Abstract
Background
Minocycline (MIN) is a tetracycline with antioxidant, anti-inflammatory, and neuroprotective properties. Given the likely involvement of inflammation and oxidative stress (IOS) in schizophrenia, MIN has been proposed as a potential adjuvant treatment in this pathology. We tested an early therapeutic window, during adolescence, as prevention of the schizophrenia-related deficits in the maternal immune stimulation (MIS) animal model.
Methods
On gestational day 15, Poly I:C or vehicle was injected in pregnant Wistar rats. A total 93 male offspring received MIN (30 mg/kg) or saline from postnatal day (PND) 35–49. At PND70, rats were submitted to the prepulse inhibition test. FDG-PET and T2-weighted MRI brain studies were performed at adulthood. IOS markers were evaluated in frozen brain tissue.
Results
MIN treatment did not prevent prepulse inhibition test behavioral deficits in MIS offspring. However, MIN prevented morphometric abnormalities in the third ventricle but not in the hippocampus. Additionally, MIN reduced brain metabolism in cerebellum and increased it in nucleus accumbens. Finally, MIN reduced the expression of iNOS (prefrontal cortex, caudate-putamen) and increased the levels of KEAP1 (prefrontal cortex), HO1 and NQO1 (amygdala, hippocampus), and HO1 (caudate-putamen).
Conclusions
MIN treatment during adolescence partially counteracts volumetric abnormalities and IOS deficits in the MIS model, likely via iNOS and Nrf2–ARE pathways, also increasing the expression of cytoprotective enzymes. However, MIN treatment during this peripubertal stage does not prevent sensorimotor gating deficits. Therefore, even though it does not prevent all the MIS-derived abnormalities evaluated, our results suggest the potential utility of early treatment with MIN in other schizophrenia domains.
Keywords: FDG-PET, inflammatory/oxidonitrosative stress, minocycline, Poly I:C, schizophrenia
Significance Statement.
Minocycline (MIN) is a tetracycline with antioxidant, anti-inflammatory, and neuroprotective properties. We aim to evaluate the potential role of MIN administration during peri-adolescence, before the onset of the symptoms, as prevention of the schizophrenia-related deficits in the maternal immune stimulation (MIS) animal model. As far as we know, this is the first study to examine the preventive effect of MIN in brain glucose metabolism and morphology, evaluated by microPET and voxel-based morphometry (VBM) approaches together with behavioral and inflammatory/oxidative stress (IOS) studies. MIN treatment during adolescence partially counteracts morphometric abnormalities and IOS deficits via iNOS and Nrf2–ARE pathways, increasing the expression of cytoprotective enzymes. However, MIN treatment during this peripubertal stage does not prevent sensorimotor gating deficit. Therefore, despite not preventing all the pathological levels assessed, MIN effectivity highlights the usefulness of anti-IOS compounds to slow down the disease course at early stages.
Introduction
Schizophrenia is a chronic multifactorial psychiatric disorder and one of the most disabling mental disorders due to its chronicity, early onset, and high rate of suicide (Correll et al., 2019). Current treatments mainly focus on alleviating the associated symptoms, but their high failure rate (Correll et al., 2019) emphasizes the importance of finding new therapeutic strategies. Recently, there has been growing interest in the prevention of schizophrenia given its well-known association with genetic and environmental risk factors (van Os et al., 2014; Lipner et al., 2019) and its neuroprogressive nature (Zhao et al., 2017). Therefore, the detection of structural and functional brain deficits prior to clinical manifestations suggests the prodrome of this disease as a potential therapeutic window to halt the disease progression or reduce its severity (Millan et al., 2016).
Inflammation and oxidative stress play a key role in the development of schizophrenia. Thus, antioxidant and anti-inflammatory drugs have been proposed as possible therapeutic strategies (Kulak et al., 2013; Muller, 2018). In this respect, minocycline (MIN) (7-dimethylamino-6-dimethyl-6-deoxytetracycline), an antibiotic currently approved for the treatment of some chronic bacterially caused conditions with anti-inflammatory, antioxidant, and neuroprotective properties (Garrido-Mesa et al., 2013b), has received particular attention. Besides its well-recognized antibiotic properties, MIN gained emergent prominence in the late 1990s when a serendipitous neuroprotective effect was discovered in a patient with depression and a non-related bacterial infection (Levine et al., 1996). Since then, its neuroprotective effects have been demonstrated in several animal models of neurological diseases (Romero-Miguel et al., 2021). Thus, it has been hypothesized that MIN might reduce free radicals and pro-inflammatory cytokines released by over-activated microglia (Zhang and Zhao, 2014). In schizophrenia, the involvement of microglia over-activation led researchers to evaluate the possible neuroprotective effect of MIN. However, until now, the results have been contradictory. Some clinical trials have suggested the beneficial effect of MIN on the negative symptoms in early-phase (Levkovitz et al., 2010; Chaudhry et al., 2012; Liu et al., 2014) and chronic schizophrenia (Khodaie-Ardakani et al., 2014; Zhang et al., 2018). This finding would represent major progress given that current treatments are not effective enough to alleviate such symptoms. Nonetheless, other clinical trials have failed to replicate these improvements (Kelly et al., 2015; Deakin et al., 2018). Therefore, although great progress has been made, the real effect of MIN in schizophrenia remains elusive, especially in the prodromal phase of the disorder.
On the preclinical side, schizophrenia-like animal models may help to decipher the extent to which MIN could be useful as a preventive therapy. In this sense, the well-validated maternal immune stimulation (MIS) animal model is based on epidemiological studies showing the association between a maternal infection during pregnancy and the increased risk of developing schizophrenia in the offspring (Brown and Derkits, 2010). Thus, the stimulation induced by an immunogenic compound (e.g., Poly I:C) during pregnancy results in a sudden release of inflammatory mediators that may trigger long-lasting brain alterations, which become evident at adulthood (Zuckerman and Weiner, 2003). Consequently, adult MIS offspring show reduced hippocampi and enlarged ventricles (Piontkewitz et al., 2011) together with metabolic reductions in cortical regions and hippocampi and metabolic increases in the nucleus accumbens, amygdala, and thalamus (Hadar et al., 2015). In fact, these structural and metabolic disturbances are similar to those observed in patients with schizophrenia (Tamminga et al., 1992; Seethalakshmi et al., 2006; Haukvik et al., 2013; Berger et al., 2017; Kim et al., 2017). Furthermore, adult MIS animals also show behavioral deficits (Hadar et al., 2015) and biochemical alterations in inflammation/oxidative stress pathways (MacDowell et al., 2017; Casquero-Veiga et al., 2019). This is particularly interesting as inflammatory and oxidative stress (IOS) imbalances play an important role in the pathophysiology of the MIS model (Moller et al., 2015; Talukdar et al., 2020) and schizophrenia (Leza et al., 2015). Moreover, the pathophysiological delay showed by this model makes it a valuable tool for testing preventive approaches. In fact, we recently demonstrated the preventive effect of periadolescent treatments with risperidone (Casquero-Veiga et al., 2019) or omega-3 poly-unsaturated fatty acids (Casquero-Veiga et al., 2021) on schizophrenia-related abnormalities at adulthood in this MIS model.
Here, we aim to evaluate the potential role of MIN administration during peri-adolescence in the MIS rat model of schizophrenia. This work includes studies of brain metabolism, morphometry, behavior, and IOS.
