Abstract
Background:
Understanding advances in the care and treatment of adults with HIV as well as remaining gaps requires comparing differences in mortality between people entering care for HIV and the general population.
Objective:
To assess the extent to which mortality among people entering HIV care in the United States is elevated over mortality among matched individuals in the general US population and trends in this mortality difference over time.
Design:
Observational cohort study
Setting:
13 US North American AIDS Cohort Collaboration on Research and Design sites
Participants:
82,766 adults entering HIV clinical care between 1999 and 2017 matched to a subset of the US population matched on calendar time, age, sex, race/ethnicity, and county using US mortality and population data compiled by the National Center for Health Statistics.
Measurements:
5-year all-cause mortality, estimated using the Kaplan-Meier estimator of the survival function.
Results:
Overall 5-year mortality was 10.6%, while mortality among the matched US population was 2.9%, for a difference of 7.7 percentage points (95% confidence interval: 7.4, 7.9). This difference decreased over time, from 11.1 percentage points among those entering care between 1999 and 2004 to 2.7 percentage points among those entering care between 2011 and 2017.
Limitations:
Matching on available covariates may have failed to account for differences in mortality due to sociodemographic factors rather than due to consequences of HIV infection and other modifiable factors.
Conclusion:
Mortality among people entering HIV care decreased dramatically between 1999 and 2017, although those entering HIV care remained at modestly higher risk of death in the years after starting care than comparable individuals in the general US population.
Primary Funding Source:
National Institutes for Health
INTRODUCTION
Understanding progress and gaps in the care and treatment of adults with HIV requires monitoring differences in mortality between people with and without HIV infection. HIV-related mortality has been declining since the introduction of effective treatment in 1996 due to improving treatment options and evolving care guidelines(1-9), but the extent to which people entering HIV care in the United States have a higher risk of mortality over the following years compared to peers in the general population over the same time period remains unclear. Prior work to elucidate temporal trends in mortality among adults with HIV over the years after entering care has not made comparison to the general US population.
To understand differences in mortality between people entering HIV care and the general population, we estimated the cumulative risk of all-cause mortality over 5 years among 82,766 people entering such care between 1999 and 2017 at US clinical sites affiliated with the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). The NA-ACCORD pooling project is representative of people in care for HIV in the US and leverages data collection mechanisms that are well-integrated into clinical HIV care (10).
Because demographic groups with historically elevated mortality also have an elevated risk of HIV infection (11), we compared mortality among people entering HIV care to mortality among the subset of the US population matched to NA-ACCORD participants on key demographic variables constructed from data compiled by the National Center for Health Statistics (NCHS). We also assessed whether the mortality difference between people entering HIV care and their matched counterparts in the general US population varied by demographic characteristics and by calendar time.
METHODS
Data sources
Population and mortality data
We obtained data from the NCHS Detailed Mortality Files on the 47,812,945 deaths occurring in the United States for the years 1999 to 2017 (12). We aggregated the number of deaths for each year for strata defined by age, sex, race/ethnicity (non-Hispanic white, non-Hispanic Black, non-Hispanic American Indian, non-Hispanic Asian/Pacific Islander, or Hispanic), and county of residence. We merged the mortality data with census data provided by the NCHS describing the estimated population size in each of the strata defined above for all US counties over the relevant time period. These merged data, containing both the number of deaths and population size by year, age, sex, race/ethnicity, and county of residence, composed our “population and mortality” data.
HIV cohort data
Data on adults entering HIV care in the United States were obtained from NA-ACCORD, the largest consortium of HIV cohorts in the US and Canada (10). NA-ACCORD includes over 20 contributing single and multisite clinical and interval epidemiologic HIV cohorts. Contributing sites report individual, standardized, data on demographics, medications, diagnoses, laboratory values, and vital status to a central Data Management Core, where they are pooled, harmonized, and undergo quality control. NA-ACCORD has demonstrated that the demographic characteristics of its US participants are similar to that of newly diagnosed persons with HIV infection captured by the US Centers for Disease Control and Prevention’s HIV surveillance system (13). Participants were enrolled in NA-ACCORD-contributing cohorts if written informed consent or a waiver of consent were obtained, and NA-ACCORD enrollment criteria include 2 HIV care visits within 12 months. For this analysis, we included data from the 13 US clinical cohorts that contribute data to NA-ACCORD. Human subjects research activities have been approved by each cohort’s local Institutional Review Boards and the Johns Hopkins School of Medicine.
Among NA-ACCORD participants, date of death was obtained through participating cohorts’ regular queries to the Social Security Death Index, the National Death Index, and state vital statistics registries. Age at entry into care, birth sex, race, ethnicity, transmission risk factor, and ZIP Code, CD4 cell count, and viral load at enrollment, were abstracted from clinical records. We harmonized covariate data between NA-ACCORD and the population and mortality file for the US population by collapsing race categories in NA-ACCORD to match those provided in the NCHS data. Moreover, in NA-ACCORD, place of residence was provided using 3- or 5-digit ZIP Codes rather than counties. To harmonize the population and mortality data for the general population described below, we mapped 5-digit ZIP Codes to counties using ZIP Code-to-county crosswalk tables provided by the US Department of Housing and Urban Development (14). When only 3-digit ZIP Codes were provided, we mapped the 3-digit ZIP Code to a list of possible counties using the same crosswalk tables.
We included 93,408 adults 18 years of age or older who newly enrolled in HIV care at a participating site in the United States during or after 1999. To identify patients newly enrolled in care at cohort entry, we selected those who enrolled after the cohort was established, did not have a recorded date of antiretroviral therapy (ART) initiation or AIDS diagnosis ≥14 days prior to the cohort enrollment date, and did not have a suppressed viral load (<75 copies/mL) measured between 30 days prior and 7 days after cohort enrollment. We additionally excluded 3 intersex patients and 10,599 patients (11%) due to missing data, including race/ethnicity (n=4813), ZIP Code at entry into care (n=6142), and dates of death (n=40), for a final analytic sample of 82,766 patients.
