Abstract
Cerebral palsy (CP) is characterized by deficits in motor function due to reduced neuromuscular control. We leveraged the guiding principles of motor learning theory to design a wearable robotic intervention intended to improve neuromuscular control of the ankle. The goal of this study was to determine the neuromuscular and biomechanical response to four weeks of exoskeleton ankle resistance therapy (exo-therapy) in children with CP. Five children with CP (12 – 17 years, GMFCS I – II, two diplegic and three hemiplegic, four males and one female) were recruited for ten 20-minute sessions of exo-therapy. Surface electromyography, three-dimensional kinematics, and metabolic data were collected at baseline and after training was complete. After completion of training and with no device on, participants walked with decreased co-contraction between the plantar flexors and dorsiflexors (−29 ± 11%, p = 0.02), a more typical plantar flexor activation profile (33 ± 13% stronger correlation to a typical soleus activation profile, p = 0.01), and increased neural control complexity (7 ± 3%, p < 0.01; measured via muscle synergy analysis). These improvements in neuromuscular control led to a more mechanically efficient gait pattern (58 ± 34%, p < 0.05) with a reduced metabolic cost of transport (−29 ± 15%, p = 0.02). The findings from this study suggest that ankle exoskeleton resistance therapy shows promise for rapidly improving neuromuscular control for children with CP, and may serve as a meaningful rehabilitative complement to common surgical procedures.
Keywords: cerebral palsy, neurorehabilitation, gait, exoskeleton, muscle synergy
Introduction
The ankle plantar flexor muscles play a key role in mechanical energy recovery while walking, extending the knee joint, preventing excessive ankle dorsiflexion during midstance, modulating center of mass vertical displacement, and providing the single-largest contribution to forward propulsion across all lower-extremity muscle groups (Gage et al., 2009; Sutherland et al., 1980). This allows the motion of the body’s center of mass to follow an arced, inverted pendular pattern that provides an effective exchange in potential and kinetic energy (Cavagna et al., 1977). Activation of the ankle plantar flexor muscles is reduced, less modulated, and often accompanied by co-activation of the antagonist dorsiflexor muscles in a majority of individuals with spastic cerebral palsy (CP) (Dietz and Berger, 2008), a movement disorder arising from injury to the brain during infancy (Graham et al., 2016). These muscle activation characteristics likely contribute directly or indirectly to reduced energy exchange (Bennett et al., 2005), elevated metabolic cost of transport (Unnithan et al., 1996), and lower levels of physical activity (Johnson et al., 2009) in CP.
Impaired neuromuscular control in children with CP may be explained in part by deficits in cortical organization (Thickbroom et al., 2001). It has been shown that the variance in muscle activity accounted for by one muscle synergy is associated with the level of motor control complexity (Cheung et al., 2012). Children with CP have greater variance accounted for by one muscle synergy while walking compared to typically developing peers, leading to the conclusion that these children use a simplified control strategy (Steele et al., 2015). This measure of motor control in children with CP plays a significant role in explaining treatment outcomes for this population (Schwartz et al., 2016).
Current standard of care for children with CP has not been successful at improving neuromuscular control (O’Brien et al., 2020). Surgical procedures, while a critical component in the management of musculoskeletal health for individuals with CP (Krigger, 2006), may have limited influence on neuromuscular control of movement (Shuman et al., 2019). Lower-limb orthoses, which serve to mitigate misalignment and contractures (Graham et al., 2016), are not specifically designed to train motor control. Physical therapy has shown limited evidence of improving motor function and walking ability (Anttila et al., 2008; Novak et al., 2013). Gait training with partial body-weight support may lead to muscle atrophy over time (Clark et al., 2007) and negatively impact muscle activation timing (McGowan et al., 2010). Functional electrical stimulation is a bottom-up approach that has failed to produce lasting changes in neuromuscular control or gain traction as an effective tool for treating CP (Khamis et al., 2018). There is an apparent gap in our ability to effectively address deficits in the neuromuscular control of walking for individuals with CP.
We recently developed an untethered, ankle exoskeleton device that provides resistance proportional to the estimated, real-time biological ankle moment to foster increased volitional engagement of the ankle plantar flexor muscles while individuals with CP walk with the device (B.C. Conner et al., 2020). It was observed that four weeks of training with this novel paradigm led to significant and rapid improvements in ankle strength and mobility-related outcomes for individuals with CP (Benjamin C. Conner et al., 2020). Changes in neuromuscular control and gait mechanics after training with this new therapeutic tool have not yet been explored.
The primary goal of this study was to explore potential underlying neuromuscular and biomechanical mechanisms behind observed improvements in mobility after training with adaptive ankle resistance, and evaluate the user experience of this task-specific gait re-training intervention. We hypothesized that repeated neuromuscular gait re-training with adaptive ankle resistance that responds directly to user input would improve the ankle plantar flexor muscle activation profile and decrease co-contraction across the ankle joint during device-free walking. We also hypothesized that these changes would reflect improved motor control complexity, as indicated by muscle synergy analysis. We further hypothesized that the combination of improved plantar flexor activation, reduced co-contraction, and more complex muscle coordination would lead to a more mechanically efficient gait pattern.
Methods
Participants
This study was approved by the Northern Arizona University Institutional Review Board (#986744). The protocol was completed at the Northern Arizona University – Phoenix Biomedical Campus (Phoenix, AZ) and registered at ClinicalTrials.gov (NCT04119063). Informed written consent was provided by a parent or legal guardian for each participant.
