Abstract
BACKGROUND:
The role of differing levels of frailty in choice of oral anticoagulants for older adults with atrial fibrillation (AF) remains unclear.
OBJECTIVE:
To examine the outcomes of direct oral anticoagulants (DOAC) versus warfarin by frailty levels
DESIGN:
1:1 propensity score-matched analysis of Medicare data, 2010-2017
SETTING:
Community
PATIENTS:
Medicare beneficiaries with AF who initiated dabigatran, rivaroxaban, apixaban, or warfarin
MEASUREMENTS:
Composite endpoint of death, ischemic stroke, or major bleeding by frailty levels, defined by a claims-based frailty index
RESULTS:
In the dabigatran-warfarin cohort (n=158,712; median follow-up, 72 days), the event rate (per 1,000 person-years) was 63.5 for dabigatran-initiators and 65.6 for warfarin-initiators (hazard ratio [HR], 0.98 [95% CI: 0.92, 1.05]; rate difference [RD], −2.2 [−6.5, 2.1]). For non-frail, pre-frail, and frail groups, HRs were 0.81 (0.68, 0.97), 0.98 (0.90, 1.08), and 1.09 (0.96, 1.23), respectively. In the rivaroxaban-warfarin cohort (n=275,944; median follow-up, 82 days), the event rate (per 1,000 person-years) was 77.8 for rivaroxaban-initiators and 83.7 for warfarin-initiators (HR, 0.98 [0.94-1.02]; RD, −3.9 [−10.3, 2.4]). For non-frail, pre-frail, and frail groups, HRs were 0.88 (0.77, 0.99), 1.04 (0.98, 1.10), and 0.96 (0.89, 1.04), respectively. In the apixaban-warfarin cohort (n=218,738; median follow-up, 84 days), the event rate (per 1,000 person-years) was 60.1 for apixaban-initiators and 92.3 for warfarin-initiators (HR, 0.68 [0.65, 0.72]; RD, −32.2 [−36.1, −28.3]). For non-frail, pre-frail, and frail groups, HRs were 0.61 (0.52, 0.71), 0.66 (0.61, 0.70), and 0.73 (0.67, 0.80), respectively.
LIMITATIONS:
Residual confounding, lack of clinical frailty assessment
CONCLUSION:
For older adults with AF, apixaban was associated with lower rates of adverse events across all frailty levels. Dabigatran and rivaroxaban were associated with lower event rates only among non-frail patients.
FUNDING SOURCE:
National Institute on Aging
INTRODUCTION
The efficacy and safety of direct oral anticoagulants (DOACs) in patients with atrial fibrillation (AF) are well established from clinical trials.(1-4) DOACs are at least as efficacious as warfarin with generally lower rates of major bleeding, fewer drug-drug or drug-food interactions, and no need for therapeutic monitoring or frequent dose adjustments. Owing to these advantages, DOACs are preferred over warfarin.(5, 6) The safety profile of DOACs may be particularly desirable for older adults with frailty,(5) who are at high risk for falls(7) and drug-related adverse events.(8) Currently, decisions about anticoagulation therapy are mainly driven by risk assessment models for ischemic stroke(9, 10) and major bleeding,(10-12) without consideration of frailty. Due to poor representation of older adults with frailty and lack of frailty assessment in clinical trials, the role of frailty in the choice between a DOAC and warfarin is uncertain(13) and anticoagulant use remains suboptimal in frail patients with AF.(14, 15)
To fill this evidence gap, we analyzed Medicare data, which contain information on prescription drug use and clinical events in older adults with AF, including those with frailty. We measured frailty using a validated claims-based frailty index (CFI).(16-19) The objective of this study was to investigate how frailty affects the association of a DOAC (dabigatran, rivaroxaban, apixaban) vs warfarin with ischemic stroke, major bleeding, and death in older adults with AF. We postulated that frailty would be an effect modifier of the benefit and safety of a DOAC vs warfarin.
METHODS
Study Design and Population
This retrospective observational study using Medicare data was approved by the Institutional Review Board of the Brigham and Women’s Hospital, Boston, Massachusetts. We emulated 3 trials, each comparing a DOAC with warfarin. We created new-user cohorts of fee-for-service Medicare beneficiaries with AF who filled a prescription for one of the 3 DOACs or warfarin from the date of approval (dabigatran cohort: October 19, 2010; rivaroxaban cohort: November 4, 2011; apixaban cohort: December 28, 2012) through December 31, 2017. Eligible beneficiaries were those who were ≥65 years old; filled their prescription for a DOAC or warfarin with no exposure in the past 183 days; had ≥1 inpatient or outpatient diagnosis of AF in the past 183 days; and had continuous enrollment in Medicare Part A, B, and D, and no enrollment in Medicare Advantage Plan in the past 183 days. We excluded those who 1) had missingness on age, sex, race, or geographical regions; 2) received hospice care in the past 183 days; 3) resided in a nursing facility at the time of drug initiation; 4) received another DOAC in the past 183 days; 5) had other indications for anticoagulation (venous thromboembolism or joint replacement); or 6) had contraindications to either DOAC or warfarin therapy (valvular disease, intracranial or retroperitoneal bleeding, chronic kidney disease stage 5 or end stage renal disease or renal transplant, or stroke within 14 days). These conditions were defined in Supplement 1. Beneficiaries who satisfied these selection criteria represent “clinical care population”. We applied additional exclusion criteria to mimic clinical trials(1-4) (e.g., any nursing facility admission, chronic kidney disease stage 4, gastrointestinal bleeding, treatment for malignancy, chronic non-steroidal anti-inflammatory drug therapy, and limited life expectancy based on the combined comorbidity score(20) ≥5 or CFI(16-19) ≥0.35, which corresponds to the predicted 1-year mortality ≥20%). This “trial population” was used in a sensitivity analysis (see diagrams in Supplement 2, Figures 1-3).
Measurement of Frailty and Baseline Covariates
Frailty was measured using a CFI,(16-19) which estimates a deficit-accumulation frailty index (range: 0-1) using 93 variables defined by diagnosis, health services, and durable medical equipment codes in the year before the drug initiation (https://dataverse.harvard.edu/dataverse/cfi). The CFI has been validated against gait speed,(17) grip strength,(17) frailty phenotype,(18) deficit-accumulation frailty index,(18) and severe disability.(18) Using accepted cutpoints,(21-23) we defined non-frailty if CFI <0.15, pre-frailty if CFI 0.15-0.24, and frailty if CFI ≥0.25.
