Abstract
Sarcopenia, and high blood pressure are highly prevalent, preventable conditions that pose significant burden for older adults and on the healthcare system. Current prevention and treatment of high blood pressure in sarcopenia, by non-pharmacological approaches remain limited and are far from optimal. Clinical trials and mechanistic studies provide encouraging evidence of a plausible therapeutic effect of progressive resistance training (PRT) on blood pressure in younger, and pre-hypertensive and hypertensive older adults. The impact of PRT on blood pressure has not been empirically tested in older adults with sarcopenia. This pilot study aims to provide effect size confidence intervals, clinical trial and intervention feasibility data, and procedural materials for a full-scale randomized controlled trial that will determine the efficacy of PRT intervention as a therapeutic strategy for blood pressure control in older adults with sarcopenia. Participants (N= 90) will be randomized to receive exercise educational materials or the PRT intervention consisting of 24 supervised exercise sessions over 12-weeks. Follow-up assessments will occur at 12-weeks and one-year later. The primary outcome is systolic blood pressure and diastolic blood pressure, analyzed separately. Microvascular mechanisms linking muscle (perfusion, strength, function) to changes in blood pressure will be explored at baseline and 12-weeks. This study will provide new evidence for the therapeutic effect of PRT as a non-pharmacological strategy for improving blood pressure. Insights gained may also inform of the potential role of muscle strength as a novel target for blood pressure control, and future exercise prescription guidelines related to muscle strengthening in high-risk older adults.
Keywords: muscle strength, blood pressure, sarcopenia, progressive resistance training, older adults
1. Introduction
Sarcopenia is an important geriatric syndrome that conservatively affects 5% to 13% of adults over 60 years of age, with prevalence rates expected to be as high as 50% in adults aged 80 years and older.1 Sarcopenia is characterized as progressive decline in skeletal muscle tissue and impairments in muscle function. With sarcopenia, there is an increased risk of having clinically low muscle strength,2–5 a discernable indicator of reduced physiological resiliency that increases vulnerability to mobility impairments, physical frailty and disability, and pre-mature mortality.6–8 Although mechanisms have yet to be clearly elucidated, low muscle strength appears to have negative effects on vascular function, and thus, blood pressure in this population.9–13 High blood pressure has been evidenced in various international cohorts of sarcopenic adults.14–19 Older adults with chronically high blood pressure are more likely to suffer from low muscular strength (and function) and deconditioning,20 and a growing body of prospective evidence suggests that low muscle strength may be a risk factor for high blood pressure in older adults.21–25
Sarcopenia and high blood pressure are both worsened by the effects of physical inactivity, which in and of itself contributes to greater impairments in muscle strength (and function), elevated blood pressure, and further decreases in physical activity.2 Emerging evidence from randomized control trials demonstrate progressive resistance training (PRT) can effectively improve muscle strength in sarcopenic adults.22, 26–28 Similarly, the feasibility, and effectiveness of PRT to counteract loss of muscle strength and improve physical function has been demonstrated in clinical trials of older adults with various functionally-limitations6, 7, 29–31, including those with frailty,32–35,36 knee osteoarthritis37–39, low bone mass,40 or osteosarcopenia,41, 42 and in institutionalized elderly adults with functional disabilities.35, 36, 43
Different lines of research have reported on the effect of PRT on lowering blood pressure in normotensive and hypertensive adults absent of cardiovascular or functionally limiting disease20, 44–49, as well as in pre-hypertensive44, 46–48 and hypertensive middle age and older adults,44, 50, 51 in whom muscle deconditioning is high20 and the potential for BP improvements are the greatest. Mechanistic studies described by our group52, 53 and others54–56 have shown encouraging evidence of a plausible therapeutic or protective effect in young and middle aged populations, such that PRT improves endothelium dependent vasodilation flow in the microcirculation leading to enhanced muscle perfusion, and reductions in arterial blood pressure. However, more evidence is needed to corroborate the impact of resistance-based exercise on blood pressure and potential mechanisms involved, in a targeted, high-risk older adult population with sarcopenia.
Despite the promising health benefits of resistance training in older adults, fewer than 25% meet national recommendations for muscle strengthening (2 times per week).57, 58 Reasons for the low prevalence rates may partly be explained by perceived informational barriers (e.g., misinformation and lack of proper training) and complexity. Thus, experimental studies that demonstrate effective promotion, and more importantly, the continuation of resistance training behavior by participants using approaches that are theoretically driven are warranted, and are likely to maximize vascular and peripheral adaptations to resistance training long-term.
The INERTIA (Impact of Interventional Exercise and Resistance Training on Blood Pressure Control In Older Adults with Sarcopenia) pilot study aims to provide effect size and confidence intervals for the change in blood pressure. It will also deliver clinical trial and intervention feasibility data and procedural materials for a full-scale randomized controlled trial that will determine the efficacy and mechanisms of action of the PRT intervention in older sarcopenic adults. Particular attention is placed on theoretical constructs used in the INERTIA study (e.g., self-efficacy, self-regulation and affective responses to training), that are based on social cognitive theory and the transtheoretical model to promote adoption of the tailored PRT program, optimize behavioral maintenance, and maximize the likelihood of achieving improvements in blood pressure outcome and muscle strength gains. Additionally, this trial will explore potential microvascular mechanisms linking muscle to blood pressure improvements. This paper describes the study design and methodology.
2. Materials and Methods
2.1. Study design
The study design is depicted in Figure 1 with each element detailed in the following sections.
Figure 1:

Study visit activities and outcomes collected for A0-C and intervention participants; FM, familiarization sessions, P-0I, post-intervention.
The INERTIA trial is a small randomized controlled trial in which 90 community-dwelling older adults aged 65 years and older, with probable sarcopenia (described below) are randomized in a 2:1 allocation procedure to the supervised progressive resistance training, or to the assessment-only control (AO-C) group, which consists of exercise educational materials mailed every three weeks for 12 weeks. All participants will return to receive follow-up assessments post-intervention (12 weeks) and 1 year later. This trial is registered in clinicaltrials.gov (NCT04255745) and approved by the University of Illinois at Chicago (UIC) Institutional Review Board. The study’s specific aims are as follows:
Aim 1. Experimentally assess the preliminary efficacy and feasibility (recruitment, retention, acceptability) of PRT on changes in blood pressure levels (primary outcome), and muscle strength at 12 weeks among participants randomized to the intervention (n=60) versus the AO-C (n=30) group.
Aim 2. Evaluate whether muscle strength mediates the effect of PRT on blood pressure at 12 weeks in intervention participants.
Aim 3. Test whether improvements in muscle strength and blood pressure are sustained one year following progressive resistance training.
Exploratory, Aim 4. Explore the PRT intervention compared with AO-C on secondary measures of cardiovascular risk (e.g., lipid profile, non-fasting glucose, insulin resistance [HOMA-IR] and inflammation [Interleukin-6, IL-6]) and physical performance (e.g., 6-meter gait speed, five time sit to stand test, 3-stage standing balance, timed up and go test) at 12 weeks and at 1 year following the PRT intervention, which may provide insights about cardiometabolic or physical benefits, if the PRT intervention is effective (or areas in need of better targeting if the intervention is not effective).
Exploratory, Aim 5: To initiate investigations of potential microvascular mechanisms (e.g., endothelial function) that link muscle (perfusion, strength, function) to improved blood pressure status (microvascular function) in a sub-sample (n=15) of intervention and AO-Cs, at baseline and at 12 weeks following PRT.
Central hypothesis:
Compared with AO-Cs, intervention participants will achieve significantly lower (improved) blood pressure levels at 12 weeks. Improvements in blood pressure may additionally be mediated by improvements in muscle strength. Additional improvements in physical performance or cardiovascular risk profiles resulting from the PRT intervention will be explored. Microvascular vasodilator function (endothelial function) will additionally be evaluated as a potential mediator of the intervention effect.
2.2. Eligibility criteria
Community-dwelling older adults ≥65 years of age will be eligible to participate (Table 1). 65 years is used as the cut point as after age 65 is when muscle mass and strength tend to decrease more rapidly in older adults, with similarities seen across all race/ethnic backgrounds.59–61 Those with significant medical (e.g., cardiovascular disease, active or currently treated cancer, lupus, multiple sclerosis, advanced/severe rheumatoid arthritis) or cognitive impairment (Alzheimer’s, dementia) or psychiatric comorbidities (e.g., psychotic or bipolar disorders) or special lifestyle circumstances (e.g., complete dependence on a cane/wheelchair, scooter, or planned relocation) will be excluded. Women and men of any racial or ethnic background who speak English, meet the inclusion criteria, and have no exclusion criteria will be enrolled.
Table 1:
Participant Eligibility Criteria
Inclusion criteria (patients will be included if meeting all of the following)
|
| Exclusion criteria (patients will be excluded if meeting any of the following) |
Medical history exclusions
|
Other exclusions
|
MoCA, Montreal Cognitive Assessment
2.3. Recruitment
The target sample size of 90 eligible and consenting participants will be enrolled over a 3.5-year period. Older adults will be recruited from the greater Chicago area by flyer posted in University campus buildings, and in nearby community churches, libraries, parks and recreational centers, barber shops/salons, and grocery or convenience stores, and cultural centers. Electronic announcements will also be posted in the University online classifieds on social media sites (e.g., Facebook). Informal research information sessions will also be scheduled at senior and community centers. Flyers or electronic announcements may additionally be sent to community partners (i.e., Chicago parks district) to optimize greater participant reach.
Recruitment at University hospital outpatient clinic facilities (e.g., Physical Therapy and Internal Medicine and Geriatrics Outpatient Clinics) will occur using a variety of methods. First, flyers, handouts, electronic announcements will be posted at outpatient clinics at or near the University hospital. Second, patients (current or past) who receive care at the Physical Therapy Outpatient Clinic may be identified and pre-screened through electronic health records (EHRs). In addition to meeting basic eligibility criteria, new and follow-up patients receiving care at the Physical Therapy Outpatient Clinic for at least 2 visits (depending on the need of care), of whom are presumed to have a higher likelihood of being compliant during the study, will be identified. Enrollment will only be made after patients have completed physical therapy sessions. Enrollment in these patients may be delayed by 6 months from the last physical therapy session if their care plan closely resembles that of the proposed intervention. Prior to enrollment, therapy program and duration of participation will be reviewed to confirm that exercises prescribed appreciably differ from those included in the proposed intervention.
Third, EHRs of patients receiving care from Internal Medicine and Geriatrics Outpatient Clinics will be pre-screened to identify potential participants meeting basic eligibility criteria (e.g., age, absence of exclusionary medical or psychiatric comorbidities). Fourth, patients identified from EHRs will be sent invitations that contain the study screening form which can be completed at home or with research staff over the phone. Participants will be asked to initiate contact if interested, with an option to opt out. If no response is received, research staff will begin calling patients 2 weeks after the invitations are sent. Provider approval is not required in order to participate in the study.
Potential participants may also be identified via various research registries, including (1) ResearchMatch.org database, an NIH sponsored initiative to improve clinical trial recruitment; (2) the “Be The New Normal™ (TNN)” Research registry, an online registry developed as a partnership between UIC and other major universities and clinical and hospital organizations within the State of Illinois and the Chicago Department of Public Health; and, (3) the UIHealthRegistry, an online research registry platform recently developed by the UIC Center for Clinical and Translational Science in collaboration with numerous academic and research institutions within Illinois.62
2.4. Screening, Enrollment and Baseline Visit
2.4.1. Screening and enrollment
Screening of participants will proceed in three steps depending on whether eligibility criteria is met: (1) screening questionnaire; (2) cognitive function assessment via The Montreal Cognitive Assessment (MoCA) questionnaire; and, (3) grip strength to determine sarcopenia status and eligibility into the study. Each step is described in further detail below.
