Abstract
OBJECTIVE:
Inadequate medication adherence is a significant limitation for achieving optimal health outcomes across chronic health conditions. Mindfulness-based interventions (MBIs) have been increasingly applied to promote medical regimen adherence as MBIs have been shown to improve patient-level barriers to adherence (i.e., depressive symptoms, cognitive impairment, stress). The purpose of this review is to investigate the state of research regarding MBIs targeting medication adherence in chronic illnesses and to identify evidence gaps to inform future studies.
METHODS:
The search reviewed 5 databases (e.g., PubMed, PsycINFO, Embase, CINAHL, Proquest Thesis/Dissertations) to identify trials that quantitatively evaluated the effect of MBIs on medication adherence. Study abstracts and full texts were screened identifying eligible studies, and findings were summarized using a narrative synthesis.
RESULTS:
A total of 497 studies were reviewed; 41 were eligible for full text review and 9 were included in narrative synthesis: seven were RCTs and two were pre-post designs. Study quality varied, with five rated moderate or high risk for bias. Clinical populations tested included living with HIV (k=3), cardiovascular disease (k=3), psychological disorders (k=2), and men who underwent a radical prostatectomy (k=1). Four studies found significant improvements in medication adherence, however only two of these studies had low risk of bias.
CONCLUSIONS:
Research on MBI’s for medication adherence is developing, but the effectiveness of MBIs remains unclear due to the nascent stage of evidence and methodological limitations of existing studies. Researchers should prioritize rigorous experimental designs, theory-driven investigations of behavioral mechanisms, and use of objective measurements of adherence.
Keywords: Cardiovascular Disease, HIV, Mindfulness, Medication Adherence, Mental Health, Systematic Review
INTRODUCTION
Despite pharmacological effectiveness for management of many chronic conditions (e.g., diabetes, human immunodeficiency virus (HIV)), half of all medical patients worldwide experience inadequate adherence to their medications as prescribed, creating a significant limitation for achieving optimal health outcomes for chronic conditions.1–4 Suboptimal adherence to prescribed medications contributes to 10% of hospitalizations and costs the U.S healthcare system between $100 billion to $289 billion annually.5–7
Medication adherence is a complex health behavior that can be defined as “the extent to which patients take medications in the manner prescribed by a clinician”.8,9 Adherence is influenced by a variety of factors on multiple levels of the healthcare system, including patient-level barriers such as psychiatric symptoms (e.g., depression, anxiety), cognitive impairments, internalized illness-related stigma, and intolerance to medication side effects.10–14 In addition, adherence is influenced by provider-level factors (e.g., mistrust in the provider-patient relationship) and structural challenges (e.g., lack of insurance, transportation and high medication costs).15–20
There is currently no single “gold standard” intervention for improving medication adherence.21,22 Further, medication adherence interventions recognized as most effective are often not cost-effective due to their multilevel nature (e.g., simultaneously targeting patient, provider, and/or policy).3,23,24 However, even for these interventions aiming to address barriers at multiple levels, effects on clinical outcomes have been moderate, at best, and evidence for long-term effects is determined to be minimal.3,23–25 In response, the Centers for Disease Control and Prevention (CDC) has called for two types of interventions to support medication adherence in chronic disease management, including those that target patient-level barriers (i.e. knowledge, beliefs, motivations), as well as those that can sustain effects beyond the intervention.24
Mindfulness-based interventions (MBIs) are an increasingly popular interventions that may offer promising approaches to addressing medication nonadherence.26,27 Mindfulness can be defined as the self-regulation of attention on immediate experience/present moment distinguished by curiosity, openness, and acceptance.28,29 MBIs include a wide range of interventions that employ mindfulness as their treatment philosophy or a central component.30 Two of the most rigorously evaluated MBIs are Mindfulness-Based Stress Reduction (MBSR) and Mindfulness-Based Cognitive Therapy (MBCT).30 Other mindfulness-informed interventions include Acceptance and Commitment Therapy (ACT) and Dialectical Behavioral Therapy (DBT).30 Multiple MBIs have been developed targeting populations with specific clinical conditions (e.g., cardiovascular disease, diabetes),30 and accumulating evidence from recent meta-analyses demonstrates the effectiveness of MBIs in improving the well-being of patients with chronic illness.31,32 Further, meta-analytic evidence on decades of research consistently suggests MBIs are effective in reducing depression,33–35 anxiety35,36 and somatic distress (e.g., sleep),37,38 as well as in improving cognitive functioning39--- all of which are patient-level barriers associated with suboptimal medication adherence among those with a chronic illness. Given its promise, theoretical models and frameworks have been proposed regarding the potential mechanisms of mindfulness for promoting medication adherence, including behavioral (e.g., sleep quality), neurological (e.g., increased default mode network connectivity, improved neurophysiological correlates of working memory) and psychological pathways (e.g., emotional regulation, psychological flexibility).40–44
Despite growing research in this area, no systematic review has been conducted to evaluate the existing body of quantitative evaluations using MBIs to promote medication adherence. As such, this systematic review aims to identify MBIs targeting medication adherence for patients with chronic health conditions, appraise study methodological quality, and narratively synthesize empirical evidence to date. Given the emerging research in this area, a systematic review can provide an overview of the state of the science, explore strengths and weaknesses in the evidence base, and inform efforts for future mindfulness research to address behavioral health needs of patients with chronic illness.
METHODS
Protocol & Registration:
This systematic review was conducted by a team consisting of a primary reviewer (WN), evidence synthesis expert and secondary reviewer (SSun), and evidence synthesis experts (SS, DO) overseeing the design and operationalization of the search. Additionally, the team included content experts on mindfulness and medication adherences (EL, BG, IK) and reported using the PRISMA-SR reporting.45 The review was preregistered on PROSPERO (ID#: CRD42018093516) on 05/2018.46
Eligibility Criteria:
Table 1 outlines the PICOS criteria (i.e., participants, interventions, comparators, outcomes, and study design) of the review. To be included, study participants needed to be engaging in a standardized medication regimen for a chronic medical condition at the time of the study. No limitations to age, gender or sexual identity, race, nationality, ethnicity or clinical condition were applied.
Table 1.
PICOS Criteria for Study Inclusion
| Population | All participants currently engaged in a standardized medication regimen for chronic medical conditions. |
| Intervention | Mindfulness-based interventions included first-generation MBPs (i.e. Mindfulness-Based Stress Reduction, Mindfulness-Based Cognitive Therapy), mindfulness-informed therapies (e.g. Acceptance and Commitment Therapy, Dialectical Behavioral Therapy) and interventions customized from “first generation” programs including those developed for specific health conditions (e.g. hypertension, substance use). |
| Comparison | Anyone not currently engaged intervention undergoing testing including waitlist controls, active control, superiority, enhanced usual control, TAU and no intervention. |
| Outcome | Self-report medication adherence instruments, medication refill/dispense records, continuous and categorical pill counts, electronic monitored adherence, biological measures (e.g. drug level), direct observed therapy. |
| Study Design | Both single and dual arm study designs including non-randomized quasi-experimental, waitlist control, quasi-experimental, non-randomized designs and randomized control trials). |
We defined MBIs such that mindfulness is an essential component to the program’s rationale and protocol.30 These included “first generation” mindfulness-based programs defined as Mindfulness-Based Stress Reduction and Mindfulness-Based Cognitive Therapy; both are termed “first generation” because they are two of the most widely tested and established interventions.30 Our definition of mindfulness-based interventions also included interventions customized from “first generation” programs, particularly those developed for specific health conditions (e.g., hypertension, substance use). In addition, we included mindfulness-informed interventions, such as Acceptance and Commitment Therapy and Dialectical Behavioral Therapy; in mindfulness-informed interventions, mindfulness is a component rather than the central therapeutic tenet differentiating them from first generation programs.30
Medication adherence was the primary outcome of interest and thus authors of primary studies needed to provide indication of medication or pharmacology compliance measurement in the abstract to be considered for full text review. Criteria regarding indices of medication measurement was operationalized by the study team to include any abstract that described monitoring of medication (e.g., medication use, medication adherence, medication dose, dosage, as prescribed), names of drugs monitored (e.g., fluvoxamine, lisinopril), or medication classification (e.g., selective serotonin reuptake inhibitors, antihypertensives, antiretrovirals) for full text review. However, for inclusion adherence was required to be monitored consistent with provider recommendations and measured by at least one of the following methods: (1) self-report instruments, (2) medication refill/dispense records, (3) continuous or categorical pill counts, (4) electronic monitoring, (4) biological measures (e.g., drug levels in lab testing), and/or (5) provider observed outcome. We did not restrict this review to studies with control or comparison groups. Pre-post trials as well as parallel-group designs were included to consider emerging evidence.