Materials and Methods
Animals
Ninety-three male Wistar rats were maintained at constant temperature (24°C ± 0.5°°C) under a 12-hour-light/-dark cycle with free access to chow/water. Two batches of animals were used: 36 animals underwent behavioral studies, and 57 animals underwent imaging and IOS studies. All animal procedures were conducted in conformity with the European Communities Council Directive 2010/63/EU and approved by the Ethics Committee for Animal Experimentation of our hospital (ES280790000087).
Drug Treatment
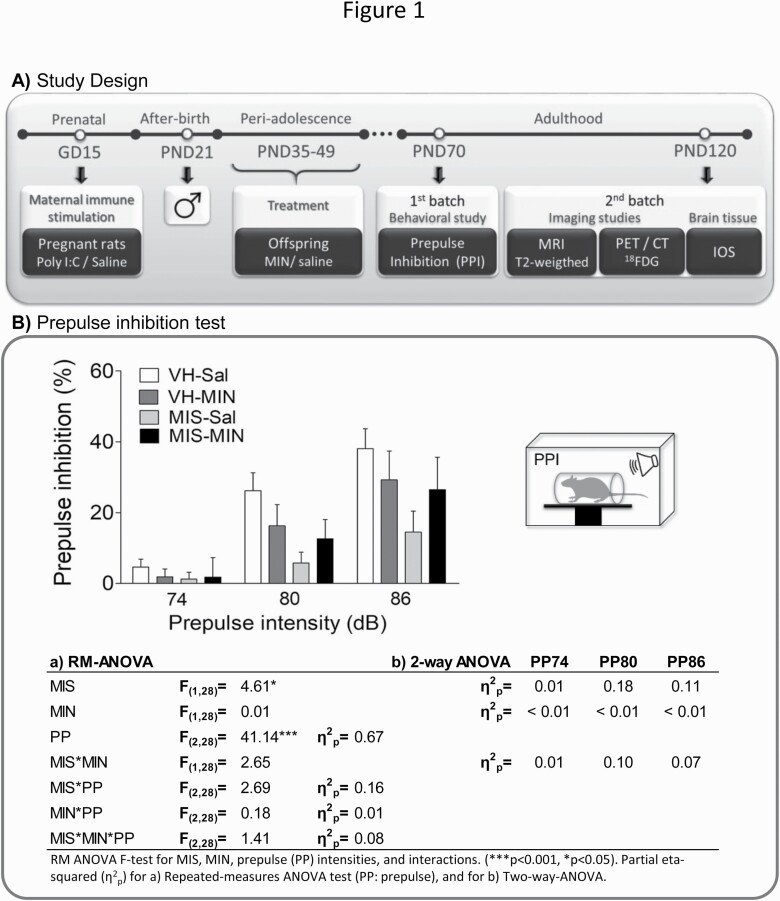
Figure 1A presents the drug treatment and study design. Prenatal Poly I:C or vehicle (VH, saline) was i.v. administered. On post-natal day (PND) 21, male offspring were weaned and housed 2-4 per cage. MIN (30 mg/kg, Sigma, M9511) (Levkovitz et al., 2007; Giovanoli et al., 2016) or saline (Sal) was i.p. injected during periadolescence (PND35–49) (Sengupta, 2013). Animals were divided into 4 groups according to the MIS condition (VH, MIS) and treatment (Sal, MIN).
Figure 1.
Study design and behavioral changes measured via PPI. (A) Representative diagram of the chronology of the experimental procedures performed during the study according to the age of the animals. Abbreviations: 18FDG, [18F]-Fluorodeoxyglucose; CT, computerized tomography; GD, gestational day; IOS, inflammatory/oxidative markers; MIN, minocycline; MRI, magnetic resonance imaging; PET, positron emission tomography; PND, post-natal day; PPI, prepulse inhibition. (B) MIN-related effects on PPI deficits in MIS rats. Each column represents the mean ± SEM of PPI (%) for each prepulse intensity (74, 80, and 86 dB) of 6–11 animals (VH-Sal 9, VH-MIN 6, MIS-Sal 11, MIS-MIN 6). The table represents the RM-ANOVA results and the effect size (partial eta-squared, η 2p) of each factor. Furthermore, effect sizes are also reported in a 2-way ANOVA approach to assess the specific effect of MIS and MIN factors on PPI without PP influence.
Behavioral Study: Prepulse Inhibition (PPI) Test
PPI of the acoustic startle response was measured at PND70. The session began with 10 minutes of acclimatization to the startle chamber (Cibertec, Spain) with 70-dB background noise followed by 5 trials of startle stimulus (pulse, 120 dB). Next, animals received 10 trials of pseudo-randomly presented stimuli: pulse (120 dB), prepulse (74, 80, or 86 dB) + pulse (120 dB), or no stimulus (background noise). Finally, 5 trials of pulse (120 dB) were conducted. Pulse and prepulse duration was 40 ms, and the prepulse-pulse interval was 100 ms, whereas the intertrial-interval ranged from 10 to 20 seconds.
Imaging Studies
Animals were scanned at adulthood (PND120) (11–17 animals/group) under sevoflurane anesthesia (4.5% induction, 2.5% maintenance in 100% O2).
Magnetic Resonance Imaging (MRI)
Animals were scanned using a 7-Tesla Biospec 70/20 scanner (Bruker, Germany) (Soto-Montenegro et al., 2014). A coronal T2-weighted spin-echo sequence was acquired with TE = 33 milliseconds, TR = 3732 milliseconds, averages 2, and slice thickness 0.4 mm. Matrix size was 256 × 256 pixels at a FOV of 3.5 × 3.5 cm2.
Positron Emission Tomography (PET)
After 45 minutes of 2-deoxy-2-[18F]fluoro-D-glucose (FDG) uptake (approximately 37 MBq, i.v.), animals were scanned for 45 minutes using a small-animal ARGUS PET/CT scanner (SEDECAL, Spain). Images were reconstructed with a 2D-OSEM algorithm (1.45 mm full width at half maximum [FWHM], voxel size 0.3875 × 0.3875 × 0.775 mm3, energy window of 400–700 keV) and corrected for decay and dead time.
Computed Tomography (CT)
Images were acquired using the above-mentioned scanner with the following parameters: 340 mA, 40 KV, 360 projections, 8 shots, and resolution of 200 µm. Images were reconstructed using a Feldkamp algorithm (isotropic voxel size of 0.121 mm) (Abella et al., 2012).
Biochemical Determinations
Frozen tissue samples (−80°C) from the prefrontal cortex, hippocampus, caudate-putamen, and amygdala (5–8 animals/group) were used for the study of biochemical determinations. IOS parameter selection was based on previous works (Leza et al., 2015; MacDowell et al., 2017; Casquero-Veiga et al., 2019, 2021).
Western Blot
Inflammatory mediators (inducible nitric oxide synthase [iNOS] and cyclooxygenase-2 [COX-2]) and the antioxidant pathway (Kelch-like ECH-associated protein 1 [KEAP1], heme oxygenase-1 [HO1], NAD(P)H:quinone oxidoreductase-1 [NQO1]) expression were measured in cytosolic extracts (MacDowell et al., 2013). Protein levels were assessed using the Bradford method.
