Abstract
JAK/STAT plays an important role in cytokine signal transduction and it is potentially involved in the proinflammatory response during the early phase of severe acute pancreatitis (SAP). However, whether JAK2 activity is upregulated and whether JAK2 inhibition plays a role in the maintenance of pancreatic homeostasis during SAP is incompletely understood. Here we show that JAK2/STAT3 activity is highly elevated in SAP and blockade of JAK2 by AG-490 protects against SAP-induced pancreatic inflammation and injury. Gene expression and ELISA studies showed that JAK2 inhibition altered the cytokine profiles in both the circulation and pancreases. Further analysis revealed that JAK2 inhibition restored the level of cytokines critical for macrophage polarization towards M2 macrophage. Our findings suggest that pharmacological targeting at JAK2/STAT signalling may be an effective choice of therapeutic interventions against SAP.
Keywords: JAK-STAT, Pancreatitis, Immunomodulation, sTREM-1
Highlights
-
•
JAK2/STAT3 pathway is activated in rat model of severe acute pancreatitis (SAP).
-
•
Inhibition of JAK2 protects against SAP-induced damage and inflammation of pancreas.
-
•
JAK2 inhibition modulates cytokine profile in rats.
-
•
JAK2 inhibition restored the level of cytokines critical for M2 macrophage polarization.
1. Introduction
Severe acute pancreatitis (SAP) is a life-threatening disorder that induces a signalling cascade leading to the rapid amplification of the proinflammatory response at early phase [[1], [2], [3]]. If inflammation remains severe and uncontrolled, SAP eventually results in a state of uncontrolled tissue inflammation to cause failure of multiple organs such as the lung [2,3]. Therefore, it is imperative to identify key signaling targets to render the inflammation under control during the early occurrence and development of SAP.
Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is a critical cascade transducing biological signaling downstream of Type I and Type II cytokine receptors [4,5]. JAK/STAT pathway therefore plays an important role in the regulation of the expression of pro-inflammatory genes in a variety of cell types [4]. Inhibitors of JAKs had been developed to block the proinflammatory action of multiple cytokines to treat inflammatory and immune-related diseases including rheumatoid arthritis and inflammatory bowel disease [6,7]. Previous studies on pancreatic STAT3 reported that STAT3 has different roles in chronic and acute pancreatitis (CP and AP) in mice, with protective effect against CP but detrimental role in local and pulmonary inflammation during AP [8,9]. However, currently it is poorly understood whether JAK inhibition can benefit pancreatic homeostasis during SAP. In addition, the direct in vivo evidences showing the activation of JAK/STAT signaling in pancreas during SAP are still lacking.
In the present study, we focused to investigate the effect of pharmacological inhibition of JAK2 signalling on pancreatic homeostasis in a rat model of SAP. We aimed to uncover whether JAK2 inhibition modulates immune response in SAP by determination of key immunoregulatory factors in both peripheral blood mononuclear cells and pancreases. Our results showed that inhibition of activated JAK2 signaling in SAP protected against SAP-induced inflammation and pancreatic tissue damage.
2. Materials and methods
2.1. Animals, chemicals and antibodies
All rats were raised in the laboratory fed with standard diet and have free access to water. All animal experiments complied with the ARRIVE guidelines [10], and were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals. The experiment was approved by the ethical committee of animal research of Bengbu Medical College (NO. 2020-206). The rats were fasted for 12 h before surgery and 30 male Sprague Dawley rats (4-week old, provided by Cavens Animal Laboratory Center, Changzhou, Jiangsu) were randomly divided into three groups: Control (n = 8), Severe Acute Pancreatitis (SAP, n = 11) and SAP plus AG-490 treatment (n = 11). The rats were anesthetized with intraperitoneal injection of 7% chloral hydrate (0.5 mL/kg). AG-490 (8 mg/kg, JAK2 inhibitor, MCE), was dissolved in DMSO and 20% SBE-β-CD (for increasing solubility), then mixed well in physiological saline solution. Phospho-JAK2 (Tyr1007/1008) antibody (1: 1000, Cell Signalling Technology, #3776), JAK2 Polyclonal antibody (1:500, Proteintech, 17670-1-AP), Phospho-STAT3 (Ser 727) antibody (1:1000, HUABIO, ET1607-39), STAT3 antibody (1:1000, Proteintech, 10253-2-AP), GAPDH antibody (1:20000, Proteintech, 60004-1-Ig).
