Abstract
Spinal cord injury (SCI) elicits chronic pain in 65% of individuals. In addition, SCI afflicts an increasing number of aged individuals, and those with SCI are predisposed to shorter lifespan. Our group previously identified that deletion of the microRNA miR-155 reduced neuroinflammation and locomotor deficits after SCI. Here, we hypothesized that aged mice would be more susceptible to pain symptoms and death soon after SCI, and that miR-155 deletion would reduce pain symptoms in adult and aged mice and improve survival. Adult (2 month-old) and aged (20 month-old) female wildtype (WT) and miR-155 knockout (KO) mice received T9 contusion SCI. Aged WT mice displayed reduced survival and increased autotomy – a symptom of spontaneous pain. In contrast, aged miR-155 KO mice after SCI were less susceptible to death or spontaneous pain. Evoked pain symptoms were tested using heat (Hargreaves test) and mechanical (von Frey) stimuli. At baseline, aged mice showed heightened heat sensitivity. After SCI, adult and aged WT and miR-155 KO mice all exhibited heat and mechanical hypersensitivity at all timepoints. miR-155 deletion in adult (but not aged) mice reduced mechanical hypersensitivity at 7 and 14 d post-SCI. Therefore, aging predisposes mice to SCI-elicited spontaneous pain and expedited mortality. miR-155 deletion in adult mice reduces evoked pain symptoms, and miR-155 deletion in aged mice reduces spontaneous pain and expedited mortality post-SCI. This study highlights the importance of studying geriatric models of SCI, and that inflammatory mediators such as miR-155 are promising targets after SCI for improving pain relief and longevity.
Keywords: Spinal cord injury, neuropathic pain, chronic pain, aging, neuroinflammation, microRNA, longevity, thermal hyperalgesia, mechanical allodynia
Introduction
Spinal cord injury (SCI) can harm systems throughout the body, causing myriad deleterious outcomes including neuropathic pain and reduced lifespan. Neuropathic pain – pain caused by nervous system dysfunction – afflicts ~65% of individuals with SCI (Siddall et al., 2003). Current first-line SCI pain therapies include pregabalin and gabapentin (Hagen and Rekand, 2015), which inhibit α2δ calcium channel function to limit synaptic transmission (Taylor et al., 2007). Unfortunately, current pain therapies are not always effective and chronic SCI pain management requires a multidisciplinary, individually-tailored approach (Finnerup and Baastrup, 2012). One potential target for pain management is excess inflammation, which persists indefinitely after SCI and worsens damage and deficits (Gaudet and Fonken, 2018). Indeed, neuroimmune reactivity far from the injury site amplifies neuropathic pain (Detloff et al., 2008). Despite our expanding understanding, no known therapies consistently relieve chronic SCI pain.
The average age of individuals sustaining SCI continues to rise – the average age at SCI increased from 29 years old in the 1970s to 43 years old since 2015 (NSCISC, 2021). In humans, mortality up to one year after SCI is 2–3% in younger adults (<65 years old) vs. 25–39% in aged persons (>65 years) (Furlan and Fehlings, 2009). Those who experience SCI at younger ages also have reduced life expectancy over 40 years post-SCI (Middleton et al., 2012). Although healthspan and longevity after SCI are major clinical issues, no known studies have addressed whether SCI affects probability of survival in aged rodents.
One potential neuroprotective strategy after SCI is reducing the pro-inflammatory microRNA miR-155 (Gaudet et al., 2018). Adult miR-155 knockout (KO) mice after SCI have reduced inflammation, and increased axon plasticity, neuroprotection, and locomotor recovery (Gaudet et al., 2016b). Aging has deleterious effects on these post-SCI outcomes (Geoffroy et al., 2016; Zhang et al., 2015). In addition, miR-155 removal protects against cellular senescence (Onodera et al., 2017). Thus, targeting miR-155 is a promising approach for ameliorating harmful local and systemic events after SCI, which could ultimately improve pain relief and longevity.
Here, we examined how age and miR-155 deletion affect survival and neuropathic pain after thoracic contusion SCI. We hypothesized that aged (vs. adult) would display worsened SCI-elicited neuropathic pain symptoms. Further, we predicted that miR-155 KO mice with SCI would show reduced pain symptoms. We noted that aged mice did not survive as well after SCI, which led to novel findings regarding geriatric SCI survival and spontaneous neuropathic pain. Our data reveal that aging and miR-155 deletion influence mouse survival and spontaneous and evoked pain symptoms after SCI.