First, interested participants will be screened for partial eligibility by questionnaire in person or by phone under an alteration of consent. Participants who screen eligible on initial screening must complete the remaining screening procedures during an in-person visit, which will tentatively serve as a baseline clinic visit. Prior to this visit, participants will be provided a copy of the consent form to review the study requirements, expectations, and assessments to be collected during the baseline visits. Participants will also be instructed to bring in prescribed medications (or bottles or a picture of bottles) to the in-person visit, which will be logged on the medical history form.
At the in-person visit, research staff will then administer the MoCA questionnaire under a waiver of documentation of consent, and those with an education-adjusted score of <22, indicative of mild cognitive impairment, will be excluded. Participants who score >22 on the MoCA will be presented with the informed consent in detail by trained staff and will be asked to provide written consent prior to the final screening. Upon providing consent, participants will then be assessed for grip strength, which will be used as the definitive measure to confirm eligibility, as per recent guidelines on sarcopenia detection.8 Low muscle strength (assessed via grip strength) is recognized as the primary parameter of sarcopenia, as it is presently the most reliable measure of muscle function.8 Participants who present with low sex-specific grip strength are considered to have ‘probable sarcopenia’ and will proceed to baseline examination. The informed decision to recruit participants with ‘probable’ versus ‘confirmed’ sarcopenia was made based on the wide range in sarcopenia prevalence reported in the literature, partly due to the inconsistent diagnostic criteria. Additionally, in clinical practice, detection of probable sarcopenia is sufficient to trigger assessment of causes and start intervention.8 Recruiting at the stage of ‘probable’ sarcopenia provides further opportunities to prevent or delay sarcopenia or its progression, and to minimize loss of strength prevention in at-risk older adults. This grip strength assessment used for screening will serve as the baseline measure. Figure 2 describes sequential steps that eligible participants will receive during the baseline visit to further confirm sarcopenia diagnosis by Dual-energy X-ray absorptiometry (DXA) scan and identify sarcopenia severity via performance-based testing.
Figure 2.
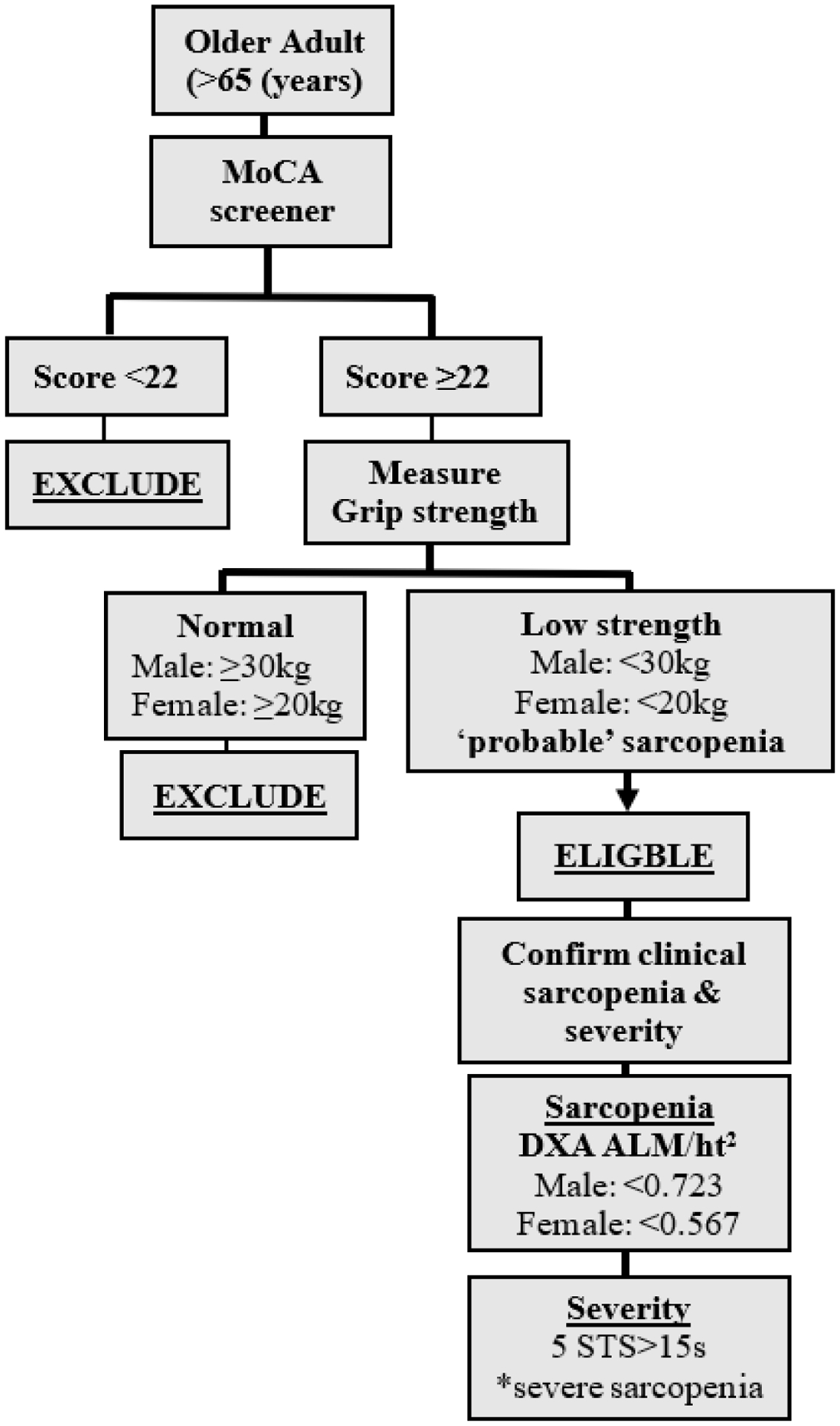
Algorithm for screening procedures, and enrollment of participants with varying degrees of sarcopenia; adapted from Cruz-Jentoft et al.8
ALM, appendicular lean mass; ht, height; MoCA, Montreal Cognitive Assessment
The consent form will include additional language asking participants to volunteer for an optional fat tissue biopsy to evaluate the microvascular system and test endothelial function. These procedures which will be carried out by a registered nurse, will be explained thoroughly during the consent process. Participants who agree to the fat biopsy procedure will be asked to provide samples at baseline and upon completion of the 12-week intervention program.
Enrollment is carried out as soon as possible but may be prolonged no longer than 12 weeks after the initial grip strength assessment.
2.4.2. Baseline visit
Baseline measurements will be collected over two separate visits, with no more than 14 days between the two visits. All clinical measurements will be collected during baseline visit 1 and muscle strength assessments during baseline visit 2. To ensure blinding of baseline data and outcome collection, randomization into either intervention arm will occur after all baseline information has been collected. At post-intervention and one-year follow-up visits (study primary and secondary endpoints), blood pressure outcome assessment will be collected without knowledge of the participant’s treatment assignment or previous visit(s) blood pressure readings.
Baseline sessions will begin with height, weight, and waist and hip circumference measurements by trained study staff following standardized procedures.63 Height and weight information will be inputted into the DXA machine for whole body composition scanning, including the detection of low muscle quantity (appendicular skeletal muscle mass relative to body size [ALM/height2]) that is used to confirm sarcopenia. Sarcopenia will be confirmed immediately after scanning, using the operational criteria (e.g., men: ALM/height2<0.723; women: ALM/height2 <0.567).8 As part of this study, we will provide participants with a copy their DXA scans; however, research staff will not provide any medical advice concerning the results of the scan, nor will results be shared the participant’s provider or in their medical record. Scans are for research purposes only.
Following, trained study staff will collect blood pressure (primary outcome) using standard protocols and instruments. Appropriate cuff bladder size will be determined based on arm circumference; thigh cuffs may be used instead of— or to confirm arm readings if the participant is obese (body mass index [BMI] >29.9 kg/m2). Blood pressure will be measured in the left arm with an automated blood pressure monitor, with the participant in seated position with legs uncrossed, and after 5 minutes of rest with no talking or distractions (i.e., phone use is not permitted). Two measurements will be taken and recorded. Participants will then undergo a battery of physical performance tests to collectively indicate whole body muscle function related to locomotion and central and peripheral nervous function (Appendix A). The five time sit to stand test will be administered to indicate severity of sarcopenia, i.e., those with slow test results (>15 seconds) in at least one of the two trials will be considered to have “severe” sarcopenia (Figure 2). The last portion of the baseline visit will include a non-fasting blood draw, and administration of self-reported questionnaires related to their medical history, physical activity, physical functioning, and mood/depression. To manage time, questionnaires may be sent home with the participant at their request, in which case research staff will schedule a phone meeting to collect responses over phone or will request the participant return in person questionnaires at baseline visit 2 or within 7 to 14 days of the previous visit.
During baseline visit 2, enrolled participants will complete a series of one-repetition maximum (1-RM) tests in a research-dedicated gym, under direct supervision of a trained exercise interventionist, and following the recommended guidelines for RM testing.64 The 1-RM test has been shown to be safe and feasible method to directly assess and optimize muscle strength gains in major muscle groups in older adult populations.64 Importantly, the 1-RM has been shown to be well-tolerated in by older adults with frailty, low bone mass, or controlled hypertension6—characteristics that may be observed in our target sarcopenic population. 1-RM exercises include a combination of upper and lower body muscle groups (knee extension, leg press, chest press, seated row or latissimus pull-down, shoulder press, and leg curl and squat). Briefly, for each of the 6 exercises, the participant will first complete a familiarization test of the exercise. Following demonstration of the exercise by the trainer, the participant will perform the exercise. The trainer will evaluate the participant for proper breathing, form and technique, and will provide mechanical corrections if necessary. The participant will then be instructed to perform 2 sets of the exercise, 10–15 repetitions, at low intensity (~20–40% of participant’s body weight). Following, the participant then performs a “warm-up” trial, wherein s/he performs 1 set of the exercise, 10 repetitions, at 50% of their perceived maximum weight. Thereafter, the participant completes the 1-RM following a 5 repetition/3 repetition/1 repetition/1 repetition (5/3/1/1) protocol, in which training load and intensity progressively increases as the number of repetitions decrease, with each attempt separated by 90- to 120-s rest intervals. 1-RM is defined as the highest load lifted through a full range of motion before two failed attempts at a given load. When the 1-RM is deemed inappropriate (e.g., patients with uncontrolled or unstable metabolic, renal or musculoskeletal disease), the 5-RM test can be used.64 The 1-RM test will be repeated at week 6 (of the 12-week intervention, intervention group only) and at the end of the intervention (intervention and controls; Table 2).