2.2. Animal model of experimental SAP
Briefly, the rat abdomen was disinfected with iodophor and shaved before the surgery. For the sham group, 1.5 cm midline incision was created in the abdomen, and the duodenum and pancreas were turned for several times. For the SAP group and treatment group, expose the pancreaticobiliary duct to the duodenum, and pierce the tip of a 5-gauge needle into the duodenal intestinal cavity and enter the pancreaticobiliary duct by opening about 0.5 cm. Meanwhile, close the pancreaticobiliary duct with arteriole forceps near the hepatic portal to prevent the pancreatic duct injection from returning to the liver. After completion, inject freshly prepared 5% sodium taurocholate (1.5 ml/kg). Clamp the pancreatic duct near the nipple with arteriole clamp for about 5 min. When there is visible edema and hemorrhage in the pancreas, the arterial clamp is removed, and the serosal layer of the duodenal puncture is repaired. After confirming the correct position of the celiac artery catheter, the incision is sutured and bandaged. 6 h post injection of sodium taurocholate, JAK inhibitor (8 mg/kg) was injected into the abdominal cavity.
2.3. Isolation of peripheral blood mononuclear cells (PBMCs)
At the end of treatment, the blood collected during terminal exsanguination from two rats from same group were pooled together to reach 10 ml and transferred into a 50 ml centrifuge tubes and diluted with PBS (1:1). Next, gently add the diluted peripheral blood to the upper layer of the ficoll pre-added to 15 ml centrifuge tubes. After centrifuge at 2200 rpm for 30 min, The white layer where PBMC is located is pipetted to clean 15 ml centrifuge tubes for further centrifuge at 1500 rpm for 10 min. Discard the supernatant and keep the cell pellet at −80°C until RNA isolation.
2.4. Histological examination
Pancreatic tissue underwent routine fixation, embedding and sectioning before proceeding to Hematoxylin & Eosin (HE) staining. For pancreatic pathology score, Kusske, Grewall and Schimidt standards were adopted to evaluate the scoring standards [[11], [12], [13]]. 10 high-powered fields were randomly selected from each slice, and the pathological score of each field was the total score of each condition (including Edema, Inflammatory cell infiltration, Bleeding and Necrosis, the score ranges from 0 to 4 depending on the severity), and the histopathological score of each slice is the average of the combined scores from 10 fields.
2.5. Western blotting
Pancreatic tissues were homogenized in RIPA lysis buffer consisting of RIPA solution, PMSF and phosSTOP phosphatase inhibitors (Roche life Sciences, 04906845001). The proteins were assayed (BCA assay) before loading to 10% acrylamine SDS gel for separation (30 μg total protein), and then transferred to PVDF membrane (Millipore, HATF00010). Before incubation in primary antibodies at 4 °C overnight, the membranes were blocked with 5% skimmed milk powder dissolved in 1 × TBST buffer at room temperature for 2 h. Next day, the membranes were washed in 1 × TBST for 3 times and subsequently incubated with secondary antibodies (1: 5000) conjugated with horseradish peroxidase (Bio-Rad, USA) for 1 h at 37 °C. The PVDF membranes were washed again in 1 × TBST for 3 times before development with enhanced chemiluminescence detected using the ChemiScope 5300 Pro system.
2.6. Real-time PCR
Samples from PBMCs and pancreatic tissues were homogenized and efficiently lysed in the Trizol reagent (Invitrogen) before the isolation of total RNA according to the manufacturer’s instruction. 1 μg total RNA was reversely transcribed using RevertAid First Strand cDNA Reverse Transcription Kit (Thermo Scientific #K1622). After mixing the cDNA template and primer with SYBR Green reagent (Thermo Scientific #F415XL), the quantitative PCR reaction was performed on the ABI-7500 real-time PCR system (Applied Biosystems) with Gapdh as the house-keeping control. Relative gene expression level was calculated using the comparative CT method. Please refer to Table 1 for the DNA sequences of primers.