Statistical methods
Participants in NA-ACCORD were followed from the date of entry into care until death or administrative censoring at 5 years after entry into care, cohort closure, or 31 December 2017 (whichever occurred first). We estimated mortality risk (15) over the 5-year period after entry into HIV care at participating sites using the Kaplan-Meier estimator (16) and standard errors using Greenwood’s variance estimator,(17) implemented using the ‘survival’ package in R.
Our goal was to compare observed cumulative mortality for eligible people entering care at an NA-ACCORD site with their expected mortality had they had the same mortality risk as people of the same age in the same year and with the same demographics (sex, race/ethnicity, and county of residence) in the general population. To compute expected mortality, we created a synthetic cohort (18) of all people in the United States of the same age in the same year matched to each eligible participant starting care at an NA-ACCORD site on sex, race/ethnicity, and county of residence using the population and mortality data described above. We computed expected mortality in each synthetic cohort using the Kaplan-Meier estimator. The overall expected mortality was the average of the expected mortality risk estimated among the synthetic cohort matched to each individual in NA-ACCORD. Technical details are provided in the Supplementary Material.
We compared mortality 5 years after entry into HIV care for participants in NA-ACCORD to mortality over the same time period among the matched US population using risk differences and ratios. We also stratified participants by calendar period of study entry (1999 – 2004, 2005 – 2010, and 2011 – 2017) and compared mortality separately within each stratum. In addition, we stratified the 5-year mortality differences across demographic variables to examine differential trends in these differences over calendar years. Throughout, standard errors for the differences and ratios were estimated using the delta method (19).
To further examine temporal trends, we also stratified 1-, 2-, and 5-year mortality among people entering care in NA-ACCORD and the matched general population by year of entry into care.
All analyses were conducted using SAS 9.4 (Cary, NC) or R 3.6.1.
Role of the funding source
This study was supported by the US National Institutes for Health, US Centers for Disease Control and Prevention, US Agency for Healthcare Research and Quality, US Health Resources and Services Administration, Grady Health System, Canadian Institutes of Health Research, Ontario Ministry of Health and Long Term Care, and the Government of Alberta, Canada. Funders played no role in the design, conduct, or reporting of this analysis.
RESULTS
Of the 82,766 eligible participants in NA-ACCORD, 84% were male, 46% were non-Hispanic Black, 16% were Hispanic, and the median age at entry into care was 42 years (interquartile range [IQR]: 33, 50) (Table 1). At entry into HIV care, eligible participants resided 324 counties across the US. All regions of the country were represented, with most participants seeking care in the Northeast/mid-Atlantic (35%) or South (34%). Until the last time period (2011 to 2017), the majority of those entering care were between the ages of 35 and 54, but the proportion of those under age 35 and ages 55 and older increased over time. Participants entered the study with a range of CD4 cell counts, and measured CD4 cell count at baseline increased over calendar years, from a median of 284 × 106 cells/L (IQR: 105, 479) among participants entering NA-ACCORD between 1999 and 2004 to 366 × 106 cells/L (IQR: 175, 558) among participants entering between 2011 and 2017.
Table 1.
Characteristics of 82,766 eligible people entering care for HIV at a US NA-ACCORD clinical site between 1999 and 2017, stratified by calendar period at entry into care.
| Overall (n = 82,766) |
Entered care between 1999 and 2004 (n = 32,588) |
Entered care between 2005 and 2010 (n = 27,104) |
Entered care between 2011 and 2017 (n = 23,074) |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | n | % | n | % | n | % | n | % |
| Race/Ethnicity | ||||||||
| NH White | 29084 | 35.1 | 11994 | 36.8 | 9099 | 33.6 | 7991 | 34.6 |
| NH Black | 38263 | 46.2 | 15133 | 46.4 | 12854 | 47.4 | 10276 | 44.5 |
