Abstract
Aims
Preterm birth has been associated with elevated blood pressure early in life; however, hypertension risks from childhood into adulthood remain unclear. We conducted a large population-based study to examine gestational age at birth in relation to hypertension risks from childhood into adulthood.
Methods and results
A national cohort study was conducted of all 4 193 069 singleton live births in Sweden during 1973–2014, who were followed up for hypertension identified from nationwide inpatient and outpatient (specialty and primary care) diagnoses from any health care encounters through 2015 (maximum age 43 years; median 22.5). Cox regression was used to examine gestational age at birth in relation to hypertension risk while adjusting for other perinatal and maternal factors, and co-sibling analyses assessed the potential influence of unmeasured shared familial (genetic and/or environmental) factors. In 86.8 million person-years of follow-up, 62 424 (1.5%) persons were identified with hypertension (median age 29.8 years at diagnosis). Adjusted hazard ratios for new-onset hypertension at ages 18–29 years associated with preterm (<37 weeks) and extremely preterm (22–27 weeks) birth were 1.28 [95% confidence interval (CI), 1.21–1.36] and 2.45 (1.82–3.31), respectively, and at ages 30–43 years were 1.25 (1.18–1.31) and 1.68 (1.12–2.53), respectively, compared with full-term birth (39–41 weeks). These associations affected males and females similarly and appeared substantially related to shared genetic or environmental factors in families.
Conclusions
In this large national cohort, preterm birth was associated with increased risk of hypertension into early adulthood. Persons born prematurely may need early preventive evaluation and long-term monitoring for the development of hypertension.
Keywords: Adult, Blood pressure, Gestational age, Hypertension, Premature birth, Preterm birth
See page 1551 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa118)
Introduction
Hypertension is a major risk factor for cardiovascular disease (CVD), stroke, and chronic kidney disease and is the world’s leading cause of disability-adjusted life-years (years of life lost due to death or lived with disability).1 , 2 Nearly one in three adults worldwide have hypertension as defined by blood pressures ≥140/90 mm Hg3 and nearly one in two using the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) definition of ≥130/80 mm Hg.4 In addition to unhealthy adult lifestyle factors, early-life exposures have been identified as potential risk factors for the development of hypertension later in life. According to the developmental origins theory, perinatal nutritional abnormalities and growth restriction may permanently alter organ structure and metabolism, resulting in early-life programming for the development of hypertension and other cardio-metabolic disorders in adulthood.5 , 6 Recent evidence has suggested that developmental programming on the background of preterm birth may have a particularly important effect on long-term outcomes.7–9 Preterm birth (gestational age <37 completed weeks) has a worldwide prevalence of 11%10 and most preterm infants now survive into adulthood.7–9 As a result, clinicians will increasingly encounter adult patients who were born preterm. A better understanding of their long-term risks of hypertension is needed to guide early preventive actions and monitoring across the life course.
Several studies have reported that preterm birth is associated with elevated blood pressure in childhood and young adulthood.11–14 However, these associations have seldom been examined further into adulthood when hypertension is more likely to manifest. In addition, it is possible that previously reported associations between preterm birth and hypertension are due to shared genetic or environmental factors in the families of those affected.7 To our knowledge, this possibility has never been evaluated. The relative contributions of shared familial factors vs. direct effects of preterm birth on the development of hypertension are currently unknown.
We addressed these knowledge gaps by conducting a national cohort study of over 4 million persons in Sweden. Our goals were to (i) provide population-based estimates for risk of hypertension from childhood into adulthood associated with gestational age at birth; (ii) assess for sex-specific differences; and (iii) perform co-sibling analyses to assess the potential influence of shared familial (genetic and/or environmental) factors. The results will help inform long-term monitoring, preventive actions, and early treatment of hypertension in the growing population of preterm-born children and adults.
Methods
Study population
The Swedish Birth Registry contains prenatal and birth information for nearly all births in Sweden since 1973.15 Using this registry, we identified 4 201 706 singleton live births during 1973–2014. Singleton births were chosen to improve internal comparability, given the higher prevalence of preterm birth and its different underlying causes among multiple births. We excluded 8637 (0.2%) births that had missing information for gestational age, leaving 4 193 069 births (99.8% of the original cohort) for inclusion in the study. This study was approved by the ethics committee of Lund University in Sweden. Participant consent was not required as this study used only de-identified registry-based secondary data.
Gestational age at birth ascertainment
Gestational age at birth was identified from the Swedish Birth Registry based on maternal report of last menstrual period in the 1970s and ultrasound estimation starting in the 1980s and later (>70% of the cohort). This was examined alternatively as a continuous variable or categorical variable with six groups: extremely preterm (22–27 weeks), very preterm (28–33 weeks), late preterm (34–36 weeks), early term (37–38 weeks), full term (39–41 weeks, used as the reference group), and post-term (≥42 weeks). In addition, the first three groups were combined to provide summary estimates for preterm birth (<37 weeks).
Outcome ascertainment
The study cohort was followed up for diagnosis of essential (primary) hypertension from birth through 31 December 2015 (maximum age 43.0 years; median 22.5). This was identified using International Classification of Diseases (ICD) codes for all primary and secondary diagnoses from any health care encounters as recorded in the Swedish Hospital, Outpatient, and Primary Care Registries (ICD-8: 400–401 during 1973–86; ICD-9: 401 during 1987–96; ICD-10: I10 during 1997–2015). The Hospital Registry started in 1964 and initially included all hospital discharge diagnoses from six populous counties in southern Sweden, but was expanded to cover nearly 80% of the national population by 1973, and >99% starting in 1987.16 The Outpatient Registry started in 2001 and contains outpatient diagnoses from all specialty clinics nationwide. The Primary Care Registry initially included all primary care diagnoses from two populous counties covering 20% of the national population starting in 1998, but was gradually expanded to include nine counties covering 72% of the national population by 2008 and onward.17
Throughout the study period, the predominant criteria for diagnosis of hypertension in adults (ages ≥18 years) were systolic blood pressure (SBP) ≥140 mm Hg and/or diastolic blood pressure (DBP) ≥90 mm Hg, or taking antihypertensive medication, based on World Health Organization and European Society of Hypertension/European Society of Cardiology guidelines.18 , 19 In children and adolescents (ages 1–17 years), the predominant criteria were SBP and/or DBP persistently ≥95th percentile for age, sex, and height.20
Other major CVD also was examined as a secondary outcome. This outcome included the earliest diagnosis of ischaemic heart disease (ICD-8/9: 410–414; ICD-10: I20-I25), heart failure (ICD-8: 427; ICD-9: 428; ICD-10: I50), or cerebrovascular disease (ICD-8/9: 430–438; ICD-10: I60–I69) identified using the same registries described above, or cardiovascular-related death (ICD-8/9: 393–459; ICD-10: I00-I99) identified using the Swedish Cause of Death Registry, which includes all deaths since 1960 with compulsory reporting nationwide.
