Abstract
Objectives:
We performed a systematic review and meta-analysis for the prevalence and risk factors of rheumatoid arthritis-related bronchiectasis (RA-BR).
Methods:
We queried PubMed and EMBASE databases to identify published literature related to prevalence and risk factors for RA-BR among patients with RA. Data extraction included study design, country, year, method of RA-BR detection, RA characteristics, numerator of RA-BR cases and denominator of patients with RA, and associations with RA-BR presence. We performed a meta-analysis using random or fixed effects models to estimate the prevalence of RA-BR among RA.
Results:
Out of a total of 253 studies, we identified 41 total studies that reported on prevalence (n=34), risk factors (n=5), or both (n=2). The included studies had heterogeneous methods to identify RA-BR. Among the 36 studies reporting prevalence, 608 RA-BR cases were identified from a total of 8,569 patients with RA. In the meta-analysis, the pooled overall prevalence of RA-BR among RA was 18.7% (95%CI 13.7–24.3%) using random effects and 3.8% (95%CI 3.3–4.2%) using fixed effects. Among studies that used high-resolution chest computed tomography (HRCT) imaging, the prevalence of RA-BR was 22.6% (95%CI 16.8–29.0%) using random effects. When only considering retrospective studies (n=12), the pooled prevalence of RA-BR among RA was 15.5% (95%CI 7.5–25.5%); among prospective studies (n=24), the pooled prevalence was 20.7% (95% CI 14.7–27.4%). Risk factors for RA-BR included older age, longer RA duration, genetics (CFTR and HLA), and undetectable circulating mannose binding lectin (MBL) biomarker.
Conclusion:
In this systematic review and meta-analysis, the prevalence of RA-BR was nearly 20% among studies with HRCT imaging, suggesting that bronchiectasis may be a common extra-articular feature of RA. Relatively few factors have been associated with RA-BR. Future studies should standardize methods to identify RA-BR cases and investigate the natural history and clinical course given the relatively high prevalence among RA.
Keywords: rheumatoid arthritis, bronchiectasis, pulmonary
INTRODUCTION
Although rheumatoid arthritis (RA) is known to affect lung structure and function(1), the prevalence of overall lung involvement among patients with RA varies significantly across studies(2). Bronchiectasis (BR) is an established extra-articular manifestation of RA, which presents as irreversible damage to the bronchi along with widening and thickening resulting in exuberant mucus production(3, 4). BR in RA patients (hereafter termed as RA-BR) can lead to decreased quality of life as well as increased risk for infection and mortality(5–7). Mortality of RA-BR has been reported to be over 7-fold higher than the general population, and 5-fold higher than RA without BR(6). RA-BR also poses a large economic burden, as both conditions are lifelong(3). Therefore, it is important to gain a better understanding of the factors that may increase risk for developing RA-BR, as well as identifying how prevalent this condition is around the world. Investigating the burden of RA-BR may lead to a better understanding of pathogenesis and improved management. The prevalence and risk factors of RA-BR are unclear in previous studies.
To address these gaps in knowledge, we focused on summarizing the prevalence of RA-BR using a systematic review and performed a quantitative meta-analysis. Additionally, we investigated the risk factors for BR presence in RA patients through a systematic review.
1. METHODS
We performed a systematic review and meta-analysis of the prevalence and risk factors of RA-BR. We used the PRISMA-P 2015 checklist and pre-registered (ID#199080 on PROSPERO).
2.1. Search strategy and study selection
We queried PubMed and EMBASE databases. The search strategy was based on the PRISMA 2019 search strategy flow diagram. We searched for “rheumatoid arthritis; AND; bronchiectasis.” No further limits were set during this search to allow for all possible results. The inclusion and exclusion criteria were assessed for study eligibility by two independent abstractors. The search was conducted on July 15, 2020.
2.2. Eligibility criteria
The following specific inclusion criteria were applied during screening: (1) peer-reviewed original science; (2) reported at least 5 patients; (3) published in English; and (4) related to both RA and BR. For the prevalence aim, we required that RA-BR be identified among a larger denominator of patients with RA. For the risk factors aim, we required that RA-BR be the outcome of interest among an RA population. The results were required to be statistically significant in the study. The following study characteristics were excluded during screening: (1) non-primary literature (i.e., review articles, editorials); (2) case reports or series involving fewer than 5 human patients; (3) published in a language other than English; (4) did not relate to both RA and BR; and (5) studies not involving humans (e.g., mouse models).
We initially screened article titles and abstracts to remove articles that obviously did not meet study criteria. After the initial screen, we conducted a full text review to verify inclusion and exclusion criteria for eligibility. All articles were reviewed independently by two individuals to verify inclusion and exclusion criteria were met. The data were extracted independently by two reviewers (LMW and LCP) into data extraction tools. A third individual (JAS) adjudicated disagreements.
2.3. Summary measures
The main objective of this review was to summarize and report on the prevalence and risk factors of RA-BR. The study population was defined as anyone with RA according to the study criteria. BR was defined according to each study criteria and needed to occur among patients with RA. None of the selected studies for review included interventions, due to a lack of interventional studies relating to this topic. All studies included were observational, and the outcome of interest was RA-BR.
The data measuring prevalence of RA-BR was extracted and confirmed by two separate reviewers. Relevant data that reported on the prevalence of BR in RA included sample size, RA disease duration, number of BR cases (numerator) among RA sample (denominator) and methods of diagnosis such as type and indication (clinical or research) of chest imaging. BR in RA cases among patients with RA-associated interstitial lung disease (RA-ILD) were also reported, if applicable. We then summarized the subset of studies that studied both bronchiectasis and ILD since RA-BR may have been secondary due to traction from ILD, rather than primary/isolated RA-BR, in these patients.
A quantitative meta-analysis was conducted using the R software package for the prevalence of RA-BR among RA cases using a random effects model for all studies meeting our initial inclusion criteria as the primary analysis. We also performed meta-analyses separately among retrospective and prospective study designs.
A series of sensitivity analyses were conducted for the meta-analyses. First, we repeated the primary analysis but used fixed effects instead of random effects. The rest of the sensitivity analyses used random effects and considered the combined, retrospective, and prospective studies, as applicable to the studies being removed. Second, we removed a large retrospective study by Shadick et al. due to having an uncertain denominator(8). Third, we removed two additional studies due to chest imaging not obtained on all patients(9, 10). Fourth, a prospective study was removed due to use of research chest radiographs instead of chest computed tomography (CT) scans(11). Fifth, we limited the analyses to studies that only used high resolution (HRCT) scans as a chest imaging modality. Finally, we removed studies with fewer than 50 patients.
Risk factors were assessed among selected articles and data were extracted by two separate reviewers. We examined all exposures that the literature investigates as a potential risk factor for BR among patients with RA. Measures of assessing risk factors were recorded including risk factor type, population type, comparison group, odds ratio (OR), relative risk (RR), frequency, and proportions. Only statistically significant findings were included in the review.
2.4. Risk of bias
To address risk of bias, two independent reviewers assessed studies for eligibility and extracted data. A third reviewer completed final assessments of studies and data extraction to assure all data included were accurate. All studies were categorized according to type of study design. We also quantified the type and presence of chest imaging and whether this was performed for clinical or research purposes.