Materials and Methods
Animals: care and surgery
Experiments were approved by University of Colorado Boulder Institutional Animal Care and Use Committee (Protocol number 1403.03) and were conducted in accordance with ARRIVE guidelines. Mice maintained on a 12:12 light/dark cycle received chow and water ad-libitum. Surgeries occurred between Zeitgeber time (ZT)−2 and −11 (ZT0 = lights on). WT and miR-155 KO mice were used as adults (2 months old [8–9 weeks]) or aged (20–21 months old) (mice from Jackson Laboratory and bred in-house: C57BL/6J, stock 000664; miR-155 KO, stock 007745). For surgery, mice received isoflurane anesthesia, and aseptic T9 laminectomy and moderate-to-severe contusion injury (midline SCI; 75 kDyn, 0 s dwell; Infinite Horizon device, Precision Systems and Instrumentation) (Gaudet et al., 2016b). To limit confounds with pain testing, analgesics and antibiotics were withheld (Gaudet et al., 2017). Mice received subcutaneous Ringer’s solution for 5 days post-operative (dpo), and post-SCI bladder voiding twicedaily. End points for euthanasia included excess loss of body mass and/or abnormal signs of declining health or discomfort, including excess autotomy (criteria: exposed muscle or bone, or expanding/non-healing large skin sores). Mice displaying minor autotomy or skin lesions were treated topically with triple antibiotic ointment (active ingredients: Bacitracin zinc (400 units/g), Neomycin sulfate (3.5 mg/g), Polymyxin B sulfate (5,000 units/g)).
Outcomes and mouse numbers
Adult WT (n = 10), adult miR-155 KO (n = 10), aged WT (n = 9 – originally n = 10, but one died during surgery under anesthesia), and aged miR-155 KO (n = 10) female mice received SCI (Force [kDyn] and displacement [μm] data: adult WT: 76.8±0.7 kDyn, 607±30 μm; aged WT: 78±1 kDyn, 630±34 μ m; adult KO: 77.3±0.9 kDyn, 658±46 μm; aged KO: 77.8±0.8 kDyn, 601±27 μm [one aged-KO data point lost]). Evoked pain symptoms were assessed prior to surgery and weekly thereafter. Mouse survival and autotomy was recorded to 42 dpo.
Behavioral testing
Neuropathic pain to evoked stimuli was assessed (Gaudet et al., 2017). Mice pre-acclimated to von Frey and Hargreaves apparati for 2–3 sessions, then had two pre-surgery tests and weekly post-surgery tests. Mice acclimated to their enclosure for 40–60 min prior to each test.
Mechanical sensory thresholds were tested using the simplified up-down (SUDO) method (Bonin et al., 2014; Gaudet et al., 2017) of von Frey testing which limits mouse stress and time out of cage. von Frey filaments (Stoelting) were pressed against the center of the plantar surface of the rat hindpaw until the filaments buckled and were held for a maximum of 3 s. von Frey testing was completed the day prior to Hargreaves to ensure accurate measurements for both tests and to minimize stress.
Heat sensory thresholds were assessed using the Hargreaves test (Hargreaves et al., 1988). An infrared source (intensity of 25) was placed under the center of their hindpaw and activated. Latency to nocifensive response was automatically recorded. Testing on left-right hindpaws was alternated (three tests per timepoint), separated by 5–10 min. Maximum response latency was 25 s.
Statistics
Most data were analyzed (SigmaPlot 13.0; Systat Software) using Student’s t- or non-parametric Mann-Whitney U test; or ANOVAs (one-, two-way, and/or repeated measure ANOVA, as appropriate). Holm-Sidak post-hoc tests were completed for tests involving >2 groups. For survival curve and autotomy curve analyses, a Kaplan-Meyer (Gehan-Breslow) test was completed with Holm-Sidak post-hoc. For categorical analysis of survival and autotomy at 42 dpo, three-way contingency tables underwent log-linear chi-square analysis. Researchers were blind to experimental group throughout testing. Data were graphed using GraphPad Prism. Data were considered significant when p<0.05. Data were plotted as mean±SEM.
Results
Aged WT mice are susceptible to death after SCI, whereas aged miR-155 KO mice show improved survival
After SCI, aged mice died (or had to be euthanized) at a higher rate: adult and aged WT and miR-155 KO mice had significantly different survival curves (Gehan-Breslow test; overall difference, p<0.005; WT: adult vs. aged, p<0.05) (Fig. 1a). At 42 dpo, there was a significant interaction between age and genotype (log-linear analysis; p<0.01) (Fig. 1b). High survival rates to 42 dpo were achieved by adult WT (survival: 10/10 mice) and adult KO mice (survival: 9/10). In contrast, aged mice showed reduced probability of survival across 42 dpo (significant effect of age; p<0.005). Specifically, aged WT mice had low survival at 42 dpo (survival: 3/9); whereas aged miR-155 KO mice showed higher probability of survival at 42 dpo (survival: 7/10; significant age-genotype interaction) (Fig. 1b).
