Abstract
Background:
Research has shown that among people with type 2 diabetes mellitus, reduction in hemoglobin A1c (HbA1c) prevents long term complications. Medically tailored meals (MTM) and telehealth-delivered medical nutrition therapy (tele-MNT) are promising strategies for patient-centered diabetes care.
Objectives:
Project MiNT will determine whether provision of MTM with and without the addition of telehealth-delivered medical nutrition therapy improves HbA1c and is cost effective for patients with type 2 diabetes mellitus.
Methods:
Patients with poorly controlled type 2 diabetes mellitus (HbA1c >8%) will be recruited from Jefferson Health. Eligible patients will be randomized to one of three arms: 1) usual care, 2) 12 weeks of home-delivered MTM, or 3) MTM + 12 months of tele-MNT. All participants (n=600) will complete three follow-up assessments at 3, 6, and 12 months. The primary outcome is change in HbA1c at 6 months. Secondary outcomes include change in HbA1c at 3 and 12 months and cost-effectiveness of the intervention at 6 and 12 months.
Conclusion:
Findings from Project MiNT will inform MTM coverage and financing decisions, how to structure services for scalability and system-wide integration, and the role of these services in reducing health disparities.
Keywords: Diabetes Mellitus, Medically Tailored Meals, Medical Nutrition Therapy, Telehealth, Hemoglobin A1c
1. Introduction
This pragmatic randomized control trial will determine whether provision of medically tailored meals with and without the addition of telehealth-delivered medical nutrition therapy improves hemoglobin A1c and is cost effective for patients with type 2 diabetes mellitus.
Diabetes mellitus (DM) impacts 30.3 million Americans, disproportionately affects minority populations [1], and is the 7th leading cause of death in the U.S [2]. National data from 2004 found that only 57% of patients with DM had adequate glycemic control [3], contributing to health disparities and greater use of unscheduled acute care services (e.g., emergency department (ED) utilization) [4–6]. Achieving glycemic control with medications and behavioral interventions is paramount to preventing the long term complications of DM. While medications are a mainstay of treatment, a focus on diet and nutrition contributes to glycemic control.
Medically tailored meals (MTM) are meals designed by a Registered Dietitian Nutritionist (RDN) to reflect appropriate dietary therapy according to evidence-based nutrition practice guidelines. They address medical diagnoses, symptoms, allergies, inability to chew or swallow, medication management, and side effects to ensure the best possible nutrition-related health outcomes. Pilot studies of MTM, including services provided by our local meal provider, demonstrated up to 50% fewer hospitalizations, reduced healthcare utilization and costs [7–9], and improved glycemic control for patients with poorly-controlled DM [7,10,11]. To date, however, no randomized control trials (RCT) have been conducted to assess the sustained impact of MTM on long-term patient outcomes for type 2 DM (T2DM).
Medical nutrition therapy (MNT) is offered as a component of diabetes self-management in many health systems and consists of individuals receiving nutrition education tailored to their unique medical needs, delivered by a RDN. While prior studies show the importance of MNT in improving DM control [12–14], patient utilization of MNT remains limited. In a study of 28,404 individuals with DM, only 9% had at least one nutrition visit within a 9-year period [12]. Individual studies of MNT delivered both in-person and via telemedicine (tele-MNT) have shown benefit for DM outcomes [15–18], yet data on the effectiveness of intensive MNT are lacking to demonstrate sustained impact of interventions.
We developed Project Meals and Nutrition Therapy (Project MiNT) to study the long term impact of MTM and MNT on outcomes for patients with T2DM. This is a pragmatic RCT funded by the National Institute of Diabetes and Digestive and Kidney Diseases (1R18DK118590). The primary outcome is change in Hemoglobin A1c (HbA1c) after 6 months of treatment with MTM and tele-MNT in patients with T2DM. Secondary outcomes include change in HbA1c at 3 and 12 months and cost effectiveness of the interventions at 6 and 12 months. We hypothesize that there will be a greater reduction in HbA1c in both treatment arms (MTM only or MTM + tele-MNT) as compared to usual care at both 3 and 6 months; however, at 12 months the effect will only be sustained in the MTM + tele-MNT arm. If successful, this study will provide robust evidence needed regarding the efficacy and cost-effectiveness of MTM and tele-MNT.
2. Study Design
This clinical trial is registered and available at Clinicaltrials.gov (NCT04264572).
We are performing a three-arm, pragmatic RCT to study the effectiveness of MTM provision with and without tele-MNT on improving outcomes for a population of patients with T2DM, all with a baseline HbA1c>8% and a majority of whom are low income and African American. The study is being implemented at Jefferson Health and includes enrollment of patients from 2 hospitals both located in Philadelphia, PA: Thomas Jefferson University Hospital and Methodist Hospital. A total of 600 eligible participants are randomized into one of three groups: (1) Usual Care, (2) MTM, or (3) MTM + tele-MNT (Figure 1). Participants are engaged for a total of 12 months.
Figure 1.
