Abstract
Spinosyns are natural broad-spectrum biological insecticides with a double glycosylated polyketide structure that are produced by aerobic fermentation of the actinomycete, Saccharopolyspora spinosa. However, their large-scale overproduction is hindered by poorly understood bottlenecks in optimizing the original strain, and poor adaptability of the heterologous strain to the production of spinosyn. In this study, we genetically engineered heterologous spinosyn-producer Streptomyces albus J1074 and optimized the fermentation to improve the production of spinosad (spinosyn A and spinosyn D) based on our previous work. We systematically investigated the result of overexpressing polyketide synthase genes (spnA, B, C, D, E) using a constitutive promoter on the spinosad titer in S. albus J1074. The supply of polyketide synthase precursors was then increased to further improve spinosad production. Finally, increasing or replacing the carbon source of the culture medium resulted in a final spinosad titer of ∼70 mg/L, which is the highest titer of spinosad achieved in heterologous Streptomyces species. This research provides useful strategies for efficient heterologous production of natural products.
Keywords: Spinosyn, Spinosad, Polyketide, Polyketide synthase, Heterologous production, Streptomyces
Abbreviations: β and ε subunits of Acc, (AccBE); β and ε subunits of PCC, (PccBE); acetyl-CoA carboxylase, (ACC); acetyl-CoA synthetase, (AcsA); biosynthetic gene cluster, (BGC); high-performance liquid chromatography, (HPLC); HPLC-high resolution mass spectrometer, (HPLC-HRMS); limit of detection, (LoD); Luria−Bertani, (LB); overlap extension-polymerase chain reaction, (OE-PCR); polyketide synthase, (PKS); propionyl-CoA carboxylase, (PCC); soya flour mannitol, (SFM); 2-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl)amino]ethanesulfonic acid, (TES)
1. Introduction
Spinosyns are natural broad-spectrum secondary metabolites and biological insecticides obtained by the aerobic fermentation of Saccharopolyspora spinosa [1]. Spinosyns are glycosylated polyketide-derived macrolides possessing a perhydro-as-indacene core that is formed via a series of intramolecular cross-bridging reactions. From fermentation broth extracts of Sa. spinosa, a series of spinosyn factors were purified and structurally characterized, the most efficient are spinosyn A and D that together comprise the commercial insecticide spinosad [2], spinosyn J and L can further be chemically catalyzed to spinetoram [3]. Spinosad and spinestoram are biodegradable pesticides that are non-toxic to mammals [[4], [5], [6]]. Therefore, they are permitted as natural pesticides for growing organic food and have won the US President's Green Chemicals Challenge Award three times (1999, 2008, and 2010).
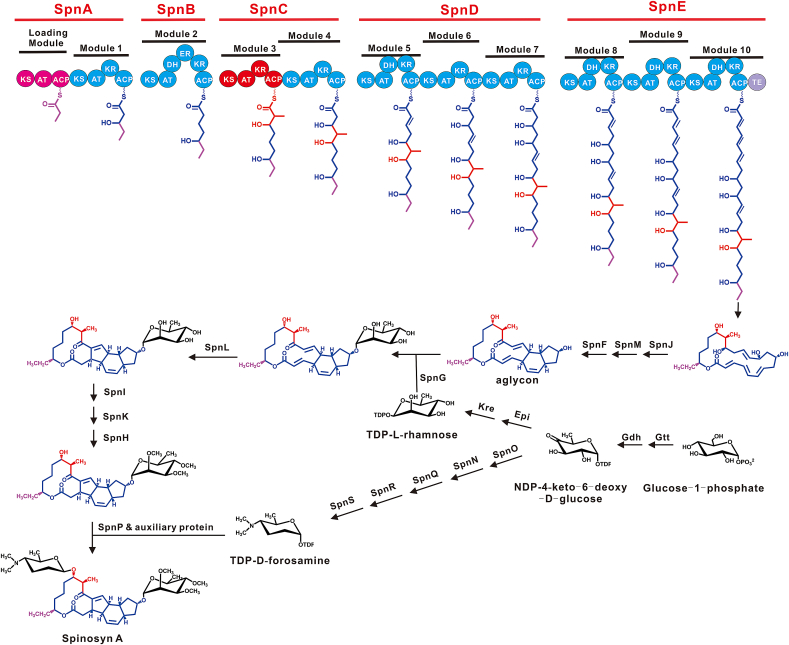
The family of spinosyns are biosynthesized by the same biosynthetic gene cluster (BGC), following a similar biosynthetic pathway; the production of multiple factors is due to the different substrates or enzymes involved in the reaction pathway; however the most productive component is spinosad. The mechanism of spinosyn A has been elucidated (Fig. 1). A load module and ten extension modules of polyketide synthase (PKS) genes (spnA, B, C, D, and E) are required to synthesize the polyketide skeleton starting from malonyl-CoA, methylmalonyl-CoA, and propionyl-CoA, which is then catalyzed by SpnJ, F, and M to form aglycone [7,8]. A rhamnose group, which is synthesized by four enzymes (Gtt, Gdh, Epi, and Kre) located outside the spinosad gene cluster, is loaded onto the C-9 group of aglycone by SpnG [9]. Subsequently, SpnL catalyzes a transannular cyclization reaction between C-3 and C-14 [7], the 2ʹ,3ʹ,4ʹ-hydroxy group of rhamnose is then O-methylated by SpnI, K, and H [10]. Finally, a forsamine group synthesized by SpnO, N, Q, R, and S [[11], [12], [13]] is loaded onto the C-17 group of aglycone by SpnP and an unknown auxiliary protein to synthesize spinosad [14]. During recent decades, great efforts have been made to improve spinosad production in Sa. spinosa by mutagenesis [15], fermentation optimization [[16], [17], [18], [19]], genomic engineering [20,21], and metabolic engineering [[22], [23], [24], [25]]. However, the titer of spinosad has not yet reached a level sufficient for industrial production; therefore, a poorly understood bottleneck may occur when optimizing the original spinosad producer Sa. spinosa.
Fig. 1.
The biosynthetic pathway of spinosyn A. KS: β-ketoacyl synthase. AT: acyltransferase, the loading and extension units including malonyl-CoA, methylmalonyl-CoA, and propionyl-CoA. DH: dehydratase. ER: enoyl reductase. KR: β-ketoreductase. ACP: Acyl carrier protein.
Since the biosynthetic pathway of spinosad is known, heterologous production can be used to unlock the bottleneck. The original BGC of spinosad was heterologously expressed in Sa. erythraea, Streptomyces coelicolor, and S. lividans to obtain the spinosad titer of 1–3 mg/L [26,27]. Metabolic engineering has also be attempted in addition to the replacement of the chassis. Huang et al. (2016) replaced native erythromycin PKS genes with the spinosad BGC in Sa. erythraea, performed several steps of metabolic engineering, and multiple rounds of ultraviolet mutagenesis to increase the spinosad titer to 830 mg/L [28]. Song et al. (2019) used five constitutive promoters to reconstitute the spinosad BGC in S. albus J1074 to obtain 1.11 mg/L spinosad [29]. We also heterologously expressed the original BGC of spinosad from Sa. spinosa in S. albus J1074 using the omics-guide strategy to increase the titer of spinosad to 1.46 mg/L by three targeted engineering steps [30]. Despite Streptomyces producing a much lower titer of spinosad than Sa. erythraea, it has great potential for heterologous production as a chassis host due to its rapid growth rate, easy genetic manipulation, and the relatively mature research methods of Streptomyces species [31]. Spinosad is a type I polyketide, and the efficient expression of PKS may be the key to its heterologous overproduction. However, previous metabolic engineering studies have not conducted in-depth studies on efficient PKS expression in heterologous hosts.
Since Sa. erythraea has been used in the large-scale production of erythromycin, which have developed mature industrial production methods, the optimized fermentation method of Sa. erythraea is likely a contributing factor to the greater production of spinosad than the model Streptomyces species. Although Song et al. (2019) confirmed that the previous culture method we used for S. albus J1074 for heterologous production of spinosad is the most efficient among several developed methods [29], it still suggests the need for designing a unique fermentation method to overcome the relatively low yield of heterologous spinosad production in Streptomyces.