Table 1.
Real-time PCR Primer sequences.
| Primer | Sequence(5'-3') | length |
|---|---|---|
| Foxp3-F | TGTGGCCTCAGTGGACAAG | 118 bp |
| Foxp3-R | TCTCCGCACAGCAAACAAG | |
| Ifng-F | AAAGGACGGTAACACGAAA | 213 bp |
| Ifng-R | TTGTGCTGGATCTGTGGGT | 114 bp |
| Il6-F | CACTTCACAAGTCGGAGGCT | 167 bp |
| Il6-R | TCTGACAGTGCATCATCGCT | |
| Tgfb1-F | AGGGCTACCATGCCAACTTC | 168 bp |
| Tgfb1-R | CCACGTAGTAGACGATGGGC | |
| Trem1-F | AGGAAGGCTTGGCAGAGGC | 215 bp |
| Trem1-R | ACAGGGTCGTTCGGAGGAT | |
| Il10-F | TAAGGGTTACTTGGGTTGC | 104 bp |
| Il10-R | AATGCTCCTTGATTTCTGG | |
| Tnfa-F | CCACCACGCTCTTCTGTCT | 148 bp |
| Tnfa-R | GCTACGGGCTTGTCACTCG | |
| Gapdh-F | ACGACCCCTTCATTGACCTC | 185 bp |
| Gapdh-R | GACATACTCAGCACCAGCAT |
2.7. Enzyme-linked immunosorbent assay (ELISA)
The blood samples from rats were centrifuged at 3000 rpm for 10 min before serum collection. Rat IL-10, TGF-β and sTREM-1 ELISA Kits were used to determine the circulating level of IL-10, TGF-β and sTREM-1 using a microplate reader (Thermo MK3). To determine the level of IL-10, TNF-α and sTREM-1 in pancreas, normal saline was added to the tissue to mash it. Supernatant was collected for ELISA assay using Kits after centrifuge at 3000 rpm for 10 min.
2.8. Statistical analysis
All the results represent mean ± SD. GraphPad Prism (Version 6.0) was used for statistical analysis. The relative expression of genes and proteins was normalized to GAPDH. Prior to statistical difference test, normality and equal variance tests were performed. The data in this study exhibit normal distribution and differences were determined by unpaired two-tailed student’s t-test. The p value < 0.05 was considered as statistically different.
3. Results
3.1. Activation of JAK-STAT pathway in the experimental rat model of SAP
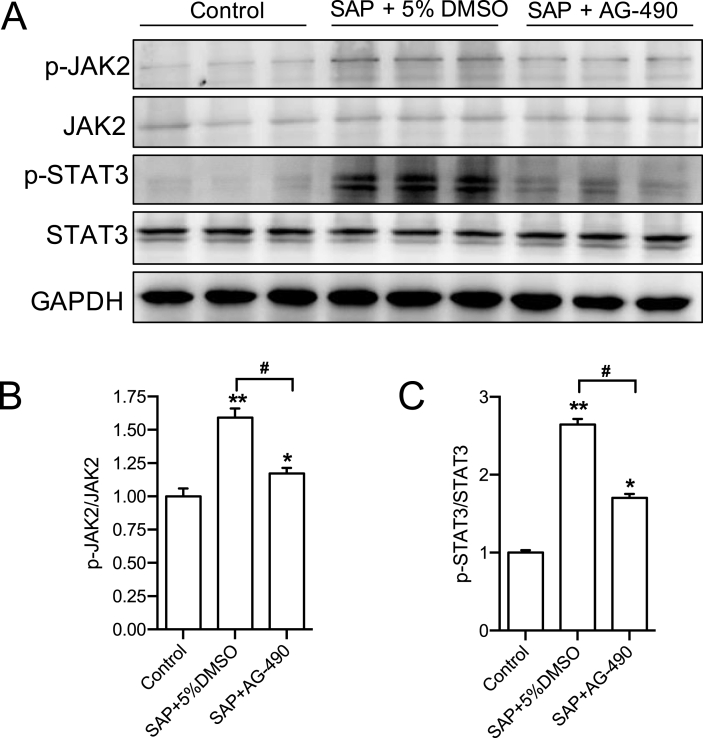