| NH American Indian | 350 | 0.4 | 131 | 0.4 | 118 | 0.4 | 101 | 0.4 |
| NH Asian/Pacific Islander | 1463 | 1.8 | 379 | 1.2 | 468 | 1.7 | 616 | 2.7 |
| Hispanic | 13606 | 16.4 | 4951 | 15.2 | 4565 | 16.8 | 4090 | 17.7 |
| Age at study entry | ||||||||
| 18-34 | 23169 | 28.0 | 7219 | 22.2 | 7564 | 27.9 | 8386 | 36.3 |
| 35-54 | 47067 | 56.9 | 21261 | 65.2 | 15221 | 56.2 | 10585 | 45.9 |
| 55+ | 12530 | 15.1 | 4108 | 12.6 | 4319 | 15.9 | 4103 | 17.8 |
| Sex | ||||||||
| Female | 13448 | 16.2 | 5412 | 16.6 | 4674 | 17.2 | 3362 | 14.6 |
| Male | 69318 | 83.8 | 27176 | 83.4 | 22430 | 82.8 | 19712 | 85.4 |
| US Region of residencea | ||||||||
| Northeast and mid-Atlantic | 29016 | 35.1 | 11018 | 33.8 | 9458 | 34.9 | 8540 | 37.0 |
| South | 27909 | 33.7 | 10591 | 32.5 | 9540 | 35.2 | 7778 | 33.7 |
| Midwest and North Central | 5332 | 6.4 | 2844 | 8.7 | 1471 | 5.4 | 1017 | 4.4 |
| West | 20509 | 24.8 | 8135 | 25.0 | 6635 | 24.5 | 5739 | 24.9 |
| CD4 cell count at entry into careb | ||||||||
| 750+ | 4483 | 5.4 | 1396 | 4.3 | 1463 | 5.4 | 1624 | 7.0 |
| 500-749 | 9250 | 11.2 | 2941 | 9.0 | 3171 | 11.7 | 3138 | 13.6 |
| 350-499 | 10047 | 12.1 | 3296 | 10.1 | 3601 | 13.3 | 3150 | 13.7 |
| 200-349 | 11083 | 13.4 | 4030 | 12.4 | 4020 | 14.8 | 3033 | 13.1 |
| <200 | 17552 | 21.2 | 7139 | 21.9 | 6242 | 23.0 | 4171 | 18.1 |
| Missing | 30351 | 36.7 | 13786 | 42.3 | 8607 | 31.8 | 7958 | 34.5 |
| Transmission risk factorc | ||||||||
| MSM | 28782 | 34.8 | 8573 | 26.3 | 9599 | 35.4 | 10610 | 46.0 |
| IDU | 14746 | 17.8 | 8279 | 25.4 | 4218 | 15.6 | 2249 | 9.7 |
NH: Non-Hispanic; MSM: Men who have sex with men; IDU: injection drug use
Region of residence was categorized by the first digit of the ZIP Code such that those beginning with 0, 1, or 2 were labeled “Northeast or mid-Atlantic,” those beginning with 3 or 7 were labeled “South”, those beginning with 4, 5, or 6 were labeled “Midwest and North Central”, and those beginning with 8 or 9 were labeled “West”
Baseline CD4 cell count (in cells/mm3) was defined as the last CD4 cell count measured between 30 days prior and 14 days after a participant’s enrollment date
Transmission risk factors were not mutually exclusive (i.e., some participants appear in both “MSM” and “IDU” rows)
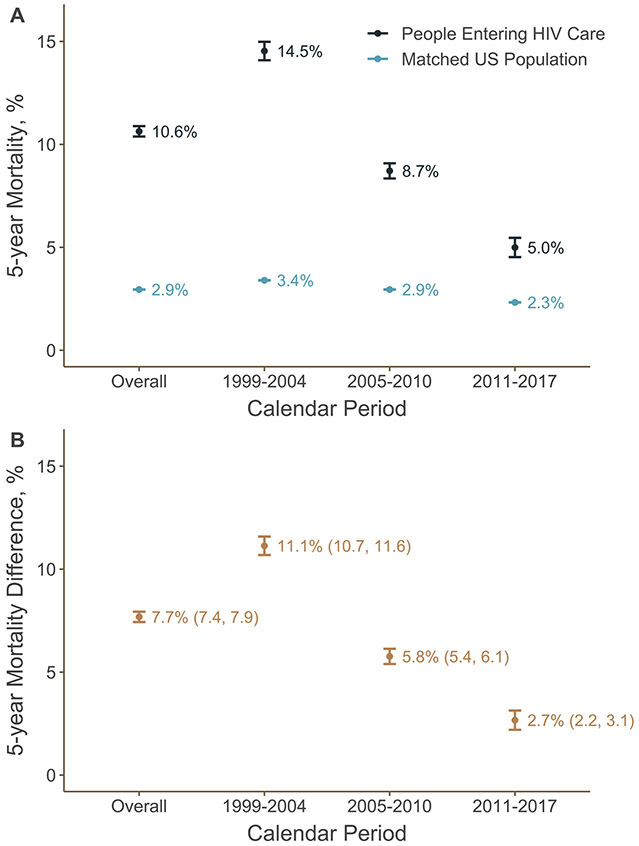
Overall, 7796 deaths occurred within 5 years of entry into care among eligible participants in NA-ACCORD. The 5-year mortality among people entering HIV care was 10.6%, while mortality among the matched US population was 2.9%. (Figure 1A). Mortality over the first 5 years after entry into HIV care declined substantially over time, from 14.5% among those entering care between 1999 and 2004 to 5.0% among those entering care between 2011 and 2017. Over the same time period, mortality declined less in the matched US population, from 3.4% between 1999 and 2004 to 2.3% between 2011 and 2017. Kaplan-Meier curves for the mortality risk functions are presented in Supplemental Figures 1 and 2.
Figure 1.
5-year mortality (Panel A) and mortality differences in percentage points (Panel B) comparing mortality among 82,766 people entering care for HIV at a US NA-ACCORD clinical site between 1999 and 2004 (n = 32,588), 2005 and 2010 (n = 27,104), and 2011 and 2017 (n = 23,074) and a matched subset of the general US population. Bars represent 95% confidence intervals.
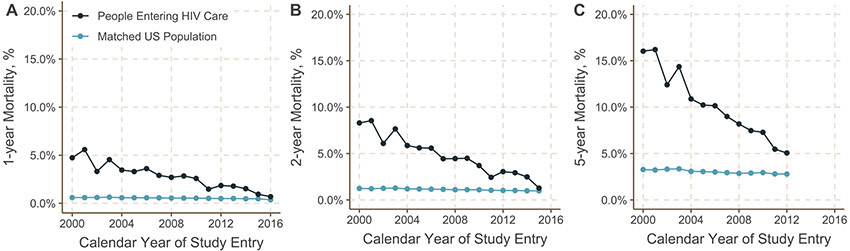
The difference in 5-year mortality between people entering HIV care and the matched US population was 7.7 percentage points (95% confidence interval [CI]: 7.4, 7.9) (Figure 1B) while the ratio was 3.60 (95% CI: 3.52, 3.69) (Supplemental Table 1). The dramatic decrease in 5-year mortality for those entering HIV care over calendar years coupled with the small decline in 5-year mortality over the same time period in the matched general population meant that the elevation in mortality for people with HIV fell over time. The mortality difference was largest among those entering care between 1999 and 2004 (difference = 11.1 percentage points; 95% CI: 10.7, 11.6) and fell to only 2.7 percentage points (95% CI: 2.2, 3.1) for those entering care between 2011 and 2017. For those entering care between 2011 and 2017, one and two year mortality continued to decline during this period, approaching the matched US population estimate by 2015 (Figure 3, Supplemental Figure 3).