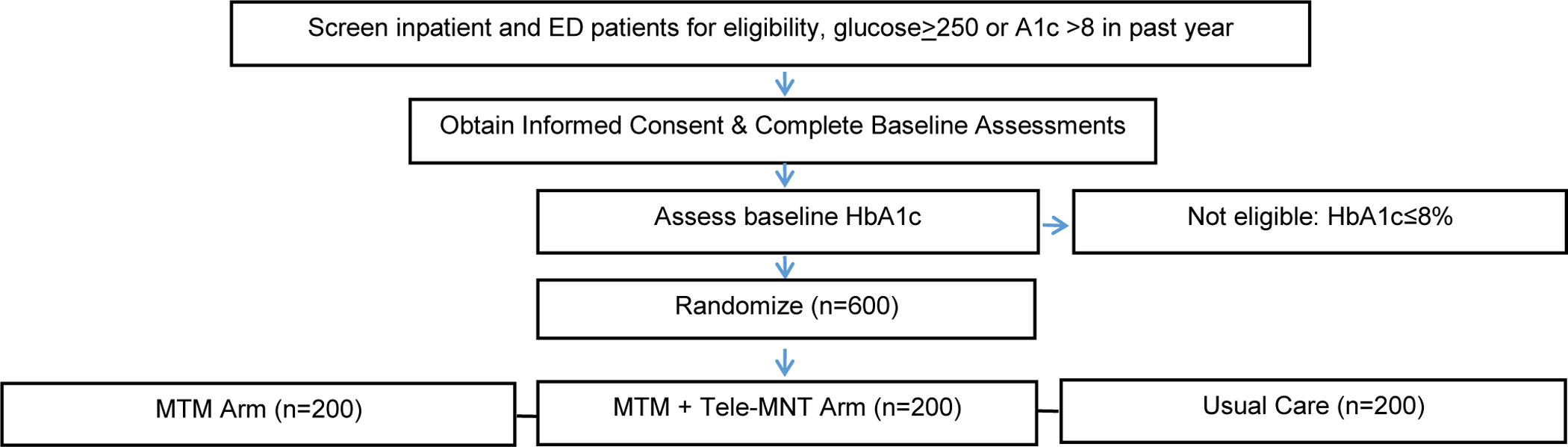
Enrollment & Randomization Flow Chart
Participants are recruited during an ED visit or hospitalization at either hospital for any complaint. Research coordinators identify all patients in the ED or hospital who have a glucose ≥ 250 or a HbA1c>8% in the past year for potential eligibility. Research coordinators then review the chart of each identified participant to further assess eligibility (Table 1). Eligible patients are approached in-person or via phone by a member of the study team to assess interest in study participation and confirm all eligibility criteria. Interested patients who meet eligibility criteria other than an HbA1c>8%, which may not yet have been assessed yet, are then consented for study participation and asked to complete baseline assessments.
Table 1.
Participant Eligibility Criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
Includes smartphone, tablet, or Windows PC
All participants are required to complete written informed consent during the index encounter, which includes teach-back questions to confirm comprehension. The consent is signed either in-person or remotely using an e-consent form in REDCap (Research Electronic Data Capture, Vanderbilt University), with remote enrollment procedures implemented to allow for enrollment that incorporates COVID-19 precautions.
Enrolled participants with a HbA1c>8% are randomized in a 1:1:1 ratio using random permuted blocks stratified by HbA1c (>8 to 10 vs. >10) and hospital discharge site (inpatient vs ED) to 1 of 3 arms: 1) Usual Care; 2) MTM; or 3) MTM + tele-MNT. The size of any particular block is randomly selected and only known by the study biostatistician. This stratification approach was selected based on the hypothesis that the interventions may have differential effects based on how poorly controlled an individual’s DM is at baseline, as well as the degree of acute decompensation of the patient at time of enrollment. A computer-generated list of random numbers is prepared in advance by the study biostatistician and loaded into the REDCap randomization tool to ensure research staff are blinded to assignment pre-randomization. All study procedures are approved by the Institutional Review Board (IRB) at Thomas Jefferson University. Study activities are regularly monitored by an external Data Safety Monitoring Board (DSMB) which meets every 6 months. Project MiNT is a nonblinded study, as it is not feasible to blind participants and research staff to assignment post enrollment. HbA1c is an objective outcome, and thus lack of blinding should not be a significant limitation.
3. Study Interventions
3.1. Usual Care
Participants in this arm receive usual services offered at Jefferson and other local health systems for patients with DM, which may include regular visits with a diabetes provider (primary care or endocrine) and standard American Diabetes Association (ADA) information pamphlets. Patients may be referred to 1) diabetes education classes and 2) nutrition counseling by dietitians and nurse practitioners by their provider. During routine office visits, providers reinforce messages about self-management and provide lists of local and national resources related to nutrition and diabetes self-management. For those referred to nutrition counseling, the standard of care at Jefferson is to begin with a single group MNT visit lasting from 60–90 minutes. Each patient’s need for additional sessions and general time-frame for follow-up is individually determined following the group session, based on patient preference. Historically, only about 2% of the Jefferson population engages in these services, thus minimizing dilution of the effect of the tele-MNT. At the 6 and 12 month follow-up periods, the study team will ask participants about their participation in any non-study individual or group nutrition counseling sessions. Participants who participate in MNT as part of usual care will still be included in study analyses.
3.2. MTM
Participants randomized to this arm receive MTM for 12 weeks. Meals are prepared and delivered by a local non-profit organization (MANNA) that has provided MTM for patients with chronic illnesses in Philadelphia and Southern New Jersey since 1990 [20]. Participants receive 21 complete, frozen meals delivered to their home each week. This includes 3 main meals per day and snacks, providing 45–60 grams of carbohydrates per meal for optimal glucose control based on ADA guidelines and 100% of overall nutritional requirements based on USDA guidelines. All participants receive MANNA’s diabetes friendly meal modification and are able to choose up to 2 other modifications to fit their individual needs and preferences. MANNA offers 11 meal modifications to address medical conditions (kidney, diabetes/heart friendly) and other preferences (mechanically soft, GI friendly, elimination of certain foods). In addition, children and any senior dependents for whom the participant is the primary caregiver receive meals for the entire 12 weeks for no additional cost, to align with MANNA’s standard of care services. The intervention duration is based primarily on current insurance policies, as we want to test a MTM timeframe that insurers would potentially consider reasonable as a covered benefit. Upon randomization to this group, a referral form is completed and sent to MANNA by the research team. MANNA staff establish a delivery time with participants, with a goal of delivering the first week of meals within two weeks of randomization. After the 12 weeks of delivered meals, participants continue with their usual diabetes care.