Studies were excluded if they: (1) did not report whether medication was taken as prescribed, (2) medication adherence was not examined as an outcome variable, or (3) used brief induction interventions (e.g., brief laboratory-based experiment followed by immediate evaluation). Publications were limited to those available in English.
Literature Search Strategy:
Literature searches were conducted in EMBASE, PubMed, CINHAL, and PsycINFO. A search of theses and dissertations was conducted using the Proquest database. In accordance with PRESS guidelines, search terms were developed in consultation with a medical library information specialist with extensive experience in evidence synthesis methods.47 Example search terms include: mindful* OR mindfulness OR “acceptance and commitment therapy” OR “dialectical behavioral therapy” OR “meditation” AND “patient compliance” OR “medication adherence” OR adheren* OR complian* OR “compliance” OR “drug intake”; for a complete list of search terms and results please see Appendix A. Supplementary Material 1. The electronic search was conducted on (1/15/2020) by a trained research assistant with the assistance of an evidence synthesis expert (Appendix A. Supplementary Material 1).
Study Selection:
A pilot screening for quality assurance (k=50) was performed to establish consensus among the two reviewers. Abstracts were then double-screened, and disagreements were resolved through consensus. Questions of eligibility specific to medication adherence or MBI criteria were resolved with the guidance of study team content experts (IK, BG, EL). All screening was completed in the open-source, online software Abstrackr (http://abstrackr.cebm).
Data Extraction:
The following data elements were extracted from selected manuscripts: (1) information specific to publication (e.g., year of publication, authors, title, journal, funding source, conflicts of interest), (2) sample characteristics (e.g., demographics, clinical condition), (3) overall study design features (e.g., randomization schema, exposures, and comparators, enrolled participants and analyzed sample sizes), (4) specific characteristics of MBIs including number of class sessions, duration of sessions, class size, delivery modality, assignment of home practice, instructor training, and intervention fidelity,48,49 (5) relevant primary outcomes (e.g., treatment satisfaction, mental and physical health outcomes), and (6) medication adherence-specific information, including the measure tool used and validity of the measure.
Risk of Bias Assessment:
Risk of bias was assessed by 2 reviewers (WN, SSun) using the Cochrane Collaboration’s Risk of Bias (RoB) tool version 2.0.50 We reviewed five domains of interest: randomization bias (e.g., sequence generation, allocation concealment), deviations from intended interventions (e.g., use of intent-to treat analysis), missingness of outcome data (i.e. attrition bias), measurement of the outcome (i.e., detection bias, bias due to knowledge of interventions received), and bias due to the selective reporting of results.50,51 Single arm trials were not reviewed using a quality assessment tool. Disagreements were resolved through consensus.
RESULTS
Study Selection:
After removing duplicates, a total of 497 papers emerged from initial literature searches and abstract review, of which 41 articles were retrieved for full-text review. In addition, content experts provided two studies for full-text review. Thirty-three studies were excluded primarily due to adherence not being a study outcome, and five were excluded as no full text was available (see Figure 1). Full-text review of these papers yielded a total of 9 studies for narrative synthesis. Figure 1 provides a flowchart of the review process.
Figure 1.
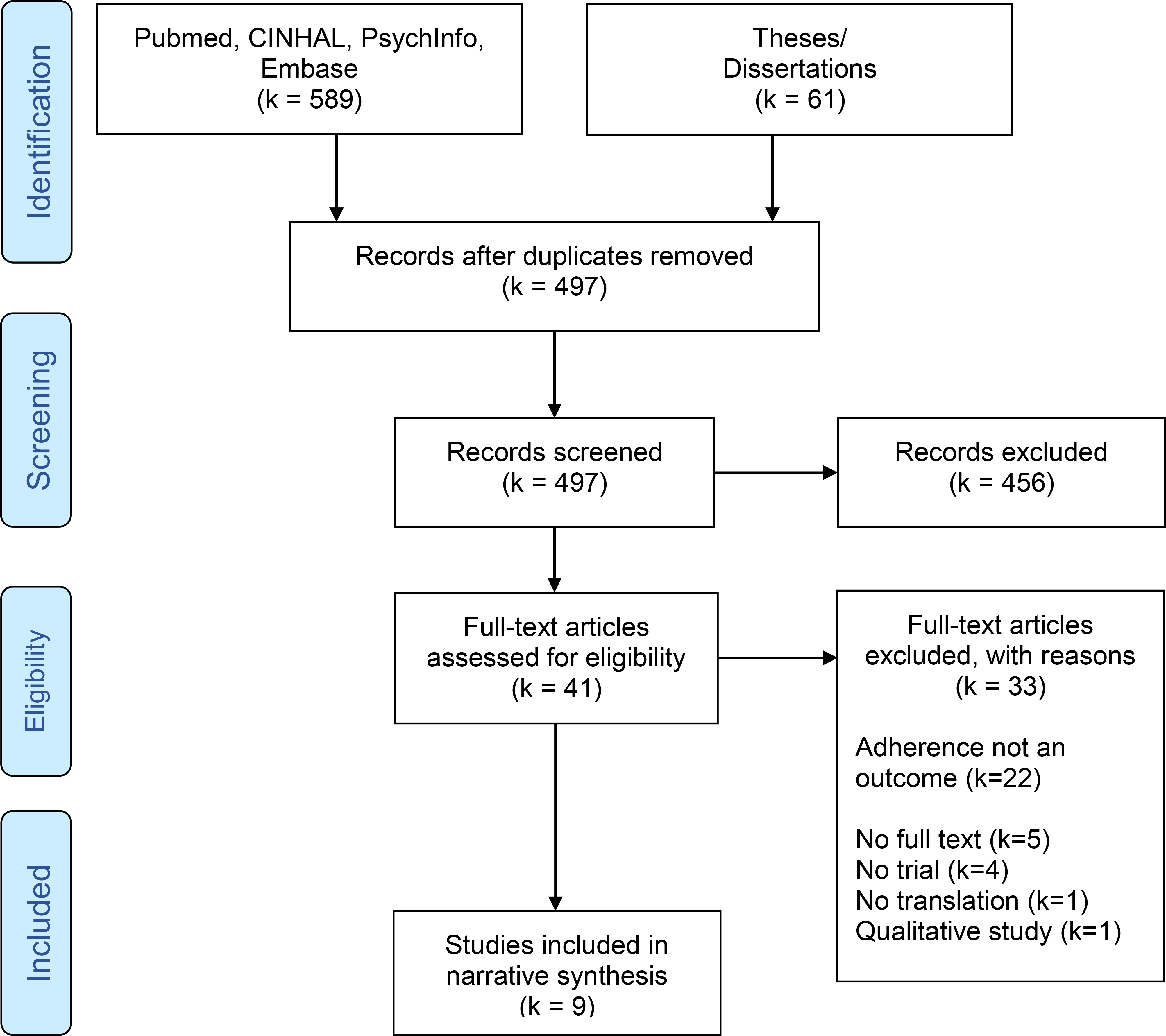
Flow Diagram of the Study Search and Selection Process
Studies are narratively summarized below (see Table 1.) and findings are reported by chronic illness conditions (i.e., people living with HIV, adults with elevated blood pressure) to provide researchers exploring the application of MBIs for chronic conditions an efficient presentation of findings, as well as to facilitate presentation of information for clinicians and patients reviewing the literature for specific clinical conditions. Given the heterogeneity of chronic health conditions as well as a wide range of control conditions (e.g., active, non-active, and single arm/without control condition), quantitative synthesis (i.e., effect size computation) was not conducted.
Study Characteristics
An overview of study designs, outcomes and narrative summaries of the main results can be found in Table 2. In terms of study location, most were conducted in the United States, (k = 6), followed by China (k = 1),52 Canada (k = 1),53 and Turkey (k = 1).54 Only one study was published before 2015 55 while five were published in 2019.
Table 2.
