein expression patterns in DRG neurons in ipsilateral DRGs, compared to contralateral DRGs, with roughly 30% of the neurons expressing TLR4 in males and females (Fig. 2B & 2C). We saw that our whole-body null animals expressed minimum amounts of TLR4 across the neuronal populations, as expected (Fig. 2B & 2C). Opposingly, we observed a male-driven sex-dependent upregulation of ipsilateral TLR4 expression in CD45+ immune cells 3 days post SNI (Fig. 2E), but no female differences. This data juxtaposes recent findings of no sex-differences in DRG immune cells after neuropathic injury (Yu et al., 2020a). Apparently, it is imperative to understand not only the cell population amount, but the expression pattern of those cells in the DRG.
Figure 2.
There are no basal or surgery-induced sex differences in TLR4 expression in sensory neurons and a surgery-induced upregulation of TLR4 in DRG immune cells 3D post SNI. A, Lumbar DRGs (L3–5) were enzymatically dissociated, stained with TLR4, NeuN, and DAPI and subjected to flow cytometry. After gating on both neurons and immune cells based on forward and side scatter, NeuN+ and CD45+ cells were further gated to TLR4+ populations. B, NeuN+/TLR4+ cells in control (contra) and surgerized DRGs (ipsi) 3D post SNI in males. C, NeuN+/TLR4+ cells in control (contra) and surgerized DRGs (ipsi) 3D post SNI in females. D, Combined male and female data; ratio of ipsilateral-to-contralateral NeuN+/TLR4+ DRG cells 3D post SNI. E, CD45+/TLR4+ cells in control (contra) and surgerized DRGs (ipsi) 3D post SNI in males. F, CD45+/TLR4+ cells in control (contra) and surgerized DRGs (ipsi) 3D post SNI in females. G, Combined male and female data; ratio of ipsilateral-to-contralateral CD45+/TLR4+ DRG cells 3D post SNI. Asterisks on the bar graphs indicate significant differences between the groups. *p < 0.05; **p < 0.01; ***p < 0.001, (n= 3–4).
3.3. Sensory Neuron TLR4 is necessary during the onset of neuropathic mechanical hypersensitivity in female mice.
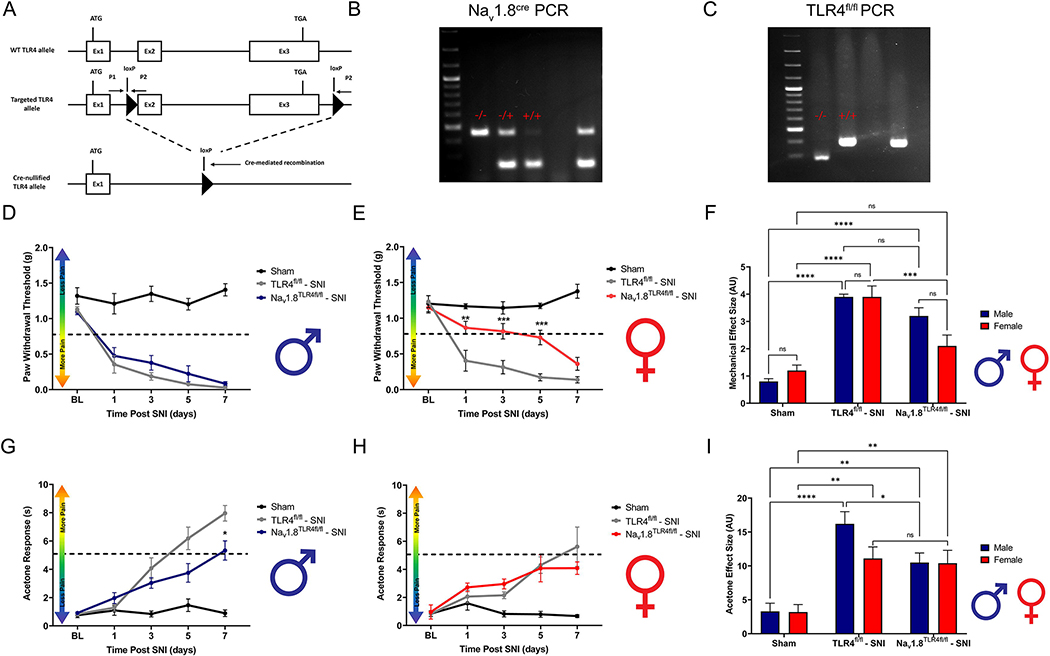
Investigating the cell-specific implications of TLR4 activation in behavioral pain phenotypes is necessary to understand the biological relevance of cell-molecule interactions. Danger signals are released by both neuronal and non-neuronal cells in response to tissue injury, where neuronally expressed TLR4 allows for rapid detection of these proteins (Liu et al., 2014). Here, we asked if there are sex differences in the response to neuropathic tissue injury by neuronally expressed TLR4. To test this, we used transgenic male and female mice that lack TLR4 on their Nav1.8+ nociceptors combined with a spared nerve injury (SNI) model of peripheral neuropathic injury (Fig. 3A). Genotypes for Nav1.8TLR4fl/fl mice are confirmed using polymerase chain reaction (PCR) and gel electrophoresis (Fig. 3B, C). We assessed the onset of neuropathic pain by measuring mechanical hypersensitivity and cold allodynia and our data reveals robust sex and genotype-dependent behavioral effects. (Mecklenburg et al., 2020)Our data demonstrates the role of TLR4 in nociceptors and their female-specific role in regulating neuropathic pain development. Male Nav1.8TLR4fl/fl mice exhibit similar mechanical withdrawal thresholds to their TLR4fl/fl littermates after SNI (Fig. 3D, Table 3). Female Nav1.8TLR4fl/fl mice exhibit reduced mechanical withdrawal thresholds days 1, 3 and 5 after SNI as compared to their TLR4fl/fl littermates (Fig. 3E, Table 3). Sexes are directly compared using effect sizes (Fig. 3F, Table 3). Interestingly, male but not female mice show reduced cold allodynia on day 3 post-surgery as compared to their TLR4fl/fl littermates (Fig. 3G–H, Table 3). Sexes are directly compared using effect sizes (Fig. 3I, Table 3). This demonstrates a distinction in the role of TLR4 in mechanical vs. thermal during neuropathic pain development. It is possible that the mechanisms which mediate cold and mechanical hypersensitivity are sexually dimorphic in nature, and a distinguished subset of transient receptor potential (TRP)+ sensory neurons in the DRG differ mechanistically between males and females in a neuropathic state, however; this was not the focus of the current study.
Figure 3.
Development of neuropathic pain is attenuated in Nav1.8TLR4fl/fl female, but not male mice. Spared-nerve injury was performed on the left hindlimb of male and female mice. A, Schematic representing the murine genetic model, Nav1.8TLR4fl/fl. B, Example PCR, and gel electrophoresis for Nav1.8cre. A – indicates homozygous WT mice, a −/+ indicates heterozygous Nav1.8cre mice, a +/+ indicates homozygous Nav1.8cre. C, Example PCR, and gel electrophoresis for TLR4fl/fl. A - indicates homozygous WT mice, a −/+ indicates heterozygous TLR4fl/fl mice, a +/+ indicates homozygous TLR4fl/fl mice. D-E, Hind paw mechanical withdrawal thresholds were measured prior to surgery and on days: 1, 3, 5, and 7 post-surgery in both male and female sham (n=8), TLR4fl/fl (n=9), Nav1.8TLR4fl/fl (n=8) mice. F, Data for males and females combined shown as mechanical effect size. G-H, Hind paw response to application of acetone were measured prior to surgery and on days: 1, 3, 5, and 7 post-surgery in both male and female sham (n=8), TLR4fl/fl (n=9) and Nav1.8TLR4fl/fl (n=8) mice. I, Data for males and females combined shown as acetone effect size. Asterisks on the line graphs indicate significant differences between the Nav1.8TLR4fl/fl and TLR4fl/fl groups. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. BL = Baseline.
Table 3.