We measured comorbidities, prescription drug use, and health care utilization during the 183 days prior to the drug initiation. We computed the CHA2DS2-VASc score(9, 10) for the ischemic stroke risk and the modified HAS-BLED score (labile international normalized ratio was unavailable in Medicare data) for the bleeding risk.(10-12) The burden of comorbidity was quantified using a combined comorbidity score.(20)
Outcomes and Follow-Up
The primary outcome was a composite endpoint of death, ischemic stroke, or major bleeding. Secondary outcomes included individual components of the primary outcome, major gastrointestinal bleeding, and intracranial bleeding. The date of death was obtained through linkage to Social Security files. Clinical events were defined using validated claims-based algorithms (Supplement 1). Ischemic stroke was defined based on the diagnosis codes in the primary position of the inpatient discharge diagnoses (positive predictive value: 85%-90%).(24, 25) Major bleeding was defined based on 1) critical site bleeding (e.g., intraarticular, intracranial, intramuscular, intraocular, intraspinal, pericardial, and retroperitoneal) in the primary position of the inpatient discharge diagnoses or 2) bleeding-related diagnosis codes in the primary position of the inpatient discharge diagnoses (positive predictive value: 86-96%)(26) with transfusion procedure codes or revenue codes. This algorithm for major bleeding has been used in previous claims-based studies.(27, 28)
Follow-up began on the day after initiating the index drug and continued until the earliest of the following events: occurrence of study outcome, disenrollment from Medicare, end of study period (December 31, 2017), discontinuation of the index drug (defined as a treatment gap [grace period] longer than 5 days), switching to a different anticoagulant, admission to a nursing facility, enrollment in hospice, initiation of dialysis, or kidney transplantation. We assumed that individuals remained exposed to the index drug as long as the treatment gap is 5 days or less, and for an additional 5 days after discontinuation (exposure risk window). Because the duration of anticoagulant action differs between a DOAC (2-3 days)(29-31) and warfarin (up to 5 days),(32) we also analyzed data by allowing a 3-day grace period and an exposure risk window of 3 days.
Statistical Analysis
The analyses were conducted for each DOAC-warfarin cohort both in the entire population and stratified by the frailty category. We compared clinical characteristics between the DOAC-treated group and warfarin-treated group. To reduce imbalance in clinical characteristics between the groups, we estimated a propensity score (PS) using a logistic regression that modeled the probability of initiating a DOAC vs warfarin as a function of age, sex, race (white, black, and other), dual eligibility, chronic conditions, CHA2DS2-VASc score, HAS-BLED score, the combined comorbidity index, CFI, prescription drug classes, emergency department visits, home health days, hospitalizations, skilled nursing facility visits, calendar year, and geographical region (states) (see Supplement 2, Table 1 for the PS models). We performed 1:1 nearest neighbor matching with a caliper of 1% of the PS for the total population and repeated matching for each frailty category. The algorithm matches patient pairs with the smallest difference in the PS first. Standardized mean difference <0.10 was considered adequate. In the matched cohort, we summarized the rates of primary and secondary outcomes and estimated HRs and 95% confidence intervals (CI) using Cox proportional hazards model. Rate differences (RD) were also estimated. We tested whether there was a heterogeneity in HR and RD estimates across frailty categories.(33) Sensitivity analyses were performed to assess the robustness of the findings under different assumptions: 1) standard dose only excluding the renal dose of DOACs; 2) 3-day grace period and 3-day exposure risk window; 3) intention-to-treat analysis not censoring individuals at the time of the index drug discontinuation or switching and following them for 365 days; and 4) trial populations. We also examined how our results would change under different scenarios of unmeasured confounding and calculated the minimum strength of the confounder-exposure and confounder-outcome associations on the risk ratio scale to fully explain non-null associations (or E-values).(34)
Analyses were conducted in the Aetion Evidence Generation Platform (including R version 3.4.2), which has been validated by accurately repeating a range of published studies(35) and by replicating(36) or predicting clinical trial findings.(37)
Role of the Funding Source
This study was funded by National Institute on Aging. The funder had no role in the design, collection, analysis, or interpretation of the data, or the decision to submit the manuscript for publication.
RESULTS
Characteristics of Study Populations
We created 3 new-user cohorts of dabigatran (n=81,863) vs warfarin (n=256,722); rivaroxaban (n=185,011) vs warfarin (n=228,028); apixaban (n=222,478) vs warfarin (n=206,031). Compared with warfarin initiators, dabigatran initiators (Supplement 2, Table 2) and rivaroxaban initiators (Supplement 2, Table 3) were younger (mean age, 76.3 vs 77.4 years in both cohorts) and healthier, as evidenced by lower mean CFI (0.19 vs 0.20 in both cohorts), lower mean CHA2DS2-VASc score (4.0 vs 4.3 in both cohorts), lower mean comorbidity score (dabigatran: 2.2 vs 2.6; rivaroxaban: 2.3 vs 2.7), and lower prevalence of comorbidities (e.g., heart failure, anemia, and acute and chronic kidney disease). Apixaban initiators (Supplement 2, Table 4) had similar age (77.3 vs 77.5 years), CFI (0.20 vs 0.20), mean CHA2DS2-VASc score (4.2 vs 4.2), mean comorbidity score (2.6 vs 2.7) to warfarin initiators. After PS matching, all characteristics were adequately balanced between DOAC and warfarin groups in the entire cohort (Table 1) and by frailty subgroups (Supplement 2, Tables 5-7). Frail patients were older and more likely to be female; had greater comorbidity score, CHA2DS2-VASc score, and HAS-BLED score; consumed more medications; and had higher health care utilization.
Table 1.