To promote the large-scale industrialization of spinosad, we performed further metabolic engineering and fermentation optimization based on our previous work. We systematically investigated the influence of using a constitutive promoter for overexpressing spnA, B, C, D, and E on the titer of spinosad in S. albus J1074. After selecting the optimal combination of PKS expression, the supply of polyketide precursor was elevated to assist in spinosad overproduction. The spinosad titer was further improved by increasing the carbon source in the culture medium and replacing it with different sugars.
2. Materials and methods
2.1. Microorganisms and flask fermentation
Streptomyces and its derivatives were cultivated as described previously [30]. Briefly, strains were cultured on soybean flour-mannitol agar plates (2% w/v soybean flour, 2% w/v mannitol, and 2% w/v agar). Spores were collected, suspended in 20% (v/v) glycerol, and stored at 80 °C. For fermentation experiments, spores were grown in trypticase soy broth and the fermentation medium was 4% (w/v) sugar (glucose, sucrose, fructose, mannitol, or maltose), 1% (w/v) glycerol, 3% (w/v) soluble starch, 1.5% (w/v) Difco soytone, 1% (w/v) beef extract, 0.65% (w/v) peptone, 0.05% (w/v) yeast extract, 0.2% (w/v) MgSO4, 0.2% (w/v) NaCl, and 0.24% (w/v) CaCO3. Unless otherwise specified, all the strains were cultured in a 250 mL Erlenmeyer flask at 30 °C and analyzed at the end of the 8-d fermentation unless otherwise specified.
2.2. Gene overexpression plasmid construction
The plasmids and primers used for strain construction are listed in Tables S1 and S2, respectively. In general, all plasmids used in this study were based on pJTU1278, which is an efficient vector for gene disruption and replacement in Streptomyces species [32]. pJTU1278-spnA was constructed for replacement of the natural promoter of the spnABC operon with promoter rpsLp-cf. Primer pair RPSLP-CF-F/RPSLP-CF-R were used to amplify promoter rpsLp-cf from pLH8 [34], and primer pairs spnA-UF/spnA-UR and spnA-DF/spnA-DR were used to amplify upstream and downstream homologous arms from S. albus J1074 (C416-M)-OE3 (OE3) [30], respectively. The amplified fragments were joined by the overlap extension polymerase chain reaction (OE-PCR) using primer pairs spnA-UF/spnA-DR. The final PCR product and pJTU1278 were digested independently with HindIII and XbaI, purified, and ligated with DNA ligase according to general molecular biology techniques to construct the plasmid pJTU1278-spnA.
pJTU1278-spnD was constructed to replace the natural promoter of spnD with promoter rpsLp-tp. Primer pair spnD-RPSTP-D/spnD-RPSTP-F were used to amplify the promoter rpsLp-tp from pLH11 [34], while spnD-UF/spnD-UR and spnD-DF/spnD-DR were used to amplify the upstream and downstream homologous arms from OE3, respectively. The amplified fragments were joined by OE-PCR using primer pairs spnD-UF/spnD-DR. The final PCR product and pJTU1278 were digested independently with KpnI and XbaI, purified, and ligated as described above to construct the plasmid pJTU1278-spnD.
pJTU1278-spnC was constructed to insert the kasOp* promoter upstream of spnC. Primer pair spnC-KasOp-F/spnC-KasOp-R were used to amplify the kasOp* promoter from pLH10 [34], with spnC-UF/spnC-UR and spnC-DF/spnC-DR used to amplify upstream and downstream homologous arms from OE3, respectively. The amplified fragments were joined by OE-PCR using primers spnC-UF/spnC-DR. The final PCR product and pJTU1278 were digested with BamHI and XbaI, purified, and ligated with DNA ligase as described above to construct the plasmid pJTU1278-spnC.
pJTU1278-spnB was constructed to insert the promoter kasOp*-rpslp-cf in front of spnB. Primer pair spnB-KORL-F/spnB-KORL-R were used to amplify the kasOp*-rpslp-cf promoter from pLH9 [34], and primer pair spnB-UF/spnB-UR and spnB-DF/spnB-DR were used to amplify the upstream and downstream homologous arms from OE3, respectively. The amplified fragments were joined by OE-PCR using primers spnB-UF/spnB-DR. The final PCR product and pJTU1278 were digested with SacI and EcoRI, purified, and ligated with DNA ligase as described above to construct the plasmid pJTU1278-spnB.
pZA001 was constructed to interrupt candicidin PKS expression; the primer pair 001-L-F/001-L-R and 001-D-F/001-D-R was used to amplify upstream and downstream homologous arms from OE3, respectively. Plasmid pJTU1278 was then digested with XbaI and HindIII. All the amplified DNA fragments and digested pJTU1278 were joined together using a Gibson assembly method to obtain plasmid pZA001.
pHT603 was constructed to replace candicidin PKS with acsA and pccBE. Primer pair 63-UF/63-UR and 63-DF/63-DR were used to amplify the upstream and downstream homologous arms from OE3, respectively. The promoter kasOp* was amplified from pLH10 by PCR using the primer pair 63KasOP-F/63 KasOP-R. Primer pairs ScoAscA-F/ScoAscA-R and ScoPccE-F/ScoPccE-R were used to amplify acsA and pccBE from S. coelicolor CH999 genomic DNA, respectively. Three primer pairs (F8-1278-1F/F8-1278-1R, F9-1278-2F/F9-1278-2R, F10-1278-3F/F10-1278-3R) were used to amplify pJTU1278. Primer pair 426-1F/426-1R was used to amplify the yeast helper fragment from the plasmid pRS426. All amplified DNA fragments were joined using DNA assembler method [33] to obtain the plasmid pHT603.
pFF209 was constructed to insert accA2 and accBE between two genes in the chromosome of S. albus J1074. Primer pairs 209-UF/209-UR and 209-DF/209-DR were used to amplify the upstream and downstream homologous arms from OE3, respectively. The rpsLp-cf promoter was amplified from pLH8 by PCR using the primer pair 209-rps-F/209-rps-R. Primer pairs ScoACCA2-F/ScoACCA2-R and ScoACCB-F/ScoACCB-R were used to amplify accA2 and accBE from S. coelicolor CH999 genomic DNA, respectively. Three pairs of primers (209-1F/209-1R, 209-2F/209-2R, 209-3F and 209-3R) were used to amplify the vector backbone from pHT603. All amplified DNA fragments were joined using DNA assembler method [33] to obtain the plasmid pFF209. All of the constructed plasmids were verified by sequencing.
2.3. Strain construction
The strains used in this study are listed in Table 1. The engineered strains were constructed by triparental conjugation as previously described [30]. Briefly, the plasmid donors Escherichia coli DH10b/plasmid and E. coli ET12567(pUB307) were grown to an OD600nm of 0.4–0.6. Cells were pelleted by centrifugation at 4000×g for 4 min, washed twice in Luria−Bertani (LB) broth, and resuspended 100 μL of LB. The fresh or frozen Streptomyces spores (stored at −40 °C) were washed twice in LB, suspended in TES (2-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl)amino]ethanesulfonic acid) buffer (0.05 M, pH 8.0) and incubated at 50 °C for 10 min to activate germination. An equal volume of double-strength germination medium (1% w/v Oxoid yeast extract, 1% w/v Difco Casamino acids and 0.01 M CaCl2) was added and the mixture was incubated at 37 °C for 2–3 h with shaking at 220 rpm. The germinated spores were pelleted by centrifugation as stated above, collected, and resuspended in 100 μL of TES buffer. Approximately 1 × 108 E. coli cells (DH10b(plasmid):ET12567(pUB307)≈1:1) were added to the prepared spores (not less than 108 spores/conjugation) and the mixture was spread onto a soya flour mannitol (SFM) agar plate containing 30 mM MgCl2. The conjugation plates were incubated for 14 h at 30 °C, then the surfaces of the plates were overlaid with 1 mL of sterile water containing 600 μg trimethoprim, 1.5 mg apramycin, and 300 μg thiostrepton. The plates were incubated for an additional 3–6 d at 30 °C, and exconjugants resistant to thiostrepton and apramycin were selected. After sporulation on SFM medium with apramycin (50 mg/L), double-crossover mutants were selected based on thiostrepton-sensitive and apramycin-resistant and verified by PCR analysis with flanking primers (Fig. S2), primer pairs A-VF/A-VR for verifying AE, primer pairs D-VF/D-VR for verifying ADE, primer pairs C-DVF/C-DVR and C-UVF/C-UVR for verifying ACDE, primer pairs B-DVF/B-DVR and B-UVF/B-UVR for verifying ABE, ABDE, and ABCDE, primer pairs 001-VF/001-VR for verifying DcanP2, primer pairs 603-DVF/603-DVR, 603-UVF/603-UVR, 209-DVF/209-DVR, 209-UVF/209-UVR for verifying ADE-AP.