Statistical values for analyses performed within Figure 3. Mechanical sensitivity and cold allodynia behavioral data were analyzed with a repeated-measures Two-Way ANOVA with Tukey’s post hoc. Effect size datasets were analyzed using Ordinary Two-Way ANOVA with Tukey’s post hoc. Statistical significance was set at p<0.05. All values deemed statistically significant are bolded.
| Dataset | Main Effect | Interactions | Multiple Comparisons | ||||||
| F (DFn, DFd) | p-value | F (DFn, DFd) | p-value | Effect | Groups | POD | p-value | ||
| Mechanical Sensitivity | Males | Genotype: F (2, 24) = 89.06 Time: F (3.068, 73.64) = 38.10 |
p<0.0001
p<0.0001 |
F (8, 96) = 9.794 | p<0.0001 | Surgery Genotype |
Mixed Sham vs TLR4fl/fl Mixed Sham vs Nav1.8TLR4LoxTB TLR4LoxTB vs Nav1.8TLR4LoxTB |
BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 |
p=0.3123 p=0.0013 p<0.0001 p<0.0001 p<0.0001 p=0.2072 p=0.0036 p<0.0001 p<0.0001 p<0.0001 p=0.7957 p=0.7581 p=0.2611 p=0.4406 p=0.2021 |
| Females | Genotype: F (2, 24) = 69.27 Time: F (3.278, 78.67) = 19.64 |
p<0.0001 | F (8, 96) = 9.249 | p<0.0001 | Surgery Genotype |
Mixed Sham vs TLR4fl/fl Mixed Sham vs Nav1.8TLR4LoxTB TLR4LoxTB vs Nav1.8TLR4LoxTB |
BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 |
p=0.9777 p=0.0012 p<0.0001 p<0.0001 p<0.0001 p=0.9158 p=0.0266 p=0.0783 p=0.0054 p<0.0001 p=0.7957 p=0.0419 p=0.0074 p=0.0010 p=0.1120 |
|
| Effect Size | Genotype: F (2, 49) = 44.27 Sex: F (1, 49) = 0.8985 |
p<0.0001 p=0.3478 |
F (2, 49) = 3.384 | p=0.0420 | Genotype | Male SNI Female SNI |
p=0.5528 p=0.0007 |
||
| Cold Allodynia | Males | Genotype: F (2, 20) = 24.29 Time: F (3.155, 63.10) = 57.32 |
p<0.0001
p<0.0001 |
F (8, 80) = 16.68 | p<0.0001 | Surgery Genotype |
Mixed Sham vs TLR4fl/fl Mixed Sham vs Nav1.8TLR4LoxTB TLR4LoxTB vs Nav1.8TLR4LoxTB |
BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 |
p=0.9239 p=0.8930 p=0.0100 p=0.0013 p<0.0001 p=0.7419 p=0.2450 p=0.0010 p=0.0340 p=0.0005 p=0.9249 p=0.3318 p=0.4565 p=0.0850 p=0.0260 |
| Females | Genotype: F (2, 20) = 21.04 Time: F (2.406, 48.12) = 10.32 |
p<0.0001
p<0.0001 |
F (8, 80) = 4.242 | p=0.0003 | Surgery Genotype |
Mixed Sham vs TLR4fl/fl Mixed Sham vs Nav1.8TLR4LoxTB TLR4LoxTB vs Nav1.8TLR4LoxTB |
BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 |
p>0.9999 p=0.7482 p=0.0029 p=0.0009 p=0.0001 p=0.9749 p=0.1593 p=0.0010 p=0.0325 p=0.0004 p=0.9718 p=0.4756 p=0.1864 p=0.9781 p=0.5727 |
|
| Effect Size | Genotype: F (2, 40) = 24.96 Sex: F (1, 40) = 2.021 |
p<0.0001 p=0.1629 |
F (2, 40) = 0.1819 | p=0.1819 | Genotype | Male SNI Female SNI |
p=0.0368 p=0.9843 |
||
3.4. Sensory Neuron TLR4 is sufficient during the onset of neuropathic mechanical hypersensitivity in female mice.
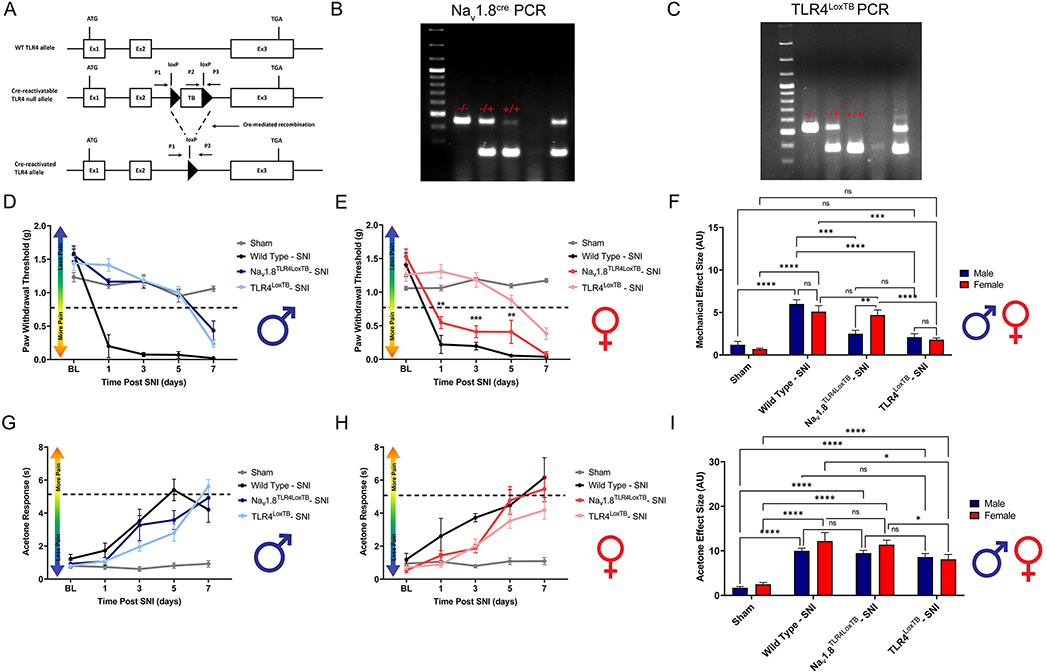
Whole body TLR4 has been shown to influence recovery from a non-terminal neuropathic injury (Stokes et al., 2013). Moreover, TLR4 knockout mice have reduced mechanical and thermal hypersensitivity in response to neuropathic injury (Piao et al., 2018). It is still unclear what specific populations of cells that express TLR4 contribute to these behavioral effects. To investigate the direct action of TLR4 on peripheral sensory neurons, we utilized Nav1.8TLR4LoxTB mice combined with an SNI model of neuropathic pain (Fig. 4A). Genotypes for Nav1.8TLR4LoxTB mice are confirmed using PCR and gel electrophoresis (Fig. 4B, C). Here, we tested the sufficiency of TLR4 expressed only on Nav1.8+ DRG neurons to produce a neuropathic behavioral pain phenotype similar to wild type mice. Analysis reveals significant genotype-dependent behavioral phenotypes and robust sex differences. Male TLR4LoxTB mice show significantly diminished mechanical withdrawal thresholds during days 1, 3 and 5 post-surgery as compared to WT littermates. Interestingly, male Nav1.8TLR4LoxTB mice do not recapitulate the severe mechanical hypersensitivity seen in WT littermates on days 1, 3 and 5 post-surgery (Fig. 4D, Table 4). Female TLR4LoxTB mice show significantly diminished mechanical withdrawal thresholds during days 1, 3 and 5 post-surgery as compared to WT littermates, however; only female Nav1.8TLR4LoxTB mice recapitulate the severe mechanical hypersensitivity seen in WT littermates on days 1, 3 and 5 post-surgery (Fig. 4E, Table 4). Sexes are directly compared using effect sizes (Fig. 4F, Table 4). Surprisingly, no sex or genotype-dependent effects are seen across time in cold allodynia development (Fig. 4G–H, Table 4). Sexes are directly compared using effect sizes (Fig. 4I, Table 4). These data suggest direct activation of neuronally expressed TLR4 by endogenous danger signals released during injury is enough to cause a neuropathic behavioral pain phenotype only in female mice.
Figure 4.
Development of neuropathic pain is phenotypically normal in Nav1.8TLR4LoxTB female, but not male mice. Spared-nerve injury was performed on the left hindlimb of male and female mice. A, Schematic representing the murine genetic model, Nav1.8TLR4LoxTB. B, Example PCR, and gel electrophoresis for Nav1.8cre. A - indicates homozygous WT mice, a −/+ indicates heterozygous Nav1.8cre mice, a +/+ indicates homozygous Nav1.8cre. C, Example PCR, and gel electrophoresis for TLR4LoxTB. A – indicates homozygous WT mice, a −/+ indicates heterozygous TLR4LoxTB mice, a +/+ indicates homozygous TLR4LoxTB mice. D-E, Hind paw mechanical withdrawal thresholds were measured prior to surgery and on days: 1, 3, 5, and 7 post-surgery in both male and female sham (n=12), wild type (n=4), TLR4LoxTB (n=9), F, Data for males and females combined shown as mechanical effect size. G-H, Hind paw response to application of acetone were measured prior to surgery and on days: 1, 3, 5, and 7 post-surgery in both male and female sham (n=12), wild type (n=4), TLR4LoxTB (n=9), Nav1.8TLR4LoxTB (n=7) mice. I, Data for males and females combined shown as acetone effect size. Asterisks on the line graphs indicate significant differences between the Nav1.8TLR4LoxTB and TLR4LoxTB groups. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. BL = Baseline.
Table 4.