Other study variables
Other perinatal and maternal characteristics that may be associated with gestational age at birth and hypertension or other CVD were identified using the Swedish Birth Registry and national census data, which were linked using an anonymous personal identification number. Adjustment variables included birth year (continuous and categorical by decade), sex, foetal growth [defined as birth weight percentile for gestational age, classified as small for gestational age (SGA; <10th percentile), appropriate for gestational age (AGA; 10th-90th percentile), or large for gestational age (LGA; >90th percentile)], birth order (1, 2, ≥3), congenital anomalies (yes/no, identified using ICD-8/9 codes 740–759 and ICD-10 codes Q00–Q99), and the following maternal characteristics: age (continuous), education level (<10, 10–12, >12 years), employment status (yes/no) and income (quartiles) in the year prior to delivery, body mass index (BMI; continuous), smoking (0, 1–9, ≥10 cigarettes/day), pre-eclampsia (ICD-8: 637; ICD-9: 624.4–624.7; ICD-10: O14-O15), and other hypertensive disorders during pregnancy (ICD-8: 400–404; ICD-9: 401–405, 642.0–642.3, 642.9; ICD-10: I10-I15, O10-O11, O13, O15-O16). Maternal smoking, pre-eclampsia, and other hypertensive disorders were included because they have been associated with preterm delivery21 and are reported risk factors for hypertension in the offspring.22–26
Maternal BMI and smoking were assessed at the beginning of prenatal care starting in 1982 and were available for 61.0% and 74.2% of women, respectively. Data were >99% complete for all other variables. Missing data for each covariate were imputed using a standard multiple imputation procedure based on the variable’s relationship with all other covariates and the outcome.27
Statistical analysis
Cox proportional hazards regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between gestational age at birth and risk of hypertension or other major CVD. These associations were examined across the age range of 0–43 years and in narrower age intervals (<18, 18–29, 30–43 years) among persons still living in Sweden without a prior diagnosis of the outcome at the beginning of the respective interval. Attained age was used as the Cox model time axis, with age at the beginning of the respective interval as ‘time zero’. Individuals were censored at emigration (i.e. moving out of Sweden and thus no longer able to be followed up for the outcome) as determined by absence of a Swedish residential address in census data (n = 259 378; 6.2%) or death as identified in the Swedish Death Registry (n = 43 478; 1.0%). Emigrants and non-emigrants had similar gestational ages at birth (median, 40 1/7 weeks for both groups), and thus it was unlikely that emigration introduced any substantial bias. Analyses were conducted both unadjusted and adjusted for covariates (as above). Sex differences were assessed by performing sex-stratified analyses and examining interactions between gestational age at birth and sex on both the additive and multiplicative scale.28 The proportional hazards assumption was assessed by examining log–log plots29 and was met in each model. Absolute risk differences and 95% CIs, attributable fraction in the exposed (AFe), and population attributable fraction (PAF) also were computed for each gestational age group compared with full term.30
Co-sibling analyses were performed to assess the potential influence of unmeasured shared familial (genetic and/or environmental) factors.9 Shared environmental factors in families may potentially include lifestyle factors such as diet, or ambient exposures such as passive smoking or air pollution. These analyses used stratified Cox regression with a separate stratum for each family as identified by the mother’s and father’s anonymous identification numbers. A total of 3 475 889 individuals (83.0% of the cohort) had at least one full sibling and were included in these analyses. In the stratified Cox model, each set of siblings had its own baseline hazard function that reflects the family’s shared genetic and environmental factors, and thus associations between gestational age at birth and the outcome were examined within the family. In addition, these analyses were further adjusted for the same covariates as in the primary analyses.
In secondary analyses, we explored for potential birth cohort effects on hypertension risks by stratifying by birth decade (1970s, 1980s, 1990s) and including follow-up to an equal attained age (20 years). We also examined hypertension risks after stratifying by spontaneous vs. medically indicated preterm birth. In addition, we examined potential interactions between gestational age at birth and foetal growth or pre-eclampsia in relation to hypertension risk. Interactions with pre-eclampsia were explored because of a previously reported higher risk of hypertension among young adults born with very low birth weight who were exposed to pre-eclampsia.14 All statistical tests were two-sided and used an α-level of 0.05. All analyses were conducted using Stata version 15.1. J.S. had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Results
Table 1 shows perinatal and maternal characteristics by gestational age at birth. Preterm infants were more likely than full-term infants to be male or first born, or have congenital anomalies; and their mothers were more likely to be at the extremes of age, have low education level or high BMI, smoke, or have pre-eclampsia or other hypertensive disorders during their pregnancy.
Table 1.
Characteristics of study participants by gestational age at birth, Sweden, 1973–2014
| Extremely preterm (22–27 weeks) | Very preterm (28–33 weeks) | Late preterm (34–36 weeks) | Early term (37–38 weeks) | Full term (39–41 weeks) | Post-term (≥42 weeks) | |
|---|---|---|---|---|---|---|
| N = 8, 324 (0.2%) | N = 44, 373 (1.1%) | N = 157, 342 (3.7%) | N = 740, 391 (17.7%) | N = 2, 896, 444 (69.1%) | N = 346, 195 (8.3%) | |
| n (%)a | n (%)a | n (%)a | n (%)a | n (%)a | n (%)a | |
| Child characteristics | ||||||
| Sex | ||||||
| Male | 4529 (54.4) | 24 734 (55.7) | 85 570 (54.4) | 381 140 (51.5) | 1 471 368 (50.8) | 188 358 (54.4) |
| Female | 3795 (45.6) | 19 639 (44.3) | 71 772 (45.6) | 359 251 (48.5) | 1 425 076 (49.2) | 157 837 (45.6) |
| Foetal growth (percentile) | ||||||
| SGA (<10th) | 73 (0.9) | 5630 (12.7) | 15 623 (9.9) | 54 405 (7.3) | 276 687 (9.6) | 66 724 (19.3) |
