Abstract
Background
Satisfaction measures such as Press Ganey (PG) scores are increasingly used to determine reimbursement.
Purpose
To investigate the relationship between PG satisfaction scores and perioperative opioid use in patients undergoing anterior cruciate ligament reconstruction (ACLR).
Methods
Patients undergoing ACLR were retrospectively identified. Perioperative opioid prescription data were collected using the electronic medical record.
Results
Positive correlations existed between immediate preoperative total morphine equivalents (TMEs) and PG scores. There was a negative correlation between “Pain Control” and preoperative TMEs.
Conclusion
PG scores were correlated with preoperative and intraoperative opioid administration but not postoperative opioid administration.
Keywords: Orthopaedics, ACL, Press Ganey, Satisfaction, Opioid, Pain Control
1. Introduction
As healthcare shifts to a patient experience-centered model, satisfaction measures such as Press Ganey (PG) scores are increasingly used to determine hospital and provider reimbursement.1,2 However, tying patient satisfaction to reimbursement may be problematic in the current climate of the opioid epidemic in the United States, as preoperative, intraoperative, and postoperative opioid consumption can influence patient satisfaction scores.3, 4, 5, 6,6, 6, 7, 8, 9, 10, 11 Orthopaedic surgeons have been cited as the third highest prescribers of narcotics, and one study reported over 70% of patients refilled opioid prescriptions in the first month after anterior cruciate ligament reconstruction (ACLR) alone.12, 13, 14 As ACLR numbers continue to increase,4,15 it is critical for orthopaedic surgeons to investigate the relationship between perioperative opioid prescription use and patient satisfaction.
Studies investigating PG scores and opioid use are currently limited to total joint arthroplasty patient populations.16, 17, 18 In total hip arthroplasty patients, only the “communication about medications” domain was significantly correlated with opioid consumption.17 No PG domains correlated with opioid consumption in patients undergoing total knee arthroplasty.18 In total shoulder arthroplasty patients, there was no difference in PG scores after an institutional change to decrease the amount of opioids prescribed at discharge.16 Using a generic measure of patient satisfaction, one study of adolescent patients undergoing ACLR found no correlation between patient satisfaction with pain control and postoperative opioid consumption.19 To our knowledge, however, no study has determined the relationship between PG satisfaction scores and perioperative opioid use in patients undergoing ACLR.
The purpose of this study was to determine the relationship between PG satisfaction scores and perioperative opioid use in patients undergoing ACLR. Specifically, this study aimed to determine the correlation between PG scores and opioid use preoperatively, intraoperatively, and postoperatively in the post-anesthesia care unit (PACU) and after discharge.
2. Methods
After obtaining Institutional Review Board approval, patients undergoing ACLR (Current Procedural Terminology code, 29888) by one of two Sports Medicine fellowship-trained orthopaedic surgeons were retrospectively reviewed at a single institution from June 2015 to May 2017. During the same timeframe, our institution's PG database was queried to determine if patients completed the Press Ganey Ambulatory Surgery (PGAS) survey postoperatively. Patients were included in the study if they: (1) underwent ACLR with or without concomitant procedures and up to one additional ligament reconstructed, (2) were greater than 12 years of age, (3) had no prior diagnosis of chronic pain, (4) were not incarcerated or a ward of the state at the time of surgery, (5) filled no greater than two preoperative opioid prescriptions within ninety days prior to surgery, (6) filled an opioid prescription at discharge, and (7) completed the PGAS survey postoperatively. An electronic medical record (EMR) review was performed to identify demographic characteristics, medical comorbidities, surgical history, and perioperative surgical center opioid and non-opioid pain medication administration. The drug name, dose, quantity dispensed, and time of administration (immediate preoperative, intraoperative, or PACU) were recorded.
A regional prescription drug monitoring database was used to identify all preoperative and postoperative outpatient opioid prescriptions filled within Maryland and the surrounding states. Opioid prescriptions were filled from July 2014 to May 2017. The drug name, date filled, dose, and quantity dispensed, and prescriber were recorded. “Preoperative” prescriptions were collected from July 2014 until the day of surgery. “Discharge” prescriptions were written on the day of surgery and filled within one week of the date of surgery. “Postoperative Refill” prescriptions were any prescription filled after the Discharge prescription and were recorded until the date that each patient completed the PGAS survey. Prescriber information and chart review was used to determine if each Postoperative Refill prescription was associated with ACLR or another procedure/condition.
Each patient in this study received a multimodal pain management protocol. Most patients received a regional nerve block (97.6%) in the form of a femoral and/or sciatic single-shot or continuous nerve block, as well as an intraoperative joint injection with either Ropivacaine 0.5% or Bupivacaine-Epinephrine 0.5% 1:200000 (Table 1, Table 2).
Table 1.
Patient demographics and surgical characteristics of PGAS responders.
| Variable | Data (N = 41) |
|---|---|
| Age, years | 24.3 ± 10.4 |
| BMI, kg/m2 | 26.5 ± 5.1 |
| Comorbid conditions | 0.3 ± 0.6 |
| Anxiety or depression | 1 (2.4%) |
| Male | 24 (58.5%) |
| Race | |
| White | 25 (61.0%) |
| Black | 7 (17.1%) |
| Other | 9 (22.0%) |
| Hispanic or Latino | 3 (7.3%) |
| Married | 6 (14.6%) |
| Insurance | |
| Government | 7 (17.1%) |
| Private | 32 (78.1%) |
| Uninsured | 2 (4.9%) |
| Employment status | |
| Employed | 14 (48.3%) |
| Unemployed | 3 (10.3%) |
| Student | 12 (41.4%) |
| Smoking status | |
| Current smoker | 2 (4.9%) |
| Former smoker | 5 (12.2%) |
| Never smoker | 34 (82.9%) |
| Alternate tobacco use | 1 (3.9%) |
| Current alcohol use | 18 (47.4%) |
| Illicit drug use | 2 (5.1%) |
| Other controlled substances filled | 9 (22.0%) |
| ASA score | |
| I | 31 (79.5%) |
| II | 8 (20.0%) |
| Regional nerve block | |
| Femoral, continuous catheter | 39 (95.1%) |
| Sciatic, single-shot | 8 (19.5%) |
| Combined femoral and sciatic | 7 (17.1%) |
| No block | 1 (2.4%) |
| Surgical characteristics | |
| Prior knee surgery | 7 (17.1%) |
| Revision ACLR | 3 (7.3%) |
| Microfracture | 1 (2.4%) |
| Other ligamenta | 1 (2.4%) |
| Meniscus surgery, any | 20 (48.8%) |
| Meniscectomy | 10 (24.4%) |
| Meniscus repair | 14 (31.1%) |
Values are given as the number of patients with the percentage in parentheses or as the mean and standard deviation.
PGAS, Press Ganey Ambulatory Surgery Survey; BMI, body mass index; TME, total milligram morphine equivalents; ASA, American Society of Anesthesiologists.