3. RESULTS
3.1. Study selection
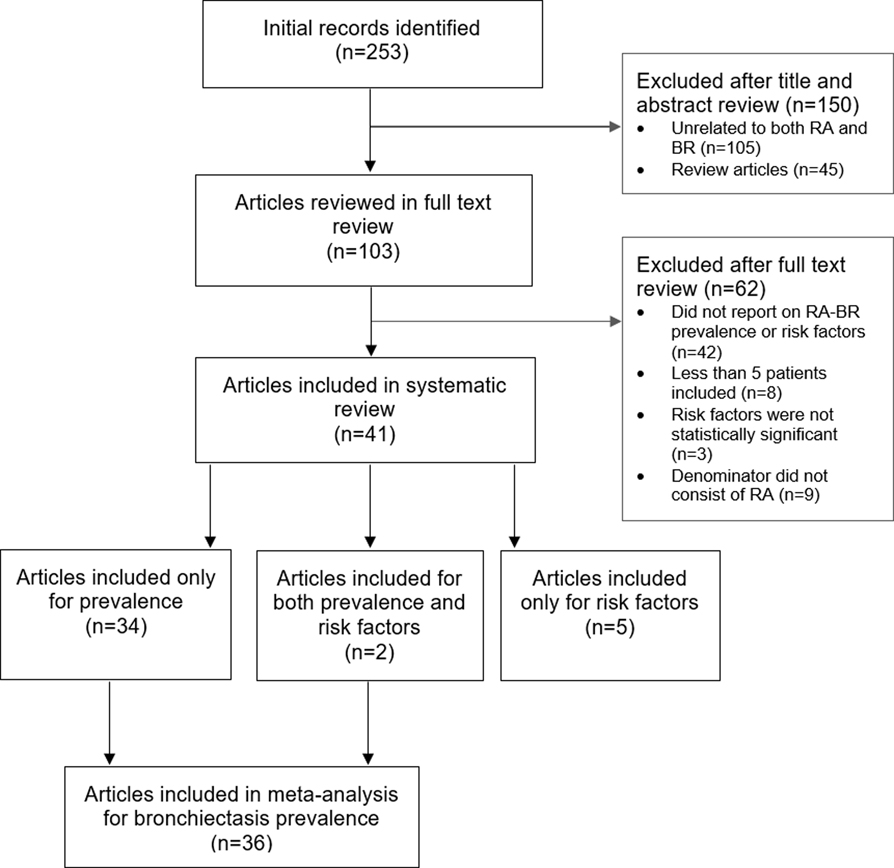
A total of 253 articles were identified through our database search. In the initial screen of titles and abstracts, we excluded 45 review articles and 105 extraneous articles (see Figure 1 for flow diagram). After screening the full text, we removed an additional 62 articles for the following reasons: did not report on RA-BR prevalence or risk factors among RA (n=42); less than 5 patients included (n=8); risk factors not statistically significant (n=3); and denominators did not consist of RA (n=9).
Fig. 1.
PRISMA flow diagram of studies assessed for bronchiectasis prevalence and risk factors among patients with rheumatoid arthritis.
In total, we included 41 full-text articles: 34 articles were included only for prevalence, 5 articles included only for risk factors, and 2 articles were included for both prevalence and risk factors. There were 36 articles reporting both RA-BR numerators and RA denominators that were included in the quantitative meta-analysis of RA-BR prevalence.
3.2. RA-BR in retrospective studies
The data collected in each retrospective study relating to prevalence are in Table 1. Dates of publication ranged from 1994 to 2020 and were conducted in France (n=5), USA (n=2), Japan (n=1), China (n=1), and Saudi Arabia (n=1). Mean RA duration ranged from 3.5 to 21.2 years. Sample size ranged from 25 to 4,000 patients with RA. All studies except Allain et al. used clinical CT or HRCT to confirm BR diagnosis(2, 8, 9, 12–19). Three studies did not conduct imaging on all patients analyzed(8–10). One study(17) specified that 270 patients were excluded due to lack of imaging. Research review of imaging modality varied considerably among studies, including blinding from clinical data, experience of radiologist, number of radiologist reviewers. Three studies did not report on the methods of RA-BR identification(8, 15, 18). One study compared prevalence of BR in RA-ILD to RA-no-ILD(18).
Table 1.
Retrospective studies reporting the prevalence of bronchiectasis among patients with rheumatoid arthritis (n=12).
| Reference | Year | Country | RA duration, years | Patients with prevalent RA-BR (numerator) | Total RA patients studied, n (denominator) | Prevalence of RA-BR in RA (%) | Chest imaging modality used | Details on review of images and other notes |
|---|---|---|---|---|---|---|---|---|
| Remy-Jardin et al. | 1994 | France | Mean 12 (SD 8) | n=23 | n=84 | 27.4% | Clinical CT | Two observers blinded to clinical history; Some of the total RA patients had suspected respiratory symptoms |
| Shadick et al. | 1994 | USA | Mean 6.4 | n=23 | n=4,000 | 0.6% | Clinical CT (not on all patients analyzed) | Not reported; Total RA patients studied is approximated |
| Cortet et al. | 1995 | France | Mean 12 (SD 8) | n=23 | n=77 | 30.0% | Clinical HRCT | Two radiologists blinded to clinical history; Some of the total RA patients reported respiratory symptoms |
| Despeaux et al. | 1996 | France | Mean 11.1 | n=12 | n=180 | 6.7% | Clinical CT (not on all patients analyzed) | Not reported; Of the total patients studied, 100 had RA and 80 had BR, but 4/80 BR patients had RA and 8/100 RA patients had BR; Some of the total patients studied reported respiratory symptoms |
| Allain et al. | 1997 | France | Mean 8.12 (SD 7.85) | n=13 | n=453 | 2.9% | Research chest radiographs (not on all patients analyzed) | Probable symptomatic RA-BR defined based on criteria from Walker WC, The lung in RA, MD Thesis, University of Edinburgh, 1966, which do not take CT findings into account; research chest CT performed for patients with chronic lower respiratory tract symptoms but no typical evidence of RA-BR on chest radiograph |
| Akira et al. | 1999 | Japan | Not reported | n=15 | n=29 | 52.0% | Clinical CT | Two experienced chest radiologists blinded to clinical history; All patients included had respiratory symptoms |
| Devouassoux et al. | 2009 | France | Mean 7.8 (SD 8.2) | n=10 | n=25 | 40.0% | Clinical HRCT | Research physicians (blinding unspecified); All patients included had respiratory symptoms |
| Kinoshita et al. | 2016 | Japan | Mean 5.0 (SD 3.9) | n=5 | n=25 | 20.0% | Clinical CT | Two chest radiologists blinded to clinical history |
| Alamoudi et al. | 2017 | Saudi Arabia | Mean 3.5 (SD 4.6) | n=31 | n=149 | 20.8% | Clinical HRCT | Two senior radiologists reviewed images (blinding unspecified); Some of the total RA patients reported respiratory symptoms (Excluded 270 patients from analysis due to no scan) |
| Zhang et al. | 2017 | China | Mean 8 (SD 9) | n=76 | n=550 | 13.8% | Clinical HRCT | Not reported; Some of the total RA patients reported respiratory symptoms RA-BR prevalence was higher in RA-ILD (18.1%) compared to RA without ILD (10.5%) |
| Duarte et al. | 2019 | UK | Mean 14 (IQR 8–29 years) | n=31 | n=1129 | 2.7% | Clinical HRCT | Expert thoracic radiologists reviewed in multidisciplinary meeting with radiologists, pulmonologists and rheumatologists (blinding unspecified); Most of the total RA patients reported respiratory symptoms |
| Huang et al. | 2020 | USA | Mean 21.2 (SD 13.2) | n=31 | n=190 | 16.3% | Clinical CT | Clinical report, cases verified by blinded review from two expert chest radiologists; Some of the total RA patients reported respiratory symptoms |
CT, computed tomography; HRCT, high-resolution computed tomography; RA, rheumatoid arthritis; RA-BR, rheumatoid arthritis-associated bronchiectasis; SD, standard deviation.
3.3. RA-BR in prospective studies
The prospective studies relating to RA-BR prevalence are shown in Table 2. Dates of publication ranged from 1994 to 2020 and were conducted in countries such as France (n=4), Turkey (n=2), Brazil (n=2), and UK (n=3). Two papers reported on the same cohort of patients(20, 21). Mean RA duration in years ranged from less than 1 to 17.1. Four studies did not report RA duration(20–23). Sample size ranged from 20 to 332 total RA patients. All used research chest CT or HRCT to identify BR diagnosis except one study which used research chest radiograph(11). Review of imaging varied among studies, such as blinding of clinical data, experience of radiologist and number of radiologist reviewers, with three studies not reporting(20, 21, 24). Six studies included data on RA-ILD(1, 23, 25–28), with two comparing BR in RA-ILD to BR in RA-no-ILD(25, 26). One study only included RA-ILD patients in the denominator of the study sample(23). Eight studies only included RA patients that did not have respiratory symptoms or airway abnormalities prior to enrollment(26, 27, 29–34), and 16 studies included patients that either reported respiratory symptoms or there was a suspicion of pulmonary abnormalities(1, 11, 20–25, 28, 35–41).
Table 2.