Figure 1.
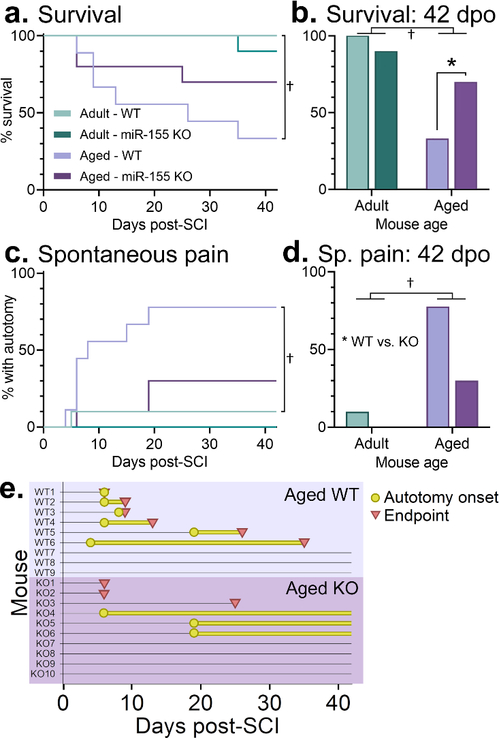
Aged WT mice with SCI had increased 6-week mortality and spontaneous pain, whereas miR-155 KO aged mice were protected from these post-SCI detriments. a. Adult WT and miR-155 KO mice and aged miR-155 KO mice had high post-SCI survival, whereas most aged WT mice died within 42 d after SCI. b. Fewer aged WT (33%, vs. 100% of adult WT) mice survived to 42 dpo; aged miR-155 KO mice had enhanced 6-week survival (70%). c. Timecourse of spontaneous pain after SCI in adult and aged WT and KO mice. Aged WT mice had higher pain incidence than adult WT mice. d. At 42 d post-SCI, aged mice had increased spontaneous pain vs. adult mice; miR-155 KO mice had reduced incidence of pain vs. WT mice (aged WT: 78%, aged KO: 30%). e. Horizontal plot of all individual aged WT and miR-155 KO mice showing onset and timing of autotomy (yellow circles and lines) and premature endpoints (red triangles). † indicates p<0.05 between adult and aged mice; * indicates p<0.05 between WT and KO mice; ANOVA with Holm-Sidak post-hoc test.
Aged mice exhibit spontaneous pain symptoms after SCI that are ameliorated by miR-155 deletion
Next, we examined the relationship between post-SCI morbidity and mortality, aging, and miR-155 deletion. After SCI, several mice exhibited autotomy – “self-severing”, or self-amputation – which is a symptom of spontaneous pain (Kauppila, 1998; Wall et al., 1979). Adult mice exhibited low rates of autotomy or early post-SCI death: one adult miR-155 KO mouse was euthanized at 28 dpo due to weight loss and ill health, and one adult WT mouse exhibited autotomy onset at 5 dpo but survived through 42 dpo.
Aged mice were more likely to display SCI-induced spontaneous pain symptoms (autotomy). Adult and aged WT and miR-155 KO mice had significantly different autotomy curves over time (Gehan-Breslow test; overall difference, p<0.001; WT: adult vs. aged, p<0.05) (Fig. 1c) and at 42 dpo (significant interaction between age and genotype; log-linear analysis; p<0.005) (Fig. 1d). miR-155 KO mice were less susceptible to developing autotomy (effect of genotype, p=0.05). 7/9 aged WT mice developed autotomy (average onset: 9 dpo; on hind paw/limb [4/7] or lower trunk skin [3/7]) and 6/7 of these reached endpoint criteria (average time from autotomy onset to euthanasia: 8 days) (Fig. 1e). In contrast, fewer aged miR-155 KO mice developed autotomy (3/10; average onset: 15 dpo); these aged KO mice presented with more mild autotomy and all aged KO mice with autotomy survived to the experimental endpoint of 42 dpo. The three aged miR-155 KO mice that died prior to 42 dpo were found dead in their cages (with no autotomy). Thus, aged mice subjected to SCI are susceptible to spontaneous pain and expedited mortality, and miR-155 deletion in aged mice ameliorated spontaneous pain symptoms and improved probability of surviving to chronic times after SCI.