One patient underwent a concomitant anterolateral ligament reconstruction with hamstring allograft.
Table 2.
Perioperative analgesia usage and TMEs of PGAS responders.
| Timeframe | N (%)a | TMEs |
|---|---|---|
| Preoperative Opioid Prescriptionb | 8 (19.5%) | 196.7 ± 775.7 |
| Day of Surgery | ||
| Immediate Preoperative | 6 (14.6%) | 2.3 ± 5.3 |
| Intraoperative Opioids | 38 (92.7%) | 14.7 ± 10.0 |
| PACU Opioids | 32 (78.0%) | 8.2 ± 5.5 |
| Perioperative Non-Opioid Pain Medications | 33 (80.5%) | – |
| NSAIDs | 22 (53.7%) | – |
| Ketorolac | 16 (39.0%) | – |
| Regional Nerve Block | 40 (97.6%) | – |
| Intraoperative Joint Injection | 37 (90.2%) | – |
| Post-Surgical Center Opioids | ||
| Discharge Opioid Prescription | 41 (100.0%) | 784.0 ± 213.8 |
| Postoperative Refill Opioid Prescription | 5 (12.2%) | 102.4 ± 300.8 |
Values are given as the number of patients with the percentage in parentheses or as the mean and standard deviation.
PGAS, Press Ganey Ambulatory Surgery Survey; TME, total milligram morphine equivalents; PACU, post-anesthesia care unit; NSAID, Non-Steroidal Anti-Inflammatory Drug.
Number (percent) of patients who filled a prescription or were administered each type of analgesia.
Preoperative opioid prescriptions were collected from July 2014 to the day of surgery.
Patients also received two weeks of daily aspirin 325 mg for deep vein thrombosis prophylaxis. The number of milligram morphine equivalents for each opioid prescribed was calculated to determine Preoperative total milligram morphine equivalents (TMEs), Immediate Preoperative TMEs, Intraoperative TMEs, PACU TMEs, Discharge TMEs, and Postoperative Refill TMEs. “Preoperative TMEs” included all outpatient opioid prescriptions recorded in the database prior to the date of surgery (July 2014 to the date of surgery). “Immediate Preoperative TMEs” refers to opioids administered while the patient was in the surgery center's preoperative holding area prior to entering the operating room. “Intraoperative TMEs” refers to opioids administered during the surgical procedure (in the operating room). “PACU TMEs” refer to opioid administered in the PACU after the procedure. “Discharge TMEs” included all prescriptions in the database written on the date of discharge and filled on the date of discharge or within one week after surgery. “Postoperative Refill TMEs” refers to postoperative outpatient opioids that were not otherwise excluded with the above postoperative opioid prescription criteria and were filled prior to the date that each patient completed the PGAS survey.
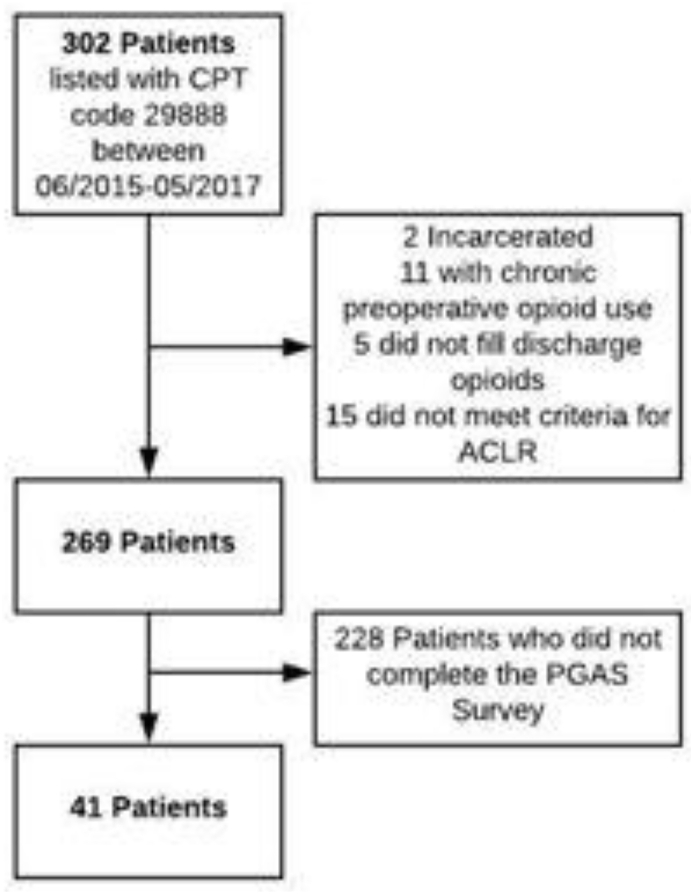
Of the 269 patients who met the initial inclusion criteria, 41 patients (15.2%) completed the PGAS survey and were included in the final analysis (Fig. 1). Our institution contracts Press Ganey, Inc. (Press Ganey Performance Solutions, Wakefield, MA) to collect patient satisfaction data. The PGAS survey contains questions concerning six domains: (1) Facility, (2) Nursing, (3) Physician, (4) Registration, (5) Personal Issues, and (6) Overall Assessment (Appendix 1).
Fig. 1.
Flow diagram depiciting the process for patient selection in this study.
Each domain consists of three to eight questions rated on a Likert scale of 1–5, with 1 being “Very Poor” and 5 being “Very Good.” At this institution, census surveying methodology is utilized so that potentially 100% of patients are being surveyed if a valid mailing or e-mail address is provided. PGAS surveys were initially sent to patients by mail within a week of surgery. Once the facility's sampling number of 120 surveys were sent each month, the method of survey administration was switched to e-mail for the remainder of the month. The e-mail was sent within a week of surgery and a second reminder e-mail was sent five days later if there was no response. The e-mail survey link closes after thirty days, but most responses are typically received within two days.
2.1. Statistical analysis
PGAS scores were converted from a 1–5 scale for each question to a 0–100 score. Domain scores were calculated and the Total PGAS score was determined by averaging the domain scores. Pearson chi-squared tests were used to evaluate categorical variables, and Wilcoxon rank sum tests were used to evaluate continuous variables between groups. Spearman's correlation coefficients were used to evaluate the relationship between PGAS scores and TMEs at each perioperative time point (Preoperative, Immediate Preoperative, Intraoperative, PACU, Discharge, and Postoperative Refill). Correlation coefficients with a rho (ρ) > 0.70 was considered a “strong” correlation, 0.50–0.70 was a “good” correlation, 0.30–0.50 was a “fair” correlation, and <0.30 was a “poor” correlation.20 All statistical analyses were performed with JMP Statistical Software (North Carolina, United States). Differences with a P < 0.05 were considered statistically significant.