Prospective studies reporting the prevalence of bronchiectasis among patients with rheumatoid arthritis (n=24).
| Reference | Year | Country | RA duration, years (unless specified) | Patients with prevalent RA-BR (numerator) | Total RA patients studied, n (denominator) | Prevalence of RA-BR in RA (%) | Chest imaging modality used | Details on review of images and other notes |
|---|---|---|---|---|---|---|---|---|
| McDonagh et al. | 1994 | United Kingdom | Median 9 (range 1–27) | n=4 | n=20 | 20.0% | Research HRCT | Two radiologists blinded to clinical history; all patients had no respiratory symptoms RA-BR prevalence was higher in RA-ILD (30.0%) compared to RA without ILD (20.0%) |
| Hassan et al. | 1995 | United Kingdom | Mean 9 (range 1–30) | n=5 | n=20 | 20.0% | Research HRCT | One radiologist blinded to clinical history; all patients had no respiratory symptoms |
| Morrison et al. | 1996 | South Africa | Mean 12.4 (range 1–50) | n=2 | n=104 | 1.9% | Research chest radiograph | Respiratory physician and radiologist blinded to clinical history; Some of the total RA patients studied reported respiratory symptoms |
| Vergnenegre et al. and Treves et al. | 1997 | France | Not reported | n=6 | n=100 | 6.0% | Research CT | Not reported; RA-BR was suspected on clinical symptoms and radiological findings; Some of the total RA patients studied reported respiratory symptoms Each paper analyzed same patients. |
| Cortet et al. | 1997 | France | Mean 12 (SD 9.2) | n=18 | n=59 | 30.5% | Research HRCT | Experienced radiologists blinded to clinical history; Some of the total RA patients studied reported respiratory symptoms Excluded 9 patients from analysis due to previous radiation therapy |
| Gabbay et al. | 1997 | Australia | Mean 13.2 (SD 8.6) months | n=2 | n=36 | 6.0% | Research HRCT | Two radiologists blinded to clinical history; additional 2 patients had traction bronchiectasis secondary to ILD |
| Despaux et al. | 1998 | France | Mean 10.6 (range 2–29) | n=23 | n=46 | 50.0% | Research HRCT | One radiologist blinded to clinical history; Some of the total RA patients studied reported respiratory symptoms |
| Perez et al. | 1998 | France | Mean 14.4 (SD 1.3) | n=15 | n=50 | 30.0% | Research HRCT | Two observers blinded to clinical history; all patients had no airway abnormalities preceding the scan |
| Demir et al. | 1999 | Turkey | Mean 5.38 (SD 2.8) | n=9 | n=34 | 26.0% | Research HRCT | One experienced radiologist blinded to clinical history; all patients had no known airways abnormalities before scan. Of 23 patients with suspected or confirmed RA-ILD, 9 (39%) had RA-BR. |
| Dawson et al. | 2001 | United Kingdom | Mean 12.7 (SD 8) | n=12 | n=150 | 9.5% | Research HRCT | Two consultant radiologists blinded to clinical history; Some of the total RA patients studied reported respiratory symptoms |
| Izumiyama et al. | 2002 | Tokyo | Mean 10.2 | n=21 | n=123 | 17.1% | Research HRCT | Two trainee clinicians and one radiologist blinded to clinical history; patients with syndromes overlapping with other rheumatic diseases were not included; Some of the total RA patients studied reported respiratory symptoms Excluded 63 patients from analysis due to no scan |
| Zrour et al. | 2005 | Tunisia | Mean 96 (SD 88) months | n=14 | n=75 | 18.7% | Research HRCT | Not reported; Respiratory symptoms reported in 33% of total RA patients studied |
| Bilgici et al. | 2005 | Turkey | Mean 8.37 (SD 8.17) | n=9 | n=52 | 17.0% | Research HRCT | Two radiologists (blinding unspecified); patients with history of airways abnormalities were not included Excluded 2 patients from analysis due to no pulmonary function tests |
| Metafratzi et al. | 2009 | Greece | Less than 1 | n=25 | n=43 | 58.0% | Research HRCT | Two observers blinded to clinical history; all patients had no known airways abnormalities before scan |
| Wilsher et al. | 2012 | New Zealand | Median 7 (range 1–12) months | n=29 | n=60 | 48.0% | Research HRCT | Two experienced independent radiologists blinded to clinical history; Respiratory symptoms reported in 30% of total RA patients studied |
| Leonel et al. | 2012 | Mexico | Not reported | n=9 | n=36 | 4.0% | Research HRCT | Two independent radiologists (blinding unspecified); All RA patients studied had clinical or radiological respiratory symptoms |
| Attar et al. | 2015 | Saudi Arabia | Mean 6.19 (SD 6.4) | n=35 | n=100 | 35.0% | Research HRCT | One senior radiologist (blinding unspecified); all patients had no known previous airways abnormalities |
| Koch et al. | 2016 | Brazil | Mean 16.7 (SD 8.8) | n=25 | n=96 | 26.0% | Research HRCT | Two radiologists alone or in consensus (blinding unspecified); Respiratory symptoms reported in 15.6% of the total RA patients studied |
| Robles-Perez et al. | 2016 | Spain | Median 12 months | n=8 | n=40 | 20.0% | Research HRCT | Reviewed by expert lung radiologist (blinding unspecified); Respiratory symptoms reported in 70% of the total RA patients studied RA-ILD was found in 2 of the cases of RA-BR |
| Matsumoto et al. | 2018 | Japan | Mean 17.1 (SD 10.2) | n=32 | n=332 | 9.6% | Research CT | Two experienced radiologists, one with 23 years’ experience reading thoracic CTs and another randomly selected, both blinded to clinical history; Some of the total RA patients studied reported respiratory symptoms RA-BR prevalence was higher in RA without ILD (9.6%) compared to RA-ILD (0.0%) |
| Bessa et al. | 2019 | Brazil | Median 204 (IQR 114–252) months | n=5 | n=21 | 23.8% | Research CT | Two readers with 5 and 21 years of experience in pulmonary imaging, blinded to clinical history; Respiratory symptoms reported in 52.4% of the total RA patients studied |
| Gautam et al. | 2020 | Pakistan | Not reported | n=2 | n=54 | 3.7% | Research HRCT | Experienced radiologists (blinding unspecified); all patients had RA-associated ILD. |
| Lucchino et al. | 2020 | Italy | Mean 13.78 (SD 11.02) months | n=5 | n=27 | 18.5% | Research HRCT | Two radiologists blinded to clinical history; patients required to have no serious comorbidities and no respiratory symptoms |
CT, computed tomography; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; IQR, interquartile range; RA, rheumatoid arthritis; RA-BR, rheumatoid arthritis-associated bronchiectasis; SD, standard deviation.
3.4. RA-BR among patients with RA and other lung diseases
Some studies reported on BR among patients with RA-associated interstitial lung disease (RA-ILD). We identified 7 studies that studied the intersection of RA-BR and RA-ILD (Table 5).One retrospective study by Zhang et al. compared BR in RA-ILD patients to RA-no-ILD, and the prevalence was higher in RA-ILD (18.1%) compared to RA without ILD (10.5%)(18). Six prospective studies included data on BR in RA-ILD patients. McDonagh et al. reported that RA-BR prevalence was higher in RA-ILD (30.0%) compared to RA without ILD (20.0%)(26), while Matsumoto et al. reported that RA-BR prevalence was higher in RA without ILD (9.6%) compared to RA-ILD (0.0%)(25). An additional two patients from a cohort of 36 had traction BR secondary to ILD, and another study found 2 cases of RA-ILD among RA-BR patients(1, 28). Demir et al. stated that of 23 patients with suspected or confirmed RA-ILD, 9 (39%), had RA-BR(27). Overall lung involvement in RA patients ranged from 7–80%, with RA-ILD being the most common(2), which could impact the overall prevalence due to varying definitions of lung abnormalities across studies.
Table 5.