Baseline heat and mechanical sensitivity: Aging increases sensitivity to heat; miR-155 deletion has no significant effect on sensory thresholds
Plantar hindpaw sensitivity to heat and mechanical stimuli was assessed using the Hargreaves test and von Frey test, respectively. For baseline heat sensitivity (Fig. 2a), aged mice (vs. adult mice) had reduced latency to respond (significant effect of age; F1,36=45.017, p<0.001). miR-155 deletion had no significant effect on baseline heat sensitivity in adult (WT: 12.5±0.3 s, miR-155 KO: 12.1±0.3 s; p>0.05) or aged (WT: 11.0±0.4 s, miR-155 KO: 11.0±0.4 s; p>0.05) mice. For mechanical sensitivity (Fig. 2d), there was no significant effect of age or miR-155 deletion (SUDO thresholds: adult WT: 9.0±0.1, adult miR-155 KO: 9.0±0.1, aged WT: 8.7±0.2, aged miR-155 KO: 8.8±0.2; p>0.05). Thus, at baseline, aged mice show hypersensitivity to heat (but not mechanical) stimuli, and miR-155 deletion does not significantly affect sensory thresholds in adult or aged mice.
Figure 2.
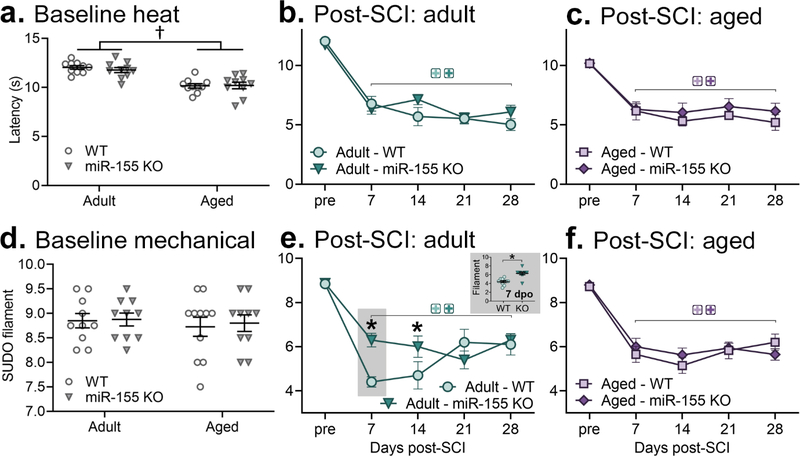
Effects of aging and miR-155 deletion on sensory thresholds at baseline and after SCI (Hargreaves heat – a-c; von Frey mechanical – d-f). a. At baseline, aged mice had reduced response latency to heat. There was no significant effect of miR-155 deletion. b-c. Both adult (b) and aged (c) mice after SCI displayed heat hyperalgesia at all times tested (7–28 dpo). miR-155 deletion did not modify SCI-elicited heat hypersensitivity. d. Baseline mechanical thresholds were not significantly altered in aged or miR-155 KO mice. e,f. Both adult and aged WT mice exhibited mechanical allodynia from 7–28 d after SCI. Adult miR-155 KO mice had ameliorated mechanical hypersensitivity at 7 and 14 dpo. † indicates p<0.05 group difference between adult and aged mice; colored + indicates p<0.05 within-genotype between pre- and post-SCI thresholds; * indicates p<0.05 between WT and KO mice.
miR-155 deletion and aging had no significant effect on SCI-elicited heat hypersensitivity
T9 75 kDyn contusion SCI in female mice elicited neuropathic pain symptoms. For heat hyperalgesia, adult WT and miR-155 KO mice showed reduced latency to nocifensive response at every timepoint examined between 7 dpo and 28 dpo (vs. adult WT or KO pre-SCI, respectively; p<0.001 at 7, 14, 21, and 28 dpo) (Fig. 2b). Adult WT and miR-155 KO heat thresholds were not significantly different at any timepoint (p>0.05).
Both aged WT and miR-155 KO mice with SCI reduced latency to response to heat (main effect of dpo; F4,48=21.99, p<0.001), but were not different from one another (no significant effect of genotype; p>0.05) (Fig. 2c). Adult and aged WT and miR-155 KO mice showed similar latencies throughout the post-SCI period. Thus, SCI induces in adult and aged female mice hindpaw heat hypersensitivity, which is not significantly modified by miR-155 deletion or by aging.
miR-155 KO adult, but not aged mice had reduced SCI-induced mechanical allodynia
Mice with SCI were tested for mechanical allodynia. Adult WT and miR-155 KO female mice had mechanical hypersensitivity that persisted from 7 to 28 dpo (both p<0.001 vs. pre-SCI) (Fig. 2e). Compared to adult WT mice, adult miR-155 KO mice at 7 and 14 dpo had higher mechanical thresholds (significant dpo x genotype interaction, F3,54=5.02, p<0.005) (SUDO thresholds: adult WT 7 dpo: 4.5±0.2, adult KO 7 dpo: 6.3±0.3; p<0.005) (adult WT 14 dpo: 4.5±0.6, adult KO 14 dpo: 6.0±0.5; p<0.05). This suggests that at these times miR-155 deletion ameliorated mechanical pain.