Statistical values for analyses performed within Figure 4. Mechanical sensitivity and cold allodynia behavioral data were analyzed with a repeated-measures Two-Way ANOVA with Tukey’s post hoc. Effect size datasets were analyzed using Ordinary Two-Way ANOVA with Tukey’s post hoc. Statistical significance was set at p<0.05. Comparisons between wild-type and sham groups can be found in Table 2. All values deemed statistically significant are bolded.
| Dataset | Main Effect | Interactions | Multiple Comparisons | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| F (DFn, DFd) | p-value | F (DFn, DFd) | p-value | Effect | Groups | POD | p-value | ||
|
| |||||||||
| Mechanical Sensitivity | Females | Genotype: F (3.28) = 55.08 Time: F (3.39, 94.92) = 67.17 |
p<0.0001
p<0.0001 |
F (12. 112) = 20.37 | p<0.0001 | Surgery Genotype |
Sham vs TLR4LoxTB Sham vs Nav1.8TLR4LoxTB TLR4LoxTB vs Nav1.8TLR4LoxTB WT vs TLR4LoxTB WT vs Nav1.8TLR4LoxTB |
BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 |
p=0.2686 p=0.1743 p=0.9994 p=0.1102 p<0.0001 p=0.0181 p=0.0026 p=0.0003 p=0.0265 p<0.0001 p=0.2939 p=0.0003 p=0.0004 p=0.1353 p=0.0299 p=0.8767 p=0.0019 p<0.0001 p<0.0001 p=0.0147 p=0.9267 p=0.2831 p=0.2463 p=0.2698 p=0.8959 |
|
| |||||||||
| Males | Genotype: F (3, 28) = 34.18 Time: F (3.612, 101.1) = 78.45 |
p<0.0001
p<0.0001 |
F (12, 112) = 9.249 | p<0.0001 | Surgery Genotype |
Sham vs TLR4LoxTB Sham vs Nav1.8TLR4LoxTB TLR4LoxTB vs Nav1.8TLR4LoxTB WT vs TLR4LoxTB WT vs Nav1.8TLR4LoxTB |
BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 |
p=0.3918 p=0.0540 p>0.9999 p=0.9731 p<0.0001 p=0.0441 p=0.7980 p=0.9997 p=0.9497 p=0.0171 p=0.7958 p=0.1310 p=0.9994 p>0.9999 p=0.5950 p=0.8051 p=0.0060 p<0.0001 p<0.0001 p=0.0110 p=0.9994 p=0.0266 p<0.0001 p<0.0001 p=0.1004 |
|
|
| |||||||||
| Effect Size | Genotype: F (3, 56) = 42.25 Sex: F (1, 56) = 0.1764 |
p<0.0001 p=0.6761 |
F (3, 56) = 5.613 | p=0.0019 | Genotype | Males: Sham vs TLR4LoxTB | p=0.5843 | ||
| Males: Sham vs Nav1.8TLR4LoxTB | p=0.2216 | ||||||||
| Males: WT vs TLR4LoxTB | p<0.0001 | ||||||||
| Males: WT vs Nav1.8TLR4LoxTB | p<0.0001 | ||||||||
| Males: Nav1.8TLR4LoxTB vs TLR4LoxTB | p=0.9959 | ||||||||
| Females: Sham vs TLR4LoxTB | p=0.3275 | ||||||||
| Females: Sham vs Nav1.8TLR4LoxTB | p<0.0001 | ||||||||
| Females: WT vs TLR4LoxTB | p=0.0002 | ||||||||
| Females: WT vs Nav1.8TLR4LoxTB | p=0.9990 | ||||||||
| Females: Nav1.8TLR4LoxTB vs TLR4LoxTB | p<0.0001 | ||||||||
| Sex | Male TLR4LoxTB vs Female TLR4LoxTB | p=0.9990 | |||||||
| Male TLR4LoxTB vs Female Nav1.8TLR4LoxTB | p=0.0004 | ||||||||
| Male Nav1.8TLR4LoxTB vs Female TLR4LoxTB | p=0.9079 | ||||||||
| Male Nav1.8TLR4LoxTB vs Female Nav1.8TLR4LoxTB | p=0.0094 | ||||||||
|
| |||||||||
| Cold Allodynia | Females | Genotype: F (3, 28) = 31.54 Time: F (2.890, 80.93) = 41.56 |
p<0.0001
p<0.0001 |
F (2, 112) = 6.852 | p<0.0001 | Surgery Genotype |
Sham vs TLR4LoxTB Sham vs Nav1.8TLR4LoxTB TLR4LoxTB vs Nav1.8TLR4LoxTB WT vs TLR4LoxTB WT vs Nav1.8TLR4LoxTB |
BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 |
p=0.6547 p=0.9513 p=0.0951 p=0.0022 p=0.0016 p=0.2301 p=0.7320 p=0.0155 p=0.0169 p=0.0042 p=0.8570 p=0.4368 p=0.9923 p=0.5887 p=0.5554 p=0.6584 p=0.4968 p=0.0233 p=0.8123 p=0.5127 p=0.4585 p=0.7330 p=0.0030 p=0.9948 p=0.9593 |
|
| |||||||||
| Males | Genotype: F (3, 28) = 38.43 Time: F (2.694, 75.42) = 44.19 |
p<0.0001
p<0.0001 |
F (12, 112) = 9.260 | p<0.0001 | Surgery Genotype |
Sham vs TLR4LoxTB Sham vs Nav1.8TLR4LoxTB TLR4LoxTB vs Nav1.8TLR4LoxTB WT vs TLR4LoxTB WT vs Nav1.8TLR4LoxTB |
BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 BL 1 3 5 7 |
p>0.9999 p=0.3785 p=0.0033 p=0.0172 p<0.0001 p=0.9660 p=0.6250 p=0.1112 p=0.0095 p=0.0033 p=0.9576 p>0.9999 p=0.5759 p=0.7327 p=0.8198 p=0.5393 p=0.6010 p=0.0320 p=0.0625 p=0.4515 p=0.8149 p=0.6146 p=0.9946 p=0.2385 p=0.8953 |
|
|
| |||||||||
| Effect Size | Genotype: F (3, 57) = 61.34 Sex: F (1, 57) = 3.348 |
p<0.0001 p=0.0725 |
F (3, 57) = 1.065 | p=0.3712 | Genotype | Males: Sham vs TLR4LoxTB | p<0.0001 | ||
| Males: Sham vs Nav1.8TLR4LoxTB | p<0.0001 | ||||||||
| Males: WT vs TLR4LoxTB | p=0.7256 | ||||||||
| Males: WT vs Nav1.8TLR4LoxTB | p=0.9843 | ||||||||
| Males: Nav1.8TLR4LoxTB vs TLR4LoxTB | p=0.8547 | ||||||||
| Females: Sham vs TLR4LoxTB | p<0.0001 | ||||||||
| Females: Sham vs Nav1.8TLR4LoxTB | p<0.0001 | ||||||||
| Females: WT vs TLR4LoxTB | p=0.0177 | ||||||||
| Females: WT vs Nav1.8TLR4LoxTB | p=0.9364 | ||||||||
| Females: Nav1.8TLR4LoxTB vs TLR4LoxTB | p=0.0183 | ||||||||
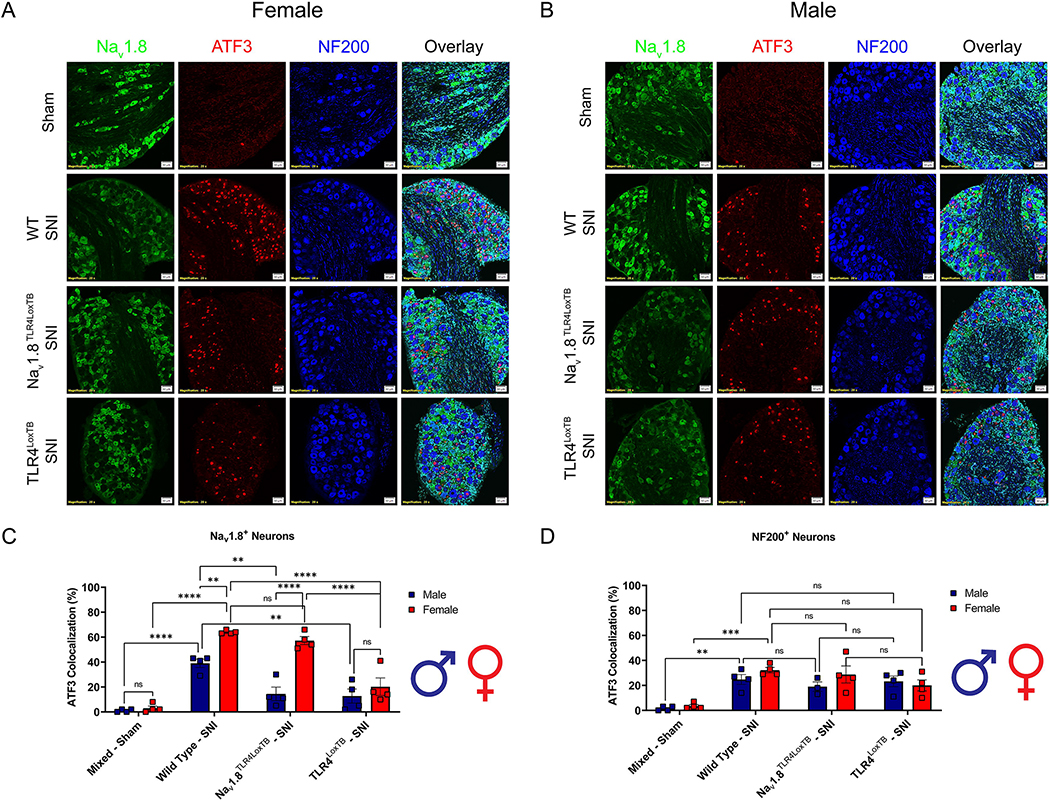
3.5. Injury marker, ATF3, in small diameter neurons is upregulated in a sex specific and TLR4 fashion
To investigate the sexual dimorphisms driving the development of neuropathic pain we used a neuronal marker of injury, ATF3, and assessed colocalization with small diameter nociceptors (Nav1.8+) and large diameter neurons (NF200+) (Fig. 5A–B, Table 6). In female Nav1.8TLR4LoxTB mice, we show significantly upregulated ATF3 expression in their Nav1.8+ DRG neurons 3 days post-SNI as compared to TLR4LoxTB mice. Conversely, in male Nav1.8TLR4LoxTB mice there is no significant upregulation of ATF3 3 days post-SNI in their Nav1.8+ DRG neurons as compared to TLR4LoxTB counterparts. There is, however, a significant upregulation in both male and female WT mice 3 days after SNI as compared to shams (Fig. 5C, Table 6). This sets a precedent that endogenous TLR4 activation on Nav1.8+ nociceptors after nerve injury regulates transcription factors (ATF3) that are important in neuronal injury in female, but not male mice. Both male and female Nav1.8TLR4LoxTB mice exhibit no significant differences in ATF3 expression after SNI as compared to TLR4LoxTB counterparts in NF200+ neurons. There is, however, a significant upregulation in both male and female WT mice 3 days after SNI as compared to shams (Fig. 5D, Table 6). This data demonstrates that TLR4 has an important role in regulating neuronal injury after neuropathic injury in both sexes, but the cell types that express TLR4 which are responsible for these effects differ between males and females. This interesting finding led us to investigate the downstream implications of TLR4 activation on these small diameter nociceptors via HMGB1 localization.