Studies that evaluated the intersection of bronchiectasis and interstitial lung disease among patients with rheumatoid arthritis (n=7).
| Reference | RA duration, years (unless specified) | Patients with prevalence RA-BR (numerator) | Total RA patients studied (denominator) | Prevalence of RA-BR in RA (%) | Comments |
|---|---|---|---|---|---|
| Gautam et al. | Not reported | n=2 | n=54 | 3.7% | All patients had RA-ILD |
| Zhang et al. | Mean 8 (SD 9) | n=76 | n=550 | 13.8% | RA-BR prevalence was higher in RA-ILD (18.1%) compared to RA without ILD (10.5%) |
| McDonagh et al. | Median 9 (range 1–27) | n=4 | n=20 | 20.0% | RA-BR prevalence was higher in RA-ILD (30.0%) compared to RA without ILD (20.0%) |
| Gabbay et al. | Mean 13.2 (SD 8.6) months | n=2 | n=36 | 6.0% | 2 patients had traction bronchiectasis secondary to ILD |
| Demir et al. | Mean 5.38 (SD 2.8) | n=9 | n=34 | 26.0% | Of 23 patients with suspected or confirmed RA-ILD, 9 (39%) had RA-BR. |
| Robles-Perez et al. | Median 12 months | n=8 | n=40 | 20.0% | RA-ILD was found in 2 of the cases of RA-BR |
| Matsumoto et al. | Mean 17.1 (SD 10.2) | n=32 | n=332 | 9.6% | RA-BR prevalence was higher in RA without ILD (9.6%) compared to RA-ILD (0.0%) |
ILD, interstitial lung disease; RA, rheumatoid arthritis; RA-BR, rheumatoid arthritis-associated bronchiectasis; SD, standard deviation.
3.5. Prevalence of RA-BR among RA meta-analysis results
A series of meta-analyses were performed to determine the prevalence of BR among patients with RA in the reported literature. Forest plots of the included studies are shown in Figure 2 for all studies, Figure 3A for retrospective studies, and Figure 3B for prospective studies.
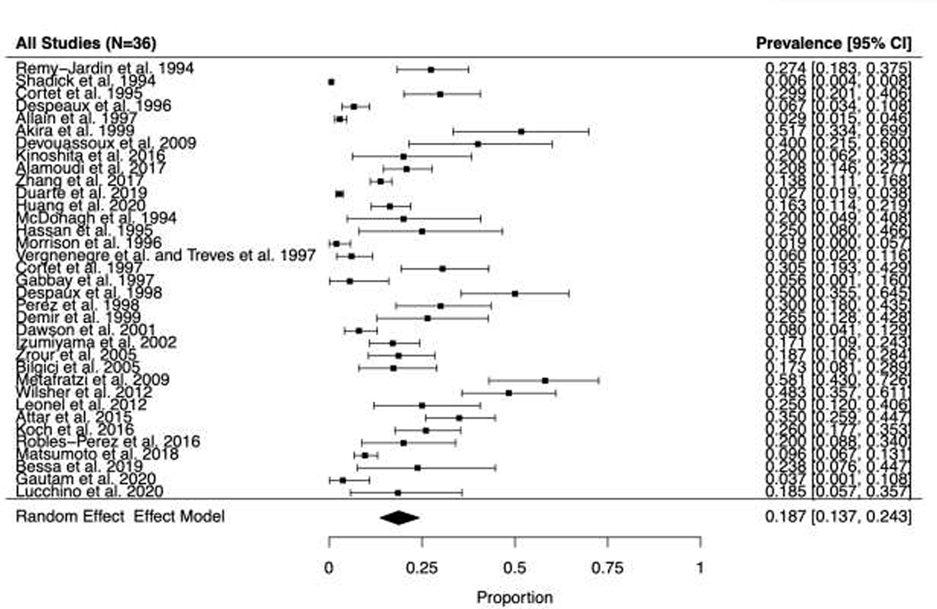
Fig. 2.
Forest plot and random effects meta-analysis for prevalence of bronchiectasis among patients with rheumatoid arthritis in all studies (n=36).
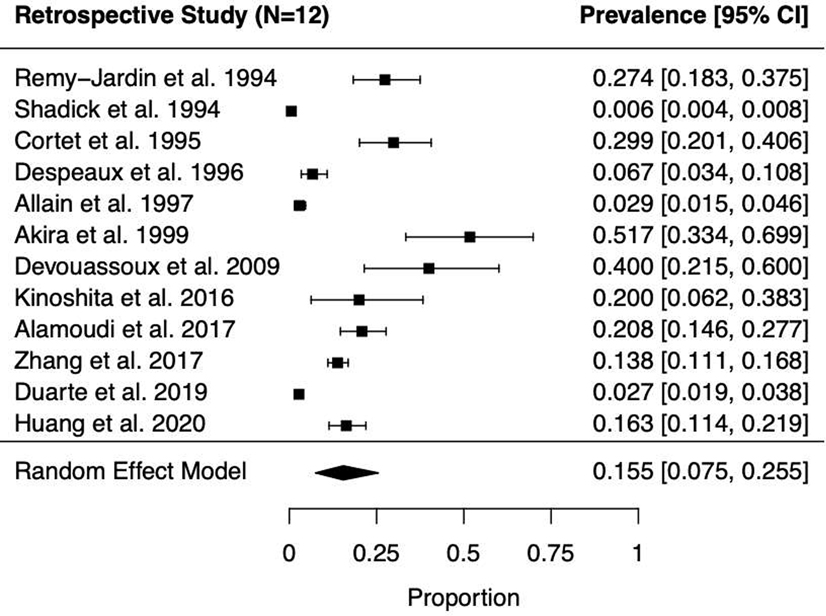
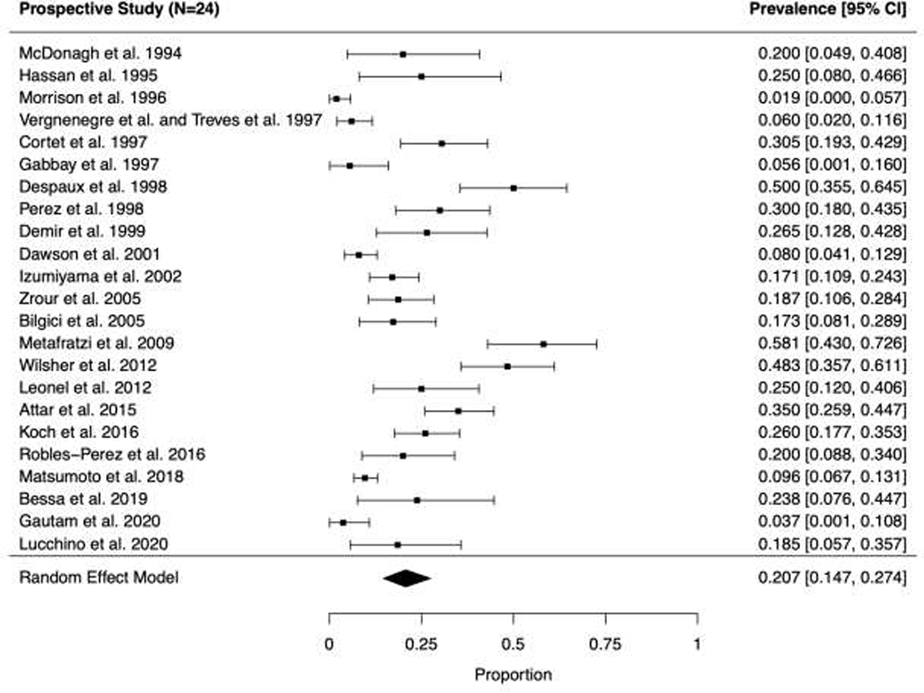
Fig. 3.
Forest plots and random effects meta-analyses of prevalence of bronchiectasis among patients with rheumatoid arthritis in (A, top panel) retrospective (n=12) and (B, bottom panel) prospective studies (n=24).
Among the 36 studies, 608 RA-BR cases were identified from a total of 8,569 patients with RA. In the primary analyses, the pooled overall prevalence of RA-BR in the random effects meta-analysis was 18.7% (95%CI 13.7–24.3%), Figure 2). Among retrospective studies (n=12) reporting RA-BR, the prevalence was 15.5% (95% CI 7.5–25.5%, Figure 3A). Among prospective studies (n=24), the prevalence of RA-BR in the meta-analysis was 20.7% (95% CI 14.7–27.4%, Figure 3B).
Prevalence was lowest in retrospective studies where RA-BR was identified through clinical care (e.g., two large retrospective studies that investigated 4,000 and 1,129 RA patients reported RA-BR prevalence of 0.6% and 2.7%, respectively(2, 8)).