Aged mice with SCI displayed mechanical allodynia between 7–28 dpo (main effect of dpo; F4,47= 55.06, p<0.001) (Fig. 2f). Both aged WT and aged miR-155 KO mice showed similar SCI-elicited reduction in mechanical thresholds (aged WT vs. aged KO post-SCI: p>0.05). Thus, adult (but not aged) miR-155 KO mice at acute-to-subacute post-SCI times showed reduced mechanical pain symptoms.
Discussion
This study explored age-related survival and pain symptoms after SCI and whether miR-155 deletion improved post-SCI outcomes in aged and adult mice. Adult mice survived at high rates after SCI, whereas aged WT, but not miR-155 KO mice were predisposed to death within six weeks post-SCI. Most aged WT (but not KO) mice exhibited SCI-elicited autotomy, a symptom of spontaneous pain. Further, aged mice at baseline had heat hypersensitivity. After SCI, adult and aged mice showed similar extent of heat hyperalgesia and mechanical allodynia; both displayed these neuropathic pain symptoms from 7 to 28 dpo. Adult (but not aged) miR-155 KO mice had ameliorated SCI-elicited mechanical pain symptoms at 7 and 14 dpo. Therefore, geriatric mice after SCI are susceptible to spontaneous pain and expedited mortality, and miR-155 removal after SCI can benefit mouse survival and improve relief of spontaneous and evoked pain.
In our study, 70% of aged WT SCI mice were euthanized within 42 d after SCI due to skin or hindlimb lesions attributable to autotomy. Autotomy is a symptom of spontaneous pain – also called “painful numbness” (Kauppila, 1998; Wall et al., 1979). Adult WT mice did not present with autotomy, suggesting that age unmasked SCI-related spontaneous pain. Thus, effective pain management is likely even more crucial after SCI with aging (as in humans, (Warner et al., 2019). Future geriatric preclinical SCI research should prospectively study survival and signs of health and discomfort. For instance, the mouse clinical frailty index (Toth, 2018) – scored from a four-minute, 31-item physical exam – could define health status of geriatric mice after SCI.
Aged miR-155 KO mice showed improved survival after SCI and did not reach end points related to autotomy or skin lesions. This implies that miR-155 deletion in aged mice reduces spontaneous pain. miR-155 deletion also promotes wound healing (van Solingen et al., 2014), which would limit post-SCI development or expansion of cutaneous sores. In addition, miR-155 influences various health-relevant body systems after SCI (Fonken et al., 2016b; Gaudet et al., 2016a; Gaudet et al., 2016b) and its removal is protective in various aging models and processes (Dinami et al., 2014; Onodera et al., 2017). Axotomy-elicited autotomy is prevented by removing the pro-inflammatory cytokine IL-1β (Gabay et al., 2011) and aging amplifies neuroinflammatory responses after inflammatory challenge (Fonken et al., 2016a), so exaggerated neuroinflammation after SCI in aged mice may exacerbate spontaneous pain. miR-155 deletion reduces expression of pro-inflammatory factors such as IL-1β and iNOS (Gaudet et al., 2018; Jablonski et al., 2016). Indeed, miR-155 is a microRNA that potently drives pro-inflammatory responses; it is increased in the SCI epicenter (Gaudet et al., 2016b) and could amplify inflammation and pain by reducing anti-inflammatory mRNA mediators. miR-155’s direct anti-inflammatory mRNA targets include Socs1, Ship1, Il13raI, and Bdnf (see (Gaudet et al., 2018) – miR-155-elicited reduction of these alone or in combination could unleash inflammation and maladaptive pain. Accordingly, miR-155 and other non-coding RNAs are potential intrinsic combinatorial targets for modifying disease outcomes. Thus, miR-155 inhibition or other anti-inflammatory treatments after SCI in aged individuals could interrupt runaway inflammation to boost pain relief and longevity.
Our preclinical outcomes related to pain and aging after SCI have important similarities and differences with SCI outcomes in humans. In our study, analgesics and antibiotics were prospectively withheld from all mice. Analgesics were not provided to limit confounds on neuropathic pain testing. For example, opioids can increase peripheral immune cell trafficking to the central nervous system (Olin et al., 2012) and activate glia (Johnston et al., 2004). Furthermore, analgesics could have unknown interactions with miR-155 deletion and confound results. There are important limitations of withholding analgesics: first, withholding analgesics does not recapitulate the clinical setting, where individuals with SCI would receive pain-relieving drugs; and second, the age-related autotomy and death after SCI observed here may have been ameliorated (or exacerbated) by peri-operative analgesia (Hook et al., 2007; Jutzeler et al., 2021; Krause et al., 2017). Although antibiotics help sustain health of mice and humans susceptible to infection after SCI (Brommer et al., 2016), prophylactic antibiotics were withheld due to their adverse effects on recovery after SCI in mice without infection (Kigerl et al., 2016) and to limit unknown potential confounds with age and genotype.