3. Results
Patient demographics and surgical characteristics are shown in Table 1. Patients were predominantly male (58.5%) with white race (61.0%) and a mean age of 24.3 ± 10.4 years.
A loss-to-follow-up analysis is shown in Appendix 2 for patients who did (“Responders,” n = 41) and did not (“Non-Responders,” n = 228) complete the PGAS survey. Non-Responders were significantly more likely to have a preoperative opioid prescription (P = 0.03) and filled significantly more preoperative TMEs compared to Responders (P = 0.03). Non-Responders also had significantly more concomitant procedures associated with the ACLR compared to Responders (P = 0.004). Concomitant procedures in Responders included meniscus repair (31.1%), meniscectomy (24.4%), microfracture (2.4%), and anterolateral ligament reconstruction with hamstring allograft (2.4%; Table 1). There were no significant differences between Responders and Non-Responders for any other patient or surgical characteristics.
Table 2 shows the Preoperative, Immediate Preoperative, Intraoperative, PACU, Discharge, and Postoperative Refill opioid analgesia and non-opioid analgesia for patients undergoing ACLR in the Responders group. Most patients (80.5%) did not have a preoperative opioid prescription, and only 6 (14.6%) patients received an opioid in the preoperative holding area. Intraoperatively, 92.7% of patients received opioids with a mean of 14.7 ± 10.0 TMEs. The majority (78.0%) received opioids after their surgery in the PACU with a mean of 8.2 ± 5.5 TMEs. Most patients also received non-opioid medications (80.5%) at the surgical center, including nonsteroidal anti-inflammatory drugs (NSAIDs; 53.7%). The most common NSAID administered was ketorolac (39.0%). Forty (97.6%) patients received regional analgesia, with 39 (95.1%) patients receiving continuous femoral nerve blocks and 8 patients (19.5%) receiving single-shot sciatic nerve blocks (Table 1). Seven (17.1%) patients received both a continuous femoral nerve block and a single-shot sciatic nerve block. Patients were discharged with a mean of 784.0 ± 213.8 TMEs (Table 2). Five patients (12.2%) refilled their opioid prescription postoperatively, with a mean of 102.4 ± 300.8 TMEs.
The Total and domain PGAS scores are shown in Table 3. Patients rated their surgical experience highly, with a mean Total PGAS Score of 91.5 ± 10.5 and domain scores ranging between 86.4 ± 13.7 for the Facility score to 96.5 ± 7.1 for the Physician score.
Table 3.
PGAS scores of ACLR patients.
| PGAS Domain or Question | Score |
|---|---|
| Facility | 86.4 ± 13.7 |
| Nursing | 92.9 ± 14.5 |
| Physician | 96.5 ± 7.1 |
| Registration | 89.0 ± 14.3 |
| Personal Issues | 88.7 ± 14.2 |
| Pain Control | 90.2 ± 17.4 |
| Overall Assessment | 95.3 ± 13.1 |
| Overall Rating of Care | 96.3 ± 11.9 |
| Likelihood of Recommendation | 93.9 ± 20.8 |
| Total | 91.5 ± 10.5 |
The values are given as the mean and standard deviation.
PGAS, Press Ganey Ambulatory Surgery Survey; ACLR, anterior cruciate ligament reconstruction.
The correlations between the PGAS Total or domain scores and the TMEs at various timeframes are shown in Table 4. There was a fair, negative correlation between the “Pain Control” question in the Personal Issues domain and Preoperative TMEs (ρ = −0.35, P = 0.03). There were several fair, positive correlations between Immediate Preoperative TMEs and Total (ρ = 0.34, P = 0.03), Nursing (ρ = 0.38, P = 0.01), and Registration (ρ = 0.31, P = 0.04) PGAS scores. There was a fair, positive correlation between the “Overall Rating of Care” question in the Overall Assessment domain and Intraoperative TMEs (ρ = 0.34, P = 0.03). There were no significant correlations between PGAS Total, domain, or question scores and TMEs at any of the postoperative time points (PACU, Discharge, and Postoperative Refill).
Table 4.
PGAS scores versus total milligram morphine equivalents.
| PGAS Domain | Timeframe of TME Administration | ρa | P value |
|---|---|---|---|
| Facility | vs. Preoperative | −0.15 | 0.35 |
| vs. Immediate Preoperative | 0.23 | 0.14 | |
| vs. Intraoperative | −0.02 | 0.88 | |
| vs. PACU | −0.27 | 0.08 | |
| vs. Discharge | 0.18 | 0.25 | |
| vs. Postoperative Refill | −0.17 | 0.29 | |
| Nursing | vs. Preoperative | −0.07 | 0.66 |
| vs. Immediate Preoperative | 0.38 | 0.01 | |
| vs. Intraoperative | 0.12 | 0.44 | |
| vs. PACU | −0.19 | 0.22 | |
| vs. Discharge | −0.09 | 0.56 | |
| vs. Postoperative Refill | −0.15 | 0.34 | |
| Physicians | vs. Preoperative | −0.09 | 0.59 |
| vs. Immediate Preoperative | 0.25 | 0.12 | |
| vs. Intraoperative | −0.02 | 0.89 | |
| vs. PACU | −0.02 | 0.90 | |
| vs. Discharge | 0.06 | 0.71 | |
| vs. Postoperative Refill | −0.13 | 0.42 | |
| Registration | vs. Preoperative | 0.004 | 0.98 |
| vs. Immediate Preoperative | 0.31 | 0.04 | |
| vs. Intraoperative | 0.26 | 0.11 | |
| vs. PACU | −0.07 | 0.66 | |
| vs. Discharge | −0.09 | 0.58 | |
| vs. Postoperative Refill | −0.05 | 0.77 | |
| Personal Issues | vs. Preoperative | −0.15 | 0.34 |
| vs. Immediate Preoperative | 0.25 | 0.11 | |
| vs. Intraoperative | 0.02 | 0.88 | |
| vs. PACU | −0.18 | 0.26 | |
| vs. Discharge | 0.02 | 0.89 | |
| vs. Postoperative Refill | −0.11 | 0.50 | |
| Pain Control | vs. Preoperative | −0.35 | 0.03 |
| vs. Immediate Preoperative | 0.15 | 0.35 | |
| vs. Intraoperative | −0.04 | 0.79 | |
| vs. PACU | −0.27 | 0.09 | |
| vs. Discharge | −0.04 | 0.78 | |
| vs. Postoperative Refill | −0.12 | 0.47 | |
| Overall Assessment | vs. Preoperative | −0.26 | 0.10 |
| vs. Immediate Preoperative | 0.19 | 0.24 | |
| vs. Intraoperative | 0.17 | 0.29 | |
| vs. PACU | −0.25 | 0.12 | |
| vs. Discharge | 0.01 | 0.96 | |
| vs. Postoperative Refill | 0.19 | 0.24 | |
| Overall Rating of Care | vs. Preoperative | −0.22 | 0.17 |
| vs. Immediate Preoperative | 0.14 | 0.40 | |
| vs. Intraoperative | 0.34 | 0.03 | |
| vs. PACU | −0.17 | 0.28 | |
| vs. Discharge | 0.24 | 0.13 | |
| vs. Postoperative Refill | 0.14 | 0.40 | |
| Likelihood of Recommendation | vs. Preoperative | −0.08 | 0.64 |
| vs. Immediate Preoperative | 0.14 | 0.40 | |
| vs. Intraoperative | 0.10 | 0.54 | |
| vs. PACU | −0.07 | 0.69 | |
| vs. Discharge | 0.02 | 0.92 | |
| vs. Postoperative Refill | 0.14 | 0.40 | |
| Total | vs. Preoperative | −0.13 | 0.42 |
| vs. Immediate Preoperative | 0.34 | 0.03 | |
| vs. Intraoperative | 0.10 | 0.52 | |
| vs. PACU | −0.23 | 0.15 | |
| vs. Discharge | 0.01 | 0.97 | |
| vs. Postoperative Refill | −0.13 | 0.40 |
PGAS, Press Ganey Ambulatory Surgery Survey; TME, total milligram morphine equivalents.