Table 2:
Cohort study data collection and outcomes for all participants
| Visit | |||||
|---|---|---|---|---|---|
| Domain | Baseline | Follow up visit | |||
| Week 6 of Intervention* | 12-week P0I | 6-months P0I | 1-year P0I | ||
| Questionnaires | |||||
| • Cognitive Function (MoCA) | X | X | |||
| • Demographics and lifestyle behaviors | X | X | |||
| • Medical History and medication inventory | X | X | |||
| • Depression (CES-D) | X | X | |||
| • Self-reported physical functioning (SF-36 PFI) | X | ||||
| • Physical activity history (CHAMPS) | X | X | X | X | |
| Physical and laboratory measurements | |||||
| • Height | X | X | X | X | |
| • Weight, waist circumference, waist-hip-ratio | X | X | X | X | |
| • Heart rate (also monitored pre and post exercise in intervention participants) | X | X | X | X | |
| • DXA scan for sarcopenia confirmation | X | X | X | X | |
| • Muscle Strength: Grip strength, 1-Repetition maximum test | X | X | X | X | |
| • Resting systolic and diastolic blood pressure (also monitored pre and post exercise in intervention participants | X | X | X | X | |
| • Cardiovascular risk factors by non-fasting blood and serum specimens: blood lipids blood glucose, Insulin resistance (HOMA-IR), inflammation (IL-6) | X | X | X | ||
| • Physical performance measures: 6-meter gait speed, five time sit to stand test, 3-stage standing balance, timed up and go | X | X | X | X | |
| • Endothelial Function via flow-induced vasodilation | X | X | |||
| Feasibility Process Measures | |||||
| • Focus group conduct* | X | ||||
| • Intervention Acceptability (monitored continuously during exercise intervention)* | |||||
| • Recruitment and intervention process measures (monitored continuously) | |||||
| • Safety of participants in the intervention arm (monitored continuously) | |||||
| • Symptoms and adverse events (monitored continuously) | |||||
Data collected in intervention arm only
B0, baseline; CES-D, Center for Epidemiologic Studies Depression Scale; CHAMPS, Community Healthy Activities Model Program for Seniors; HOMA-IR, homeostasis model assessment-estimated insulin resistance; IL-6, interleukin-6; MoCA, Montreal Cognitive Assessment; P0I, post-intervention; SF-36 PFI, Short-Form Physical Function Index
2.5. Randomization and blinding
Following informed consent and baseline data collection, participants will be randomized to the PRT intervention or AO-C group using a 2:1 allocation procedure (computerized random numbers). Randomization scheme table will be generated using permuted block method with stratification on baseline body mass index and gender, and the procedure will be done using REDCap (Research Electronic Data Capture) system, which ensures allocation concealment. Randomization will be performed by a designated study staff person (e.g., research coordinator) who is responsible for overseeing data management and data quality assurance, but does not have the ability to influence its execution. By design, treatment assignments are identifiable to participants and the exercise interventionist (trainer) and select study staff who are responsible for scheduling visits, but the investigators, safety monitoring committee members, outcome assessors, and data analyst will be blinded throughout the trial. Further, to ensure unbiased ascertainment between the intervention and control participants, the trainer (interventionist) will not be an outcome assessor and will be masked to all outcome measures obtained by outcome assessors during blind assessments.
2.6. Assessment-only controls (AO-C)
AO-C participants will receive exercise educational material developed based on the NIH/National Institutes on Aging Go4Life® every 3 weeks during the 12-week intervention timeline. A total of 4 mailings will be sent to controls during the 12-week exercise intervention. Materials include non-specific at-home exercises focused on endurance, balance, strengthening, and flexibility with minimal equipment and items commonly found at home (chair, tennis ball, soup cans). With each exercise mailing, an exercise log will be included, which asks participants to document the type and duration of exercise performed each day, along with any comments they wish to share. Control particpiants will be encouraged to complete an exercise log for every day exercise is perfromed (regardless of whether it is from the exercise materials received or other exercise, or if no exercises performed) for the following three weeks. However, participants in the control group will not receive any specific instructions with respect to exercise conduct nor will they receive an exercise prescription from study personnel. Exercise logs will be returned in a pre-stamped envelope, or research staff may collect responses over phone no more than 14 days from the last dated exercise log.
2.7. Intervention format, structure and content delivery
Participants randomized to the exercise intervention will complete 12 weeks (up to 24 sessions) of supervised progressive resistance training. Up to two make-up sessions will also be provided at the end of the 12-week period.
2.7.1. Theoretical basis
The theoretical underpinnings of the intervention will be derived from Social Cognitive Theory65 and the Transtheoretical Model of Behavior Change.66 Social Cognitive Theory emphasizes initiation and maintenance of behaviors are determined by a complex interaction of individual [e.g., self-efficacy], social [e.g., support network] and environmental factors, whereas the Transtheoretical Model of Behavior Change66 describes behavior change as a dynamic process that moves, at variable speed, through stages of readiness to change.
The fundamental tenet of resistance training is the ability to induce a marginal overload within the context of a higher-intensity stimulus (defined as degree of momentary effort, and not necessarily the amount of resistance). Following progressive overload, recovery, adaption, and maintenance are key to affect and improve muscle strength and function. With this respect, initial self-efficacy for performing the behavior (i.e., lifting heavy resistance), includes developing confidence in the ability to both, move the resistance on a leg press machine, as well as move the resistance while keeping to repetition form and duration, and to be able to consistently create an overload at every several training session either by increasing repetitions, resistance, time under load, or a combination of these factors.64, 67 Further, confidence is needed to effectively perform training under these factors, not only for one exercise, but for multiple exercises targeting various muscle groups. Performing these exercises with precision (e.g., form) and uniformity for each movement and repetition requires cognitive (knowing what to do), motivational (wanting to perform), and behavioral (execution) factors.67 Confidence in these realms, as well as awareness and self-evaluation of progress is gained over time, and with practice and with frequent assessment feedback by the trainer. Moreover, self-regulation, in the form of consistent planning and goal-setting is necessary to elicit maintenance of resistance training behaviors. With resistance training, sessions need to be scheduled and rest days in between sessions must be planned in order to elicit appropriate neuromuscular learning and adaptations. Rest days also allow performance and progress to be assessed (from both the participant and trainer’s perspective). Thus, each session needs to be monitored by a trainer, who can facilitate self-regulation approaches with a cognitive and behavior focus, evaluate effort and progress, and discuss with the participant, new resistance or repetition goals for subsequent workouts while also providing/encouraging self-feedback that are relate back to participant’s short-term and long-term goals.67 Importantly, because evidence of progress in resistance training is specific and quantifiable, it can contribute to positive ‘affect,’ (and thus, positive feedback) to reinforce various facets of self-regulation. For example, anticipation of making progress in a training session can lead to a participant feeling positive affect when actual progress has been made, which may further strengthen self-regulation skills and positive self-feedback and actual performance. It is therefore critical that in addition to monitoring actual performance and training efficiency, trainers are aware of how to adapt the protocol, while still maintaining its integrity, to better align with a participant’s goals, which in turn, may increase motivation, enhance positive affect, and greater likelihood of reaching performance goals.67
In INERTIA, the trainer (interventionist) will work closely with each participant to elicit outcome expectations, perform realistic, individualized goal setting, and develop a customized action plan in the form of a mutually agreed-upon contract. To improve adherence during the intervention, the trainer will attend to the presence of any disincentives and relapse warning signs and will apply motivational interviewing techniques to help participants explore and resolve ambivalence about making changes in exercise behavior, promote positive affect at all stages of the training session to reinforce self-regulation (e.g., anticipatory affect for initiating in resistance training, affect while engaged in resistance training, and affect when a session is completed).67
2.7.2. Structure
The exercise intervention delivered in the INERTIA study is based on the American Heart Association (AHA) and the American College of Sports Medicine (ACSM) recommendations for PRT for high risk older adults.48, 64 The PRT program utilizes a goal and progress-based (overload, recovery, and adaptation) approach with foundational underpinnings of social and transtheoretical behavior change constructs, to optimize exercise initiation, and enhance training efficiency and likelihood of exercise program completion. Further, each participant’s training program will be tailored according to their physical and functional abilities assessed at baseline, as well as personal preferences.
The PRT intervention will be performed at a University exercise gym and will be delivered by a trained research staff member with an exercise physiology background and strength and conditioning certification. The PRT protocol will include a combination of seven upper- and lower-extremity exercises (chest press, seated leg press, seated latissimus pull-down, knee/leg extension, shoulder press, leg curls, and calf-raises) performed in the order described, using pressurized weight machines, where applicable. To optimize self-regulation, participants will attend exercise sessions on 2 non-consecutive days a week. Each exercise session will begin with a 10-minute warm-up of low-intensity aerobic exercise (treadmill walking or stationary bike) and flexibility and dynamic stretching targeting major muscle groups. Training sessions will be approximately 1 hour and 30 minutes (including warm up and cool down). Trainers may deliver a training session to a maximum of 2 participants at a time to promote social support (and thus, self-efficacy) and to maximize pragmatic and feasibility of study design.
2.7.3. Intervention delivery
Training regimen will gradually progress by workload and by relative perceived level of exertion (RPE), via the Borg scale protocol,68 to monitor exercise intensity during the 12-week training program. Since the objective of the training program is to improve muscle strength, the resistance training intensity and number of repetitions performed with each set will be inverse as the participant progresses. To elaborate, once overload is reached (that is, the “upper limit” of the prescribed repetition range), the trainer will gradually increase the number of repetitions (i.e., 12–15 reps), and then the training load weight (resistance) by up to 5% (e.g., 2 to 5 lb increase in weight for arm exercises, 5 to 10 lb increase in leg exercises). Resistance will be incremented only when a subject completes 12 reps for at least two of the three total sets at a given resistance, which will be complemented by a reduction in the number of repetitions per set. Finally, once overload of the prescribed loaded weight is achieved, then increasing the number of sets per exercise, and decreasing the rest period between sets or exercises will follow. Each set is designed to be performed to the point of muscle fatigue but not failure in order to avoid over exertion of muscles and likelihood of injury or debilitating residual muscle soreness.69
The ultimate goal is for each participant to progress and maintain their workload to 3 sets of 8–12 repetitions performed at 80% of the initial 1-RM (RPE of 8–10). Each participant will start the 12-week training with 1 to 2 sets/exercise, resistance at 40–50% of their 1-RM, 10–15 repetitions per set. On week 2, participants will be instructed to perform 2 sets of 8–12 repetitions with a moderate-intensity training load of 60–65% 1-RM. By week 3, participants will perform 3 sets of 8–12 repetitions with a moderate-to-vigorous intensity training load of 60–65% 1-RM. By week 4, participants will perform 3 sets of 8 to 12 repetitions with a moderate-intensity load of 70–75% 1-RM. By week 5, participants will perform 3 sets of 8 to 12 repetitions with a moderate-intensity load of 80% 1-RM. Weeks 6 through 12 will be dedicated towards maintaining or working towards this optimal load of 3 sets of 8 to 12 repetitions with a moderate-intensity load of 80% 1-RM. Once a participant reaches the optimal training load, emphasis will be placed on “transfer of training” and maintenance of learned training behaviors, so that participants can later train on their own in the long-term, as appropriate with the participant’s goals. Rest session between sets will be between 2–3 minutes, up to 5 minutes if needed (i.e., in severely deconditioned participants). Finally, each training session will include calf-raise exercises to evaluate muscle endurance, with the goal of seeing how many calf raises a participant can do until they reach a RPE of 6–7 or a maximum of 25 calf raises per set (whichever is first). Participants will complete 2 sets using body weight only, with one hand placed on the wall for balance and may progress to holding free weights when appropriate. Each session will end with a 5-minute cool-down of low-intensity static stretching, and flexibility exercises. Participants will be asked to fill out a brief exercise acceptability questionnaire at the end of each session. To reinforce resistance training behaviors, participants will be offered the opportunity to train in the research gym once a month during the surveillance period (12-weeks to one-year follow up visit). During this time, exercise trainers will be present to monitor safety and answer questions but will not provide any formal exercise instruction (additional details described in section 2.9).
2.8. Participant Safety
2.8.1. Exercise Contraindications
Per ACSM and AHA guidelines,48, 64 the contraindication for exercise for this high-risk population is having a resting (pre-exercise) systolic blood pressure ≥200 mmHg OR a diastolic blood pressure ≥110 mmHg. Several considerations will be evaluated to determine if a person is eligible to participate in a planned exercise session visit. First, blood pressure will be assessed before starting each exercise session (two trials) and after each session, with additional measurements taken as needed.64 The first reading will take place after 5 minutes of rest, and the second reading after 3 minutes of rest. If either of the readings meets the contraindication threshold for blood pressure, the trainer will instruct the participant to rest for 6–10 minutes before rechecking the blood pressure for a third trial. If the third blood pressure reading meets the contraindication cut points, then the participant will be monitored before being sent home. If the third trial blood pressure reading comes down but remains high (i.e., systolic blood pressure, 190 to 200 mmHg OR a diastolic blood pressure, 100–110 mmHg), then the trainer will instruct the participant to complete the aerobic-training warm up before checking the blood pressure for a fourth trial. If the blood pressure remains within range permissible for exercising safely, the trainer will instruct the participant to perform one set of exercises, and then reassess blood pressure after 5 minutes of rest to ensure that the participant can safely continue the training session. All participants will have their blood pressure assessed after completion of the exercise training and cool down. It is prudent to maintain post-exercise systolic blood pressure ≤ 220mmHg, and/or diastolic blood prssure ≤ 105 mmHg.64 If a particpant has a blood pressure reading above either threshold, they will be instructed to repeat the aerobic cool down and rest for 5 minutes before another blood pressure reading is taken. Participants will continued to be monitored until their blood pressure readings are reduced to below contraindications levels.64 If a participant consistently has blood pressure readings that meets contraindications for starting exercise at 3 separate visits, they may be deemed ineligeable and excluded from the study for safety reasons. Additionaly, trainers will make note of whether blood pressure medications were taken prior to their visit in those who reported use of antihypertensive medicaton at baseline. During all training sessions, and regardless of pre-exercise blood pressure, trainers will closely monitor each participant for signs and symptoms of exercise intolerance such as excessive fatigue, dizziness or light-headedness, chronotropic incompetence, and signs or symptoms of ischemia.