Table 1.
Genotypic features of the Streptomyces and Escherichia coli strains used in this study.
| Strain | Features | Source |
|---|---|---|
| S. albus J1074 (C416-M)-OE3 | Based on S. albus J1074 (C4I6-M) in which a BAC plasmid containing the spinosad biosynthetic gene cluster is integrated into the chromosome of S. albus J1074, an extra spnI gene was overexpressed under the control of rpsLp-cf, gtt and epi were co-overexpressed under the control of kasOp*, gdh and kre were co-overexpressed under the control of kasOp*, spnO and spnN were co-overexpressed under the control of kasOp*, spnQ and spnR were co-overexpressed under the control of rpsLp-cf, spnS was overexpressed under the control of rpsLp-tp, and the PKS gene spnE was overexpressed by kasOp*. | Tan et al., 2017 [30] |
| S. coelicolor CH999 | proA1, argA1 redE60 Δact::ermE SCP−, SCP2− | McDaniel et al., [43] |
| AE | The PKS operon spnABC was overexpressed under the control of the rpsLp-cf promoter in S. albus J1074 (C416-M)-OE3 | This study |
| ADE | The PKS gene spnD was overexpressed under the control of the rpsLp-tp promoter in AE | This study |
| ACDE | The PKS gene spnC was individually overexpressed under the control of the kasOp* promoter in ADE | This study |
| ABDE | The PKS operon spnBC was overexpressed under the control of the kasOp*- rpsLp-cf promoter in ADE | This study |
| ABCDE | The PKS genes spnB and spnC were overexpressed under the control of kasOp*-rpsLp-cf, and kasOp* promoters in ADE, respectively | This study |
| ABE | The PKS operon spnBC was overexpressed under the control of the kasOp*- rpsLp-cf promoter in AE | This study |
| DcanP2 | Deletion part of candicidin PKS gene in ADE | This study |
| ADE-AP | Extra acsA and pccBE were overexpressed under the control of kasOp*, additional accA2 and accBE were overexpressed under the control of rpsLp-cf in ADE, PKS of candicidin BGC in ADE was replaced by acsA and pccBE to interrupt the expression of the candicidin PKS gene | This study |
| E. coli DH10b | F− mcrA Δ(mrr-hsdRMS-mcrBC) | Gibco-BRL |
| E. coli ET12567(pUB307) | dam dcm hsdS/pUB307 | Flett et al., 1997 [44] |
2.4. Extraction and analysis of spinosads
Spinosads were extracted from the fermentation cultures (1 mL) by mixing with 2 mL of acetonitrile, vortexed for 20 min, incubated for 30 min at room temperature, and centrifuged at 3500×g for 10 min to remove cell debris. The supernatant was filtered using a 0.22 μm syringe filter and injected into a C18-reversed phase HPLC column (5 μm, 250 × 4.6 mm, Waters, Milford, USA) at 25 °C using isocratic elution with acetonitrile: methanol: 0.05% ammonium acetate buffer (4.5:4.5:1, v/v/v) at a flow rate of 1 mL/min and detection at 250 nm (HPLC with a UV detector, SPD-20A, Shimadzu, Kyoto, Japan), or a C18-reversed phase HPLC column (5 μm, 250 × 4.6 mm, Agilent, California, USA) at same separation condition and detection at 250 nm (HPLC, 1260DAD, Thermo scientific, Waltham, USA coupled with a DAD detector, DAD3000, Dionex, Sunnyvale, USA). Spinosad titer from Streptomyces and its derivatives was determined by comparison with standard spinosyn A and D.
2.5. Extraction and analysis of acyl-CoAs by LC-MS
After 3 d fermentation, 10 mL of S. ablus J1074 broth was centrifuged at 3500×g for 10 min at 4 °C to collect the bacteria, then was frozen with liquid nitrogen and stored at −80 °C for subsequent extraction of acyl-CoAs. Three biological replicates were used. When extracting the acyl-CoAs, the sample was chilled in ice, then two zirconia glass beads (2 mm), 100 μL of zirconia glass beads (1 mm), and 1 mL of ice-cold monopotassium phosphate buffer (67 mM, pH 4.9) were added to the sample, and the bacteria were ground twice using a grinder (JXFSTPRP-24L, Jingxin, Shanghai, China, 60 Hz, stop for 10 s after 60 s) that was precooled at −40 °C. Next, 500 μL of precooled isopropanol was added, and the sample was ground again. After grinding, 1 mL of precooled acetonitrile and 60 μL of saturated ammonium sulfate solution (room temperature) was added, and the sample was ground once. After grinding, the tube was placed on ice for 10 min, centrifuged at 12,000×g at 4 °C for 10 min, the supernatant transferred into a new tube, lyophilized and then stored at −80 °C for subsequent analysis. The sample was resuspended in 200 μL of 50% methanol, vortexed for 1 min, and then water bath sonicated for 2 min. The sample was centrifuged at 12,000 ×g at 4 °C for 10 min and the supernatant used for acyl-CoA analysis using a HPLC-high resolution mass spectrometer (HPLC-HRMS, UltiMate 3000 system, Dionex, Sunnyvale, USA coupled with a Q Exactive Orbitrap mass analyzer, Thermo Fisher, Waltham, USA).
Chromatographic separation was achieved at 25 °C on a SeQuant ZIC-pHILIC column (150 × 2.1 mm, 5 μm; Merck, Darmstadt, Germany) coupled with a 20 mm SeQuant ZIC-pHILIC guard column at a flow rate of 0.2 mL/min. A linear gradient of solvent A (5% acetonitrile, 95% 15 mm NH4HCO3 pH 8.5) and solvent B (acetonitrile) was performed as follows: 0–1 min at 90% B, 1–13 min 90%–30% B, 13–16 min 30% B, 16–17 min 30%–90% B, followed by 8 min of re-equilibration at 90% B. The injection volume was 1 μL and the autosampler was set at 4 °C during the analysis. The Q Exactive mass spectrometer was operated in electrospray ionization (ESI) negative mode. Source parameters were optimized with a spray voltage of 3.2 kV (−). The other parameters were set as follows: capillary temperature, 320 °C; auxiliary gas temperature, 300 °C; sheath gas, 40 Arb; auxiliary gas, 10 Arb; sweep gas, 0 Arb, and the S-lens RF level was set at 50. The Q Exactive detector was operated in full scan mode plus data-dependent MS2 (dd MS2) mode. In the full scan mode, the resolution was set at 70,000. The automatic gain control (AGC) target and maximum injection time (IT) were set at 1 × 106 ion capacity and 100 ms, respectively. In data-dependent MS2 (dd MS2) mode, the resolution was set to 17,500. The AGC target and maximum IT were set at 2 × 105 ion capacity and 50 ms, respectively. The inclusion list was on. All targeted metabolites m/z at [M − H]- were included in the list and prepared to fragment. The scan range was set at m/z 100–1500. The normalized collision energies (NCE) were 20%, 40%, and 60%. The isolation window was set at 1.2 Da. The apex trigger was set at 5–15 s, the loop count was set at 3, and the dynamic exclusion was set at 5 s.