3.3. MTM + tele-MNT
Participants in this arm receive MTM services exactly as described above, as well as tele-MNT over a period of 12 months. All video visits are conducted synchronously between participant and the study RDN using the MyChart application [21]. Participants receive technology support as needed from Jefferson telehealth support staff for setting up MyChart and initiating a telehealth visit. The tele-MNT intervention is designed based on evidence-based recommendations for MNT and informed by social cognitive theory. Based on Academy of Nutrition and Dietetics recommendations, each participant’s MNT includes the following core features: nutrition assessment, intervention, care coordination, monitoring and evaluation [12]. Sessions also include motivational interviewing to help participants overcome challenges related to their individual food choices and understand how choices affect their blood glucose values. In the first three months, sessions focus on supporting individuals who are not selecting, preparing, or purchasing their own meals, as participants will be receiving MANNA meals during this time. As the end of MTM services approaches, the intervention shifts to focus on how to transition from MTM to self-directed healthy eating. The curriculum includes a set of core features and covers a list of specific evidence-based topics [12,13,22], while also being individualized to address the needs and preferences of each participant (Table 2) [22].
Table 2.
Individual Tele-MNT Meeting Structure
| Individual Visits (Months 1–6) | Length | Required? |
|---|---|---|
| Month 1, Visit 1: Nutrition Basics | 35–60 minutes | Yes |
| Month 1, Visit 2: Check in | 15–20 minutes | Yes |
| Month 2, Visit 1: Understanding Carbohydrates | 35–60 minutes | Yes |
| Month 2, Visit 2: Check in | 15–20 minutes | Yes |
| Month 3, Visit 1: Protein | 35–60 minutes | Yes |
| Month 3, Visit 2: Check in | 15–20 minutes | Yes |
| Month 4, Visit 1: Breakfast Ideas | 35–60 minutes | Yes |
| Month 4, Visit 2: Check in | 15–20 minutes | Yes |
| Month 5, Visit 1: Lunch and Snack Ideas | 35–60 minutes | Yes |
| Month 5, Visit 2: Check in | 15–20 minutes | Yes |
| Month 6, Visit 1: Meal Planning | 35–60 minutes | Yes |
| Month 6, Visit 2: Check in | 15–20 minutes | Yes |
The visit schedule includes bi-monthly one-on-one visits that make up 315 minutes of direct patient time in the first 6 months. Each month, one visit focuses on education delivery while the other visit checks-in on participant progress. Visits may last 15–60 minutes. In months 7–12, participants are invited to attend a monthly, 60-minute group session via Zoom with a set, rotating topic to reinforce topics discussed in individual sessions (Table 3).
Table 3.
Group Tele-MNT Meeting Structure
| Group Visits (Months 7–12) | Length | Required? |
|---|---|---|
| Basic Meal Planning | 60 minutes | No |
| Physical Activity and Moving More | 60 minutes | No |
| Eating Out and Special Occasions | 60 minutes | No |
| More on Meal Planning | 60 minutes | No |
| Making Good Food Choices | 60 minutes | No |
| Importance of Consistent Sleep and Eating Patterns | 60 minutes | No |
4. Assessment Protocol
Data collection occurs at four different time points throughout the study: baseline, 3 months, 6 months, and 12 months (Table 4). All baseline assessments are conducted prior to randomization. Assessments may be administered in-person or over the phone at all time points.
Table 4.
Measures and Collection Schedule
| Measure | ||||
|---|---|---|---|---|
| Baseline | 3-month | 6-month | 12-month | |
| Demographics, family support | + | |||
| Medical history/comorbidities | + | + | + | + |
| HbA1c (primary outcome) | + | + | + | + |
| Healthcare Costs | + | + | + | |
| Health Utility & quality-adjusted life (EQ-5D-5L) [24] | + | + | + | |
| Intervention Costs | + | + | + | |
| Adherence to Refills and Medications Scale for Diabetes (ARMS-D) [26] | + | + | + | |
| Diabetes Self-Care (SDSCA) [27] | + | + | + | |
| Diabetes Self-Efficacy (Diabetes Self-Efficacy Scale) [28] | + | + | + | |
| Diabetes Treatment Satisfaction (DTSQ) [29] | + | + | + | + |
| Diabetes Quality of Life (DQoL) [30] | + | + | + | |
| Dietary Assessment (DietID) [31] | + | + | + | + |
| Readiness to Change | + | + | + | + |
| Food Insecurity (U.S. Household Food Security Survey: Two-Item Screener) [33] | + | + | ||
| Weight | + | + | + | + |
| Fidelity and Satisfaction | + | + | + |
4.1. Primary Measure – HbA1c
Participants’ HbA1c is assessed using non-fasting blood samples collected at assessment points (baseline, 3 months, 6 months, 12 months).
4.2. Secondary Measures
4.2.1. Healthcare Costs
The initial protocol was to receive participant health care utilization from the Healthshare Exchange of Southeast Pennsylvania (HSX) [23], the regional health information exchange. HSX captures electronic health records for outpatient care, emergency department care, inpatient care, post-acute care and prescription drugs. More granular detail was needed than could be obtained through HSX, so the protocol was modified to obtain Jefferson billing and claims data for this measure.