We recruited six individuals diagnosed with hemiplegic or diplegic CP based on the following inclusion criteria: Gross Motor Function Classification System (GMFCS) levels I – II, the ability to walk with or without a walker for at least six minutes, age between 10 – 21 years, and the ability to follow directions. Individuals were excluded from the study if they had orthopedic surgery within the past six months, botulinum toxin injections to the lower limbs within the past four months, or any conditions that would prevent safe participation. Data from five participants are reported in this study due to a motion capture recording equipment failure for one participant and the resulting absence of experimental biomechanics data needed for this analysis (Table 1).
Table 1.
Participant characteristics
| Gender | Age (y:m) | Height (cm) | Body mass (kg) | GMFCS levela | Hemi- or diplegic | More-affected side | Gait typeb | |
|---|---|---|---|---|---|---|---|---|
| P1 | M | 16:11 | 173.5 | 54.4 | II | Di | Right | Crouch |
| P2 | M | 13:11 | 143.0 | 42.0 | II | Hemi | Right | Crouch |
| P3 | M | 14:10 | 160.5 | 59.0 | I | Hemi | Right | Crouch |
| P4 | F | 16:5 | 160.5 | 68.5 | I | Hemi | Left | Stiff knee gait |
| P5 | M | 11:8 | 131.5 | 30.2 | II | Di | Right | Crouch |
GMFCS: Gross Motor Function Classification System.
Gait type: ‘Crouch’ gait defined by those parameters set by Gage et al [2].
Wearable Resistance Platform
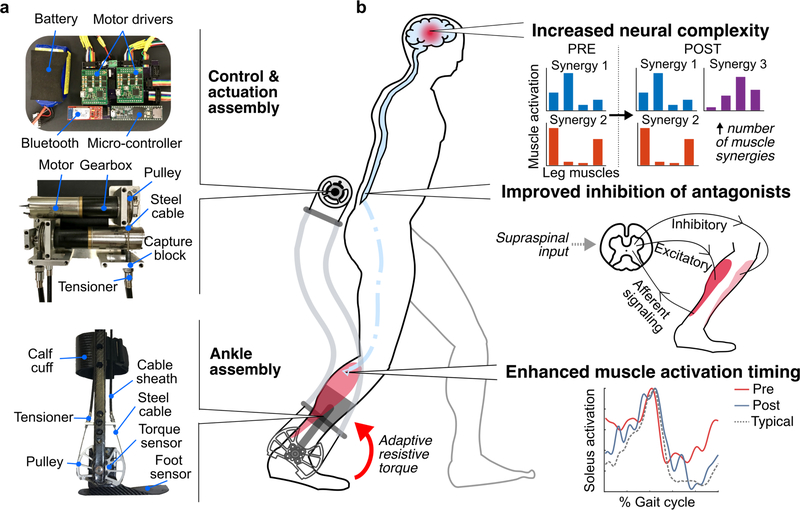
Our exoskeleton ankle resistance therapy (exo-therapy) utilized a custom battery-powered ankle exoskeleton that provided resistance to plantar flexion (Fig. 1a). The device had an actuation and control assembly that was worn at the waist, and ankle assemblies worn bilaterally. Both hemiplegic and diplegic participants wore a bilateral exoskeleton for consistency. Detection of gait events and real-time estimates of ankle moment from the embedded force sensors allowed the device to provide proportional resistance to ankle plantar flexion (B.C. Conner et al., 2020). An onboard microcontroller with Bluetooth allowed for remote control via a custom MATLAB graphical user interface and visualization of the estimated ankle movement and exoskeleton torque, allowing us to assess user performance in real-time; participants were blinded to this real-time visualization of their performance.
Figure 1. Wearable adaptive resistance training.
a) Pictures of the exoskeleton used to deliver adaptive ankle resistance b) Schematic depiction of the proposed mechanisms underlying the neuromuscular response to wearable adaptive resistance training, including increased neural complexity (as indicated by an increase in the number of muscle synergies recruited for walking), improved inhibition of antagonist muscles about the ankle, and enhanced muscle activation timing of the plantar flexor muscles for more typical activation timing (experimental data from P1).
Pre- and Post-Assessment
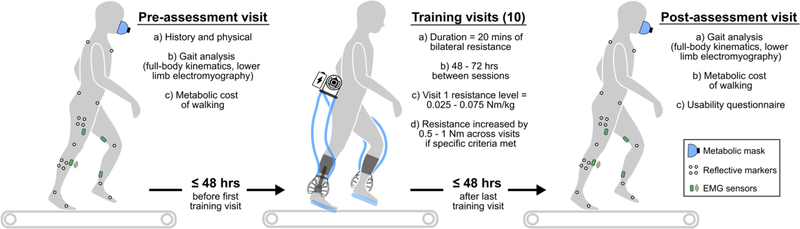
A separate pre-assessment visit was completed within 48 hours before a participant’s first training session, and a separate post-assessment visit was completed within 48 hours after a participant’s last training session (Fig 2). During the pre-assessment, participants were evaluated by a licensed physical therapist to determine physical characteristics, GMFCS level and each participant’s more- and less-affected lower-limbs. For both the pre- and post-assessments, wireless surface electromyography (EMG) sensors (Noraxon; 1000 Hz) were placed on the soleus (SOL), tibialis anterior (TA), vastus lateralis (VL), and semitendinosus (ST) according to SENIAM recommendations for consistency between visits (Hermens et al., 1999). Participants were outfitted with 38 reflective markers for measuring 3D kinematics of the legs, pelvis, and trunk using eight motion capture cameras (Vicon; 100 Hz). Participants walked on a treadmill at a self-selected speed while being monitored by a laboratory technician. Preferred walking speed was identified by asking participants to choose a speed that they would normally walk at school or home. The treadmill speed was then increased and decreased to confirm preferred walking speed.
