Abstract
Aims
Guidelines-recommended criteria for identifying severe aortic stenosis (AS) are based on small, homogenous cohorts of patients, leading to potentially inconsistent or missed diagnosis. We used a large cohort of patients with varying degrees of AS to (i) characterize its progression; (ii) evaluate the influence of demographic and echocardiographic variables; and (iii) derive haemodynamically consistent cut-off values.
Methods and results
We identified 916 patients with mild to severe AS who had undergone >1 echocardiographic study (N = 2547). For each study, aortic valve area (AVA), peak transaortic velocity (V max), and mean pressure gradient (ΔP) were extracted. Annual rates of AVA change were determined by a linear mixed-effects model. To determine the prevalence of inconsistent diagnosis of severe AS, AVA was plotted against ΔP and V max, with quadrants defined using guidelines-recommended cut-offs. The rate of AVA change was −0.070 ± 0.003 cm2/year and was more rapid in men than women and in Whites than African Americans. AVA = 1 cm2 corresponded to ΔP = 32 mmHg and V max = 3.7 m/s, causing discrepancies in defining severe AS in 480 (19%) and 458 (18%) studies, respectively. Conversely, ΔP = 40 mmHg corresponded to AVA = 0.89 cm2 and V max = 4.0 m/s corresponded to AVA = 0.92 cm2, confirming the inconsistency of the guidelines. Notably, discrepancy rate was higher in 206 patients with low flow (SVi < 35 mL/m2): 40% vs. 16% in the remaining patients.
Conclusion
Our findings demonstrated gender- and race-related differences in AS progression and underscored the need to refine the multiparametric criteria for diagnosis of severe AS to minimize internal inconsistencies, which are high with the current cut-offs and amplified in patients with low stroke volumes.
Keywords: valvular heart disease, Doppler echocardiography
Introduction
Aortic stenosis (AS) is the most common valvular heart disease in the developed world and its prevalence is growing due to longer life expectancies. Prior studies of the natural history of AS were performed on small, homogenous cohorts of patients and there is limited data on factors affecting the progression of AS. There have been many studies aiming to prospectively ascertain risk factors for more rapid progression, but no conclusive data has been produced.
With the emerging utilization of transcatheter aortic valve (AV) replacement procedures, now with expansion into the low- and moderate-risk population,1–4 the need to accurately assess the severity of AS in order to optimally manage and time interventions is critical. Currently, quantitative assessment of AS is done almost entirely using transthoracic echocardiography (TTE), which also provides information regarding leaflet number, mobility, and degree of calcification. Current guidelines recommend obtaining AS peak jet velocity (V max), mean transvalvular pressure gradient (ΔP), and AV area (AVA) by continuity equation. Severe AS is defined by an AVA <1 cm2, V max ≥4 m/s, or ΔP ≥ 40 mmHg.5 , 6 We, along with others, have observed that these three parameters can at times be haemodynamically inconsistent with an AVA < 1 cm2 corresponding to a V max <4 m/s and a ΔP < 40 mmHg, even in the setting of normal left ventricular (LV) systolic function.7 , 8 For optimal interventional planning, it is important to have internally consistent criteria for grading severe AS, which would avoid ambiguous diagnosis.
This study was designed to use a large historic cohort of patients with varying degrees of AS to: (i) characterize the progression of AS, as reflected by changes in AVA and the influence of demographic factors, including age, gender, and race, as well as baseline echocardiographic variables; and (ii) determine the relationship between V max, ΔP, and AVA, in order to derive with high statistical confidence haemodynamically consistent cut-off values for these parameters that would better define severe AS.
Methods
Patient population
Using diagnostic finding codes, we identified 916 patients from our echocardiographic database, dating from its beginning in 1994 through 2018 with mild to severe AS, who had multiple TTE studies. Patients with prosthetic AVs were excluded. There was no minimum or maximum duration of time set between studies.
Study parameters
Age, gender, and body surface area at the time of the initial study were obtained from the database. AV parameters were extracted from the digital TTE report, including LV outflow tract (LVOT) diameter measured in mid-systole in the parasternal long-axis view. In our standard TTE assessment of AS, the AV and LVOT velocity time integrals (VTIs) are routinely measured offline (Philips Xcelera, Andover, MA, USA) from the apical three- and five-chamber views. It is our laboratory’s standard protocol to align the continuous-wave Doppler of the AV VTI as close to parallel with flow as possible, use the highest V max and the highest AV VTI, from which ΔP is derived. A Pedof transducer was used in the majority of patients. When there was more than one measurement of the LVOT diameter or the LVOT VTI saved in the digital report, the average was used in the calculation of AVA.