2.8.2. Other safety-related precautions
In addition to our eligibility criteria, which includes medical history questionnaire, participants will be carefully screened, and individuals for whom the intervention is deemed medically inappropriate or unsafe will be excluded. Provider approval is not required before potentially eligible participants are contacted for screening or are enrolled. However, participants will be systematically asked at baseline and at the 12-week follow up clinic visit to report any changes in health or possible adverse events occurring since last contact. Participants will also be asked to report any changes in their health or adverse events occurring during one-year follow-up surveillance period (from 12-weeks to one-year follow up). At each exercise session, intervention participants will report any changes in self-reported health or adverse events. Trainers supervising exercise sessions will also closely observe and document if any developments or changes in health status become apparent during exercise sessions. Participants who are diagnosed with any pertinent exclusionary condition (e.g., coronary heart disease or cardiovascular event, stroke) following randomization and prior to completion of the 12-week intervention program may not continue in the trial. If an event or medical diagnosis occurs after the 12-week post intervention visit, participants may remain in the study. An adverse event is defined as any untoward medical or psychological event experienced by a participant during or as a result of his/her participation in the study that represents a new symptom or an exacerbation of an existing condition whether or not considered study-related based on appropriate medical judgment.70 Adverse events discovered outside these planned evaluations (e.g., during intervention encounters) will be duly noted and followed up with, as needed, to assure participant safety.
2.9. Retention
Various retention activities will be followed to minimize loss to follow up and maintain contact of all participants. These strategies will include: 1) careful selection of the research team, including exercise trainers (interventionists) who have completed standardized training to deliver the resistance training intervention, use of motivational interviewing techniques, implement trial-specific protocols, and apply problem solving techniques as appropriate to their study roles; 2) legally adequate informed consent and detailed consenting process by trained research staff; 3) careful eligibility screening including assessment of willingness and motivation to adhere to data collection and treatment requirements; 5) prudent participant incentives including transportation compensation and flexible scheduling; 6) promotion of INERTIA study identity; 7) ongoing monitoring of recruitment and retention; and 8) regular meetings between research staff and exercise trainers to review participant adherence 9) up-to-date contact log for the participant and two emergency contacts. Additionally, reminder emails, phone calls, or text messages by research staff will go out 24 to 48 hours prior to the scheduled clinic visit. All methods of communication will be tracked in a phone log in a secure database, and reasons for absence or cancellations will be documented. Further, all research staff carrying out recruitment will follow a detailed informed consent process that will include a careful review of the study requirements, explanation of the study protocol including the randomized assignment to the intervention or treatment, details and expectations of the treatment, and will stress importance to completing baseline, and all follow-up assessment visits regardless of whether treatment adherence is less than optimal. Research staff will reach out to non-compliant participants and engage in strategies to re-engage participants into the study.
Additional retention activities will be performed to promote ongoing support, participation, and adherence to physical activity during the intervention and surveillance period (12-weeks to one-year follow up), such as raffle tickets for session attendance or completion of data collection forms, birthday cards, and quarterly newsletters. All retention activity content will be carefully tailored to the study population and designed to ensure that materials do not contain information related to the intervention or study outcomes. At the end of the 12-week post-intervention follow up visit, general information regarding community and local facilities (gyms, recreational centers), or other national resources available will be given to intervention and control participants to reinforce or encourage strength training exercises during the surveillance period (12-weeks to one-year follow up) and after completion of study (e.g., after the 1 year follow up visit). In intervention participants, follow-up calls will be made by research staff once a month during the surveillance period (12-weeks to one-year follow up) to answer any questions, report whether resistance training or other exercise behaviors being maintained and the duration and frequency, and document barriers to maintaining training. During the surveillance period, all participants (intervention and control) will be offered the option to attend the research gym to conduct resistance training exercises one time per month, with monitoring and assistance by a trainer as needed. However, no formal prescription or instructions will be provided. Blood pressure measurements will be taken prior to—and after exercise completion, and other precautionary steps to ensure participant safety will be followed during the optional training sessions, as previously described.
2.10. Study measures and data collection schedule (Table 2)
2.10.1. Primary and secondary outcomes
The primary outcomes are the change from baseline in systolic and diastolic blood pressure analyzed separately. Brachial Artery Blood Pressure will be measured on the left arm in the seated position using an automated oscillometric cuff at each time point (HEM-907XL, Omron Corporation, Japan) following the AHA guidelines.71 Net change in blood pressure will also be calculated, as the difference (intervention minus control) of the changes (from baseline) in these mean values.
2.10.2. Secondary outcomes
Cardiovascular risk factors including lipid profile, non-fasting glucose, insulin resistance (HOMA-IR) and inflammation (Interleukin-6, IL-6) measured at the University Clinical Research Center Laboratory by a trained nurse at 12-weeks and one year will be secondary outcomes. Additional secondary outcome measures will include physical performance measures that are considered to be diagnostic components of sarcopenia, and logically, will be significantly impacted by the intervention. These meaures include hand grip strength (kg) 6-meter gait speed (meters/second) peformed at usual walking speed, with walking aids (e.g., cane, walker) permitted as necessary, five time sit to stand test, 3-stage standing balance, and the timed up and go test. Additional details regarding cutpoints and assessment criteria are described in Appendix A. The Short Physical Performance Battery, a composite score from gait speed, five time sit to stand test, and balance test will also be used to objectively assess overall physical functioning status.72 Each performance measure will be rigorously measured to evaluate its effect size to inform a future larger trial that definitively tests whether selective performance measures, or the composite Short Physical Performance Battery score reduces blood pressure in sarcopenic adults.
2.10.3. Exploratory Aim: microvascualr mechanisms
To understand physiologically, the effects of the exercise intervention on blood pressure, 10 intervention participants and 5 AO-C participants (ntotal=15) will be asked during the informed consent process, to volunteer to have a small amount of fat extracted (~1–2 ml) before and immediately after 12-week intervention period to measure the microvascular system and test endothelial function.73, 74 This involves tissue extraction from subcutaneous fat (~1–2 ml) after the skin is locally anesthetized, dissection of resistance arterioles, followed by flow-induced vasodilation (FID), to promote vasodilation and examine endothelial function under varying experimentally-induced pressures. Since fat tissue is relatively avascular and will be obtained under direct inspection, there is minimal risk of excessive bleeding or other complications. Participants will be instructed to keep the area dry for 24–48 hours after which waterproof bandage applied after the procedures can be removed.
For those who agree and consent to this optional procedure, an additional 60 minutes will be added to baseline (visit 1) and at the 12-week post-intervention clinic visit. Alternatively, participants may opt to complete the biopsy sampling procedure on a separate clinic visit following completion of baseline and the 12-week clinic visit testing. 1-RM testing at baseline and the 12-week clinic visit will be scheduled prior to—or at least 48 hours after biopsy sampling to minimize risk of wound reopening. The fat biopsy procedure is performed by a trained registered nurse, and with minimal discomfort to the participant. After extraction, vessels will be prepared for FID using methods previously described.73, 74 Briefly, the single vessels will be maintained in an organ perfusion chamber and prepared for at an intraluminal pressure of 20 mmHg for 30 min, after which experiments will be performed under controlled conditions to pre-constrict vessels and dilate with endothelin-1 (ET-1; 100–200 pM)75, 76 and in the absence and presence of the eNOS inhibitor L-NAME (10−4 M) or polyethyleneglycol catalase (PEG-CAT; 500 U/ml) to block nitric oxide production and reactive oxygen species during FID. Some vessels will be denuded (confirmed with the absence of dilation to acetylcholine) to confirm the endothelial contribution of endothelium.
2.10.4. Potential mediators
Measures of muscle strength will be examined as mechanistic outcomes and potential mediators of the intervention effect on blood pressure change. Muscle strength measures that will specifically be tested as mediators include hand grip strength and lower-extremity strength (e.g., seated leg press, leg extension, leg/knee curls by the 1-RM, or the five time sit to stand performance test). Microvascular vasodilator function (endothelial function), which has been correlated with changes in blood pressure in well-functioning older adults,73, 74, 77 will additionally be evaluated as potential mediators of the intervention effect.
2.10.5. Potential effect modifiers
To complement the primary and secondary findings, we will explore for whom and under what condition (effect modifiers) treatment effects occur. Data will be collected on measures of potential moderators from the medical history intake form (e.g., gender, age, race/ethnicity, education, body mass index (or similar markers of body composition), smoking status, employment status, alcohol intake, living situation (alone, married, roommate), general diet habits, previous history of falls (12 months prior) and history of comorbidity (chronic obstructive pulmonary disorder, diabetes, arthritis). Data on the of medications to regulate blood pressure or cholesterols (strength, dose) will be evaluated to address confounding by indication. Baseline physical performance measures (e.g., gait speed), depression measured via the Center for Epidemiologic Studies Depression Scale (CES-D),78 self-reported physical functioning via the RAND modification of the Short-Form Physical Function Index (SF-36 PFI) questionnaire,79 and physical activity history by the CHAMPS questionnaire are additional variables that will be evaluated as potential effect modifiers of intervention response.
2.10.6. Process measures
Data will be collected on the recruitment and screening process; e.g., the proportion and representativeness of patients who are eligible at initial and subsequent screenings, recruitment source from which participant interest was received, reasons for exclusions, demographics of patients who screen ineligible or decline participation throughout the study period. We also will assess the proportion of participant interest from each recruitment site, and for clinic sites specifically, the proportion and representativeness of providers willing to assist in recruiting or screening their patients, and the representativeness of patients who are found eligible from these sites, complete clinic and follow-up visits, and reasons for missed exercise sessions or dropout.
In intervention participants, data on intervention acceptability will be obtained after each exercise session, which will be used adherence monitoring and for individual feedback. Acceptability will be evaluated for each session by tracking mean participant satisfaction, using a brief questionnaire developed by the PI, that asks 1) I found the exercises fun to do; 2) I was able to do the exercises without difficulty; 3) the exercises were worth my time to do; the exercises were easy to do; 4) the directions for doing the exercises were clear; and, 5) I received the guidance I needed from the research staff, with a Likert-type response options (from 1=Strongly disagree to 4 = Strongly agree). A mean score of 3.0 or greater will be set, a priori, as the study criterion to indicate intervention acceptability. Additionally, for participants assigned to the intervention group, the 12-week assessment visit will include a one-hour long focus group with no more than nine intervention participants in a group (including those who did not complete the intervention) to elicit participants’ feedback regarding their overall satisfaction with this pilot study and with the intervention, perceived benefits, relevance, feasibility, preferences, and problems encountered, and suggestions for improvement in a subsequent full trial.