Propensity Score-Matched Clinical Care Population with Atrial Fibrillation Treated with Direct Oral Anticoagulants vs Warfarin*
| Characteristics | Dabigatran Cohort | Rivaroxaban Cohort | Apixaban Cohort | |||
|---|---|---|---|---|---|---|
| Warfarin (n=79,365) |
Dabigatran (n=79,365) |
Warfarin (n=137,972) |
Rivaroxaban (n=137,972) |
Warfarin (n=109,369) |
Apixaban (n=109,369) |
|
| Age, years | 76.4 (7.1) | 76.4 (7.1) | 76.8 (7.3) | 76.8 (7.2) | 77.3 (7.4) | 77.3 (7.4) |
| Female | 49.9 | 49.9 | 49.9 | 49.9 | 50.6 | 50.4 |
| White race | 91.7 | 91.7 | 91.2 | 91.2 | 91.5 | 91.5 |
| Combined comorbidity index | 2.2 (2.1) | 2.2 (2.2) | 2.4 (2.3) | 2.4 (2.3) | 2.7 (2.5) | 2.7 (2.5) |
| CFI | 0.19 (0.06) | 0.19 (0.07) | 0.20 (0.07) | 0.20 (0.07) | 0.20 (0.07) | 0.20 (0.07) |
| CHA2DS2-VASc score | 4.1 (1.6) | 4.1 (1.6) | 4.1 (1.6) | 4.1 (1.6) | 4.2 (1.6) | 4.2 (1.6) |
| HAS-BLED score | 2.0 (0.7) | 2.0 (0.7) | 2.1 (0.7) | 2.1 (0.7) | 2.1 (0.7) | 2.1 (0.7) |
| Medical history | ||||||
| Acute kidney disease | 5.5 | 5.5 | 8.3 | 8.2 | 10.8 | 10.8 |
| Acute myocardial infarction | 3.3 | 3.2 | 4.4 | 4.3 | 5.4 | 5.4 |
| Anemia | 18.2 | 18.3 | 19.6 | 19.6 | 21.4 | 21.5 |
| Cancer | 13.4 | 13.5 | 13.8 | 13.9 | 14.0 | 13.8 |
| Cardioversion | 3.8 | 3.9 | 2.9 | 2.9 | 3.0 | 3.1 |
| Chronic kidney disease | 9.7 | 9.8 | 11.9 | 11.9 | 16.4 | 16.5 |
| Chronic lung disease | 19.6 | 19.6 | 21.3 | 21.2 | 22.1 | 22.1 |
| Dementia | 5.1 | 5.1 | 5.6 | 5.6 | 6.1 | 6.0 |
| Diabetes | 33.0 | 32.9 | 33.7 | 33.7 | 34.7 | 34.8 |
| Falls | 0.6 | 0.6 | 0.4 | 0.4 | 0.4 | 0.4 |
| Fractures | 0.7 | 0.7 | 1.3 | 1.3 | 1.1 | 1.1 |
| GI bleeding (inpatient) | 1.5 | 1.5 | 2.0 | 2.0 | 2.6 | 2.5 |
| Heart failure (inpatient) | 12.2 | 12.2 | 14.0 | 14.0 | 15.8 | 15.9 |
| Hypertension | 62.3 | 62.7 | 66.5 | 66.5 | 69.5 | 69.6 |
| Ischemic heart disease | 37.7 | 37.7 | 37.7 | 37.6 | 39.0 | 39.0 |
| Stroke (inpatient) | 4.6 | 4.6 | 4.8 | 4.7 | 5.6 | 5.5 |
| Transient ischemic attack | 5.1 | 5.1 | 4.4 | 4.5 | 4.6 | 4.6 |
| Medications | ||||||
| ACE inhibitors | 25.3 | 25.3 | 26.0 | 26.1 | 26.7 | 26.6 |
| Angiotensin receptor blockers | 7.1 | 7.1 | 5.4 | 5.3 | 5.0 | 4.9 |
| Antiarrhythmic agents | 20.4 | 20.5 | 17.3 | 17.3 | 17.0 | 17.0 |
| Antiplatelet agents | 14.6 | 14.8 | 14.0 | 13.9 | 14.5 | 14.4 |
| Benzodiazepines | 7.2 | 7.2 | 13.0 | 13.0 | 14.6 | 14.6 |
| Beta-blockers | 62.0 | 62.2 | 63.8 | 63.9 | 65.6 | 65.6 |
| Calcium channel blockers | 2.3 | 2.3 | 2.1 | 2.1 | 2.0 | 2.1 |
| Dementia drugs | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 |
| Diuretics | 49.1 | 49.0 | 50.8 | 50.7 | 52.6 | 52.6 |
| H2-blockers | 5.8 | 5.8 | 6.1 | 6.1 | 6.4 | 6.4 |
| Hypnotics | 8.6 | 8.7 | 7.2 | 7.1 | 6.5 | 6.4 |
| Insulin | 5.8 | 5.8 | 6.6 | 6.7 | 7.5 | 7.5 |
| Metformin | 15.3 | 15.0 | 15.4 | 15.5 | 15.5 | 15.6 |
| NSAIDs | 13.6 | 13.6 | 12.5 | 12.5 | 11.5 | 11.5 |
| Opioids | 29.0 | 29.1 | 30.3 | 30.1 | 30.0 | 30.2 |
| Proton pump inhibitors | 26.9 | 26.9 | 28.3 | 28.1 | 29.1 | 29.1 |
| SSRIs/SNRIs | 16.9 | 16.8 | 17.6 | 17.5 | 18.1 | 18.1 |
| Statins | 57.8 | 57.9 | 58.3 | 58.3 | 59.7 | 59.8 |
| Health care utilization in 183 days | ||||||
| Emergency department visits | 0.36 (0.78) | 0.35 (0.76) | 0.40 (0.82) | 0.40 (0.79) | 0.42 (0.83) | 0.42 (0.78) |
| Home health services | 0.11 (0.46) | 0.11 (0.44) | 0.07 (0.36) | 0.07 (0.35) | 0.04 (0.28) | 0.05 (0.28) |
| Hospitalizations | 0.49 (0.77) | 0.50 (0.77) | 0.54 (0.81) | 0.54 (0.79) | 0.57 (0.84) | 0.57 (0.82) |
| Skilled nursing facility stays | 0.08 (0.52) | 0.08 (0.50) | 0.06 (0.43) | 0.06 (0.44) | 0.03 (0.30) | 0.03 (0.31) |
| Geographic region | ||||||
| Northeast | 19.9 | 19.8 | 19.3 | 19.3 | 19.8 | 19.8 |
| Midwest | 22.6 | 22.5 | 25.9 | 25.9 | 26.9 | 27.0 |
| South | 40.1 | 40.3 | 37.2 | 37.1 | 35.5 | 35.7 |
| West | 17.3 | 17.3 | 17.5 | 17.6 | 17.7 | 17.4 |
| Other | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
Abbreviations: ACE, angiotensin converting enzyme; CFI, claims-based frailty index; GI, gastrointestinal; NSAID, non-steroidal anti-inflammatory drug; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Data are presented in proportions or mean (standard deviation). Standardized mean difference <0.1 for all variables (see Supplementary Table 2 for the entire list of variables).
Among the 3 PS-matched cohorts (Table 1), the apixaban cohort had older mean age (dabigatran, 76.4 years; rivaroxaban, 76.8 years; apixaban, 77.3 years), greater mean CFI (dabigatran, 0.19; rivaroxaban, 0.20; apixaban, 0.20), greater mean CHA2DS2-VASc score (dabigatran, 4.1; rivaroxaban, 4.1; apixaban, 4.2), greater mean HAS-BLED score (dabigatran, 2.0; rivaroxaban, 2.1; apixaban, 2.1), mean comorbidity score (dabigatran, 2.2; rivaroxaban, 2.4; apixaban, 2.7), and higher prevalence of comorbidities (e.g., acute myocardial infarction, anemia, acute and chronic kidney disease, gastrointestinal bleeding, and heart failure) and medication use (e.g., benzodiazepines). The apixaban cohort showed highest use of emergency department visits and hospitalizations and lowest use of home health and skilled nursing facility services.