Narrative Synthesis of Nine Included Mindfulness-Based Interventions with Results.
| Author/Year | Participants/Avg. Age | Study Design/Follow-up | Study Condition/ (n) | Adherence Measure(s) | Additional Variables* | Summary of Reported Results |
|---|---|---|---|---|---|---|
|
| ||||||
| Carey, M. 2019 | Adult PLW HIV M age: 47.5 50% female |
RCT 10-week 3-month |
Intervention: MBSR (n=20) Control: Health coaching (n=22) |
Biomarker: Viral Load Self-report: Custom measure** Direct measure: Pill counts |
Sexual Risk Behavior Depression Distress Mindfulness Impulsivity |
No significant difference in pill count or self-report adherence. Viral suppression (>500 copies/mL) increased for MBSR and Control at 10 weeks trending toward significance but not sustained at 3 months. Reductions in depression, anxiety, distress, & impulsivity did not differ significantly between groups at follow-up. |
| Cetin, N. 2018 | Adults w/ Schizophrenia M age:21–60 31% female |
RCT 10-week |
Intervention: MBSR (n=55) Control: not listed (n=80) |
Self-report: Medication Adherence Rating Scale | Cognitive Insight | Adherence scores in the MBSR v Control were statistically significant at 10 weeks (p <.05). Cognitive insight total score and self-expression subscale were significantly improved compared to control (p<.05). |
| Duncan, L. 2012 | Adult PLW HIV M age: 48 16% female |
RCT 3-month 6-month |
Intervention: MBSR (n=40) Control: WLC (n=36) |
Self-report: 3-day AIDS Clinical Trials Group Self-report: Visual Analogue Scale-30 day |
ART-Side Effects Quality of Life Cognitive Function Aggression Mindfulness Self-regulation |
No significant differences in adherence. MBSR vs Control reported significant reductions in ART symptoms at 3 months (p=.04) & 6 month (p=.025), however there were no significant improvements in any other outcomes compared with control. |
| Eunjoo, A. 2019 | Adults w/ elevated BP (> 120/80) M age: 60 75% female |
RCT 13-week |
Intervention: MAP (n=20) Control: Health education (n=16) |
Self-report: Brief medication questionnaire | Systolic BP Direct Diastolic BP Direct Diet/Nutrition Physical Activity |
No significant increase in adherence. Significant reductions in diastolic (p=.005) & systolic (p=.003) BP were observed for MAP vs Control at 13-weeks. |
| Khoury, B. 2015 | Adults w/ early psychosis M age:29 33% female |
1-arm 10-week 3-month |
Intervention: CAM (n=12) | Self-report: Medication Adherence Rating Scale | Anxiety Cognitive Insight Emotional Regulation Interpersonal Behavior Mindfulness Psychiatric Symptoms Distress |
No significant difference in adherence. For the total sample, post treatment effects on adherence outcomes were not significant at 10 weeks. The study found that anxiety, self-neglect and somatic concerns significantly decreased at 3-month follow-up (p< .05). |
| Liang, H. 2019 | Patients w/ acute myocardial infarction after PCI M age: 55 32.5% female |
RCT 10-week |
Intervention: MBSR (n=58) Control: Nursing education (n=58) |
Self-report: Medication compliance scale adapted** | Anxiety Depression Life Satisfaction Sleep Quality Nursing Satisfaction |
Medication adherence, drug abuse, and unauthorized drug withdrawal significantly improved in MBSR vs Control (p<.001). Anxiety, depression significantly reduced at 10 week (p<.05) / 3 month vs Control and Quality of sleep and quality of life significantly improved vs Control (p<.05). |
| Loucks. E.B. 2020 | Adults w/ elevated BP (> 120/80) M age: 60 61.2% female |
1-arm 3-month 6-months 12-months |
Intervention: MB-BP (n=48) | Direct measure: Electronic Monitoring Caps (eCaps) | Systolic BP Direct Diastolic BP Direct Medication Usage Diet/Nutrition Body Mass Index Physical Activity Mindfulness Emotional Regulation Distress |
Among participants (n=16) who elected to use the eCap monitoring medication adherence significantly decreased (p<0.001) from 91% to 87% at 3-month follow-up returning to baseline levels at 6 & 12 months. Changes at 3, 6, & 12 months were observed in dietary behavior (p<.0001) and at 6 & 12 months in emotional regulation (p<.05). The number of participants decreasing medication use did not significantly differ from those who increased use at 3, 6, & 12 months. |
| Nelson, C. 2019 | Men in penile rehabilitation post prostatectomy M age: 60 0% female |
RCT 4-month 8-month |
Intervention: ACT (n=26) Control: Injection training (n=27) |
Direct Measure: # of reported syringes used at follow-up | Depression Treatment Regret Sexual Bother Sexual Self-Esteem Sexual Confidence Treatment Satisfaction |
ACT significantly more adherent to injection use at 4 month (p=.02). Injection use significantly increased at 4 (p=.0001) & 8 months (p=.003) vs Control. ACT vs Control showed significantly lower prostate cancer treatment regret at 4-months (p=.02). |
| Webb, L. 2018 | Adolescent PLW HIV M age: 18 45.8% female |
RCT 3-month |
Intervention: MBSR (n=38) Control: Health education (n=34) |
Biomarker: Viral Load, CD4 count Self-report: 6-item self-report measure adapted** |
Cognitive Function Expressive Attention Mindfulness Aggression Distress Quality of Life Self-Regulation |
At 3-month follow-up VL decreased significantly for participants in the intervention group with high VL at baseline (p=.04) and did not increase significantly among patients with a low VL at baseline. MBSR participants had significantly higher mindfulness (p=.03), problem solving to coping (p=.03), life satisfaction (p=.05) and lower aggression (p=.002) vs Control at 3-months. |
RCT= Randomized Controlled Trial; PLW HIV: People living with HIV; MBSR= Mindfulness-Based Stress Reduction; WLC=Waitlist Control; MAP=Mindfulness Awareness Program; BP: Blood pressure; CAM=Compassion, Acceptance, and Mindfulness intervention; MB-BP: Mindfulness-Based Blood Pressure Study; PCI: percutaneous coronary intervention; ACT=Acceptance and Commitment Therapy; CD4: cluster of differentiation 4.
For a listing of measures used to assess all variables beyond medication adherence please see Appendix 1.
Investigators customized measures for medication adherence from previous measures. Measures where based off validated instruments (e.g. Medication Adherence Rating Scale and Morisky Medication Adherence Scale).
Eight of the nine included studies (k = 8) were published in peer-reviewed journals and one study was a dissertation.56 With respect to study design, seven were RCTs, and two studies were single-arm trials.57,58 Among the seven RCTs, six used active control groups and one compared the intervention to a waitlist control.55 Sample sizes ranged from 12 to 135 participants (see Table 2). Seven of nine studies were in-person programs, one study used a customized phone delivery,59 and one used both in-person and phone delivery.60 Two studies were individual therapies, specifically Acceptance and Commitment Therapy and Mindfulness-Based Stress Reduction (MBSR) customized for 1:1 phone delivery.59,60 All others (k = 7) were group-based mindfulness interventions with the majority (k=4) testing MBSR, followed by customized mindfulness interventions (k=2), and one study of “Compassion, Acceptance and Mindfulness” (CAM) training. Intervention length was 7 weeks on average (range = [1, 13] weeks), with most falling between 4–8 weeks (k = 4).60 Session length/contact time with the provider was on average 83 minutes, (range = [5, 150] minutes) (see Table 3).
Table 3.
Summary of Nine Mindfulness-Based Interventions by Treatment Fidelity Monitoring Domains
| Study/Year | MBP | Session Length (min) | Class frequency | Modality/Format | Facilitator Description | Class Attendance Reported | Home Practice Assigned | Mindfulness Measures Assessed |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| |
Design |
Training |
Receipt |
Enactment |
||||
| Carey, M. P. 2019 | MBSR | 30 | 1 per week 8 weeks | phone/individual | Certified MBSR teacher | Y | Y | FFMQ (15-item) |
| Cetin, N. 2018 | MBSR | 60 | 2 per week 4 weeks | in-person/group | Clinician/ “attended MBSR” | N | Y | None |
| Duncan, L. 2012 | MBSR | 150 | 1 per week 9 weeks | in-person/group | Certified MBSR teacher | Y | Y | FFMQ (15-item) |
| Eunjoo, A. 2019 | MAP | 120 | 1 per week 6 weeks | in-person/group | “Certified Mindfulness Teacher” | N | Y | None |
| Khoury, B. 2015 | CAM | 75 | 1 per week 8 weeks | in-person/group | Clinician | Y | N | FMI-short (Freiburg Mindfulness Inventory) |
| Liang, H. 2019 | MBSR | 60 | 7 classes in 1 week | in-person/group | Nurse trained in MBSR | N | N | None |
| Loucks, E.B. 2020 | MBBP | 150 | 1 per week 9 weeks | in-person/group | Certified MBSR teacher | Y | Y | FFMQ (15-item) |
| Nelson, C. 2019 | ACT | 5–45** | 1 session 2 weeks for 13 weeks | phone/1:1 delivery | Psychologist/Clinician | N/A* | N/A* | None |
| Webb, L. 2018 | MBSR | 120 | 1 per week 9 weeks | in-person/group | MBSR trained/ not certified | Y | N | MAAS |
RCT= Randomized Controlled Trial; MBSR= Mindfulness-Based Stress Reduction; WLC=Waitlist Control; MAP=Mindfulness Awareness Program; CAM=Compassion, Acceptance, and Mindfulness intervention; MBBP= Mindfulness-Based Blood Pressure Reduction Study; ACT=Acceptance and Commitment Therapy; FFMQ=Five Facet Mindfulness Questionnaire; FMI= Freiburg Mindfulness Inventory; MAAS=Mindful Attention and Awareness Scale.