Figure 3.
Mortality at 1 year (Panel A), 2 years (Panel B), and 5 years (Panel C) after entering HIV care among 82,766 people entering care for HIV at a US NA-ACCORD clinical site between 1999 and 2017 compared to mortality among a matched subset of the general US population, stratified by year of entry into care. The gap in 1-year mortality appears to close by the later years examined (panel A), but 5-year mortality remains elevated over the entire calendar period (panel C), suggesting that there may be factors that influence the effectiveness of care (e.g., treatment discontinuation, disengagement from care) and, therefore, increase mortality among those with HIV after the first year.
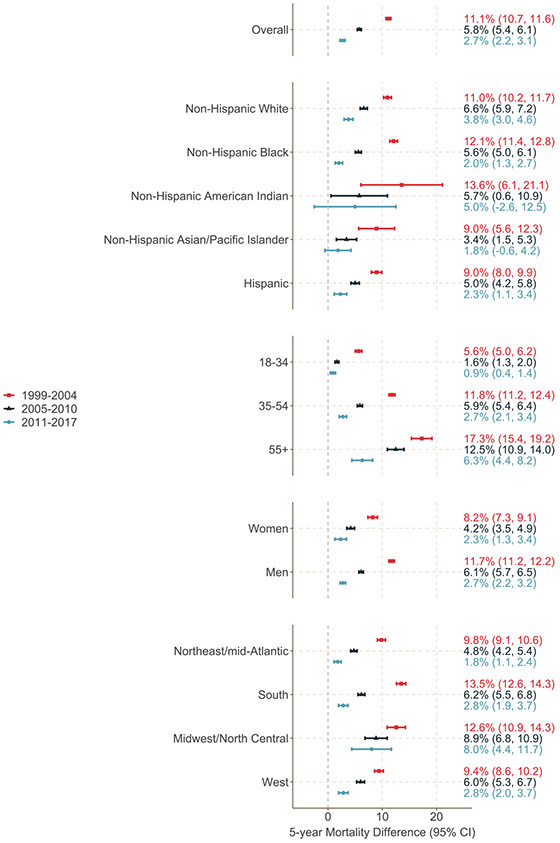
Patterns in mortality differences were similar across demographic groups defined by race, ethnicity, age, and sex (Figure 2; Supplemental Table 2). Notably, by the most recent calendar period, 5-year mortality among participants entering care between the ages of 18 and 34 was only modestly elevated compared to mortality among their peers in the matched US population (mortality difference: 0.9 percentage points; 95% CI: 0.4, 1.4). For older people entering HIV care (55+), the mortality difference also declined substantially over time but remained substantial in the most recent calendar period (6.3 percentage points; 95% CI 4.4, 8.2).
Figure 2.
Percentage point differences in 5-year mortality among 82,766 people entering care for HIV at a US NA-ACCORD clinical site between 1999 and 2004 (n = 32,588; red squares and bars), 2005 and 2010 (n = 27,104; black triangles and bars), and 2011 and 2017 (n = 23,074; blue circles and bars) and a matched subset of the general US population, overall and stratified by race/ethnicity, age, sex, and region.
5-year mortality among non-Hispanic Black people entering care at participating sites fell considerably over the calendar periods from 16.5% in the earliest period (1999 and 2004) to 4.9% in the most recent period (2011 and 2017), while mortality among non-Hispanic white people entering HIV care fell less dramatically (from 13.8% to 6.0% over the same period) (Supplemental Figure 4). Notably, by the last calendar time period, 5-year mortality among people entering care at study sites was lower among Black people than white people. Mortality in the matched general US population fell among both groups, though remained higher for non-Hispanic Black people than non-Hispanic white people in all calendar periods, such that the disparity in mortality between people entering HIV care and the general population was smaller for non-Hispanic Black people than non-Hispanic white people in the most recent calendar period.
Among those entering care between 1999 and 2004, the largest mortality differences between those with and without HIV were seen in the South (difference: 13.5 percentage points; 95% CI: 12.6, 14.3) (Supplemental Table 2). However, the South also saw the greatest reduction in disparity over time such that the difference in mortality between 2011 and 2017 was only 2.8 percentage points (95% CI: 1.9, 3.7).
The trends in 5-year mortality differences over calendar periods were significantly (p<0.05) different from 0 for all subgroups except non-Hispanic American Indians, for whom the sample size was small (Supplemental Table 2). Ratios comparing mortality by subgroup are presented in Supplemental Table 3.
Patterns in mortality and mortality differences, both overall and stratified by age group, were similar when results were stratified by individual year of study entry rather than calendar period (Figure 3 and Supplemental Figures 4 and 5). Mortality differences were smaller when compared after the first year or 2 years in care than at 5 years. Mortality differences and trends over time were attenuated when analysis was limited to participants entering care with CD4 cell counts >500 × 106 cells/L (n=13,673) (Supplemental Figure 6).
DISCUSSION
People entering HIV care in the United States had higher mortality over the subsequent years than people of similar demographics and geographic context in the general US population, but this disparity in mortality declined over calendar years between 1999 and 2017. By the latest calendar period examined (2011 – 2017), 5-year mortality for someone starting care for HIV was only 2.7 percentage points higher than 5-year mortality among people of the same age, sex, race/ethnicity, and county in the general population. In later years of the study, mortality risks for people in NA-ACCORD after 1 year in care were similar to 1-year mortality risks in the general population.