| AGA (10th–90th) | 8015 (96.3) | 37 019 (83.4) | 128 530 (81.7) | 601 039 (81.2) | 2 326 431 (80.3) | 253 586 (73.2) |
| LGA (>90th) | 236 (2.8) | 1724 (3.9) | 13 189 (8.4) | 84 947 (11.5) | 293 326 (10.1) | 25 885 (7.5) |
| Birth order | ||||||
| 1 | 4140 (49.7) | 22 780 (51.3) | 78 019 (49.6) | 297 655 (40.2) | 1 219 056 (42.1) | 172 702 (49.9) |
| 2 | 2384 (28.6) | 12 525 (28.2) | 47 050 (29.9) | 271 130 (36.6) | 1 087 624 (37.5) | 111 055 (32.1) |
| ≥3 | 1800 (21.6) | 9068 (20.4) | 32 273 (20.5) | 171 606 (23.2) | 589 764 (20.4) | 62 438 (18.0) |
| Congenital anomalies | 335 (4.0) | 1877 (4.2) | 5542 (3.5) | 23 518 (3.2) | 76 064 (2.6) | 8971 (2.6) |
| Maternal characteristics | ||||||
| Age (years) | ||||||
| <20 | 356 (4.3) | 2058 (4.6) | 6476 (4.1) | 22 069 (3.0) | 84 019 (2.9) | 12 962 (3.7) |
| 20–24 | 1575 (18.9) | 8957 (20.2) | 33 190 (21.1) | 139 115 (18.8) | 580 841 (20.0) | 76 287 (22.0) |
| 25–29 | 2436 (29.3) | 13 725 (30.9) | 51 191 (32.5) | 243 257 (32.9) | 1 018 871 (35.2) | 121 285 (35.0) |
| 30–34 | 2271 (27.3) | 11 831 (26.7) | 41 555 (26.4) | 211 859 (28.6) | 821 668 (28.4) | 92 813 (26.8) |
| 35–39 | 1314 (15.8) | 6199 (14.0) | 20 167 (12.8) | 100 963 (13.6) | 330 847 (11.4) | 36 883 (10.7) |
| ≥40 | 372 (4.5) | 1603 (3.6) | 4763 (3.0) | 23 128 (3.1) | 60 198 (2.1) | 5965 (1.7) |
| Education (years) | ||||||
| <10 | 1401 (16.8) | 7322 (16.5) | 24 405 (15.5) | 104 171 (14.1) | 367 829 (12.7) | 48 594 (14.0) |
| 10–12 | 3920 (47.1) | 21 112 (47.6) | 74 269 (47.2) | 338 702 (45.7) | 1 304 778 (45.0) | 157 018 (45.4) |
| >12 | 3003 (36.1) | 15 939 (35.9) | 58 668 (37.3) | 297 518 (40.2) | 1 223 837 (42.3) | 140 583 (40.6) |
| Employment | 6780 (81.5) | 36 817 (83.0) | 132 269 (84.1) | 623 726 (84.2) | 2 482 940 (85.7) | 296 859 (85.7) |
| Income (quartile) | ||||||
| 1st (highest) | 2543 (30.5) | 12 011 (27.1) | 39 853 (25.3) | 188 738 (25.5) | 724 186 (25.0) | 81 698 (23.6) |
| 2nd | 2162 (26.0) | 11 205 (25.2) | 39 700 (25.2) | 184 320 (24.9) | 729 883 (25.2) | 86 587 (25.0) |
| 3rd | 1696 (20.4) | 10 549 (23.8) | 38 485 (24.5) | 181 913 (24.6) | 723 742 (25.0) | 90 269 (26.1) |
| 4th (lowest) | 1923 (23.1) | 10 608 (23.9) | 39 304 (25.0) | 185 420 (25.0) | 718 633 (24.8) | 87 641 (25.3) |
| Body mass index | ||||||
| <18.5 | 140 (1.7) | 1146 (2.6) | 4808 (3.1) | 21 784 (2.9) | 65 600 (2.3) | 4649 (1.3) |
| 18.5–24.9 | 6111 (73.4) | 34 215 (77.1) | 121 37 (77.1) | 567 018 (76.6) | 2 279 549 (78.7) | 275 218 (79.5) |
| 25.0–29.9 | 1456 (17.5) | 6189 (13.9) | 21 663 (13.8) | 107 959 (14.6) | 404 340 (14.0) | 46 600 (13.5) |
| ≥30.0 | 617 (7.4) | 2823 (6.4) | 9534 (6.1) | 43 630 (5.9) | 146 991 (5.1) | 19 728 (5.7) |
| Smoking (cigarettes/day) | ||||||
| 0 | 6091 (73.2) | 31 407 (70.8) | 114 671 (72.9) | 565 076 (76.3) | 2 216 387 (76.5) | 247 302 (71.4) |
| 1–9 | 1737 (20.9) | 10 211 (23.0) | 33 688 (21.4) | 138 352 (18.7) | 567 341 (19.6) | 87 934 (25.4) |
| ≥10 | 496 (6.0) | 2755 (6.2) | 8983 (5.7) | 36 963 (5.0) | 112 716 (3.9) | 10 959 (3.2) |
| Pre-eclampsia | 1032 (12.4) | 7869 (17.7) | 16 095 (10.2) | 39 382 (5.3) | 94 720 (3.3) | 11 833 (3.4) |
| Other hypertensive disorders | 111 (1.3) | 790 (1.8) | 2241 (1.4) | 8897 (1.2) | 24 746 (0.9) | 2347 (0.7) |
Number and percentage of participants in the specified gestational age group with the respective characteristic.
AGA, appropriate for gestational age; LGA, large for gestational age; SGA, small for gestational age.
Gestational age at birth and risk of hypertension
In 86.8 million person-years of follow-up, 62 424 (1.5%) persons were identified with hypertension, yielding an overall incidence rate of 71.89 per 100 000 person-years across ages 0–43 years. The corresponding incidence rates were 88.83 among those born preterm, 74.76 among those born at early term, and 69.61 among those born full term (Table 2). The median age for the entire cohort (i.e. including those with or without hypertension) at the end of follow-up was 22.5 years (mean 22.2 ± 12.2), and the median age at diagnosis of hypertension was 29.8 years (mean 27.1 ± 11.0). A total of 1 250 847 persons were followed up to ages ≥30 years, of whom 39 597 (3.2%) were ever diagnosed with hypertension, and 316 463 persons were followed up to ages ≥40 years, of whom 16 055 (5.1%) were ever diagnosed with hypertension.
Table 2.
Associations between gestational age at birth and risk of hypertension, Sweden, 1973–2015
| Cases | Ratea | Unadjusted | Adjustedb
|
Risk difference (95% CI)c | AFe | PAF | ||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | P | ||||||
| Attained ages 0–43 years | ||||||||
| Preterm (<37 weeks) | 3639 | 88.8 | 1.34 (1.29, 1.39) | 1.25 (1.20, 1.29) | <0.001 | 19.2 (16.3, 22.2) | 21.6% | 1.7% |
| Extremely preterm (22–27 weeks) | 97 | 117.6 | 2.39 (1.96, 2.92) | 1.83 (1.50, 2.23) | <0.001 | 48.0 (24.6, 71.4) | 40.8% | 0.1% |
| Very preterm (28–33 weeks) | 785 | 97.0 | 1.50 (1.40, 1.61) | 1.34 (1.25, 1.44) | <0.001 | 27.4 (20.6, 34.2) | 28.3% | 0.5% |
| Late preterm (34–36 weeks) | 2757 | 86.0 | 1.28 (1.23, 1.33) | 1.21 (1.16, 1.26) | <0.001 | 16.4 (13.1, 19.7) | 19.1% | 1.2% |
| Early term (37–38 weeks) | 11 006 | 74.8 | 1.18 (1.15, 1.20) | 1.11 (1.09, 1.14) | <0.001 | 5.2 (3.6, 6.7) | 6.9% | 1.4% |
| Full term (39–41 weeks) | 41 779 | 69.6 | Reference | Reference | Reference | |||
| Post-term (≥42 weeks) | 6000 | 75.1 | 0.89 (0.87, 0.92) | 0.95 (0.93, 0.98) | <0.001 | 5.5 (3.5, 7.5) | 7.3% | 0.9% |
| Per additional week (trend) | 0.94 (0.94, 0.95) | 0.96 (0.96, 0.97) | <0.001 | |||||
| Attained ages <18 years | ||||||||
| Preterm (<37 weeks) | 781 | 28.0 | 1.29 (1.20, 1.39) | 1.24 (1.15, 1.34) | <0.001 | 6.3 (4.2, 8.3) | 22.3% | 1.8% |
| Extremely preterm 22–27 weeks) | 31 | 49.0 | 2.29 (1.61, 3.26) | 1.47 (1.03, 2.09) | 0.03 | 27.3 (10.0, 44.5) | 55.6% | 0.2% |
| Very preterm (28–33 weeks) | 191 | 34.2 | 1.58 (1.37, 1.82) | 1.45 (1.26, 1.68) | <0.001 | 12.5 (7.6, 17.4) | 36.5% | 0.8% |
| Late preterm (34–36 weeks) | 559 | 25.8 | 1.19 (1.09, 1.29) | 1.18 (1.08, 1.28) | <0.001 | 4.0 (1.9, 6.2) | 15.6% | 0.9% |
| Early term (37–38 weeks) | 2542 | 25.0 | 1.15 (1.10, 1.20) | 1.09 (1.04, 1.14) | <0.001 | 3.2 (2.2, 4.3) | 13.0% | 2.9% |
| Full term (39–41 weeks) | 8802 | 21.7 | Reference | Reference | Reference | |||
| Post-term (≥42 weeks) | 757 | 15.0 | 0.69 (0.64, 0.74) | 0.81 (0.75, 0.87) | <0.001 | −6.7 (−7.9, −5.5) | (30.9%)d | (3.4%)e |
| Per additional week (trend) | 0.93 (0.92, 0.94) | 0.95 (0.95, 0.96) | <0.001 | |||||
| Attained ages 18–29 years | ||||||||