Proteins were loaded into electrophoresis gel and then blotted onto a membrane with a semi-dry transfer system. Blots were blocked with 5% bovine serum albumin (BSA) (Sigma, Spain) for 1 hour at room temperature and probed overnight at 4°C with rabbit anti-iNOS (sc-650, 1:750 BSA 2%; SCBT), goat anti-COX2 (sc-1747, 1:750 BSA 2.5%; SCBT), mouse anti-KEAP1 (MAB3024, 1:1000; R&D), rabbit anti-HO1 (ab68477, 1:1000; Abcam), goat anti-NQO1 (sc16464, 1:750; BSA 1%, SCBT), and mouse anti-β-actin (A5441, 1:10 000; Sigma). Primary antibodies were incubated for 1.5 hours at room temperature with horseradish peroxidase-linked secondary antibodies. Binding was detected by an Odyssey Fc System (LI-COR, Germany). All western blots were performed at least 3 times in separate assays.
Lipid Peroxidation
Lipid peroxidation was determined by the thiobarbituric acid test for malondialdehyde as described (Das and Ratty, 1987).
Nuclear Erythroid-Related Factor Activity
Activation of nuclear erythroid-related factor (NRF2) was measured in nuclear extracts of tissue samples through a commercial ELISA-based kit (600590, Cayman Chemical).
Antioxidant Activity
Tissue samples were sonicated in 400 µL phosphate-buffered saline (pH = 7) with a protease inhibitor cocktail (Complete, Roche). Homogenates were centrifuged at 10 000 g for 15 minutes at 4°C. Supernatants were used to determine the activity of superoxide dismutase (SOD) (K028-H1, Arbor Assay), catalase (CAT) (K033-H1, Arbor Assay), and glutathione peroxidase (GPx) (703102, Cayman Chemical), and concentration of glutathione (GSH) (K006-H1, Arbor Assay). Results were expressed in U/mg of protein for the enzymes and in µM/µg of protein for GSH.
Data Processing and Analysis
PPI Data
The percent of PPI for each prepulse intensity was calculated as follows: 100 – ([prepulse + pulse)/pulse) × 100]). Note that prepulse + pulse is the startle response of the 10 PPI trials for each intensity, and pulse is the mean startle response of the 10 pulse-alone trials. Data were analyzed by means of repeated-measurements ANOVA.
MRI and PET Data Processing
A voxel-based morphometry (VBM) approach was performed in MRI data (Casquero-Veiga et al., 2019). Briefly, T2 images were preprocessed, realigned, and resliced to (Valdes-Hernandez et al., 2011) rat brain template space using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12). A custom brain template was created based on these data (Avants and Gee, 2004), and all resliced images were registered to the template. Then we obtained gray matter (GM) and cerebrospinal fluid (CSF) segmented and modulated images using, first, the probabilistic maps of the Valdes-Hernandez template as priors, and second, the Jacobian determinants from the spatial normalization process. Modulated images were smoothed by a 10-mm FWHM Gaussian filter and then used for statistical analyses.
In addition, raw T2-images were registered to a common CT reference using the algorithms described in (Gasull-Camos et al., 2017). Then, 5 manual regions of interest (ROIs) were segmented on each MRI image in the hippocampus, ventricles, prefrontal cortex (PFC), cortex, and whole brain according to (Paxinos and Watson, 2008).
PET image post-processing and intensity normalization were performed as described in (Gasull-Camos et al., 2017). Briefly, all PET images were registered to the same stereotactic space as MRI images and normalized in intensity to a cluster of brain regions that do not show statistically significant differences between the groups, following a data-driven approach (Borghammer et al., 2009). Then, images were smoothed by a Gaussian kernel of 2.5 times the voxel size of FWHM and masked to eliminate extracerebral voxels from the voxel-based analysis.
Furthermore, 6 manual ROIs (whole brain, caudate-putamen, hippocampus, cortex, PFC, and amygdala) were segmented on a registered MRI according to (Paxinos and Watson, 2008) and applied to all PET smoothed images.
Biochemical Analysis Processing
Digital images of western blots were analyzed using densitometry (ImageJ, NIH). Values were normalized to the loading control (β-actin) and expressed as a percentage variation from control. Data from the activity assays were normalized to the protein content of each sample.
Voxel-Based Analyses
The statistical analyses were conducted using SPM12. Groups were compared using 2-way ANOVAs, and results were obtained setting a threshold of P < .01 uncorrected at voxel-level significance but cluster-based corrected by false discovery rate to avoid type II error. A 1000-voxel clustering threshold was applied to minimize type I error. PET results were obtained setting a threshold of P < .05 uncorrected at voxel-level significance but cluster-based corrected. A 50-voxel clustering threshold was applied.
Statistical Analysis of ROI and Biochemical Data
The normality and homoscedasticity of each variable were tested using Shapiro-Wilk’s and Levene’s test, respectively. Heteroscedastic or abnormally distributed data were transformed through a 2-step approach (Templeton, 2011) to ensure both homoscedasticity and normality. Data were analyzed by means of 2-way ANOVAs followed by Bonferroni post-hoc test (P < .05). Data were expressed as mean ± SEM.
Results
Behavioral Study Results
Figure 1B shows the PPI results. RM-ANOVA reveals a significant MIS and prepulse effect. However, it does not reveal a statistically significant 3-way interaction (prepulse, MIS, and MIN).
Brain Volumetric Results
Manual ROI Analysis
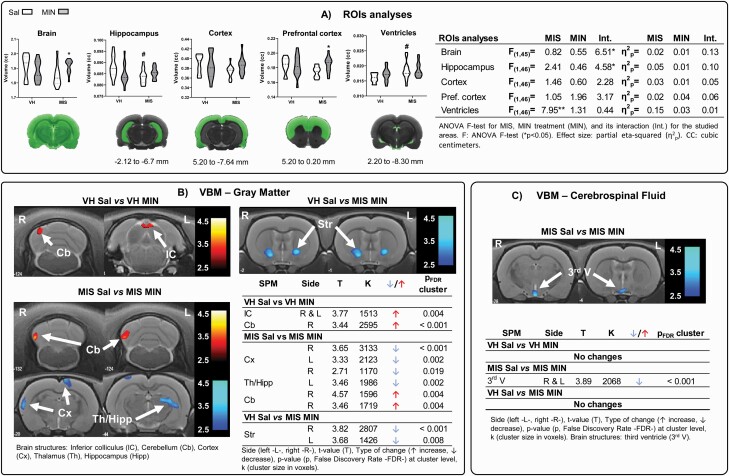
A significant MIS effect was found in ventricular and hippocampal regions, with increased volume in ventricles and reduced volume in the hippocampus in MIS vs VH animals (Figure 2A). No effects of MIN were observed. An interaction between MIS and MIN was found in the whole brain and hippocampus. Finally, post-hoc tests showed a significant increase of the whole brain and prefrontal cortex in MIS animals after MIN treatment.
Figure 2.
Volumetric changes measured via MRI. (A) ROI analysis: violin plots of global and regional volumetric changes in adulthood after MIN treatment during adolescence in saline and MIS animals (VH-Sal 11, VH-MIN 12, MIS-Sal 13, MIS-MIN 14). Two-way ANOVA followed by Bonferroni post-hoc test (*P < .05 vs saline-treated animals; #P < .05 vs VH-saline animals). Voxel-based SPM in (B) gray matter (GM) and (C) cerebrospinal fluid (CSF). VBM results in T-maps overlaid on a T2-MR template showing MIN pharmacological effect in VH and MIS animals. The color bars represent the T values corresponding to GM enlargements (warm) and shrinkages (cold). Tables show MIN-related effects on brain volumetric changes in the voxel-based analysis in the VH and MIS animals.