In addition, using the extracted ΔP and V max, a predicted AVA (pAVA) was derived from extracted ΔP and V max using the Gorlin equation, assuming a heart rate of 80 bpm, systolic ejection period of 0.33 s, and a cardiac output of 6 L/min.
AS progression
The annual rate of AVA change (ΔAVA) was determined using the linear mixed-effects (LME) model based on repeated measurements of AVA from subsequent echocardiograms of the same patient. These LME models are an extension of linear regression models. Linear regression models can only deal with data wherein all observations are independent, while LME models can handle data where there is dependence, such as our patients’ data where repeated measurements of the same patient are correlated. This is achieved by grouping the observations by patient, and allowing the observations within a group to be correlated, while the means of groups are independent and can be modelled by linear regression. Random variables are factors used to model the observations within a group and can be different for each group. On the other hand, fixed variables are factors used to model the group means and are invariant for all groups. Since LME models contain both random and fixed variables, they are called mixed-effect models.
In our study, to estimate the annual AVA progression rate for each risk factor, we built an LME model consisting of the risk factor and the time interval between the measurement. The fixed effects included the risk factors, the time interval, and their interaction term. The random variables included the time interval and a random intercept in order to account for the differing baseline AVA and progression rates due to individual characteristics that are not explained by the risk factors. The significance of the risk factors upon the progression rate is reflected by the P-value of the interaction term in the fixed effects. Since all the risk factors are categorical, the P-value using Student’s t-test was used to determine statistical significance of the progression rate, compared to the reference level of each risk factor.
To determine whether there were demographic parameters associated with more rapid AS progression, subgroup analysis was performed. Subgroups included: age quantiles (below and above the median value), gender, race (self-identified as listed in the electronic medical records: African American, white, and other), initial AVA (mild >1.5 cm2 , moderate 1.0–1.5 cm2 , and severe <1.0 cm2 as defined by the current valvular guidelines5 , 6), LV systolic function (normal or reduced, as defined by LV ejection fraction cut-off of 50%). Reduced LV systolic function was determined using the diagnostic finding codes of mild, moderate, and severe systolic dysfunction.
Severity discrepancies
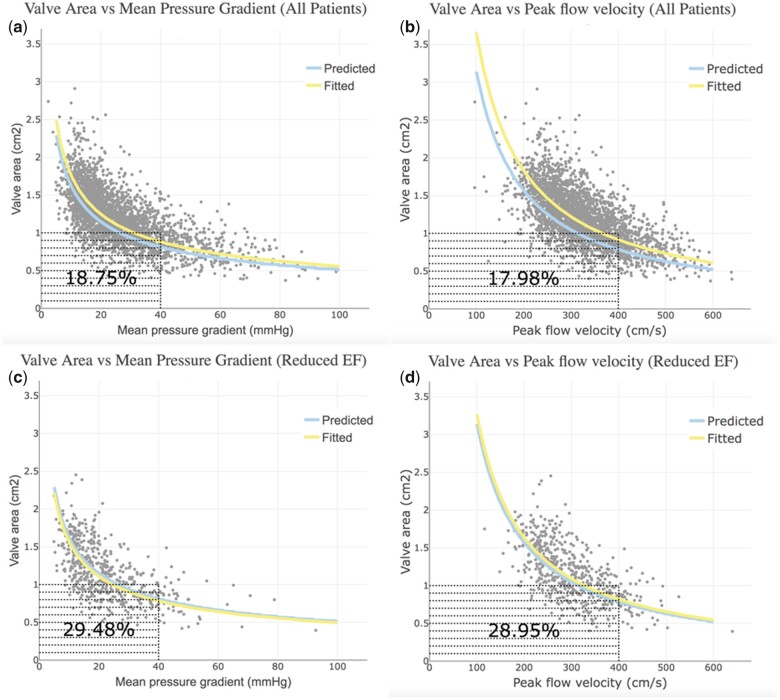