The standard solutions were prepared at six individual calibration concentrations between 0.1 and 5 mg/L for acetyl-CoA and propionyl-CoA; or 0.2–5 mg/L for malonyl-CoA and methylmalonyl-CoA.
2.6. Measurements of malonyl-CoA using an ELISA kit
Cell suspensions of the strains cultured in 10 mL of fermentation medium for 3 d were removed by centrifuging at 4000×g for 10 min. The cells were then re-suspended in 4 mL of ddH2O, and the cell wall was destroyed by ultrasonication, after which the suspensions were centrifuged at 4000×g for 10 min. The concentration of malonyl-CoA in the supernatant was measured using a microbial malonyl-CoA ELISA kit (Shanghai FANKEL Industrial Co., Ltd., Shanghai, China) according to the manufacturer's instructions.
2.7. Detection of residual glucose
The fermentation broth was centrifuged and diluted 10 times using sterile water, and the residual glucose concentration was detected by the enzyme electrode method with a glucose analyzer (SBA-40e, Biology Institute Shandong Academy of Sciences, Shandong, China) based on a standard curve of glucose. The analyzer uses immobilized glucose oxidase to convert glucose into gluconic acid and H2O2, with the electrode detecting the amount of H2O2 produced.
3. Results
3.1. Overexpression of PKS genes using a constitutive promoter in OE3
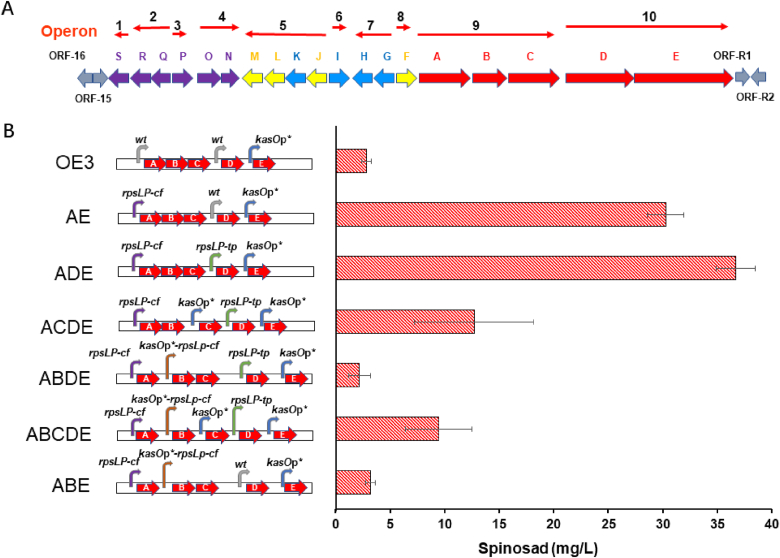
Previous studies have shown that the original BGC of spinosad contains ten operons; the PKS genes are controlled by two promoters, spnA, B and C are co-expressed by one promoter located before spnA, and spnD and E are co-expressed by another promoter located in before spnD (Fig. 2A) [29,30]. In our previous work, the expression of spnE was found to be enhanced by inserting a strong constitutive promoter (kasOp*) between spnD and spnE with the spinosad titer increasing from 686 μg/L to 1460 μg/L [30], showing that the overexpression of PKS significantly contributes to the overproduction of spinosad in heterologous hosts. In our current study, through the rejuvenation of our former engineered strain OE3, the period of fermentation was extended from 5 to 8 d resulting in an increased spinosad titer from 1.46 mg/L to 2.81 mg/L (Fig. S1). Subsequent results show that further extension of the fermentation period did not increase the production of spinosad. Therefore, 8 d fermentation was used for genetically modified strains in this study unless otherwise specified.
Fig. 2.
Metabolic engineering of spinosad PKS promoters to increase the titer of spinosad in S. albus J1074. (A) The spinosad biosynthetic genes are located in different operons. Each red arrow indicates the gene(s) in the same operon. (B) The production of spinosad in different engineered strains overexpressing PKS genes using different constitutive promoters. Each sample was performed in triplicate with the error bars stated as mean ± SD. wt: wild-type (natural) promoter. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Previously, we have quantified the expressing strength of 20 promoters in S. albus [34], three promoters showed similar strength with kasOp* [35], a engineered promoter of kasOp that encodes a SARP family regulator and is an activator of a cryptic type I PKS responsible for coelimycin P1 production in S. coelicolor A3. Two of them are rpsLp promoter from Cellulomonas flavigena and Tsukamurella paurometabola that controlling the expression of the housekeeping gene, 30S ribosomal protein S12, named as rpsLp-cf and rpsLp-tp, respectively [36], the last one is a kasOp* derived promoter (kasOp*-rpsLP-cf) which combing kasOp* promoter, RiboJ insulator and the ribosomal binding site of rpsLp-cf [37]. To investigate the influence of PKS overexpressing on the production of spinosad in heterologous S. albus J1074, we attempt to control the expression of every PKS gene using these four strong constitutive promoters. Replacement of the original promoter of spnABC with the rpsLP-cf promoter in OE3 to construct the strain AE (Fig. S2A) resulted in spinosad titer increasing by ∼11-fold to 30.26 ± 1.69 mg/L compared with OE3 (Fig. 2B). When the original promoter of spnD was replaced with the rpsLP-tp promoter to construct the ADE strain (Fig. S2C), the spinosad titer increased by 21.22% to 36.68 ± 1.81 mg/L compared with AE (Fig. 2B). These results showed that the use of a strong constitutive promoter to overexpress PKS is an efficient engineering strategy to increase spinosad titer.
Although in the ADE strain, five PKS genes were controlled by strong constitutive promoter, spnABC still co-expressed as an operon which controlled by rpsLP-cf promoter. To investigate whether individual expression of each PKS gene using the constitute promoter can further enhance the production of spinosad in heterologous host S. albus J1074, we attempt to express spnB and spnC individually using different strong constitute promoter. When the ADE strain was engineered to insert strong independent constitutive kasOp* and kasOp*-rpsLP-cf promoters before spnC and spnB genes, strain ACDE and ABDE were constructed, respectively (Figs. S2B and E). As a result, the titer of spinosad decreased to 12.71 ± 5.46 mg/L in ACDE and 2.18 ± 1.00 mg/L in ABDE (Fig. 2B). These results suggest that the overexpression of spnB and spnC may be harmful to the overproduction of spinosad when the spnABC operon is destroyed. Since ACDE showed a smaller negative effect on the reduction of spinosad production compared with ABDE, the balanced expression of spnA and spnBC seems is more important than that of spnAB and spnC for spinosad overproduction.
To verify that the disruption of the balanced expression of the spnABC operon leads to a decrease in the production of spinosad in the heterologous strain, two strains (ABCDE and ABE) were constructed (Fig. S2E). When all five PKS genes were individually overexpressed by a strong constitutive promoter (ABCDE), the titer of spinosad decreased from 12.71 ± 5.46 mg/L to 9.43 ± 3.05 mg/L compared to ACDE (Fig. 2B). In the ABE strain, the PKS operon spnBC was co-overexpressed under the control of the kasOp*-rpsLp-cf promoter based on the AE strain. The titer of spinosad in ABE was 3.21 ± 0.45 mg/L compared with 30.26 ± 1.69 mg/L in AE (Fig. 2B). These results reinforce the conclusion that disruption of the spnABC operon is detrimental to spinosad overproduction, while the destruction of the co-expression of spnA and spnBC causes a significant decrease in production. Overall, the results clearly indicated that balanced overexpression of PKS using one strong constitutive promoter to co-overexpress the spnABC operon and two other strong constitutive promoters to individually overexpress spnD and spnE (ADE strain), significantly increased the production of spinosad.