To show the direct evidence supporting upregulated JAK2 activity during SAP, we first determined the phosphorylation level of JAK2/STAT3 in pancreatic tissues. Western blotting results showed that, compared with the control group, while the total expression of JAK2 and STAT3 was not changed, the levels of p-JAK2 and p-STAT3 were significantly increased in the pancreas of the SAP model (Fig. 1 A and B). Inhibition of JAK2 by its inhibitor AG-490 significantly reduced the elevated ratio of p-JAK2/JAK2 and p-STAT3/STAT3 (Fig. 1 A and B). These results demonstrate that JAK2-STAT3 pathway is activated during SAP and suggest that JAK2 may mediate the pathological alteration of pancreas.
Fig. 1.
The expression and activity of JAK2 and STAT3 in pancreatic tissues of SAP rats. (A) The protein level of p-JAK2, JAK2, p-STAT3, STAT3 and GAPDH in rat pancreases were determined by western blotting. (B) The quantitative analysis result of p-JAK2/JAK2 ratio. n = 8–11. (C) The quantitative analysis result of p-STAT3/STAT3 ratio. n = 8–11, all data are expressed as mean ± SD. *P < 0.05, **P < 0.01 vs Control. #P < 0.05 vs SAP+5% DMSO, unpaired two-tailed t-test. SAP: severe acute pancreatitis.
3.2. Inhibition of JAK2 protects against pancreatic damage and reduces inflammation
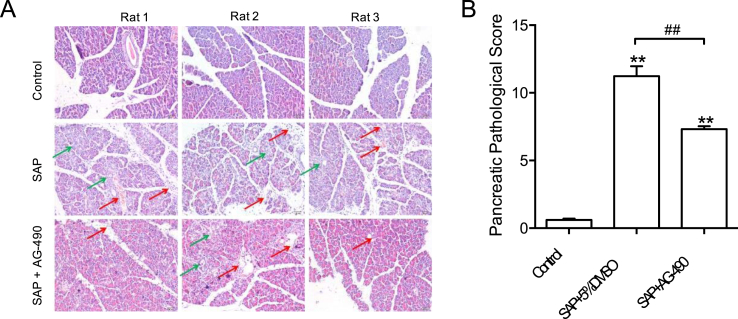
To examine whether inhibition of JAK2 protects against SAP-induced damage and inflammation of pancreases, H&E staining of pancreatic sections was performed to assess pancreatic pathology. The pathological analysis of the pancreas of the rats in each group showed that the rats in the sham operation group had neither edema in the pancreatic lobules nor leukocyte infiltration, bleeding, necrosis, or rupture (Fig. 2A). In the SAP model group, the pancreatic lobular space was widened; edema, bleeding and necrosis occurred in the acinar cells. In addition, the integrity of the acinar was damaged, and the acinar fluid overflowed (Fig. 2A). The JAK2 inhibitor AG-490 suppressed the infiltration of proinflammatory cells, lobular edema, bleeding, and necrosis (Fig. 2A). The overall pathological score of pancreases was also significantly reduced by AG-490 treatment (Fig. 2B). These observations support the notion that JAK2 inhibition provides beneficial effects to pancreas in SAP.
Fig. 2.