VBM Analysis
MIN treatment produced significant GM enlargements in the cerebellum and inferior colliculus in VH animals (Figure 2B–C; supplementary Figure 1A). In addition, MIN enlarged the GM in areas of the cerebellum and brainstem and decreased it in the cortex and the hippocampal-thalamic area. Also, striatal shrinkage became evident in MIN-treated MIS animals compared with the VH-saline group (Figure 2B). Also, MIN diminished the third ventricle in MIS animals (Figure 2C).
Brain Metabolic Results
Manual ROI Analysis
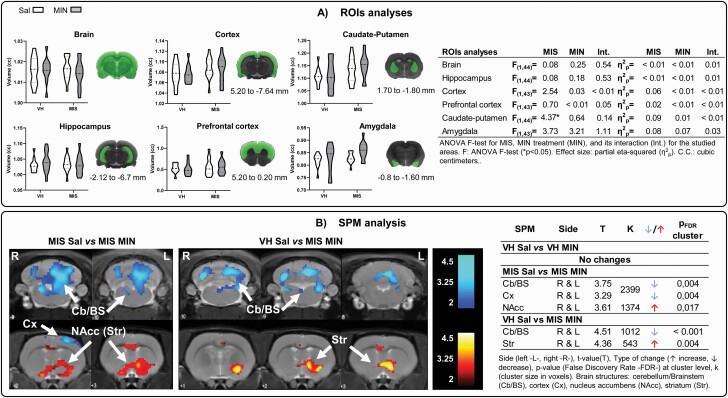
A significant MIS effect was found in the caudate-putamen, with increased metabolism observed. No statistically significant MIN or interaction effects were observed (Figure 3A).
Figure 3.
Brain metabolic changes measured via PET. (A) ROI analysis: ROIs were placed by identifying the 3D coordinates of each structure on the rat brain atlas (Paxinos and Watson, 2008) and locating the corresponding position in the MRI. Violin plots show the metabolic changes at adulthood after minocycline treatment during adolescence (VH-Sal 11, VH-Min 11, MIS-Sal 14, MIS-Min 12). Two-way ANOVA followed by Bonferroni post-hoc test was performed (*P < .05, MIS effect). Table shows minocycline-related effects on brain metabolism in Sal and MIS animals. (B) SPM analysis: colored PET overlays on the MR reference indicate reduced (blue) and increased (red) FDG uptake after minocycline treatment in MIS animals and compared with control animals (effectivity effect). The color bars represent the T value.
SPM Analysis
In VH animals, MIN did not modify brain metabolism (Figure 3B; supplementary Figure 1B). In MIS animals, MIN significantly diminished metabolism in the brainstem, cortex, and cerebellum, and it increased FDG uptake in the cingulate cortex and nucleus accumbens. Furthermore, similar results were obtained when comparing MIS-MIN animals with the VH-saline group.
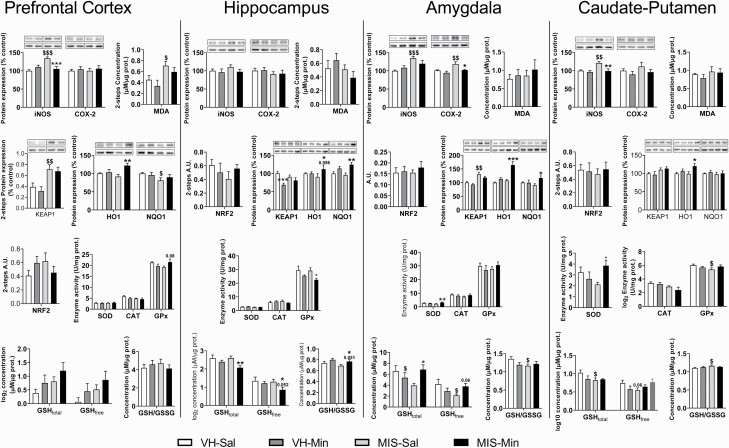
Oxidative/Inflammatory Parameters
Table 1 and Figure 4 show the results of IOS analyses.
Table 1.
Minocycline-Related Effects on the Expression of Inflammatory and Oxidative Markers in VH and MIS Offspring
| Inflammatory/oxidative markers | MIS | Treatment | Interaction | |||
|---|---|---|---|---|---|---|
| iNOS | ||||||
| Prefrontal cortex | F(1,28) = 9.80** | η 2p = 0.26 | F(1,28) = 4.090.053 | η 2p = 0.13 | F(1,28) = 17.52*** | η 2p = 0.39 |
| Hippocampus | F(1,28) = 0.75 | η 2p = 0.03 | F(1,28) = 1.60 | η 2p = 0.05 | F(1,28) = 0.44 | η 2p = 0.01 |
| Amygdala | F(1,28) = 13.55*** | η 2p = 0.33 | F(1,28) = 0.25 | η 2p = 0.01 | F(1,28) = 3.52 | η 2p = 0.11 |
| Caudate-putamen | F(1,28) = 7.72** | η 2p = 0.22 | F(1,28) = 8.87** | η 2p = 0.24 | F(1,28) = 4.75* | η 2p = 0.14 |
| COX2 | ||||||
| Prefrontal cortex | F(1,28) = 0.02 | η 2p < 0.01 | F(1,28) = 0.57 | η 2p = 0.20 | F(1,28) = 0.02 | η 2p < 0.01 |
| Hippocampus | F(1,28) = 1.49 | η 2p = 0.05 | F(1,28) = 0.02 | η 2p < 0.01 | F(1,28) < 0.001 | η 2p < 0.01 |
| Amygdala | F(1,28) = 8.94** | η 2p = 0.24 | F(1,28) = 7.42* | η 2p = 0.21 | F(1,28) = 1.40 | η 2p = 0.05 |
| Caudate-putamen | F(1,28) = 1.06 | η 2p = 0.04 | F(1,28) = 2.22 | η 2p = 0.07 | F(1,28) = 0.09 | η 2p < 0.01 |
| Oxidative markers | ||||||
| KEAP1 | ||||||
| Prefrontal cortex | F(1,27) = 20.08*** | η 2p = 0.43 | F(1,27) = 0.54 | η 2p = 0.20 | F(1,27) = 0.07 | η 2p < 0.01 |
| Hippocampus | F(1,28) = 0.05 | η 2p < 0.01 | F(1,28) = 13.49** | η 2p = 0.33 | F(1,28) = 3.76 | η 2p = 0.12 |
| Amygdala | F(1,28) = 28.63*** | η 2p = 0.51 | F(1,28) = 3.52 | η 2p = 0.11 | F(1,28) = 0.22 | η 2p < 0.01 |
| Caudate-putamen | F(1,28) = 5.42* | η 2p = 0.16 | F(1,28) < 0.001 | η 2p < 0.01 | F(1,28) = 0.33 | η 2p = 0.01 |
| NRF2 | ||||||
| Prefrontal cortex | F(1,27) = 0.14 | η 2p < 0.01 | F(1,27) = 0.01 | η 2p < 0.01 | F(1,27) = 3.41 | η 2p = 0.11 |
| Hippocampus | F(1,26) = 0.53 | η 2p = 0.02 | F(1,26) = 0.05 | η 2p < 0.01 | F(1,26) = 1.70 | η 2p = 0.06 |
| Amygdala | F(1,28) = 0.13 | η 2p < 0.01 | F(1,28) = 0.51 | η 2p = 0.02 | F(1,28) = 0.15 | η 2p < 0.01 |
| Caudate-putamen | F(1,26) = 0.53 | η 2p < 0.01 | F(1,26) = 0.05 | η 2p < 0.01 | F(1,26) = 1.70 | η 2p < 0.01 |
| HO1 | ||||||
| Prefrontal cortex | F(1,28) = 0.69 | η 2p = 0.02 | F(1,28) = 7.81** | η 2p = 0.22 | F(1,28) = 4.93* | η 2p = 0.15 |
| Hippocampus | F(1,28) = 0.03 | η 2p < 0.01 | F(1,28) = 2.00 | η 2p = 0.07 | F(1,28) = 1.99 | η 2p = 0.07 |