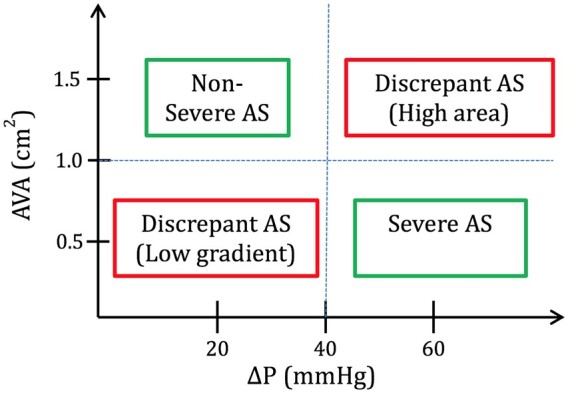
AVA and the pAVA were plotted against both ΔP and V max (Figure 1). TTE studies that had AVA ≥1.0 cm2 and ΔP <40 mmHg were considered as true non-severe AS by both area and haemodynamic criteria (upper left quadrant). Those TTEs with an AVA <1 cm2 and ΔP ≥40 mmHg were considered as true severe AS by both area and haemodynamic criteria (lower right quadrant). The other two quadrants represent discrepancies in the current AS severity partition values. Those in the lower left quadrant have severe AS by AVA but not by ΔP. Those in the upper right quadrant have severe AS by ΔP but not by AVA. Since LV systolic dysfunction has been implicated in ‘low-gradient AS’, these plots were created for the entire cohort and then, separately, for those with LV systolic dysfunction. The same discrepancy analysis was performed by plotting AVA against V max with a cut-off of 4 m/s.
Figure 1.
Aortic valve area plotted against mean pressure gradient defining discrepant classifications.
Results
Baseline characteristics
There were 916 patients with a total of 2547 TTEs. The mean time between two consecutive examinations was 17 ± 14 months. Systolic and diastolic blood pressures were 135 ± 22 and 68 ± 13 mmHg, respectively. The median age at the time of the initial TTE was 75 years (interquartile range 67–82 years) and 56% were female. There were 377 (41%), 262 (29%), and 67 (7%) self-identified as African American, White, and other race designations, respectively, while race data were missing from the electronic medical record in 210 (23%) patients, who were excluded from the race-related analysis. The majority (84%) of the patients had normal LV systolic function on their initial TTE.
Rates of AS progression
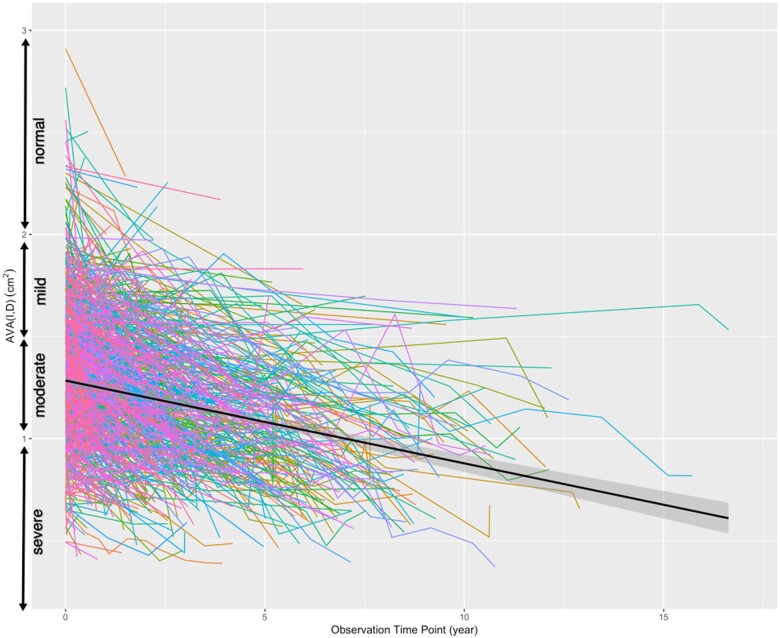
The overall rate of AS progression by change in AVA was −0.070 ± 0.003 cm2/year (Figure 2). Table 1 shows the baseline demographic and echocardiographic parameters of the entire study group, as well as the different subgroups. There was no difference in AVA progression between the two age quantiles. However, AS progression was significantly more rapid in men compared to women. Likewise, there were also racial differences: African American patients had a significantly slower AVA narrowing, compared to White patients. There was an inverse relationship between initial severity of AS and progression rate with the most rapid progression noted in the patients with mild AS on the initial TTE. This is despite the fact that more than half of the patients with mild AS (161/292) never progressed past mild AS, and showed a mean progression rate of −0.0133 ± 0.0057 cm2/year, which was considerably slower than in the entire study group (P = 0.02). Patients with and without systolic dysfunction had similar rates of AS progression.