JAK2 inhibition protects against SAP-induced pancreatic damage and inflammation. (A) Representative H&E staining photos of pancreases showing JAK2 inhibitor AG-490 reduced tissue edema (Green arrows) and inflammation (Red arrows). (B) Summarized pancreatic histological score showing the beneficial effect of AG-490 on pancreatic homeostasis. n = 8–11, all data are expressed as mean ± SD. **P < 0.01 vs Control. ##P < 0.01 vs SAP+5% DMSO, unpaired two-tailed t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. JAK2 inhibition suppressed proinflammatory gene expression in PMBCs and pancreas
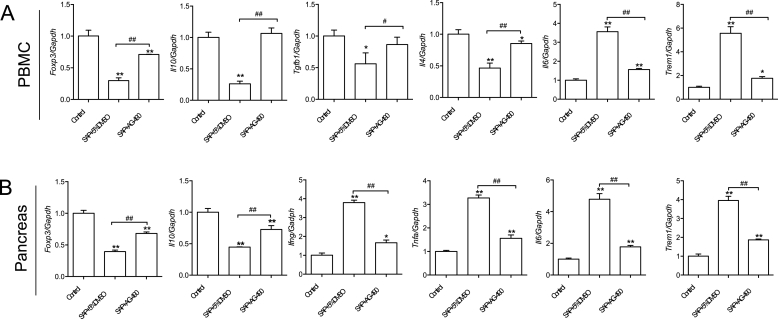
To reveal the effect of AG-490 on pancreatic homeostasis during SAP at the molecular level, the present study focused on the detection of the expression of proinflammatory genes in both PMBC and pancreases. Compared with the control group, the mRNA expression of genes (Foxp3, Il10, Tgfb1 and Il4) involved in regulatory T cell activation and macrophage polarization was significantly reduced in isolated PMBCs from the SAP model group, whereas the mRNA level of Il6 (IL-6) and Trem1 (sTREM-1) was significantly increased (Fig. 3A). After injection of AG-490, the expression of Foxp3, Il10, Tgfb1 and Il4 was significantly increased, reaching a level comparable to those in the control group. Importantly, the expression of proinflammatory genes Il6 and Trem1 was effectively brought down by AG-490 (Fig. 3A) (see Fig. 4).
Fig. 3.
Gene expression profile in PBMCs and pancreases. (A) The qPCR data representing gene expression level of Foxp3, Il10, Tgfb1, Il4, Il6 and Trem1 in isolated PBMCs. (B). The qPCR data representing gene expression level of Foxp3, Il10, Ifng, Tnfa, Il6 and Trem1 in pancreatic tissues. n = 4–5, all data are expressed as mean ± SD. *P < 0.05, **P < 0.01 vs Control. #P < 0.05, ##P < 0.01 vs SAP+5% DMSO, unpaired two-tailed t-test.
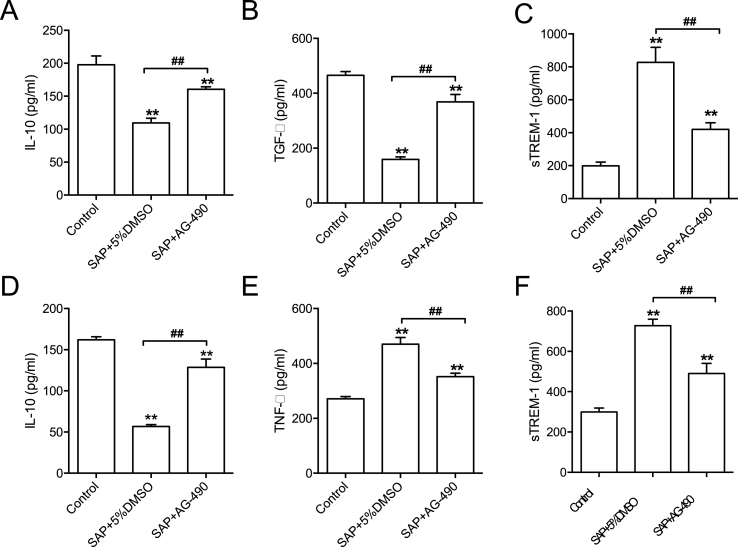
Fig. 4.