Figure 5.
The injury marker, ATF3, is upregulated in small diameter nociceptors in a sex and genotype dependent manner via TLR4 expression. A, Female sham (mixed genotypes), WT, Nav1.8TLR4LoxTB, and TLR4LoxTB lumbar (L3–5) DRGs were immunostained 3D post SNI with DAPI (teal), Nav1.8 (green), ATF3 (red) and NF200 (blue; representative images from n=4 mice). B, Male sham (mixed genotypes), WT, Nav1.8TLR4LoxTB, and TLR4LoxTB lumbar (L3–5) DRGs were immunostained 3D post SNI with DAPI (teal), Nav1.8 (green), ATF3 (red) and NF200 (blue; representative images from n=4 mice). C, Quantification of ATF3 colocalization with Nav1.8+ neurons of both sexes (n=4 per group). D, Quantification of ATF3 colocalization with NF200+ neurons of both sexes (n=4 per group). Scale bar: 50 μm. Magnification: 20x. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. ns = not significant.
Table 6.
Statistical values for analyses performed within Figure 6. HMGB1 localization datasets were analyzed using Ordinary Two-Way ANOVA with Tukey’s post hoc. Significance was set at p<0.05 for all datasets. Bolded values are statistically significant.
| Dataset | Main Effect | Interactions | Multiple Comparisons | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| F (DFn, DFd) | p-value | F (DFn, DFd) | p-value | Effect | Groups | p-value | ||
|
| ||||||||
| HMGB1 Localization | Nav1.8+ Neurons | Genotype: F (3, 24) = 8.720 Sex: F (1, 24) = 3.194 |
p=0.0004 p=0.0866 |
F (3, 24) = 2.697 | p=0.0684 | Genotype | Males: Sham vs TLR4LoxTB | p=0.9996 |
| Males: Sham vs Nav1.8TLR4LoxTB | p>0.9999 | |||||||
| Males: WT vs TLR4LoxTB | p=0.4787 | |||||||
| Males: WT vs Nav1.8TLR4LoxTB | p=0.7364 | |||||||
| Males: Nav1.8TLR4LoxTB vs TLR4LoxTB | p=0.9994 | |||||||
| Females: Sham vs TLR4LoxTB | p>0.9999 | |||||||
| Females: Sham vs Nav1.8TLR4LoxTB | p=0.0958 | |||||||
| Females: WT vs TLR4LoxTB | p=0.0005 | |||||||
| Females: WT vs Nav1.8TLR4LoxTB | p=0.2405 | |||||||
| Females: Nav1.8TLR4LoxTB vs TLR4LoxTB | p=0.0948 | |||||||
|
| ||||||||
| NF200+ Neurons | Genotype: F (3, 24) = 4.233 Sex: F (1, 24) = 0.01456 |
p=0.0155 p=0.9050 |
F (3,24) = 0.252 | p=0.8593 | Genotype | Males: Sham vs TLR4LoxTB | p=0.9202 | |
| Males: Sham vs Nav1.8TLR4LoxTB | p=0.1687 | |||||||
| Males: WT vs TLR4LoxTB | p=0.6859 | |||||||
| Males: WT vs Nav1.8TLR4LoxTB | p=0.9784 | |||||||
| Males: Nav1.8TLR4LoxTB vs TLR4LoxTB | p=0.4483 | |||||||
| Females: Sham vs TLR4LoxTB | p=0.9914 | |||||||
| Females: Sham vs Nav1.8TLR4LoxTB | p=0.3730 | |||||||
| Females: WT vs TLR4LoxTB | p=0.1849 | |||||||
| Females: WT vs Nav1.8TLR4LoxTB | p=0.8830 | |||||||
| Females: Nav1.8TLR4LoxTB vs TLR4LoxTB | p=0.5369 | |||||||
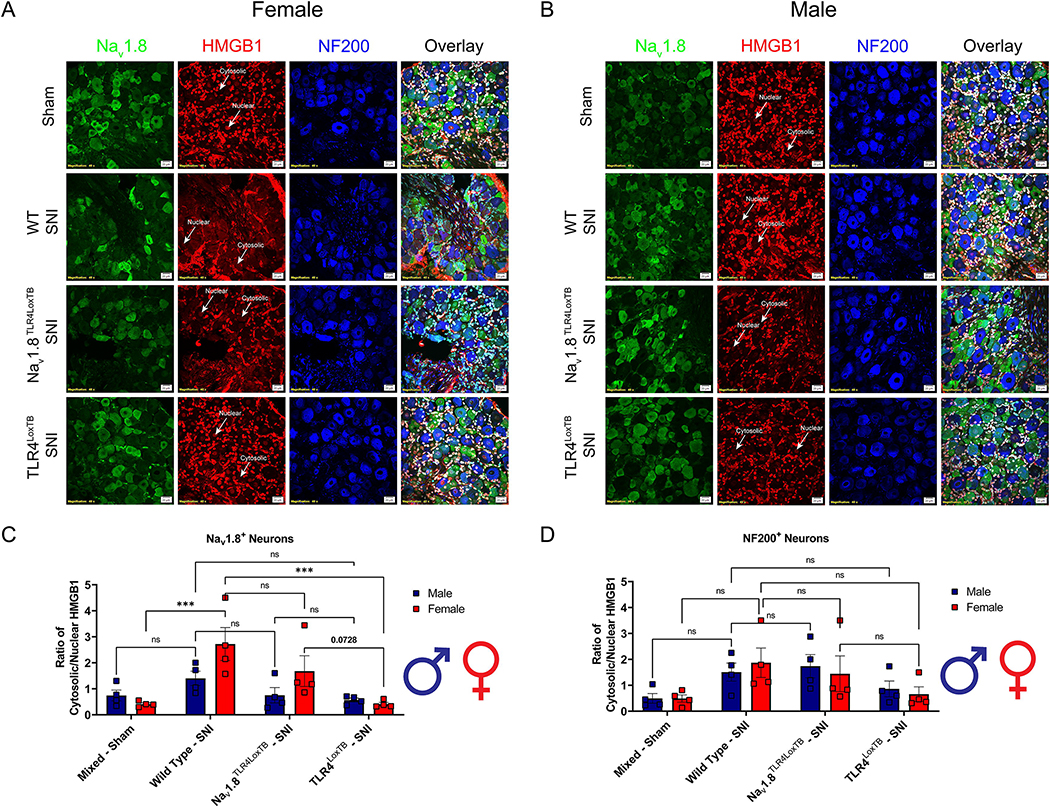
3.6. TLR4 expression in small diameter neurons mediates HMGB1 translocation from the nucleus to the cytosol of the cell
We demonstrated the sufficiency of TLR4 to upregulate the injury marker, ATF3, in Nav1.8+ DRG neurons in female, but not male mice. Now that we have established a significant role for TLR4 in regulating neuronal injury, we further investigated the downstream implication of TLR4 signaling in these populations of small and large diameter DRG neurons. Here, we immunoassayed lumbar DRGs (L3–5) from female and male Nav1.8TLR4LoxTB, TLR4LoxTB, and WT mice 3 days post-SNI for cellular localization of HMGB1, a DAMP, in both small (Nav1.8+) and large (NF200+) diameter neurons (Fig. 6A, B). We found there to be a trend of increased cytosolic HMGB1 in female Nav1.8+ DRG neurons of Nav1.8TLR4LoxTB mice as compared to TLR4LoxTB mice. Interestingly, male Nav1.8TLR4LoxTB mice do not show significantly increased cytosolic HMGB1 in their Nav1.8+ DRG neurons 3 days after SNI as compared to TLR4LoxTB mice. These results are recapitulated only in female WT mice, indicating direct activation of TLR4 on Nav1.8+ DRG neurons through endogenous DAMP signaling is sufficient to induce mobilization of HMGB1 to the cytosol of the cell (Fig. 6C, Table 6). We find no significant differences between both male and female Nav1.8TLR4LoxTB and TLR4LoxTB mice regarding cytosolic HMGB1 localization in NF200+ large diameter DRG neurons 3 days after SNI (Fig. 6D, Table 6). These highly sex and genotype dependent results indicate that endogenous activation of TLR4 on sensory neurons in the DRG of female mice after neuropathic injury initiates cytosolic mobilization of HMGB1. This, taken together with the upregulation of ATF3 expression in nociceptors indicates females utilize a TLR4-dependent pathway to facilitate pain behaviors during a neuropathic injury.