We also observed increased 42 dpo mortality in aged mice after SCI (vs. adults; mostly due to autotomy-related euthanasia). In humans, senescence is associated with accelerated mortality after SCI: mortality up to one year after SCI was 2–3% in younger adults (<65 years old) vs. 25–39% in aged persons (>65 years) (Furlan and Fehlings, 2009). Unfortunately, those who experience SCI at younger ages also have reduced life expectancy over 40 years post-SCI (Middleton et al., 2012). Severe chronic pain is associated with increased risk of mortality (Torrance et al., 2010), especially when pain is concomitant with functional limitations (Smith et al., 2018); however, the link between pain and expedited mortality after SCI is muddled by co-occurring socioeconomic and health factors (Krause et al., 2017).
Our study showed that aged mice (20–21 months old) had increased baseline sensitivity to heat, but not mechanical stimulation. Senescence in mice occurs at 18+ months and a mouse’s lifespan averages 24 months. Combining our results with the findings of others, mice appear to have adult-typical thermal thresholds through 6–12 months (Karl et al., 2017), increased hypersensitivity at 12–21 months (our data and (Azkona et al., 2016), and reduced sensitivity by 24 months (Wang et al., 2006).
This is the first known study to reveal that SCI elicits neuropathic pain in aged rodents. SCI evokes neuropathic pain symptoms in adult mice (Watson et al., 2014) and rats (Detloff et al., 2008; Gaudet et al., 2017). Future studies should address whether SCI in aged rodents elicits neuropathic pain in both sexes (as in adult mice; (McFarlane et al., 2020). Clinical pain should be modeled by incorporating complementary behavioral measures of affective-cognitive components and spontaneous pain (Kramer et al., 2016).
Adult miR-155 KO mice had reduced SCI-elicited mechanical allodynia. Potential mechanisms underlying ameliorated pain symptoms in miR-155 KO mice after SCI include decreased intraspinal neuroinflammation that could dampen pain signaling (Detloff et al., 2008; Gaudet et al., 2016b); preserved descending pain control (Millan, 2002); reduced maladaptive nociceptive activity (Gerke et al., 2003); or diminished neuroimmune interface activation (Ghasemlou et al., 2015). Studies using peripheral pain models have similarly identified a pain-promoting role for miR-155 (e.g., (Tan et al., 2015).
Conclusions
We explored the effects of aging and miR-155 deletion after SCI on survival and neuropathic pain symptoms. Our data suggest that aging predisposes mice to spontaneous pain and autotomy, and premature death after SCI, and that miR-155 removal may be effective after SCI for reducing health detriments and neuropathic pain. More broadly, our results imply that quenching or shifting harmful neuroimmune cascades after SCI could improve health and analgesia. Future preclinical and clinical studies should further examine geriatric models of SCI, and should leverage novel immunomodulatory strategies to benefit health outcomes, longevity, and pain management.
Highlights.
Aged 20 month-old mice were prone to death and pain after spinal cord injury (SCI).
Aged miR-155 knockout SCI mice were protected from increased pain and mortality.
Aged mice showed baseline heat hyperalgesia.
SCI elicited mechanical and heat pain in both adult (2 month-old) and aged mice.
miR-155 deletion in adults ameliorated mechanical allodynia at 7 and 14 d post-SCI.
Acknowledgements:
The authors thank the Muenzinger and Wilderness Place husbandry staff for excellent animal care, the Biological Sciences Initiative (BSI) and Undergraduate Research Opportunities Program (UROP) at CU-Boulder for undergraduate research support, and the Light Microscopy Core Facility at CU-Boulder. At University of Texas at Austin, the authors thank the staff at the Animal Resource Center, and Bethaney Watson and Barbara Landberg for their superb administrative support.
Funding sources: This work was supported by the Craig H. Neilsen Foundation (SFM), the Wings for Life Foundation (ADG/LRW), and the National Institutes of Health (LKF; grant R01 AG062716).