Figure 2. Study visit allocation.
Participants completed 12 total visits: a pre-assessment, ten training visits, and a post-assessment.
Oxygen and carbon dioxide levels were assessed using a metabolic measurement system (TrueOne 2400, Parvo Medics). Metabolic measurements were taken while participants stood for two minutes, followed by six minutes of walking at their preferred treadmill speed, or until oxygen consumption levels stabilized. Once a steady state of oxygen consumption was reached, kinematic and EMG data were recorded for 30 seconds of walking. During the post-assessment, if the participant’s preferred speed differed from his or her preferred speed during the pre-assessment, all measurements were taken at the pre-assessment preferred speed so that pre- and post-assessment speeds were matched. For metabolic cost measurements at a participants’ final preferred speed, see (Benjamin C. Conner et al., 2020).
During the post-assessment visit, a usability questionnaire was completed with study participants to determine 1) if they would like to train with the device again, 2) if the level of difficulty was sufficient, and 3) if they had any additional comments about their experience. Parents or guardians were then asked to comment on their experience with the training intervention. A summary of questionnaire results are reported in Supplemental Material.
Exoskeleton Ankle Resistance Therapy (Exo-therapy)
Training consisted of ten 20-minute treadmill walking sessions, separated by 48 – 72 hours (Fig 2), with ankle resistance at each participant’s preferred speed. One to two rest periods were provided during each session, depending on participant preference. The nominal resistance setpoint was initialized between 0.025 – 0.075 Nm/kg, which represents the maximal amount of resistance that would be applied during a gait cycle (i.e., the amount of resistance applied when the peak biological ankle moment was reached, typically at push-off). Participants were instructed to focus on engaging their ankle plantar-flexor muscles, with an emphasis on their more-affected limb. Verbal coaching was provided as needed. Following the completion of each session, participants were asked to rank their level of soreness on the following scale: none, mild, moderate, severe, and extremely severe. If participants had both 1) a perceived level of soreness between none and moderate, and 2) reached their prescribed level of resistance for more than 50% of the training session, then the prescribed resistance level was increased by 0.5 – 1 Nm for the next training session. An interval of 0.5 – 1 Nm was used based on pilot testing of sufficient magnitudes to induce a noticeable change in difficulty level.
Data Processing & Statistical Analysis
An OpenSim musculoskeletal model was scaled to the anthropometrics of each participant (Delp et al., 2007). Joint angles across 10 continuous gait cycles were calculated in OpenSim via inverse kinematics (pre- and post-musculoskeletal gait model videos can be found in Supplementary Vid. 1). Participant EMG signals were bandpass filtered (4th order Butterworth, 20 – 400 Hz band-pass cutoff), rectified, and low-pass filtered (4th order Butterworth, 10 Hz low-pass cutoff), and then time normalized from 0 – 100% of the gait cycle (Lerner et al., 2017). The EMG curves were then averaged over the same 10 gait cycles and normalized to the maximal activity recorded during walking within a visit for each respective muscle (Kang et al., 2017). For consistency across participants with diplegia and hemiplegia, all EMG measures were assessed for the more-affected limb only.
Co-contraction between the soleus and tibialis anterior was calculated using a co-contraction index (CCI) (Rudolph et al., 2000), which captures the temporal and magnitude components of an EMG signal (Knarr et al., 2012), as in Equation 1:
| Eq. 1 |
where i represents the individual time points of the time-normalized gait cycle (0 – 100%, or 101 total data points), LEMG represents the normalized magnitude of the less active muscle at time point i, and MEMG represents the normalized magnitude of the more active muscle at time point i.
We assessed the relationship between the soleus EMG profile for our participants and the typical (control) soleus EMG profile from unimpaired individuals by calculating the Pearson product moment correlation coefficient (R) (Mukaka, 2012). The typical soleus EMG curve were from unimpaired individuals walking at a non-dimensional speed of 0.25 (Hof et al., 2002), which was very close to the non-dimensional speed of our participants (0.21); non-dimensional walking speed was calculated by dividing walking speed by the square root of gravity multiplied by leg length (Hof, 2018).
The variance in muscle activity accounted for by one muscle synergy (VAF1) was calculated using non-negative matrix factorization (NNMF) (Lee and Seung, 1999) (Steele et al., 2015), as in Equation 2:
| Eq. 2 |
where EMG represents a matrix containing the normalized and averaged EMG data recorded for each muscle; W represents the relative activation level in a synergy and is a 4 × 1 matrix, with a separate row for each muscle; and C represents the activation level of a synergy over the gait cycle.