Figure 6.
HMGB1 translocation following neuropathic injury is upregulated in a sex and genotype dependent manner in small diameter nociceptors and is mediated by TLR4. A, Female sham (mixed genotypes), WT, Nav1.8TLR4LoxTB, and TLR4LoxTB lumbar (L3–5) DRGs were immunostained 3D post SNI with DAPI (teal), Nav1.8 (green), HGMB1 (red) and NF200 (blue; representative images from n=4 mice). White arrows point to an example of nuclear or cytosolic localization of HMGB1. B, Male sham (mixed genotypes), WT, Nav1.8TLR4LoxTB, and TLR4LoxTB lumbar (L3–5) DRGs were immunostained 3D post SNI with DAPI (teal), Nav1.8 (green), HGMB1 (red) and NF200 (blue; representative images from n=4 mice). White arrows point to an example of nuclear or cytosolic localization of HMGB1. C, Quantification of cytosolic localization of HMGB1 in Nav1.8+ neurons of both sexes (n=4 per group). D, Quantification of cytosolic localization of HMGB1 in NF200+ neurons of both sexes (n=4 per group). Scale bar: 20 μm. Magnification: 40x. *p < 0.05; **p < 0.01; ****p < 0.0001. ns = not significant.
4. Discussion
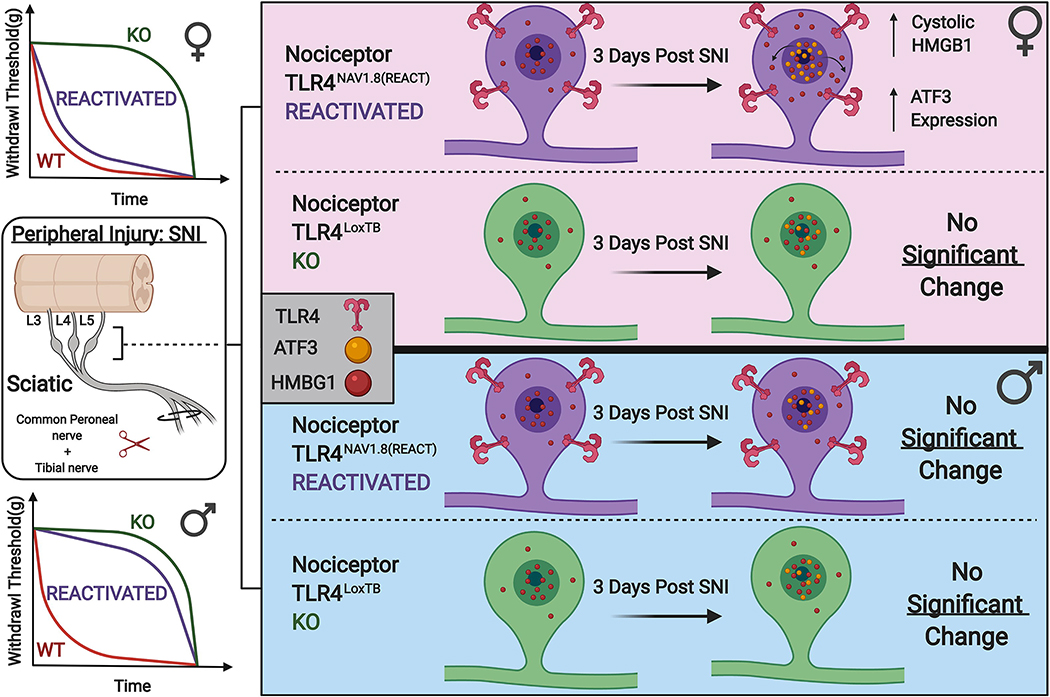
In the nociceptive system, bi-directional communication between neuronal and non-neuronal cells is a core mechanism in mediating the response to injury (Iwata and Shinoda, 2019; Szabo-Pardi et al., 2021). Emerging evidence suggests that both sexes utilize myeloid-derived immune cells in the central and peripheral nervous system to drive cytokine production and subsequent neuronal sensitization (Peng et al., 2016; Yu et al., 2020b). Currently, little is known about direct neuronal response to activation by endogenous DAMP signaling during injury. Here, we report that female mice utilize a specific pathway where direct activation of TLR4 by endogenous danger signals on small diameter nociceptors in the DRG leads to upregulation of stress-induced ATF3 and DAMPs (HMGB1) in the lumbar DRG. Additionally, these molecular changes lead to a delay in the onset of neuropathic pain associated with peripheral nerve injury in a TLR4 dependent manner (Fig. 7). Our genetic and histological experiments demonstrate a robust sexual dimorphism wherein peripheral nociceptors expressing TLR4 mediate the early onset of neuropathic mechanical hypersensitivity in female mice, in addition to regulating molecular changes in peripheral nervous tissue. Moreover, TLR4 expression on Nav1.8+ DRG neurons is both necessary and sufficient for the behavioral response to neuropathic injury. Use of this genetic model has allowed us to elucidate a novel pathway to neuronal injury in female mice. Our results support the idea that TLR4 signaling on peripheral nociceptors plays an important role in direct sensitization through the up-regulation of stress-induced transcription factors and DAMPs in these cells. In our view, this identifies a novel mechanism of direct neuronal sensitization in female mice crucial for the early onset of neuropathic pain. This provides novel evidence for the dissection of the sexually dimorphic response to tissue injury (Anwar et al., 2019).
Figure 7.
Schematic model indicated that TLR4 expressed on Nav1.8+ nociceptors in the lumbar DRGs (L3–5) plays an important role in regulating the localization of HMGB1 in response to neuropathic injury in females, but not males. This is both behaviorally and molecularly significant, as female mice lacking TLR4 expression on their Nav1.8+ nociceptors exhibit reduced mechanical hypersensitivity following SNI. Moreover, re-expression of TLR4 only on these Nav1.8 nociceptors recapitulates a behavioral phenotype similar to that of wild type mice. This effect is not present in male mice of either genotype.
Women are found to have a higher incidence of neuropathic pain as compared to males, in addition to lowered efficacy of common therapeutics (DiBonaventura et al., 2017). As such, there exists a need to understand the core mechanisms that differentiate male and female nociceptive circuitry. Compared with the well-documented behavioral phenotypes seen after SNI, we show that male and female wild type mice exhibit no differences in the magnitude of both mechanical hypersensitivity and cold allodynia (Bravo-Caparros et al., 2019; Sorge et al., 2015). Interestingly, evidence suggests a fundamental disconnect between the mechanisms involved in the onset of neuropathic pain (Mogil, 2020). For example, male mice lacking chemokine receptor 1 (CX3CR1) expressing immune cells exhibit delayed development of mechanical hypersensitivity; however, female mice remain unaffected (Peng et al., 2016). It has also been shown that neuronally expressed NOD-like receptor protein 3 (NLRP3) inflammasome has sex specific effects in pain, where females are dependent on NLRP3 in sensory neurons to facilitate mechanical hypersensitivity (Cowie et al., 2019). NLRP3 inflammasome activation is enhanced via intracellular cascades initiated through TLR4 activation (Bauernfeind et al., 2009). Importantly, inflammasome activation in tissue injury increases production of IL-1β, which plays a prominent role in pain, specifically mechanical hypersensitivity (Chen et al., 2015). Sensory neurons express large amounts of interleukin (IL) −1β receptor, which leads us to believe that autocrine signaling through the TLR4 and NLRP3 pathways in sensory neurons presents a point of sexually divergent mechanisms of pain sensitization.