Abbreviations
- ANOVA
analysis of variance
- dpo
days post-operative
- KO
knockout
- miR
microRNA
- SCI
spinal cord injury
- SEM
standard error of the mean
- SUDO
Simplified Up-Down method (von Frey)
- WT
wildtype
- ZT
Zeitgeber time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azkona G, Saavedra A, Aira Z, Aluja D, Xifró X, Baguley T, Alberch J, Ellman JA, Lombroso PJ, Azkue JJ, Pérez-Navarro E, 2016. Striatal-enriched protein tyrosine phosphatase modulates nociception: evidence from genetic deletion and pharmacological inhibition. Pain 157, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Bories C, De Koninck Y, 2014. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Molecular pain 10, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brommer B, Engel O, Kopp MA, Watzlawick R, Muller S, Pruss H, Chen Y, DeVivo MJ, Finkenstaedt FW, Dirnagl U, Liebscher T, Meisel A, Schwab JM, 2016. Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain : a journal of neurology 139, 692–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM, 2008. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Experimental neurology 212, 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinami R, Ercolani C, Petti E, Piazza S, Ciani Y, Sestito R, Sacconi A, Biagioni F, le Sage C, Agami R, Benetti R, Mottolese M, Schneider C, Blandino G, Schoeftner S, 2014. miR-155 drives telomere fragility in human breast cancer by targeting TRF1. Cancer research 74, 4145–4156. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Baastrup C, 2012. Spinal cord injury pain: mechanisms and management. Current pain and headache reports 16, 207–216. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, D’Angelo HM, Norden DM, Weber MD, Barrientos RM, Godbout JP, Watkins LR, Maier SF, 2016a. The Alarmin HMGB1 Mediates Age-Induced Neuroinflammatory Priming. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 7946–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Gaudet AD, Gaier KR, Nelson RJ, Popovich PG, 2016b. MicroRNA-155 deletion reduces anxiety- and depressive-like behaviors in mice. Psychoneuroendocrinology 63, 362–369. [DOI] [PubMed] [Google Scholar]
- Furlan JC, Fehlings MG, 2009. The impact of age on mortality, impairment, and disability among adults with acute traumatic spinal cord injury. Journal of neurotrauma 26, 1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay E, Wolf G, Shavit Y, Yirmiya R, Tal M, 2011. Chronic blockade of interleukin-1 (IL-1) prevents and attenuates neuropathic pain behavior and spontaneous ectopic neuronal activity following nerve injury. European journal of pain (London, England) 15, 242–248. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Ayala MT, Schleicher WE, Smith EJ, Bateman EM, Maier SF, Watkins LR, 2017. Exploring acute-to-chronic neuropathic pain in rats after contusion spinal cord injury. Experimental neurology 295, 46–54. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Fonken LK, 2018. Glial Cells Shape Pathology and Repair After Spinal Cord Injury. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 15, 554–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet AD, Fonken LK, Gushchina LV, Aubrecht TG, Maurya SK, Periasamy M, Nelson RJ, Popovich PG, 2016a. miR-155 Deletion in Female Mice Prevents Diet-Induced Obesity. Scientific reports 6, 22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet AD, Fonken LK, Watkins LR, Nelson RJ, Popovich PG, 2018. MicroRNAs: Roles in Regulating Neuroinflammation. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 24, 221–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet AD, Mandrekar-Colucci S, Hall JC, Sweet DR, Schmitt PJ, Xu X, Guan Z, Mo X, Guerau-de-Arellano M, Popovich PG, 2016b. miR-155 Deletion in Mice Overcomes Neuron-Intrinsic and Neuron-Extrinsic Barriers to Spinal Cord Repair. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 8516–8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy CG, Hilton BJ, Tetzlaff W, Zheng B, 2016. Evidence for an Age-Dependent Decline in Axon Regeneration in the Adult Mammalian Central Nervous System. Cell reports 15, 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke MB, Duggan AW, Xu L, Siddall PJ, 2003. Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience 117, 715–722. [DOI] [PubMed] [Google Scholar]
- Ghasemlou N, Chiu IM, Julien JP, Woolf CJ, 2015. CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proceedings of the National Academy of Sciences of the United States of America 112, E6808–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen EM, Rekand T, 2015. Management of Neuropathic Pain Associated with Spinal Cord Injury. Pain and therapy 4, 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J, 1988. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88. [DOI] [PubMed] [Google Scholar]