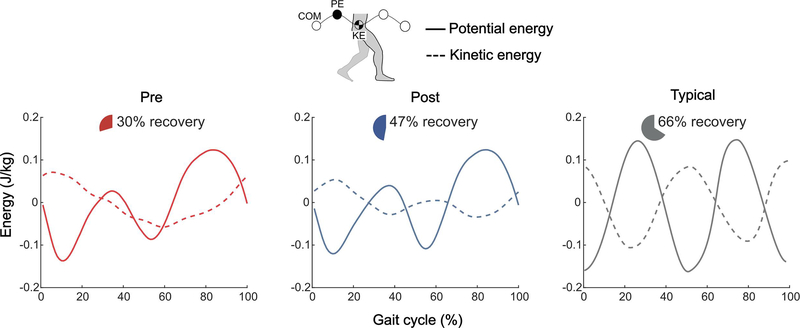
The mechanical efficiency of each individual’s pre- and post-training gait patterns was calculated using energy recovery analysis (Fig. 4) (Bennett et al., 2005). Center of mass mechanical energy recovery is significantly lower in children with CP (Bennett et al., 2005), which may contribute to the higher metabolic cost of walking in this population (Rose et al., 1990). This measure was quantified as the exchange between kinetic and potential energy of the center of mass (COM) movement (Eq. 5) by considering the external work (Wext, Eq. 3) on the COM and the work done by the COM (Wne, Eq. 3) (Bennett et al., 2005).
| Eq. 3 |
| Eq. 4 |
| Eq. 5 |
where i represents the individual time points of the time-normalized gait cycle (0 – 100%, or 101 total data points), ΔPE represents the change in potential energy of the COM between i and i + 1, and ΔKE represents the change in kinetic energy of the COM between i and i + 1.
Figure 4. Center of mass mechanical efficiency.
a) Group average pre-exo-therapy (red), group average post-exo-therapy (blue), and typical unimpaired (gray) potential energy (PE, solid lines) and kinetic energy (KE, dashed lines) curves. Pie charts indicate the group average center of mass (COM) energy exchange recovery percentage; For efficient energy exchange and increased recovery, potential and kinetic energies should be 180º out of phase with equal and opposite amplitudes. The typical, unimpaired curve was adopted from Bennett et al [6].
Metabolic cost of transport was calculated using participants’ expired gas data (TrueOne 2400, Parvo Medics, Salt Lake City, UT, USA). First, steady state regions for both quiet standing and walking data were determined by Kendall’s tau-b approach (Schwartz, 2007), which has been shown to contribute to a five-fold reduction in variability of measured oxygen costs while walking for individuals with CP (Schwartz, 2007). Metabolic cost of each region was calculated using Brockway’s standard equation (Brockway, 1987), and a net metabolic cost (W) was determined by subtracting the metabolic cost of quiet standing from the metabolic cost of walking. Net metabolic cost was then divided by body mass (kg) and walking speed (m/s) for a final measure of body-mass-normalized metabolic energy required to walk a unit distance (i.e., metabolic cost of transport, J/kg-m).
Primary outcome measures included CCI, relationship of experimental soleus EMG profile to a typical profile, energy recovery, and metabolic cost of transport. Secondary outcome measure included VAF1. All outcome variables were assessed at matched speeds (i.e., pre vs. post-training, both at the initial preferred speed).
We assessed all outcome measure data for normality and the presence of outliers. Normality was tested using the Kolmogorov-Smirnov test with small sample Lilliefors correction (Ghasemi and Zahediasl, 2012). Outliers were defined as any data point below the first quartile or above the third quartile of the 1.5 interquartile range (Tukey, 1977). No data met the definition as an outlier. We evaluated our outcome measures using two-tailed paired t-tests. Multiple comparisons were corrected for with a Holm-Bonferroni correction for our primary outcome variables. Significance level was set at α = 0.05. Cohen’s d (d) was used to calculate effect size, where 0.2 was considered a small effect, 0.5 a medium effect, and 0.8 a large effect (Cohen, 1988).
Supplementary Analysis
To provide better context for any changes in motor control complexity after exo-therapy, we retrospectively matched our participants by age and GMFCS level to patients with motor control complexity data who underwent two of the most common procedures for children with CP – single event multi-level surgery (SEMLS) and selective dorsal rhizotomy (SDR). See Supplemental Material for further details on this analysis.
Results
All participants were able to continue walking at or above their initial walking speed through progressive increases in plantar flexor resistance and indicated an interest in training with the device again. The average level of resistance increased by 0.015 ± 0.012 Nm/kg across the training sessions; a majority of the participants indicated the levels of resistance were “just right”, with two participants indicating that it “could have been harder”.
Neuromuscular control
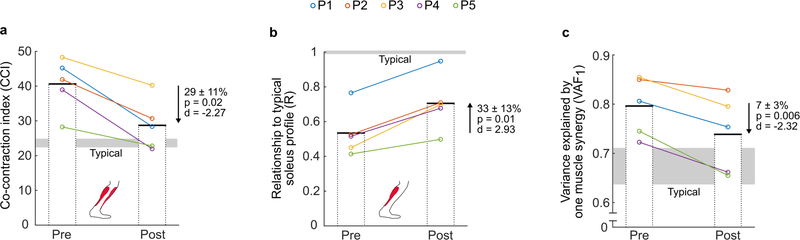
Following exo-therapy, more-affected limb co-contraction between the soleus and tibialis anterior decreased by 29 ± 11% (p = 0.02, d = −2.27; Fig. 3a) and the relationship (R) between experimental and the typical soleus curve increased by 33 ± 13% (p = 0.01, d = 2.93; Fig. 3b; see Supplementary Fig. 1 for individual participant EMG curves) during walking at the same speed. The variance in muscle activity of the soleus, tibialis anterior, vastus lateralis, and semitendinosus explained by one muscle synergy decreased by 7 ± 3% (p = 0.006, d = −2.32; Fig. 3c). When compared to age- and GMFCS-matched control data (Supplementary Table 1), exo-therapy resulted in greater reduction in VAF1 than both SEMLS (p = 0.0004) and SDR procedures (p = 0.0003) (Supplementary Fig. 2).