Serum and pancreatic ELISA data. (A-C) Serum level of IL-10, TGF-β and sTREM-1. (D-E) Levels of IL-10, TNF-αand sTREM-1 in pancreatic tissues. n = 4–5, all data are expressed as mean ± SD. **P < 0.01 vs Control. ##P < 0.01 vs SAP+5% DMSO, unpaired two-tailed t-test.
Similar to the gene expression pattern seen in PBMCs, in contrast to the elevated expression level of proinflammatory genes (Ifng-encoding IFN-γ, Tnfa, Il6 and Trem1), the level of genes (Foxp3 and Il10) holding anti-inflammatory function was marked reduced in pancreas (Fig. 3B). Strikingly, the administration of AG-490 reversed SAP-induced effects on the expression of aforementioned genes (Fig. 3B).
3.4. JAK2 inhibition balanced the relative levels of proinflammatory and anti-inflammatory cytokines
To further verify the immunomodulatory effect of JAK2 inhibition, we assayed the levels of key cytokines in both the circulation and pancreases using ELISA. Compared with the control group, the content of IL-10 and TGF-β was decreased whereas the content of sTREM-1 was increased. By contrast, injection of AG-490 restored the circulating levels of IL-10 and TGF-β and reduced the amount of sTREM-1 in the blood stream. In the pancreases, TNF-α and sTREM-1 were markedly elevated while the level of IL-10 was downregulated, which was also reversed by AG-490 treatment. Taken together, the profiling of these key cytokines suggests that JAK2 plays a regulatory function in immune response to regulate resolution of inflammatory signaling.
4. Discussion
JAK2, a non-receptor tyrosine kinase downstream of cytokine receptors, regulates expression of a wide range of proinflammatory genes via control of STAT transcription factors [14]. The activity of JAK2 is tightly controlled at multiple levels by cytokines, growth factor and hormones [15,16]. Severe acute pancreatitis (SAP) is an inflammatory disease with elevated level of a myriad of proinflammatory cytokines [17]. Our findings of the anti-inflammatory effect of JAK2 inhibition through modulation of cytokine profile in SAP reveal a new molecular link bridging JAK2 with pancreatic inflammation.
In the present study, we observed that AG-490 at a concentration (8 mg/kg) did not cause signs of toxicity and the AG-490-administered rats appeared improved vitality when compared to the sham treated rats. Previous studies done in mice also reported that administration of AG490 was very well tolerated and treated showed no signs of toxicity and appeared healthier compared to control group [18,19]. These findings suggest the use of AG-490 is generally safe and will not cause significant toxicity in vivo.
We aimed to reveal whether JAK2/STAT3 activity is regulated under SAP. The present results showed that phosphorylated level of both JAK2 and STAT3 was increased in the pancreas during SAP, and such increase was abolished by AG-490 administration. Our in vivo experiments also found that inhibition of JAK2 activity has a beneficial effect on pancreas. These results are consistent with the previous reports on the bad role of hyperactivity of JAK/STAT pathway in other forms of pancreatitis [20,21]. Further studies on the cellular mediator (s), for example - macrophage phenotype, in pancreases for inhibiting JAK2 activity will help uncover any unknown pathophysiological roles of JAK2 in SAP.
We next sought to investigate the potential molecular basis of JAK2 inhibition-mediated anti-inflammatory property. We used qPCR and ELISA approaches to examine the expression of a panel of key factors involved in macrophage polarization. The results indicated that JAK2 may modulate macrophage polarization by repressing the expression of IL-10 and TGF-β, key cytokines for alternatively activated macrophages that participate in the resolution of tissue inflammation and repair [22,23]. We also determined factors and cytokines that serve as the markers of classically activated macrophages [24]. TNF-α and IL-6 are potent proinflammatory cytokines signaling through NF-κB and JAK/STAT pathways [25,26] and sTREM-1 amplifies monocyte-mediated inflammatory responses by stimulation of the release of pro-inflammatory mediators [27,28]. AG-490 treatment abolished the upregulated level of IL-6 and sTREM-1. Interestingly, the promoter regions of IL-6 and sTREM-1 all contain putative binding sites for STATs, indicating a forward regulatory mechanism between JAK/STAT and the target genes. Moreover, the reduced level of Foxp3, the master regulator of regulatory T cells [29], was restored by JAK2 inhibition, suggesting that JAK2 also holds regulatory function for T cells maturation. Altogether, these findings indicate JAK2 is a crucial immunomodulatory kinase in SAP although future mechanistic studies are needed to reveal the detailed mechanisms by which JAK2 regulates the differentiation and activation of critical immune cells.