This decline in mortality among people with HIV likely reflects advances in HIV care and treatment (13,20), new guidelines indicating earlier treatment (21), greater engagement in care, higher levels of viral suppression (22), a trend towards linking people with HIV to care earlier in the course of infection (i.e., at higher CD4 cell counts) (23), and evolving patient characteristics in the cohort over time. These trends, specifically CD4 cell count at entry into care, engagement in care, and time to viral suppression, have been shown to differ by population subgroup (24-27). The confluence of these factors limits our ability to assign causes to the observed patterns. For example, the dramatic decline in mortality among Black people entering HIV care may have been due to improvements in care or could reflect trends in the demographic and clinical features of Black people enrolled in participating sites over time (such as age or timing of presentation to care).
Even in the era of safe, simple, and effective ART, mortality in the years after entering care for HIV may remain higher than mortality among similar people in the general population for at least four reasons. First, though universal immediate treatment (regardless of CD4 cell count) has been recommended in the US since 2012 (28), not everyone entering care for HIV able to start treatment immediately (23,29,30). For example, a recent analyses have reported that, even in high income countries, the median CD4 cell count at ART initiation was below 400 × 106 cells/L for both men and women (26, 31). In the universal treatment era, delays in starting ART are typically due to delays in HIV diagnosis and linkage to care. Second, treatment alone is not a panacea. Although new treatments are vastly improved, they remain imperfect, are only effective when adhered to, and may increase the risk of adverse events with prolonged exposure. With the important exception of long lasting ART, the majority of antiretroviral drugs must be taken every day. Nonadherence, either fleeting or sustained, or treatment discontinuation due to disengagement from care, limits the ability of these drugs to curb mortality. Moreover, treatment itself may play a role in other disease processes (32-38). Third, even in the presence of effective ART, HIV may play a role in non-AIDS related comorbidities and mortality, particularly among people who enter care with advanced immune suppression or at older ages (39-42). Fourth, people with HIV may have higher prevalence of other risk factors for mortality, such as smoking(43-45), substance use(46,47), and comorbidities(48-50).
Previous studies have illustrated that life expectancy among people with HIV is approaching, yet remains below, that of the general population (5,7,8,51), and that heterogeneity in life expectancy between subgroups exists(6). These studies typically compared life expectancy at a specific age (e.g., age 20) between people with and without HIV. But people are diagnosed with HIV at all ages, and it is unclear what an elevation in life expectancy at age 20 means for someone diagnosed with HIV in their 30s, 40s, or beyond. Unlike prior work that compared life expectancy, here we directly compared mortality for people entering HIV care with mortality among their age- (and other covariate-) matched peers in the general US population.
Comparing mortality between people starting HIV care and people of the same age in the general population directs focus to the time after entry into care, when clinicians can intervene on factors that may reduce mortality. Thus, this comparison can be used to guide a set of actionable interventions and assess the effects of such interventions. For example, using the current results as a reference, we could assess how the disparity in mortality between people with HIV and the general population might narrow or widen under new treatments or new strategies to address comorbidities. With appropriate data, additional comparisons that examine mortality after HIV seroconversion (rather than entry into care) could inform broader public health interventions, such as those aimed at accelerating diagnosis and linkage to care(52).
This study had limitations. Estimates of mortality among those entering care for HIV were limited to those entering care at participating NA-ACCORD sites, which may not be representative of all sites offering HIV clinical care in the United States(53). If people who inject drugs or other groups at higher risk for mortality are over represented in NA-ACCORD, our mortality estimates among people in NA-ACCORD may be higher than mortality among all people starting HIV care in the US. However, cohorts contributing to NA-ACCORD are diverse and representative of many HIV care settings(13). Moreover, we compared mortality after matching age, sex, race/ethnicity, and county to decrease differences in mortality associated with factors other than HIV(54), but both NA-ACCORD and data from NCHS lacked granular information on socioeconomic status and neighborhood. Moreover, we did not have information on smoking, substance use, or comorbidities, which may have been more prevalent in people with HIV(43,47,48) and likely varied over time. In addition, comparison of risks for some strata, such as NH American Indians, were based on small sample sizes in NA-ACCORD, leading to imprecise results.
Furthermore, while we matched on county of residence, counties are large and often diverse, meaning that county is likely to be an inadequate proxy for the attributes of “place” that affect mortality risk(55). This means that some of the difference in mortality between people entering HIV care and the matched general US population could be due to residual differences in geography not captured by the matching variables. Because HIV risk is likely to be spatially correlated with mortality within counties, but county is the most granular spatial unit available for this analysis, our reported mortality differences may overestimate the true difference in mortality between people entering HIV care and similar individuals in the general population. This issue may have been exacerbated when participants in NA-ACCORD had only 3-digit Zip Codes available. In these instances, participants were matched to residences of all counties intersecting the large area covered by the 3-digit Zip Code, which may have been heterogeneous with regard to mortality risk.
County of residence was also subject to misclassification, particularly if participants relocated during the course of the study or did not have long-term permanent residences. Such misclassification could produce bias in the estimated mortality differences, though we expect the proportion of patients affected by this misclassification to be small. Finally, NA-ACCORD may have failed to capture all deaths among participants. Linkage to the National Death Index and state vital status registries likely mitigates this limitation, but any delays in linking to these registries could distort trends in mortality.