2.11. Statistical analysis
2.11.1. Analytic plan
Baseline characteristics across the intervention and AO-C participants will be presented (e.g., means, medians, proportions, missingness), and Analysis of variance (ANOVA) and chi-squared tests for continuous and categorical variables will be used to test statistically significant differences in participant characteristics between intervention and AO-C groups. Multiple linear mixed effects model or Generalized estimated equations (GEE) will be used to examine the intervention effect on blood pressure (systolic or diastolic) over time, adjusting for covariates and confounders that may affect the outcome. The main fixed effect estimates except for the random error part to test the hypotheses in the models are expressed as, BP = β0 + β1 * Time + β2 * Group + β3 * Group * Time + βk * covariates, where group represents intervention or A0-C group. From this model the BP change over time of each group can be estimated, and the two groups’ change trends can be compared by testing the significance of the interaction effect, β3. Baseline characteristics will be evaluated as potential confounders to be included in the mixed effect model. Additional factors that could be on the mechanistic path between the intervention and blood pressure, that meet criteria for consideration as a potential confounder, will be added to the multivariable-adjusted model as a final step after some model comparisons (such as the cardiovascular secondary endpoints; e.g., lipid profile, non-fasting glucose, insulin resistance and inflammation). The covariates’ multicollinearity check will be performed using variance inflation factor (VIF) statistics when the selected variables are under possible concerns for high correlations. Separate models using GEE will be conducted, substituting continuously measured blood pressure outcomes for 2017 AHA/ACC blood pressure categories: Normal BP, <120/<80 mm Hg; Elevated BP, 120–129/<80 mm Hg; Stage I, 130–139 or 80–89 mm Hg; Stage II, ≥140 or ≥90 mm Hg.80
The missing data quantity and mechanism will be reviewed to consider any imputation procedures when it is not proper to analyze using only observed data assuming Missing Completely at Random (MCAR). Imputation methods can be chosen and utilized with enough information from the sample depending on missing at random (MAR) or not at random (MNAR). Multiple imputation and Maximum likelihood method requires the missing at random at least for instance.
Despite limitations in power, we will conduct mediation analyses to explore changes in muscle strength measures in pathophysiology of blood pressure and their potential mediating effects on treatment response using the longitudinal single mediator models. Separate analyses will explore potential mediation by individual endothelial function or inflammation. There are different approaches for the longitudinal mediation analysis with different assumptions to be considered. As one of the auto-regressive model approaches contemporaneous mediation [i.e., changes in mediators and change in blood pressure (or secondary outcomes) from baseline to 12-weeks months and longitudinal changes from 12-weeks to one-year] will be examined by MacKinnon’s product of coefficients test.81 The asymptotic standard error will be generated using the multivariate delta method.82 Because our primary interest is to evaluate mediation by muscle strength, we will test strength as a mediator separately in a single-mediator model. To determine the extent of mediated effect, the percentage of total effect mediated will be calculated for each significant mediator. We will additionally conduct moderation analyses to explore differences in intervention effect by gender or sex and other phenotypes (psychosocial or lifestyle or comorbidity factors). These analyses will follow the same general analytic approach as described above for primary and secondary outcomes, with the inclusion of appropriate moderator main effects and moderator by-group interaction terms.
Responses from focus groups will be transcribed verbatim and saved as text files and entered into ATLAS.ti (SCOLARI, 2003) for data analysis. “Classical content analyses,” frameworks will be used to analyze data. Evaluation of unexpected themes and recurring themes particularly focused on health concerns, injuries, or barriers and enablers that may be used to ensure adherence, acceptability and tolerance to the intervention will be identified. The list of themes will serve as data for intervention refinement in future R01 applications.
In exploratory analyses, FID dose-response comparisons will be analyzed using a two-way repeated measures ANOVA, with vessel diameter as the primary covariate, followed by pairwise comparisons using a Bonferroni adjustment [pressure gradient × treatment (baseline, L-NAME, and PEG–CAT)].
2.11.2. Sample size, power and data interpretation
An efficacy-based power analysis is beyond the scope of this pilot study. Thus, a CI approach will be used to base decisions concerning whether a definitive hypothesis-testing trial is warranted largely on the primary outcome, change in resting blood pressure and the CI width in reference to conventional effect size standards of clinical significance. Power analysis is based on the primary outcome of interest, mean change in blood pressure for unadjusted tests. The proposed sample size of n=60 for intervention and 30 for AO-C group has been caculated after considering a projected 15% drop-out rate, but will ensure power 80% with the significance level 0.05 to detect about the medium effect size, d=0.6 which corresponds to the mean blood pressure (systolic or diastolic blood pressure) change difference 1.3 with standard deviation (SD) for both groups 2.0. The total sample size 90 (76 after 15% drop-out) also may detect a difference in linear regression slopes 0.59 between the two groups with the SD of an outcome 2. All power calculation were based on the two-sided test with significance level 0.05. With this level of precision assured in calculating the sample size, we will be unlikely to miss a between-group differences in mean blood pressure that has a Cohen’s d=0.6 (medium effect) or larger because the confidence intervals for these scenarios (A-B, Figure 3) because of a low probability that the lower confidence interval will overlap the null—a finding sufficiently convincing to justify a larger, definitively powered full trial. In scenarios C-E, we will consider the blood pressure results in conjunction with secondary cardiovascular risk factors (e.g., lipid profile, non-fasting glucose, insulin resistance and inflammation, in addition to diabetes, smoking status, family history of cardiovascular disease, medication use, BMI or total body fat, visceral fat) and secondary physical performance measures expressed individually (grip strength, 6-meter gait speed, five time sit to stand test, 3-stage standing balance, timed up and go test) or as a composite (e.g., Short Physical Performance Battery), with results from other studies to decide whether a future full trial is warranted. Finally, if the pilot study leads to scenario F, in which the upper confidence limit is >d=0.2 (small effect), then the true treatment effect is unlikely to be of clinical interest, and hence, we will conclude that the pilot study outcome does not warrant a full trial, without substantial modification to the study design or approach. A lack of consistent findings, regardless of isolated p values, will be cause for caution against proceeding to a full trial without further research. We will assess findings on secondary cardiovascular risk factors or performance measures and subgroups as well as process evaluation data to determine if modifications to the design, intervention, or target population should be made prior to a larger trial and/or if additional pilot trials (e.g., with further intervention tailoring to particular subgroups) are indicated instead.
Figure 3.
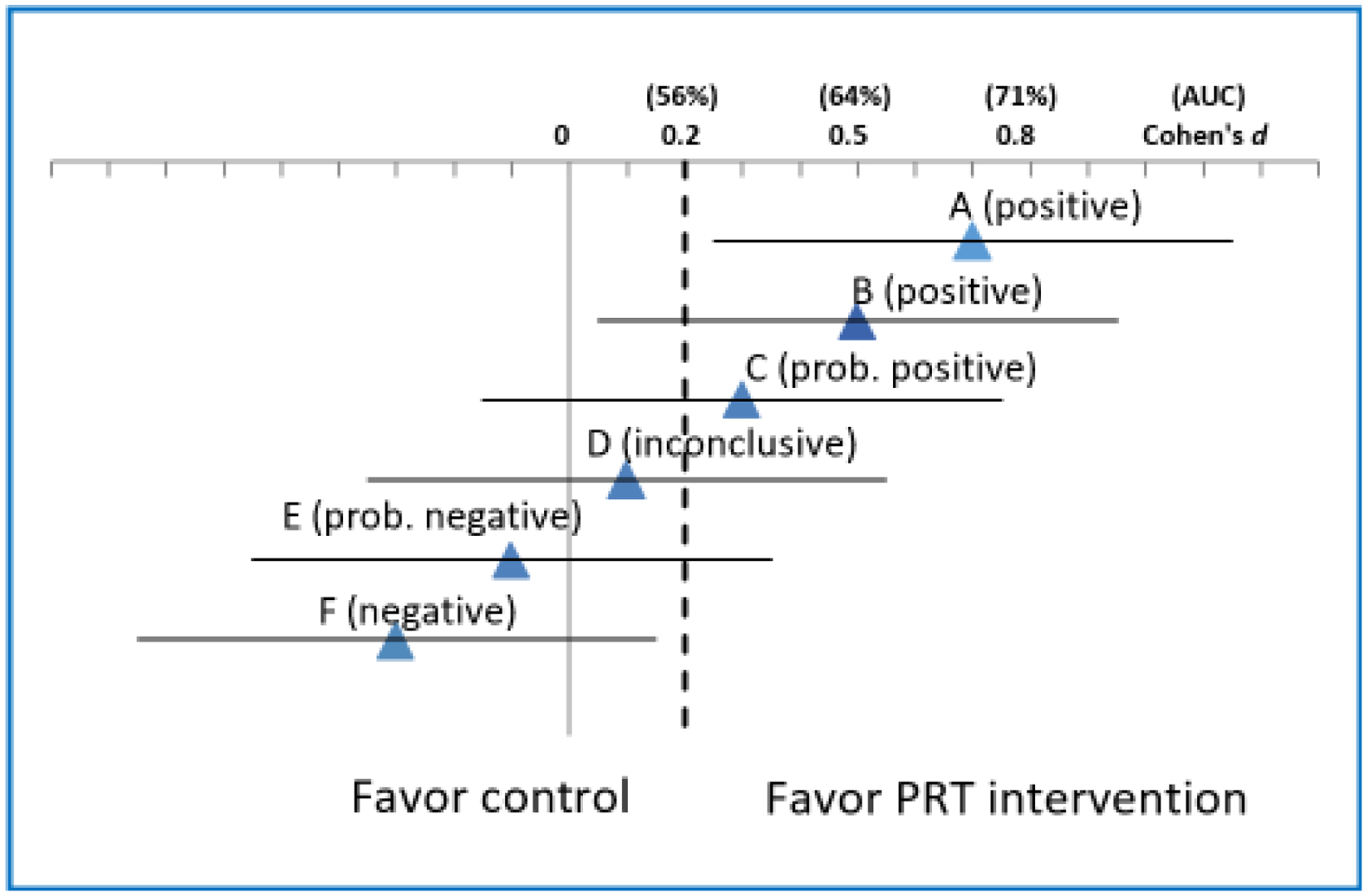
Illustrative scenarios (A-F) of pilot study results for blood pressure.
Effect size estimates expressed in Cohen’s d
2.12. Quality control
2.12.1. Data management
The raw data will be collected as hard copies (reporting of assessment results, questionnaires) and then transferred into electronic form in the REDCap database. Where practical, study data will be directly entered into the REDCap database by research staff. Additionally, REDCap allows for questionnaires to be sent to participants (and received) securely through email. Participants will be informed that questionnaires or other mailings may be received through email. Study data will be managed on-site by research staff at UIC using REDCap and may be additionally back up using a UIC secured BOX account. All research staff will be trained in the use of REDCap for entry of study data. Appropriate logic and limits checking (including cross-form validity checks) and missing value checks will be implemented in REDCap to facilitate accurate and consistent data entry. All datasets will be cleaned, verified and archived, and then read into SAS (version 9.4; SAS Institute Inc., Cary, NC) datasets, which also will be archived. One official copy of all study data and a master data dictionary will be maintained and updated regularly. All analytic and tracking databases will be stored in a password-protected, encrypted network drive with continuous backups. For the protection of participant confidentiality, unique anonymous study IDs will be used for data storing, tracking and reporting. Protected health information will be stored separately from all other study data, will only be accessible by select study staff (i.e., research coordinators) and will be used and disclosed in accordance with the Health Insurance Portability and Accountability Act regulations. Regular reports will be produced on (1) participant accrual and reasons for declining to participate and follow-up completion/ retention in relation to study goals and timeline; (2) the randomization process and group comparability on the balancing variables; (3) key baseline characteristics of the sample; (4) intervention exposure and adherence; and (5) protocol violations. No interim looks are anticipated but quality control and data entry errors and omissions will be continuously monitored and remediated. Any observed delays in these processes or data irregularities will be followed up and resolved in a timely manner.