- Hook MA, Liu GT, Washburn SN, Ferguson AR, Bopp AC, Huie JR, Grau JW, 2007. The impact of morphine after a spinal cord injury. Behavioural brain research 179, 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski KA, Gaudet AD, Amici SA, Popovich PG, Guerau-de-Arellano M, 2016. Control of the Inflammatory Macrophage Transcriptional Signature by miR-155. PloS one 11, e0159724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR, 2004. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 7353–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutzeler CR, Bourguignon L, Tong B, Ronca E, Bailey E, Harel NY, Geisler F, Ferguson AR, Kwon BK, Cragg JJ, Grassner L, Kramer JLK, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmacological Management of Acute Spinal Cord Injury: A longitudinal multi-cohort observational study. medRxiv, 2021.2005.2028.21257947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl F, Grießhammer A, Üçeyler N, Sommer C, 2017. Differential Impact of miR-21 on Pain and Associated Affective and Cognitive Behavior after Spared Nerve Injury in B7-H1 ko Mouse. Frontiers in molecular neuroscience 10, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppila T, 1998. Correlation between autotomy-behavior and current theories of neuropathic pain. Neuroscience and biobehavioral reviews 23, 111–129. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Hall JC, Wang L, Mo X, Yu Z, Popovich PG, 2016. Gut dysbiosis impairs recovery after spinal cord injury. The Journal of experimental medicine 213, 2603–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JL, Minhas NK, Jutzeler CR, Erskine EL, Liu LJ, Ramer MS, 2016. Neuropathic pain following traumatic spinal cord injury: Models, measurement, and mechanisms. Journal of neuroscience research. [DOI] [PubMed] [Google Scholar]
- Krause JS, Cao Y, Clark JMR, 2017. Pain Intensity, Interference, and Medication Use After Spinal Cord Injury: Association With Risk of Mortality After Controlling for Socioeconomic and Other Health Factors. Archives of physical medicine and rehabilitation 98, 2464–2470. [DOI] [PubMed] [Google Scholar]
- McFarlane K, Otto TE, Bailey WM, Veldhorst AK, Donahue RR, Taylor BK, Gensel JC, 2020. Effect of Sex on Motor Function, Lesion Size, and Neuropathic Pain after Contusion Spinal Cord Injury in Mice. Journal of neurotrauma 37, 1983–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton JW, Dayton A, Walsh J, Rutkowski SB, Leong G, Duong S, 2012. Life expectancy after spinal cord injury: a 50-year study. Spinal cord 50, 803–811. [DOI] [PubMed] [Google Scholar]
- Millan MJ, 2002. Descending control of pain. Progress in neurobiology 66, 355–474. [DOI] [PubMed] [Google Scholar]
- NSCISC, 2021. National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. In: Center NSCIS (Ed.). University of Alabama at Birmingham, Birmingham, AL. [Google Scholar]
- Olin M, Oh S, Roy S, Peterson P, Molitor T, 2012. Morphine induces splenocyte trafficking into the CNS. J Neuroimmune Pharmacol 7, 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera Y, Teramura T, Takehara T, Obora K, Mori T, Fukuda K, 2017. miR-155 induces ROS generation through downregulation of antioxidation-related genes in mesenchymal stem cells. Aging cell 16, 1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ, 2003. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103, 249–257. [DOI] [PubMed] [Google Scholar]
- Smith D, Wilkie R, Croft P, Parmar S, McBeth J, 2018. Pain and mortality: mechanisms for a relationship. Pain 159, 1112–1118. [DOI] [PubMed] [Google Scholar]
- Tan Y, Yang J, Xiang K, Tan Q, Guo Q, 2015. Suppression of microRNA-155 attenuates neuropathic pain by regulating SOCS1 signalling pathway. Neurochemical research 40, 550–560. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Angelotti T, Fauman E, 2007. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy research 73, 137–150. [DOI] [PubMed] [Google Scholar]
- Torrance N, Elliott AM, Lee AJ, Smith BH, 2010. Severe chronic pain is associated with increased 10 year mortality. A cohort record linkage study. European journal of pain (London, England) 14, 380–386. [DOI] [PubMed] [Google Scholar]
- Toth LA, 2018. Identifying and Implementing Endpoints for Geriatric Mice. Comp Med 68, 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Solingen C, Araldi E, Chamorro-Jorganes A, Fernández-Hernando C, Suárez Y, 2014. Improved repair of dermal wounds in mice lacking microRNA-155. Journal of cellular and molecular medicine 18, 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, Tomkiewicz MM, 1979. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain 7, 103–113. [DOI] [PubMed] [Google Scholar]
- Wang S, Davis BM, Zwick M, Waxman SG, Albers KM, 2006. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiology of aging 27, 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner FM, Cragg JJ, Jutzeler CR, Finnerup NB, Werhagen L, Weidner N, Maier D, Kalke YB, Curt A, Kramer JLK, 2019. Progression of Neuropathic Pain after Acute Spinal Cord Injury: A Meta-Analysis and Framework for Clinical Trials. Journal of neurotrauma 36, 1461–1468. [DOI] [PubMed] [Google Scholar]
- Watson JL, Hala TJ, Putatunda R, Sannie D, Lepore AC, 2014. Persistent atlevel thermal hyperalgesia and tactile allodynia accompany chronic neuronal and astrocyte activation in superficial dorsal horn following mouse cervical contusion spinal cord injury. PloS one 9, e109099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Bailey WM, Braun KJ, Gensel JC, 2015. Age decreases macrophage IL-10 expression: Implications for functional recovery and tissue repair in spinal cord injury. Experimental neurology 273, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