| Preterm (<37 weeks) | 1260 | 126.5 | 1.43 (1.35, 1.52) | 1.28 (1.21, 1.36) | <0.001 | 37.9 (30.7, 45.0) | 29.9% | 2.7% |
| Extremely preterm (22–27 weeks) | 43 | 274.4 | 3.24 (2.40, 4.38) | 2.45 (1.82, 3.31) | <0.001 | 185.8 (103.8, 267.9) | 67.7% | 0.2% |
| Very preterm (28–33 weeks) | 267 | 138.5 | 1.57 (1.40, 1.78) | 1.33 (1.17, 1.50) | <0.001 | 49.9 (33.3, 66.6) | 36.0% | 0.7% |
| Late preterm (34–36 weeks) | 950 | 120.6 | 1.36 (1.28, 1.45) | 1.25 (1.17, 1.33) | <0.001 | 32.0 (24.2, 39.8) | 26.5% | 1.8% |
| Early term (37–38 weeks) | 3878 | 110.3 | 1.25 (1.21, 1.30) | 1.14 (1.10, 1.19) | <0.001 | 21.8 (18.0, 25.6) | 19.7% | 4.6% |
| Full term (39–41 weeks) | 12 895 | 88.6 | Reference | Reference | Reference | |||
| Post-term (≥42 weeks) | 1489 | 72.8 | 0.80 (0.76, 0.85) | 0.97 (0.91, 1.02) | 0.20 | −15.8 (−19.8, −11.8) | (17.9%)d | (2.2%)e |
| Per additional week (trend) | 0.92 (0.92, 0.93) | 0.96 (0.95, 0.96) | <0.001 | |||||
| Attained ages 30–43 years | ||||||||
| Preterm (<37 weeks) | 1598 | 518.9 | 1.30 (1.23, 1.37) | 1.25 (1.18, 1.31) | <0.001 | 112.8 (86.7, 138.8) | 21.7% | 1.6% |
| Extremely preterm (22–27 weeks) | 23 | 653.5 | 1.65 (1.10, 2.49) | 1.68 (1.12, 2.53) | 0.01 | 247.3 (−19.8, 514.5) | 37.8% | <0.1% |
| Very preterm (28–33 weeks) | 327 | 564.2 | 1.41 (1.27, 1.57) | 1.34 (1.21, 1.50) | <0.001 | 158.1 (96.6, 219.5) | 28.0% | 0.4% |
| Late preterm (34–36 weeks) | 1248 | 506.4 | 1.27 (1.20, 1.34) | 1.22 (1.15, 1.29) | <0.001 | 100.2 (71.5, 128.8) | 19.8% | 1.2% |
| Early term (37–38 weeks) | 4586 | 448.9 | 1.14 (1.10, 1.17) | 1.11 (1.07, 1.15) | <0.001 | 42.8 (28.6, 56.9) | 9.5% | 1.8% |
| Full term (39–41 weeks) | 20 082 | 406.2 | Reference | Reference | Reference | |||
| Post-term (≥42 weeks) | 3754 | 416.9 | 1.00 (0.96, 1.03) | 0.97 (0.94, 1.00) | 0.08 | 10.8 (−3.7, 25.2) | 2.6% | 0.4% |
| Per additional week (trend) | 0.97 (0.96, 0.97) | 0.97 (0.96, 0.97) | <0.001 | |||||
Incidence rate per 100 000 person-years.
Adjusted for child characteristics (birth year, sex, foetal growth, birth order, congenital anomalies) and maternal characteristics (age, education, employment, income, BMI, smoking, pre-eclampsia, other hypertensive disorders).
Incidence rate difference per 100 000 person-years.
Prevented fraction among the exposed.
Population prevented fraction.
AFe, attributable fraction in the exposed; HR, hazard ratio; PAF, population attributable fraction.
Across all attained ages (0–43 years), gestational age at birth was inversely associated with hypertension risk. Each additional week of gestation was associated with a 4% lower risk (adjusted HR per additional week, 0.96; 95% CI, 0.96–0.97; P < 0.001; Table 2). Preterm and early term birth were associated with 1.25-fold (95% CI, 1.20–1.29) and 1.11-fold (95% CI, 1.09–1.14) risks of hypertension, respectively, relative to full-term birth. Persons born extremely preterm had a 1.8-fold risk (adjusted HR, 1.83; 95% CI, 1.50–2.23: P < 0.001).
Preterm and extremely preterm birth accounted for 19.2 (95% CI, 16.3–22.2) and 48.0 (24.6–71.4) additional hypertension cases per 100 000 person-years, respectively, compared with full-term birth (Table 2, risk differences). An estimated 21.6% and 40.8% of hypertension cases among those born preterm or extremely preterm, and 1.7% and 0.1% cases in the entire population, were associated with exposure to preterm or extremely preterm birth, respectively (Table 2, AFe and PAF). An estimated 728 excess hypertension cases in this population were associated with preterm birth (1 excess case at the attained ages examined per 288 preterm births).
In analyses of narrower age intervals at diagnosis, preterm birth was associated with significantly increased risks of hypertension at ages <18 years (adjusted HR, 1.24; 95% CI, 1.15–1.34; P < 0.001), 18–29 years (1.28; 95% CI, 1.21–1.36; P < 0.001), and 30–43 years (1.25; 95% CI, 1.18–1.31; P < 0.001). Figure 1 shows adjusted HRs and risk differences for hypertension by attained age for different gestational age groups compared with full-term birth. Relative risks were fairly stable across ages 0–43 years, but risk differences associated with preterm birth increased at older attained ages when hypertension is more prevalent.
Figure 1.
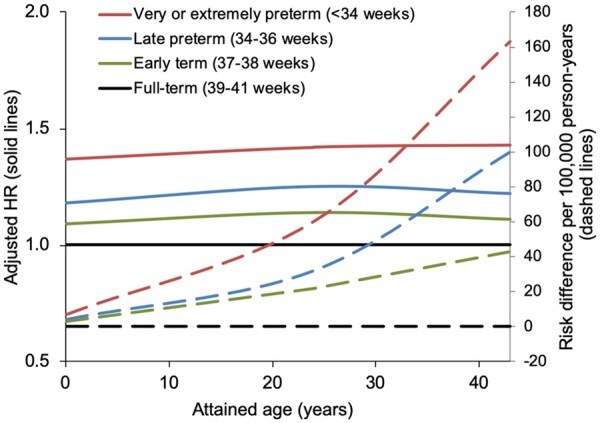
Adjusted hazard ratios (solid lines) and risk differences per 100 000 person-years (dashed lines) for hypertension by gestational age at birth compared with full-term birth, Sweden, 1973–2015.
Sex-stratified analyses are shown in Supplementary material online, Table S1 in the Appendix. Increased risks of hypertension were observed among males and females at ages 0–43 years (e.g. adjusted HR comparing preterm with full term, males: 1.27; 95% CI, 1.22–1.33; P < 0.001; females: 1.21; 95% CI, 1.15–1.27; P < 0.001). Across narrower age intervals, similar patterns were observed among males and females compared with the overall analyses. No interactions between gestational age at birth and sex in relation to hypertension risk were found on either the additive or multiplicative scale (Supplementary material online, Table S2). The absence of additive interaction suggests that preterm birth accounted for a similar number of hypertension cases among males and females.
Gestational age at birth and risk of other major cardiovascular disease
Gestational age at birth also was inversely associated with risk of other major CVD across all ages examined (Table 3). Adjusted HRs at ages 0–43 years associated with preterm and extremely birth were 1.57 (95% CI, 1.50–1.63) and 5.68 (4.89–6.60), respectively. In total, 29.8% of major CVD cases were ischaemic heart disease, 27.1% were heart failure, 39.9% were cerebrovascular disease, and 3.2% were cardiovascular-related death. Preterm and extremely preterm birth accounted for 22.0 (95% CI, 19.8–24.3) and 163.2 (134.1–192.2) additional major CVD cases per 100 000 person-years, respectively, compared with full-term birth (Table 3, risk differences). An estimated 40.4% and 83.4% of cases among those born preterm or extremely preterm, and 4.2% and 0.7% in the entire population, were associated with exposure to preterm or extremely preterm birth, respectively (Table 3, AFe and PAF). An estimated 918 excess cases in this population were associated with preterm birth (1 excess case per 229 preterm births). Exclusion of persons with congenital anomalies had little effect on these risk estimates (e.g. preterm vs. full term: adjusted HR at ages 0–43 years, 1.57; 95% CI, 1.51–1.64; P < 0.001).