3. Results/Discussion
There is a paucity of epidemiologic studies, and an absence of intervention studies on the effect of low muscle strength on blood pressure in older adults with sarcopenia and effective non-pharmaceutical treatment approaches. Low muscle strength is considered to be the most powerful indication of sarcopenia.60, 83, 84 Low muscle strength also shares many pathological mechanisms with high blood pressure, including arterial stiffness,14, 85, 86, reduced hemodynamics,87, 88 and impaired microvascular function,86, 89–91 and a growing body of evidence from prospective studies suggests that low muscle strength may be a risk factor for high blood pressure in older adults16, 18, 21–25.
Consequently, sarcopenia and high blood pressure are both influenced by decreased physical activity92 and chronic inflammation16, 93–96— factors that together, may further potentiate muscle strength deconditioning,97 and increase blood pressure98–100 in sarcopenic adults. Physical activity is considered fundamentally important to prevention and management of sarcopenia and blood pressure. Regular engagement in resistance training activities that progressively increase in challenge (overload) is suggested to be the cornerstone of sarcopenia treatment, with emerging evidence from randomized control trials demonstrating improvements in muscle strength by PRT in sarcopenic older adult populations (mean effect size range 0.3 to 0.4 across 4 studies).22, 26–28 More consistent findings of the feasibility effectiveness of moderate intensity PRT to counteract muscle strength losses have been demonstrated in older adults with prevalent musculoskeletal conditions37–42, and most notably, in frail populations.32–35, 101, 102 For example, in nonagenarians participating in the Health Enhancing Strength Training in Nonagenarians (‘STRONG’) study, Serra-Rexach and colleagues36 reported that short duration (8 weeks) of light to moderate intensity resistance training (e.g., 30–70% of 1-repetition maximum) significantly increased leg muscle strength, with partial maintenance of strength gains 4 weeks post intervention training, indicating that some aspects of neuromuscular performance are maintained following PRT. Seyennes et al43 reported on the dose-response relationships of PRT, where higher (80% of 1-repetition maximum) versus lower intensity (40% of 1-repetition maximum) and volume were associated with greater strength and functional adaptations to PRT in frail adults. More recently, evidence in older men from the Franconian Osteopenia and Sarcopenia Trial (‘FrOST’), showed that supervised high intensity PRT combined with modestly dosed protein supplementation for 36 weeks resulted in significant gains in hip/leg extensor strength and lean body mass.42 However, the contribution of the PRT on body composition gains independent of protein supplementation and whether favorable effects on muscle would be found in older women and if training behaviors were maintained long-term following completion of supervised training remain unclear. Nevertheless, in many functionally impaired populations, gains in strength have subsequently translated into improvements in functional performance and reduced disability.29, 34, 43, 101, 103, 104, underscoring the importance of targeting this aging biomarker to improve physiological resiliency105 and reduce vulnerabilities to adverse health events in older adults.
Though traditionally not considered in the context of cardiovascular therapy, findings from systematic reviews have reported on clinically meaningful reductions in blood pressure by PRT in normotensive and hypertensive healthy adults,20, 44–49 with reported antihypertensive effectiveness comparable to- or greater than aerobic endurance.44 The greatest reduction in systolic blood pressure/diastolic blood pressure were evidenced among pre-hypertensive (~3/3 mmHg)44, 46–48 and hypertensive adults (~6–10/5–8 mm Hg)44, 50, 51, underlining the potential value of PRT resistance training as an adjunct therapy for the prevention and control of blood pressure in these preclinical populations. However, evidence of PRT in hypertensive individuals is limited and most studies on performed in mostly healthy, middle-aged adults, suggesting prudency in the interpretation and generalizability of these results to older, deconditioned populations.44, 47 Nonetheless, pathophysiological mechanisms supporting the blood-pressure lowing effects of PRT suggest resistance training promotes increased muscle perfusion and angiogenesis, leading to improvements endothelial function (EF) and arterial blood pressure.106, 107 Vasculoprotective effects of PRT involving enhanced bioavailability of NO, consequent to the reductions in oxidative stress have further been demonstrated.52–54, 76, 107.
The impact of PRT on blood pressure has not been empirically tested in a high-risk population specifically identified with sarcopenia. However, findings from previous studies reinforce the critical need to investigate the feasibility and effectiveness of PRT on blood pressure in older adults with clinical muscle weakness of whom are also are at a higher risk for adverse consequences of high blood pressure and related cardiovascular morbidities. The current pilot study is mechanistically driven and utilizes a mixed-methods design and an evidence-based PRT intervention to simultaneously target muscle strength (and function) deficits and improve blood pressure in older adults with sarcopenia, with the potential to maintain improvements in muscle and blood pressure levels after one-year. This trial will further explore reversibility resulting from the intervention by examining the potential mediating effects of muscle strength on blood pressure, as well as plausible physiological mechanisms by which PRT may mediate blood pressure response during and immediately after treatment. This pilot study specifically aims to provide critical data on the feasibility, and potential efficacy of the PRT intervention in older adults with sarcopenia. Data collected will be need to: (1) justify whether a full trial is warranted, (2) estimate the accrual and attrition rates, (3) determine whether adequate adherence to the PRT intervention is achievable and acceptable in the target population, (4) identify the need and potential for further improvement in the intervention and trial procedures, and (5) explore potential mechanisms and effect modifiers to guide further investigation. If proven efficacious, this pilot will enable us to refine the design and approach of a full-scale trial that will be adequately powered to test the efficacy of PRT in improving blood pressure control among older adults with sarcopenia. Even though translation is out of the scope for a pilot study preliminary data from this trial will allow for a more comprehensive, definitive clinical study designed to better understand, mechanistically, the therapeutic effects of PRT on intravascular pressure and ultimately blood pressure. Furthermore, it is worth noting that PRT delivery falls is within scope of practice of physical therapists and other rehabilitation specialists. Thus, process measures from the pilot and (if warranted) subsequent efficacy trial also will set the foundation for future trials aiming to investigate intervention’s potential for implementation into clinical rehabilitation settings—a model that has been successfully used for translation of other lifestyle interventions, such as fall prevention.108, 109
4. Conclusion
Sarcopenia, and high blood pressure are highly prevalent, preventable conditions that disproportionately affect older adults2, 5, 110, 111. Given the projected growth of the older adult population and increased life expectancy,112 the co-existence of these two conditions is imminent. The current study proposes timely research questions and addresses an important area of research due to the growing problem of high blood pressure in older adults and the need for effective non-pharmaceutical approaches to better manage blood pressure and sarcopenia in old age. This line of investigation is expected to contribute new evidence regarding muscle strength as a novel, non-pharmacological target for blood pressure management in adults with sarcopenia (and similar comorbidities), using an approach with demonstrated health benefits beyond blood pressure control. It will further inform public health recommendations and future exercise prescription guidelines related to muscle strengthening in older adults.
Funding:
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K01HL148503. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- 1-RM
one-repetition maximum test
- ACSM
American College of Sports Medicine
- AHA
American Heart Association
- ANOVA
Analysis of variance
- AO-C
assessment only controls
- ALM
appendicular lean muscle mass
- BMI
body mass index
- CES-D
Center for Epidemiologic Studies Depression Scale
- CHAMPS
Community Healthy Activities Model Program for Seniors
- DXA
Dual-energy X-ray absorptiometry
- EHR
electronic health records
- FID
flow-induced vasodilation
- GEE
Generalized estimated equations
- HOMA-IR
homeostasis model assessment-estimated insulin resistance
- INERTIA
Impact of Interventional Exercise and Resistance Training on Blood Pressure Control In Older Adults with Sarcopenia
- IL-6
Interleukin-6
- MoCA
Montreal Cognitive Assessment
- PRT
progressive resistance training
- REDCap
Research Electronic Data Capture
- RPE
relative perceived level of exertion
- SF-36 PFI
Short-Form Physical Function Index
- UIC
University of Illinois at Chicago
Appendix Table A.
Physical Performance measures and assessment criteria
| Performance Test | Methods | Evaluation Criteria |
|---|---|---|
| Hand grip strength (kg) | The maximum strength (kg) of dominant hand using a Jamar dynamometer will be recorded. | Two trials. Clinically meaningful weakness will defined as a grip strength <30kg in men and <20kg in women113. Meeting low strength cutpoint in either trial indicates eligibility. |
| Timed Up and Go Test (“TUG”)114, 115 | stands up from a chair, walks 3 meters, turns, walks back (usual pace), and sits down | Two trials, average of 2 trials reported 13.5 seconds indicate greater risk for falls116 |
| Lower leg-muscle strength and endurance | Isokinetic knee extensor and flexor strength and endurance will be evaluated on the right leg, at an angular velocity of 60°/s using a Biodex System 4 dynamometer (Shirley, NY); endurance (total muscular work) tested with 30 repetitions at maximal effort at 180°/s. | Best of the 5 maximal voluntary efforts per test will be reported as peak torque at 60° in Newton meter (Nm) units |
| 6 meter Gait Speed* (meters/second) | Participant instructed to walk over 6-meter course at the usual pace, which will be timed | One trial. Mobility impairment is defined as gait speed ≤0.8 m/s113 |
| *Standing balance | maintain their feet side-by-side, semi-tandem and tandem; 10 seconds each | 10 seconds each timing stops when feet move, participant grasps the interviewer for support, or end of 10 sec elapsed |
| *Chair stands: | participants stand, arms folded across the chest from a straightbacked, non-padded chair, and perform five complete sit to stand tests as fast as they can. | Two trials, >15 seconds to complete 5 chair stands indicates severe sarcopenia; inability to complete five repetitions without assistance or use of upper extremity support indicates failure of the test |
Short Physical Performance Battery (SPPB), composite score of standing balance, chair stands and gait speed, performance tests are scored between 0 and 4, and a summary score ranging from 0 to 12 is computed. Scores approaching 12 reflect higher levels of physical functioning39
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interests:
Dr. Ma serves as a paid scientific consultant for Health Mentor, Inc. (San Jose, CA).