Clinical Outcomes Associated with Dabigatran vs Warfarin by Frailty Levels
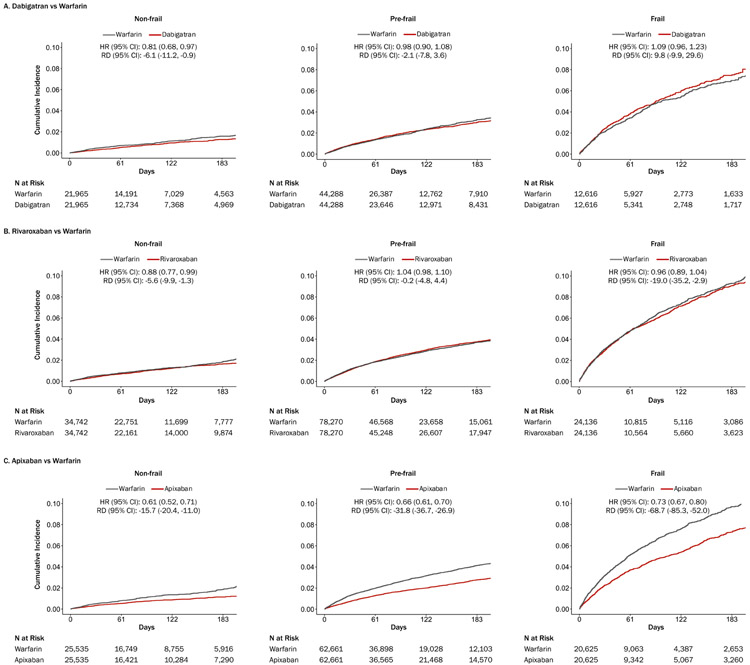
Over a median follow-up (interquartile range) of 72 (33, 143) days, the rate of the composite endpoint of death, ischemic stroke, or major bleeding (per 1,000 person-years) was 63.5 for dabigatran initiators and 65.6 for warfarin initiators (HR [95% CI], 0.98 [0.92, 1.05]; RD [95% CI] per 1,000 person-years, −2.2 [−6.5, −2.1]) (Table 2 and Supplement 2, Table 8). There was evidence for heterogeneity by frailty on the HR scale (Pheterogeneity=0.027), not on the RD scale (Pheterogeneity=0.231). Dabigatran was associated with a lower rate of the composite endpoint than warfarin for non-frail subgroup (HR, 0.81 [0.68, 0.97]), but not for pre-frail or frail subgroup (Table 2 and Figure 1A).
Table 2.
Frailty and the Association of Direct Oral Anticoagulants vs Warfarin with Clinical Outcomes in Propensity Score-Matched Clinical Care Population with Atrial Fibrillation*
| Outcomes | Dabigatran Cohort (Rate per 1,000 PYs) | Rivaroxaban Cohort (Rate per 1,000 PYs) | Apixaban Cohort (Rate per 1,000 PYs) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Warfarin (n=79,365) |
Dabigatran (n=79,365) |
HR (95% CI) |
RD (95% CI) |
Warfarin (n=137,972) |
Rivaroxaban (n=137,972) |
HR (95% CI) |
RD (95% CI) |
Warfarin (n=109,369) |
Apixaban (n=109,369) |
HR (95% CI) |
RD (95% CI) |
|
| Composite event | ||||||||||||
| Total | 65.6 | 63.5 | 0.98 (0.92, 1.05) |
−2.2 (−6.5, 2.1) |
83.7 | 77.8 | 0.98 (0.94, 1.02) |
−5.9 (−9.4, −2.4) |
92.3 | 60.1 | 0.68 (0.65, 0.72) |
−32.2 (−36.1, −28.3) |
| Non-frail | 31.4 | 25.4 | 0.81 (0.68, 0.97) |
−6.1 (−11.2, −0.9) |
37.2 | 31.6 | 0.88 (0.77, 0.99) |
−5.6 (−9.9, −1.3) |
37.6 | 21.9 | 0.61 (0.52, 0.71) |
−15.7 (−20.4, −11.0) |
| Pre-frail | 64.1 | 62.1 | 0.98 (0.90, 1.08) |
−2.1 (−7.8, 3.6) |
76.7 | 76.5 | 1.04 (0.98, 1.10) |
−0.2 (−4.8, 4.4) |
86.0 | 54.2 | 0.66 (0.61, 0.70) |
−31.8 (−36.7, −26.9) |
| Frail | 160.2 | 170.0 | 1.09 (0.96, 1.23) |
9.8 (−9.9, 29.6) |
219.8 | 200.8 | 0.96 (0.89, 1.04) |
−19.0 (−35.2, −2.9) |
226.2 | 157.6 | 0.73 (0.67, 0.80) |
−68.7 (−85.3, −52.0) |
| P heterogeneity | 0.027 | 0.231 | 0.026 | 0.040 | 0.080 | <0.001 | ||||||
| Death | ||||||||||||
| Total | 30.8 | 31.2 | 1.03 (0.93, 1.13) |
0.4 (−2.5, 3.4) |
42.7 | 37.0 | 0.91 (0.86, 0.97) |
−5.7 (−8.2, −3.2) |
48.2 | 37.9 | 0.82 (0.77, 0.88) |
−10.3 (−13.2, −7.3) |
| Non-frail | 12.5 | 11.1 | 0.89 (0.67, 1.18) |
−1.3 (−4.6, 2.0) |
16.4 | 11.7 | 0.72 (0.60, 0.88) |
−4.8 (−7.5, −2.0) |
16.5 | 11.8 | 0.74 (0.58, 0.92) |
−4.7 (−7.9, −1.5) |
| Pre-frail | 28.5 | 28.1 | 1.00 (0.87, 1.14) |
−0.4 (−4.2, 3.4) |
37.4 | 32.6 | 0.90 (0.83, 0.98) |
−4.8 (−7.9, −1.7) |
43.0 | 30.9 | 0.74 (0.68, 0.82) |
−12.2 (−15.7, −8.6) |
| Frail | 84.3 | 98.7 | 1.19 (1.01, 1.40) |
14.5 (−0.2, 29.1) |
129.7 | 116.5 | 0.94 (0.85, 1.04) |
−13.2 (−25.5, −0.9) |
131.8 | 110.5 | 0.88 (0.79, 0.98) |
−21.4 (−34.5, −8.2) |
| P heterogeneity | 0.127 | 0.118 | 0.066 | 0.414 | 0.058 | 0.001 | ||||||
| Ischemic stroke | ||||||||||||
| Total | 7.2 | 5.1 | 0.72 (0.58, 0.89) |
−2.1 (−3.5, −0.8) |
8.2 | 5.6 | 0.71 (0.61, 0.83) |
−2.6 (−3.6, −1.5) |
10.1 | 6.1 | 0.63 (0.54, 0.74) |
−4.0 (−5.3, −2.7) |
| Non-frail | 3.9 | 3.0 | 0.78 (0.46, 1.31) |
−0.9 (−2.7, 0.9) |
4.6 | 3.8 | 0.87 (0.61, 1.24) |
−0.8 (−2.3, 0.7) |
5.0 | 4.2 | 0.89 (0.60, 1.32) |
−0.8 (−2.6, 1.1) |
| Pre-frail | 6.7 | 5.7 | 0.86 (0.65, 1.15) |
−1.1 (−2.8, 0.7) |
8.6 | 6.5 | 0.78 (0.65, 0.95) |
−2.1 (−3.5, −0.6) |
10.4 | 6.9 | 0.68 (0.56, 0.83) |
−3.6 (−5.3, −1.9) |
| Frail | 8.4 | 8.1 | 0.99 (0.58, 1.69) |
−0.2 (−4.6, 4.2) |
14.0 | 9.4 | 0.68 (0.49, 0.95) |
−4.6 (−8.4, −0.8) |
17.4 | 11.2 | 0.67 (0.49, 0.92) |
−6.2 (−10.8, −1.7) |
| P heterogeneity | 0.819 | 0.945 | 0.607 | 0.149 | 0.457 | 0.024 | ||||||
| Major bleeding | ||||||||||||
| Total | 30.7 | 29.0 | 0.97 (0.88, 1.07) |
−1.7 (−4.6, 1.3) |
36.1 | 37.6 | 1.09 (1.03, 1.17) |
1.4 (−1.0, 3.8) |
37.4 | 18.1 | 0.51 (0.46, 0.55) |
−19.3 (−21.7, −17.0) |
| Non-frail | 16.7 | 12.3 | 0.74 (0.58, 0.96) |
−4.4 (−8.1, −0.7) |
18.8 | 17.4 | 0.95 (0.80, 1.13) |
−1.5 (−4.6, 1.7) |
18.9 | 6.8 | 0.37 (0.29, 0.49) |
−12.1 (−15.2, −9.0) |
| Pre-frail | 31.5 | 29.9 | 0.97 (0.85, 1.10) |