Table 3.
Associations between gestational age at birth and risk of other major cardiovascular disease, Sweden, 1973–2015
| Cases | Ratea | Unadjusted | Adjustedb
|
Risk difference (95% CI)c | AFe | PAF | ||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | P | ||||||
| Attained ages 0–43 years | ||||||||
| Preterm (<37 weeks) | 2380 | 54.5 | 1.71 (1.63, 1.78) | 1.57 (1.50, 1.63) | <0.001 | 22.0 (19.8, 24.3) | 40.4% | 4.2% |
| Extremely preterm (22–27 weeks) | 174 | 195.7 | 6.83 (5.89, 7.93) | 5.68 (4.89, 6.60) | <0.001 | 163.2 (134.1, 192.2) | 83.4% | 0.7% |
| Very preterm (28–33 weeks) | 578 | 67.0 | 2.12 (1.95, 2.30) | 1.88 (1.73, 2.05) | <0.001 | 34.5 (29.0, 39.9) | 51.5% | 1.4% |
| Late preterm (34–36 weeks) | 1628 | 47.7 | 1.48 (1.41, 1.56) | 1.38 (1.31, 1.45) | <0.001 | 15.2 (12.9, 17.6) | 31.9% | 2.3% |
| Early term (37–38 weeks) | 5881 | 37.4 | 1.19 (1.16, 1.23) | 1.14 (1.11, 1.17) | <0.001 | 4.9 (3.8, 6.0) | 13.1% | 2.9% |
| Full term (39–41 weeks) | 20 779 | 32.5 | Reference | Reference | Reference | |||
| Post-term (≥42 weeks) | 2873 | 34.0 | 0.96 (0.93, 1.00) | 0.98 (0.94, 1.02) | 0.38 | 1.5 (0.2, 2.9) | 4.5% | 0.5% |
| Per additional week (trend) | 0.92 (0.92, 0.93) | 0.93 (0.93, 0.94) | <0.001 | |||||
| Attained ages <18 years | ||||||||
| Preterm (<37 weeks) | 1308 | 45.1 | 2.31 (2.17, 2.44) | 1.99 (1.88, 2.11) | <0.001 | 25.6 (23.1, 28.1) | 56.8% | 7.8% |
| Extremely preterm 22–27 weeks) | 149 | 222.9 | 10.94 (9.30, 12.86) | 8.00 (6.80, 9.42) | <0.001 | 203.4 (167.6, 239.2) | 91.2% | 1.6% |
| Very preterm (28–33 weeks) | 362 | 62.4 | 3.17 (2.86, 3.53) | 2.60 (2.34, 2.89) | <0.001 | 42.8 (36.4, 49.3) | 68.7% | 2.9% |
| Late preterm (34–36 weeks) | 797 | 35.4 | 1.81 (1.69, 1.95) | 1.61 (1.49, 1.73) | <0.001 | 15.9 (13.4, 18.4) | 44.9% | 4.0% |
| Early term (37–38 weeks) | 2643 | 24.9 | 1.27 (1.22, 1.33) | 1.20 (1.15, 1.26) | <0.001 | 5.4 (4.4, 6.4) | 21.7% | 5.3% |
| Full term (39–41 weeks) | 8216 | 19.5 | Reference | Reference | Reference | |||
| Post-term (≥42 weeks) | 939 | 18.1 | 0.93 (0.87, 1.00) | 0.94 (0.88, 1.01) | 0.09 | −1.5 (−2.7, −0.2) | (7.5%)d | (0.8%)e |
| Per additional week (trend) | 0.88 (0.87, 0.88) | 0.90 (0.89, 0.90) | <0.001 | |||||
| Attained ages 18–29 years | ||||||||
| Preterm (<37 weeks) | 625 | 57.9 | 1.34 (1.23, 1.45) | 1.26 (1.16, 1.36) | <0.001 | 14.6 (9.9, 19.2) | 25.1% | 2.1% |
| Extremely preterm (22–27 weeks) | 15 | 85.4 | 2.00 (1.21, 3.32) | 1.74 (1.05, 2.89) | 0.03 | 42.0 (−1.2, 85.2) | 49.2% | 0.1% |
| Very preterm (28–33 weeks) | 126 | 60.1 | 1.39 (1.16, 1.66) | 1.27 (1.07, 1.52) | 0.007 | 16.7 (6.2, 27.3) | 27.9% | 0.5% |
| Late preterm (34–36 weeks) | 484 | 56.8 | 1.31 (1.19, 1.44) | 1.24 (1.13, 1.36) | <0.001 | 13.5 (8.3, 18.6) | 23.7% | 1.6% |
| Early term (37–38 weeks) | 1952 | 51.0 | 1.18 (1.12, 1.24) | 1.12 (1.06, 1.18) | <0.001 | 7.6 (5.2, 10.1) | 15.0% | 3.3% |
| Full term (39–41 weeks) | 6830 | 43.3 | Reference | Reference | Reference | |||
| Post-term (≥42 weeks) | 852 | 39.3 | 0.90 (0.84, 0.97) | 0.98 (0.91, 1.05) | 0.59 | −4.0 (−6.9, −1.2) | (9.3%)d | (1.1%)e |
| Per additional week (trend) | 0.95 (0.94, 0.96) | 0.97 (0.96, 0.98) | <0.001 | |||||
| Attained ages 30–43 years | ||||||||
| Preterm (<37 weeks) | 447 | 116.0 | 1.24 (1.13, 1.37) | 1.22 (1.11, 1.34) | <0.001 | 21.7 (10.7, 32.8) | 18.8% | 1.4% |
| Extremely preterm (22–27 weeks) | 10 | 221.9 | 2.43 (1.30, 4.51) | 2.41 (1.30, 4.49) | 0.005 | 127.6 (−9.9, 265.2) | 57.5% | 0.1% |
| Very preterm (28–33 weeks) | 90 | 123.5 | 1.32 (1.08, 1.63) | 1.29 (1.04, 1.58) | 0.02 | 29.3 (3.7, 54.9) | 23.7% | 0.4% |
| Late preterm (34–36 weeks) | 347 | 112.6 | 1.21 (1.08, 1.35) | 1.18 (1.06, 1.32) | 0.002 | 18.4 (6.3, 30.5) | 16.3% | 0.9% |
| Early term (37–38 weeks) | 1286 | 99.7 | 1.08 (1.01, 1.14) | 1.08 (1.01, 1.14) | 0.02 | 5.5 (−0.5, 11.5) | 5.5% | 1.0% |
| Full term (39–41 weeks) | 5733 | 94.2 | Reference | Reference | Reference | |||
| Post-term (≥42 weeks) | 1082 | 100.5 | 1.04 (0.98, 1.12) | 1.01 (0.95, 1.08) | 0.76 | 6.2 (−0.2, 12.7) | 6.2% | 1.0% |
| Per additional week (trend) | 0.98 (0.97, 0.99) | 0.97 (0.96, 0.99) | <0.001 | |||||
Adjusted for child characteristics (birth year, sex, foetal growth, birth order, congenital anomalies) and maternal characteristics (age, education, employment, income, BMI, smoking, pre-eclampsia, other hypertensive disorders).
Incidence rate per 100 000 person-years.
Incidence rate difference per 100 000 person-years.
Prevented fraction among the exposed.
Population prevented fraction.
AFe, attributable fraction in the exposed; HR, hazard ratio; PAF, population attributable fraction.