Bold print indicates statistical significance.
Bivariate analysis with Spearman's rank correlation coefficient (ρ) compared each PGAS domain or question with the TMEs at each timeframe (Preoperative, Intraoperative, Immediate Preoperative + PACU, Discharge, and Postoperative Refill).
There were no significant differences in PGAS scores based on the type of regional anesthesia administered (Table 5), except for patients who received a sciatic nerve block having higher mean Facility domain scores compared with those who did not have a sciatic nerve block (P = 0.049).
Table 5.
PGAS converted scores versus nerve block status.
| PGAS Domain or Question | Nerve Block Statusa | PGAS Scora | P value |
|---|---|---|---|
| Facility | Femoral | 85.67 ± 13.70 | 0.11 |
| No Femoral | 100.00 ± 0.00 | ||
| Sciatic | 95.00 ± 8.86 | 0.049 | |
| No Sciatic | 84.28 ± 13.95 | ||
| Combined Femoral + Sciatic | 94.29 ± 9.32 | 0.11 | |
| No Combined Femoral + Sciatic | 84.74 ± 14.00 | ||
| Nursing | Femoral | 92.52 ± 14.78 | 0.19 |
| No Femoral | 100.00 ± 0.00 | ||
| Sciatic | 97.27 ± 4.56 | 0.39 | |
| No Sciatic | 91.82 ± 15.89 | ||
| Combined Femoral + Sciatic | 96.88 ± 4.77 | 0.60 | |
| No Combined Femoral + Sciatic | 92.06 ± 15.71 | ||
| Physicians | Femoral | 96.31 ± 7.27 | 0.39 |
| No Femoral | 100.00 ± 0.00 | ||
| Sciatic | 99.22 ± 2.21 | 0.26 | |
| No Sciatic | 95.83 ± 7.76 | ||
| Combined Femoral + Sciatic | 99.11 ± 2.36 | 0.35 | |
| No Combined Femoral + Sciatic | 95.96 ± 7.68 | ||
| Registration | Femoral | 88.73 ± 14.55 | 0.75 |
| No Femoral | 93.75 ± 8.84 | ||
| Sciatic | 89.06 ± 11.93 | 0.74 | |
| No Sciatic | 88.95 ± 14.97 | ||
| Combined Femoral + Sciatic | 89.29 ± 12.87 | 0.88 | |
| No Combined Femoral + Sciatic | 88.91 ± 14.75 | ||
| Personal Issues | Femoral | 88.14 ± 14.35 | 0.15 |
| No Femoral | 100.00 ± 0.00 | ||
| Sciatic | 92.03 ± 10.13 | 0.59 | |
| No Sciatic | 87.92 ± 15.06 | ||
| Combined Femoral + Sciatic | 90.89 ± 10.38 | 0.88 | |
| No Combined Femoral + Sciatic | 88.27 ± 14.98 | ||
| Pain Control | Femoral | 89.74 ± 17.69 | 0.34 |
| No Femoral | 100.00 ± 0.00 | ||
| Sciatic | 92.50 ± 10.35 | 0.94 | |
| No Sciatic | 89.70 ± 18.79 | ||
| Combined Femoral + Sciatic | 91.43 ± 10.69 | 0.72 | |
| No Combined Femoral + Sciatic | 90.00 ± 18.59 | ||
| Overall Assessment | Femoral | 95.09 ± 13.34 | 0.52 |
| No Femoral | 100.00 ± 0.00 | ||
| Sciatic | 97.92 ± 5.89 | 0.67 | |
| No Sciatic | 94.70 ± 14.25 | ||
| Combined Femoral + Sciatic | 97.62 ± 6.30 | 0.79 | |
| No Combined Femoral + Sciatic | 94.85 ± 14.07 | ||
| Overall Rating of Care | Femoral | 96.15 ± 12.22 | 0.64 |
| No Femoral | 100.00 ± 0.00 | ||
| Sciatic | 100.00 ± 0.00 | 0.31 | |
| No Sciatic | 95.45 ± 13.19 | ||
| Combined Femoral + Sciatic | 100.00 ± 0.00 | 0.35 | |
| No Combined Femoral + Sciatic | 95.59 ± 13.01 | ||
| Likelihood of Recommendation | Femoral | 93.59 ± 21.24 | 0.64 |
| No Femoral | 100.00 ± 0.00 | ||
| Sciatic | 93.75 ± 17.68 | 0.80 | |
| No Sciatic | 93.94 ± 21.68 | ||
| Combined Femoral + Sciatic | 92.86 ± 18.90 | 0.69 | |
| No Combined Femoral + Sciatic | 94.12 ± 21.37 | ||
| Total | Femoral | 91.08 ± 10.64 | 0.19 |
| No Femoral | 98.96 ± 1.47 | ||
| Sciatic | 95.08 ± 5.46 | 0.40 | |
| No Sciatic | 90.58 ± 11.30 | ||
| Combined Femoral + Sciatic | 94.68 ± 5.76 | 0.52 | |
| No Combined Femoral + Sciatic | 90.80 ± 11.20 |
Bivariate analysis with the Wilcoxon rank sum test between PGAS Total and domain scores based on regional nerve block status. PGAS scores are presented as the mean and standard deviation.
PGAS, Press Ganey Ambulatory Surgery Survey.
Bold print indicates statistical significance.
Femoral (aontinuous catheter, n = 39) versus No Femoral (n = 2), Sciatic (single-shot, n = 8) versus No Sciatic (n = 33), Combined Femoral + Sciatic (n = 7) versus No Combined Femoral + Sciatic (n = 34).