Figure 2.
‘Spaghetti plot’ of the progression of aortic stenosis. The colour lines show the historical data of the individual patients. The time of the first echocardiographic study is considered as zero time and follow-up studies are plotted accordingly based on the time intervals from the first study. The solid black line is the overall progression fitted by the linear mixed-effects model and the shadowed area represents the 95% confidence interval.
Table 1.
Baseline demographic and echocardiographic parameters: relationship with aortic stenosis progression rate
| Variables | Levels | N | Rate (cm2/year) | P value |
|---|---|---|---|---|
| AVA baseline level | Mild | 292 | −0.106 ± 0.005 | |
| Moderate | 466 | −0.068 ± 0.004 | <0.0001 | |
| Severe | 158 | −0.032 ± 0.009 | <0.0001 | |
| ΔP baseline level | Mild | 506 | −0.076 ± 0.003 | |
| Moderate | 345 | −0.068 ± 0.005 | 0.23 | |
| Severe | 65 | −0.055 ± 0.015 | 0.20 | |
| V max baseline level | Non-severe | 843 | −0.073 ± 0.003 | |
| Severe | 73 | −0.049 ± 0.014 | 0.10 | |
| LV function baseline level | Abnormal | 143 | −0.066 ± 0.005 | |
| Normal | 759 | −0.071 ± 0.003 | 0.43 | |
| Gender | Female | 512 | −0.065 ± 0.004 | |
| Male | 404 | −0.078 ± 0.004 | 0.02 | |
| Age group at baseline (years) | >75 | 468 | −0.074 ± 0.004 | |
| ≤75 | 448 | −0.067 ± 0.004 | 0.21 | |
| Race | African American | 377 | −0.062 ± 0.004 | |
| White | 262 | −0.075 ± 0.005 | 0.03 |
Severity discrepancies
AVA was plotted against ΔP (Figure 3A) and V max (Figure 3B), showing that a substantial number of patients’ AS grades were discrepant because they did not consistently meet the guidelines criteria for severe AS, as they were in the lower left quadrants corresponding to AVA ≤1 cm2 but ΔP <40 mmHg (n = 480, 19%) or V max <4 m/s (n = 458, 18%). For the subgroup of patients with reduced LV ejection fraction (n = 249, 455 studies), the incidence of discrepancy was even higher for ΔP and V max (Figure 3C and D). Based on the curve fits of our data, AVA = 1.0 cm2 corresponded to ΔP = 32 mmHg and V max = 3.7 m/s. Conversely, ΔP = 40 mmHg corresponded to AVA = 0.89 cm2 and V max = 4.0 m/s corresponded to AVA = 0.92 cm2. The predicted Gorlin AVA curve fits based on both ΔP and V max yielded smaller AVAs than those seen clinically (P < 0.001) and confirmed the haemodynamic inconsistency of the guidelines recommended cut-offs.
Figure 3.
Plots of aortic valve area vs mean pressure gradient and aortic valve area vs peak flow velocity. (A and B) The entire patient cohort, while (C and D) Only patients with reduced left ventricle ejection fraction. Each dot indicates a transthoracic study. The blue curve shows the valve area computed using Gorlin equation, while the yellow curve represents the valve area fitted from the data set. The number shown in the left lower quadrant of each panel is the percentage of discrepant studies.