Co-sibling analyses
Co-sibling analyses to control for unmeasured shared familial factors resulted in substantially lower risk estimates for hypertension that were no longer statistically significant (Table 4). For example, comparing preterm with full-term births, the adjusted HRs for hypertension at ages 0–43 years were 1.25 (95% CI, 1.20–1.29) in the primary analysis vs. 1.07 (0.98–1.16) in the co-sibling analysis; at ages 18–29 years were 1.28 (95% CI, 1.21–1.36) vs. 1.11 (0.98–1.26); and at ages 30–43 years were 1.25 (95% CI, 1.18–1.31) vs. 1.05 (0.90–1.22). Adjusted HRs for other major CVD also were substantially attenuated in co-sibling analyses, but remained significantly elevated at ages 0–43 years (1.36; 95% CI, 1.24–1.50) and <18 years (1.71; 1.49–1.96).
Table 4.
Co-sibling analyses of gestational age at birth and risk of hypertension or other major cardiovascular disease, Sweden, 1973–2015
| Hypertension |
Other major cardiovascular disease |
|||||
|---|---|---|---|---|---|---|
| Cases | HR (95% CI)a | P | Cases | HR (95% CI)a | P | |
| Attained ages 0–43 years | ||||||
| Preterm (<37 weeks) | 2697 | 1.07 (0.98, 1.16) | 0.13 | 1874 | 1.36 (1.24, 1.50) | <0.001 |
| Early term (37–38 weeks) | 8601 | 1.04 (1.00, 1.10) | 0.08 | 4845 | 1.04 (0.98, 1.11) | 0.16 |
| Full term (39–41 weeks) | 32 807 | Reference | 17 019 | Reference | ||
| Per additional week | 0.99 (0.97, 1.00) | 0.02 | 0.96 (0.95, 0.97) | <0.001 | ||
| Attained ages <18 years | ||||||
| Preterm (<37 weeks) | 629 | 1.12 (0.93, 1.34) | 0.24 | 1, 071 | 1.71 (1.49, 1.96) | <0.001 |
| Early term (37–38 weeks) | 2195 | 1.05 (0.95, 1.17) | 0.32 | 2284 | 1.11 (1.02, 1.21) | 0.01 |
| Full term (39–41 weeks) | 7639 | Reference | 7073 | Reference | ||
| Per additional week | 0.99 (0.96, 1.02) | 0.44 | 0.92 (0.91, 0.94) | <0.001 | ||
| Attained ages 18–29 years | ||||||
| Preterm (<37 weeks) | 1045 | 1.11 (0.98, 1.26) | 0.11 | 511 | 1.11 (0.94, 1.30) | 0.23 |
| Early term (37–38 weeks) | 3413 | 1.05 (0.98, 1.13) | 0.17 | 1652 | 0.99 (0.90, 1.08) | 0.82 |
| Full term (39–41 weeks) | 11 497 | Reference | 5941 | Reference | ||
| Per additional week | 0.98 (0.96, 0.99) | 0.02 | 1.00 (0.98, 1.02) | 0.81 | ||
| Attained ages 30–43 years | ||||||
| Preterm (<37 weeks) | 1023 | 1.05 (0.90, 1.22) | 0.54 | 292 | 1.03 (0.82, 1.31) | 0.78 |
| Early term (37–38 weeks) | 2993 | 1.06 (0.97, 1.15) | 0.22 | 909 | 0.97 (0.85, 1.12) | 0.69 |
| Full term (39–41 weeks) | 13 671 | Reference | 4005 | Reference | ||
| Per additional week | 0.98 (0.96, 1.00) | 0.09 | 1.00 (0.97, 1.03) | 0.82 | ||
Adjusted for shared familial (genetic and/or environmental) factors, and additionally for specific child characteristics (birth year, sex, foetal growth, birth order, congenital anomalies) and maternal characteristics (age, education, employment, income, BMI, smoking, pre-eclampsia, other hypertensive disorders).
HR, hazard ratio.
Secondary analyses
We explored for potential birth cohort effects on hypertension risks by stratifying by birth decade and including follow-up to age 20 years. Preterm birth was associated with an increased risk of hypertension in each birth cohort, compared with full-term birth (P < 0.01). However, this risk declined in the latest birth decade (adjusted HR by birth year, 1973–79: 1.68; 95% CI, 1.15–2.46; 1980–89: 1.68; 95% CI, 1.31–2.15; 1990–95: 1.24; 95% CI, 1.10–1.38; Supplementary material online, Table S3).
We further examined hypertension risks after stratifying by spontaneous (71.4%) or medically indicated (28.6%) preterm birth, which was systematically recorded starting in 1990 (maximum age 26 years at end of follow-up). Both spontaneous and medically indicated preterm birth was associated with increased risk of hypertension compared with full-term birth (adjusted HRs, 1.17; 95% CI, 1.08–1.27; P < 0.001; and 1.35; 95% CI, 1.23–1.48; P < 0.001, respectively), but medically indicated preterm birth was the stronger risk factor (P = 0.02 for difference in HRs).
Both preterm birth and SGA were independently associated with an increased risk of hypertension at ages 0–43 years in the fully adjusted model (preterm vs. full term: HR, 1.25; 95% CI, 1.20–1.29; SGA vs. AGA: HR, 1.15; 95% CI, 1.13–1.18), although preterm birth was the stronger risk factor (P < 0.001 for difference in HRs). However, no interactions were found (i.e. the association between preterm birth and hypertension risk did not vary significantly in those born SGA vs. AGA; Supplementary material online, Table S4).
Interactions between gestational age at birth and maternal pre-eclampsia are shown in Supplementary material online, Table S5. Both preterm birth and pre-eclampsia were independent risk factors for hypertension across ages 0–43 years. The highest risk was observed among those exposed to both preterm birth and pre-eclampsia (adjusted HR, 1.75; 95% CI, 1.61–1.91; P < 0.001, relative to those born full term and not exposed to pre-eclampsia). However, no interactions between gestational age at birth and pre-eclampsia were found.
Sensitivity analysis
In a sensitivity analysis, we restricted to persons born in 2001 or later when nationwide outpatient data were available throughout the duration of follow-up. All results were similar to those from the entire cohort for comparable ages. For example, the adjusted HR for hypertension at ages <15 years associated with preterm birth was 1.23 (95% CI, 1.12–1.36) among those born in 2001 or later, compared with 1.25 (1.15–1.36) in the entire cohort. These findings suggest that the overall results were not strongly influenced by the unavailability of outpatient data in earlier years.
Discussion
In this large national cohort study, gestational age at birth was inversely associated with risk of hypertension as well as other major CVD. After adjusting for other perinatal and maternal factors, persons born preterm or extremely preterm had >1.2- and 1.8-fold risks of hypertension, respectively. These associations persisted from childhood into early adulthood and affected males and females similarly. They appeared largely explained by shared genetic or environmental factors in families. Persons born preterm or extremely preterm also had >1.5- and 5.6-fold risks of other major CVD. The absolute risks for hypertension and other CVD associated with preterm birth were low at the young ages examined in this cohort but increased in adulthood when these conditions are more prevalent.