Only mindfulness informed program included in the analysis session length can vary for ACT. N/A was selected as homework and attendance reporting can vary for mindfulness informed programs.
Medication adherence was a primary outcome for three of the studies,54–56 and a secondary outcome for the remaining studies. To measure adherence, five studies (see Table 2) used self-report adherence measures only (e.g., Medication Adherence Rating Scale), two used objective measures only (e.g., syringe counts, pill counts),58,60 one used a combination of biomarker (e.g., viral load) and self-report adherence,61 and one used a multi-model approach (i.e., combination of three measures: pill counts, viral load as biomarker, and self-report).62 Patient-level psychological factors (i.e., barriers and facilitators) linked to adherence were measured and included depression (k=3),52,60,62 anxiety (k=2),52,62 cognitive function/insight (k=4),53–55,61 psychological distress (k=4),53,58,61,62 and quality of life (k=2).55,61 In addition, one study investigated the effect of MBIs on the number of medication-related side effects that occurred and the negative experiences associated with those side effects.63 Two studies investigated treatment satisfaction as secondary outcomes.52,60 For those studies where medication adherence was the primary outcome, patient-level psychological factors were examined as secondary outcomes. Four of the nine studies were proof of concept designs to test acceptability and feasibility of a mindfulness intervention.56,59,60,64
Risk of Bias Within Studies
A summary of the quality assessment of included studies is provided in Figure 2. Six included RCTs were assessed via standard Cochrane Collaboration’s Risk of Bias (RoB) tool version 2.0; one study employed a cluster randomization design and was assessed with Cochrane’s RoB 2.0 additional recommendations.52,55,56,59–61 Among these seven studies, two studies were determined low risk, 60,62 two had ‘some concerns’,55,56 and three were identified as high risk (see Figure 2).52,54,65 The main reason for high risk was due to bias specific to outcome measurement, as all three trials provided minimal descriptions of assessment procedures (e.g., blinding) and relied on self-report measures for adherence outcomes. This lack of detail in combination with participant knowledge of intervention allocation made it unclear whether assessment of the outcome may have been influenced by knowledge of the intervention received.52,54,65 For complete details of Risk of Bias domains and reviewer ratings please see Appendix B. Supplementary Material 2.
Fig. 2.
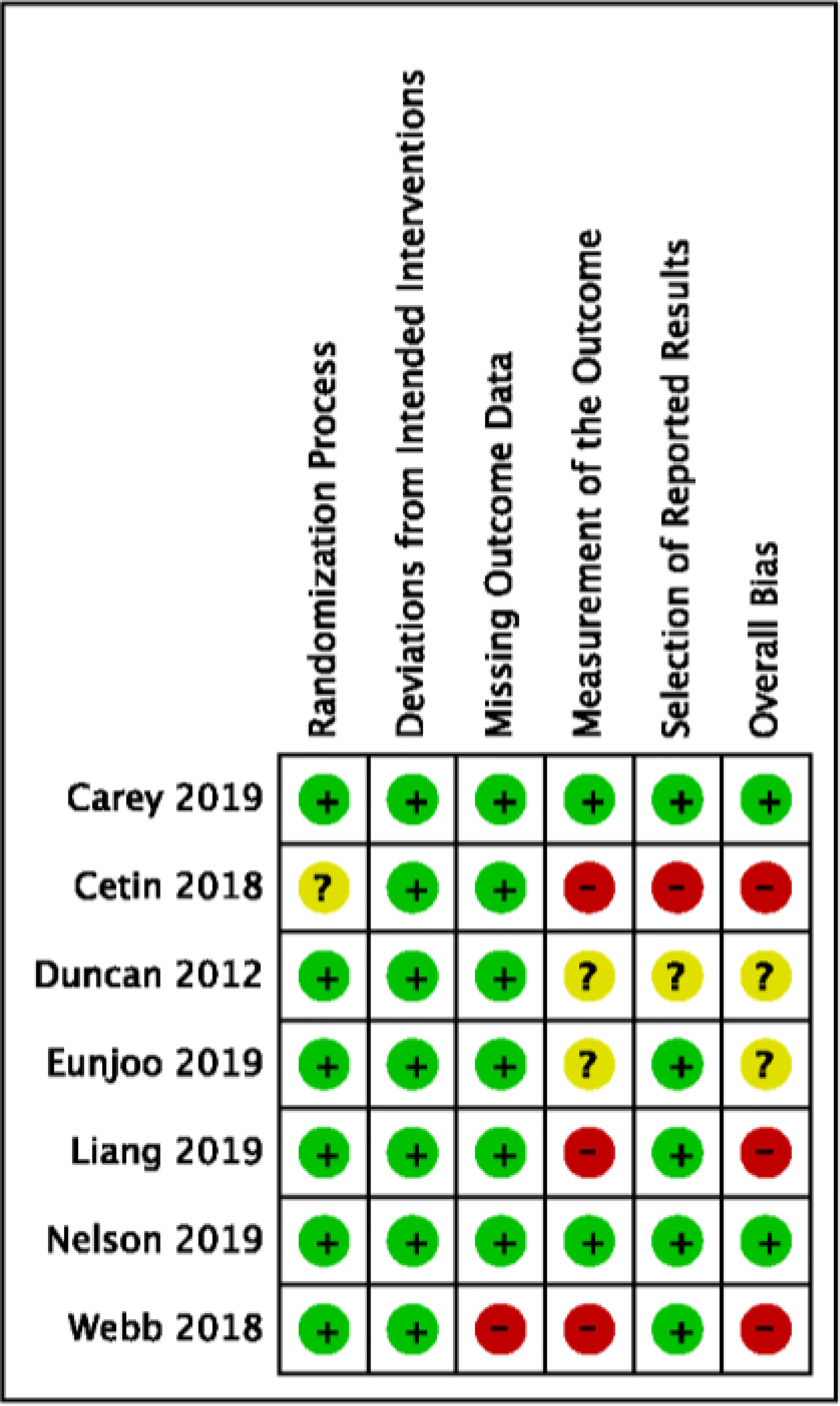
Risk of Bias Evaluation by Included Study
People living with HIV (k = 3)
Three interventions were conducted among people living with HIV: two with adults and one with youth. All were RCTs and comparison conditions varied with two using active controls59,61 and one waitlist control (see Table 2).55 Attrition was reported in all trials varying widely across trials: Carey et al. (N=44) reported 4% attrition in the MBSR group vs 6% in control at 3 months,62 Duncan et al. (N=76) reported 7% attrition in the MBSR group vs 8% in control at 6 months,55 and Webb et al. (N=72) reported 47% attrition in the MBSR group vs 41% in control at 3 months.61
Table 3 presents characteristics of the included MBIs. All interventions were MBSR, Webb et al. and Duncan et al. following the standard MBSR curriculum delivered in-person using a group format (i.e., 9-week 2.5 hours sessions) while Carey et al. customized MBSR for 1:1 phone delivery.55,59,61 All the studies were delivered by trained MBSR instructors and documented class attendance.55,59,61
Results of studies on medication adherence as well as a complete list of all measures utilized are narratively summarized in Table 2. Only Webb et al. found significant effects of MBSR when compared with control at follow-up as measured through viral load, considered a direct measure of medication adherence, among people living with HIV.61 Viral load was log scaled and dichotomized at 2.0 (i.e., 100 copies/mL) cutoff as high/low, results indicated significant changes from baseline to 3-month follow up in the MBSR group (p=.04) compared with non-significant findings in waitlist control (p= .21).61 Specifically, of the 18 intervention participants with high viral load at baseline, 8 (44%) exhibited low viral load at 3-month follow-up.61 However, these results should be reviewed with caution as dropout rates at 3-month follow-up were 45% for the overall sample. In addition, in Carey et al., patients in both MBSR and active control (i.e., time-matched health coaching) there was no group by time interaction observed for any of the medication adherence outcomes at follow-up (i.e., self-report, unannounced pill counts, viral load suppression).59
Regarding potential mediators associated with HIV medication non-adherence in people living with HIV, two studies indicated significant changes post program compared with controls (see Table 2.). Duncan et al. (2012) found significant reductions in average antiretroviral medication side effects in the intervention group compared with waitlist control at three (.33; 95% CI [0.01, 0.66], p= .044) and six months (.38; 95% CI [0.05, 0.71], p= .025).63 While in Webb et al., participants in the intervention group significantly improved in coping skills (0.49; 95% CI [ .05, .92]; p= .03) as well as life satisfaction (0.57; 95% CI [ .01, 1.13]; p = .05), and had significantly lower levels of aggression (−.89; 95% CI [−1.41, −.37]; p = .002) compared with the health education control at 3-months.61 In Carey et al., patients in both MBSR and health education control improved in adherence related factors (i.e., depression, anxiety), however no significant group by time interaction was observed.