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- 1.von Haehling S, Morley JE and Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. Journal of cachexia, sarcopenia and muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrucci L, de Cabo R, Knuth ND and Studenski S. Of Greek heroes, wiggling worms, mighty mice, and old body builders. J Gerontol A Biol Sci Med Sci. 2012;67:13–6. doi: 10.1093/gerona/glr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S and Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. Journal of the American Medical Directors Association. 2011;12:249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM and Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–7. doi: 10.1093/gerona/61.1.72 [DOI] [PubMed] [Google Scholar]
- 5.Walston JD. Sarcopenia in older adults. Current opinion in rheumatology. 2012;24:623–7. doi: 10.1097/BOR.0b013e328358d59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fragala MS, Cadore EL, Dorgo S, Izquierdo M, Kraemer WJ, Peterson MD and Ryan ED. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. Journal of strength and conditioning research / National Strength & Conditioning Association. 2019;33:2019–2052. doi: 10.1519/jsc.0000000000003230. [DOI] [PubMed] [Google Scholar]
- 7.Fragala MS, Kenny AM and Kuchel GA. Muscle Quality in Aging: a Multi-Dimensional Approach to Muscle Functioning with Applications for Treatment. Sports medicine (Auckland, NZ). 2015. doi: 10.1007/s40279-015-0305-z. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y and Sayer AA. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.König M, Buchmann N, Seeland U, Spira D, Steinhagen-Thiessen E and Demuth I. Low muscle strength and increased arterial stiffness go hand in hand. Scientific Reports. 2021;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson MD, Duchowny K, Meng Q, Wang Y, Chen X and Zhao Y. Low normalized grip strength is a biomarker for cardiometabolic disease and physical disabilities among US and Chinese adults. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2017;72:1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latroche C, Gitiaux C, Chretien F, Desguerre I, Mounier R and Chazaud B. Skeletal Muscle Microvasculature: A Highly Dynamic Lifeline. Physiology (Bethesda, Md). 2015;30:417–27. doi: 10.1152/physiol.00026.2015. [DOI] [PubMed] [Google Scholar]
- 12.Golbidi S and Laher I. Exercise and the aging endothelium. Journal of diabetes research. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson TJ. Assessment and treatment of endothelial dysfunction in humans. Journal of the American College of Cardiology. 1999;34:631–8. [DOI] [PubMed] [Google Scholar]
- 14.Sanada K, Miyachi M, Tanimoto M, Yamamoto K, Murakami H, Okumura S, Gando Y, Suzuki K, Tabata I and Higuchi M. A cross-sectional study of sarcopenia in Japanese men and women: reference values and association with cardiovascular risk factors. Eur J Appl Physiol. 2010;110:57–65. doi: 10.1007/s00421-010-1473-z. [DOI] [PubMed] [Google Scholar]
- 15.dos Santos EP, Gadelha AB, Safons MP, Nobrega OT, Oliveira RJ and Lima RM. Sarcopenia and sarcopenic obesity classifications and cardiometabolic risks in older women. Archives of gerontology and geriatrics. 2014;59:56–61. doi: 10.1016/j.archger.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Han K, Park YM, Kwon HS, Ko SH, Lee SH, Yim HW, Lee WC, Park YG, Kim MK and Park YM. Sarcopenia as a determinant of blood pressure in older Koreans: findings from the Korea National Health and Nutrition Examination Surveys (KNHANES) 2008–2010. PloS one. 2014;9:e86902. doi: 10.1371/journal.pone.0086902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chin SO, Rhee SY, Chon S, Hwang YC, Jeong IK, Oh S, Ahn KJ, Chung HY, Woo JT, Kim SW, Kim JW, Kim YS and Ahn HY. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PloS one. 2013;8:e60119. doi: 10.1371/journal.pone.0060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SH, Park JH, Song PS, Kim DK, Kim KH, Seol SH, Kim HK, Jang HJ, Lee JG, Park HY, Park J, Shin KJ, Kim D and Moon YS. Sarcopenic obesity as an independent risk factor of hypertension. J Am Soc Hypertens. 2013;7:420–5. doi: 10.1016/j.jash.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O and Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc. 2014;62:253–260. doi: 10.1111/jgs.12652 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inder JD, Carlson DJ, Dieberg G, McFarlane JR, Hess NC and Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hypertension research : official journal of the Japanese Society of Hypertension. 2016;39:88–94. doi: 10.1038/hr.2015.111. [DOI] [PubMed] [Google Scholar]
- 21.Ochi M, Kohara K, Tabara Y, Kido T, Uetani E, Ochi N, Igase M and Miki T. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis. 2010;212:327–32. doi: 10.1016/j.atherosclerosis.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Martel GF, Hurlbut DE, Lott ME, Lemmer JT, Ivey FM, Roth SM, Rogers MA, Fleg JL and Hurley BF. Strength training normalizes resting blood pressure in 65- to 73-year-old men and women with high normal blood pressure. Journal of the American Geriatrics Society. 1999;47:1215–21. doi: 10.1111/j.1532-5415.1999.tb05202.x [DOI] [PubMed] [Google Scholar]
- 23.Maslow AL, Sui X, Colabianchi N, Hussey J and Blair SN. Muscular strength and incident hypertension in normotensive and prehypertensive men. Medicine and science in sports and exercise. 2010;42:288–95. doi: 10.1249/MSS.0b013e3181b2f0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artero EG, Lee DC, Lavie CJ, Espana-Romero V, Sui X, Church TS and Blair SN. Effects of muscular strength on cardiovascular risk factors and prognosis. Journal of cardiopulmonary rehabilitation and prevention. 2012;32:351–8. doi: 10.1097/HCR.0b013e3182642688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Artero EG, Lee DC, Ruiz JR, Sui X, Ortega FB, Church TS, Lavie CJ, Castillo MJ and Blair SN. A prospective study of muscular strength and all-cause mortality in men with hypertension. Journal of the American College of Cardiology. 2011;57:1831–7. doi: 10.1016/j.jacc.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan BH, Hewitt J, Keogh JW, Bermeo S, Duque G and Henwood TR. Impact of resistance training on sarcopenia in nursing care facilities: A pilot study. Geriatric nursing (New York, NY). 2016;37:116–21. doi: 10.1016/j.gerinurse.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Chen HT, Chung YC, Chen YJ, Ho SY and Wu HJ. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. Journal of the American Geriatrics Society. 2017;65:827–832. doi: 10.1111/jgs.14722. [DOI] [PubMed] [Google Scholar]
- 28.Stoever K, Heber A, Eichberg S and Brixius K. Influences of Resistance Training on Physical Function in Older, Obese Men and Women With Sarcopenia. Journal of geriatric physical therapy (2001). 2018;41:20–27. doi: 10.1519/jpt.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 29.Westcott WL. Resistance training is medicine: effects of strength training on health. Current sports medicine reports. 2012;11:209–16. doi: 10.1249/JSR.0b013e31825dabb8. [DOI] [PubMed] [Google Scholar]
- 30.Braith RW and Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation. 2006;113:2642–50. doi: 10.1161/circulationaha.105.584060. [DOI] [PubMed] [Google Scholar]
- 31.Valenzuela T Efficacy of progressive resistance training interventions in older adults in nursing homes: a systematic review. Journal of the American Medical Directors Association. 2012;13:418–28. doi: 10.1016/j.jamda.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA and Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA : the journal of the American Medical Association. 1990;263:3029–34. [PubMed] [Google Scholar]
- 33.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA and Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–75. doi: 10.1056/nejm199406233302501. [DOI] [PubMed] [Google Scholar]
- 34.Chandler JM, Duncan PW, Kochersberger G and Studenski S. Is lower extremity strength gain associated with improvement in physical performance and disability in frail, community-dwelling elders? Arch Phys Med Rehabil. 1998;79:24–30. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan DH, Roberson PK, Smith ES, Price JA and Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. Journal of the American Geriatrics Society. 2007;55:20–8. doi: 10.1111/j.1532-5415.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 36.Serra-Rexach JA, Bustamante-Ara N, Hierro Villarán M, González Gil P, Sanz Ibáñez MJ, Blanco Sanz N, Ortega Santamaría V, Gutiérrez Sanz N, Marín Prada AB, Gallardo C, Rodríguez Romo G, Ruiz JR and Lucia A. Short-term, light- to moderate-intensity exercise training improves leg muscle strength in the oldest old: a randomized controlled trial. Journal of the American Geriatrics Society. 2011;59:594–602. doi: 10.1111/j.1532-5415.2011.03356.x. [DOI] [PubMed] [Google Scholar]
- 37.King LK, Birmingham TB, Kean CO, Jones IC, Bryant DM and Giffin JR. Resistance training for medial compartment knee osteoarthritis and malalignment. Medicine and science in sports and exercise. 2008;40:1376–84. doi: 10.1249/MSS.0b013e31816f1c4a. [DOI] [PubMed] [Google Scholar]
- 38.Kryger AI and Andersen JL. Resistance training in the oldest old: consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scandinavian journal of medicine & science in sports. 2007;17:422–30. doi: 10.1111/j.1600-0838.2006.00575.x. [DOI] [PubMed] [Google Scholar]
- 39.Jan MH, Lin JJ, Liau JJ, Lin YF and Lin DH. Investigation of clinical effects of high- and low-resistance training for patients with knee osteoarthritis: a randomized controlled trial. Physical therapy. 2008;88:427–36. doi: 10.2522/ptj.20060300. [DOI] [PubMed] [Google Scholar]
- 40.Liu-Ambrose TY, Khan KM, Eng JJ, Lord SR, Lentle B and McKay HA. Both resistance and agility training reduce back pain and improve health-related quality of life in older women with low bone mass. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;16:1321–9. doi: 10.1007/s00198-005-1842-3. [DOI] [PubMed] [Google Scholar]
- 41.Kemmler W, Kohl M, Jakob F, Engelke K and von Stengel S. Effects of High Intensity Dynamic Resistance Exercise and Whey Protein Supplements on Osteosarcopenia in Older Men with Low Bone and Muscle Mass. Final Results of the Randomized Controlled FrOST Study. Nutrients. 2020;12. doi: 10.3390/nu12082341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kemmler W, Weineck M, Kohl M, von Stengel S, Giessing J, Fröhlich M and Schoene D. High Intensity Resistance Exercise Training to Improve Body Composition and Strength in Older Men With Osteosarcopenia. Results of the Randomized Controlled Franconian Osteopenia and Sarcopenia Trial (FrOST). Frontiers in sports and active living. 2020;2:4. doi: 10.3389/fspor.2020.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seynnes O, Fiatarone Singh MA, Hue O, Pras P, Legros P and Bernard PL. Physiological and functional responses to low-moderate versus high-intensity progressive resistance training in frail elders. J Gerontol A Biol Sci Med Sci. 2004;59:503–9. doi: 10.1093/gerona/59.5.m503. [DOI] [PubMed] [Google Scholar]
- 44.MacDonald HV, Johnson BT, Huedo-Medina TB, Livingston J, Forsyth KC, Kraemer WJ, Farinatti PT and Pescatello LS. Dynamic Resistance Training as Stand-Alone Antihypertensive Lifestyle Therapy: A Meta-Analysis. Journal of the American Heart Association. 2016;5. doi: 10.1161/jaha.116.003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carpio-Rivera E, Moncada-Jimenez J, Salazar-Rojas W and Solera-Herrera A. Acute Effects of Exercise on Blood Pressure: A Meta-Analytic Investigation. Arquivos brasileiros de cardiologia. 2016;106:422–33. doi: 10.5935/abc.20160064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cornelissen VA, Fagard RH, Coeckelberghs E and Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension. 2011;58:950–8. doi: 10.1161/hypertensionaha.111.177071. [DOI] [PubMed] [Google Scholar]
- 47.Cornelissen VA and Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. Journal of the American Heart Association. 2013;2:e004473. doi: 10.1161/jaha.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, Gulanick M, Laing ST and Stewart KJ. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–84. doi: 10.1161/circulationaha.107.185214. [DOI] [PubMed] [Google Scholar]
- 49.Vincent KR, Vincent HK, Braith RW, Bhatnagar V and Lowenthal DT. Strength training and hemodynamic responses to exercise. The American journal of geriatric cardiology. 2003;12:97–106. [DOI] [PubMed] [Google Scholar]
- 50.Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL and Nelson ME. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes care. 2002;25:2335–41. doi: 10.2337/diacare.25.12.2335 [DOI] [PubMed] [Google Scholar]
- 51.Cononie CC, Graves JE, Pollock ML, Phillips MI, Sumners C and Hagberg JM. Effect of exercise training on blood pressure in 70- to 79-yr-old men and women. Medicine and science in sports and exercise. 1991;23:505–11. [PubMed] [Google Scholar]
- 52.Franklin NC, Robinson AT, Bian JT, Ali MM, Norkeviciute E, McGinty P and Phillips SA. Circuit resistance training attenuates acute exertion-induced reductions in arterial function but not inflammation in obese women. Metabolic syndrome and related disorders. 2015;13:227–34. doi: 10.1089/met.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson AT, Franklin NC, Norkeviciute E, Bian JT, Babana JC, Szczurek MR and Phillips SA. Improved arterial flow-mediated dilation after exertion involves hydrogen peroxide in overweight and obese adults following aerobic exercise training. Journal of hypertension. 2016;34:1309–16. doi: 10.1097/hjh.0000000000000946. [DOI] [PubMed] [Google Scholar]
- 54.Heffernan KS, Fahs CA, Iwamoto GA, Jae SY, Wilund KR, Woods JA and Fernhall B. Resistance exercise training reduces central blood pressure and improves microvascular function in African American and white men. Atherosclerosis. 2009;207:220–6. doi: 10.1016/j.atherosclerosis.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 55.Korthuis RJ. Skeletal muscle circulation. Colloquium Series on Integrated Systems Physiology: From Molecule to Function. 2011;3:1–144. [Google Scholar]
- 56.Yung LM, Laher I, Yao X, Chen ZY, Huang Y and Leung FP. Exercise, vascular wall and cardiovascular diseases: an update (part 2). Sports medicine (Auckland, NZ). 2009;39:45–63. doi: 10.2165/00007256-200939010-00004. [DOI] [PubMed] [Google Scholar]
- 57.Keadle SK, McKinnon R, Graubard BI and Troiano RP. Prevalence and trends in physical activity among older adults in the United States: A comparison across three national surveys. Preventive medicine. 2016;89:37–43. doi: 10.1016/j.ypmed.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. http://www.health.gov/PAGuidelines/guidelines/default.aspx
- 59.Murphy RA, Ip EH, Zhang Q, Boudreau RM, Cawthon PM, Newman AB, Tylavsky FA, Visser M, Goodpaster BH and Harris TB. Transition to sarcopenia and determinants of transitions in older adults: a population-based study. J Gerontol A Biol Sci Med Sci. 2014;69:751–8. doi: 10.1093/gerona/glt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M and Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64. doi: 10.1093/gerona/61.10.1059 [DOI] [PubMed] [Google Scholar]
- 61.Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, Miles TP and Visser M. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2003;51:323–30. [DOI] [PubMed] [Google Scholar]
- 62.University of Illinois at Chicago (UIC) Center for Clinical and Translational Science.