In response to this, we sought to characterize endogenous DAMP signaling and chose to focus on HMGB1, specifically, as it has been implicated by numerous studies in chronic pain development (Campana et al., 2009; O’Connor et al., 2003; Zhang et al., 2015). HMGB1 is localized in the nucleus of cells in homeostasis, however; with adequate stimulation, hyperacetylation of the protein causes translocation to the cytosolic and subsequent release in both neuronal and non-neuronal cells (Lotze and Tracey, 2005). Secreted HMGB1 acts on a variety of receptors, namely the receptor for advanced glycation and end products (RAGE), TLR2, TLR4 and TLR5 all of which are involved in immunomodulation and neuroinflammation (Das et al., 2016; Frasnelli et al., 2015). Interestingly, HMGB1 is capable of upregulating canonical NLRP3 activation, both of which are dependent on the nuclear factor (NF)-κB pathway which is directly linked to TLR4 signaling (Chi et al., 2015). Downstream consequences of neuronally-expressed TLR4 activation are poorly understood, however; evidence does suggest that neurons are able to produce cytokines through its activation (Leow-Dyke et al., 2012). Moreover, it has been demonstrated that activation of TLRs on sensory neurons does promote the production of neuropeptides and chemokines which may facilitate neuronal sensitization (Diogenes et al., 2011). To our surprise, we have identified a robust sexual dimorphism in the lumbar DRG 3 days after SNI. Wild type female mice exhibit elevated cytosolic localization of HMGB1 in Nav1.8 expressing nociceptors. Cytosolic localization of HMGB1 after injury is decreased in both male and female TLR4 knockouts, but only females with TLR4 reactivated in their Nav1.8 neurons see an increase that trends towards a wild type phenotype. Moreover, this same population of peripheral nociceptors in females express higher levels of neuronal injury measured by upregulated ATF3 expression. We demonstrate that this increase in ATF3 expression is linked to TLR4 expression, as both male and female TLR4 knockouts have lower expression of ATF3 after injury. ATF3 has been shown to be a negative regulator of TLR4 activity by dampening the activity of NF-κB. Although co-expression of ATF3 and HMGB1 were not performed, it is a potential that the increase in ATF3 localization within Nav1.8+ neurons is due to endogenous TLR4 activation from tissue injury (Kwon et al., 2015; Rao et al., 2015). These data provide evidence that neuronally-expressed HMGB1 is involved in the onset of neuropathic pain in females, however; in future experiments it may be necessary to distinguish specific subsets of nociceptor populations (isolectin b4 (IB4) vs. calcitonin gene related peptide (CGRP)) that express HMGB1 to enhance granularity as to the specific circuitry involved. Nonetheless, this novel finding leads us to believe that females utilize more neuronally-driven mechanisms to facilitate chronic pain development.
To better understand the implications of direct neuronal activation through endogenous TLR4 signaling, we use two recently developed transgenic lines wherein TLR4 is either deleted (Nav1.8TLR4fl/fl) or reactivated (Nav1.8TLR4LoxTB) constitutively in a cre dependent manner (Jia et. Al., 2020). Evidence suggests TLR4 plays a major role in regulating the onset of neuropathic mechanical hypersensitivity, but not it’s persistence (Hu et al., 2018). Here, we investigate the nocifensive response to SNI in both male and female mice using mechanical and thermal measures. Interestingly, removal of TLR4 only on Nav1.8+ nociceptors delay the onset of mechanical hypersensitivity but not cold allodynia in female mice. Additionally, re-expression of TLR4 on these same nociceptors confers a phenotype that recapitulates a wild type behavioral response to neuropathic injury only in females. Lastly, whole body knockouts of TLR4 in both sexes behave similarly, conferring necessity during the onset of neuropathic pain in both sexes. Together, these data indicate that neuronally-expressed TLR4 is both necessary and sufficient during the onset of neuropathic mechanical hypersensitivity. The involvement of TLR4 signaling in thermal hyperalgesia is unclear. TLR4 deficiency has been shown to reduce mechanical allodynia, but not thermal hyperalgesia in a model of trigeminal neuropathic pain (Hu et al., 2018). Conversely, administration of a TLR4 antagonist, lipopolysaccharide (LPS)-RS, after chronic constriction injury (CCI) reduced both mechanical allodynia and thermal hyperalgesia (Jurga et al., 2016). We report no significant differences in cold allodynia, measured by behavioral response to acetone application, throughout the entirety of our behavioral experiments, independent of both sex and genotype. Interestingly, a recent study highlights how over 80% of cold-sensitive neurons in the DRG do not express Nav1.8 (Luiz et al., 2019). As we study the role of TLR4 in the Nav1.8 expressing neuronal population, it is a potential that co-expression between TLR4 and cold sensitive neurons (TRPM8) is not high enough to confer biological relevant behavioral changes in a murine model of neuropathic pain.
A major unanswered question from this work is how the spontaneous ectopic activity of Nav1.8+ DRG neurons is affected by direct TLR4 activity following neuropathic injury. Our histological data provides some insights as to what molecular changes these neurons undergo soon after injury, however; changes in their excitability are unknown. Following SNI, there is a large increase in the spontaneous activity of DRG neurons in addition to afferent fibers in the injured sciatic nerve (Seltzer et al., 1990; Wall and Gutnick, 1974). As seen in our model and consistent with others, whole body knockouts of TLR4 exhibit attenuated pain responses following injury (Piao et al., 2018; Tanga et al., 2005). While we currently cannot pinpoint the effects of direct TLR4 activation on DRG neuron excitability, there is evidence in the brain where TLR4 activation leads to increased neuronal excitability in the dentate gyrus after injury (Li et al., 2015b). Although not directly translatable, this does provide compelling evidence that TLR4 can modulate neuronal excitability. Therefore, understanding the role of TLR4 in regulating the spontaneous activity and subsequent excitability of DRG nociceptors will yield significant insights as to how the transient changes in behavior we see evolves into long-term nociceptor plasticity. This is a research endeavor we plan on pursuing as a follow-up to the present study.
In conclusion, we have identified a novel and sexually dimorphic mechanism of neuronal injury in a murine model of neuropathic pain. Female, but not male mice utilize endogenous TLR4 activation on their Nav1.8 expressing nociceptors to facilitate the onset of mechanical hypersensitivity following SNI. Additionally, TLR4 expression on Nav1.8 expressing nociceptors plays an important role in regulating neuronal localization of HMGB1 in female mice. Taken together, these data indicate a unique mechanism of neuronal injury and behavioral output following peripheral injury in female mice. This work is poised as an initial endeavor into the sexually dimorphic mechanisms that regulate pain plasticity.
Table 5.
Statistical values for analyses performed within Figure 5. ATF3 localization datasets were analyzed using Ordinary Two-Way ANOVA with Tukey’s post hoc. Significance was set at p<0.05 for all datasets. Bolded values are statistically significant.