4. Discussion
As increased emphasis is placed on the patient experience to determine hospital and provider reimbursement,1,2,21 understanding the impact of perioperative pain management on patient satisfaction metrics is essential.4, 5, 6,6,11,21 In this study, PGAS scores correlated with preoperative and intraoperative opioid administration, but not with the postoperative administration. In the context of the current opioid epidemic, surgeons should be aware of the potential impact of preoperative and intraoperative opioid administration on satisfaction scores. However, this study indicates that surgeons can prescribe opioids in the postoperative period according to the individual patient needs.
Prior studies exploring the relationship of PG scores and perioperative opioid use are limited to patients undergoing total joint arthroplasty. Bloom et al.16 found no significant change in PG scores of total shoulder arthroplasty patients after an institutional change to reduce opioid prescriptions at discharge. Etcheson et al.17,18 showed no significant correlations between PG scores and opioid administration, except for one positive correlation between postoperative morphine milliequivalents and “communication about medications” in hip arthroplasty patients. The same institution showed that the total opioid administration was not significantly correlated with any PG domains in patients undergoing total knee arthroplasty.17 Both authors concluded that surgeons should follow pain management guidelines when controlling postoperative pain, rather than overtreating pain for fear of reimbursement penalties from patient satisfaction scores. While the current study did not show any strong correlations, fair correlations existed between PGAS scores and perioperative opioid administration at several time points, with the exception of the postoperative period.
As may be expected, greater Preoperative TMEs were negatively correlated with the “Pain Control” question in the Personal Issues domain. This is consistent with prior research showing worse outcomes and greater pain control challenges in orthopaedic patients with opioid tolerance.7,8,22,23 Forlenza et al.24 performed a retrospective review of patients undergoing ACLR and reported preoperative opioid use was predictive of continued opioid use six months after surgery and worse outcomes at one year of follow-up. The authors also determined the threshold patient-reported outcome measure scores that indicated patients reached a satisfactory state, showing preoperative opioid use was associated with 69% lower odds of achieving satisfactory pain levels at one year postoperatively. The current study assessed pain control at a much earlier postoperative timeframe but showed a consistent inverse relationship between preoperative opioid use and patient satisfaction. While the Total and domain PGAS scores may not be significantly altered by a single question related to pain control, surgeons can use this information to set appropriate expectations for opioid tolerant patients.
While outpatient preoperative opioid use negatively impacts satisfaction with pain control, opioid administration in the preoperative holding area prior to surgery (Immediate Preoperative TMEs) was found to be associated with significantly higher Total and domain PGAS scores. This has not been previously reported in ACLR patients to our knowledge. The literature surrounding preemptive analgesia with opioids in other areas of orthopaedics have shown variable results. In a study of total joint arthroplasty patients, Cooper et al. found that patients who received preemptive opioids immediately prior to surgery experienced more pain, consumed more opioids postoperatively, and had impaired function early after surgery.25 However, several studies in a variety of orthopaedic surgery populations have shown that preemptively controlling pain prior to surgery can provide better pain control and less opioid use postoperatively.26, 27, 28, 29, 30, 31 Additional studies in ACLR patients using varied methods of measuring satisfaction will be needed to further elucidate the relationship between preemptive opioid analgesia and patient satisfaction.
The finding that Intraoperative TMEs are positively correlated with the “Overall Rating of Care” question in the Overall Assessment domain is novel in the ACLR literature but has been explored in other areas of orthopaedics with variable results. In their retrospective analysis of total hip arthroplasty patients, Maher et al. showed increased intraoperative opioid administration was associated with improved Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey responses related to pain control.32 Several other studies have shown similar findings, where perioperative pain control using patient satisfaction surveys was significantly associated with intraoperative opioid administration.6,7 When administered along with primary anaesthetic agents, opioid medications significantly augment the likelihood of unconsciousness by reducing nociception-induced arousal, likely resulting in a better intraoperative experience for the patient.33 Alternatively, multiple studies have shown conflicting results where increased intraoperative opioid doses can result in worse postoperative pain control and more postoperative side effects, such as nausea and vomiting.15,34, 35, 36 It is unclear if the orthopaedic surgeon should have any role in decision-making regarding intraoperative opioid administration, but further study seems warranted.
In this study, there were no significant correlations between PGAS scores and TMEs at any of the postoperative time points (PACU, Discharge, and Postoperative Refill), which is consistent with prior orthopaedic literature. In their studies of total arthroplasty patients, Etcheson et al. found no association between PG scores and opioid administration in the immediate postoperative period, with the exception of one positive correlation with “communication about medications” in hip arthroplasty patients.17,18 In a population of total shoulder arthroplasty patients, Bloom et al. found no difference in PG scores after an institutional change to decrease the amount of opioids prescribed at discharge.16 The results of this study show that ACLR patients exhibit similar findings, which indicates that surgeons can prescribe opioid medications for the treatment of postoperative pain according to the individual patient needs without concern for reimbursement penalties from patient satisfaction surveys.
Regional analgesia is considered an important aspect of pain control in patients undergoing ACLR. Prior research has shown improved pain control, lower analgesia requirements, and improved patient satisfaction with combined femoral and sciatic nerve blocks in patients undergoing ACLR.37 Further studies showed improvements in pain control with combined femoral and sciatic blocks were short lived, however, as patients had no differences in pain control, opioid consumption, or patient satisfaction during postoperative days 1–3.38 While the number of patients limited comparisons in the current study, patients who had a sciatic nerve block had significantly higher Facility domain scores. Further study is warranted to determine the optimal pain management strategy for ACLR.
4.1. Limitations
There were several limitations in the current study. The low response rate and potential for non-response bias has been commonly cited in studies utilizing PG databases. The PGAS response rate of 15.2% is on the upper end of previously reported response rates between 8.9% and 16.5%.25,39,40 The loss-to-follow-up analysis showed several significant differences between responders and non-responders, which may indicate the PGAS survey is subject to non-response bias.40,41 Most notably, non-responders filled significantly more preoperative TMEs compared with responders, which could have affected the results of the current study. Despite the universally low response rates for PG surveys, Press Ganey Associates, Inc., has concluded that only 30 surveys are needed to draw meaningful conclusions about a practice.42 While the sample size is relatively small in the current study, the 41 patients included in the study represent the entire ACLR cohort that completed the PGAS survey over a two-year period and meets the threshold of 30 surveys to publicly report and rank our institution's hospital satisfaction scores. Additionally, a sample size of 41 patients provides 80% power to detect a Spearman's correlation of 0.41 with an alpha level of 0.05. Therefore, the study was appropriately powered to show a fair or better correlation between the PGAS score and TMEs. Second, this study collected postoperative refill data only until each patient's date of PGAS survey completion. This could introduce a time-dependent bias where patients who filled out their PGAS survey later would potentially have more postoperative opioid refills. Accordingly, a fair positive correlation (ρ = 0.34, P = 0.03) between Postoperative Refill TMEs and time to survey completion was found. Ideally, patients would have completed the survey on the same postoperative day, but this was limited by the retrospective nature of the study. However, this variability in survey completion time in our study is likely more reflective of typical survey behavior. Third, although the regional prescription drug monitoring program tracks Maryland and the surrounding states, it does not document all opioid prescriptions across the country. This could underestimate the true opioid burden in our study. Additionally, the preoperative and postoperative opioids represent prescriptions that were filled but does not measure the quantity of opioids used. Finally, the amount of postoperative narcotics prescribed in this study reflect the time before the introduction of opioid-limiting regulations, and it is possible that the results may differ if fewer opioids are prescribed postoperatively.