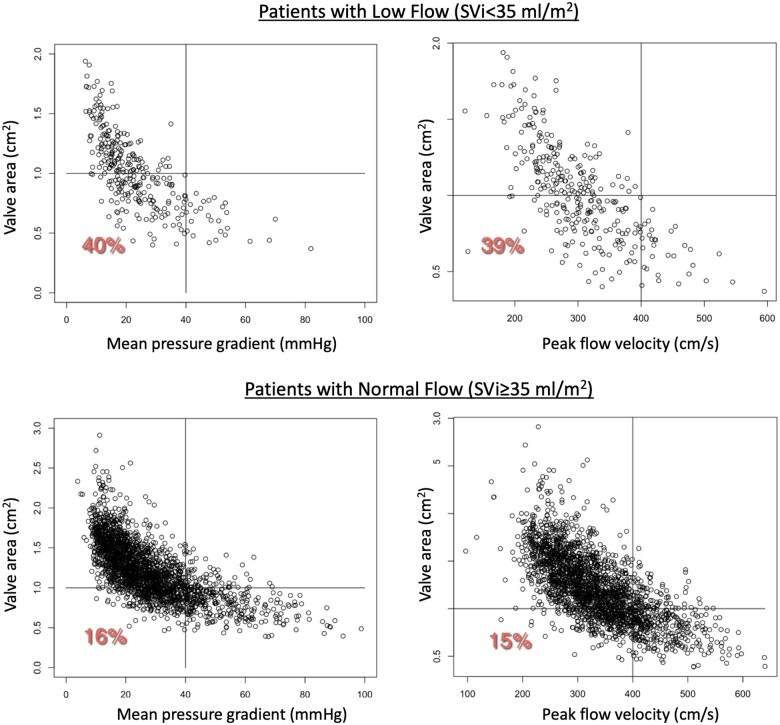
In addition, to elucidate the potential effects of low flow on our findings, we identified patients in our study cohort with low stroke volume (defined by stroke volume index < 35 mL/m2), which turned out to be 206/916 (22.5%). We repeated our analysis for this subgroup, and separately for the remaining 710 patients. We found that of the total of 18.75% of the studies that fell into the lower left quadrant in the main analysis (Figure 3), 5% were due to the patients with stroke volume index <35 mL/m2, while the remaining 13.75% represented patients who were not part of this subgroup. Specifically, of the above 206 patients, 82 patients (40%) had inconsistent criteria for severe AS, as they were in the lower left quadrant, corresponding to AVA ≤1 cm2 but had ΔP <40 mmHg. Similarly, 80 of the 206 patients (39%) were in the lower left quadrant corresponding to AVA ≤1 cm2 but had V max <4 m/s. In contrast, of the remaining 710 patients, 114 (16%%) were in the ‘discrepant’ category by pressure gradient and 107 (15%%) were ‘discrepant’ by peak flow velocity (Figure 4).
Figure 4.
Results of sub-analysis for 206 patients with low flow, defined by stroke volume index <35 mL/m2 (top), compared to the remaining 710 patients from our study cohort (bottom). Data are presented in the same format as in Figure 3. See text for detail.
Discussion
The main findings in this study were (i) the overall rate of AS progression by change in AVA was −0.070 ± 0.003 cm2/year and was more rapid in men than women and in White patients than in African Americans. We also found that AVA of 1 cm2 corresponded to ΔP = 32 mmHg and V max = 3.7 m/s, therefore, causing discrepancies in the prevalence of severe AS when different measures were used to categorize AS severity, based on the current guidelines.
In a study published in 1997, of 123 patients with asymptomatic moderate or greater AS, the annual rate of AVA narrowing was 0.12 ± 0.19 cm2/year, i.e. considerably faster than in our study cohort.9 This difference may be explained by the fact that our cohort included patients with mild AS, some of which never progressed past mild grade. Another consideration may be that our cohort included more contemporary patients treated using more aggressive cardiovascular risk factor management, although there is no conclusive data on the effects of improving atherosclerotic risk factors on the progression of AS.
Large, retrospective cross-sectional studies using diagnostic codes and discharge diagnoses suggest racial and gender differences in the prevalence of AS and AS-related health care utilization, but there is a paucity of data on whether race and gender impact the rate of AS progression. A nationwide survey of inpatients found a higher prevalence of AS in white and male patients but did not follow patients longitudinally.10 In our study, AS progression was also found to be more rapid in men and white patients. The racial difference may be partially attributed to the higher prevalence of bicuspid AV in the white population,11 while explaining the gender difference would need further studies. The inverse relationship between initial severity of AS and progression rate likely reflects the fact that patients with more severe stenosis during baseline studies could not progress much more, as further changes would be physiologically implausible.