Patients with severe mental illness (k = 2)
Two studies investigated MBIs for psychotic disorders: Cetin et al. an RCT testing MBSR for patients with schizophrenia66 (n=135) and Khoury et al., a single-arm, non-randomized trial testing a customized for patients with early psychosis (n=12) call an called “Compassion, Acceptance and Mindfulness training” (CAM). Both studies reported no attrition at follow up, potentially due to their inpatient setting.64
Cetin et al. modified MBSR to two classes per week over the course of 4 weeks, while the CAM intervention was 6 weeks with 2-hour sessions. 64,66 Both studies assigned home practice and neither reported class attendance (see Table 3.).64
Cetin et al. found significant effects (t(134)= −3.44, p< .05) on the average change in the Medication Adherence Rating Scale among MBSR participants (1.76 ± .42) compared with standard care control (1.50 ± .50). In addition, MBSR participants averaged significantly greater improvements in cognitive insight (t(134)=3.13, p< .05) post-program (4.89 ± 6.05) compared with standard care control (1.68 ± 5.67).66 Khoury et al. reported no effect on self-reported antipsychotic medication adherence however anxiety, a potential mediator on adherence, was significantly reduced at 3-month follow-up (d= .92, p< .05).64
Adults with elevated blood pressure (>120/80 mmHg) (k = 2)
Two studies investigated the effects of mindfulness interventions on blood pressure. One single-arm, non-randomized trial tested pre-post change of adherence within a customized MBI targeted for patients (n=48) with hypertension, called the Mindfulness-Based Blood Pressure Reduction program (MB-BP) while the second was a dissertation using an RCT design testing a customized MBI called the Mindfulness Awareness Program (MAP) against health education control for adults (n=36) with hypertension.56,58 MB-BP assessed participants at 3, 6, and 12 months with 10% attrition reported at 12-months.58 The MAP intervention assessed participants at 13-weeks post-program with a 22% attrition reported.56 Please see Table 2. for additional study design information.
MAP consisted of an 8-week intervention with 1-hour group sessions and the intervention was facilitated by a “certified mindfulness teacher” and the study did not document class attendance.56 MB-BP was taught by a certified MBSR instructor with expertise in cardiovascular disease prevention. The study reported treatment fidelity metrics including class attendance, home practice, intervention dose using a 10% quality audit, and adverse events (see Table 3. for additional MBI details).58
In the MAP intervention medication adherence was a secondary outcome, measured using self-report, and no significant effects were found. The study also did not investigate outcomes linked to non-adherence (e.g., psychological distress). The MB-BP study assessed medication adherence using eCap electronic monitoring systems (Information Mediary Corp. Ottawa, Canada). Adherence data were analyzed for a smaller sample of participants who were taking antihypertensive medication (n=16, 33% of the total study sample).58,67 The data were calculated at baseline as percent of days monitored with the correct number of pills taken during specific ranges of data collection (i.e. 4–6 weeks of data before or 4–12 weeks after) during each assessment period.58 If the patient was on multiple hypertensive medications they were asked to use the electronic monitoring on the medication they had been using the longest. Results indicated that adherence decreased from 95% at baseline to 87% (0.87, 95% CI [.82, .93], p=0.004) at follow-up, which then returned to baseline levels of adherence (i.e., 91%) at all subsequent follow-ups. Distress, a risk factor for non-adherence assessed using the Perceived Stress Scale, was significantly reduced compared with baseline levels at all subsequent follow-up (i.e., 3-month) and sustained through 12-month follow-up (20.7; 95% CI [18.6, 22.8], p= .012) please see Table 2. for further details.58
Patients with acute myocardial infarction after PCI (k = 1)
One RCT tested an MBSR group intervention customized for 7-day delivery versus health education among patients (n=116) with acute myocardial infarction after a percutaneous coronary intervention.52 The intervention was taught by a nurse practitioner trained in MBSR and attrition was 0% in both groups. The intervention was offered with 1-hour sessions every day. Assignment of home practice and class attendance were not reported (see Table 3.).
Medication adherence was assessed using a 4-item customized adherence measure with unclear validation (see Table 2.). MBSR participants reported significantly higher average scores regarding how often they took medication consistent with provider recommendations (3.44 ± .62) compared with health education (2.89 ± .59) at post-program follow-up (t(115)=4.894, p< .001).52 In addition, Liang et al. reported that MBSR significantly impacted theorized mediators for non-adherence compared with health education. Specifically, participants in the MBSR group had significantly reduced average anxiety scores (47.18 ± 7.37) compared with controls (50.41 ± 7.65) at follow up (t(115)=2.316, p= .02) and the MBSR group had significant reductions in average depressive symptomatology (45.53 ± 6.88) compared with the health education group (48.66 ± 6.74) at 10-weeks (t(115)=2.475, p= .01). The intervention group also reported improvements in average life satisfaction (MBSR= 27.38 ± 5.20, Health Education=23.52 ± 4.71) post intervention (t(115)=4.190, p<.001) as well as enhanced sleep quality (MBSR= 9.92 ± 1.78, Health Education=9.23 ± 1.94) compared with controls (t(115)=1.996, p= .048); all items were measured using self-report for complete list of measures please see Table 2.52
Patients with erectile dysfunction post radical prostatectomy (k = 1)
One RCT tested ACT versus time-matched educational programming among men who had undergone a radical prostatectomy (n=53) and were in treatment for erectile dysfunction using prescribed injectable medication defined as 2 weekly injections for 3 of 4 weeks a month (i.e., at least 24 injections in a 4-month study period) see Table 2.60 The intervention was 13-weeks and was facilitated by a trained psychologist consisting of seven therapeutic contacts: two in-person meetings and five phone calls (see Table 3). Attrition was 19% in ACT and 15% in control at 4-months, and 43% in ACT and 61% in control at 8-months.
Medication adherence was a secondary outcome assessed by tabulating the prescribed number of syringes minus the total number of unused syringes, a process described as comparable to standard pill counts (see Table 2.).60 Results indicated that in the ACT group was 4.4 times as likely to be adherent to injection use at 4-month follow-up with 44% of men meeting injection adherence (i.e., ≥ 2 time per week) compared with only 10% in the active control (Risk Ratio=4.4, p= .02). In addition, the ACT group reported lower treatment regret on average (5.2 ± 2.4) compared with enhanced monitoring controls (7.7 ± 3.7) at 4-months (d= .74, p=.02) both shown to influence medication adherence; however, these effects were not sustained at 8-month follow-up.