5. Conclusion
In conclusion, this is the first study to assess the relationship between PGAS scores and perioperative opioid administration in patients undergoing ACLR. While patient satisfaction metrics are meant to improve patient care, linking hospital reimbursement to the patient experience could have the unintended consequence of incentivizing surgeons to prescribe more opioids than necessary with the intent to improve pain control. The current study shows several correlations between PGAS scores and preoperative and intraoperative opioid administration, particularly in the immediate preoperative period, but not postoperative opioid administration. Surgeons performing ACLR should remain aware of the association between satisfaction scores and opioid administration in the preoperative and intraoperative periods. This relationship represents a potential conflict for the prescribing surgeon, particularly in the context of the current opioid epidemic. However, our results indicate that surgeons can prescribe opioid medication for postoperative pain according to individual patient needs without concern for reimbursement penalties from poor satisfaction survey results.
Disclaimers
None.
Funding
This work was supported by a grant from The James Lawrence Kernan Hospital Endowment Fund, Incorporated and the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory R&D (BLRD) Service Career Development Award Number IK2 BX004879.
Institutional Review Board (IRB)
University of Maryland, Baltimore IRB approval was obtained for this project. HP-00062261-5.
CRediT authorship contribution statement
Leah E. Henry: Conceptualization, Writing – original draft, Writing – review & editing. Tina Zhang: Conceptualization, Writing – original draft, Writing – review & editing. Ali Aneizi: Writing- Original Draft, Writing- Review & Editing, Methodology, Data curation, Formal analysis. Tristan B. Weir: Writing- Original Draft, Writing- Review & Editing, Methodology, Data curation, Formal analysis. Matheus B. Schneider: Writing- Original Draft, Writing- Review & Editing. Sean J. Meredith: Writing- Original Draft, Writing- Review & Editing. Natalie L. Leong: Writing- Original Draft, Writing- Review & Editing, Supervision. Jonathan D. Packer: Writing- Original Draft, Writing- Review & Editing, Supervision. R. Frank Henn: Writing – original draft, Writing- Review & Editing, Methodology, Data curation, Formal analysis, Project administration, Supervision, Funding acquisition, Validation.
Acknowledgements
J. Kathleen Tracy, Ph.D.; Andrew G. Dubina, MD; Julio J. Jauregui, MD; Vidushan Nadarajah, MD; Patrick M.J. Sajak: Joshua M. Abzug, MD; Farshad Adib, MD, Craig H. Bennett, MD; S. Ashfaq Hasan, MD; Vincent Ng, MD; Cameran I. Burt, Shaun H. Medina; Keyan Shasti; Dominic J. Ventimiglia; Alexander J. Wahl; and Michael P. Smuda for their assistance with data collection.
Contributor Information
Leah E. Henry, Email: Leah.Henry@som.umaryland.edu.
Tina Zhang, Email: Tzhang1@som.umaryland.edu.
Ali Aneizi, Email: Ali.Aneizi@som.umaryland.edu.
Tristan B. Weir, Email: Tweir@som.umaryland.edu.
Matheus B. Schneider, Email: Matheus.schneider@som.umaryland.edu.
Sean J. Meredith, Email: Smeredith@som.umaryland.edu.
Natalie L. Leong, Email: Nleong@som.umaryland.edu.
Jonathan D. Packer, Email: Jpacker@som.umaryland.edu.
R. Frank Henn, III, Email: fhenn@som.umaryland.edu.
Appendix 1. Press Ganey Ambulatory Surgery (PGAS) Survey Domains with Associated Questions
| PGAS Domain | Question | Answer |
|---|---|---|
| Facility | - Comfort of the registration waiting area | (1) Very Poor (2) Poor (3) Fair (4) Good (5) Very Good |
| - Comfort of your room or resting area | ||
| - Comfort of the waiting area for your family | ||
| - Attractiveness of the Surgery Center | ||
| - Cleanliness of the Surgery Center | ||
| Nursing | - Friendliness/courtesy of the nurses | |
| - Your confidence in the skill of the nurses | ||
| - Information nurses gave you on the day of your procedure | ||
| - Nurses' concern for your comfort after the procedure | ||
| - Nurses' courtesy toward family who accompanied you (if applicable) | ||
| - Skill of the nurse starting IV | ||
| - Instructions nurses gave you about caring for yourself at home | ||
| - Information nurses gave your family about your surgery or procedure | ||
| Physicians | - Friendliness/courtesy of the physician | |
| - Explanation the physician gave you about what the surgery or procedure would be like | ||
| - Information the physician provided about what was done during your surgery or procedure | ||
| - Your confidence in the skill of the physician | ||
| Registration | - If you spoke with Ambulatory Services staff by phone, helpfulness of the person you spoke with before your procedure | |
| - How easy it was to get an appointment for surgery when you wanted | ||
| - Helpfulness of the person at the registration desk | ||
| - Information you received prior to surgery (i.e., time of surgery, how to prepare) | ||
| Personal Issues | - Response to concerns/complaints made during your stay | |
| - Our concern for your privacy | ||
| - Degree to which your pain was controlled (“Pain Control”) | ||
| - Information provided about delays (if you experienced delays) | ||
| Overall Assessment | - Overall rating of care received during your visit (“Overall Rating of Care”) | |
| - Likelihood of your recommending our Ambulatory Services to others (“Likelihood of Recommendation”) | ||
| - Degree to which staff worked together to care for you |
Appendix 2. Loss-to-Follow-up Analysis Between PGAS Responders and Non-Responders
| Variable | Responders (N = 41) | Non-Responders (N = 228) | P value |
|---|---|---|---|
| Age, years | 24.3 ± 10.4 | 24.4 ± 10.1 | 0.71 |
| BMI, kg/m2 | 26.5 ± 5.1 | 26.6 ± 5.8 | 0.90 |
| Number of Procedures | 1.7 ± 0.7 | 2.0 ± 0.7 | 0.004 |
| Comorbid conditions, n | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.82 |
| Male | 24 (58.5%) | 138 (60.5%) | 0.81 |
| Race | |||
| White | 25 (61.0%) | 112 (49.1%) | 0.17 |
| Black | 7 (17.1%) | 72 (31.6%) | |