| Amygdala | F(1,28) = 17.60*** | η 2p = 0.39 | F(1,28) = 25.28*** | η 2p = 0.48 | F(1,28) = 9.60** | η 2p = 0.26 |
| Caudate-putamen | F(1,28) = 1.12 | η 2p = 0.04 | F(1,28) = 5.66* | η 2p = 0.17 | F(1,28) = 1.42 | η 2p = 0.05 |
| NQO1 | ||||||
| Prefrontal cortex | F(1,28) = 4.110.052 | η 2p = 0.13 | F(1,28) = 0.14 | η 2p < 0.01 | F(1,28) = 1.03 | η 2p = 0.36 |
| Hippocampus | F(1,28) = 0.38 | η 2p = 0.01 | F(1,28) = 13.80*** | η 2p = 0.33 | F(1,28) = 1.78 | η 2p = 0.06 |
| Amygdala | F(1,28) = 0.28 | η 2p = 0.01 | F(1,28) = 3.16 | η 2p = 0.10 | F(1,28) = 3.54 | η 2p = 0.11 |
| Caudate-putamen | F(1,28) = 0.28 | η 2p = 0.01 | F(1,28) = 0.8 | η 2p = 0.01 | F(1,28) = 0.02 | η 2p < 0.01 |
| SOD | ||||||
| Prefrontal cortex | F(1,28) = 0.42 | η 2p = 0.02 | F(1,28) = 0.01 | η 2p < 0.01 | F(1,28) = 0.25 | η 2p = 0.01 |
| Hippocampus | F(1,27) = 5.19* | η 2p = 0.16 | F(1,27) = 0.38 | η 2p = 0.01 | F(1,27) = 0.59 | η 2p = 0.02 |
| Amygdala | F(1,28) = 0.01 | η 2p < 0.01 | F(1,28) = 2.72 | η 2p = 0.09 | F(1,28) = 5.84* | η 2p = 0.17 |
| Caudate-putamen | F(1,28) = 0.01 | η 2p < 0.01 | F(1,28) = 1.35 | η 2p = 0.05 | F(1,28) = 5.84* | η 2p = 0.17 |
| CAT | ||||||
| Prefrontal cortex | F(1,28) = 2.12 | η 2p = 0.07 | F(1,28) = 2.25 | η 2p = 0.07 | F(1,28) = 0.59 | η 2p = 0.02 |
| Hippocampus | F(1,28) = 0.001 | η 2p < 0.01 | F(1,28) = 0.41 | η 2p = 0.01 | F(1,28) = 2.78 | η 2p = 0.10 |
| Amygdala | F(1,28) = 0.54 | η 2p = 0.02 | F(1,28) = 0.04 | η 2p < 0.01 | F(1,28) = 2.08 | η 2p = 0.07 |
| Caudate-putamen | F(1,27) = 4.97* | η 2p = 0.18 | F(1,27) = 2.53 | η 2p = 0.04 | F(1,27) = 0.06 | η 2p = 0.01 |
| GSHfree | ||||||
| Prefrontal cortex | F(1,28) = 3.23 | η 2p = 0.10 | F(1,28) = 2.34 | η 2p = 0.08 | F(1,28) = 0.01 | η 2p < 0.01 |
| Hippocampus | F(1,28) = 1.72 | η 2p = 0.06 | F(1,28) = 3.50 | η 2p = 0.11 | F(1,28) = 1.01 | η 2p = 0.03 |
| Amygdala | F(1,28) = 0.94 | η 2p = 0.03 | F(1,28) = 0.11 | η 2p < 0.01 | F(1,28) = 5.64* | η 2p = 0.17 |
| Caudate-putamen | F(1,28) = 0.81 | η 2p = 0.03 | F(1,28) = 0.26 | η 2p = 0.01 | F(1,28) = 3.40 | η 2p = 0.11 |
| GSHtotal | ||||||
| Prefrontal cortex | F(1,28) = 3.41 | η 2p = 0.11 | F(1,28) = 2.59 | η 2p = 0.09 | F(1,28) = 0.003 | η 2p < 0.01 |
| Hippocampus | F(1,28) = 1.43 | η 2p = 0.05 | F(1,28) = 7.80** | η 2p = 0.22 | F(1,28) = 1.59 | η 2p = 0.05 |
| Amygdala | F(1,28) = 0.48 | η 2p = 0.02 | F(1,28) = 1.04 | η 2p = 0.04 | F(1,28) = 6.10* | η 2p = 0.18 |
| Caudate-putamen | F(1,26) = 2.16 | η 2p = 0.08 | F(1,26) = 0.98 | η 2p = 0.04 | F(1,26) = 1.85 | η 2p = 0.07 |
| GSHfree/GSSG | ||||||
| Prefrontal cortex | F(1,28) = 0.002 | η 2p < 0.01 | F(1,28) = 0.05 | η 2p < 0.01 | F(1,28) = 1.27 | η 2p = 0.04 |
| Hippocampus | F(1,28) = 2.07 | η 2p = 0.07 | F(1,28) = 6.47* | η 2p = 0.18 | F(1,28) = 0.12 | η 2p < 0.01 |
| Amygdala | F(1,27) = 1.91 | η 2p = 0.07 | F(1,27) = 0.83 | η 2p = 0.03 | F(1,27) = 3.09 | η 2p = 0.10 |
| Caudate-putamen | F(1,28) = 2.80 | η 2p = 0.09 | F(1,28) = 0.04 | η 2p < 0.01 | F(1,28) = 1.76 | η 2p = 0.06 |
| GPx | ||||||
| Prefrontal cortex | F(1,27) = 0.03 | η 2p < 0.01 | F(1,27) = 0.14 | η 2p < 0.01 | F(1,27) = 4.43* | η 2p = 0.14 |
| Hippocampus | F(1,27) = 0.74 | η 2p = 0.03 | F(1,27) = 7.35* | η 2p = 0.21 | F(1,27) = 0.43 | η 2p = 0.02 |
| Amygdala | F(1,28) = 0.08 | η 2p < 0.01 | F(1,28)<0.001 | η 2p < 0.01 | F(1,28) = 1.38 | η 2p = 0.05 |
| Caudate-putamen | F(1,28) = 1.52 | η 2p = 0.05 | F(1,28) = 0.04 | η 2p < 0.01 | F(1,28) = 4.010.054 | η 2p = 0.13 |
| MDA | ||||||
| Prefrontal cortex | F(1,27) = 6.56* | η 2p = 0.25 | F(1, 27) = 0.53 | η 2p = 0.06 | F(1,27) = 0.53 | η 2p < 0.01 |
| Hippocampus | F(1,27) = 2.03 | η 2p = 0.70 | F(1,27)<0.001 | η 2p < 0.01 | F(1,27) = 1.62 | η 2p = 0.06 |
| Amygdala | F(1,28) = 0.46 | η 2p = 0.02 | F(1,28) = 0.49 | η 2p = 0.02 | F(1,28) = 0.03 | η 2p < 0.01 |
| Caudate-putamen | F(1,27) = 1.10 | η 2p = 0.04 | F(1,27) = 0.39 | η 2p = 0.01 | F(1,27) = 0.14 | η 2p < 0.01 |
Abbreviations: CAT, catalase; COX2, cyclooxygenase-2; F, ANOVA F-test (*P < .05, **P < .01, ***P < .001); GSH, glutathione; GSSG, oxidized glutathione; GPx, glutathione peroxidase; HO1, heme oxygenase 1; iNOS: inducible nitric oxide synthase; KEAP1, Kelch like ECH associated protein 1; MDA, malondialdehyde; NRF2, nuclear factor-erythroid factor 2-related factor 2; NQO1, NAD(P)H:quinone oxidoreductase-1; SOD, superoxide dismutase; η 2p, partial eta-squared. Each column represents the ANOVA F-test and η 2p for MIS, minocycline treatment, and its interaction for the studied areas.