- 63.Lohman TG, Rocheand AF Martorell R. Anthropometric Standardization Reference Manual Human Kinetics Champaign, Illinois; 1988. [Google Scholar]
- 64.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription: Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 65.Bandura A Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, N.J.: Prentice Hall; 1986. [Google Scholar]
- 66.Prochaska JO and DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–5. [DOI] [PubMed] [Google Scholar]
- 67.Winett RA, Williams DM and Davy BM. Initiating and maintaining resistance training in older adults: a social cognitive theory-based approach. Br J Sports Med. 2009;43:114–119. doi: 10.1136/bjsm.2008.049361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borg GA. Psychophysical bases of perceived exertion. Medicine and science in sports and exercise. 1982;14:377–381. [PubMed] [Google Scholar]
- 69.Thompson PD, Arena R, Riebe D and Pescatello LS. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Current sports medicine reports. 2013;12:215–7. doi: 10.1249/JSR.0b013e31829a68cf. [DOI] [PubMed] [Google Scholar]
- 70.National Institutes of Health and National Heart LaBI. NHLBI Adverse Event and Unanticipated Problem Reporting Policy. AccessedJune 29, 2021. https://www.nhlbi.nih.gov/grants-and-training/policies-and-guidelines/nhlbi-adverse-event-and-unanticipated-problem-reporting-policy
- 71.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG and Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 72.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA and Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- 73.Mahmoud AM, Szczurek MR, Blackburn BK, Mey JT, Chen Z, Robinson AT, Bian JT, Unterman TG, Minshall RD, Brown MD, Kirwan JP, Phillips SA and Haus JM. Hyperinsulinemia augments endothelin-1 protein expression and impairs vasodilation of human skeletal muscle arterioles. Physiol Rep. 2016;4. doi: 10.14814/phy2.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mahmoud AM, Ali MM, Miranda ER, Mey JT, Blackburn BK, Haus JM and Phillips SA. Nox2 contributes to hyperinsulinemia-induced redox imbalance and impaired vascular function. Redox Biol. 2017;13:288–300. doi: 10.1016/j.redox.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grizelj I, Cavka A, Bian JT, Szczurek M, Robinson A, Shinde S, Nguyen V, Braunschweig C, Wang E, Drenjancevic I and Phillips SA. Reduced flow-and acetylcholine-induced dilations in visceral compared to subcutaneous adipose arterioles in human morbid obesity. Microcirculation. 2015;22:44–53. doi: 10.1111/micc.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phillips SA, Hatoum OA and Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. American journal of physiology Heart and circulatory physiology. 2007;292:H93–100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- 77.Parker BA, Smithmyer SL, Jarvis SS, Ridout SJ, Pawelczyk JA and Proctor DN. Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. American journal of physiology Heart and circulatory physiology. 2007;292:H1148–56. doi: 10.1152/ajpheart.00729.2006. [DOI] [PubMed] [Google Scholar]
- 78.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 79.Syddall HE, Martin HJ, Harwood RH, Cooper C and Aihie Sayer A. The SF-36: a simple, effective measure of mobility-disability for epidemiological studies. The journal of nutrition, health & aging. 2009;13:57–62. doi: 10.1007/s12603-009-0010-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 81.MacKinnon DP, Fritz MS, Williams J and Lockwood CM. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behav Res Methods. 2007;39:384–389. doi: 10.3758/bf03193007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bishop YM, Fienberg SE, Holland PW, Light RJ and Mosteller F. Book Review: Discrete multivariate analysis: Theory and practice. Appl Psychol Meas. 1977;1:297–306. [Google Scholar]
- 83.Santilli V, Bernetti A, Mangone M and Paoloni M. Clinical definition of sarcopenia. Clinical cases in mineral and bone metabolism : the official journal of the Italian Society of Osteoporosis, Mineral Metabolism, and Skeletal Diseases. 2014;11:177–80. [PMC free article] [PubMed] [Google Scholar]
- 84.Ferrucci L, Guralnik JM, Buchner D, Kasper J, Lamb SE, Simonsick EM, Corti MC, Bandeen-Roche K and Fried LP. Departures from linearity in the relationship between measures of muscular strength and physical performance of the lower extremities: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52:M275–85. [DOI] [PubMed] [Google Scholar]
- 85.Srikanthan P and Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. The Journal of clinical endocrinology and metabolism. 2011;96:2898–903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 86.Abbatecola AM, Chiodini P, Gallo C, Lakatta E, Sutton-Tyrrell K, Tylavsky FA, Goodpaster B, de Rekeneire N, Schwartz AV, Paolisso G and Harris T. Pulse wave velocity is associated with muscle mass decline: Health ABC study. Age (Dordr). 2012;34:469–78. doi: 10.1007/s11357-011-9238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Figueroa A, Park SY, Seo DY, Sanchez-Gonzalez MA and Baek YH. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause. 2011;18:980–4. doi: 10.1097/gme.0b013e3182135442. [DOI] [PubMed] [Google Scholar]
- 88.Coelho Junior HJ, Aguiar Sda S, Goncalves Ide O, Sampaio RA, Uchida MC, Moraes MR and Asano RY. Sarcopenia Is Associated with High Pulse Pressure in Older Women. Journal of aging research. 2015;2015:109824. doi: 10.1155/2015/109824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME and Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. American journal of physiology Heart and circulatory physiology. 2011;300:H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Boer MP, Meijer RI, Wijnstok NJ, Jonk AM, Houben AJ, Stehouwer CD, Smulders YM, Eringa EC and Serne EH. Microvascular dysfunction: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Microcirculation. 2012;19:5–18. doi: 10.1111/j.1549-8719.2011.00130.x. [DOI] [PubMed] [Google Scholar]
- 91.Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, Spurgeon H and Vaitkevicius P. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension. 2001;38:429–33. doi: 10.1161/01.hyp.38.3.429 [DOI] [PubMed] [Google Scholar]
- 92.Liu X, Zhang D, Liu Y, Sun X, Han C, Wang B, Ren Y, Zhou J, Zhao Y, Shi Y, Hu D and Zhang M. Dose-Response Association Between Physical Activity and Incident Hypertension: A Systematic Review and Meta-Analysis of Cohort Studies. Hypertension. 2017;69:813–820. doi: 10.1161/hypertensionaha.116.08994. [DOI] [PubMed] [Google Scholar]
- 93.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA and Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–9. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schaap LA, Pluijm SM, Deeg DJ and Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. The American journal of medicine. 2006;119:526 e9–17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 95.Mattace-Raso FU, Verwoert GC, Hofman A and Witteman JC. Inflammation and incident-isolated systolic hypertension in older adults: the Rotterdam study. Journal of hypertension. 2010;28:892–5. doi: 10.1097/HJH.0b013e328336ed26. [DOI] [PubMed] [Google Scholar]
- 96.Kampus P, Muda P, Kals J, Ristimae T, Fischer K, Teesalu R and Zilmer M. The relationship between inflammation and arterial stiffness in patients with essential hypertension. International journal of cardiology. 2006;112:46–51. doi: 10.1016/j.ijcard.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 97.Lightfoot AP, McCormick R, Nye GA and McArdle A. Mechanisms of skeletal muscle ageing; avenues for therapeutic intervention. Curr Opin Pharmacol. 2014;16:116–21. doi: 10.1016/j.coph.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 98.Dumurgier J, Elbaz A, Ducimetiere P, Tavernier B, Alperovitch A and Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. Bmj. 2009;339:b4460. doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA and Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA : the journal of the American Medical Association. 2006;295:2018–26. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 100.Smith SC Jr., Anderson JL, Cannon RO 3rd, Fadl YY, Koenig W, Libby P, Lipshultz SE, Mensah GA, Ridker PM and Rosenson R. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the clinical practice discussion group. Circulation. 2004;110:e550–3. doi: 10.1161/01.cir.0000148981.71644.c7. [DOI] [PubMed] [Google Scholar]
- 101.Peterson MD, Rhea MR, Sen A and Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing research reviews. 2010;9:226–37. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Binder EF, Yarasheski KE, Steger-May K, Sinacore DR, Brown M, Schechtman KB and Holloszy JO. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2005;60:1425–31. doi: 10.1093/gerona/60.11.1425 [DOI] [PubMed] [Google Scholar]
- 103.Binder EF, Schechtman KB, Ehsani AA, Steger-May K, Brown M, Sinacore DR, Yarasheski KE and Holloszy JO. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. Journal of the American Geriatrics Society. 2002;50:1921–8. doi: 10.1046/j.1532-5415.2002.50601.x [DOI] [PubMed] [Google Scholar]
- 104.Liu CJ and Latham NK. Progressive resistance strength training for improving physical function in older adults. The Cochrane database of systematic reviews. 2009:Cd002759. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferrucci L, Levine ME, Kuo P-L and Simonsick EM. Time and the metrics of aging. Circ Res. 2018;123:740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anderson TJ and Phillips SA. Assessment and prognosis of peripheral artery measures of vascular function. Prog Cardiovasc Dis. 2015;57:497–509. doi: 10.1016/j.pcad.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 107.Phillips SA, Das E, Wang J, Pritchard K and Gutterman DD. Resistance and aerobic exercise protects against acute endothelial impairment induced by a single exposure to hypertension during exertion. Journal of applied physiology (Bethesda, Md : 1985). 2011;110:1013–20. doi: 10.1152/japplphysiol.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Centers for Disease Control and Prevention and National Center for Injury Prevention and Control. Tools to Impleement the Otago Exercise Program: A Program to Reduce Falls. First Edition. 1–92. [Google Scholar]
- 109.Shubert TE, Smith ML, Jiang L and Ory MG. Disseminating the Otago Exercise Program in the United States: Perceived and Actual Physical Performance Improvements From Participants. Journal of applied gerontology : the official journal of the Southern Gerontological Society. 2018;37:79–98. doi: 10.1177/0733464816675422. [DOI] [PubMed] [Google Scholar]
- 110.Guo F, He D, Zhang W and Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. Journal of the American College of Cardiology. 2012;60:599–606. doi: 10.1016/j.jacc.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 111.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW and Turner MB. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/cir.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 112.Methodology, Assumptions, and Inputs for the 2017 National Population Projections: Projections for the United States: 2017 to 2060. Centers for Disease Control and Prevention. 2018. [Google Scholar]
- 113.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M and Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Podsiadlo D and Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. 1991;39:142–8. [DOI] [PubMed] [Google Scholar]
- 115.Shumway-Cook A, Brauer S and Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Physical therapy. 2000;80:896–903. [PubMed] [Google Scholar]
- 116.Rockwood K, Awalt E, Carver D and MacKnight C. Feasibility and measurement properties of the functional reach and the timed up and go tests in the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2000;55:M70–3. doi: 10.1093/gerona/55.2.m70 [DOI] [PubMed] [Google Scholar]