Understanding differences in mortality between people entering HIV care and the matched US population is critical to monitor opportunities to improve HIV care. While these differences have declined dramatically in the era of modern treatments, gaps remain. These gaps could reflect the effects of prolonged immune deficiency in those who present late to care or persistent immune activation and subsequent end-stage chronic diseases even among those successfully treated. Antiretroviral medications have adverse effects that may contribute to mortality risk and the interplay between HIV and aging-related comorbidities and coinfections may accentuate differences in mortality, especially in older populations.(56-60) The uptake by clinicians and patients of standard preventative interventions, such as smoking cessation and lipid and cancer screening, may have lagged in people with HIV especially in earlier time periods and improved over time. Quantifying the elevation in mortality observed for people in care for HIV in the era of modern treatments will inform efforts to address both AIDS and non-AIDS related consequences of HIV infection and long-term ART.
Supplementary Material
ACKNOWLEDGEMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Centers for Disease Control and Prevention. This work was supported by National Institutes of Health through an administrative supplement to the University of North Carolina at Chapel Hill Center for AIDS Research (P30 AI50410) as well as grants K01AI125087, R01AI157758, R01HD093602, U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01DA011602, R01DA012568, R01 AG053100, R24AI067039, R34DA045592, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA03629, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24AA020794,U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR002378, Z01CP010214 and Z01CP010176; contracts CDC-200-2006-18797 and CDC-200-2015-63931 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; the Grady Health System; grants CBR-86906, CBR-94036, HCP-97105 and TGF-96118 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care, and the Government of Alberta, Canada. Additional support was provided by the National Institute Of Allergy And Infectious Diseases (NIAID), National Cancer Institute (NCI), National Heart, Lung, and Blood Institute (NHLBI), Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute for Mental Health (NIMH) and National Institute on Drug Abuse (NIDA), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Nursing Research (NINR), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
The authors thank Ms. Lauren Zalla, Ms. Arti Virkud, and Ms. Grace Mulholland for helpful comments on earlier versions of this manuscript.
Footnotes
Publisher's Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to Annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Contributor Information
Jessie K. Edwards, Department of Epidemiology, University of North Carolina at Chapel Hill, NC.
Stephen R. Cole, Department of Epidemiology, University of North Carolina at Chapel Hill, NC.
Tiffany L. Breger, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Jacqueline E. Rudolph, Department of Epidemiology, Emory University, Atlanta, GA.
Lindsey M. Filiatreau, Department of Epidemiology, University of North Carolina at Chapel Hill, NC.
Kate Buchacz, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, GA.
Elizabeth Humes, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Peter F. Rebeiro, Department of Biostatistics, Vanderbilt University School of Medicine, Nashville, TN.
Gypsyamber D’Souza, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
M. John Gill, Department of Medicine, University of Calgary, Calgary, Alberta.
Michael J. Silverberg, Kaiser Permanente Northern California, Oakland, CA.
W. Christopher Mathews, Department of Medicine, University of California San Diego, San Diego, CA.
Michael A. Horberg, Kaiser Permanent Mid-Atlantic Permanente Research Institute, Rockville, MD.
Jennifer Thorne, School of Medicine, Johns Hopkins University, Baltimore, MD.
H. Irene Hall, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, GA.
Amy Justice, Yale School of Public Health, New Haven, CT and Veterans Affairs Connecticut Healthcare System, West Haven, CT.
Vincent C. Marconi, School of Medicine, Emory University, Atlanta, GA and Rollins School of Public Health, Emory University, Atlanta, GA.
Viviane D. Lima, Faculty of Medicine, University of British Columbia, Vancouver, Canada.
Ronald J. Bosch, Harvard TH Chan School of Public Health, Boston, MA.
Timothy R. Sterling, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN.
Keri N. Althoff, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Richard D. Moore, School of Medicine, Johns Hopkins University, Baltimore, MD.
Michael Saag, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL.
Joseph J. Eron, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC.
REFERENCES
- 1.Eron JJ, Cooper DA, Steigbigel RT, Clotet B, Gatell JM, Kumar PN, et al. Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. The Lancet Infectious Diseases. 2013;13(7):587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cain LE, Robins JM, Lanoy E, Logan R, Costagliola D, Hernán M a. When to Start Treatment? A Systematic Approach to the Comparison of Dynamic Regimes Using Observational Data. The International Journal of Biostatistics. 2010January;6(2):Article 18–Article 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CD4+ Count–Guided Interruption of Antiretroviral Treatment. New England Journal of Medicine. 2006November30;355(22):2283–96. [DOI] [PubMed] [Google Scholar]
- 4.Clotet B, Feinberg J, van Lunzen J, Khuong-Josses M-A, Antinori A, Dumitru I, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. The Lancet. 2014June;383(9936):2222–31. [DOI] [PubMed] [Google Scholar]
- 5.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS one. 2013January;8(12):e81355–e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Althoff KN, Chandran A, Zhang J, Arevalo WM, Gange SJ, Sterling TR, et al. Life-Expectancy Disparities Among Adults With HIV in the United States and Canada: The Impact of a Reduction in Drug- and Alcohol-Related Deaths Using the Lives Saved Simulation Model. Am J Epidemiol. 2019December31;188(12):2097–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP, Klein DB, et al. Narrowing the Gap in Life Expectancy Between HIV-Infected and HIV-Uninfected Individuals With Access to Care. J Acquir Immune Defic Syndr. 201601;73(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus JL, Leyden WA, Alexeeff SE, Anderson AN, Hechter RC, Hu H, et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000-2016. JAMA Netw Open. 2020June1;3(6):e207954–e207954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosh KA, Johnson AS, Hernandez AL, Prejean J, Taylor J, Wingard R, et al. Vital Signs: Deaths Among Persons with Diagnosed HIV Infection, United States, 2010-2018. MMWR Morb Mortal Wkly Rep. 2020November20;69(46):1717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gange SJ, Kitahata MM, Saag MS, Bangsberg DR, Bosch RJ, Brooks JT, et al. Cohort Profile: The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol. 2007April1;36(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). Racial/ethnic disparities in diagnoses of HIV/AIDS--33 states, 2001-2004. MMWR Morb Mortal Wkly Rep. 2006February10;55(5):121–5. [PubMed] [Google Scholar]
- 12.National Center for Health Statistics. Centers for Disease Control and Prevention. – Restricted-Use Vital Statistics Data. https://www.cdc.gov/nchs/nvss/nvss-restricted-data.htm.Accessed 22 March 2021.