−1.6 (−5.6, 2.4) |
33.9 | 39.5 | 1.22 (1.12, 1.33) |
5.5 (2.4, 8.7) |
35.6 | 18.2 | 0.53 (0.47, 0.60) |
−17.4 (−20.5, −14.4) |
| Frail | 71.9 | 67.7 | 0.98 (0.81, 1.17) |
−4.2 (−17.0, 8.6) |
82.2 | 80.3 | 1.03 (0.91, 1.16) |
−1.9 (−11.8, 8.2) |
83.4 | 40.7 | 0.51 (0.44, 0.60) |
−42.7 (−52.2, −33.2) |
| P heterogeneity | 0.158 | 0.591 | 0.010 | 0.006 | 0.071 | <0.001 | ||||||
| Major GI bleeding | ||||||||||||
| Total | 15.7 | 22.0 | 1.44 (1.27, 1.63) |
6.3 (4.0, 8.7) |
19.7 | 26.0 | 1.40 (1.29, 1.52) |
6.2 (4.4, 8.1) |
20.6 | 10.1 | 0.52 (0.46, 0.58) |
−10.6 (−12.3, −8.8) |
| Non-frail | 7.5 | 9.2 | 1.23 (0.88, 1.72) |
1.7 (−1.1, 4.5) |
8.9 | 12.0 | 1.42 (1.13, 1.79) |
3.1 (0.8, 5.5) |
9.1 | 3.5 | 0.40 (0.28, 0.58) |
−5.6 (−7.8, −3.4) |
| Pre-frail | 17.1 | 23.3 | 1.39 (1.18, 1.64) |
6.2 (3.0, 9.4) |
18.6 | 27.4 | 1.56 (1.39, 1.74) |
8.8 (6.3, 11.3) |
19.9 | 10.3 | 0.55 (0.47, 0.64) |
−9.6 (−11.9, −7.3) |
| Frail | 46.3 | 50.1 | 1.13 (0.90, 1.41) |
3.8 (−6.8, 14.5) |
48.3 | 55.8 | 1.22 (1.05, 1.43) |
7.6 (−0.4, 15.6) |
49.7 | 25.3 | 0.54 (0.44, 0.66) |
−24.4 (−31.8, −17.0) |
| P heterogeneity | 0.329 | 0.116 | 0.044 | 0.005 | 0.291 | <0.001 | ||||||
| Intracranial bleeding | ||||||||||||
| Total | 8.4 | 3.5 | 0.42 (0.33, 0.53) |
−4.9 (−6.2, −3.6) |
9.2 | 5.6 | 0.62 (0.53, 0.72) |
−3.6 (−4.7, −2.5) |
10.0 | 5.0 | 0.51 (0.43, 0.60) |
−5.1 (−6.3, −3.8) |
| Non-frail | 5.9 | 1.8 | 0.31 (0.17, 0.55) |
−4.1 (−6.0, −2.2) |
6.6 | 3.6 | 0.54 (0.38, 0.75) |
−3.0 (−4.7, −1.4) |
6.5 | 2.5 | 0.39 (0.25, 0.60) |
−4.1 (−5.9, −2.2) |
| Pre-frail | 8.0 | 3.6 | 0.45 (0.32, 0.61) |
−4.4 (−6.2, −2.7) |
8.5 | 5.6 | 0.66 (0.54, 0.81) |
−2.9 (−4.3, −1.5) |
9.2 | 5.3 | 0.58 (0.47, 0.73) |
−3.9 (−5.5, −2.4) |
| Frail | 10.5 | 6.9 | 0.68 (0.40, 1.15) |
−3.6 (−8.1, 0.9) |
17.8 | 10.1 | 0.58 (0.43, 0.79) |
−7.7 (−11.9, −3.5) |
18.0 | 8.3 | 0.47 (0.33, 0.66) |
−9.7 (−14.1, −5.4) |
| P heterogeneity | 0.138 | 0.936 | 0.550 | 0.100 | 0.234 | 0.046 | ||||||
Abbreviations: CI, confidence interval; GI, gastrointestinal; HR, hazard ratio; PY, person-years; RD, rate difference.
Individuals were classified as non-frail (claims-based frailty index <0.15), pre-frail (0.15-0.24), and frail (≥0.25). The composite endpoint (primary endpoint) was defined as any of death, ischemic stroke, or major bleeding (which includes major GI bleeding and intracranial bleeding).
Figure 1. Frailty and Cumulative Incidence Plots of a Composite Endpoint of Death, Ischemic Stroke, or Major Bleeding in Older Adults with Atrial Fibrillation Newly Treated with Direct Oral Anticoagulants vs Warfarin.
Abbreviations: CI, confidence interval; HR, hazard ratio; RD, rate difference. In the propensity score-matched cohort of 158,730 Medicare beneficiaries newly treated with dabigatran vs warfarin (panel A), dabigatran was associated with a modest reduction in the composite endpoint of death, ischemic stroke, or major bleeding only in non-frail subgroup. In the propensity score-matched cohort of 275,944 Medicare beneficiaries newly treated with rivaroxaban vs warfarin (panel B), rivaroxaban was associated with a modest reduction in the composite endpoint in non-frail subgroup. In the propensity score-matched cohort of 218,738 Medicare beneficiaries newly treated with apixaban vs warfarin (panel C), apixaban was associated with a reduction in the composite endpoint in non-frail, pre-frail, and frail subgroups. Frailty status was defined using a validated claims-based frailty index: non-frail if <0.15, pre-frail if 0.15-0.24, and frail if ≥0.25.
For secondary endpoints, dabigatran initiators showed lower rates of ischemic stroke (HR [95% CI], 0.72 [0.58, 0.89]; RD per 1,000 person-years [95% CI], −2.1 [−3.5, −0.8]) and intracranial bleeding (HR, 0.42 [0.33, 0.53]; RD, 4.9 [−6.2, −3.6]); similar rates of death (HR, 1.03 [0.93, 1.13]; RD, −0.4 [−2.5, 3.4]) and major bleeding (HR, 0.97 [0.88, 1.07]; RD, −1.7 [−4.6, 1.3]); and a higher rate of major gastrointestinal bleeding (HR, 1.44 [1.27, 1.63]; RD, 6.3 [4.0, 8.7]) (Table 2 and Supplement 2, Table 8). There was no statistically significant evidence for heterogeneity by frailty on either HR or RD scale for these secondary endpoints (Table 2).