4.2.2. Health Utility and Quality-adjusted Life
Preference-based health related quality of life is measured using the EQ-5D-5L, which assesses 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, on a scale from 1–5 [24]. EQ-5D-5L responses will be converted into a health utility using validated values sets developed by the EuroQol Research Foundation. An area under the curve approach will be applied to calculate participants’ quality-adjusted life year (QALY) during the trial [25].
4.2.3. Intervention Costs
The costs of delivering usual care are obtained from administrative data within the Jefferson Health system. The cost of medically-tailored meals is derived from MANNA’s per meal cost. Cost of delivering tele-MNT is obtained from data logs which capture time preparing and delivering sessions.
4.2.4. Adherence to Refills and Medications
The Adherence to Refills and Medications Scale for people with T2DM (ARMS-D) is used to assess participants’ self-reported adherence to diabetes medications [26]. The survey consists of 14 questions on a 4-point scale to assess frequency of medication-related activities.
4.2.5. Diabetes Self-Care
Self-care is measured using The Summary of Diabetes Self-Care Activities (SDSCA) [27], made up of 11 questions, all scored on a 7-point scale. The SDSCA assess five regimen areas: General Diet, Specific Diet, Exercise, Blood-Glucose Testing, Foot Care, and Smoking Status. Scores are calculated for each of the five regimen areas by finding the mean number of days each task was performed within each regimen.
4.2.6. Diabetes Self-Efficacy
Self-efficacy is measured using The Diabetes Self Efficacy Scale [28], made up of 8 questions, all scored on a 10-point Likert scale. Each questions asks about the respondent’s confidence in performing an activity relative to diabetes. The score for the scale is the mean of the eight items.
4.2.7. Diabetes Treatment Satisfaction
Treatment satisfaction is measured using the Diabetes Treatment Satisfaction Questionnaire (DTSQs) instrument [29]. The DTSQ is made up of 6 questions assessing treatment satisfaction and 2 items assessing perceived frequency of hyperglycemia and hypoglycemia. All items are scored on a 7-point Likert scale assessing either frequency or satisfaction.
4.2.8. Diabetes Quality of Life
Quality of life is measured using the Diabetes Quality of Life (DQoL) instrument [30], made up of 15 questions, all scored on a 5-point Likert scale assessing either frequency or satisfaction.
4.2.9. Dietary Assessment
Participant self-reported dietary habits are assessed using the DietID tool, powered by Diet Quality Photo Navigation [31]. As opposed to a detailed recall or log of food consumed, DietID uses images to establish dietary patterns and diet quality, resulting in a DietID quality score and a Healthy Eating Index score.
4.2.10. Readiness to Change
Readiness to change is measured using two questions based on motivational interviewing practices [32]. The two items assess readiness to make changes to manage diabetes and readiness to make changes to diet on an 11-point Likert scale.
4.3.11. Food Insecurity
Food insecurity is measured using a brief, two-question screener based on the 18-item U.S. Household Food Security Survey (HFSS). The two-item assessment has been validated for use as a screening tool in healthcare settings [33]. A response of “often true” or “sometimes true” to either question identifies a household at risk for food insecurity.
4.2.11. Weight
Weight is measured using study-specific calibrated scales, in bare feet, at baseline assessment (Etekcity Digital Body Scale). Built-in hospital bed scales or weight recorded in the EMR may be used if participants are unable to stand on scale. Follow-up weight measures may be captured using participants’ personal scales, or measurements recorded in the EMR.
4.3. Fidelity and Satisfaction
Process evaluation measures allow for a better understanding of intervention fidelity and participant satisfaction, which inform continuous quality improvement. MTM fidelity is being assessed by the number of deliveries each participant receives in the 12-week period, as well as using participant self-reported information regarding intake of MANNA meals as well as other supplemental food. Meal delivery information is tracked by the meal provider and shared with the study team on a monthly basis. At the 3 month follow-up, participants who received meals are asked about their satisfaction with the meals and to assess how frequently they strayed from the study-provided meals. Similarly, tele-MNT visit participation is tracked using an EMR-based platform and dietitian attendance tracking. At the 6 month follow-up, participants who completed tele-MNT visits are asked about their satisfaction with the sessions. Participant feedback that does not change the study design will be considered for ongoing program strengthening.
5. Analysis Plan
5.1. Sample Size & Power
Power was calculated to detect a difference in mean change of 0.5 in our primary endpoint of HbA1c for MTM compared to usual care, as well as MTM + tele-MNT compared to usual care, at 6 months. An absolute change of 0.5 was determine as the minimal clinically important difference in HbA1c based on 1) clinical guidelines indicating a change in HbA1c of 0.5 is clinically significant [34–36] and 2) the fact that numerous studies have powered for this outcome [37,38]. Statisticians fixed the two-sided type 1 error rate α to be 0.05. Since othe trial is multi-armed and two primary hypotheses are being tested, Bonferroni multiple comparison adjustment is performed to control the familywise error rate, which equates to using a smaller value for α (0.025) in the power calculations. The standard deviation of change in HbA1c over a 6-month period ranges from 0.9 to 1.5 in the literature [39,40]. Assumptions include a SD of 1.4 for our calculation and that 20% of participants either drop out or will not provide data on the primary outcome, based on observed rates in the literature [41]. A sample of 200 randomized participants (160 with complete data for analysis) per arm provides greater than 80% power to detect a difference in mean changes of 0.5.