| Other | 9 (22.0%) | 44 (19.3%) | |
| Hispanic or Latino | 3 (7.3%) | 14 (6.3%) | 0.81 |
| Married | 6 (14.6%) | 37 (16.5%) | 0.76 |
| Insurance | |||
| Government | 7 (17.1%) | 61 (26.8%) | 0.29 |
| Private | 32 (78.1%) | 162 (71.1%) | |
| Uninsured | 2 (4.9%) | 5 (2.2%) | |
| Employment status | |||
| Employed | 14 (48.3%) | 46 (32.6%) | 0.27 |
| Unemployed | 3 (10.3%) | 18 (12.8%) | |
| Student | 12 (41.4%) | 77 (54.6%) | |
| Smoking status | |||
| Current smoker | 2 (4.9%) | 12 (5.3%) | 0.59 |
| Former smoker | 5 (12.2%) | 17 (7.5%) | |
| Never smoker | 34 (82.9%) | 199 (87.3%) | |
| Alternate tobacco use | 1 (3.9%) | 5 (3.6%) | 0.95 |
| Current alcohol use | 18 (47.4%) | 88 (41.5%) | 0.50 |
| Current or former illicit drug use | 2 (5.1%) | 21 (10.0%) | 0.35 |
| Other controlled substances filled | 9 (22.0%) | 62 (27.2%) | 0.48 |
| Preoperative opioid prescription | 8 (19.5%) | 84 (37.0%) | 0.03 |
| Opioids, TMEs | |||
| Preoperative | 196.7 ± 775.7 | 486.2 ± 2595.9 | 0.03 |
| Discharge | 784.0 ± 213.8 | 780.4 ± 229.2 | 0.73 |
| Postoperative refill | 184.8 ± 348.6 | 1632.7 ± 16790.1 | 0.31 |
| ASA score | |||
| I | 31 (79.5%) | 149 (68.4%) | 0.16 |
| II | 8 (20.0%) | 69 (31.7%) | |
| Regional nerve block | |||
| Femoral, continuous catheter or | 39 (95.1%) | 220 (96.5%) | 0.67 |
| single-shot | |||
| Sciatic, single-shot | 8 (19.5%) | 56 (24.6%) | 0.48 |
| Combined femoral + sciatic | 7 (17.1%) | 51 (22.4%) | 0.45 |
| Surgical characteristics | |||
| Prior knee surgery | 7 (17.1%) | 51 (22.4%) | 0.45 |
| Revision ACLR | 3 (7.3%) | 26 (11.6%) | 0.42 |
| Prior knee surgeries, n | 0.2 ± 0.5 | 0.3 ± 0.7 | 0.42 |
| Microfracture | 1 (2.4%) | 11 (4.8%) | 0.50 |
Values are given as the number of patients with the percentage in parentheses or as the mean and standard deviation.
PGAS, Press Ganey Ambulatory Surgery Survey; BMI, body mass index; TME, total milligram morphine equivalents; ASA, American Society of Anesthesiologists.
Bold print indicates statistical significance.
References
- 1.Weiss A.J., Elixhauser A. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Trends in operating room procedures in U.S. Hospitals, 2001–2011: statistical brief #171.http://www.ncbi.nlm.nih.gov/books/NBK201926/ Accessed. [Google Scholar]
- 2.Zywiel M.G., Mahomed A., Gandhi R., Perruccio A.V., Mahomed N.N. Measuring expectations in orthopaedic surgery: a systematic review. Clin Orthop Relat Res. 2013;471(11):3446–3456. doi: 10.1007/s11999-013-3013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballantyne J.C., Murinova N., Krashin D.L. Opioid guidelines are a necessary response to the opioid crisis. Clin Pharmacol Ther. 2018;103(6):946–949. doi: 10.1002/cpt.1063. [DOI] [PubMed] [Google Scholar]
- 4.Carrico J.A., Mahoney K., Raymond K.M. The association of patient satisfaction-based incentives with primary care physician opioid prescribing. J Am Board Fam Med. 2018;31(6):941–943. doi: 10.3122/jabfm.2018.06.180067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung C.W., Ching Wong S.S., Qiu Q., Wang X. Oral oxycodone for acute postoperative pain: a review of clinical trials. Pain Physician. 2017;20(2S):SE33–SE52. [PubMed] [Google Scholar]
- 6.Mistry J.B., Chughtai M., Elmallah R.K. What influences how patients rate their hospital after total hip arthroplasty? J Arthroplasty. 2016;31(11):2422–2425. doi: 10.1016/j.arth.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 7.Maher D.P., Wong W., Woo P. Perioperative factors associated with HCAHPS responses of 2,758 surgical patients. Pain Med. 2015;16(4):791–801. doi: 10.1111/pme.12651. [DOI] [PubMed] [Google Scholar]
- 8.Meredith S.J., Nadarajah V., Jauregui J.J. Preoperative opioid use in knee surgery patients. J Knee Surg. 2019;32(7):630–636. doi: 10.1055/s-0038-1666868. [DOI] [PubMed] [Google Scholar]
- 9.Trasolini N.A., McKnight B.M., Dorr L.D. The opioid crisis and the orthopedic surgeon. J Arthroplasty. 2018;33(11):3379–3382. doi: 10.1016/j.arth.2018.07.002. e1. [DOI] [PubMed] [Google Scholar]
- 10.Wickramatilake S., Zur J., Mulvaney-Day N., Klimo MC von, Selmi E., Harwood H. How states are tackling the opioid crisis. Publ Health Rep. 2017;132(2):171–179. doi: 10.1177/0033354916688206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller M. Patient satisfaction, prescription drug abuse, and potential unintended consequences. J Am Med Assoc. 2012;307(13):1377. doi: 10.1001/jama.2012.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anthony C.A., Westermann R.W., Bedard N. Opioid demand before and after anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(13):3098–3103. doi: 10.1177/0363546517719226. [DOI] [PubMed] [Google Scholar]
- 13.Morris B.J., Mir H.R. The opioid epidemic: impact on orthopaedic surgery. J Am Acad Orthop Surg. 2015;23(5):267–271. doi: 10.5435/JAAOS-D-14-00163. [DOI] [PubMed] [Google Scholar]
- 14.Volkow N.D. Characteristics of opioid prescriptions in 2009. J Am Med Assoc. 2011;305(13):1299. doi: 10.1001/jama.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guignard B., Bossard A.E., Coste C. Acute opioid tolerance. Anesthesiology. 2000;93(2):409–417. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Bloom D.A., Manjunath A.K., Gotlin M.J. Institutional reductions in opioid prescribing do not change patient satisfaction on Press Ganey surveys after total shoulder arthroplasty. J Shoulder Elbow Surg. 2021;30(4):858–864. doi: 10.1016/j.jse.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Etcheson J.I., Gwam C.U., George N.E., Caughran A.T., Mont M.A., Delanois R.E. Does the amount of opioid consumed influence how patients rate their experience of care after total knee arthroplasty? J Arthroplasty. 2018;33(11):3407–3411. doi: 10.1016/j.arth.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Etcheson J.I., Gwam C.U., George N.E., Virani S., Mont M.A., Delanois R.E. Opioids consumed in the immediate post-operative period do not influence how patients rate their experience of care after total hip arthroplasty. J Arthroplasty. 2018;33(4):1008–1011. doi: 10.1016/j.arth.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 19.Beck J.J., Cline K., Sangiorgio S., Serpa R., Shifflett K.A., Bowen R.E. Prospective study of acute opioid use after adolescent anterior cruciate ligament reconstruction shows No effect from patient- or surgical-related factors. J Am Acad Orthop Surg. 2020;28(7):293–300. doi: 10.5435/JAAOS-D-18-00766. [DOI] [PubMed] [Google Scholar]