Figure 4.
Minocycline-related effects on the expression of IOS markers in VH and MIS animals. Inflammatory markers expression (iNOS, COX2), antioxidant enzyme activity (GPx, CAT, SOD), and oxidative stress markers (MDA, NRF2, KEAP1, HO1, NQO1, GSHfree, GSHtotal, GSSG) in prefrontal cortex, hippocampus, caudate-putamen, and amygdala (n = 7–8 animals). Representative bands of iNOS, COX2, KEAP1, HO1, and NQO1 (upper bands) and the loading control, β-actin (lower bands), are shown above their corresponding graph bars. Each column represents the mean ± SEM of 5–8 animals. Two-way ANOVA followed by a pairwise interaction contrast (*P < .05, **P < .01, ***P < .001, vs Sal-treated animals, &P < .05, &&P < .01, &&&P < .001 vs VH animals).
Prefrontal Cortex
A significant MIS effect was found in iNOS, KEAP1, and malondialdehyde and an almost significant effect was found in NQO1 (P = .052). A significant MIN effect appeared in HO1 and an almost significant effect in iNOS (P = .053). An interaction was found in iNOS, HO1, and GPx.
Hippocampus
A significant MIS effect was found in SOD. A significant MIN effect appeared in KEAP1, NQO1, GSHtotal, GSHfree/oxidized glutathione (GSSG), and GPx. No interaction was found.
Amygdala
ANOVA showed a significant MIS effect in iNOS, COX2, KEAP1, and HO1. A significant MIN effect appeared in HO1 and COX2. An interaction was found in HO1, SOD, GSHfree, and GSHtotal.
Caudate-Putamen
A significant MIS effect was found in iNOS, KEAP1, and CAT. A significant MIN effect appeared in iNOS and HO1. An interaction was found in iNOS and SOD and almost significant in GPx (P = 0.054).
Discussion
In this study, MIN treatment during periadolescence in the MIS model (1) prevents structural abnormalities in the third ventricle, (2) modulates brain metabolism in the cerebellum and nucleus accumbens, (3) reduces the expression of some brain IOS markers, and (4) does not prevent the behavioral deficit in PPI. To our knowledge, this is the first work to tackle this topic using FDG-PET, together with VBM, adapted to rodents.
Furthermore, we observed specific MIN-related effects in VH animals (ie, GM enlargements in the cerebellum and inferior colliculus, reduced levels of iNOS and COX-2, and increases in HO1 and NQO1). However, long-term treatment with minocycline is generally safe and well-tolerated in humans (Garrido-Mesa et al., 2013a; Zheng et al., 2019). Furthermore, no pathological implication has been associated with the changes observed in VH animals and, thus, they may be assumable given the potential benefits that minocycline-derived protection may have for the patient.
MIN Does Not Prevent Sensorimotor Gating Deficits
Sensorimotor gating deficits are manifested in the rat MIS model at adulthood as loss of PPI (Hadar et al., 2015; Bikovsky et al., 2016). As expected, our adult MIS animals showed deficits in PPI. However, MIN treatment during adolescence was not effective in halting the progression into a schizophrenia-like behavioral phenotype after infection-mediated neurodevelopmental disturbances. It is noteworthy that the evaluation of other behavioral domains could have provided a broader perspective of the preventive potential of MIN, given the different neurodevelopmental trajectories followed by each specific symptom (Piontkewitz et al., 2012). Therefore, while no successful results were obtained in PPI, MIN could potentially prevent other schizophrenia-like traits shown by the MIS model.
Several preclinical studies have reported that acute MIN treatment decreases hyperlocomotion (Zhang et al., 2007; Giovanoli et al., 2016), improves visual-spatial memory (Levkovitz et al., 2007; Fujita et al., 2008), and improves PPI impairments in MIS models (Mattei et al., 2014; Zhu et al., 2014a, 2014b). In addition, a recent study showed the preventive effect of MIN in a mouse double-hit model of schizophrenia (Giovanoli et al., 2016), showing that MIN administration during the peripubertal stage blocked central inflammatory responses to stress and improved PPI deficits but only in those animals exposed to prenatal immune activation and peripubertal stress. However, as in our study, these improvements did not occur in animals only exposed to MIS (Giovanoli et al., 2016). Several reasons could explain these outcomes: (1) the different animal models used (rats or mice); (2) the regimen of MIN administration, including dose (3–35 mg/kg) and period of time (ie, during adolescence [PND30-50] (Zhu et al., 2014a; Giovanoli et al., 2016) or beginning at late adolescence [PND60] (Mattei et al., 2014); and (3) behavioral studies were conducted after a 2-week drug washout period, whereas examinations in previous studies took place shortly after MIN treatment. This is important, since MIN acts over activated microglia, suggesting the need for activated microglia to obtain a MIN-related benefit on the brain. Thus far, MIN has demonstrated some beneficial effects when it is administered as an adjuvant to risperidone or clozapine. In this sense, MIN improved the negative symptoms in early-phase (Liu et al., 2014) or persistent schizophrenia (Khodaie-Ardakani et al., 2014; Kelly et al., 2015), and it also improved working memory, anxiety, and depression (Kelly et al., 2015), but its preventive potential has only recently been explored in humans. In this respect, only 1 study addresses a 6-month intervention with MIN added to regular treatment in individuals at risk of developing schizophrenia or psychosis (Qurashi et al., 2017), although no results have been reported yet.