3.6. Sensitivity analysis results
Sensitivity results of meta-analyses are shown in are shown in Table 3. In the meta-analysis using fixed effects, the prevalence of RA-ILD among RA was 3.8% (95%CI 3.3–4.2%). The remaining analyses used random effects as a variation of the primary analysis. After removing a large study with an uncertain denominator, the prevalence was 19.6% (95%CI 14.7–25.1%). After removing four studies(8–11) that did not obtain chest imaging on all patients or used of research chest radiograph instead of chest CT scans, the prevalence was 21.9% (95% CI 16.9–27.4%). When we restricted the analysis to include studies that only conducted HRCT scans (n=24), the prevalence was 22.6% (95% CI 16.8–29.0%). After limiting to studies that had fewer than 50 patients (n=23), the prevalence was 14.4% (95% CI 9.3–20.3%).
Table 3.
Sensitivity results of meta-analyses.
| Analysis | Number of studies included | Prevalence estimate | 95%CI |
|---|---|---|---|
| Combined | |||
| Main analysis | 36 | 0.187 | [0.137, 0.243] |
| Main analysis with fixed effects | 36 | 0.038 | [0.033, 0.042] |
| Shadick et al. removed due to uncertain denominator | 35 | 0.196 | [0.147, 0.251] |
| Removed Shadick et al., Despeaux et al., and Allain et al. due to chest imaging not obtained on all patients | 33 | 0.210 | [0.159, 0.266] |
| Removed Shadick et al., Despeaux et al., and Allain et al. due to chest imaging not obtained on all patients, and Morrison et al. due to use of research chest radiograph instead of chest CT scans | 32 | 0.219 | [0.169, 0.274] |
| Inclusion of studies with only HRCT scans | 24 | 0.226 | [0.168, 0.290] |
| Studies removed with less than 50 patients | 23 | 0.144 | [0.093, 0.203] |
| Retrospective | |||
| Main analysis | 12 | 0.155 | [0.075, 0.255] |
| Main analysis with fixed effects | 12 | 0.021 | [0.017, 0.025] |
| Shadick et al. removed due to uncertain denominator | 11 | 0.177 | [0.095, 0.277] |
| Removed Shadick et al., Despeaux et al., and Allain et al. due to chest imaging not obtained on all patients | 9 | 0.218 | [0.126, 0.327] |
| Inclusion of studies with only HRCT scans | 5 | 0.183 | [0.068, 0.335] |
| Studies removed with less than 50 patients | 9 | 0.108 | [0.046, 0.193] |
| Prospective | |||
| Main analysis | 24 | 0.207 | [0.147, 0.274] |
| Main analysis with fixed effects | 24 | 0.166 | [0.148, 0.185] |
| Removed Morrison et al. due to use of research chest radiograph as a chest imaging modality instead of chest CT scans | 23 | 0.220 | [0.160, 0.286] |
| Inclusion of studies with only HRCT scans | 19 | 0.241 | [0.176, 0.312] |
| Studies removed with less than 50 patients | 14 | 0.172 | [0.103, 0.254] |
3.7. Risk factors for RA-BR
A total of seven studies included in our review found statistically significant risk factors for RA-BR presence among patients with RA(3, 30, 42–46), included in Table 4. Older age and African American ethnicity were the only statistically significant demographic risk factors reported(3, 30). Six genetic risk factors were reported among four studies including CFTR mutations and HLA genetic variants. Hillarby et al. reported that the proportion of HLA-DQB1*0601 in RA-BR was 31%, compared to 4.0% in RA-no-BR (p=0.0001) and also found statistically significant differences in proportion among HLA-DQA and HLA-DQB variants(44). Toussirot et al. found the proportion of HLA-DRB1*0401 to be higher among RA-BR patients (39%) compared to RA-no-BR (17%) (p<0.001)(45). Two studies found significant differences in CFTR for RA-BR risk(42,43). Undetectable circulating mannose binding lectin was the only biomarker reported to be associated with developing BR in RA patients (37.5%) when compared to RA-no-BR (8.9%) (p=0.005)(46). Additionally, biologic disease-modifying antirheumatic drug use was reported as having statistically lower odds of RA-BR compared no use(46).
Table 4.
Demographics, genetics, biomarkers, and medications statistically associated with bronchiectasis among patients with rheumatoid arthritis (n=9 studies).
| Reference | Year | Country | Study design | Risk factor group | Comparison group | Patients with RA-BR | Patients with RA-no-BR | Results | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||
| Attar et al. | 2015 | Saudi Arabia | Prospective cross-sectional | Older age (continuous variable) | N/A | n=35 | n=65 | Mean age of RA-BR: 58.6 years; Mean age of RA-no-BR: 47.0 years (p<0.001) | BR confirmed by senior radiologist evaluating HRCT scan |
| McShane et al. | 2012 | USA | Cross-sectional | African American | European American | n=12 | N/A | Proportion of RA-BR in AA group: 28.6% Proportion of RA-BR in EA group: 6.2% (p<0.05) |
All analyzed patients had BR. BR was confirmed by dedicated chest radiologist blinded to the study. RA was more likely to be the underlying etiology of BR for African Americans. |
| Rheumatoid arthritis characteristics | |||||||||
| Attar et al. | 2015 | Saudi Arabia | Prospective cross-sectional | Longer RA disease duration (continuous variable) | N/A | n=35 | n=65 | Mean disease duration RA-BR: 8.57 years Mean disease duration RA-no-BR: 4.92 years (p=0.006) |
BR confirmed by senior radiologist evaluating HRCT scan |
| Genetics | |||||||||
| Puechal et al. | 1999 | France | Cross-sectional | CFTR mutation F508 | No CFTR mutation F508 | n=26 | n=29 | Proportion of CFTR mutation F508 in RA-BR group: 15.4%; Proportion of CFTR mutation F508 in RA-no-BR: 0% (p<0.05) |
BR confirmed by two independent reviewers of HRCT scan |
| Hillarby et al. | 1993 | England | Prospective cross-sectional | HLA-DQA1*0501 | No HLA-DQA1*0501 | n=41 | n=148 | Proportion of HLA-DQA1*0501 in RA-BR: 40%; Proportion of HLA-DQA1 *0501 in RA-no-BR: 22% (p=0.039) |
BR confirmed by surgery or bronchography |
| Hillarby et al. | 1993 | England | Prospective cross-sectional | HLA-DQB1*0201 | No HLA-DQB1*0201 | n=41 | n=148 | Proportion of HLA-DQB1*0201 in RA-BR: 40%; Proportion of HLA-DQB1*0201 in RA-no-BR: 22% (p=0.039) |
BR confirmed by surgery or bronchography |
| Hillarby et al. | 1993 | England | Prospective cross-sectional | HLA-DQB1*0601 | No HLA-DQB1*0601 | n=41 | n=148 | Proportion of HLA-DQB1*0601 in RA-BR: 31%; Proportion of HLA-DQB1*0601 in RA-no-BR: 4.0% (p=0.0001) |
BR confirmed by surgery or bronchography |
| Toussirot et al. | 2000 | France | Prospective cross-sectional | HLA-DRB1*0401 | No HLA-DRB1*0401 | n=15 | n=25 | Proportion of HLA-DRB1*0401 in RA-BR: 39%; Proportion of HLA-DRB1*0401 in RA-no-BR: 17%; (p<0.0001) |
BR confirmed by CT scan |
| Puechal et al. | 2010 | France | Cross-sectional | CFTR mutation | No CFTR mutation | n=30 | n=19 | CFTR mutation: OR 5.30 for RA-BR (p=5×10−5) | BR confirmed by two independent reviewers of HRCT scan |
| Biomarkers | |||||||||
| Makin et al. | 2019 | Israel | Retrospective cohort | Undetectable mannose binding lectin (MBL) | Detectable MBL | n=17 | n=114 | Proportion of undetectable MBL in RA-BR: 37.5%; Proportion of undetectable MBL in RA-no-BR: 8.9% (p=0.005) |
BR confirmed by evaluation of HRCT scan; MBL is a pattern recognition molecule of the innate immune system that binds to sugars on microbial surfaces to activate complement pathways |
| Medications | |||||||||
| Makin et al. | 2019 | Israel | Retrospective cohort | No bDMARD use | bDMARD use | n=16 | n=115 | Proportion of bDMARD use in RA-BR: 25%; Proportion of bDMARD use RA-no-BR: 75% (p=0.0002) |
BR confirmed by evaluation of HRCT scan; finding could be due to reverse causation where clinicians were avoiding bDMARDs in patients with known RA-BR |
bDMARD, biologic disease-modifying antirheumatic drug; BR, bronchiectasis; CFTR, cystic fibrosis transmembrane conductance regulator; HLA, human leukocyte antigen; MBL, mannose binding lectin; N/A, not applicable; RA, rheumatoid arthritis.