3.2. Overproduction of polyketide precursors to enhance the production of spinosad
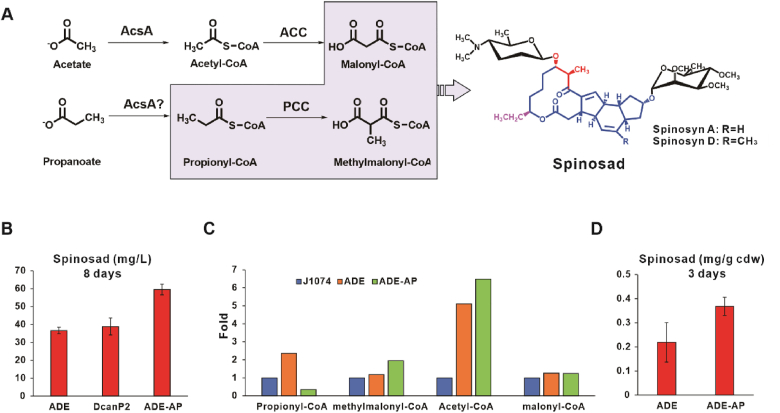
A sufficient supply of polyketide precursors is considered an important factor for the overproduction of spinosad in the engineered strain. The first committed step in polyketide biosynthesis involves the biotin-dependent carboxylation of acyl-CoA and is catalyzed by acyl-CoA carboxylases (ACCases), such as acetyl-CoA carboxylase (ACC) and propionyl-CoA carboxylase (PCC) producing malonyl-CoA, and methylmalonyl-CoA respectively (Fig. 3A) [38]. For spinosad synthesis, the precursors of polyketide include malonyl-CoA, methylmalonyl-CoA, and propionyl-CoA (Fig. 1) and their supply in the host should be considered for optimal production. LC–MS analysis of acyl-CoAs is problematic since acyl-CoAs are structurally complex, easily degraded with observed signal deterioration, and show severe peak tailing with poor detection limits [39,40]. The limit of detection (LoD) of the developed method for detecting acyl-CoAs was evaluated using commercial standards. The LoD was 0.1 mg/L and 0.2 mg/L for malonyl-CoA and methylmalonyl-CoA, and acetyl-CoA and propionyl-CoA respectively (Fig. S3A). Meanwhile, only a small amount of acetyl-CoA, propionyl-CoA, and methylmalonyl-CoA was detected in S. albus J1074 (Fig. S3B). We speculate that the absence of malonyl-CoA was due to its low extraction efficiency, which was previously reported to be <20% [41]. We, therefore, employed the ELISA platform to quantify malonyl-CoA and found its concentration in S. albus J1074 to be 0.155 ± 0.006 ng/g cdw.
Fig. 3.
Overproduction of polyketide precursors to enhance Spinosad production. (A) Biosynthetic pathway for polyketide precursors. AcsA: acetyl-CoA synthetase. PCC: propionyl-CoA carboxylase. ACC: acetyl-CoA carboxylase. (B) Spinosad production in engineered strains. Each sample was performed in triplicate with error bars representing mean ± SD. (C) Fold change in acyl-CoAs in S. albus J1074, ADE, and ADE-AP. All strains were fermented for 3 days, the concentration of all acyl-CoAs was calculated according to the content per gram of cell dry weight (μg/g cdw). The concentration of S. albus J1074 was defined as “1”, and the concentration of ADE and ADE-AP was expressed as their fold change of S. albus J1074. Each sample was performed in triplicate, and the average value was used to calculate the fold change. (D) Spinosad production in ADE and ADE-AP following three-day fermentation. The samples are from the same batch of samples shown in panel C. Each sample was performed in triplicate with the error bars indicating mean ± SD.
Considering that acyl-CoAs serve as the common precursors for nearly all polyketides, knockout of other PKS genes in S. albus J1074 may optimize acyl-CoAs levels required for spinosad biosynthesis. Therefore, based on the ADE strain, candicidin expression, which was previously detected by transcriptomics (unpublished), was disrupted to construct the DcanP2 strain (Fig. S2D). Results revealed that the spinosad titer in DcanP2 was 38.89 ± 4.75 mg/L (Fig. 3B), indicating that disruption of candicidin expression slightly enhances spinosad synthesis. Therefore, to further increase the supply of polyketide precursors, ACC and PCC should be overexpressed to increase the levels of malonyl-CoA and methylmalonyl-CoA, respectively (Fig. 3A). The enzymes of ACC and PCC in S. albus J1074 have not been identified; however, the components and mechanism of these enzymes in S. coelicolor have been determined [42]. In S. coelicolor, ACC and PCC share the same biotinylated subunit, AccA2, while the β and ε subunits are specific to each complex. Acetyl-CoA synthetase (AcsA) synthesizes malonyl-CoA using the substrate acetyl-CoA, and may can also produce propionyl-CoA (Fig. 3A). Accordingly, AcsA, AccA2, the β and ε subunits of ACC (AccBE), and that of PCC (PccBE) should be overexpressed to increase the supply of precursors (malonyl-CoA, methylmalonyl-CoA, and propionyl-CoA) for spinosad. Therefore, acsA, accA2, accBE, and pccBE were inserted into the chromosome of ADE to form ADE-AP (Fig. S2F), the titer of spinosad in ADE-AP was 59.59 ± 3.00 mg/L, which is 62.46% greater than that of ADE (Fig. 3B).
To verify whether the increased spinosad in ADE-AP is related to the polyketide precursor concentration, we quantified the concentration of acyl-CoAs in ADE and ADE-AP. Considering that the extraction efficiency and detector response differed for each acyl-CoA, the concentration of acyl-CoAs in ADE and ADE-AP was measured as their fold changes of S. albus J1074 in 3-day fermentation. This method of relative quantitation revealed higher concentrations in all acyl-CoAs in ADE compared to in S. albus J1074, an effect that was particularly apparent for acetyl-CoA (Fig. 3C). Hence, the expression of spinosad genes appears to impact the carbon metabolism of host. Comparing ADE with ADE-AP, the concentration of propionyl-CoA was 6.8-fold higher in ADE than in ADE-AP, while that of methylmalonyl-CoA in ADE was lower than that in ADE-AP (Fig. 3C), indicating that PCC overexpression resulted in more propionyl-CoA being converted to methylmalonyl-CoA. Additionally, the concentration of acetyl-CoA was 1.3-fold higher in ADE-AP than in ADE (Fig. 3C), indicating that AcsA is functioning efficiently. Although we observed no difference in malonyl-CoA concentration between ADE and ADE-AP (Fig. 3C), the spinosad titer increased from 0.21 ± 0.10 mg/g cdw in ADE to 0.34 ± 0.07 mg/g cdw in ADE-AP (Fig. 3D), indicating that ADE-AP utilized more malonyl-CoA to synthesize spinosad. We, therefore, speculated that the overexpression of ACC increases the abundance of malonyl-CoA available for spinosad synthesis. Collectively, these results indicate that a targeted increase in spinosad precursors leads to efficient overproduction of spinosad.
3.3. The relationship between residual sugar in the medium with spinosad production
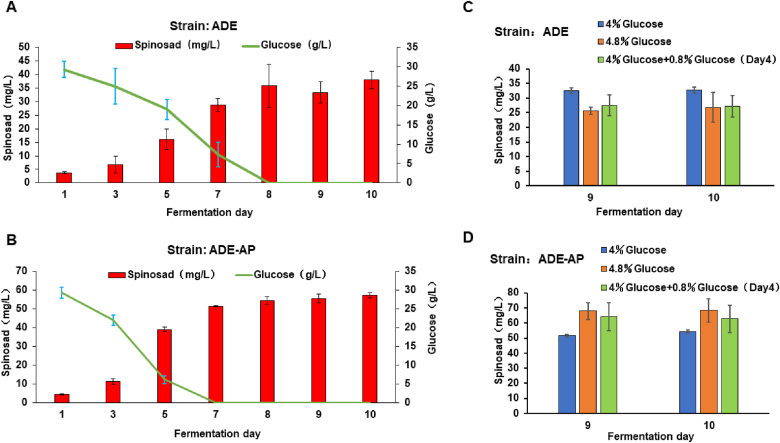
The selection of an efficient supply of carbon atoms as building blocks of polyketides and spinosad synthesis may be closely related to sugar type and concentration, which is the main carbon source in the culture medium. Residual sugar was detected in the samples on days 1, 3, 5, 7, 8, 9, and 10 of fermentation in both ADE and ADE-AP. In the ADE strain, the spinosad titer increased from day 1 to day 8, while the concentration of residual glucose during this period decreased from 29.4 to 0 g/L (Fig. 4A). Meanwhile the residual sugar on the first day was much lower than the original concentration (40 g/L), which may be due to the carbonization of glucose during the sterilization process preventing their reaction with glucose oxidase on the detector. From day 8 to day 10, the production of spinosad stopped increasing (the fluctuating titer of spinosad may be due to evaporation of the culture medium and subsequent calculation errors). Similar results were observed in the ADE-AP strain (Fig. 4B) with increased spinosad production from days 1–7 and decreased residual glucose concentration in the medium from 29.3 to 0 g/L. Hence, with no available glucose in the culture medium, spinosad production did not increase after day 7. These results showed that spinosad production is negatively correlated with the concentration of residual glucose in the culture medium.