MIN Prevents Volumetric Abnormalities in the Third Ventricle
Two of the volumetric traits induced by MIS are increased ventricular volume and decreased hippocampal volume (Piontkewitz et al., 2011; Hadar et al., 2018; Casquero-Veiga et al., 2019). Here, MIN treatment during peri-adolescence was effective in preventing this enlargement at the third ventricle level, although no hippocampal recovery was observed. Studies addressing structural brain changes in patients with early-phase schizophrenia showed a prevention of GM loss in the mid-posterior cingulate cortex and precentral gyrus after 1 year of MIN treatment as an adjuvant to patients’ usual treatment with antipsychotic medication (Chaves et al., 2010). However, the BeneMin trial, performed in a study population comprising people within 5 years of a schizophrenia diagnosis, failed to show GM volume changes after 1 year of treatment (Deakin et al., 2018), which was related to a plateau in GM volume by the time of recruitment. These contradictory results highlight the necessity of future studies considering patients at early stages in which MIN could be effective. However, MIN’s side effects (Utari et al., 2010), as well as the fact that not all at-risk people complete the transition to pathology, give the cost/benefit ratio of MIN particular relevance in selecting this kind of preventive strategy. Therefore, further research in this topic is essential to consider MIN as a plausible strategy in schizophrenia prevention.
At the preclinical side, while several animal studies investigated the MIN effects through MRI techniques, many were performed in animal models of vascular neurological diseases (Tang et al., 2016; Dai et al., 2019), but none of them were conducted in schizophrenia models. Interestingly, one of these studies reported reductions in edema, microglia activation, and lateral ventricular volume, as in our study, in association with MIN use in a model of germinal matrix hemorrhage (Tang et al., 2016). Moreover, the protective effects of MIN were related to the activation of cannabinoid receptor 2 (CB2R) (Tang et al., 2016). In this respect, recent studies highlighted the potential of CB2R alterations in the neurobiology of psychiatric disorders (Ortega-Alvaro et al., 2011; Rodriguez-Munoz et al., 2017). In fact, first episode of psychosis (FEP) and patients with chronic schizophrenia have shown reduced levels of CB2R (Bioque et al., 2013), and the deletion of CB2R in mice has been related to schizophrenia-like behaviors (Ortega-Alvaro et al., 2011). In addition, CB2R are expressed in perivascular microglia, a special microglia with fast turnover and a crucial role in processes such as viral entry to the CNS (Nunez et al., 2004). In this context, our results suggest that MIN treatment may increase CB2R in the microglia, inducing a reduction of edema and lateral ventricular volume in our MIS model. However, further research is necessary to study the effect of MIN on the cannabinoid system in this animal model.
In addition, our VBM analysis revealed that MIN induced significant structural enlargements in the inferior colliculus (IC) in the VH animals, with a similar tendency observed for the MIS animals (supplementary Figure 1), the brainstem in the MIS animals, and the cerebellum in both groups. Reductions in the volume of the IC (Kang et al., 2008) and the cerebellum (Yeganeh-Doost et al., 2011) have been reported in patients with schizophrenia, and both structures have been associated with the auditory-cognitive dysfunction seen in this pathology (Horga et al., 2014; Cierpka et al., 2017). Moreover, abnormal glutamatergic neurotransmission was also shown in these structures (Schmitt et al., 2010; Yeganeh-Doost et al., 2011), and MIN acts on the glutamatergic system by modulating the N-methyl-D-aspartate receptor (Garrido-Mesa et al., 2013a), which is altered in schizophrenia (Anderson and Maes, 2013). We have found that MIN partially counteracts the structural shrinkage in these brain areas, probably by enhancing the glutamatergic neurotransmission (Sanchez-Perez et al., 2005; Chaves et al., 2009), reinforcing the idea that MIN could be useful for alleviating the auditory hallucinations in schizophrenia (Shergill et al., 2000).
Surprisingly, MIN-treated MIS animals showed striatal shrinkage compared with VH-saline animals. Striatal alterations are a well-known trait of schizophrenia patients (Ebdrup et al., 2010; Stegmayer et al., 2014; Okada et al., 2016) and also in subjects in the MIS model (Casquero-Veiga et al., 2019). When we explored whether these alterations could be influenced by the MIN treatment, we observed similar results with a less restrictive significance threshold than the one shown before in VH and MIS animals (supplementary Figure 1), suggesting that MIN is not able to counteract this striatal GM reduction compared with antipsychotics such as haloperidol (Konradi and Heckers, 2001; Andersson et al., 2002) or risperidone (Massana et al., 2005; Casquero-Veiga et al., 2019).
MIN Modulates the Cerebellum and Nucleus Accumbens Metabolism
PET studies in schizophrenia patients have shown cortical and subcortical dysfunctions, with reductions in glucose metabolism at global and regional scales compared with controls (Kim et al., 2017). To date, no FDG-PET study has evaluated the effect of MIN on brain metabolism. Nevertheless, huge efforts have been made in understanding the role of microglia and brain plasticity changes in acute psychosis and schizophrenia by using translocator protein-PET tracers (De Picker et al., 2017). However, results are contradictory: while activated microglia has been detected in the frontal and temporal lobes and the hippocampus in patients with ultra-high risk of psychosis (Bloomfield et al., 2016) and schizophrenia (van Berckel et al., 2008; Doorduin et al., 2009), other authors could not find microglia changes in patients at various clinical stages (Kenk et al., 2015; Coughlin et al., 2016; Collste et al., 2017; Di Biase et al., 2017). The intrinsic limitations of translocator protein-PET could be responsible for these inconclusive data.
In our study, treatment with MIN during adolescence reduced brain metabolism in the cerebellum and increased FDG uptake in the nucleus accumbens in MIS animals, while it did not modify the brain metabolism in VH animals. The cerebellum plays an important role in the pathophysiology of schizophrenia (Moberget et al., 2018). Thus, disruptions in cerebrocerebellar functional connectivity may partially underlie the psychotic symptoms and cognitive deficits seen in schizophrenia (Andreasen et al., 1998) as well as the onset and severity of neurodevelopmental psychiatric disorders (Sathyanesan et al., 2019; Dong et al., 2020). In vivo PET neuroimaging studies in controls and schizophrenia patients showed that the cerebellum is activated in a variety of mental activities, apart from its classical involvement in motor activity (Andreasen and Pierson, 2008). Preclinical FDG-PET studies showed reduced metabolism in the cerebellum at adulthood after risperidone treatment during adolescence (Casquero-Veiga et al., 2019) or during acute deep-brain stimulation in the medial PFC (Bikovsky et al., 2016), both in the MIS model. In this sense, our results support the claim that MIN treatment during adolescence could modify cerebellar physiological activity, which could be related to the antidepressant properties attributed to this compound (Pae et al., 2008).
Unexpectedly, MIN treatment in our study increased FDG uptake in the nucleus accumbens in the MIS animals, which may be related to the striatal constriction produced by MIN and previously discussed. Abnormalities of the mesolimbic dopaminergic and reward systems have been reported in psychiatric disorders, such as schizophrenia or depression (Dubol et al., 2018). In the MIS model, abnormal increased glucose metabolism in the nucleus accumbens was also found, and it correlated with high levels of dopamine in this area compared with saline offspring (Hadar et al., 2015). However, we found that MIN treatment during adolescence did not revert the abnormally enhanced metabolic activity in the nucleus accumbens, suggesting a permanence of the exacerbated dopaminergic activity in the mesolimbic system. This fact could be responsible for our not obtaining any improvement in the PPI in the MIS animals, since the nucleus accumbens is involved in sensorimotor gating disruption (Geyer et al., 2001). Therefore, while MIN would not counteract the PPI deficits, schizophrenia could benefit from its use for the treatment of negative symptoms as adjunctive therapy (Khodaie-Ardakani et al., 2014; Liu et al., 2014; Deakin et al., 2018).