4. DISCUSSION
In this systematic review and meta-analysis, the prevalence of RA-BR among RA was 18.7% among the 36 included studies, emphasizing that BR may be a common and underrecognized extra-articular feature of RA. However, given that most studies incorporated chest imaging, this may include subclinical involvement. Despite how common RA-BR may be, we only identified 7 studies that reported statistically significant risk factors for RA-BR, emphasizing the relative lack of literature regarding risk factors of BR in RA patients.
When studies were separated based on study design (prospective vs. retrospective) the prevalence was 15.5% vs. 20.7%, respectively. This difference could be attributed to the use of either research scans or clinical scans that results in overestimation of BR diagnosis. Wiater et al. recently conducted a similar systematic review on RA-BR prevalence and reported similar results: prevalence of 21.1% overall; clinical prevalence was (2.69%) and prevalence among those with imaging was 24.9%(5). Our results extend these by performing additional sensitivity analyses and also systemically reviewing the current literature related to risk factors for RA-BR.
Our results also indicate heterogeneous study designs and methods related to identification and case definitions of RA-BR. Studies varied considerably related to key factors such as prospective vs. retrospective design, sample size, diagnostic tools, and demographic or clinical features of included patients. For example, prevalence ranged from 0.6% to 58.0% across all studies included, 0.6% to 52.0% in retrospective studies, and 1.9% to 58% in prospective studies. Smaller studies of both prospective and retrospective methods generally reported higher prevalence of RA-BR, while most larger studies reported lower prevalence with regards to our pooled prevalence of 18.7%. The prevalence largely varied among clinical and research scans, use of CT, HRCT, or radiographic methods. Additionally, many studies did not report on whether experienced chest radiologists were involved in the detection of RA-BR caes. The inclusion or exclusion of reported respiratory symptoms prior to BR diagnosis in RA patients may have resulted in either an underestimation of BR diagnosis or, for the latter, identification of subclinical BR. Secondary or co-existing pulmonary conditions such as RA-ILD are another possible explanation, and 7 included studies reported on this(1, 18, 23, 25–28), three of which found a higher prevalence of BR in RA-ILD patients compared to RA without known ILD(18, 26, 27). The significant variation in prevalence highlights the need for longitudinal studies with larger RA cohorts to investigate the prevalence rates of BR in RA patients, taking into account the current diagnostic methods for BR (chest CT vs. HRCT vs. clinical symptoms), RA disease duration, and population characteristics related to respiratory symptoms and pre-test clinical suspicion for pulmonary involvement. There are also may be a selection bias in prospective studies where patients may be more amenable to participate if they have respiratory symptoms. Conversely, healthy individuals may be more amenable to participate in research studies. Thus, the results of our prevalence studies should be interpreted with caution and more work is needed to establish the true clinical and subclinical prevalence. However, our results do emphasize that BR is under-recognized and under-studied. RA-BR may have important implications related to the growing literature implicating airways disease in RA pathogenesis(47–51). RA-BR are also known to have increased risk for infection mortality(6, 52–54). Therefore, our results emphasize the many gaps in the literature related to RA-BR that deserve future study.
There were only 7 studies that reported statistical associations of risk factors with presence of RA-BR among RA patients. Based on our findings, the strongest evidence for possible risk involved genetics, but three(43–45) of the four studies were conducted >20 years ago, which demonstrates the need for updated research focusing on larger sample size and standardized diagnostic methods. The 6 genetic risk factors linked to BR development were reported in four studies(42–45) which focused on CFTR and HLA variants. A strength of Hillarby et al. study was its larger sample size when compared to all other risk factor groups, but its method of BR diagnosis using either surgery or bronchography could create inconsistent reports of true BR(44). Puechal et al. had a fairly small sample size in both studies looking at CFTR mutation, but its method of BR diagnosis was most reliable in comparison to the other studies of genetic risk factors in that it used HRCT scans reviewed by two independent reviewers(42). Additionally, Attar et al. confirmed BR diagnosis using HRCT scans reviewed by a senior radiologist when reporting on age and RA disease duration(30). Disease duration and age of RA patients were identified as risk factors in the study by Attar et al. where BR in RA patients had a greater mean RA disease duration by 3.65 years, as well as older mean age by 11.6 years(30). This emphasizes the need to focus on and categorize patient age and disease duration when reporting the etiology and prevalence of BR in RA. The two studies that found increased odds of BR in RA among patients with African American ethnicity (compared to European)(3) and no use of biologic DMARDs(46) should be investigated further. The former association may be surprising given the higher prevalence of CFTR mutations in those with European ancestry. The latter association could be due to possible reverse causation and RA since clinicians may avoid biologic DMARDs due to the risk of infection. The biomarker mannose binding lectin (MBL) was found to be undetectable in a greater proportion of RA-BR patients compared to RA-no-BR(46). MBL is a pattern recognition molecule of the innate immune system that binds to sugars on microbial surfaces to activate complement pathways, suggesting that innate immunity and complement system may be involved in RA-BR. However, this was a small single study so this finding should be replicated. Identification of other biomarkers are needed to elucidate the pathogenesis of RA-BR and develop screening strategies for clinical use.
Although our systematic review did not focus on non-statistically significant risk factors and followed strict inclusion criterion, it is important to mention some of the proposed risk factors in other studies. Smoking is known to increase the risk of RA as well as various obstructive lung diseases(7, 55), but there is no current evidence suggesting smoking as a risk factor for BR development in RA(44). On the contrary, Kaushik et al. reported a higher proportion of non-smokers in the RA-BR group compared to RA alone, and those with RA only had a higher smoking pack-year mean than those with RA-BR(55). Elevated inflammatory markers were also mentioned as a potential risk factor in two studies(30, 56), one of which found elevated CCP and RF antibodies in a higher proportion of RA-BR patients compared to RA-no-BR, but this was not statistically significant(56). Further research is needed to clarify the link between these risk factors and development of BR in RA patients.
The sample sizes and scans not conducted on all patients may influence the estimation of individual prevalence rates. For example, the largest study approximated the total RA patients studied as 4,000, but clinical CT was not conducted on all patients analyzed(8), which could explain why the prevalence was only 0.6%. As expected, the prevalence increased when we removed this study (to 19.6%) and again when we removed studies that did not conduct imaging on all patients (to 21.0%)(9, 10).
As mentioned previously, most of the smaller studies had higher prevalence rates. After 13 studies with 50 or fewer patients were removed, the prevalence decreased to 14.4%(1, 14–16, 22, 26–29, 31, 34, 36, 41), representing a significant portion of the overall prevalence studies. The studies with some of the largest sample sizes in comparison to others, reported a lower prevalence of RA-BR than our pooled prevalence. Duarte et al. reported a prevalence of 2.75% among 1,129 RA patients(2), and Matsumoto et al. reported a 9.6% prevalence among 332 RA patients(25). Due to differing study design methods and reporting of other variables, it is unclear whether and which factors could have impacted the results. For example, individual patient demographics, including RA disease duration and reported respiratory symptoms pose a limitation due to inconsistencies across included studies. Additionally, it was important to include any data on suspected RA-associated interstitial lung disease (RA-ILD) which could interfere with the accuracy of reported lung abnormalities, specifically relating to BR.
Recommended chest imaging modality for BR diagnosis is vital for accurate diagnosis, and 12 of the studies did not use HRCT scans(8–12, 14, 16, 19–21, 25, 41). When these studies were removed, prevalence increased to 22.6%. There was significant heterogeneity in the methods used to interpret imaging scans and identify RA-BR cases across all studies. Five of the studies did not report details on image interpretation.(8, 9, 18, 20, 21, 24). This emphasizes the need for a reliable and consistent method of BR diagnosis among RA patients.