Fig. 4.
Investigating the relationship between residual glucose in the medium and spinosad production. (A) Concentration of residual sugar and production of spinosad on days 1, 3, 5, 7, 8, 9, and 10 of fermentation in ADE. (B) Concentration of residual sugar and production of spinosad on days 1, 3, 5, 7, 8, 9, and 10 of fermentation in ADE-AP. (C) Production of spinosad in ADE when extra glucose was added in the culture medium. 4% glucose +0.8% glucose (Day 4) indicates the addition of 0.8% glucose to the fermentation medium after 4 d. (D) Production of spinosad in ADE-AP when extra glucose was added in the culture medium. Each sample was performed in triplicate with the error bars stated as mean ± SD.
The effect of glucose concentration on spinosad titer was measured by transferring the same amount of cells grown in the seed media to culture media containing two different concentrations of glucose (4% and 4.8%). The additional 0.8% glucose was either added to the fermentation medium from the beginning or after 4 d. The spinosad titer decreased in 4.8% glucose media in the ADE strain regardless of when the additional glucose was added (Fig. 4C), while the opposite was observed in the ADE-AP strain (Fig. 4D). A maximum of 68.39 ± 9.20 mg/L was achieved when an additional 0.8% glucose was added to the culture medium of ADE-AP at the beginning of fermentation, which is 25.49% higher than that at 4% glucose. We propose that the difference in performance between the two strains may be due to differing efficiency in the use of glucose. In the ADE strain, additional glucose seems to prevent carbon atoms from flowing toward spinosad synthesis. However, the overexpression of ACC, PCC and AcsA in ADE-AP allows a range of carbon sources to be involved in spinosad synthesis.
3.4. The production of spinosad with different sugars
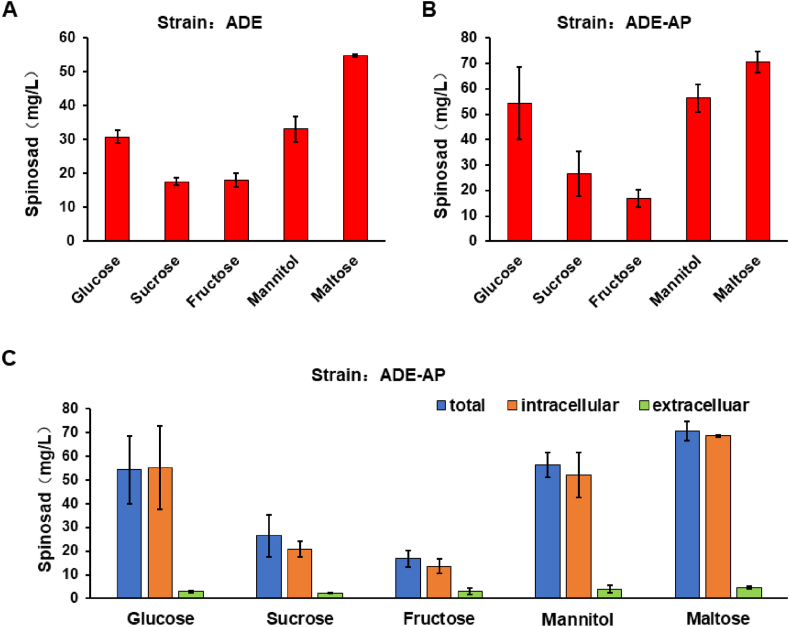
The supplementation of glucose in different engineered strains of S. albus J1074 shows variable titer of spinosad suggesting that it is not a common efficient strategy to increase its production. Four kinds of frequently used sugar sources (sucrose, fructose, mannitol, and maltose) were chosen to replace glucose in the fermentation culture medium to explore whether alternative sugar sources can readily increase the production of spinosad in engineered heterologous strains. The replacement of glucose with sucrose or fructose reduced the production of spinosad in ADE strains, while the replacement of glucose with mannitol slightly increased spinosad titer from 30.88 ± 1.81 mg/L to 33.12 ± 3.74 mg/L (Fig. 5A). Notably, the replacement of glucose with maltose shows a significant increasing from 30.88 ± 1.81 mg/L to 54.96 ± 0.37 (Fig. 5A). These results suggest that maltose is beneficial for increasing the production of spinosad in ADE.
Fig. 5.
The production of spinosad in the culture medium with different sugar. (A) The production of spinosad in the culture medium with five kind of sugar in ADE. (B) The production of spinosad in the culture medium with five kind of sugar in ADE-AP. (C) The intracellular and extracellular production of spinosad in the culture medium with five kind of sugar in ADE-AP. Each sample was performed in triplicate with the error bars stated as mean ± SD.
Similarly, the replacement of glucose with sucrose and fructose in the ADE-AP strain also reduced the production of spinosad from 54.30 ± 14.21 mg/L to 26.52 ± 8.79 mg/L, and 16.84 ± 3.49 mg/L, respectively. Meanwhile the replacement of glucose with mannitol and maltose increased spinosad titer from 54.30 ± 14.21 mg/L to 56.41 ± 5.30 mg/L, and 70.61 ± 3.99 mg/L, respectively (Fig. 5B). These results reinforced that fructose or sucrose are not suitable sugars for production of spinosad, while maltose is beneficial for increasing the production of spinosad in engineered S. albus. The titer of spinosad in ADE-AP fermenting maltose-based culture medium (70.61 ± 3.99 mg/L) is very close to the previous titer (68.39 ± 9.20 mg/L) achieved in culture medium with 4.8% glucose. These results suggest a bottleneck may exist that limits the accumulation of more spinosad in ADE-AP. Nearly all of the spinosad synthesized in ADE-AP was an intracellular product under all culture conditions (the detection of tiny extracellular product may be caused by cell rupture) (Fig. 5C), indicating that the spinosad titer may not continue to increase may due to limited intracellular space or intracellular tolerance to spinosad.
4. Discussion
We systematically investigated the influence of using a constitutive promoter for overexpressing each PKS gene on the titer of spinosad in S. albus J1074. The engineered strain ADE, which uses one strong constitutive promoter to co-overexpress spnABC operon and two other strong constitutive promoters to individually overexpress spnD and spnE significantly increased the titer of spinosad by ∼12-fold. When the supply of polyketide precursor (acyl-CoAs) was increased by overexpressing ACC, PCC, and AcsA, a higher titer of spinosad in the engineered strain ADE-AP than ADE was achieved. Additional glucose or replacement with maltose as the carbon source in the culture medium resulted in the titer of spinosad increasing to ∼70 mg/L, which is the highest titer achieved in heterologous Streptomyces in the literature and an improvement of our previous work [27,29,30]. Based on the original OE3 strain [30], the production of heterologously expressed spinosad in S. albus J1074 was increased by approximately 50-fold.
In this study, the most significant improvement in spinosad production is the use of a strong constitutive promoter that has been characterized in S. albus to control the expression of the spinosad PKS gene. We speculated that this effect was mainly due to the use of biological elements that the heterologous host can recognize to efficiently express heterologous products. In our previous work [30], the efficiency of spinosad production in S. albus J1074 using its original promoter was low due to the incompatibility of the heterologous host and biological elements. This is a universal problem in heterologous expression of natural products, especially for complex molecules such as polyketides. Our successful strategy herein may help follow-up research to solve the incompatibility of the heterologous host and biological elements.