| Dataset | Main Effect | Interactions | Multiple Comparisons | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| F (DFn, DFd) | p-value | F (DFn, DFd) | p-value | Effect | Groups | p-value | ||
|
| ||||||||
| ATF3 Localization | Nav1.8+ Neurons | Genotype: F (3, 24) = 55.51 Sex: F (1, 24) = 43.95 |
p<0.0001
p<0.0001 |
F (3, 24) = 10.03 | p=0.0002 | Genotype | Males: Sham vs TLR4LoxTB | p=0.4919 |
| Males: Sham vs Nav1.8TLR4LoxTB | p=0.3250 | |||||||
| Males: WT vs TLR4LoxTB | p=0.0031 | |||||||
| Males: WT vs Nav1.8TLR4LoxTB | p=0.0064 | |||||||
| Males: Nav1.8TLR4LoxTB vs TLR4LoxTB | p>0.9999 | |||||||
| Females: Sham vs TLR4LoxTB | p=0.1037 | |||||||
| Females: Sham vs Nav1.8TLR4LoxTB | p<0.0001 | |||||||
| Females: WT vs TLR4LoxTB | p<0.0001 | |||||||
| Females: WT vs Nav1.8TLR4LoxTB | p=0.9360 | |||||||
| Females: Nav1.8TLR4LoxTB vs TLR4LoxTB | p<0.0001 | |||||||
| Sex | Male TLR4LoxTB vs Female TLR4LoxTB | p=0.8948 | ||||||
| Male TLR4LoxTB vs Female Nav1.8TLR4LoxTB | p<0.0001 | |||||||
| Male Nav1.8TLR4LoxTB vs Female TLR4LoxTB | p=0.9720 | |||||||
| Male Nav1.8TLR4LoxTB vs Female Nav1.8TLR4LoxTB | p<0.0001 | |||||||
|
| ||||||||
| NF200+ Neurons | Genotype: F (3, 23) = 17.49 Sex: F (1, 23) = 2.067 |
p<0.0001 p=0.1639 |
F (3,23) = 1.088 | p=0.3741 | Genotype | Males: Sham vs TLR4LoxTB | p=0.0035 | |
| Males: Sham vs Nav1.8TLR4LoxTB | p=0.0402 | |||||||
| Males: WT vs TLR4LoxTB | p>0.9999 | |||||||
| Males: WT vs Nav1.8TLR4LoxTB | p=0.9166 | |||||||
| Males: Nav1.8TLR4LoxTB vs TLR4LoxTB | p=0.9797 | |||||||
| Females: Sham vs TLR4LoxTB | p=0.0355 | |||||||
| Females: Sham vs Nav1.8TLR4LoxTB | p=0.0007 | |||||||
| Females: WT vs TLR4LoxTB | p=0.1901 | |||||||
| Females: WT vs Nav1.8TLR4LoxTB | p=0.9888 | |||||||
| Females: Nav1.8TLR4LoxTB vs TLR4LoxTB | p=0.5423 | |||||||
Highlights:
TLR4 on sensory neurons drive the early development of neuropathic pain in females
ATF3 in Nav1.8 neurons is higher in females early after neuropathic injury
Neuronal HMGB1 activation is mediated by TLR4 expression in females after injury
TLR4 does not contribute to the development of cold allodynia after injury
Acknowledgments:
We would like to thank all current and former lab members for their assistance in the generation of this manuscript. Graphics and graphical abstract were created with Biorender.com.
Funding:
This research was supported by NIH grant: K22NS096030 (MDB), The American Pain Society Future Leaders Grant (MDB), and the Rita Allen Foundation Award in Pain (MDB), The University of Texas Rising STARs Award (MDB). The authors declare no competing financial interests.
Abbreviations:
- TLR
Toll-like receptor
- WT
Wild-type
- HMGB1
High mobility group box-1
- ATF3
Activating transcription factor-3
- DRG
Dorsal root ganglia
- PRRs
Pattern recognition receptors
- DAMPs
Danger-associated molecular patterns
- SNI
Spared nerve injury
- FBS
Fetal bovine serum
- SEM
Standard error of the mean
- ECM
Extracellular matrix
- TRP
Transient receptor potential
- NLRP3
NOD-like receptor protein 3
- RAGE
Receptor for advanced glycation and end products
- IB4
Isolectin B4
- CGRP
Calcitonin gene related peptide
- LPS
Lipopolysaccharide
- CCI
Chronic constriction injury
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agalave NM, Mody PH, Szabo-Pardi TA, Jeong HS, Burton MD, 2021a. Neuroimmune Consequences of eIF4E Phosphorylation on Chemotherapy-Induced Peripheral Neuropathy. Front Immunol 12, 642420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agalave NM, Rudjito R, Farinotti AB, Khoonsari PE, Sandor K, Nomura Y, Szabo-Pardi TA, Urbina CM, Palada V, Price TJ, Erlandsson Harris H, Burton MD, Kultima K, Svensson CI, 2021b. Sex-dependent role of microglia in disulfide high mobility group box 1 protein-mediated mechanical hypersensitivity. Pain 162, 446–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agalave NM, Svensson CI, 2015. Extracellular high-mobility group box 1 protein (HMGB1) as a mediator of persistent pain. Mol Med 20, 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allette YM, Due MR, Wilson SM, Feldman P, Ripsch MS, Khanna R, White FA, 2014. Identification of a functional interaction of HMGB1 with Receptor for Advanced Glycation End-products in a model of neuropathic pain. Brain Behav Immun 42, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar MA, Shah M, Kim J, Choi S, 2019. Recent clinical trends in Toll-like receptor targeting therapeutics. Med Res Rev 39, 1053–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E, 2009. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183, 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestall SM, Hulse RP, Blackley Z, Swift M, Ved N, Paton K, Beazley-Long N, Bates DO, Donaldson LF, 2018. Sensory neuronal sensitisation occurs through HMGB-1-RAGE and TRPV1 in high-glucose conditions. J Cell Sci 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Caparros I, Perazzoli G, Yeste S, Cikes D, Baeyens JM, Cobos EJ, Nieto FR, 2019. Sigma-1 Receptor Inhibition Reduces Neuropathic Pain Induced by Partial Sciatic Nerve Transection in Mice by Opioid-Dependent and- Independent Mechanisms. Front Pharmacol 10, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana L, Bosurgi L, Bianchi ME, Manfredi AA, Rovere-Querini P, 2009. Requirement of HMGB1 for stromal cell-derived factor-1/CXCL12-dependent migration of macrophages and dendritic cells. J Leukoc Biol 86, 609–615. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, 1994. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53, 55–63. [DOI] [PubMed] [Google Scholar]
- Chen H, Jiang YS, Sun Y, Xiong YC, 2015. p38 and interleukin-1 beta pathway via toll-like receptor 4 contributed to the skin and muscle incision and retraction-induced allodynia. J Surg Res 197, 339–347. [DOI] [PubMed] [Google Scholar]
- Chi W, Chen H, Li F, Zhu Y, Yin W, Zhuo Y, 2015. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-kappaB pathway in acute glaucoma. J Neuroinflammation 12, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Barrett LB, Williams EK, Strochlic DE, Lee S, Weyer AD, Lou S, Bryman GS, Roberson DP, Ghasemlou N, Piccoli C, Ahat E, Wang V, Cobos EJ, Stucky CL, Ma Q, Liberles SD, Woolf CJ, 2014. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Yoon YW, Na HS, Kim SH, Chung JM, 1994. Behavioral Signs of Ongoing Pain and Cold Allodynia in a Rat Model of Neuropathic Pain. Pain 59, 369–376. [DOI] [PubMed] [Google Scholar]
- Cowie AM, Menzel AD, O’Hara C, Lawlor MW, Stucky CL, 2019. NOD-like receptor protein 3 inflammasome drives postoperative mechanical pain in a sex-dependent manner. Pain 160, 1794–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C, 2018. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep 67, 1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N, Dewan V, Grace PM, Gunn RJ, Tamura R, Tzarum N, Watkins LR, Wilson IA, Yin H, 2016. HMGB1 Activates Proinflammatory Signaling via TLR5 Leading to Allodynia. Cell Rep 17, 1128–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ, 2000. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158. [DOI] [PubMed] [Google Scholar]
- DiBonaventura MD, Sadosky A, Concialdi K, Hopps M, Kudel I, Parsons B, Cappelleri JC, Hlavacek P, Alexander AH, Stacey BR, Markman JD, Farrar JT, 2017. The prevalence of probable neuropathic pain in the US: results from a multimodal general-population health survey. J Pain Res 10, 2525–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM, 2011. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res 90, 759–764. [DOI] [PubMed] [Google Scholar]
- Feldman P, Due MR, Ripsch MS, Khanna R, White FA, 2012. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J Neuroinflammation 9, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasnelli SC, de Medeiros MC, Bastos Ade S, Costa DL, Orrico SR, Rossa Junior C, 2015. Modulation of immune response by RAGE and TLR4 signalling in PBMCs of diabetic and non-diabetic patients. Scand J Immunol 81, 66–71. [DOI] [PubMed] [Google Scholar]
- Hanamsagar R, Hanke ML, Kielian T, 2012. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol 33, 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler SN, Ahmad FB, Burgos-Vega CC, Boitano S, Vagner J, Price TJ, Dussor G, 2019. Protease activated receptor 2 (PAR2) activation causes migraine-like pain behaviors in mice. Cephalalgia 39, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu TT, Wang RR, Tang YY, Wu YX, Yu J, Hou WW, Lou GD, Zhou YD, Zhang SH, Chen Z, 2018. TLR4 deficiency abrogated widespread tactile allodynia, but not widespread thermal hyperalgesia and trigeminal neuropathic pain after partial infraorbital nerve transection. Pain 159, 273–283. [DOI] [PubMed] [Google Scholar]