4.1. Strengths and Limitations
Strengths of our systematic review and meta-analysis were the use of pre-specified inclusion and exclusion criteria as well as extensive research and review of all relevant published articles on BR and RA. Several sensitivity analyses were conducted to provide further support and clarity on what may or may not impact resultsA limitation included the inability to clearly identify the underlying causes of bronchiectasis. Bronchiectasis could be isolated or secondary to architectural distortion from fibrotic lung disease. Patients with isolated bronchiectasis may be more likely to require treatment aimed at primary airway dysfunction including mucociliary clearance, antibiotics, bronchodilators, and perhaps azithromycin. Bronchiectasis secondary to traction due to bronchiectasis may be less likely to be complicated by these issues so may be clinically distinct. Therefore, we summarized the specific studies that studied both RA and ILD. These findings highlight the need for more standardized assessments for bronchiectasis in RA, either isolated or due to traction from ILD, among other causes. We limited inclusion criteria to statistically significant risk factors, but it is possible overall trends could have been identified for risk factors that were not statistically significant in the individual studies. Another limitation is our use of 2 reviewers during the process of literature review, and may have resulted in risk of assessment bias. Additionally, after the search, we may have left out recently published relevant studies.
5. Conclusion
The prevalence of RA-BR in this systematic review and meta-analysis was 18.7%, emphasizing that bronchiectasis is a common extra-articular feature of RA. There were relatively few risk factors identified for RA-BR presence among RA, including older age, longer RA duration, genetics (CFTR and HLA), and undetectable MBL. Future studies should standardize methods to identify RA-BR cases and investigate the natural history and clinical course given the relatively high prevalence that we report.
Funding
Dr. Sparks is supported by the National Institutes of Health (NIH)National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers K23 AR069688, R03 AR075886, L30 AR066953, P30 AR070253, and P30 AR072577), the Rheumatology Research Foundation (R Bridge Award), and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Doyle is supported by the NIH/National Heart, Lung, and Blood Institute (grant numbers K23 HL119558, R03 HL148484). The funders had no role in the decision to publish or preparation of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the NIH.
Declaration of Competing Interest
Dr. Sparks reports research support from Amgen and Bristol-Myers Squibb and consultancy fees from Bristol-Myers Squibb, Gilead, Inova, Janssen, Optum, and Pfizer. Dr. Doyle reports research support from Bristol Myers Squibb, consulting fees from Boehringer Ingelheim, and has been involved in clinical trials funded by Bristol Myers Squibb and Genentech. Dr. Shadick reports research support from Bristol-Myers Squibb, Amgen, Eli Lilly, Mallinckrodt, and Sanofi and consulting fees from Bristol-Myers Squibb. All other authors report no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Robles-Perez A, Luburich P, Rodriguez-Sanchon B, Dorca J, Nolla JM, Molina-Molina M, et al. Preclinical lung disease in early rheumatoid arthritis. Chronic respiratory disease. 2016;13(1):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duarte AC, Porter JC, Leandro MJ. The lung in a cohort of rheumatoid arthritis patients-an overview of different types of involvement and treatment. Rheumatology. 2019;58(11):2031–8. [DOI] [PubMed] [Google Scholar]
- 3.McShane PJ, Naureckas ET, Strek ME. Bronchiectasis in a diverse US population: effects of ethnicity on etiology and sputum culture. Chest. 2012;142(1):159–67. [DOI] [PubMed] [Google Scholar]
- 4.Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chronic respiratory disease. 2017;14(4):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiater R, Hakansson KEJ, Ulrik CS. A causal relationship between rheumatoid arthritis and bronchiectasis? A systematic review and meta-analysis. Chronic respiratory disease. 2021;18:1479973121994565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swinson DR, Symmons D, Suresh U, Jones M, Booth J. Decreased survival in patients with co-existent rheumatoid arthritis and bronchiectasis. Br J Rheumatol. 1997;36(6):689–91. [DOI] [PubMed] [Google Scholar]
- 7.Park WH, Kim SS, Shim SC, Song ST, Jung SS, Kim JH, et al. Visual Assessment of Chest Computed Tomography Findings in Anti-cyclic Citrullinated Peptide Antibody Positive Rheumatoid Arthritis: Is it Associated with Airway Abnormalities? Lung. 2016;194(1):97–105. [DOI] [PubMed] [Google Scholar]
- 8.Shadick NA, Fanta CH, Weinblatt ME, O’Donnell W, Coblyn JS. Bronchiectasis. A late feature of severe rheumatoid arthritis. Medicine. 1994;73(3):161–70. [PubMed] [Google Scholar]
- 9.Despaux J, Polio JC, Toussirot E, Dalphin JC, Wendling D. Rheumatoid arthritis and bronchiectasis. A retrospective study of fourteen cases. Revue du rhumatisme. 1996;63(11):801–8. [PubMed] [Google Scholar]
- 10.Allain J, Saraux A, Guedes C, Valls I, Devauchelle V, Le Goff P. Prevalence of symptomatic bronchiectasis in patients with rheumatoid arthritis. Revue du rhumatisme. 1997;64(10):531–7. [PubMed] [Google Scholar]
- 11.Morrison SC, Mody GM, Benatar SR, Meyers OL. The lungs in rheumatoid arthritis--a clinical, radiographic and pulmonary function study. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1996;86(7):829–33. [PubMed] [Google Scholar]
- 12.Remy-Jardin M, Remy J, Cortet B, Mauri F, Delcambre B. Lung changes in rheumatoid arthritis: CT findings. Radiology. 1994;193(2):375–82. [DOI] [PubMed] [Google Scholar]
- 13.Cortet B, Flipo RM, Remy-Jardin M, Coquerelle P, Duquesnoy B, Remy J, et al. Use of high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Annals of the rheumatic diseases. 1995;54(10):815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akira M, Sakatani M, Hara H. Thin-section CT findings in rheumatoid arthritis-associated lung disease: CT patterns and their courses. Journal of computer assisted tomography. 1999;23(6):941–8. [DOI] [PubMed] [Google Scholar]
- 15.Devouassoux G, Cottin V, Liote H, Marchand E, Frachon I, Schuller A, et al. Characterisation of severe obliterative bronchiolitis in rheumatoid arthritis. The European respiratory journal. 2009;33(5):1053–61. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita S, Aoki T, Takahashi H, Oki H, Hayashida Y, Saito K, et al. Thin-section chest CT findings in polymyalgia rheumatica: a comparison between with and without rheumatoid arthritis. Clinical imaging. 2016;40(3):382–5. [DOI] [PubMed] [Google Scholar]
- 17.Alamoudi OSB, Attar SM. Pleuropulmonary manifestation in patients with rheumatoid arthritis in Saudi Arabia. Annals of thoracic medicine. 2017;12(4):266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Li H, Wu N, Dong X, Zheng Y. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clinical rheumatology. 2017;36(4):817–23. [DOI] [PubMed] [Google Scholar]
- 19.Huang S, Doyle TJ, Hammer MM, Byrne SC, Huang W, Marshall AA, et al. Rheumatoid arthritis-related lung disease detected on clinical chest computed tomography imaging: Prevalence, risk factors, and impact on mortality. Semin Arthritis Rheum. 2020;50(6):1216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vergnenegre A, Pugnere N, Antonini MT, Arnaud M, Melloni B, Treves R, et al. Airway obstruction and rheumatoid arthritis. The European respiratory journal. 1997;10(5):1072–8. [DOI] [PubMed] [Google Scholar]
- 21.Treves R, Pugnere N, Bonnet C, Bertin P, Arnaud M, Vergnenegre A, et al. A prospective study of pulmonary symptoms in 188 patients with rheumatoid arthritis. Revue du rhumatisme. 1997;64(6):435. [PubMed] [Google Scholar]
- 22.Leonel D, Lucia C, A M, Martha-Alicia H, Blanca M. Pulmonary function test: its correlation with pulmonary high-resolution computed tomography in patients with rheumatoid arthritis. Rheumatology international. 2012;32(7):2111–6. [DOI] [PubMed] [Google Scholar]