We also observed that unbalanced expression of PKS genes is harmful to spinosad overproduction. Specifically, disrupting the original spnABC operon with one or two strong promoter(s) resulted in decreased spinosad production implying that a finely balanced co-expression of spnA, spnB, and spnC is required. Therefore, we suggest that the original organization of the operon is suitable for expression, unless the expression level of the gene is particularly low, such as spnE in our previous work [30]. In our work, we observed that the concentration of acetyl-CoA is significantly increased in ADE-AP than S. albus J1074, although we overexpressed ACC, the concentration of malonyl-CoA did not significantly increase, implying that the efficiency of ACC was weak. Therefore, selecting an alternative host with a sufficient supply of malonyl-CoA, as well as other acyl-CoAs, may further increase the spinosad titer in future work.
Optimization of the culture medium is an efficient strategy to increase the titer of heterologous production, and our work shows that supplementation or replacement of the carbon source is useful for increasing the production of spinosad. Further optimization of the culture medium was not performed as we speculate the current bottleneck of spinosad heterologous production in S. albus J1074 is due to the limitation of intracellular space or intracellular tolerance. Therefore, the next rational engineering strategy will focus on secreting spinosad through introducing an appropriate transporter, or adding a specific spinosad extractant that has little effect on cell growth during fermentation. Overall, this research provides an efficient strategy to solve the low production of spinosad in heterologous S. albus J1074. This approach may be applied for increasing heterologous production of other natural products.
CRediT authorship contribution statement
Ziheng An: Methodology, Investigation, Writing – original draft. Hui Tao: Methodology, Investigation. Yong Wang: Investigation. Bingqing Xia: Investigation. Yang Zou: Investigation. Shuai Fu: Investigation. Fang Fang: Investigation. Xiao Sun: Investigation. Renqiong Huang: Investigation. Yao Xia: Investigation. Zixin Deng: Conceptualization, Supervision. Ran Liu: Conceptualization, Investigation, Writing – review & editing, Supervision. Tiangang Liu: Conceptualization, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Wuhan University have applied patents based on this work.
Acknowledgements
This work was supported by the National Key R&D Program of China [grant number 2018YFA0900400], the National Natural Science Foundation of China [grant number 31670090], and J1 Biotech Co., Ltd.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.synbio.2021.09.008.
Contributor Information
Ran Liu, Email: ran.liu@siat.ac.cn.
Tiangang Liu, Email: liutg@whu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mertz F.P., Yao R.C. Saccharopolyspora spinosa sp. nov. isolated from soil collected in a sugar mill rum still. Int J Syst Bacteriol. 1990;40(1):34–39. doi: 10.1099/00207713-40-1-34. [DOI] [PubMed] [Google Scholar]
- 2.Huang K.X., Xia L., Zhang Y., Ding X., Zahn J.A. Recent advances in the biochemistry of spinosyns. Appl Microbiol Biotechnol. 2009;82(1):13–23. doi: 10.1007/s00253-008-1784-8. [DOI] [PubMed] [Google Scholar]
- 3.Sparks T.C., Crouse G.D., Dripps J.E., Anzeveno P., Martynow J., Deamicis C.V. Neural network-based QSAR and insecticide discovery: spinetoram. J Comput Aided Mol Des. 2008;22(6–7):393–401. doi: 10.1007/s10822-008-9205-8. [DOI] [PubMed] [Google Scholar]
- 4.Raymond-Delpech V., Matsuda K., Sattelle B.M., Rauh J.J., Sattelle D.B. Ion channels: molecular targets of neuroactive insecticides. Invertebr Neurosci. 2005;5(3–4):119–133. doi: 10.1007/s10158-005-0004-9. [DOI] [PubMed] [Google Scholar]
- 5.Millar N.S., Denholm I. Saccharopolyspora spinosa sp. nov. isolated from soil collected in a sugar mill rum still nicotinic acetylcholine receptors: targets for commercially important insecticides. Invertebr Neurosci. 2007;7(1):53–66. doi: 10.1007/s10158-006-0040-0. [DOI] [PubMed] [Google Scholar]
- 6.Salgado V.L., Sheets J.J., Watson G.B., Schmidt A.L. Studies on the mode of action of spinosad: the internal effective concentration and the concentration dependence of neural excitation. Pestic Biochem Physiol. 1998;60(2):103–110. doi: 10.1006/pest.1998.2333. [DOI] [Google Scholar]
- 7.Kim H.J., Ruszczycky M.W., Choi S.H., Liu Y.N., Liu H.W. Enzyme-catalysed [4+2] cycloaddition is a key step in the biosynthesis of spinosyn A. Nature. 2011;473(7345):109–112. doi: 10.1038/nature09981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldron C., Matsushima P., Rosteck P.R., Jr., Broughton M.C., Turner J., Madduri K. Cloning and analysis of the spinosad biosynthetic gene cluster of Saccharopolyspora spinosa. Chem Biol. 2001;8(5):487–499. doi: 10.1016/s1074-5521(01)00029-1. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y.L., Chen Y.H., Lin Y.C., Tsai K.C., Chiu H.T. Functional characterization and substrate specificity of spinosyn rhamnosyltransferase by in vitro reconstitution of spinosyn biosynthetic enzymes. J Biol Chem. 2009;284(11):7352–7363. doi: 10.1074/jbc.M808441200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H.J., White-Phillip J.A., Ogasawara Y., Shin N., Isiorho E.A., Liu H.W. Biosynthesis of spinosyn in Saccharopolyspora spinosa: synthesis of permethylated rhamnose and characterization of the functions of SpnH, SpnI, and SpnK. J Am Chem Soc. 2010;132(9):2901–2903. doi: 10.1021/ja910223x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong L., Zhao Z., Liu H.W. Characterization of SpnQ from the spinosyn biosynthetic pathway of Saccharopolyspora spinosa: mechanistic and evolutionary implications for C-3 deoxygenation in deoxysugar biosynthesis. J Am Chem Soc. 2006;128(44):14262–14263. doi: 10.1021/ja0649670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Z., Hong L., Liu H.W. Characterization of protein encoded by spnR from the spinosyn gene cluster of Saccharopolyspora spinosa: mechanistic implications for forosamine biosynthesis. J Am Chem Soc. 2005;127(21):7692–7693. doi: 10.1021/ja042702k. [DOI] [PubMed] [Google Scholar]
- 13.Hong L., Zhao Z., Melancon C.E., 3rd, Zhang H., Liu H.W. In vitro characterization of the enzymes involved in TDP-D-forosamine biosynthesis in the spinosyn pathway of Saccharopolyspora spinosa. J Am Chem Soc. 2008;130(14):4954–4967. doi: 10.1021/ja0771383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isiorho E.A., Jeon B.S., Kim N.H., Liu H.W., Keatinge-Clay A.T. Structural studies of the spinosyn forosaminyltransferase. SpnP. Biochemistry. 2014;53(26):4292–4301. doi: 10.1021/bi5003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Y., Lu W., Wen J. Improvement of Saccharopolyspora spinosa and the kinetic analysis for spinosad production. Appl Biochem Biotechnol. 2009;152(3):440–448. doi: 10.1007/s12010-008-8281-5. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Xue C., Zhao F., Li D., Yin J., Zhang C. Suitable extracellular oxidoreduction potential inhibit rex regulation and effect central carbon and energy metabolism in Saccharopolyspora spinosa. Microb Cell Factories. 2014;13:98. doi: 10.1186/s12934-014-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan Z., Zhao C., Guo W., Guan X., Zhang X. Optimization of culture medium for maximal production of spinosad using an artificial neural network - genetic algorithm modeling. J Mol Microbiol Biotechnol. 2015;25(4):253–261. doi: 10.1159/000381312. [DOI] [PubMed] [Google Scholar]