Prior investigators have found subsets of patients with varying progression rates, and there have been many studies aiming to prospectively ascertain risk factors for more rapid progression. Aortic sclerosis and early AS pathogenesis seem to follow the same risk profile as atherosclerosis, including smoking, hypertension, hyperlipidaemia, and diabetes. The propagation phase to the later stages of AS appears to be mediated by different, pro-calcific factors. Similar to coronary artery disease, there has been a special interest in identifying modifiable risk factors such as cholesterol, as targeted therapies exist, but to date, no disease modulating therapies have been validated.12–17 It is one of our future aims to link our electronic medical records and echocardiography database to determine whether any of these factors, if treated, would mitigate the progression of AS.
Severe AS portends poor prognosis even when asymptomatic, and valve replacement has been shown to improve outcomes.9 , 18–21 As access to transcatheter interventions for AS has become more readily available, the ability to accurately assess AS by echocardiography and identify those patients with severe AS who may benefit the most from intervention is crucial.
Assessment of AS with TTE was first described in 1980 using the modified Bernoulli equation to estimate the peak pressure across the AV, in order to determine whether AS was moderate or severe, and was subsequently validated against invasive data.22 Using echocardiographic parameters also allows the calculation of AVA based on the principle of conservation of mass. The AVA is routinely derived using the continuity equation, as the flow through the LVOT is equal to that through the AV. By measuring the LVOT diameter to calculate cross-sectional area of the LVOT and the AV VTIs, the AVA is derived and has been extensively validated against invasive AVA measurements.23–25
Current multiparametric guidelines define severe AS by an AVA <1 cm2, V max ≥4 m/s or ΔP ≥40 mmHg.5 , 6 Low-gradient AS, defined by AVA <1 cm2 and ΔP <40, has been traditionally classified based on flow and LV systolic function. The most frequent cause of low-gradient AS is a low outflow state secondary to LV systolic dysfunction. The prevalence of low-flow, low-gradient AS has been observed in 5–10% of patients with severe AS,26 but in clinical practice, discordant AVA and ΔP are seen far more frequently. Our sub-analysis of patients with low flow showed that a considerable number of patients with severe AS and inconsistent criteria have low stroke volume. In fact, using the Gorlin formula and assuming a normal cardiac output of 6 L/min, systolic ejection period of 0.33 s, and a heart rate of 80 bpm, an AVA of 1 cm2 corresponds to a ΔP of 26 mmHg7 which explains the higher frequency of grading discrepancies observed by others and confirmed in our study. The ideal multiparametric grading scheme ought to be internally consistent. We found the current severe cut-off value of AVA = 1.0 cm2 corresponded to ΔP = 32 mmHg and V max = 3.7 m/s, indicating the need to refine the criteria for severe AS.
Limitations
The limitations of this study include its single-centre nature, which makes our findings not necessarily generalizable to the experience of other laboratories. The retrospective study design has well-known limitations of lack of standardization in potentially important variables, such as time intervals between consecutive examinations, equipment upgrades over the years, and different methods of assessment of LV systolic function.
Conclusions
In summary, the knowledge gleaned in this study of a large cohort of patients with varying degrees of AS shed new light onto the temporal progression of this pathology and uncovered previously unknown gender- and racial differences, which need to be confirmed in future studies. Our findings indicate the need to refine the multiparametric criteria for accurate diagnosis of severe AS, and provide alternative cut-offs, since we found that current AS severity cut-offs result in high rates of internal haemodynamic inconsistencies, that are further amplified in patients with low-flow AS. This information may help address the growing need for accurate determination of the optimal timing of intervention for severe AS,27 , 28 which is now available to a growing number of patients with the widespread adoption of transcatheter valve replacement.
Conflict of interest: none declared.