Figure 3. Neuromuscular outcome measures.
Individual (color-coded circles) and group mean (bars) pre- and post-exo-therapy neuromuscular variables, including a) co-contraction index (CCI) between the soleus and tibialis anterior; b) the relationship of the experimental soleus activation profile to a typical activation profile at a non-dimensional speed of 0.25; c) variance in muscle activity of the soleus, tibialis anterior, vastus lateralis, and semitendinosus that could be explained by one muscle synergy (VAF1). ‘Typical’ values were calculated using a publicly available dataset [30] of individuals walking at a non-dimensional speed of 0.25.
Gait efficiency
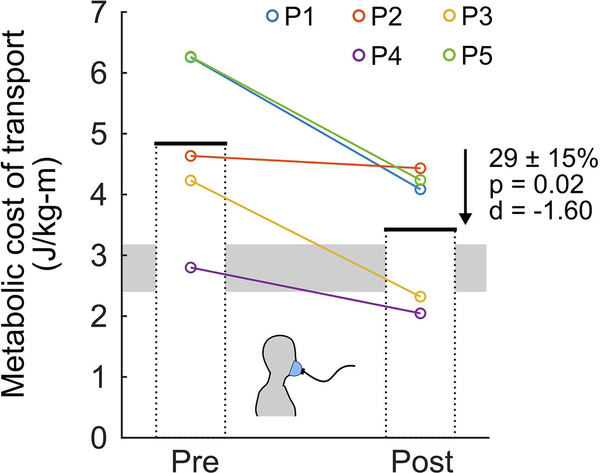
Whole-body center of mass potential-kinetic energy exchange recovery increased by 58 ± 34% (p = 0.04, d = 1.57; Fig. 4; see Supplementary Fig. 3 for individual energy curves) when walking at the same preferred speed, with a pre-assessment average of 30% and a post-assessment average of 47% (typically developing average is 66%). Metabolic cost of transport decreased by 29 ± 15% (p = 0.02, d = −1.60; Fig. 5, see Supplemental Fig. 4 for individual metabolic curves) when walking at the same speed.
Figure 5. Metabolic cost.
Individual (color-coded circles) and group mean (bars) pre- and post-exo-therapy metabolic cost of transport, representing the body-mass-normalized metabolic energy required to walk a unit distance. Both pre- and post-measures were collected at the pre-exo-therapy preferred walking speed on the treadmill.
Discussion
Our results support the hypotheses that there would be improvements in neuromuscular control after this exo-therapy training protocol (Fig. 3), translating to improvements in mechanical and metabolic efficiency (Figs. 4,5). In children with CP, ankle power is significantly reduced (Olney et al., 1990), potentially due to ineffective muscle activation profiles and co-contraction. In our prior validation study of the resistance controller, we observed a significant decrease in ankle co-contraction when individuals walked with resistance (B.C. Conner et al., 2020). The present study found that there was a carry-over of this desirable reduction in co-contraction to walking without the device following exo-therapy. We theorize that reductions in co-contraction may be due to improvement in reciprocal inhibition (Fig. 3a), which is impaired in children with CP (Leonard et al., 2008). We also observed a significant increase in the similarity of our participants’ more-affected limb soleus muscle activity profiles compared to the activation pattern from unimpaired individuals (Fig. 3b), reflecting improved activation timing.
We found a modest, but significant reduction in the variance in muscle activity that can be explained by one muscle synergy (VAF1) following exo-therapy (Fig. 3c), which suggests the potential for improved motor control complexity. Smaller VAF1 in children with CP has been shown to be a strong predictor of positive treatment outcomes, independent of the treatment (Schwartz et al., 2016).
Exo-therapy was designed to capitalize on the core principles of motor learning, including task-specificity, repetition, and active engagement (Winstein et al., 2014). Our wearable system allowed participants to train within the task-specific, functional context of walking, and achieve repetitive, high-volume practice. Applying resistance to plantar flexion necessitated active user engagement to maintain speed on the treadmill. Additionally, the adaptive nature of the proportional joint-moment control scheme made it so that resistance was immediately responsive to user input, creating an experience that fostered active engagement while still being feasible for various levels of motor function (i.e., GMFCS levels I and II). This was supported by the positive user experience when training with the device.
Afferent signals from load receptors play a critical role in muscle activation timing while walking (Bastiaanse et al., 2000), and the phase-specific resistance of this intervention may have served as a supraphysiologic signal for the plantar flexors to fire at the appropriate time. Motor control theory also dictates that the modular recruitment of muscles (i.e., muscle synergies) become more specific with greater biomechanical constraints (Ting et al., 2015). With resistance as a new biomechanical constraint, there may have been a demand for greater precision in motor control by the participants, necessitating increased neural complexity as indicated by the significant changes in VAF1. These features of exo-therapy may have worked together to facilitate rapid motor learning for improved neuromuscular control. Exo-therapy may have potential to facilitate the treatment of walking disability across the broad spectrum of neurological disorders.