- 23.Gautam M, Masood MJ, Arooj S, Mahmud ME, Mukhtar MU. Rheumatoid Arthritis Related Interstitial Lung Disease: Patterns of High-resolution Computed Tomography. Cureus. 2020;12(2):e6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zrour SH, Touzi M, Bejia I, Golli M, Rouatbi N, Sakly N, et al. Correlations between high-resolution computed tomography of the chest and clinical function in patients with rheumatoid arthritis. Prospective study in 75 patients. Joint bone spine. 2005;72(1):41–7. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto T, Iwano S, Takahashi N, Asai S, Watanabe T, Asai N, et al. Association between chest computed tomography findings and respiratory adverse events in rheumatoid arthritis patients undergoing long-term biological therapy. International journal of rheumatic diseases. 2019;22(4):626–35. [DOI] [PubMed] [Google Scholar]
- 26.McDonagh J, Greaves M, Wright AR, Heycock C, Owen JP, Kelly C. High resolution computed tomography of the lungs in patients with rheumatoid arthritis and interstitial lung disease. British journal of rheumatology. 1994;33(2):118–22. [DOI] [PubMed] [Google Scholar]
- 27.Demir R, Bodur H, Tokoglu F, Olcay I, Ucan H, Borman P. High resolution computed tomography of the lungs in patients with rheumatoid arthritis. Rheumatology international. 1999;19(1–2):19–22. [DOI] [PubMed] [Google Scholar]
- 28.Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, et al. Interstitial lung disease in recent onset rheumatoid arthritis. American journal of respiratory and critical care medicine. 1997;156(2 Pt 1):528–35. [DOI] [PubMed] [Google Scholar]
- 29.Lucchino B, Di Paolo M, Gioia C, Vomero M, Diacinti D, Mollica C, et al. Identification of Subclinical Lung Involvement in ACPA-Positive Subjects through Functional Assessment and Serum Biomarkers. International journal of molecular sciences. 2020;21(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attar SM, Alamoudi OS, Aldabbag AA. Prevalence and risk factors of asymptomatic bronchiectasis in patients with rheumatoid arthritis at a tertiary care center in Saudi Arabia. Annals of thoracic medicine. 2015;10(3):176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metafratzi ZM, Georgiadis AN, Ioannidou CV, Alamanos Y, Vassiliou MP, Zikou AK, et al. Pulmonary involvement in patients with early rheumatoid arthritis. Scandinavian journal of rheumatology. 2007;36(5):338–44. [DOI] [PubMed] [Google Scholar]
- 32.Bilgici A, Ulusoy H, Kuru O, Celenk C, Unsal M, Danaci M. Pulmonary involvement in rheumatoid arthritis. Rheumatology international. 2005;25(6):429–35. [DOI] [PubMed] [Google Scholar]
- 33.Perez T, Remy-Jardin M, Cortet B. Airways involvement in rheumatoid arthritis: clinical, functional, and HRCT findings. American journal of respiratory and critical care medicine. 1998;157(5 Pt 1):1658–65. [DOI] [PubMed] [Google Scholar]
- 34.Hassan WU, Keaney NP, Holland CD, Kelly CA. High resolution computed tomography of the lung in lifelong non-smoking patients with rheumatoid arthritis. Annals of the rheumatic diseases. 1995;54(4):308–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortet B, Perez T, Roux N, Flipo RM, Duquesnoy B, Delcambre B, et al. Pulmonary function tests and high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Annals of the rheumatic diseases. 1997;56(10):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Despaux J, Manzoni P, Toussirot E, Auge B, Cedoz JP, Wendling D. Prospective study of the prevalence of bronchiectasis in rheumatoid arthritis using high-resolution computed tomography. Revue du rhumatisme. 1998;65(7–9):453–61. [PubMed] [Google Scholar]
- 37.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax. 2001;56(8):622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izumiyama T, Hama H, Miura M, Hatakeyama A, Suzuki Y, Sawai T, et al. Frequency of broncho-bronchiolar disease in rheumatoid arthritis: an examination by high-resolution computed tomography. Modern rheumatology. 2002;12(4):311–7. [DOI] [PubMed] [Google Scholar]
- 39.Wilsher M, Voight L, Milne D, Teh M, Good N, Kolbe J, et al. Prevalence of airway and parenchymal abnormalities in newly diagnosed rheumatoid arthritis. Respiratory medicine. 2012;106(10):1441–6. [DOI] [PubMed] [Google Scholar]
- 40.Koch MC, Pereira IA, Nobre LF, Neves FS. Computed tomography of pulmonary changes in rheumatoid arthritis: carcinoembryonic antigen (CEA) as a marker of airway disease. Rheumatology international. 2016;36(4):531–9. [DOI] [PubMed] [Google Scholar]
- 41.Bessa EJC, Ribeiro FMC, Pinheiro G, Lopes AJ. Does the nitrogen single-breath washout test contribute to detecting pulmonary involvement in rheumatoid arthritis? A pilot study. BMC research notes. 2019;12(1):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puechal X, Bienvenu T, Genin E, Berthelot JM, Sibilia J, Gaudin P, et al. Mutations of the cystic fibrosis gene in patients with bronchiectasis associated with rheumatoid arthritis. Annals of the rheumatic diseases. 2011;70(4):653–9. [DOI] [PubMed] [Google Scholar]
- 43.Puechal X, Fajac I, Bienvenu T, Desmazes-Dufeu N, Hubert D, Kaplan JC, et al. Increased frequency of cystic fibrosis deltaF508 mutation in bronchiectasis associated with rheumatoid arthritis. The European respiratory journal. 1999;13(6):1281–7. [DOI] [PubMed] [Google Scholar]
- 44.Hillarby MC, McMahon MJ, Grennan DM, Cooper RG, Clarkson RW, Davies EJ, et al. HLA associations in subjects with rheumatoid arthritis and bronchiectasis but not with other pulmonary complications of rheumatoid disease. Br J Rheumatol. 1993;32(9):794–7. [DOI] [PubMed] [Google Scholar]
- 45.Toussirot E, Despaux J, Wendling D. Increased frequency of HLA-DRB1*0401 in patients with RA and bronchiectasis. Annals of the rheumatic diseases. 2000;59(12):1002–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makin K, Easter T, Kemp M, Kendall P, Bulsara M, Coleman S, et al. Undetectable mannose binding lectin is associated with HRCT proven bronchiectasis in rheumatoid arthritis (RA). PloS one. 2019;14(4):e0215051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ford JA, Liu X, Chu SH, Lu B, Cho MH, Silverman EK, et al. Asthma, Chronic Obstructive Pulmonary Disease, and Subsequent Risk for Incident Rheumatoid Arthritis Among Women: A Prospective Cohort Study. Arthritis Rheumatol. 2020;72(5):704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedlander HM, Ford JA, Zaccardelli A, Terrio AV, Cho MH, Sparks JA. Obstructive lung diseases and risk of rheumatoid arthritis. Expert Rev Clin Immunol. 2020;16(1):37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang S, He X, Doyle TJ, Zaccardelli A, Marshall AA, Friedlander HM, et al. Association of rheumatoid arthritis-related autoantibodies with pulmonary function test abnormalities in a rheumatoid arthritis registry. Clin Rheumatol. 2019;38(12):3401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaccardelli A, Liu X, Ford JA, Cui J, Lu B, Chu SH, et al. Elevated Anti-Citrullinated Protein Antibodies Prior to Rheumatoid Arthritis Diagnosis and Risks for Chronic Obstructive Pulmonary Disease or Asthma. Arthritis Care Res (Hoboken). 2021;73(4):498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaccardelli A, Liu X, Ford JA, Cui J, Lu B, Chu SH, et al. Asthma and elevation of anti-citrullinated protein antibodies prior to the onset of rheumatoid arthritis. Arthritis Res Ther. 2019;21(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Soyza A, McDonnell MJ, Goeminne PC, Aliberti S, Lonni S, Davison J, et al. Bronchiectasis Rheumatoid Overlap Syndrome Is an Independent Risk Factor for Mortality in Patients With Bronchiectasis: A Multicenter Cohort Study. Chest. 2017;151(6):1247–54. [DOI] [PubMed] [Google Scholar]
- 53.Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, et al. Incidence and mortality of obstructive lung disease in rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken). 2013;65(8):1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Md Yusof MY, Iqbal K, Darby M, Lettieri G, Vital EM, Beirne P, et al. Effect of rituximab or tumour necrosis factor inhibitors on lung infection and survival in rheumatoid arthritis-associated bronchiectasis. Rheumatology (Oxford). 2020;59(10):2838–46. [DOI] [PubMed] [Google Scholar]
- 55.Kaushik VV, Hutchinson D, Desmond J, Lynch MP, Dawson JK. Association between bronchiectasis and smoking in patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2004;63(8):1001–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perry E, Kelly C, Eggleton P, De Soyza A, Hutchinson D. The lung in ACPA-positive rheumatoid arthritis: an initiating site of injury? Rheumatology. 2014;53(11):1940–50. [DOI] [PubMed] [Google Scholar]