5.2. Primary Analysis
The primary objective of this trial is to assess the effectiveness of MTM compared to usual care, and of MTM + tele-MNT compared to usual care, in reducing HbA1c at 6 months. Mixed effects linear regression will be used to model the repeated, longitudinal measurements of HbA1c. Fixed effects in the model will be randomization assignment (usual care, MTM, and MTM + tele-MNT), time (baseline vs 3 months vs 6 months vs 12 months) and time by randomization interaction. The randomization stratification variables will be included as covariates. Additional baseline covariates (BMI, prior health utilization, age, sex, self-efficacy, self-care, quality of life, treatment satisfaction, medication adherence, family support, and food insecurity) will also be included if bivariate associations with HbA1c are significant at the p <0.2 level. A compound symmetric or first-order auto-regressive correlation structure will be assumed to account for correlation among repeated measurements.
For the primary outcome assessment, we will perform two linear contrasts, estimating the difference in the mean change from baseline in each intervention arm compared with the mean change from baseline in the control group, and test the null hypothesis that these differences equal zero. Both tests are performed at the alpha=0.025 level. To estimate the effect of actually receiving treatment, for the primary hypotheses related to the effectiveness of MTM compared to usual care, and of MTM + tele-MNT compared to usual care, in reducing HbA1c, we will implement an innovative approach specifically designed to address the issue of noncompliance, called a contamination adjusted intent-to-treat (CA ITT) analysis [42]. Under this framework, the RCT is treated as an instrumental variable (IV), with treatment assignment as the instrument. The effect of treatment assignment on the outcome observed is adjusted by the percentage of assigned participants who ultimately receive the treatment. The CA ITT analysis can only be implemented if one has information on the treatment that an individual actually received.
5.3. Secondary and Exploratory Analyses
For any continuous endpoint measured in all groups, groups will be compared with respect to change from baseline to 3, 6, and 12 months using the same mixed effects model approach as for the primary endpoint. Exploratory analyses will consider whether the effect of treatment differs by the following variables: sex, race/ethnicity, baseline HbA1c, BMI, diabetes self-efficacy, medication adherence, and level of food insecurity. Separate mixed effect models will be fit for each potential effect modifier where the model is extended to allow for separate treatment effects by level of the modifier through the use of interaction terms.
5.4. Cost-effectiveness Analysis
Cost-effectiveness at 6 and 12 months will be evaluated from a healthcare payor perspective [43,44]. For each strategy (MTM, MTM + tele-MNT, and usual care), intervention costs, healthcare costs, and QALYs will be calculated. Strategies will be ranked in terms of increasing total cost (intervention + healthcare costs) and the least costly strategy will serve as the reference case. Next, incremental cost-effectiveness ratios (ICERs) will be calculated to determine the extra cost per QALY of a more expensive strategy compared to the adjacent less expensive strategy. In a secondary analysis, ICERs in which the unit of benefit is HbA1c will be calculated. In the secondary cost effectiveness analysis, the ICER will be interpreted as the cost to reduce HbA1c. Univariate and probabilistic sensitivity analyses will be conducted to examine uncertainty and conclusions.
5.5. Missing Data
While all effort will be made to collect follow-up data on all randomized participants, there will undoubtedly be missing data due to the patient population having one or more chronic illnesses, and some may die or be lost to follow up. To avoid biasing the results and wasting data, we will use an analytic approach for the primary outcome (mixed effect linear regression with maximum likelihood estimation) that produces unbiased estimates under the assumption that data are missing at random. For cost effectiveness analyses, we will receive Jefferson administrative data for all enrolled participants regardless of whether they are otherwise lost to follow up, thus facilitating capture of complete utilization data. Health utility data will be captured from patient surveys and we will use multiple imputation to estimate the health utility of patients that dropout.
6. Discussion
Patients with DM report needing better access to healthy food and nutrition education to manage their condition [45,46], and data suggest that provision of MTM and MNT improve short term outcomes for patients with DM [7,10,11,15–18]. Yet uptake of these services is generally low, due to patient accessibility barriers and insurance coverage limitations. This is the first large RCT to rigorously assess the impact of MTM and MNT on outcomes for patients with diabetes over 12 months. Findings will have important implications for providing patient-centered cost-effective treatment options.
Project MiNT will assess the impact of MTM, with and without tele-MNT, for patients with T2DM. We hypothesize that there will be a greater reduction in HbA1c in both treatment arms as compared to usual care at both 3 and 6 months; however, at 12 months the effect will only be sustained in the MTM + tele-MNT arm. Sustained benefit will be due to continued participation in MNT. We also aim to assess the cost-effectiveness of each intervention arm, compared to usual care. Rigorous data incorporating formal cost effectiveness analyses are needed to support more widespread coverage of these nutrition interventions as routine benefits.
Project MiNT has some important strengths to highlight. Project MiNT uses home-based interventions, which were designed to overcome patient accessibility and scheduling barriers. While this trial was designed before COVID-19, the importance of these interventions was highlighted with the onset of the pandemic. Provision of home delivered meals and the use of technology to conduct video-based nutrition therapy made it possible to continue all study activities despite widespread shutdown of in-person activities. Using existing Jefferson Health technology platforms and capabilities, all study activities including enrollment, consent, and interventions, can be completed remotely. In addition, it is a pragmatic trial, and thus these interventions have already been incorporated into the real world setting, supporting their ability to be continued upon trial completion if demonstrated to be cost effective.