DISCUSSION
To our knowledge, this is the first systematic review assessing mindfulness interventions targeting medication adherence for chronic health conditions. Overall, findings indicated mixed results and documented a field that remains understudied with room for growth. Due to the preliminary nature of the research and the high levels of study heterogeneity, specifically across the clinical populations evaluated (e.g., patient characteristics), intervention design, control conditions, and adherence measures, we elected to utilize a narrative synthesis to summarize results rather than meta-analyses.68 Promising findings included four studies reporting significant effects,52,60,61,66 and five reporting significant effects on potential mediators of adherence including reducing treatment regret, decreasing medication side effects and bother related to side effects, as well as improving cognitive functioning.52,60,61,64,66 However, four of the nine studies were designed to test the acceptability and feasibility of MBIs and were not designed as efficacy trials to evaluate adherence as a primary outcome measure.56,58–60 In addition, three of nine studies found no intervention effects on medication adherence,55,56,64 and one study found patients’ adherence decreased post-intervention intervention but returned to non-significant baseline levels at 3, 6, and 12-month follow-ups.58 Based on our methodological review of the seven studies with a comparison group, only two studies were considered to have low risk for bias.59,60
As such, it remains difficult to ascertain the effect of mindfulness interventions on medication adherence. Although we found some documented evidence for improvements in adherence and adherence-related risk factors among included studies, continued research is needed to understand the impact of MBIs on patients taking their medications as prescribed. The paucity of research on MBIs effect on medication adherence is surprising not just because multiple theoretical frameworks have been proposed, but also due to the clinical reality that patients engaging in mindfulness training will likely be on a medication regimen as a part of a whole person approach, making the practical implications of concurrent treatment an important area for future research.40–43 In other words, will patients view mindfulness intervention as an alternative to pharmacological treatment, thereby lowering adherence, or will they consider mindfulness training an integrative component of a treatment regimen for improving or sustaining adherence?40–43 As MBIs become more common components of chronic disease treatment (i.e., as providers are increasingly recommending the use of MBIs as part of treatment regimens more broadly) and patients’ interest in mindfulness as a complementary therapy continues to grow, effective integration of MBIs and pharmacotherapies to provide a comprehensive, whole person approach to care remains an important area for research.31,32,69–71 This review reveals an emerging body of evidence and growing interest in understanding the impact MBIs have on medication adherence, but the effect MBIs may have remains unclear due to the nascent stage of the scientific literature and methodological limitations in the existing body of work.
As the field continues to investigate the potential relationship between MBIs and medication adherence, expanding the evidence base toward translation of research into practice will require more rigorous and comprehensive experimental testing.48,72 This should begin with the inclusion of more objective measures of medication adherence such as biomarkers, electronic monitoring systems, and pill counts, as over half of studies identified in this review relied on a variety of self-report metrics with unclear validation. In a recent meta-analysis examining the correlation of electronic monitoring adherence and self-report questionnaires, self-report adherence was found to be moderately correlated with electronic device measured adherence, however, self-reported adherence can be inflated, is subject to recall bias, and measures are singular to clinical conditions, thereby limiting the ability to compare across study populations.73
Moving forward, another area to improve experimental testing concerns the mechanistic targets of the MBIs. Although seven studies (78%) reported potential mechanisms (e.g., medication side-effects, stress, anxiety), many of the studies did not select secondary outcomes based on a specified theory driven approach. This is consistent with previous literature, as a recent review found that only 3% of studies funded by the National Institutes of Health (NIH) to improve medication adherence targeted theorized mechanisms of behavior change.21
Finally, this review found that there was a consistent lack of reporting on essential study procedures and this lack of reporting resulted in a higher risk of bias indicating a lower quality of evidence. For example, 56% of studies provided minimal details for assessment procedures, including blinding of participants or study staff. Also, despite the inclusion of specific pharmacotherapies as intervention targets, there comprehensive adverse effect monitoring was not sufficiently reported: only two studies reported adverse events and only one documented the process of monitoring.58,62
Building off findings from our review and the current state of the evidence, we offer three recommendations for research moving forward. First, due to the variety of measurement tools utilized across studies, experts investigating mindfulness and medication adherence may benefit from the creation of a Core Outcome Set of measures, which include minimum reporting guidelines for medication adherence to help direct the field moving forward. A consensus will enable development of suggested sets of measurement procedures that represent the minimum outcomes and processes that should be reported in a clinical trial when investigating mindfulness interventions for medication adherence.74 In this review, we found that medication usage (i.e., taking medication) and medication adherence (i.e., taking medication as prescribed) was conflated in excluded studies and that few included studies used a multi-modal approach for measuring adherence,75 instead favoring single, self-report measures of adherence at times customized by the investigators reducing measure validity. In addition, minimal descriptions of the adherence behavior being targeted was provided (i.e., implementation of a regimen versus initiation persistence) in studies.76 Minimal reporting on established definitions and suggested measures for adherence across trials is critical for evidence synthesis of research, as consistent reporting allows for comparable outcomes and reductions in outcome-reporting bias.77,78 In addition, since developing a Core Outcome Set involves multiple stakeholders (e.g., policymakers, clinicians), interventions will more effectively test outcomes deemed important through consensus.74,79
Second, we encourage applying the NIH Science of Behavior Change recommendations to investigate mechanisms consistent with existing theory.21 Consistent with the Science of Behavior Change network’s suggestion for health behavior research to develop “systematic, rigorous and reproducible method for identifying the mechanisms that drive successful behavior change,”21 researchers examining MBIs for medication adherence should examine mechanisms identified by proposed theoretical frameworks.40–43 Successful identification of mediators linking MBIs to adherence can clarify pathways of change, set the stage for more effective testing and replication of research over time, and help to produce more efficient and effective MBIs to benefit patients with suboptimal adherence helping them achieve related health benefits.
Third, existing tools for reporting mindfulness interventions should be utilized more widely in MBI research. Comprehensive and clear documentation of mindfulness interventions, including treatment fidelity measurement, adverse event monitoring, and instructor training improves the validity of the study, offers targeted improvements to study design, contributes to more effective and accurate comparisons, and provides clarity for replication of interventions.80,81 However, in this review as well as in the field, MBIs have lacked reporting on fidelity, adverse events, and instructor training.82–86 Currently tools for minimum reporting of mindfulness interventions have been created (e.g., Treatment Fidelity Tool for Mindfulness-based Interventions),49 and include guidance for reporting adverse events, treatment fidelity, documenting intent-to-treat analysis, and pre-registering study protocols.84,86
Limitations:
This review has several limitations. First, the lack of standardized nomenclature for medication usage versus adherence applied in mindfulness research was a complicating factor in searching and screening studies for review. With guidance from content experts, our study used an approach to identify relevant evidence on medication adherence in accordance with accepted practice in that field. As with all systematic reviews, we anticipate that some primary studies that captured relevant outcomes may have been missed during abstract screening. However, even for those studies that did capture potentially relevant outcomes, the differential usage of terminology and reporting of primary studies would have hindered aggregation and interpretation. Taken together, this limitation is an important finding in our study, that clear reporting guidelines are needed to improve future comparative effectiveness studies on mindfulness-based interventions and medication adherence. Secondly, the heterogeneity of intervention designs and clinical populations, as well as the lack of standardization in the evaluation and operationalization of medication adherence, prohibited quantitative synthesis. Last, as in all reviews, results from this synthesis might be limited by the search procedures and inability to identify/include all potentially relevant studies, including non-English language publications.
CONCLUSION:
This systematic review identified and appraised the existing body of research on the effects of mindfulness interventions on medication adherence. Overall, findings indicated mixed results and documented a field that remains understudied. As mindfulness interventions are implemented across clinical conditions, it remains important to consider their effects within the larger pantheon of clinical treatment options; both as singular treatments (e.g., Mindfulness-Based Cognitive Therapy for depression) and as adjunctive therapies (e.g., in combination with pharmacotherapy for cardiovascular disease).33,36,87 Importantly, more rigorous experimental testing is needed investigate the impact of mindfulness interventions on medication adherence as a primary outcome. Future research will offer important evidence for clinicians recommending meditation to patients and for insurance companies contemplating including mindfulness interventions as reimbursable programs. The recommendations in this review are meant to catalyze continued research and future efficacy trials investigating mindfulness interventions delivered adjunctively with medication treatment and to consider how mindfulness training can support the patient-provider partnership for the shared goal of promoting optimal health and well-being.
Supplementary Material
Studies included patients with HIV, cardiovascular disease, and schizophrenia.
Four studies found significant improvements in medication adherence at follow-up
Most studies were reliant on self-report measurement of medication adherence
Only 2 of the 9 included studies were deemed low risk of bias
Efficacy trials testing mindfulness interventions for medication adherence are needed
ACKNOWLEDGEMENTS:
This study would like to thank Dr. Elena Salmoriago-Blotcher for her contributions in the preliminary development of this work. In addition, we would like to acknowledge the important contributions of the Brown University Medical Librarian Erin Anthony as well as Gaelen Adam, Senior Research Associate at the Center for Evidence Synthesis and Health, both of whom contributed to the development and refinement of the search strategy. Work by Dr. Shufang Sun was also supported by the National Institute of Health (K23AT011173).