- 18.Zhao F., Zhang C., Yin J., Shen Y., Lu W. Coupling of spinosad fermentation and separation process via two-step macroporous resin adsorption method. Appl Biochem Biotechnol. 2015;176(8):2144–2156. doi: 10.1007/s12010-015-1704-1. [DOI] [PubMed] [Google Scholar]
- 19.Bai Y., Zhou P.P., Fan P., Zhu Y.M., Tong Y., Wang H.B. Four-stage dissolved oxygen strategy based on multi-scale analysis for improving spinosad yield by Saccharopolyspora spinosa ATCC49460. Microb Biotechnol. 2015;8(3):561–568. doi: 10.1111/1751-7915.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C., Zhang X., Chen Z., Wen Y., Song Y. Strain construction for enhanced production of spinosad via intergeneric protoplast fusion. Can J Microbiol. 2009;55(9):1070–1075. doi: 10.1139/w09-064. [DOI] [PubMed] [Google Scholar]
- 21.Jin Z.H., Xu B., Lin S.Z., Jin Q.C., Cen P.L. Enhanced production of spinosad in Saccharopolyspora spinosa by genome shuffling. Appl Biochem Biotechnol. 2009;159(3):655–663. doi: 10.1007/s12010-008-8500-0. [DOI] [PubMed] [Google Scholar]
- 22.Luo Y., Kou X., Ding X., Hu S., Tang Y., Li W. Promotion of spinosad biosynthesis by chromosomal integration of the Vitreoscilla hemoglobin gene in Saccharopolyspora spinosa. Sci China Life Sci. 2012;55(2):172–180. doi: 10.1007/s11427-012-4276-0. [DOI] [PubMed] [Google Scholar]
- 23.Pan H.X., Li J.A., He N.J., Chen J.Y., Zhou Y.M., Shao L. Improvement of spinosad production by overexpression of gtt and gdh controlled by promoter PermE* in Saccharopolyspora spinosa SIPI-A2090. Biotechnol Lett. 2011;33(4):733–739. doi: 10.1007/s10529-010-0481-8. [DOI] [PubMed] [Google Scholar]
- 24.Jha A.K., Pokhrel A.R., Chaudhary A.K., Park S.W., Cho W.J., Sohng J.K. Metabolic engineering of rational screened Saccharopolyspora spinosa for the enhancement of spinosyns A and D production. Mol Cell. 2014;37(10):727–733. doi: 10.14348/molcells.2014.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X., Zhang C., Wang M., Lu W. Genome-scale metabolic network reconstruction of Saccharopolyspora spinosa for spinosad production improvement. Microb Cell Factories. 2014;13(1):41. doi: 10.1186/1475-2859-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J., Long Q., Zhao Z., Chen L., He W., Hong J. Engineering the erythromycin-producing strain Saccharopolyspora erythraea HOE107 for the heterologous production of polyketide antibiotics. Front Microbiol. 2020;11:593217. doi: 10.3389/fmicb.2020.593217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao C., Huang Y., Guo C., Yang B., Zhang Y., Lan Z. Heterologous expression of spinosyn biosynthetic gene cluster in Streptomyces species is dependent on the expression of rhamnose biosynthesis genes. J Mol Microbiol Biotechnol. 2017;27(3):190–198. doi: 10.1159/000477543. [DOI] [PubMed] [Google Scholar]
- 28.Huang J., Yu Z., Li M.H., Wang J.D., Bai H., Zhou J. High level of spinosad production in the heterologous host Saccharopolyspora erythraea. Appl Environ Microbiol. 2016;82(18):5603–5611. doi: 10.1128/AEM.00618-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song C., Luan J., Cui Q., Duan Q., Li Z., Gao Y. Enhanced heterologous spinosad production from a 79-kb synthetic multioperon assembly. ACS Synth Biol. 2019;8(1):137–147. doi: 10.1021/acssynbio.8b00402. [DOI] [PubMed] [Google Scholar]
- 30.Tan G.Y., Deng K., Liu X., Tao H., Chang Y., Chen J. Heterologous biosynthesis of spinosad: an omics-guided large polyketide synthase gene cluster reconstitution in Streptomyces. ACS Synth Biol. 2017;6(6):995–1005. doi: 10.1021/acssynbio.6b00330. [DOI] [PubMed] [Google Scholar]
- 31.Liu R., Deng Z., Liu T. Streptomyces species: ideal chassis for natural product discovery and overproduction. Metab Eng. 2018;50:74–84. doi: 10.1016/j.ymben.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 32.He Y., Wang Z., Bai L., Liang J., Zhou X., Deng Z. Two pHZ1358-derivative vectors for efficient gene knockout in Streptomyces. J Microbiol Biotechnol. 2010;20(4):678–682. doi: 10.4014/jmb.0910.10031. [DOI] [PubMed] [Google Scholar]
- 33.Shao Z., Zhao H. Manipulating natural product biosynthetic pathways via DNA assembler. Curr Protoc Chem Biol. 2014;6(2):65–100. doi: 10.1002/9780470559277.ch130191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q., Xiao L., Zhou Y., Deng K., Tan G., Han Y. Development of Streptomyces sp. FR-008 as an emerging chassis. Synth Syst Biotechnol. 2016;1(3):207–214. doi: 10.1016/j.synbio.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W., Li X., Wang J., Xiang S., Feng X., Yang K. An engineered strong promoter for Streptomycetes. Appl Environ Microbiol. 2013;79(14):4484–4492. doi: 10.1128/AEM.00985-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao Z., Rao G., Li C., Abil Z., Luo Y., Zhao H. Refactoring the silent spectinabilin gene cluster using a plug-and-play scaffold. ACS Synth Biol. 2013;2(11):662–669. doi: 10.1021/sb400058n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai C., Zhang Y., Zhao X., Hu Y., Xiang S., Miao J. Exploiting a precise design of universal synthetic modular regulatory elements to unlock the microbial natural products in Streptomyces. Proc Natl Acad Sci U S A. 2015;112(39):12181–12186. doi: 10.1073/pnas.1511027112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynen F. New experiments of biotin enzymes. CRC Crit Rev Biochem. 1979;7(2):103–119. doi: 10.3109/10409237909105428. [DOI] [PubMed] [Google Scholar]
- 39.Abranko L., Williamson G., Gardner S., Kerimi A. Comprehensive quantitative analysis of fatty-acyl-Coenzyme A species in biological samples by ultra-high performance liquid chromatography-tandem mass spectrometry harmonizing hydrophilic interaction and reversed phase chromatography. J Chromatogr A. 2018;1534:111–122. doi: 10.1016/j.chroma.2017.12.052. [DOI] [PubMed] [Google Scholar]
- 40.Zimmermann M., Thormann V., Sauer U., Zamboni N. Nontargeted profiling of coenzyme A thioesters in biological samples by tandem mass spectrometry. Anal Chem. 2013;85(17):8284–8290. doi: 10.1021/ac401555n. [DOI] [PubMed] [Google Scholar]
- 41.Perera M.A., Choi S.Y., Wurtele E.S., Nikolau B.J. Quantitative analysis of short-chain acyl-coenzymeAs in plant tissues by LC-MS-MS electrospray ionization method. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(5–6):482–488. doi: 10.1016/j.jchromb.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 42.Diacovich L., Peiru S., Kurth D., Rodriguez E., Podesta F., Khosla C. Kinetic and structural analysis of a new group of Acyl-CoA carboxylases found in Streptomyces coelicolor A3(2) J Biol Chem. 2002;277(34):31228–31236. doi: 10.1074/jbc.M203263200. [DOI] [PubMed] [Google Scholar]
- 43.McDaniel R., Ebert-Khosla S., Hopwood D.A., Khosla C. Engineered biosynthesis of novel polyketides. Science. 1993;262(5139):1546–1550. doi: 10.1126/science.8248802. [DOI] [PubMed] [Google Scholar]
- 44.Flett F., Mersinias V., Smith C.P. High efficiency intergeneric conjugal transfer of plasmid DNA from Escherichia coli to methyl DNA-restricting streptomycetes. FEMS Microbiol Lett. 1997;155(2):223–229. doi: 10.1111/j.1574-6968.1997.tb13882.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.