This study had several limitations. First, we did not include a direct control group to isolate the effects of exo-therapy independent of structured treadmill walking, which should be considered when interpreting our findings. However, the 20 minutes of training completed during each visit was likely not a significant departure from typical walking volume. To the best of our knowledge, no prior studies of treadmill-only training in CP have reported similar findings to those reported here. Second, as a feasibility and preliminary assessment study, we had a limited sample size. Despite the limited sample, there were very large effect sizes, and the number of participants closely matches those of other device-centered neurorehabilitation studies (Burdea et al., 2013; Kang et al., 2017). Third, the device’s calf cuff prevented recording of gastrocnemius activity, and given its unique role during walking (Gottschall and Kram, 2003), the response of this muscle to training should be explored in future studies. Finally, proper engagement with the exoskeleton device required some verbal cues. However, the use of verbal cues is consistent with other studies that have investigated robotic gait training paradigms for children with CP (Druzbicki et al., 2013; Smania et al., 2011; Wallard et al., 2018), and seems to be a necessary feature for teaching individuals effective engagement with a novel device.
Conclusion
The findings from this study indicate the potential for treadmill training with exo-therapy to positively affect neuromuscular function of the ankle musculature in children with CP. We observed improved soleus activation timing and coordination, improved energy transfer, reduced co-contraction, and increased complexity of neuromuscular control. This novel training modality could supplement the current standard of care for individuals with CP, offering increased access to targeted neuromuscular rehabilitation.
Supplementary Material
Supplementary Analysis 1. Description of supplemental retrospective comparison.
Supplementary Figure 1. Individual soleus activation curves.
Supplementary Figure 2. Retrospective comparison of changes in motor control complexity.
Supplementary Figure 3. Individual energy recovery curves.
Supplementary Figure 4. Individual metabolic cost of transport curves.
Supplementary Table 1. Individual matched-control participant characteristics.
Supplementary User Experience results
Supplementary Video 1. Pre- and post-intervention scaled musculoskeletal models in OpenSim at preferred walking speed.
Acknowledgments
This research was supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R03HD094583. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported in part by the University of Arizona College of Medicine – Phoenix MD/PhD Program. The authors would like to thank Nushka Remec, Emily Frank, and Elizabeth Orum for their assistance with data collections, and James Babers and Leah Liebelt for their assistance with device manufacturing. The authors would also like to thank the participants and their families for their involvement in the study.
Conflict of interest statement
ZFL is a named inventor on a pending utility patent application that describes the robotic device utilized in the study. ZFL is a co-founder of a company seeking to commercialize the device. No other authors have competing interests to declare.
Abbreviations:
- CP
Cerebral palsy
- SEMLS
Single event multi-level surgery
- SDR
Selective dorsal rhizotomy
- GMFCS
Gross Motor Function Classification System
- Exo-therapy
Exoskeleton ankle resistance therapy
- EMG
Electromyography
- SOL
Soleus
- TA
Tibialis anterior
- VL
Vastus lateralis
- ST
Semitendinosus
- CCI
Co-contraction index
- R
Pearson product moment correlation coefficient
- VAF1
Variance in muscle activity accounted for by one muscle synergy
- NNMF
Non-negative matrix factorization
- COM
Center of mass
- PE
Potential energy
- KE
Kinetic energy
- ER
Mechanical energy recovery
- d
Cohen’s d
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anttila H, Autti-Rämö I, Suoranta J, Mäkelä M, Malmivaara A, 2008. Effectiveness of physical therapy interventions for children with cerebral palsy: A systematic review. BMC Pediatr. 8, 14. 10.1186/1471-2431-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaanse CM, Duysens J, Dietz V, 2000. Modulation of cutaneous reflexes by load receptor input during human walking. Exp. Brain Res. 135, 189–198. 10.1007/s002210000511 [DOI] [PubMed] [Google Scholar]
- Bennett BC, Abel MF, Wolovick A, Franklin T, Allaire PE, Kerrigan DC, 2005. Center of Mass Movement and Energy Transfer During Walking in Children With Cerebral Palsy. Arch. Phys. Med. Rehabil. 86, 2189–2194. 10.1016/J.APMR.2005.05.012 [DOI] [PubMed] [Google Scholar]
- Brockway JM, 1987. Derivation of formulae used to calculate energy expenditure in man. Hum. Nutr. Clin. Nutr 41, 463–71. [PubMed] [Google Scholar]
- Burdea GC, Cioi D, Kale A, Janes WE, Ross SA, Engsberg JR, 2013. Robotics and gaming to improve ankle strength, motor control, and function in children with cerebral palsy--a case study series. IEEE Trans. Neural Syst. Rehabil. Eng. 21, 165–173. 10.1109/TNSRE.2012.2206055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna GA, Heglund NC, Taylor CR, 1977. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am. J. Physiol. - Regul. Integr. Comp. Physiol 2. 10.1152/ajpregu.1977.233.5.r243 [DOI] [PubMed] [Google Scholar]