In conclusion, the present study provides the first line of evidences that inhibition of JAK2 modulates immune and inflammatory responses and preserves pancreatic homeostasis in SAP. The suppressive effect of JAK2 inhibitor on inflammation is highly likely attributed to immunomodulatory function of JAK2. Thus, the presented results suggest that pharmacological targeting at JAK2/STAT signalling hold therapeutic potential against acute pancreatitis.
Data availability statement
Data are available upon reasonable request.
Author statement
Z.Q. and Z.W. performed the majority of the experiments, data analysis, and contributed to manuscript preparation. P.Y. assisted in the animal experiments. Z.B., L.L. provided technical supports and made critical comments on the manuscript. F.C., H.J., F.Z contributed to the study design and the preparation of the manuscript. F.X. supervised the study and provided with materials and contributed to the experimental design and wrote the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by Key Project of Translational Medicine, Bengbu Medical College, Anhui Province (BYTM2019017) and China Primary Health Care Foundation (YLGX-JZ-2020012)
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.101133.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mayer J., Rau B., Gansauge F., Beger H.G. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000;47:546–552. doi: 10.1136/gut.47.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beger H.G., Rau B.M. Severe acute pancreatitis: clinical course and management. World J. Gastroenterol. 2007;13:5043–5051. doi: 10.3748/wjg.v13.i38.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zerem E. Treatment of severe acute pancreatitis and its complications. World J. Gastroenterol. 2014;20:13879–13892. doi: 10.3748/wjg.v20.i38.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villarino A.V., Kanno Y., O'Shea J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017;18:374–384. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schindler C., Levy D.E., Decker T. JAK-STAT signaling: from interferons to cytokines. J. Biol. Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz D.M., Kanno Y., Villarino A., Ward M., Gadina M., O'Shea J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017;17 doi: 10.1038/nrd.2017.267. 78–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Shea J.J., Schwartz D.M., Villarino A.V., Gadina M., McInnes I.B., Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shigekawa M., Hikita H., Kodama T., Shimizu S., Li W., Uemura A. Pancreatic STAT3 protects mice against caerulein-induced pancreatitis via PAP1 induction. Am. J. Pathol. 2012;181:2105–2113. doi: 10.1016/j.ajpath.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H., Neuhöfer P., Song L., Rabe B., Lesina M., Kurkowski M.U. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J. Clin. Invest. 2013;123:1019–1031. doi: 10.1172/JCI64931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilkenny C., Browne W., Cuthill I.C., Emerson M., Altman D.G. NC3Rs Reporting Guidelines Working Group, Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br. J. Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt J., Lewandrowsi K., Warshaw A.L., Compton C.C., Rattner D.W. Morphometric characteristics and homogeneity of a new model of acute pancreatitis in the rat. Int. J. Pancreatol. 1992;12:41–51. doi: 10.1007/BF02927069. [DOI] [PubMed] [Google Scholar]
- 12.Grewal H.P., Mohey el Din A., Gaber L., Kotb M., Gaber A.O. Amelioration of the physiologic and biochemical changes of acute pancreatitis using an anti-TNF-alpha polyclonal antibody. Am. J. Surg. 1994;167 doi: 10.1016/0002-9610(94)90076-0. 214–8– discussion 218–9. [DOI] [PubMed] [Google Scholar]
- 13.Kusske A.M., Rongione A.J., Ashley S.W., McFadden D.W., Reber H.A. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284–288. doi: 10.1016/s0039-6060(96)80299-6. discussion 289. [DOI] [PubMed] [Google Scholar]