Clinical Outcomes Associated with Rivaroxaban vs Warfarin by Frailty Levels
Over a median follow-up (IQR) of 82 (33, 156) days, the rate of the composite endpoint (per 1,000 person-years) was 77.8 for rivaroxaban initiators and 83.7 for warfarin initiators (HR [95% CI], 0.98 [0.94, 1.02]; RD [95% CI] per 1,000 person-years, −5.9 [−9.4, −2.4]) (Table 2 and Supplement 2, Table 9). On the HR scale, rivaroxaban was associated with a lower rate of the composite endpoint than warfarin for non-frail subgroup (HR, 0.88 [0.77, 0.99]) but not for pre-frail or frail subgroup (Pheterogeneity=0.026) (Table 2 and Figure 1B). On the RD scale, rivaroxaban was associated with fewer composite events among non-frail (RD, −5.6 [−9.9, −1.3]) and frail (RD, −19.0 [−35.2, −2.9]) subgroups (Pheterogeneity=0.040).
For secondary endpoints, rivaroxaban initiators had lower rates of death (HR [95% CI], 0.91 [0.86, 0.97]; RD [95% CI], −5.7 [−8.2, −3.2]), ischemic stroke (HR, 0.71 [0.61, 0.83]; RD, −2.6 [−3.6, −1.5]), and intracranial bleeding (HR, 0.62 [0.53, 0.72]; RD, −3.6 [−4.7, −2.5]); and higher rates of major bleeding (HR, 1.09 [1.03-1.17]; RD, 1.4 [−1.0, 3.8]) and major gastrointestinal bleeding (HR, 1.40 [1.29, 1.52]; RD, 6.2 [4.4, 8.1]) (Table 2 and Supplement 2, Table 9). There was statistically significant evidence for heterogeneity by frailty for major bleeding and major gastrointestinal bleeding on both the HR and RD scales (Table 2). The positive association between rivaroxaban and bleeding events seemed stronger for pre-frail subgroup. No statistically significant heterogeneity was found for death, ischemic stroke, and intracranial bleeding.
Clinical Outcomes Associated with Apixaban vs Warfarin by Frailty Levels
Over a median follow-up (interquartile range) of 84 (33, 157) days, the rate of the composite endpoint (per 1,000 person-years) was 60.1 for apixaban initiators and 92.3 for warfarin initiators (HR [95% CI], 0.68 [0.65, 0.72]; RD [95% CI] per 1,000 person-years, −32.2 [−36.1, −28.3]) (Table 2 and Supplement 2, Table 10). This beneficial association for apixaban seemed consistent across frailty subgroups on the HR scale (Pheterogeneity=0.080) but greater for frail subgroup on the RD scale (Pheterogeneity<0.001) (RD for non-frail, pre-frail, and frail groups: −15.7 [−20.4, −11.0], −31.8 [−36.7, −26.9], and −68.7 [−85.3, −52.0], respectively) (Table 2 and Figure 1C).
For secondary endpoints, apixaban initiators had lower rates of death (HR [95% CI], 0.82 [0.77, 0.88]; RD [95% CI], −10.3 [−13.2, −7.3]), ischemic stroke (HR, 0.63 [0.54, 0.74]; RD, −4.0 [−5.3, −2.7]), major bleeding (HR, 0.51 [0.46, 0.55]; RD, −19.3 [−21.7, −17.0]), major gastrointestinal bleeding (HR, 0.52 [0.46, 0.58]; RD, −10.6 [−12.3, −8.8]), and intracranial bleeding (HR, 0.51 [0.43, 0.60]); RD, −5.1 [−6.3, −3.8]) (Table 2 and Supplement 2, Table 10). While the associations were consistent across frailty subgroups on the HR scale, RD estimates were greater as frailty increased (Pheterogeneity<0.05 for all secondary endpoints) (Table 2).
Sensitivity Analyses
Sensitivity analyses showed that the HR estimates under different design and analytic scenarios were qualitatively consistent with the primary analysis results (Supplement 2, Tables 11-13 and Supplement 2, Figures 4-6). The HR estimates for the composite endpoints varied minimally under different scenarios. There were greater variations in the HR estimates for some individual endpoints. In general, compared with the primary analysis estimates, HR estimates from sensitivity analyses were similar or larger when we compared standard DOAC doses with warfarin; similar when we used a 3-day grace period and exposure risk window; and attenuated when we applied the intention-to-treat approach. Applying more restrictive exclusion criteria of a clinical trial tended to produce somewhat stronger associations. The associations of dabigatran vs warfarin and of rivaroxaban vs warfarin were sensitive to the presence of moderate or strong unmeasured confounding, whereas the association of apixaban vs warfarin was robust to most scenarios of unmeasured confounding (Supplement 2, Table 14). E-values for non-null associations were 1.77 for dabigatran vs warfarin in non-frail subgroup (HR, 0.81); 1.53 for rivaroxaban vs warfarin in non-frail subgroup (HR, 0.88); and 2.08-2.66 for apixaban vs warfarin (HR ranging from 2.08 to 0.66).
DISCUSSION
Real-world database studies of anticoagulation therapy in AF using Medicare(27, 28, 38-40) and commercial insurance claims(40-45) have shown results that are generally consistent with clinical trials.(1-3) However, lack of information on frailty in real-world databases and clinical trials does not allow evaluation of the benefits and harms of DOACs vs warfarin across frailty levels. While frailty has long been a key clinical concept that should be taken into consideration before initiating high-risk medical or surgical treatments, there is little empirical evidence from clinical trials to guide how the treatment should be modified based on frailty information.(13, 46) Our study exemplifies how CFI can be used to evaluate heterogeneity in the emulated treatment effectiveness by frailty in a Medicare claims-based comparative effectiveness and safety study.
We found that apixaban was associated with a 32% relative reduction in the hazard of the composite endpoint of death, ischemic stroke, or major bleeding compared with warfarin in the overall population and consistent 27-39% relative reductions across frailty subgroups. Although dabigatran and rivaroxaban were not associated with a lower hazard of the composite endpoint than warfarin in the overall population, we found a 12-19% relative reduction in the hazard for non-frail subgroup and little or no reductions for pre-frail or frail subgroups. The strength of association on the RD scale was proportional to the absolute event rates across frailty categories. In the dabigatran cohort, HRs of 0.81 (non-frail) and 1.09 (frail) corresponded to 6.1 fewer and 9.8 excess composite endpoints per 1,000 person-years, respectively. In the rivaroxaban cohort, HRs of 0.88 (non-frail) and 0.96 (frail) were comparable to 5.6 and 19.0 fewer composite endpoints, respectively. In the apixaban cohort, HRs of 0.61 (non-frail) and 0.73 (frail) were consistent with 15.7 and 68.7 fewer composite endpoints, respectively. This beneficial association for apixaban vs warfarin in the frail subgroup appeared to be mainly driven by a large reduction in major bleeding, which occurred 4 to 5 times more than ischemic stroke in our study. Because older adults with frailty are predisposed to decline in renal function, higher renal clearance of dabigatran (80-85%) and rivaroxaban (66%) vs apixaban (27%) can result in higher plasma drug concentration, thereby increasing bleeding risk.(47) Our findings are consistent with the results from a previous commercial claims database study of frail older adults with AF (major bleeding at 1 year of follow-up, dabigatran vs warfarin: HR [95% CI], 0.92 [0.62, 1.37]; rivaroxaban vs warfarin: HR 1.06 [0.81, 1.39]; apixaban vs warfarin: HR, 0.61 [0.39, 0.93]).(48) Our study provides more precise estimates by frailty using Medicare data.