References
- 1.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–20. [DOI] [PubMed] [Google Scholar]
- 2.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–31. [DOI] [PubMed] [Google Scholar]
- 3.Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387:2218–25. [DOI] [PubMed] [Google Scholar]
- 4.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–705. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2017;30:372–92. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438–88. [DOI] [PubMed] [Google Scholar]
- 7.Carabello BA. Clinical practice. Aortic stenosis. N Engl J Med 2002;346:677–82. [DOI] [PubMed] [Google Scholar]
- 8.Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J 2008;29:1043–8. [DOI] [PubMed] [Google Scholar]
- 9.Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997;95:2262–70. [DOI] [PubMed] [Google Scholar]
- 10.Beydoun HA, Beydoun MA, Liang H, Dore GA, Shaked D, Zonderman AB et al. Sex, race, and socioeconomic disparities in patients with aortic stenosis (from a Nationwide Inpatient Sample). Am J Cardiol 2016;118:860–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandra S, Lang RM, Nicolarsen J, Gayat E, Spencer KT, Mor-Avi V et al. Bicuspid aortic valve: inter-racial difference in frequency and aortic dimensions. JACC Cardiovasc Imaging 2012;5:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahler RC, Desser DR, Finkelhor RS, Brener SJ, Youssefi M. Factors leading to progression of valvular aortic stenosis. Am J Cardiol 1999;84:1044–8. [DOI] [PubMed] [Google Scholar]
- 13.Briand M, Lemieux I, Dumesnil JG, Mathieu P, Cartier A, Despres JP et al. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J Am Coll Cardiol 2006;47:2229–36. [DOI] [PubMed] [Google Scholar]
- 14.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005;111:3316–26. [DOI] [PubMed] [Google Scholar]
- 15.Palta S, Pai AM, Gill KS, Pai RG. New insights into the progression of aortic stenosis: implications for secondary prevention. Circulation 2000;101:2497–502. [DOI] [PubMed] [Google Scholar]
- 16.Pohle K, MäFfert R, Ropers D, Moshage W, Stilianakis N, Daniel WG et al. Progression of aortic valve calcification: association with coronary atherosclerosis and cardiovascular risk factors. Circulation 2001;104:1927–32. [DOI] [PubMed] [Google Scholar]
- 17.Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611–7. [DOI] [PubMed] [Google Scholar]
- 18.Campo J, Tsoris A, Kruse J, Karim A, Andrei AC, Liu M et al. Prognosis of severe asymptomatic aortic stenosis with and without surgery. Ann Thorac Surg 2019;108:74–9. [DOI] [PubMed] [Google Scholar]
- 19.Carabello BA. Selection of patients with aortic stenosis for operation: the asymptomatic patient and the patient with poor LV function. Adv Cardiol 2002;39:49–60. [DOI] [PubMed] [Google Scholar]
- 20.Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005;111:3290–5. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki A, Tajiri K, Ishizu T, Nakayama M, Hoshi T, Suzuki S et al. Effect of asymptomatic severe aortic stenosis on outcomes of individuals aged 80 and older. J Am Geriatr Soc 2018;66:1800–4. [DOI] [PubMed] [Google Scholar]
- 22.Hatle L, Angelsen BA, Tromsdal A. Non-invasive assessment of aortic stenosis by Doppler ultrasound. Br Heart J 1980;43:284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callahan MJ, Tajik AJ, Su-Fan Q, Bove AA. Validation of instantaneous pressure gradients measured by continuous-wave Doppler in experimentally induced aortic stenosis. Am J Cardiol 1985;56:989–93. [DOI] [PubMed] [Google Scholar]
- 24.Oh JK, Taliercio CP, Holmes DR Jr, Reeder GS, Bailey KR, Seward JB et al. Prediction of the severity of aortic stenosis by Doppler aortic valve area determination: prospective Doppler-catheterization correlation in 100 patients. J Am Coll Cardiol 1988;11:1227–34. [DOI] [PubMed] [Google Scholar]
- 25.Smith MD, Dawson PL, Elion JL, Booth DC, Handshoe R, Kwan OL et al. Correlation of continuous wave Doppler velocities with cardiac catheterization gradients: an experimental model of aortic stenosis. J Am Coll Cardiol 1985;6:1306–14. [DOI] [PubMed] [Google Scholar]
- 26.Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol 2012;60:1845–53. [DOI] [PubMed] [Google Scholar]
- 27.Kang DH, Park SJ, Lee SA, Lee S, Kim DH, Kim HK et al. Early surgery or conservative care for asymptomatic aortic stenosis. N Engl J Med 2020;382:111–9. [DOI] [PubMed] [Google Scholar]
- 28.Lancellotti P, Vannan MA. Timing of intervention in aortic stenosis. N Engl J Med 2020;382:191–3. [DOI] [PubMed] [Google Scholar]