- 14.Levine R.L., Pardanani A., Tefferi A., Gilliland D.G. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat. Rev. Canc. 2007;7:673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard S.R. Mechanistic insights into regulation of JAK2 tyrosine kinase. Front. Endocrinol. 2017;8:361. doi: 10.3389/fendo.2017.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seif F., Khoshmirsafa M., Aazami H., Mohsenzadegan M., Sedighi G., Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017;15 doi: 10.1186/s12964-017-0177-y. 23–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malmstrøm M.L., Hansen M.B., Andersen A.M., Ersbøll A.K., Nielsen O.H., Jørgensen L.N. Cytokines and organ failure in acute pancreatitis: inflammatory response in acute pancreatitis. Pancreas. 2012;41:271–277. doi: 10.1097/MPA.0b013e3182240552. [DOI] [PubMed] [Google Scholar]
- 18.Davoodi-Semiromi A., Wasserfall C.H., Xia C.Q., Cooper-DeHoff R.M., Wabitsch M., Clare-Salzler M. The tyrphostin agent AG490 prevents and reverses type 1 diabetes in NOD mice. PloS One. 2012;7 doi: 10.1371/journal.pone.0036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumano K., Nakao A., Nakajima H., Miike S., Kurasawa K., Saito Y. Blockade of JAK2 by tyrphostin AG-490 inhibits antigen-induced eosinophil recruitment into the mouse airways. Biochem. Biophys. Res. Commun. 2000;270:209–214. doi: 10.1006/bbrc.2000.2403. [DOI] [PubMed] [Google Scholar]
- 20.Komar H.M., Serpa G., Kerscher C., Schwoegl E., Mace T.A., Jin M. Inhibition of Jak/STAT signaling reduces the activation of pancreatic stellate cells in vitro and limits caerulein-induced chronic pancreatitis in vivo. Sci. Rep. 2017;7:1787–1810. doi: 10.1038/s41598-017-01973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe T., Kudo M., Strober W. Immunopathogenesis of pancreatitis. Mucosal Immunol. 2017;10:283–298. doi: 10.1038/mi.2016.101. [DOI] [PubMed] [Google Scholar]
- 22.Abdelaziz M.H., Abdelwahab S.F., Wan J., Cai W., Huixuan W., Jianjun C. Alternatively activated macrophages; a double-edged sword in allergic asthma. J. Transl. Med. 2020;18 doi: 10.1186/s12967-020-02251-w. 58–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yunna C., Mengru H., Lei W., Weidong C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020;877:173090. doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- 24.Leopold Wager C.M., Wormley F.L. Classical versus alternative macrophage activation: the Ying and the Yang in host defense against pulmonary fungal infections. Mucosal Immunol. 2014;7:1023–1035. doi: 10.1038/mi.2014.65. [DOI] [PubMed] [Google Scholar]
- 25.Bishehsari F., Sharma A., Stello K., Toth C., O'Connell M.R., Evans A.C. TNF-alpha gene (TNFA) variants increase risk for multi-organ dysfunction syndrome (MODS) in acute pancreatitis. Pancreatology. 2012;12:113–118. doi: 10.1016/j.pan.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright S.W., Lovelace-Macon L., Hantrakun V., Rudd K.E., Teparrukkul P., Kosamo S. sTREM-1 predicts mortality in hospitalized patients with infection in a tropical, middle-income country. BMC Med. 2020;18:159. doi: 10.1186/s12916-020-01627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tammaro A., Derive M., Gibot S., Leemans J.C., Florquin S., Dessing M.C. TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacol. Ther. 2017;177:81–95. doi: 10.1016/j.pharmthera.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 28.Feng C.-W., Chen N.-F., Sung C.-S., Kuo H.-M., Yang S.-N., Chen C.-L. Therapeutic effect of modulating TREM-1 via anti-inflammation and autophagy in Parkinson's disease. Front. Neurosci. 2019;13:769. doi: 10.3389/fnins.2019.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudensky A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.