- 13.Althoff KN, Buchacz K, Hall HI, Zhang J, Hanna DB, Rebeiro P, et al. U.S. Trends in Antiretroviral Therapy Use, HIV RNA Plasma Viral Loads, and CD4 T-Lymphocyte Cell Counts Among HIV-Infected Persons, 2000 to 2008. Annals of Internal Medicine. 2012September4;157(5):325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.HUD USPS ZIP Code Crosswalk Files ∣ HUD USER [Internet]. [cited 2020 Jun 3]. Available from: https://www.huduser.gov/portal/datasets/usps_crosswalk.html
- 15.Cole SR, Hudgens MG, Brookhart MA, Westreich D. Risk. American Journal of Epidemiology. 2015February;181(4):246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958June;53(282):457–81. [Google Scholar]
- 17.Greenwood M. The Natural Duration of Cancer. Reports of Public Health and Related Subjects. 1926;33. [Google Scholar]
- 18.Rudolph JE, Cole SR, Edwards JK, Whitsel EA, Serre ML, Richardson DB. Using Animations of Risk Functions to Visualize Trends in US All-Cause and Cause-Specific Mortality, 1968–2016. Am J Public Health. 2019January24;109(3):451–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oehlert GW. A Note on the Delta Method. The American Statistician. 1992February1;46(1):27–9. [Google Scholar]
- 20.Trickey A, May MT, Vehreschild J-J, Obel N, Gill MJ, Crane HM, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. The Lancet HIV. 2017August1;4(8):e349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crepaz N, Song R, Lyss S, Hall HI. Trends in Time From HIV Diagnosis to First Viral Suppression Following Revised US HIV Treatment Guidelines, 2012–2017. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2020September1;85(1):46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nance RM, Delaney JAC, Simoni JM, Wilson IB, Mayer KH, Whitney BM, et al. HIV Viral Suppression Trends Over Time Among HIV-Infected Patients Receiving Care in the United States, 1997 to 2015. Ann Intern Med. 2018August21;169(6):376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesko CR, Cole SR, Zinski A, Poole C, Mugavero MJ. A systematic review and meta-regression of temporal trends in adult CD4(+) cell count at presentation to HIV care, 1992-2011. Clin Infect Dis. 2013October;57(7):1027–37. [DOI] [PubMed] [Google Scholar]
- 24.Althoff KN, Gebo KA, Gange SJ, Klein MB, Brooks JT, Hogg RS, et al. CD4 count at presentation for HIV care in the United States and Canada: Are those over 50 years more likely to have a delayed presentation? AIDS Res Ther. 2010December15;7(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanna DB, Buchacz K, Gebo KA, Hessol NA, Horberg MA, Jacobson LP, et al. Trends and Disparities in Antiretroviral Therapy Initiation and Virologic Suppression Among Newly Treatment-Eligible HIV-Infected Individuals in North America, 2001–2009. Clin Infect Dis. 2013April15;56(8):1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JS, Humes EA, Hogan BC, Buchacz K, Eron JJ, Gill MJ, Sterling TR, Rebeiro PF, Lima VD, Mayor A, Silverberg MJ. CD4 count at entry into HIV care and at antiretroviral therapy prescription in the US, 2005–2018. Clinical Infectious Diseases. 2020December31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beer L, Bradley H, Mattson CL, Johnson CH, Hoots B, Shouse RL. Trends in racial and ethnic disparities in antiretroviral therapy prescription and viral suppression in the United States, 2009–2013. J Acquir Immune Defic Syndr. 2016December1;73(4):446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Cock KM, El-Sadr WM. When to Start ART in Africa — An Urgent Research Priority. New England Journal of Medicine. 2013March7;368(10):886–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fatukasi TV, Cole SR, Moore RD, Mathews WC, Edwards JK, Eron JJ. Risk factors for delayed antiretroviral therapy initiation among HIV-seropositive patients. PLoS ONE. 2017;12(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cocohoba J, Wang QJ, Cox C, Gange SJ, Cohen M, Glesby M, et al. Consistency of Initial Antiretroviral Therapy With HIV Treatment Guidelines in a US Cohort of HIV-Infected Women. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2008March1;47(3):377–83. [DOI] [PubMed] [Google Scholar]
- 31.The IeDEA and COHERE Cohort Collaborations. Global Trends in CD4 Cell Count at the Start of Antiretroviral Therapy: Collaborative Study of Treatment Programs. Clinical Infectious Diseases. 2018March5;66(6):893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gresele P, Falcinelli E, Momi S, Francisci D, Baldelli F. Highly active antiretroviral therapy-related mechanisms of endothelial and platelet function alterations. Reviews in cardiovascular medicine. 2014January;15Suppl 1:S9–20. [DOI] [PubMed] [Google Scholar]
- 33.The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination Antiretroviral Therapy and the Risk of Myocardial Infarction. New England Journal of Medicine. 2003November20;349(21):1993–2003. [DOI] [PubMed] [Google Scholar]
- 34.Keiser O, Fellay J, Opravil M, Hirsch HH, Hirschel B, Bernasconi E, et al. Adverse events to antiretrovirals in the Swiss HIV Cohort Study: effect on mortality and treatment modification. Antiviral Therapy. 2007;8. [PubMed] [Google Scholar]