Footnotes
COMPETING INTERESTS STATEMENT:
The authors have no competing interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography:
- 1.van Heuckelum M, van den Ende CHM, Houterman AEJ, Heemskerk CPM, van Dulmen S, van den Bemt BJF. The effect of electronic monitoring feedback on medication adherence and clinical outcomes: A systematic review. PloS one. 2017;12(10):e0185453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008(2):Cd000011. [DOI] [PubMed] [Google Scholar]
- 3.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Annals of internal medicine. 2012;157(11):785–795. [DOI] [PubMed] [Google Scholar]
- 4.Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. The American journal of medicine. 2012;125(9):882–887.e881. [DOI] [PubMed] [Google Scholar]
- 5.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2003;60(7):657–665. [DOI] [PubMed] [Google Scholar]
- 6.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. [DOI] [PubMed] [Google Scholar]
- 7.Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ open. 2018;8(1):e016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kronish IM, Ye S. Adherence to cardiovascular medications: lessons learned and future directions. Progress in cardiovascular diseases. 2013;55(6):590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramer JA, Roy A, Burrell A, et al. Medication Compliance and Persistence: Terminology and Definitions. Value in Health. 2008;11(1):44–47. [DOI] [PubMed] [Google Scholar]
- 10.Enhancing medication adherence the public health dilemma. In: Bosworth HB, ed. London: :: SpringerHealthcare; 2012. [Google Scholar]
- 11.Grenard JL, Munjas BA, Adams JL, et al. Depression and Medication Adherence in the Treatment of Chronic Diseases in the United States: A Meta-Analysis. Journal of general internal medicine. 2011;26(10):1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell NL, Boustani MA, Skopelja EN, Gao S, Unverzagt FW, Murray MD. Medication adherence in older adults with cognitive impairment: a systematic evidence-based review. Am J Geriatr Pharmacother. 2012;10(3):165–177. [DOI] [PubMed] [Google Scholar]
- 13.Sweeney SM, Vanable PA. The Association of HIV-Related Stigma to HIV Medication Adherence: A Systematic Review and Synthesis of the Literature. AIDS Behav. 2016;20(1):29–50. [DOI] [PubMed] [Google Scholar]
- 14.Dibonaventura M, Gabriel S, Dupclay L, Gupta S, Kim E. A patient perspective of the impact of medication side effects on adherence: Results of a cross-sectional nationwide survey of patients with schizophrenia. BMC psychiatry. 2012;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surratt HL, Kurtz SP, Levi-Minzi MA, Chen M. Environmental Influences on HIV Medication Adherence: The Role of Neighborhood Disorder. American journal of public health. 2015;105(8):1660–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajesh R, Sudha V, Varma D, Sonika S. Association between Medication Adherence Outcomes and Adverse Drug Reactions to Highly Active Antiretroviral Therapy in Indian Human Immunodeficiency Virus-Positive Patients. J Young Pharm. 2012;4(4):250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abel WM, Efird JT. The Association between Trust in Health Care Providers and Medication Adherence among Black Women with Hypertension. Frontiers in public health. 2013;1:66–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang JG, Roh D, Kim C-H. Association between Therapeutic Alliance and Adherence in Outpatient Schizophrenia Patients. Clin Psychopharmacol Neurosci. 2019;17(2):273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Lazaro CI, García-González JM, Adams DP, et al. Adherence to treatment and related factors among patients with chronic conditions in primary care: a cross-sectional study. BMC Family Practice. 2019;20(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gast A, Mathes T. Medication adherence influencing factors-an (updated) overview of systematic reviews. Systematic reviews. 2019;8(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edmondson D, Falzon L, Sundquist KJ, et al. A systematic review of the inclusion of mechanisms of action in NIH-funded intervention trials to improve medication adherence. Behaviour research and therapy. 2018;101:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwadry-Sridhar FH, Manias E, Lal L, et al. Impact of interventions on medication adherence and blood pressure control in patients with essential hypertension: a systematic review by the ISPOR medication adherence and persistence special interest group. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2013;16(5):863–871. [DOI] [PubMed] [Google Scholar]
- 23.E. S Adherence to Long-Term Therapies: Evidence for Action. World Health Organization;2003. [Google Scholar]
- 24.Neiman AB, Ruppar T, Ho M, et al. CDC Grand Rounds: Improving Medication Adherence for Chronic Disease Management - Innovations and Opportunities. MMWR Morbidity and mortality weekly report. 2017;66(45):1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010(3):Cd005182. [DOI] [PubMed] [Google Scholar]
- 26.Baer RA. Mindfulness-based treatment approaches: clinicians guide to evidence base and applications. Massechusets: Academy; 2006. [Google Scholar]
- 27.Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How Does Mindfulness Meditation Work? Proposing Mechanisms of Action From a Conceptual and Neural Perspective. Perspectives on Psychological Science. 2011;6(6):537–559. [DOI] [PubMed] [Google Scholar]
- 28.Bishop SR, Lau M, Shapiro S, et al. Mindfulness: A proposed operational definition. Clin Psychol-Sci Pr. 2004;11(3):230–241. [Google Scholar]
- 29.Bishop SR, Lau M, Shapiro S, et al. Mindfulness: A Proposed Operational Definition. Clinical Psychology: Science & Practice. 2004;11(3):230–241. [Google Scholar]
- 30.Crane RS, Brewer J, Feldman C, et al. What defines mindfulness-based programs? The warp and the weft. Psychol Med. 2017;47(6):990–999. [DOI] [PubMed] [Google Scholar]
- 31.Scott-Sheldon LAJ, Gathright EC, Donahue ML, et al. Mindfulness-Based Interventions for Adults with Cardiovascular Disease: A Systematic Review and Meta-Analysis. Ann Behav Med. 2020;54(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott-Sheldon LAJ, Balletto BL, Donahue ML, et al. Mindfulness-Based Interventions for Adults Living with HIV/AIDS: A Systematic Review and Meta-analysis. AIDS Behav. 2019;23(1):60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being. AHRQ Publication No. 13(14)-EHC116-EF.January20142014. [PubMed] [Google Scholar]
- 34.Zhang Q, Zhao H, Zheng Y. Effectiveness of mindfulness-based stress reduction (MBSR) on symptom variables and health-related quality of life in breast cancer patients-a systematic review and meta-analysis. Support Care Cancer. 2018. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg SB, Tucker RP, Greene PA, et al. Mindfulness-based interventions for psychiatric disorders: A systematic review and meta-analysis. Clinical psychology review. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Vibe M, Bjørndal A, Fattah S, Dyrdal G, Halland E, Tanner-Smith E. Mindfulness-based stress reduction (MBSR) for improving health, quality of life and social functioning in adults: a systematic review and meta-analysis. Campbell Systematic Reviews. 2017;13. [Google Scholar]
- 37.Crowe M, Jordan J, Burrell B, Jones V, Gillon D, Harris S. Mindfulness-based stress reduction for long-term physical conditions: A systematic review. Aust N Z J Psychiatry. 2016;50(1):21–32. [DOI] [PubMed] [Google Scholar]
- 38.Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lao SA, Kissane D, Meadows G. Cognitive effects of MBSR/MBCT: A systematic review of neuropsychological outcomes. Consciousness and cognition. 2016;45:109–123. [DOI] [PubMed] [Google Scholar]
- 40.Loucks EB, Schuman-Olivier Z, Britton WB, et al. Mindfulness and Cardiovascular Disease Risk: State of the Evidence, Plausible Mechanisms, and Theoretical Framework. Current Cardiology Reports. 2015;17(12):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salmoirago-Blotcher E, Carey MP. Can Mindfulness Training Improve Medication Adherence? Integrative Review of the Current Evidence and Proposed Conceptual Model. Explore (NY). 2018;14(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moitra E, Gaudiano BA. A psychological flexibility model of medication adherence in psychotic-spectrum disorders. Journal of Contextual Behavioral Science. 2016;5(4):252–257. [Google Scholar]
- 43.Larouche E, Hudon C, Goulet S. Potential benefits of mindfulness-based interventions in mild cognitive impairment and Alzheimer’s disease: an interdisciplinary perspective. Behavioural brain research. 2015;276:199–212. [DOI] [PubMed] [Google Scholar]
- 44.Kerrigan D, Grieb SM, Ellen J, Sibinga E. Exploring the dynamics of ART adherence in the context of a mindfulness instruction intervention among youth living with HIV in Baltimore, Maryland. AIDS Care. 2018:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R. William EBL Nardi, Springs Stacey, Operario Don, Kronish Ian M., Guadiano Brandon A., Shufang Sun. Systematic review of mindfulness interventions and medication adherences. PROSPERO: International prospective register of systematic reviews. 2018. [Google Scholar]
- 47.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–46. [DOI] [PubMed] [Google Scholar]
- 48.Van Dam NT, van Vugt MK, Vago DR, et al. Mind the Hype: A Critical Evaluation and Prescriptive Agenda for Research on Mindfulness and Meditation. Perspectives on psychological science : a journal of the Association for Psychological Science. 2018;13(1):36–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kechter A, Amaro H, Black DS. Reporting of Treatment Fidelity in Mindfulness-Based Intervention Trials: A Review and New Tool using NIH Behavior Change Consortium Guidelines. Mindfulness (N Y). 2019;10(2):215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 51.Eldridge S, Campbell MK, Campbell MJ, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): additional considerations for cluster-randomized trials. 2016.