While most insurance, including Medicare and Medicaid, do not systematically provide coverage of home-delivered MTM for most beneficiaries, several recent national changes support the potential for more widespread integration of MTM as a covered benefit. These include a report from the National Quality Forum focused on the importance of addressing food insecurity and housing instability [47], expansion by the Affordable Care Act of the ability of states to use waivers to cover the cost of home-delivered meals for Medicaid recipients [48], establishment of a bipartisan Food is Medicine working group by the House of Representatives focused on advancing policies to ensure access to MTM for chronically-ill people [49], and passage of the Chronic Care Act by Congress in 2018 which provides two potential reimbursement mechanisms to support covering both MTM and tele-MNT. If this trial demonstrates that MTM or MTM + tele-MNT improves T2DM outcomes in a cost-effective manner, regional payers have committed to consider expanding their coverage of these services based on study findings.
Sustainability of medically tailored nutrition services (food and education) will be crucial for improved health and well-being in the long-term. While temporary services, including medically tailored meals and nutrition education, may influence positive health outcomes over a set period of time, the ultimate goal is to support patients in becoming self-sufficient through increased knowledge and positive behavior change. Like traditional medical management, nutrition needs and practices should be assessed regularly over time to support the ever-changing needs and situations of all patients.
As in all research, this study has limitations. We are only recruiting patients from Philadelphia and the surrounding areas, as they must live within the MANNA service area for study inclusion [19]. Although the population of patients with poorly-controlled T2DM at Jefferson is racially and ethnically diverse and includes a high proportion of low-income individuals, it is possible that findings will have limited generalizability to patient populations in other areas of the country. Due to the current abilities of the study team, we are only enrolling English-speaking participants. In addition, because we are offering a telemedicine intervention, we exclude patients who do not have a device that can support videoconferencing visits. While this limits the generalizability of our findings to a certain group of patients with poorly controlled diabetes with access to devices, in our experience we have found that only a small proportion, including low-income older adults, do not have access to a smartphone, tablet or computer that can support telemedicine (<10% according to internal Jefferson data). If we demonstrate cost effectiveness of these interventions, it is our hope that these findings, combined with results from other demonstration projects across the country, will provide sufficient data to support widespread policy change. Finally, we are unable to assess the impact of tele-MNT alone on outcomes for patients, and this may need future study if MTM + tele-MNT together is demonstrated to be effective.
Project MiNT’s immediate goal is to provide patient-centered care, through food and education, to improve outcomes for patients with type 2 diabetes. The results of the study will add to the evidence-base for diabetes care while also reframing what medical care can look like. The study design, which utilizes existing health system infrastructure and local partnerships, is unique in its ability to promote sustainable, scalable, patient-centered care.
Highlights:
Glycemic control is paramount to preventing long term complications of diabetes
Diet and nutrition education can contribute to long term glycemic control
Medically tailored meals may improve outcomes and lower cost of care
Tele-medical nutrition therapy may overcome barriers to patient engagement
Opportunities exist to expand insurance coverage of meals and nutrition therapy
8. Funding
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant 1R18DK118590. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors have no competing interests to disclose.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Schiller J, Lucas J, Peregoy J, Summary Health Statistics for U.S. Population: National Health Interview Survey, 2011. National Center for Health Statistics., Vital Heal. Stat 10 (2012). [PubMed] [Google Scholar]
- [2].DHHS, National Diabetes Statistics Report, 2020, Natl. Diabetes Stat. Rep (2020) 2. [Google Scholar]
- [3].Shaya FT, Yan X, Lin PJ, Simoni-Wastila L, Bron M, Baran R, Donner TW, US trends in glycemic control, treatment, and comorbidity burden in patients with diabetes, J. Clin. Hypertens 12 (2010) 826–832. doi: 10.1111/j.1751-7176.2010.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Peek ME, Cargill A, Huang ES, Diabetes health disparities: A systematic review of health care interventions, Med. Care Res. Rev 64 (2007). doi: 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].AHRQ, Diabetes Disparities Among Racial and Ethnic Minorities, Agency Healthc. Res. Qual (2001) 1–6. [Google Scholar]
- [6].Yang W, Dall TM, Halder P, Gallo P, Kowal SL, Hogan PF, Petersen M, Economic costs of diabetes in the U.S. in 2012, Diabetes Care 36 (2013) 1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gurvey J, Rand K, Daugherty S, Dinger C, Schmeling J, Laverty N, Examining health care costs among MANNA clients and a comparison group, J. Prim. Care Community Heal 4 (2013) 311–317. doi: 10.1177/2150131913490737. [DOI] [PubMed] [Google Scholar]
- [8].Troyer JL, McAuley WJ, McCutcheon ME, Cost-effectiveness of medical nutrition therapy and therapeutically designed meals for older adults with cardiovascular disease., J. Am. Diet. Assoc 110 (2010) 1840–1851. doi: 10.1016/j.jada.2010.09.013. [DOI] [PubMed] [Google Scholar]