While our study suggests that frailty may inform choice between a DOAC and warfarin for older adults with AF, there are important caveats in applying our results to clinical practice. We emulated 3 target trials, each comparing a DOAC with warfarin. Thus, the 3 populations had notable differences, and direct comparison across the trials among DOACs was not possible. The warfarin group in the apixaban cohort had the highest rate of the composite endpoint (92.3 per 1,000 person-years) and the warfarin group in the dabigatran cohort had the lowest rate (65.6 per 1,000 person-years). Because PS matching created a target population that resembles the DOAC initiators within each cohort, our emulated treatment effectiveness estimate represents the effectiveness of the DOAC vs warfarin among the patients treated with the DOAC (“treatment effect among the treated”).
We defined frailty using a validated CFI because a clinical frailty assessment was unavailable in the Medicare database. Since our CFI was developed using a deficit-accumulation frailty index as the reference standard,(16-18) the CFI value is interpretable as a frailty index value calculated from a clinical assessment (non-frail: <0.15, pre-frail: 0.15-0.24, mildly frail: 0.25-0.34, moderately frail: 0.35-0.44, and severely frail: ≥0.45).(21-23) However, due to lack of time and resources, frailty assessment is not routinely performed in clinical practice.(46) Busy clinicians can adopt the Clinical Frailty Scale,(49) a clinical judgment-based scale that can be administered within 3 minutes. A Clinical Frailty Scale category of 5 or higher corresponds to a frailty index ≥0.25. Alternatively, an online frailty index calculator is available (https://www.bidmc.org/research/research-by-department/medicine/gerontology/calculator) to estimate a frailty index based on a comprehensive geriatric assessment.
Strengths and Limitations
The main strengths of our study include novel application of a CFI to evaluate heterogeneity of treatment effectiveness by frailty in a claims-based pharmacoepidemiologic study; emulation of a clinical trial design(50) by applying inclusion and exclusion criteria of a trial, new-user, active comparator design, and PS matching to achieve balance in a large number of covariates; sensitivity analyses to test robustness of our findings to various study design assumptions; and generalizability of our results to older Medicare beneficiaries in routine practice.
Our study also has limitations. In a claims-based study, we were unable to measure clinical variables (e.g., International Normalized Ratio, creatinine, and aspirin use) that influence the effectiveness and safety of oral anticoagulants. Despite PS matching, unmeasured confounding remains possible. The different associations by CFI might reflect a different degree of unmeasured or residual confounding by frailty subgroups. Our sensitivity analysis suggests that our results from the dabigatran and rivaroxaban cohorts were sensitive to moderate unmeasured confounding, while the results from the apixaban cohort were robust to strong unmeasured confounding. Moreover, the short median duration of follow-up (dabigatran: 72 days; rivaroxaban: 82 days; apixaban: 84 days), measured from prescription fill records, did not allow assessment of long-term associations. Given these limitations, the quality of evidence from our observational study can be rated moderate for the apixaban results and low for the dabigatran and rivaroxaban results.
Conclusions
Our study provides evidence to guide choice of a DOAC vs warfarin for older adults with AF. Only apixaban was consistently associated with lower rates of the composite endpoint of death, ischemic stroke, and major bleeding than warfarin across all frailty levels. Our novel approach to evaluate heterogeneity of the emulated effectiveness of drug therapy using CFI can be extended to claims-based pharmacoepidemiologic studies to generate evidence that is unavailable from clinical trials.
Supplementary Material
ACKNOWLEDGMENTS
Funding Support: This study was funded by R01AG062713 from the National Institute on Aging (NIA) and the Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) to Dr. Kim from NIA, American Federation for Aging Research, John A. Hartford Foundation, and Atlantic Philanthropies.
Footnotes
- Dr. Pawar is the Director, Real World Investigator at Sanofi, U.S.
- Dr. Gagne is a principal scientist at Exponent. He was the principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Eli Lilly and Company, Indianapolis, Indiana and Novartis Pharmaceuticals Corporation, Basel, Switzerland, unrelated to the topic of this study. He was a consultant to Aetion, Inc., New York, New York, and Optum, Inc., Eden Prairie, Minnesota.
- Dr. Schneeweiss is a consultant to Aetion, Inc., New York, New York, a software manufacturer of which he also owns equity. He is an investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Boehringer Ingelheim, Ingelheim am Rhein, Germany, unrelated to the topic of this study.
- The other authors declare no competing interests.
- Study protocol: Unavailable
- Statistical code: Unavailable
- Dataset: Medicare data files can be accessed through ResDAC (www.resdac.org) under data use agreement.