- 52.Liang H, Liu L, Hu H. The effects of mindfulness-based stress reduction on the mental states, sleep quality, and medication compliance of patients with acute myocardial infarction after percutaneous coronary intervention. International Journal of Clinical and Experimental Medicine. 2019;12(12):13514–13523. [Google Scholar]
- 53.Khoury B, Lecomte T, Comtois G, Nicole L. Third-wave strategies for emotion regulation in early psychosis: A pilot study. Early Intervention in Psychiatry. 2015;9(1):76–83. [DOI] [PubMed] [Google Scholar]
- 54.Çetin N, Aylaz R. The effect of mindfulness-based psychoeducation on insight and medication adherence of schizophrenia patients. Archives of psychiatric nursing. 2018;32(5):737–744. [DOI] [PubMed] [Google Scholar]
- 55.Duncan LG, Moskowitz JT, Neilands TB, Dilworth SE, Hecht FM, Johnson MO. Mindfulness-based stress reduction for HIV treatment side effects: a randomized, waitlist controlled trial. J Pain Symptom Manage. 2012;43(2):161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.An E Mindfulness and Lifestyle Education for Blood Pressure Reduction in Hypertension [Ph.D.]. Ann Arbor, University of California, Los Angeles; 2019. [Google Scholar]
- 57.Khoury B, Lecomte T, Comtois G, Nicole L. Third-wave strategies for emotion regulation in early psychosis: a pilot study. Early intervention in psychiatry. 2015;9(1):76–83. [DOI] [PubMed] [Google Scholar]
- 58.Loucks EB, Nardi WR, Gutman R, et al. Mindfulness-Based Blood Pressure Reduction (MB-BP): Stage 1 single-arm clinical trial. PloS one. 2019;14(11):e0223095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carey M, Dunne E, Norris A, et al. Telephone-Delivered Mindfulness Training to Promote Medication Adherence and Reduce Sexual Risk Behavior Among Persons Living with HIV: An Exploratory Clinical Trial. AIDS and behavior. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson CJ, Saracino RM, Napolitano S, Pessin H, Narus JB, Mulhall JP. Acceptance and Commitment Therapy to Increase Adherence to Penile Injection Therapy-Based Rehabilitation After Radical Prostatectomy: Pilot Randomized Controlled Trial. Journal of Sexual Medicine. 2019;16(9):1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Webb L, Perry-Parrish C, Ellen J, Sibinga E. Mindfulness instruction for HIV-infected youth: a randomized controlled trial. AIDS Care. 2018;30(6):688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carey MP, Dunne EM, Norris A, et al. Telephone-Delivered Mindfulness Training to Promote Medication Adherence and Reduce Sexual Risk Behavior Among Persons Living with HIV: An Exploratory Clinical Trial. AIDS Behav. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duncan LG, Moskowitz JT, Neilands TB, Dilworth SE, Hecht FM, Johnson MO. Mindfulness-based stress reduction for HIV treatment side effects: A randomized, waitlist controlled trial. Journal of Pain and Symptom Management. 2012;43(2):161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khoury B, Lecomte T, Comtois G, Nicole L. Third-wave strategies for emotion regulation in early psychosis: A pilot study. Early Intervention in Psychiatry. 2015;9(1):76–83. [DOI] [PubMed] [Google Scholar]
- 65.Webb L, Perry-Parrish C, Ellen J, Sibinga E. Mindfulness instruction for HIV-infected youth: a randomized controlled trial. AIDS Care. 2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cetin N, Aylaz R. The effect of mindfulness-based psychoeducation on insight and medication adherence of schizophrenia patients. Arch Psychiatr Nurs. 2018;32(5):737–744. [DOI] [PubMed] [Google Scholar]
- 67.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu R, Gartlehner G, Grant M, et al. AHRQ Methods for Effective Health Care Conducting Quantitative Synthesis When Comparing Medical Interventions: AHRQ and the Effective Health Care Program. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. [PubMed] [Google Scholar]
- 69.Misitzis A Increased Interest for Mindfulness Online. International journal of yoga. 2020;13(3):247–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schultchen D, Terhorst Y, Holderied T, et al. Stay Present with Your Phone: A Systematic Review and Standardized Rating of Mindfulness Apps in European App Stores. Int J Behav Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA internal medicine. 2014;174(3):357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salmoirago-Blotcher E, Carey MP. Can Mindfulness Training Improve Medication Adherence? Integrative Review of the Current Evidence and Proposed Conceptual Model. Explore: The Journal of Science and Healing. 2018;14(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi L, Liu J, Fonseca V, Walker P, Kalsekar A, Pawaskar M. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health and quality of life outcomes. 2010;8(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gargon E, Williamson PR, Altman DG, Blazeby JM, Tunis S, Clarke M. The COMET Initiative database: progress and activities update (2015). Trials. 2017;18(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simoni JM, Huh D, Wang Y, et al. The validity of self-reported medication adherence as an outcome in clinical trials of adherence-promotion interventions: Findings from the MACH14 study. AIDS and behavior. 2014;18(12):2285–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kronish IM, Thorpe CT, Voils CI. Measuring the multiple domains of medication nonadherence: findings from a Delphi survey of adherence experts. Transl Behav Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barbosa-Leiker C, Kostick M, Lei M, et al. Measurement invariance of the perceived stress scale and latent mean differences across gender and time. Stress and health : journal of the International Society for the Investigation of Stress. 2013;29(3):253–260. [DOI] [PubMed] [Google Scholar]
- 78.Williamson PR, Altman DG, Blazeby JM, et al. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gargon E, Williamson PR, Altman DG, Blazeby JM, Clarke M. The COMET Initiative database: progress and activities from 2011 to 2013. Trials. 2014;15:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23(5):443–451. [DOI] [PubMed] [Google Scholar]
- 81.Gould LF, Dariotis JK, Greenberg MT, Mendelson T. Assessing Fidelity of Implementation (FOI) for School-Based Mindfulness and Yoga Interventions: A Systematic Review. Mindfulness (N Y). 2016;7(1):5–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baer R Mindfulness Training as a Clinical Intervention: A Conceptual and Empirical Review. Clinical Psychology: Science and Practice. 2003;10:125–143. [Google Scholar]
- 83.Davidson RJ, Kaszniak AW. Conceptual and methodological issues in research on mindfulness and meditation. The American psychologist. 2015;70(7):581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coronado-Montoya S, Levis AW, Kwakkenbos L, Steele RJ, Turner EH, Thombs BD. Reporting of Positive Results in Randomized Controlled Trials of Mindfulness-Based Mental Health Interventions. PloS one. 2016;11(4):e0153220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuyken W, Warren FC, Taylor RS, et al. Efficacy of Mindfulness-Based Cognitive Therapy in Prevention of Depressive Relapse: An Individual Patient Data Meta-analysis From Randomized Trials. JAMA Psychiatry. 2016;73(6):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goldberg SB, Tucker RP, Greene PA, Simpson TL, Kearney DJ, Davidson RJ. Is mindfulness research methodology improving over time? A systematic review. PloS one. 2017;12(10):e0187298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Levine GN, Lange RA, Bairey-Merz CN, et al. Meditation and Cardiovascular Risk Reduction: A Scientific Statement From the American Heart Association. J Am Heart Assoc. 2017;6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