- [9].Berkowitz SA, Terranova J, Hill C, Ajayi T, Linsky T, Tishler LW, DeWalt DA, Meal delivery programs reduce the use of costly health care in dually eligible medicare and medicaid beneficiaries, Health Aff 37 (2018) 535–542. doi: 10.1377/hlthaff.2017.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Palar K, Napoles T, Hufstedler LL, Seligman H, Hecht FM, Madsen K, Ryle M, Pitchford S, Frongillo EA, Weiser SD, Comprehensive and Medically Appropriate Food Support Is Associated with Improved HIV and Diabetes Health, J. Urban Heal 94 (2017) 87–99. doi: 10.1007/s11524-016-0129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Seligman HK, Lyles C, Marshall MB, Prendergast K, Smith MC, Headings A, Bradshaw G, Rosenmoss S, Waxman E, A pilot food bank intervention featuring diabetes-appropriate food improved glycemic control among clients in three states, Health Aff 34 (2015) 1956–1963. doi: 10.1377/hlthaff.2015.0641. [DOI] [PubMed] [Google Scholar]
- [12].Briggs Early K, Stanley K, Position of the Academy of Nutrition and Dietetics: The Role of Medical Nutrition Therapy and Registered Dietitian Nutritionists in the Prevention and Treatment of Prediabetes and Type 2 Diabetes, J. Acad. Nutr. Diet 118 (2018) 343–353. doi: 10.1016/j.jand.2017.11.021. [DOI] [PubMed] [Google Scholar]
- [13].Franz MJ, MacLeod J, Evert A, Brown C, Gradwell E, Handu D, Reppert A, Robinson M, Academy of Nutrition and Dietetics Nutrition Practice Guideline for Type 1 and Type 2 Diabetes in Adults: Systematic Review of Evidence for Medical Nutrition Therapy Effectiveness and Recommendations for Integration into the Nutrition Care Process, J. Acad. Nutr. Diet 117 (2017) 1659–1679. doi: 10.1016/j.jand.2017.03.022. [DOI] [PubMed] [Google Scholar]
- [14].Franz MJ, Powers MA, Leontos C, Holzmeister LA, Kulkarni K, Monk A, Wedel N, Gradwell E, The evidence for medical nutrition therapy for type 1 and type 2 diabetes in adults, J. Am. Diet. Assoc 110 (2010) 1852–1889. doi: 10.1016/j.jada.2010.09.014. [DOI] [PubMed] [Google Scholar]
- [15].Marincic PZ, Hardin A, Salazar MV, Scott S, Fan SX, Gaillard PR, Diabetes Self-Management Education and Medical Nutrition Therapy Improve Patient Outcomes: A Pilot Study Documenting the Efficacy of Registered Dietitian Nutritionist Interventions through Retrospective Chart Review, J. Acad. Nutr. Diet 117 (2017) 1254–1264. doi: 10.1016/j.jand.2017.01.023. [DOI] [PubMed] [Google Scholar]
- [16].Su D, McBride C, Zhou J, Kelley MS, Does nutritional counseling in telemedicine improve treatment outcomes for diabetes? A systematic review and meta-analysis of results from 92 studies, J. Telemed. Telecare 22 (2016) 333–347. doi: 10.1177/1357633X15608297. [DOI] [PubMed] [Google Scholar]
- [17].Myers EF, Spence LA, Leslie B, Brauer PM, Spahn JM, Snetselaar L, Nutrition and Telephone Counseling, Top. Clin. Nutr 25 (2010) 88–108. doi: 10.1097/tin.0b013e3181db7dd9. [DOI] [Google Scholar]
- [18].B. J Oakes A, Garmo V, Bone L, Longo D, Segal J, Identifying and Prioritizing the Barriers and Facilitators to the Self-Management of Type 2 Diabetes Mellitus: A Community-Centered Approach, Patient 10 (2017) 773–783. [DOI] [PubMed] [Google Scholar]
- [19].MANNA, Where We Serve, (n.d.).
- [20].Home - MANNA, (n.d.).
- [21].MyChart | Powered by Epic, (n.d.).
- [22].Care D, Suppl SS, Lifestyle management: Standards of medical care in Diabetesd2018, Diabetes Care 41 (2018) S38–S50. doi: 10.2337/dc18-S004. [DOI] [PubMed] [Google Scholar]
- [23].HealthShare Exchange |, (n.d.). https://www.healthshareexchange.org/ (accessedDecember 8, 2020).
- [24].Janssen MF, Lubetkin EI, Sekhobo JP, Pickard AS, The use of the EQ-5D preference-based health status measure in adults with Type 2 diabetes mellitus, Diabet. Med 28 (2011) 395–413. doi: 10.1111/j.1464-5491.2010.03136.x. [DOI] [PubMed] [Google Scholar]
- [25].Craig BM, Rand K, Choice Defines QALYs A US Valuation of the EQ-5D-5L, 2018. www.lww-medicalcare.com (accessedDecember 8, 2020). [DOI] [PubMed]
- [26].Kripalani S, Risser J, Gatti ME, Jacobson TA, Adherence to Refills and Medications Scale, PsycTESTS® (2009) 18080. https://search.proquest.com/docview/1876877068?accountid=14529%0Ahttps://oceano.biblioteca.deusto.es/openurl/DEUSTO/DEUSTO_SP? [DOI] [PubMed] [Google Scholar]
- [27].Toobert DJ, Hampson SE, Glasgow RE, The Summary of Diabetes Self-Care, Diabetes Care J 23 (2000) 943–950. [DOI] [PubMed] [Google Scholar]
- [28].J. L Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent D, Outcomes Measures for Health Education and Other Care Interventions, SAGE Publications, Inc., Thousand Oaks, CA, 1996. [Google Scholar]
- [29].Bradley C, Speight J, Patient perceptions of diabetes and diabetes therapy: Assessing quality of life, Diabetes. Metab. Res. Rev 18 (2002) 64–69. doi: 10.1002/dmrr.279. [DOI] [PubMed] [Google Scholar]
- [30].Burroughs TE, Desikan R, Waterman BM, Gilin D, McGill J, Development and Validation of the Diabetes Quality of Life Brief Clinical Inventory, Diabetes Spectr 17 (2004) 41–49. doi: 10.2337/diaspect.17.1.41. [DOI] [Google Scholar]
- [31].Katz DL, Rhee LQ, Katz CS, Aronson DL, Frank GC, Gardner CD, Willett WC, Dansinger ML, Dietary assessment can be based on pattern recognition rather than recall, Med. Hypotheses 140 (2020) 109644. doi: 10.1016/j.mehy.2020.109644. [DOI] [PubMed] [Google Scholar]
- [32].Welch G, Rose G, Ernst D, Motivational interviewing and diabetes: What is it, how is it used, and does it work?, Diabetes Spectr 19 (2006) 5–11. doi: 10.2337/diaspect.19.1.5. [DOI] [Google Scholar]
- [33].Hager ER, Quigg AM, Black MM, Coleman SM, Heeren T, Rose-Jacobs R, Cook JT, Ettinger de Cuba SA, Casey PH, Chilton M, Cutts DB, Meyers AF, Frank DA, Development and validity of a 2-item screen to identify families at risk for food insecurity, Pediatrics 126 (2010) e26–32. doi: 10.1542/peds.2009-3146. [DOI] [PubMed] [Google Scholar]
- [34].Type 2 diabetes: newer agents Type 2 diabetes: newer agents for blood glucose control in type 2 diabetes, 2010. www.nice.org.uk (accessedDecember 10, 2020).