- 20.Hazra A., Gogtay N. Biostatistics series module 6: correlation and linear regression. Indian J Dermatol. 2016;61(6):593. doi: 10.4103/0019-5154.193662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J.S., Hu H.M., Brummett C.M. Postoperative opioid prescribing and the pain scores on hospital consumer assessment of healthcare providers and Systems survey. J Am Med Assoc. 2017;317(19):2013. doi: 10.1001/jama.2017.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devin C.J., Lee D.S., Armaghani S.J. Approach to pain management in chronic opioid users undergoing orthopaedic surgery. J Am Acad Orthop Surg. 2014;22(10):614–622. doi: 10.5435/JAAOS-22-10-614. [DOI] [PubMed] [Google Scholar]
- 23.Smith S.R., Bido J., Collins J.E., Yang H., Katz J.N., Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg. 2017;99(10):803–808. doi: 10.2106/JBJS.16.01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forlenza E.M., Lavoie-Gagne O., Lu Y. Preoperative opioid use predicts prolonged postoperative opioid use and inferior patient outcomes following anterior cruciate ligament reconstruction. Arthrosc J Arthrosc Relat Surg. 2020;36(10):2681–2688. doi: 10.1016/j.arthro.2020.06.014. e1. [DOI] [PubMed] [Google Scholar]
- 25.Cooper H.J., Lakra A., Maniker R.B., Hickernell T.R., Shah R.P., Geller J.A. Preemptive analgesia with oxycodone is associated with more pain following total joint arthroplasty. J Arthroplasty. 2019;34(12):2878–2883. doi: 10.1016/j.arth.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Bian Y., Wang L., Qian W. Role of parecoxib sodium in the multimodal analgesia after total knee arthroplasty: a randomized double-blinded controlled trial. Orthop Surg. 2018;10(4):321–327. doi: 10.1111/os.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamal P.K., Shrestha A.B., Shrestha R.R. Efficacy of preemptive gabapentin for lower extremity orthopedic surgery under subarachnoid block. JNMA J Nepal Med Assoc. 2015;53(200):210–213. [PubMed] [Google Scholar]
- 28.Khalili G., Janghorbani M., Saryazdi H., Emaminejad A. Effect of preemptive and preventive acetaminophen on postoperative pain score: a randomized, double-blind trial of patients undergoing lower extremity surgery. J Clin Anesth. 2013;25(3):188–192. doi: 10.1016/j.jclinane.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Labrum J., Ilyas A. Preemptive analgesia in thumb basal joint arthroplasty: immediate postoperative pain with preincision versus postincision local anesthesia. J Hand Microsurg. 2017;9(2):80–83. doi: 10.1055/s-0037-1603734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montazeri K., Kashefi P., Honarmand A. Pre-emptive gabapentin significantly reduces postoperative pain and morphine demand following lower extremity orthopaedic surgery. Singap Med J. 2007;48(8):748–751. [PubMed] [Google Scholar]
- 31.Panah Khahi M., Yaghooti A.A., Marashi S.H., Nadjafi A. Effect of pre-emptive gabapentin on postoperative pain following lower extremity orthopaedic surgery under spinal anaesthesia. Singap Med J. 2011;52(12):879–882. [PubMed] [Google Scholar]
- 32.Maher D.P., Woo P., Wong W., Zhang X., Yumul R., Louy C. Perioperative factors associated with Hospital Consumer Assessment of Healthcare Providers and Systems responses of total hip arthroplasty patients. J Clin Anesth. 2016;34:232–238. doi: 10.1016/j.jclinane.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 33.Brown E.N., Pavone K.J., Naranjo M. Multimodal general anesthesia: theory and practice. Anesth Analg. 2018;127(5):1246–1258. doi: 10.1213/ANE.0000000000003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frauenknecht J., Kirkham K.R., Jacot‐Guillarmod A., Albrecht E. Analgesic impact of intra‐operative opioids vs. opioid‐free anaesthesia: a systematic review and meta‐analysis. Anaesthesia. 2019;74(5):651–662. doi: 10.1111/anae.14582. [DOI] [PubMed] [Google Scholar]
- 35.Hayhurst C.J., Durieux M.E. Differential opioid tolerance and opioid-induced hyperalgesia. Anesthesiology. 2016;124(2):483–488. doi: 10.1097/ALN.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 36.Lee M., Silverman S.M., Hansen H., Patel V.B., Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145–161. [PubMed] [Google Scholar]
- 37.Jansen T.K., Miller B.E., Arretche N., Pellegrini J.E. Will the addition of a sciatic nerve block to a femoral nerve block provide better pain control following anterior cruciate ligament repair surgery? AANA J (Am Assoc Nurse Anesth) 2009;77(3):213–218. [PubMed] [Google Scholar]
- 38.Harbell M.W., Cohen J.M., Kolodzie K. Combined preoperative femoral and sciatic nerve blockade improves analgesia after anterior cruciate ligament reconstruction: a randomized controlled clinical trial. J Clin Anesth. 2016;33:68–74. doi: 10.1016/j.jclinane.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Rane A.A., Tyser A.R., Presson A.P., Zhang C., Kazmers N.H. Patient satisfaction in the hand surgery clinic: an analysis of factors that impact the press Ganey survey. J Hand Surg Am. 2019;44(7):539–547. doi: 10.1016/j.jhsa.2019.03.015. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tyser A.R., Abtahi A.M., McFadden M., Presson A.P. Evidence of non-response bias in the Press-Ganey patient satisfaction survey. BMC Health Serv Res. 2016;16(a):350. doi: 10.1186/s12913-016-1595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Compton J., Glass N., Fowler T. Evidence of selection bias and non-response bias in patient satisfaction surveys. Iowa Orthop J. 2019;39(1):195–201. [PMC free article] [PubMed] [Google Scholar]
- 42.Zusman E.E. HCAHPS replaces Press Ganey survey as quality measure for patient hospital experience. Neurosurgery. 2012;71(2):N21–N24. doi: 10.1227/01.neu.0000417536.07871. [DOI] [PubMed] [Google Scholar]