- 35.Han WM, Wattanachanya L, Apornpong T, Jantrapakde J, Avihingsanon A, Kerr SJ, et al. Bone mineral density changes among people living with HIV who have started with TDF-containing regimen: A five-year prospective study. PLOS ONE. 2020March25;15(3):e0230368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant PM, Cotter AG. Tenofovir and Bone Health. Curr Opin HIV AIDS. 2016May;11(3):326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerchberger AM, Sheth AN, Angert CD, Mehta CC, Summers NA, Ofotokun I, et al. Weight Gain Associated With Integrase Stand Transfer Inhibitor Use in Women. Clin Infect Dis. 2020July27;71(3):593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourgi K, Jenkins CA, Rebeiro PF, Shepherd BE, Palella F, Moore RD, et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. Journal of the International AIDS Society. 2020;23(4):e25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips AN, Neaton J, Lundgren JD. The Role of HIV in Serious Diseases Other than AIDS. AIDS. 2008November30;22(18):2409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warriner AH, Burkholder GA, Overton ET. HIV-related metabolic comorbidities in the current ART era. Infectious disease clinics of North America. 2014September;28(3):457–76. [DOI] [PubMed] [Google Scholar]
- 41.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS medicine. 2008October;5(10):e203–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deeks SG. HIV Infection, Inflammation, Immunosenescence, and Aging. Annual Review of Medicine. 2011;62(1):141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahmanian S, Wewers ME, Koletar S, Reynolds N, Ferketich A, Diaz P. Cigarette Smoking in the HIV-Infected Population. Proc Am Thorac Soc. 2011June1;8(3):313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crothers K, Goulet JL, Rodriguez-Barradas MC, Gibert CL, Oursler KAK, Goetz MB, et al. IMPACT OF CIGARETTE SMOKING ON MORTALITY IN HIV-POSITIVE AND HIV-NEGATIVE VETERANS. AIDS Educ Prev. 2009June;21(3 Suppl):40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013March;56(5):727–34. [DOI] [PubMed] [Google Scholar]
- 46.Deren S, Cortes T, Dickson VV, Guilamo-Ramos V, Han BH, Karpiak S, et al. Substance Use Among Older People Living With HIV: Challenges for Health Care Providers. Front Public Health [Internet]. 2019. [cited 2020 Oct 27];7. Available from: https://www.frontiersin.org/articles/10.3389/fpubh.2019.00094/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartzler B, Dombrowski JC, Crane HM, Eron JJ, Geng EH, Christopher Mathews W, et al. Prevalence and predictors of substance use disorders among HIV care enrollees in the United States. AIDS Behav. 2017April;21(4):1138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities Among US Patients With Prevalent HIV Infection—A Trend Analysis. J Infect Dis. 2017December19;216(12):1525–33. [DOI] [PubMed] [Google Scholar]
- 49.Whiteside YO, Selik R, An Q, Huang T, Karch D, Hernandez AL, et al. Comparison of Rates of Death Having any Death-Certificate Mention of Heart, Kidney, or Liver Disease Among Persons Diagnosed with HIV Infection with those in the General US Population, 2009-2011. Open AIDS J. 2015February27;9:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adih WK, Selik RM, Hall HI, Babu AS, Song R. Associations and Trends in Cause-Specific Rates of Death Among Persons Reported with HIV Infection, 23 U.S. Jurisdictions, Through 2011. Open AIDS J. 2016July29;10:144–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008July26;372(9635):293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008July2;300(1):51–9. [DOI] [PubMed] [Google Scholar]
- 53.Lesko CR, Cole SR, Hall HI, Westreich D, Miller WC, Eron JJ, et al. The effect of antiretroviral therapy on all-cause mortality, generalized to persons diagnosed with HIV in the USA, 2009-11. International journal of epidemiology. 2016January;dyv352–dyv352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong C, Althoff K, Gange SJ. Identifying the Appropriate Comparison Group for HIV-infected Individuals. Curr Opin HIV AIDS. 2014July;9(4):379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark CR, Williams DR. Understanding County-Level, Cause-Specific Mortality: The Great Value—and Limitations—of Small Area Data. JAMA. 2016December13;316(22):2363–5. [DOI] [PubMed] [Google Scholar]
- 56.Drozd DR, Kitahata MM, Althoff KN, Zhang J, Gange SJ, Napravnik S, et al. Increased Risk of Myocardial Infarction in HIV-Infected Individuals in North America Compared to the General Population. J Acquir Immune Defic Syndr. 2017August15;75(5):568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silverberg MJ, Lau B, Achenbach CJ, Jing Y, Althoff KN, D’Souza G, et al. Cumulative Incidence of Cancer Among Persons With HIV in North America. Annals of Internal Medicine. 2015October6;163(7):507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Althoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert C, Oursler KK, et al. Comparison of Risk and Age at Diagnosis of Myocardial Infarction, End-Stage Renal Disease, and Non-AIDS-Defining Cancer in HIV-Infected Versus Uninfected Adults. Clin Infect Dis. 2015February15;60(4):627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shiels MS, Althoff KN, Pfeiffer RM, Achenbach CJ, Abraham AG, Castilho J, et al. HIV Infection, Immunosuppression, and Age at Diagnosis of Non-AIDS-Defining Cancers. Clin Infect Dis. 2017February15;64(4):468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Althoff KN, Gebo KA, Moore RD, Boyd CM, Justice AC, Wong C, et al. Contributions of traditional and HIV-related risk factors on non-AIDS-defining cancer, myocardial infarction, and end-stage liver and renal diseases in adults with HIV in the USA and Canada: a collaboration of cohort studies. The Lancet HIV. 2019February1;6(2):e93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Messer LC. Invited Commentary: Measuring Social Disparities in Health--What Was the Question Again? American Journal of Epidemiology. 2008February25;167(8):900–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.