To our knowledge, this is the largest population-based study of preterm birth in relation to long-term hypertension risks. Prior studies have linked preterm birth with elevations in blood pressure measured cross-sectionally early in life. A meta-analysis of 10 studies with 1342 preterm- and 1738 term-born individuals aged 6–22 years reported that preterm birth was associated with higher SBP by 2.5 mm Hg on average (95% CI, 1.7–3.3).31 A meta-analysis of 27 studies with 17 030 preterm- and 295 261 term-born young adults (mean age 19.6 years) found that preterm birth was associated with higher SBP by 4.2 mm Hg (95% CI, 2.8–5.7) and higher DBP by 2.6 mm Hg (95% CI, 1.2–4.0), with stronger effects among women.13 Another pooled analysis of nine cohorts with 1571 young adults born at very low birth weight (<1500 g) and 777 controls reported similar findings: preterm birth was associated with higher SBP by 3.4 mm Hg (95% CI, 2.2–4.6) and higher DBP by 2.1 mm Hg (1.3–3.0 mm Hg), with slightly stronger effects among women.14 In a Swedish cohort of 636 552 adults, preterm birth was associated with increased prescription of antihypertensive medications at ages 25–37 years.11 A UK study examined single-day blood pressure measurements in 7847 adults aged 44–45 years and reported that each additional week of gestation was associated with a lower SBP by 0.53 mm Hg on average (95% CI, 0.32–0.75); however, there were too few preterm births to examine more specific gestational age subgroups.32
The present study contributes to this evidence by providing population-based risk estimates longitudinally from childhood into adulthood in a national cohort of over 4 million persons. The findings show that those born preterm or early term have increased risks of new-onset hypertension in adulthood. Early term birth has seldom been examined in relation to hypertension. Heterogeneity of outcomes by gestational age among term births has increasingly been recognized, warranting separate examination of early term births.9 , 33 , 34 Increased risks of hypertension among persons born at early term may potentially contribute to the increased CVD mortality previously reported in this subgroup.9 , 33
These findings have several potential underlying mechanisms. Premature birth interrupts foetal angiogenesis during a critical developmental period, leading to reduced capillary density and increased arterial stiffness.35–37 Prematurity also has been associated with persistently elevated levels of antiangiogenic factors [e.g. soluble endoglin (sENG) and soluble fms-like tyrosine kinase-1 (sFlt-1)], which are correlated with resting and ambulatory blood pressure.38 Increases in placental-derived sENG and sFlt-1 have been linked with capillary rarefaction,39 , 40 which is a major determinant of vascular resistance and may contribute to the development of hypertension.41 , 42 Imaging studies have further shown that preterm-born adults have disproportionately reduced size and function of the heart and vascular system, including the microvasculature.43 Co-sibling analyses in the present study further suggest a role of shared familial factors. Family- and twin-based studies have suggested that genetic factors inherited primarily from the mother influence gestational age at birth, with an estimated heritability of 25–40%.44–46 In addition, heritability estimates for blood pressure range from 31% to 68%, with numerous genetic loci identified.47–49 Additional clinical and genetic studies are needed to elucidate possible shared genetic or environmental factors that might link premature birth with the development of hypertension.
The associations we found between preterm birth and hypertension may have several clinical implications. Hypertension may contribute to other long-term sequelae of preterm birth previously reported, including ischaemic heart disease and chronic kidney disease.50 , 51 Our findings suggest that preterm-born children and adults may need early preventive evaluation and long-term clinical follow-up for timely detection and treatment of hypertension. Long-term monitoring of renal function and proteinuria may also be needed in this patient population.51 Preventive actions should include targeted counselling to reduce other modifiable risk factors, including obesity, physical inactivity, smoking, and alcohol use.52–54 Medical records and history-taking for patients of all ages should include gestational age at birth to help trigger preventive counselling and anticipatory screening in those born prematurely.55–57 Effective strategies to reduce preterm birth are also urgently needed, including better access to preconception and prenatal care among high-risk women.58
A key strength of the present study is its national cohort design with follow-up into adulthood. Availability of highly complete nationwide birth and medical registry data, including primary care diagnoses, helped minimize potential selection or ascertainment biases. The large sample size enabled higher statistical power than prior studies and assessment of more narrowly defined gestational age groups. We were able to control for multiple perinatal and maternal factors and assess the potential influence of unmeasured shared familial factors using co-sibling analyses.
This study also had several limitations. First, detailed blood pressure measurements were unavailable to verify diagnoses. However, high (>95%) positive predictive values for hypertension and most CVD outcomes have been reported in the Swedish registries,16 and the Swedish national health system also helps reduce disparities in health care access, which may improve ascertainment of these outcomes in the general population. Second, although primary care diagnoses were included, they were available for 72% of the Swedish population,17 resulting in under-reporting. It is possible that persons born prematurely were more likely to be diagnosed with hypertension because of greater contact with the health care system (i.e. detection bias). However, this is less likely to occur in adulthood, where associations with new-onset hypertension were still observed. Third, information was unavailable on lifestyle factors during follow-up such as physical inactivity, poor diet, obesity, smoking, or alcohol use. These factors are potentially important modifiers of the association between preterm birth and hypertension and warrant further investigation in studies with access to such data. Fourth, despite up to 43 years of follow-up, this was still a relatively young cohort. Additional follow-up will be needed to examine prematurity in relation to hypertension at older ages in this or other large cohorts. Lastly, because of ongoing changes in neonatal and paediatric care, these findings may not be generalizable to later birth cohorts, which will need to be followed up for hypertension in future studies when such data become available.
In summary, this is the largest population-based study to date of gestational age at birth in relation to the long-term risk of hypertension. We found that persons born prematurely had increased risks of hypertension from childhood into early adulthood, although the absolute risks were low at these attained ages. Future interventions should prioritize the reduction of environmental risk factors for preterm birth and hypertension that are shared within families. Children and adults born prematurely may need early preventive evaluation and long-term monitoring for the development of hypertension, especially those born at the earliest gestational ages.
Funding
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health [R01 HL139536 to C.C. and K.S.]; the Swedish Research Council; the Swedish Heart-Lung Foundation; and ALF project grant, Region Skåne/Lund University, Sweden. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Conflict of interest: none declared.
Supplementary Material
References
- 1.Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, Ali R, Alvis-Guzman N, Azzopardi P, Banerjee A, Barnighausen T, Basu A, Bekele T, Bennett DA, Biadgilign S, Catala-Lopez F, Feigin VL, Fernandes JC, Fischer F, Gebru AA, Gona P, Gupta R, Hankey GJ, Jonas JB, Judd SE, Khang YH, Khosravi A, Kim YJ, Kimokoti RW, Kokubo Y, Kolte D, Lopez A, Lotufo PA, Malekzadeh R, Melaku YA, Mensah GA, Misganaw A, Mokdad AH, Moran AE, Nawaz H, Neal B, Ngalesoni FN, Ohkubo T, Pourmalek F, Rafay A, Rai RK, Rojas-Rueda D, Sampson UK, Santos IS, Sawhney M, Schutte AE, Sepanlou SG, Shifa GT, Shiue I, Tedla BA, Thrift AG, Tonelli M, Truelsen T, Tsilimparis N, Ukwaja KN, Uthman OA, Vasankari T, Venketasubramanian N, Vlassov VV, Vos T, Westerman R, Yan LL, Yano Y, Yonemoto N, Zaki ME, Murray CJ. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA 2017;317:165–182. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1345–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global Disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016;134:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ. The fetal origins of hypertension. J Hypertens Suppl 1996;14:S117–S120. [PubMed] [Google Scholar]
- 6.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–128. [PubMed] [Google Scholar]
- 7.Raju TNK, Pemberton VL, Saigal S, Blaisdell CJ, Moxey-Mims M, Buist S. Adults Born Preterm Conference Speakers and Discussants. Long-term healthcare outcomes of preterm birth: an executive summary of a conference sponsored by the National Institutes of Health. J Pediatr 2017;181:309–318 e1. [DOI] [PubMed] [Google Scholar]
- 8.Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and mortality in young adulthood. JAMA 2011;306:1233–1240. [DOI] [PubMed] [Google Scholar]
- 9.Crump C, Sundquist J, Winkleby MA, Sundquist K. Gestational age at birth and mortality from infancy into mid-adulthood: a national cohort study. Lancet Child Adolesc Health 2019;3:408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–2172. [DOI] [PubMed] [Google Scholar]
- 11.Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of hypertension among young adults who were born preterm: a Swedish national study of 636,000 births. Am J Epidemiol 2011;173:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson S, Iliadou A, Bergvall N, Tuvemo T, Norman M, Cnattingius S. Risk of high blood pressure among young men increases with the degree of immaturity at birth. Circulation 2005;112:3430–3436. [DOI] [PubMed] [Google Scholar]
- 13.Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics 2013;131:e1240–e1263. [DOI] [PubMed] [Google Scholar]
- 14.Hovi P, Vohr B, Ment LR, Doyle LW, McGarvey L, Morrison KM, Evensen KA, van der Pal S, Grunau RE, Collaboration A, Brubakk AM, Andersson S, Saigal S, Kajantie E. Blood pressure in young adults born at very low birth weight: adults born preterm international collaboration. Hypertension 2016;68:880–887. [DOI] [PubMed] [Google Scholar]
- 15.Statistics Sweden. The Swedish Medical Birth Register. https://www.socialstyrelsen.se/register/halsodataregister/medicinskafodelseregistret/inenglish (20 May 2019).