- Cheung VCK, Turolla A, Agostini M, Silvoni S, Bennis C, Kasi P, Paganoni S, Bonato P, Bizzi E, 2012. Muscle synergy patterns as physiological markers of motor cortical damage. Proc. Natl. Acad. Sci. U. S. A 109, 14652–6. 10.1073/pnas.1212056109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BC, Pierce JR, Manini TM, Ploutz-Snyder LL, 2007. Effect of prolonged unweighting of human skeletal muscle on neuromotor force control. Eur. J. Appl. Physiol 100, 53–62. 10.1007/s00421-007-0399-6 [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power for the Behavioral Sciences, 2nd ed. Lawrence Erlbaum Associates, Hillsdale. [Google Scholar]
- Conner BC, Luque J, Lerner ZF, 2020. Adaptive Ankle Resistance from a Wearable Robotic Device to Improve Muscle Recruitment in Cerebral Palsy. Ann. Biomed. Eng. 48, 1309–1321. 10.1007/s10439-020-02454-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner Benjamin C., Remec NM, Orum EK, Frank EM, Lerner Z, 2020. Wearable adaptive resistance training improves ankle strength, walking efficiency and mobility in cerebral palsy: a pilot clinical trial. IEEE Open J. Eng. Med. Biol 1–1. 10.1109/OJEMB.2020.3035316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG, 2007. OpenSim: Open-Source Software to Create and Analyze Dynamic Simulations of Movement. IEEE Trans. Biomed. Eng. 54, 1940–1950. 10.1109/TBME.2007.901024 [DOI] [PubMed] [Google Scholar]
- Dietz V, Berger W, 2008. Cerebral Palsy and Muscle Transformation. Dev. Med. Child Neurol 37, 180–184. 10.1111/j.1469-8749.1995.tb11987.x [DOI] [PubMed] [Google Scholar]
- Druzbicki M, Rusek W, Snela S, Dudek J, Szczepanik M, Zak E, Durmala J, Czernuszenko A, Bonikowski M, Sobota G, Drużbicki M, Rusek W, Snela S, Dudek J, Szczepanik M, Zak E, Durmala J, Czernuszenko A, Bonikowski M, Sobota G, Druzbicki M, Rusek W, Snela S, Dudek J, Szczepanik M, Zak E, Durmala J, Czernuszenko A, Bonikowski M, Sobota G, 2013. Functional effects of robotic-assisted locomotor treadmill therapy in children with cerebral palsy. J. Rehabil. Med 45, 358–363. 10.2340/16501977-1114 [DOI] [PubMed] [Google Scholar]
- Gage JR, Schwartz MH, Koop SE, Novacheck TF, 2009. The identification and treatment of gait problems in cerebral palsy, 2nd ed. Mac Keith Press, London. [Google Scholar]
- Ghasemi A, Zahediasl S, 2012. Normality tests for statistical analysis: a guide for non-statisticians. Int. J. Endocrinol. Metab. 10, 486–9. 10.5812/ijem.3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschall JS, Kram R, 2003. Energy cost and muscular activity required for propulsion during walking. J. Appl. Physiol. 94, 1766–1772. 10.1152/japplphysiol.00670.2002 [DOI] [PubMed] [Google Scholar]
- Graham HK, Rosenbaum P, Paneth N, Dan B, Lin J-P, Damiano DL, Becher JG, Gaebler-Spira D, Colver A, Reddihough DS, Crompton KE, Lieber RL, 2016. Cerebral palsy. Nat. Rev. Dis. Prim. 2, 15082. 10.1038/nrdp.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hägg G, 1999. European Recommendations for Surface ElectroMyoGraphy Results of the SENIAM project. Roessingh Research and Development. [Google Scholar]
- Hof AL, 2018. Scaling and Normalization, in: Wolf SI, Muller B (Eds.), Handbook of Human Motion. Springer, New York, pp. 295–305. [Google Scholar]
- Hof AL, Elzinga H, Grimmius W, Halbertsma JPK, 2002. Speed dependence of averaged EMG profiles in walking. Gait Posture 16, 78–86. 10.1016/s0966-6362(01)00206-5 [DOI] [PubMed] [Google Scholar]
- Johnson DL, Miller F, Subramanian P, Modlesky CM, 2009. Adipose tissue infiltration of skeletal muscle in children with cerebral palsy. J. Pediatr. 154, 715–20. 10.1016/j.jpeds.2008.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Martelli D, Vashista V, Martinez-Hernandez I, Kim H, Agrawal SK, 2017. Robot-driven downward pelvic pull to improve crouch gait in children with cerebral palsy. Sci. Robot 2, eaan2634. 10.1126/scirobotics.aan2634 [DOI] [PubMed] [Google Scholar]
- Khamis S, Herman T, Krimus S, Danino B, 2018. Is functional electrical stimulation an alternative for orthotics in patients with cerebral palsy? A literature review. Eur. J. Paediatr. Neurol. 22, 7–16. 10.1016/j.ejpn.2017.10.004 [DOI] [PubMed] [Google Scholar]
- Knarr BA, Zeni JA, Higginson JS, 2012. Comparison of electromyography and joint moment as indicators of co-contraction. J. Electromyogr. Kinesiol. 22, 607–611. 10.1016/J.JELEKIN.2012.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krigger KW, 2006. Cerebral Palsy: An Overview. Am. Fam. Physician 73, 91–100. [PubMed] [Google Scholar]
- Lee DD, Seung HS, 1999. Learning the parts of objects by non-negative matrix factorization. Nature 401, 788–791. 10.1038/44565 [DOI] [PubMed] [Google Scholar]
- Leonard CT, Moritani T, Hirschfeld H, Forssberg H, 2008. Deficits in reciprocal inhibition of children with cerebral palsy as revealed by H reflex testing. Dev. Med. Child Neurol. 32, 974–984. 10.1111/j.1469-8749.1990.tb08120.x [DOI] [PubMed] [Google Scholar]