- Huck NA, Siliezar-Doyle J, Haight ES, Ishida R, Forman TE, Wu S, Shen H, Takemura Y, Clark JD, Tawfik VL, 2021. Temporal Contribution of Myeloid-Lineage TLR4 to the Transition to Chronic Pain: A Focus on Sex Differences. J Neurosci 41, 4349–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR, 2008. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur J Neurosci 28, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata K, Shinoda M, 2019. Role of neuron and non-neuronal cell communication in persistent orofacial pain. J Dent Anesth Pain Med 19, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Lee S, Tierney JA, Elmquist JK, Burton MD, Gautron L, 2021. TLR4 Signaling Selectively and Directly Promotes CGRP Release from Vagal Afferents in the Mouse. eNeuro 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Vianna CR, Fukuda M, Berglund ED, Liu C, Tao C, Sun K, Liu T, Harper MJ, Lee CE, Lee S, Scherer PE, Elmquist JK, 2014. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun 5, 3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Parada CA, Levine JD, 2003. Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain 105, 143–150. [DOI] [PubMed] [Google Scholar]
- Jurga AM, Rojewska E, Piotrowska A, Makuch W, Pilat D, Przewlocka B, Mika J, 2016. Blockade of Toll-Like Receptors (TLR2, TLR4) Attenuates Pain and Potentiates Buprenorphine Analgesia in a Rat Neuropathic Pain Model. Neural Plast 2016, 5238730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ, 2006. Persistent postsurgical pain: risk factors and prevention. Lancet 367, 1618–1625. [DOI] [PubMed] [Google Scholar]
- Klawitter M, Hakozaki M, Kobayashi H, Krupkova O, Quero L, Ospelt C, Gay S, Hausmann O, Liebscher T, Meier U, Sekiguchi M, Konno S, Boos N, Ferguson SJ, Wuertz K, 2014. Expression and regulation of toll-like receptors (TLRs) in human intervertebral disc cells. Eur Spine J 23, 1878–1891. [DOI] [PubMed] [Google Scholar]
- Kwon JW, Kwon HK, Shin HJ, Choi YM, Anwar MA, Choi S, 2015. Activating transcription factor 3 represses inflammatory responses by binding to the p65 subunit of NF-kappaB. Sci Rep 5, 14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow-Dyke S, Allen C, Denes A, Nilsson O, Maysami S, Bowie AG, Rothwell NJ, Pinteaux E, 2012. Neuronal Toll-like receptor 4 signaling induces brain endothelial activation and neutrophil transmigration in vitro. J Neuroinflammation 9, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Adamek P, Zhang H, Tatsui CE, Rhines LD, Mrozkova P, Li Q, Kosturakis AK, Cassidy RM, Harrison DS, Cata JP, Sapire K, Zhang H, Kennamer-Chapman RM, Jawad AB, Ghetti A, Yan J, Palecek J, Dougherty PM, 2015a. The Cancer Chemotherapeutic Paclitaxel Increases Human and Rodent Sensory Neuron Responses to TRPV1 by Activation of TLR4. J Neurosci 35, 13487–13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Korgaonkar AA, Swietek B, Wang J, Elgammal FS, Elkabes S, Santhakumar V, 2015b. Toll-like receptor 4 enhancement of non-NMDA synaptic currents increases dentate excitability after brain injury. Neurobiol Dis 74, 240–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XJ, Zhang Y, Liu T, Xu ZZ, Park CK, Berta T, Jiang D, Ji RR, 2014. Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res 24, 1374–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes DM, Malek N, Edye M, Jager SB, McMurray S, McMahon SB, Denk F, 2017. Sex differences in peripheral not central immune responses to pain-inducing injury. Sci Rep 7, 16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ, 2005. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5, 331–342. [DOI] [PubMed] [Google Scholar]
- Luiz AP, MacDonald DI, Santana-Varela S, Millet Q, Sikandar S, Wood JN, Emery EC, 2019. Cold sensing by NaV1.8-positive and NaV1.8-negative sensory neurons. Proc Natl Acad Sci U S A 116, 3811–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Greenquist KW, Lamotte RH, 2006. Inflammatory mediators enhance the excitability of chronically compressed dorsal root ganglion neurons. J Neurophysiol 95, 2098–2107. [DOI] [PubMed] [Google Scholar]
- Man L-L, Liu F, Wang Y-J, Song H-H, Xu H-B, Zhu Z-W, Zhang Q, Wang Y-J, 2015. The HMGB1 signaling pathway activates the inflammatory response in Schwann cells. Neural Regen Res 10, 1706–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AM, Toelle TR, Rowbotham DJ, Schaefer CP, Dukes EM, 2006. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain 10, 127–135. [DOI] [PubMed] [Google Scholar]
- Mecklenburg J, Zou Y, Wangzhou A, Garcia D, Lai Z, Tumanov AV, Dussor G, Price TJ, Akopian AN, 2020. Transcriptomic sex differences in sensory neuronal populations of mice. Sci Rep 10, 15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, 2020. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 21, 353–365. [DOI] [PubMed] [Google Scholar]
- Nicotra L, Loram LC, Watkins LR, Hutchinson MR, 2012. Toll-like receptors in chronic pain. Exp Neurol 234, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor KA, Hansen MK, Rachal Pugh C, Deak MM, Biedenkapp JC, Milligan ED, Johnson JD, Wang H, Maier SF, Tracey KJ, Watkins LR, 2003. Further characterization of high mobility group box 1 (HMGB1) as a proinflammatory cytokine: central nervous system effects. Cytokine 24, 254–265. [DOI] [PubMed] [Google Scholar]
- Peng J, Gu N, Zhou L, U BE, Murugan M, Gan WB, Wu LJ, 2016. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun 7, 12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao Y, Gwon DH, Kang DW, Hwang TW, Shin N, Kwon HH, Shin HJ, Yin Y, Kim JJ, Hong J, Kim HW, Kim Y, Kim SR, Oh SH, Kim DW, 2018. TLR4-mediated autophagic impairment contributes to neuropathic pain in chronic constriction injury mice. Mol Brain 11, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao J, Qian X, Li G, Pan X, Zhang C, Zhang F, Zhai Y, Wang X, Lu L, 2015. ATF3-mediated NRF2/HO-1 signaling regulates TLR4 innate immune responses in mouse liver ischemia/reperfusion injury. Am J Transplant 15, 76–87. [DOI] [PubMed] [Google Scholar]
- Ross JL, Queme LF, Lamb JE, Green KJ, Jankowski MP, 2018. Sex differences in primary muscle afferent sensitization following ischemia and reperfusion injury. Biol Sex Differ 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudjito R, Agalave NM, Farinotti AB, Lundback P, Szabo-Pardi TA, Price TJ, Harris HE, Burton MD, Svensson CI, 2021. Sex- and cell-dependent contribution of peripheral high mobility group box 1 and TLR4 in arthritis-induced pain. Pain 162, 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y, 1990. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43, 205–218. [DOI] [PubMed] [Google Scholar]
- Seth P, Rudd RA, Noonan RK, Haegerich TM, 2018. Quantifying the Epidemic of Prescription Opioid Overdose Deaths. American journal of public health 108, 500–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS, 2011. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 31, 15450–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS, 2015. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 18, 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JA, Cheung J, Eddinger K, Corr M, Yaksh TL, 2013. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J Neuroinflammation 10, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo-Pardi TA, Agalave NM, Burton MD, 2021. The role of microglia versus peripheral macrophages in maladaptive plasticity after nerve injury. Neural Regen Res 16, 1202–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajerian M, Sahbaie P, Sun Y, Leu D, Yang HY, Li W, Huang TT, Kingery W, David Clark J, 2015. Sex differences in a Murine Model of Complex Regional Pain Syndrome. Neurobiol Learn Mem 123, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA, 2005. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A 102, 5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Gutnick M, 1974. Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma. Exp Neurol 43, 580–593. [DOI] [PubMed] [Google Scholar]
- Wan W, Cao L, Khanabdali R, Kalionis B, Tai X, Xia S, 2016. The Emerging Role of HMGB1 in Neuropathic Pain: A Potential Therapeutic Target for Neuroinflammation. J Immunol Res 2016, 6430423–6430423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangzhou A, Paige C, Ray PR, Dussor G, Price TJ, 2021. Diversity of receptor expression in central and peripheral mouse neurons estimated from single cell RNA sequencing. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woller SA, Ravula SB, Tucci FC, Beaton G, Corr M, Isseroff RR, Soulika AM, Chigbrow M, Eddinger KA, Yaksh TL, 2016. Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: The role of TLR4 in the evolution of a persistent pain state. Brain Behav Immun 56, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang W, Chao ZY, Feng DY, 2015. Role of Toll-like receptor/MYD88 signaling in neurodegenerative diseases. Rev Neurosci 26, 407–414. [DOI] [PubMed] [Google Scholar]
- Yamasoba D, Tsubota M, Domoto R, Sekiguchi F, Nishikawa H, Liu K, Nishibori M, Ishikura H, Yamamoto T, Taga A, Kawabata A, 2016. Peripheral HMGB1-induced hyperalgesia in mice: Redox state-dependent distinct roles of RAGE and TLR4. J Pharmacol Sci 130, 139–142. [DOI] [PubMed] [Google Scholar]
- Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, Basbaum AI, 2020a. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 11, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, Basbaum AI, 2020b. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nature Communications 11, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang H, Zi T, 2015. Overexpression of microRNA-141 relieves chronic constriction injury-induced neuropathic pain via targeting high-mobility group box 1. Int J Mol Med 36, 1433–1439. [DOI] [PubMed] [Google Scholar]