REFERENCES
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 4.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104. 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 5.Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–93. 10.1093/eurheartj/ehy136 [DOI] [PubMed] [Google Scholar]
- 6.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019. 10.1016/j.jacc.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 7.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullinan S, O'Mahony D, O'Sullivan D, Byrne S. Use of a frailty index to identify potentially inappropriate prescribing and adverse drug reaction risks in older patients. Age Ageing. 2016;45(1):115–20. 10.1093/ageing/afv166 [DOI] [PubMed] [Google Scholar]
- 9.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 10.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33(12):1500–10. 10.1093/eurheartj/ehr488 [DOI] [PubMed] [Google Scholar]
- 11.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–100. 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 12.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57(2):173–80. 10.1016/j.jacc.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson C, Todd O, Clegg A, Gale CP, Hall M. Management of atrial fibrillation for older people with frailty: a systematic review and meta-analysis. Age Ageing. 2019;48(2):196–203. 10.1093/ageing/afy180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perera V, Bajorek BV, Matthews S, Hilmer SN. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing. 2009;38(2):156–62. 10.1093/ageing/afn293 [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre MC, St-Onge M, Glazer-Cavanagh M, Bell L, Kha Nguyen JN, Viet-Quoc Nguyen P, et al. The Effect of Bleeding Risk and Frailty Status on Anticoagulation Patterns in Octogenarians With Atrial Fibrillation: The FRAIL-AF Study. Can J Cardiol. 2016;32(2):169–76. 10.1016/j.cjca.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–7. 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Glynn RJ, Avorn J, Lipsitz LA, Rockwood K, Pawar A, et al. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2018. 10.1093/gerona/gly197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DH, Patorno E, Pawar A, Lee H, Schneeweiss S, Glynn RJ. Measuring Frailty in Administrative Claims Data: Comparative Performance of Four Claims-Based Frailty Measures in the U.S. Medicare Data. J Gerontol A Biol Sci Med Sci. 2020;75(6):1120–5. 10.1093/gerona/glz224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gautam N, Bessette L, Pawar A, Levin R, Kim DH. Updating International Classification of Diseases Ninth Revision to Tenth Revision of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci. 2020. 10.1093/gerona/glaa150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–59. 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–7. 62/7/722 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Shi SM, McCarthy EP, Mitchell S, Kim DH. Changes in Predictive Performance of a Frailty Index with Availability of Clinical Domains. J Am Geriatr Soc. 2020;68(8):1771–7. 10.1111/jgs.16436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi SM, McCarthy EP, Mitchell SL, Kim DH. Predicting Mortality and Adverse Outcomes: Comparing the Frailty Index to General Prognostic Indices. J Gen Intern Med. 2020. 10.1007/s11606-020-05700-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33(10):2465–70. [DOI] [PubMed] [Google Scholar]
- 25.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiol Drug Saf. 2008;17(1):20–6. 10.1002/pds.1518 [DOI] [PubMed] [Google Scholar]
- 26.Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560–6. 10.1002/pds.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham DJ, Reichman ME, Wernecke M, Hsueh YH, Izem R, Southworth MR, et al. Stroke, Bleeding, and Mortality Risks in Elderly Medicare Beneficiaries Treated With Dabigatran or Rivaroxaban for Nonvalvular Atrial Fibrillation. JAMA Intern Med. 2016;176(11):1662–71. 10.1001/jamainternmed.2016.5954 [DOI] [PubMed] [Google Scholar]
- 28.Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131(2):157–64. 10.1161/CIRCULATIONAHA.114.012061 [DOI] [PubMed] [Google Scholar]
- 29.PRADAXA (dabigatran etexilate mesylate) Capsules. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022512s028lbl.pdfAccessed February 7, 2019.
- 30.XARELTO (rivaroxaban) Tablets. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202439s021lbl.pdfAccessed February 7, 2019.
- 31.ELIQUIS (apxiaban) Tablets. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdfAccessed February 7, 2019.
- 32.Coumadin Tablets (Warfarin Sodium Tablets, USP) Crystalline. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/009218s108lbl.pdfAccessed February 7, 2019. [Google Scholar]
- 33.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2(2):121–45. 10.1177/096228029300200202 [DOI] [PubMed] [Google Scholar]
- 34.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–74. 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 35.Wang SV, Verpillat P, Rassen JA, Patrick A, Garry EM, Bartels DB. Transparency and Reproducibility of Observational Cohort Studies Using Large Healthcare Databases. Clin Pharmacol Ther. 2016;99(3):325–32. 10.1002/cpt.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fralick M, Kesselheim AS, Avorn J, Schneeweiss S. Use of Health Care Databases to Support Supplemental Indications of Approved Medications. JAMA Intern Med. 2018;178(1):55–63. 10.1001/jamainternmed.2017.3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SC, Solomon DH, Rogers JR, Gale S, Klearman M, Sarsour K, et al. Cardiovascular Safety of Tocilizumab Versus Tumor Necrosis Factor Inhibitors in Patients With Rheumatoid Arthritis: A Multi-Database Cohort Study. Arthritis Rheumatol. 2017;69(6):1154–64. 10.1002/art.40084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez I, Baik SH, Pinera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med. 2015;175(1):18–24. 10.1001/jamainternmed.2014.5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amin A, Keshishian A, Trocio J, Dina O, Le H, Rosenblatt L, et al. Risk of stroke/systemic embolism, major bleeding and associated costs in non-valvular atrial fibrillation patients who initiated apixaban, dabigatran or rivaroxaban compared with warfarin in the United States Medicare population. Curr Med Res Opin. 2017;33(9):1595–604. 10.1080/03007995.2017.1345729 [DOI] [PubMed] [Google Scholar]
- 40.Lip GYH, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K, et al. Effectiveness and Safety of Oral Anticoagulants Among Nonvalvular Atrial Fibrillation Patients. Stroke. 2018;49(12):2933–44. 10.1161/STROKEAHA.118.020232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lauffenburger JC, Farley JF, Gehi AK, Rhoney DH, Brookhart MA, Fang G. Effectiveness and safety of dabigatran and warfarin in real-world US patients with non-valvular atrial fibrillation: a retrospective cohort study. J Am Heart Assoc. 2015;4(4). 10.1161/JAHA.115.001798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abraham NS, Noseworthy PA, Yao X, Sangaralingham LR, Shah ND. Gastrointestinal Safety of Direct Oral Anticoagulants: A Large Population-Based Study. Gastroenterology. 2017;152(5):1014–22 e1. 10.1053/j.gastro.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 43.Norby FL, Alonso A. Comparative effectiveness of rivaroxaban in the treatment of nonvalvular atrial fibrillation. J Comp Eff Res. 2017;6(6):549–60. 10.2217/cer-2017-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Go AS, Singer DE, Toh S, Cheetham TC, Reichman ME, Graham DJ, et al. Outcomes of Dabigatran and Warfarin for Atrial Fibrillation in Contemporary Practice: A Retrospective Cohort Study. Ann Intern Med. 2017;167(12):845–54. 10.7326/M16-1157 [DOI] [PubMed] [Google Scholar]
- 45.Seeger JD, Bykov K, Bartels DB, Huybrechts K, Zint K, Schneeweiss S. Safety and effectiveness of dabigatran and warfarin in routine care of patients with atrial fibrillation. Thromb Haemost. 2015;114(6):1277–89. 10.1160/TH15-06-0497 [DOI] [PubMed] [Google Scholar]
- 46.Walston J, Bandeen-Roche K, Buta B, Bergman H, Gill TM, Morley JE, et al. Moving Frailty Toward Clinical Practice: NIA Intramural Frailty Science Symposium Summary. J Am Geriatr Soc. 2019;67(8):1559–64. 10.1111/jgs.15928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turagam MK, Velagapudi P, Flaker GC. Stroke prevention in the elderly atrial fibrillation patient with comorbid conditions: focus on non-vitamin K antagonist oral anticoagulants. Clin Interv Aging. 2015;10:1431–44. 10.2147/CIA.S80641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez BK, Sood NA, Bunz TJ, Coleman CI. Effectiveness and Safety of Apixaban, Dabigatran, and Rivaroxaban Versus Warfarin in Frail Patients With Nonvalvular Atrial Fibrillation. J Am Heart Assoc. 2018;7(8). 10.1161/JAHA.118.008643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franklin JM, Patorno E, Desai RJ, Glynn RJ, Martin D, Quinto K, et al. Emulating Randomized Clinical Trials With Nonrandomized Real-World Evidence Studies: First Results From the RCT DUPLICATE Initiative. Circulation. 2021;143(10):1002–13. 10.1161/CIRCULATIONAHA.120.051718 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.