- 16.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundquist J, Ohlsson H, Sundquist K, Kendler KS. Common adult psychiatric disorders in Swedish primary care where most mental health patients are treated. BMC Psychiatry 2017;17:235.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.1993 guidelines for the management of mild hypertension: memorandum from a World Health Organization/International Society of Hypertension meeting. Guidelines Sub-Committee. J Hypertens 1993;11:905–918. [DOI] [PubMed] [Google Scholar]
- 19.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De CR, Dean V, Dickstein K, Filippatos G, Funck BC, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti RE, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti RE, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis Aj NP, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van ZP, Waeber B, Williams B; Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 20.Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, Invitti C, Litwin M, Mancia G, Pall D, Rascher W, Redon J, Schaefer F, Seeman T, Sinha M, Stabouli S, Webb NJ, Wühl E, Zanchetti A. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens 2016;34:1887–1920. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics 2012;129:e1552–e1561. [DOI] [PubMed] [Google Scholar]
- 23.Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey Smith G, Sattar N, Nelson SM, Lawlor DA. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation 2010;122:1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miettola S, Hartikainen AL, Vaarasmaki M, Bloigu A, Ruokonen A, Jarvelin MR, Pouta A. Offspring’s blood pressure and metabolic phenotype after exposure to gestational hypertension in utero. Eur J Epidemiol 2013;28:87–98. [DOI] [PubMed] [Google Scholar]
- 25.Hogberg L, Cnattingius S, Lundholm C, D’Onofrio BM, Langstrom N, Iliadou AN. Effects of maternal smoking during pregnancy on offspring blood pressure in late adolescence. J Hypertens 2012;30:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jonge LL, Harris HR, Rich-Edwards JW, Willett WC, Forman MR, Jaddoe VW, Michels KB. Parental smoking in pregnancy and the risks of adult-onset hypertension. Hypertension 2013;61:494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 28.VanderWeele TJ. Causal interactions in the proportional hazards model. Epidemiology 2011;22:713–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grambsch PM. Goodness-of-fit and diagnostics for proportional hazards regression models. Cancer Treat Res 1995;75:95–112. [DOI] [PubMed] [Google Scholar]
- 30.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3rd ed. New York: Wiley; 2003. [Google Scholar]
- 31.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 2012;59:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper R, Atherton K, Power C. Gestational age and risk factors for cardiovascular disease: evidence from the 1958 British birth cohort followed to mid-life. Int J Epidemiol 2009;38:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crump C, Sundquist K, Winkleby MA, Sundquist J. Early-term birth (37-38 weeks) and mortality in young adulthood. Epidemiology 2013;24:270–276. [DOI] [PubMed] [Google Scholar]
- 34.Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA 2013;309:2445–2446. [DOI] [PubMed] [Google Scholar]
- 35.Ingelfinger JR, Nuyt AM. Impact of fetal programming, birth weight, and infant feeding on later hypertension. J Clin Hypertens (Greenwich) 2012;14:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonamy AK, Andolf E, Martin H, Norman M. Preterm birth and carotid diameter and stiffness in childhood. Acta Paediatr 2008;97:434–437. [DOI] [PubMed] [Google Scholar]
- 37.Bonamy AK, Martin H, Jorneskog G, Norman M. Lower skin capillary density, normal endothelial function and higher blood pressure in children born preterm. J Intern Med 2007;262:635–642. [DOI] [PubMed] [Google Scholar]
- 38.Lewandowski AJ, Davis EF, Yu G, Digby JE, Boardman H, Whitworth P, Singhal A, Lucas A, McCormick K, Shore AC, Leeson P. Elevated blood pressure in preterm-born offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension 2015;65:607–614. [DOI] [PubMed] [Google Scholar]
- 39.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 2004;95:884–891. [DOI] [PubMed] [Google Scholar]
- 41.Shore AC, Tooke JE. Microvascular function in human essential hypertension. J Hypertens 1994;12:717–728. [PubMed] [Google Scholar]
- 42.Antonios TF, Rattray FM, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart 2003;89:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewandowski AJ, Augustine D, Lamata P, Davis EF, Lazdam M, Francis J, McCormick K, Wilkinson AR, Singhal A, Lucas A, Smith NP, Neubauer S, Leeson P. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 2013;127:197–206. [DOI] [PubMed] [Google Scholar]
- 44.Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG 2000;107:375–381. [DOI] [PubMed] [Google Scholar]
- 45.Wilcox AJ, Skjaerven R, Lie RT. Familial patterns of preterm delivery: maternal and fetal contributions. Am J Epidemiol 2008;167:474–479. [DOI] [PubMed] [Google Scholar]
- 46.Svensson AC, Sandin S, Cnattingius S, Reilly M, Pawitan Y, Hultman CM, Lichtenstein P. Maternal effects for preterm birth: a genetic epidemiologic study of 630,000 families. Am J Epidemiol 2009;170:1365–1372. [DOI] [PubMed] [Google Scholar]
- 47.Hottenga JJ, Boomsma DI, Kupper N, Posthuma D, Snieder H, Willemsen G, de Geus EJ. Heritability and stability of resting blood pressure. Twin Res Hum Genet 2005;8:499–508. [DOI] [PubMed] [Google Scholar]
- 48.Kupper N, Willemsen G, Riese H, Posthuma D, Boomsma DI, de Geus EJ. Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension 2005;45:80–85. [DOI] [PubMed] [Google Scholar]
- 49.Padmanabhan S, Newton-Cheh C, Dominiczak AF. Genetic basis of blood pressure and hypertension. Trends Genet 2012;28:397–408. [DOI] [PubMed] [Google Scholar]
- 50.Crump C, Howell EA, Stroustrup A, McLaughlin MA, Sundquist J, Sundquist K. Association of preterm birth with risk of ischemic heart disease in adulthood. JAMA Pediatr 2019;173:736.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crump C, Sundquist J, Winkleby MA, Sundquist K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ 2019;365:l1346.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staessen JA, Wang J, Bianchi G, Birkenhager WH. Essential hypertension. Lancet 2003;361:1629–1641. [DOI] [PubMed] [Google Scholar]
- 53.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA 2009;302:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crump C, Sundquist J, Winkleby MA, Sundquist K. Interactive effects of physical fitness and body mass index on the risk of hypertension. JAMA Intern Med 2016;176:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crump C. Medical history taking in adults should include questions about preterm birth. BMJ 2014;349:g4860.. [DOI] [PubMed] [Google Scholar]
- 56.Crump C. Birth history is forever: implications for family medicine. J Am Board Fam Med 2015;28:121–123. [DOI] [PubMed] [Google Scholar]
- 57.Crump C, Sundquist K, Sundquist J. Adult outcomes of preterm birth. Prev Med 2016;91:400–401. [DOI] [PubMed] [Google Scholar]
- 58.Shapiro-Mendoza CK, Barfield WD, Henderson Z, James A, Howse JL, Iskander J, Thorpe PG. CDC grand rounds: public health strategies to prevent preterm birth. MMWR Morb Mortal Wkly Rep 2016;65:826–830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