- Lerner ZF, Damiano DL, Bulea TC, 2017. The Effects of Exoskeleton Assisted Knee Extension on Lower-Extremity Gait Kinematics, Kinetics, and Muscle Activity in Children with Cerebral Palsy. Sci. Rep. 7, 13512. 10.1038/S41598-017-13554-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan CP, Neptune RR, Clark DJ, Kautz SA, 2010. Modular control of human walking: Adaptations to altered mechanical demands. J. Biomech 43, 412–419. 10.1016/J.JBIOMECH.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaka MM, 2012. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 24, 69–71. [PMC free article] [PubMed] [Google Scholar]
- Novak I, Mcintyre S, Morgan C, Campbell L, Dark L, Morton N, Stumbles E, Wilson S-A, Goldsmith S, 2013. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev. Med. Child Neurol. 55, 885–910. 10.1111/dmcn.12246 [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Lichtwark GA, Carroll TJ, Barber LA, 2020. Impact of Lower Limb Active Movement Training in Individuals With Spastic Type Cerebral Palsy on Neuromuscular Control Outcomes: A Systematic Review. Front. Neurol 11, 581892. 10.3389/fneur.2020.581892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney SJ, MacPhail HA, Hedden DM, Boyce WF, 1990. Work and Power in Hemiplegic Cerebral Palsy Gait. Phys. Ther 70, 431–438. 10.1093/ptj/70.7.431 [DOI] [PubMed] [Google Scholar]
- Rose J, Gamble JG, Burgos A, Medeiros J, Haskell WL, 1990. Energy expenditure index of walking for normal children and for children with cerebral palsy. Dev. Med. Child Neurol. 32, 333–340. 10.1111/j.1469-8749.1990.tb16945.x [DOI] [PubMed] [Google Scholar]
- Rudolph KS, Axe MJ, Snyder-Mackler L, 2000. Dynamic stability after ACL injury: who can hop? Knee Surgery, Sport. Traumatol. Arthrosc 8, 262–269. 10.1007/s001670000130 [DOI] [PubMed] [Google Scholar]
- Schwartz MH, 2007. Protocol changes can improve the reliability of net oxygen cost data. Gait Posture 26, 494–500. 10.1016/j.gaitpost.2007.07.014 [DOI] [PubMed] [Google Scholar]
- Schwartz MH, Rozumalski A, Steele KM, 2016. Dynamic motor control is associated with treatment outcomes for children with cerebral palsy. Dev. Med. Child Neurol. 58, 1139–1145. 10.1111/dmcn.13126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman BR, Goudriaan M, Desloovere K, Schwartz MH, Steele KM, 2019. Muscle synergies demonstrate only minimal changes after treatment in cerebral palsy. J. Neuroeng. Rehabil 16, 46. 10.1186/s12984-019-0502-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smania N, Bonetti P, Gandolfi M, Cosentino A, Waldner A, Hesse S, Werner C, Bisoffi G, Geroin C, Munari D, 2011. Improved gait after repetitive locomotor training in children with cerebral palsy. Am. J. Phys. Med. Rehabil 90, 137–149. 10.1097/PHM.0b013e318201741e [DOI] [PubMed] [Google Scholar]
- Steele KM, Rozumalski A, Schwartz MH, 2015. Muscle synergies and complexity of neuromuscular control during gait in cerebral palsy. Dev. Med. Child Neurol. 57, 1176–1182. 10.1111/dmcn.12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland DH, Cooper L, Daniel D, 1980. The role of the ankle plantar flexors in normal walking. J. Bone Joint Surg. Am 62, 354–63. [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Archer SA, Nagarajan L, Mastaglia FL, 2001. Differences in sensory and motor cortical organization following brain injury early in life. Ann. Neurol. 49, 320–327. 10.1002/ana.68 [DOI] [PubMed] [Google Scholar]
- Ting LH, Chiel HJ, Trumbower RD, Allen JL, McKay JL, Hackney ME, Kesar TM, 2015. Neuromechanical Principles Underlying Movement Modularity and Their Implications for Rehabilitation. Neuron 86, 38–54. 10.1016/J.NEURON.2015.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW, 1977. Exploratory Data Analysis. Addison-Wesley, Reading. [Google Scholar]
- Unnithan VB, Dowling JJ, Frost G, Bar-Or O, 1996. Role of cocontraction in the O2 cost of walking in children with cerebral palsy. Med. Sci. Sports Exerc. 28, 1498–504. 10.1097/00005768-199612000-00009 [DOI] [PubMed] [Google Scholar]
- Wallard L, Dietrich G, Kerlirzin Y, Bredin J, 2018. Effect of robotic-assisted gait rehabilitation on dynamic equilibrium control in the gait of children with cerebral palsy. Gait Posture 60, 55–60. 10.1016/j.gaitpost.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Winstein C, Lewthwaite R, Blanton SR, Wolf LB, Wishart L, 2014. Infusing motor learning research into neurorehabilitation practice: a historical perspective with case exemplar from the accelerated skill acquisition program. J. Neurol. Phys. Ther 38, 190–200. 10.1097/NPT.0000000000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Analysis 1. Description of supplemental retrospective comparison.
Supplementary Figure 1. Individual soleus activation curves.
Supplementary Figure 2. Retrospective comparison of changes in motor control complexity.
Supplementary Figure 3. Individual energy recovery curves.
Supplementary Figure 4. Individual metabolic cost of transport curves.
Supplementary Table 1. Individual matched-control participant characteristics.
Supplementary User Experience results
Supplementary Video 1. Pre- and post-intervention scaled musculoskeletal models in OpenSim at preferred walking speed.