- [35].Garber AJ, Treat-to-target trials: uses, interpretation and review of concepts, Obe. Metab 16 (2014) 193–205. doi: 10.1111/dom.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lenters-Westra E, Schindhelm RK, Bilo HJG, Groenier KH, Slingerland RJ, Differences in interpretation of haemoglobin A1c values among diabetes care professionals, Neth. J. Med 72 (2014) 462–466. [PubMed] [Google Scholar]
- [37].Hirst JA, Stevens RJ, Farmer AJ, Changes in HbA1c Level over a 12-Week Follow-up in Patients with Type 2 Diabetes following a Medication Change, (n.d.). doi: 10.1371/journal.pone.0092458. [DOI] [PMC free article] [PubMed]
- [38].Crawford P, Res A, Effectiveness of Cinnamon for Lowering Hemoglobin A1C in Patients with Type 2 Diabetes: A Randomized, Controlled Trial, (n.d.). doi: 10.3122/jabfm.2009.05.080093. [DOI] [PubMed]
- [39].Davis NJ, Tomuta N, Schechter C, Isasi CR, Segal-Isaacson CJ, Stein D, Zonszein J, Wylie-Rosett J, Comparative Study of the Effects of a 1-Year Dietary Intervention of a Low-Carbohydrate Diet Versus a Low-Fat Diet on Weight and Glycemic Control in Type 2 Diabetes, (2009). doi: 10.2337/dc08-2108. [DOI] [PMC free article] [PubMed]
- [40].Spencer MS, Rosland A-M, Kieffer EC, Sinco BR, Valerio M, Palmisano G, Anderson M, Ricardo Guzman J, Heisler M, Effectiveness of a Community Health Worker Intervention Among African American and Latino Adults With Type 2 Diabetes: A Randomized Controlled Trial, Am. J. Public Heal. Spencer Al. | Peer Rev. | Res. Pract 101 (2011) 2253. doi: 10.2105/AJPH.2010.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Delahanty LM, Riggs M, Klioze SS, Chew RD, England RD, Digenio A, Maximizing retention in long-term clinical trials of a weight loss agent: use of a dietitian support team, (2016). doi: 10.1002/osp4.57. [DOI] [PMC free article] [PubMed]
- [42].Sussman JB, Hayward RA, Using instrumental variables to adjust for treatment contamination in randomised controlled trials, BMJ 340 (2010) 1181–1184. doi: 10.1136/bmj.c2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shepard DS, Cost-effectiveness in Health and Medicine. By Gold MR, Siegel JE, Russell LB, and Weinstein MC (eds). New York: Oxford University Press, 1996, J. Ment. Health Policy Econ 2 (1999) 91–92. doi:. [DOI] [Google Scholar]
- [44].Glick HA, Doshi JA, Sonnad SS, Polsky D, Economic Evaluation in Clinical Trials, Oxford University Press, 2014. doi: 10.1093/med/9780199685028.001.0001. [DOI] [Google Scholar]
- [45].Rising KL, Hudgins A, Reigle M, Hollander JE, Carr BG, “I’m Just a Patient”: Fear and Uncertainty as Drivers of Emergency Department Use in Patients With Chronic Disease, Ann. Emerg. Med 68 (2016) 536–543. doi: 10.1016/j.annemergmed.2016.03.053. [DOI] [PubMed] [Google Scholar]
- [46].Reyes J, Tripp-Reimer T, Parker E, Muller B, Laroche H, Factors Influencing Diabetes Self-Management Among Medically Underserved Patients With Type II Diabetes, Glob. Qual. Nurs. Res 4 (2017). doi: 10.1177/2333393617713097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].National Quality Forum, A Framework for State Medicaid Programs to Address Social Determinants of Health: Food Insecurity and Housing Instability, (2017) 57. http://www.qualityforum.org/Publications/2017/12/Food_Insecurity_and_Housing_Instability_Final_Report.aspx. Accessed5/22/2018.
- [48].Malinda Ellwood C, Downer S, Broad Leib E, Greenwald R, Farthing-Nichol D, Luk E, Food Is Medicine Opportunities in Public and Private Health Care for Supporting Nutritional Counseling and Medically-Tailored, Home-Delivered Meals, n.d. http://www.chlpi.org/wp-content/uploads/2013/12/ (accessedDecember 15, 2020). [Google Scholar]
- [49].Bipartisan Members of Congress Launch Food is Medicine Working Group to Highlight Impacts of Hunger on Health | U.S. House of Representatives, (n.d.). https://mcgovern.house.gov/news/documentsingle.aspx?DocumentID=397179 (accessedDecember 15, 2020).