MIN May Prevent Brain Inflammatory and Oxidative Alterations
MIN was first shown to be an effective anti-inflammatory and antioxidant drug more than 2 decades ago, when 2 studies demonstrated minocycline’s ability to attenuate iNOS expression (Amin et al., 1996; Lee et al., 2004). Here, MIS animals showed higher levels of pro-inflammatory molecules, mainly in the PFC, amygdala, and caudate-putamen, as previously reported (Casquero-Veiga et al., 2019). The 2-week MIN treatment during rat adolescence downregulated iNOS expression in the PFC as well as caudate-putamen and COX-2 expression in the amygdala, with similar trends in the hippocampus and amygdala for iNOS activity. Therefore, our results indicate that MIN exerts its anti-inflammatory action mainly through the iNOS pathway, like antipsychotics with anti-inflammatory activity such as risperidone (Casquero-Veiga et al., 2019). Previous preclinical studies have also demonstrated the iNOS downregulation induced by MIN in different animal models (Wu et al., 2002; Zheng et al., 2014) as well as other pro-inflammatory molecules, such as NF-κB, in cell culture studies (Sun et al., 2015; Tian et al., 2017). Remarkably, only 1 study has explored MIN as a preventive strategy; it operated with a mouse double-hit model of schizophrenia, also showing beneficial effects of MIN in microglia activation and interleukin-1β expression in the hippocampus and PFC (Giovanoli et al., 2016). Clinically, there are only 2 studies that have suggested the benefit of MIN in the regulation of negative symptoms and its association with pro-inflammatory cytokine levels (Zhang et al., 2018, 2019). Thus, the addition of MIN to risperidone during a treatment course of 3 months reduced the serum levels of IL-1β, IL-6, and TNF-α together with a significant improvement in negative symptoms (Zhang et al., 2018, 2019).
Furthermore, we found that MIN increased the activity/concentration of KEAP1 in the PFC, HO1, and NQO1 in the amygdala, HO1 in the caudate-putamen, and HO1 and NQO1 in the hippocampus only in the MIS animals. One possible explanation for the changes in the MIS animals may be related to the presence of high basal levels of oxidative stress: the bactericidal actions of minocycline prevail over its anti-inflammatory consequences, and hence its antioxidant effect may become more evident in a context of oxidative/nitrosative damage. These molecules are part of the Keap1-Nrf2-ARE (Antioxidant Response Elements) pathway, which is involved in the cellular defense system against oxidative stress. Previous preclinical studies have shown the acute effect of MIN in the modulation of oxidative damage by increasing the Nrf2 pathway (Sakata et al., 2012; Tian et al., 2017). Furthermore, MIN increased antioxidant molecules such as CAT, SOD, and HO1 after nigrostriatal dopaminergic damage in a Parkinson’s Disease model (Kumar et al., 2016). However, as far as we know, no study has evaluated the preventive effect of MIN during adolescence on oxidative parameters at adulthood in either the MIS model, schizophrenia patients, or those identified to have a high risk of psychosis. Therefore, our results suggest that MIN improves the oxidative stress imbalance induced by MIS, mainly through increasing the expression of the Nrf2-ARE pathway.
Limitations
This study is subject to some limitations. First, we have evaluated only male animal subjects. The influence of MIS on males is well-validated, which is not the case in females, probably because females develop symptoms later compared with males and due to the need to control the estrous cycle. In this regard, additional studies will be necessary to investigate the effect of sex in the MIS model. Second, the voxel-level statistical analyses in SPM methods were not corrected for multiple comparisons. In this sense, Bonferroni correction results are too conservative for voxel-based image analyses because they consider independence of the voxels, which is not accurate due to the constant presence of spatial correlation between nearby voxels (Lieberman and Cunningham, 2009; Verger et al., 2018). However, we applied cluster-level corrections to prevent type I errors.
Conclusions
Our study demonstrates that MIN treatment during adolescence partially counteracts certain structural abnormalities and inflammatory/oxidative stress deficits via iNOS and Nrf2-ARE pathways in the MIS model, increasing the expression of cytoprotective enzymes. However, MIN treatment during this peripubertal stage does not prevent sensorimotor gating deficits in this model. Therefore, even though it does not prevent all the MIS-derived abnormalities evaluated, our results suggest the potential utility of early treatment with MIN in other schizophrenia domains.
Supplementary Material
Acknowledgments
M.L.S. was supported by the Ministerio de Ciencia, Innovación y Universidades, Instituto de Salud Carlos III (projects PI14/00860 and PI17/01766, and grant CPII14/00005), co-financed by European Regional Development Fund (ERDF), “A way of making Europe”, CIBER of Mental Health (CIBERSAM), Delegación del Gobierno para el Plan Nacional sobre Drogas (2017/085), Fundación Mapfre and Fundación Alicia Koplowitz. M.C.-V. was supported by Fundación Tatiana Pérez de Guzmán el Bueno. D.R.-M. was supported by Consejería de Educación e Investigación, Comunidad de Madrid, co-funded by European Social Fund “Investing in your future” (grant, PEJD-2018-PRE/BMD-7899). N.L.-R. was supported by Instituto de investigación Sanitaria Gregorio Marañón. A.R.-M. was supported by Consejería de Educación e Investigación, Comunidad de Madrid, co-funded by European Social Fund “Investing in your future” (grant, PEJ15/BIO/TL-0216). The CNIC was supported by the Spanish Ministerio de Ciencia, Innovación y Universidades (MCIU) and the Pro-CNIC Foundation. J.C.L. was supported by the Spanish Ministry of Economy, Industry and Competitiveness (MINECO-EU-FEDER) SAF2016-75500-R and CIBERSAM. E.B.D., S.T.-S., and J.A.G.-P. were supported by grants: co-financed by the “Fondo Europeo de Desarrollo Regional” (FEDER)-UE “A way to build Europe” from the “Ministerio de Economía y Competitividad” (MINECO: RTI2018-099778-B-I00) and the “Ministerio de Salud-Instituto de Salud Carlos III (PI18/01691); from the “Plan Nacional sobre Drogas, Ministerio de Sanidad, Consumo y Bienestar Social” (2019I041); from the “Programa Operativo de Andalucía FEDER, Iniciativa Territorial Integrada ITI 2014–2020 Consejería Salud, Junta de Andalucía” (PI-0080-2017 and PI-0009-2017); from the Consejería de Salud de la Junta de Andalucía (PI-0134-2018); from the University of Cádiz (PR2019-046); from the “Instituto de Investigación e Innovación en Ciencias Biomédicas de Cádiz-INiBICA” (IN-C22; LI19/06IN-CO22); from the “Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía” (CTS-510); from the “CIBERSAM” (CB/07/09/0033).
Statement of Interest
None.
References
- Abella M, Vaquero JJ, Sisniega A, Pascau J, Udías A, García V, Vidal I, Desco M (2012) Software architecture for multi-bed FDK-based reconstruction in X-ray CT scanners. Comput Methods Programs Biomed 107:218–232. [DOI] [PubMed] [Google Scholar]
- Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, Patel IR, Abramson SB (1996) A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci U S A 93:14014–14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson C, Hamer RM, Lawler CP, Mailman RB, Lieberman JA (2002) Striatal volume changes in the rat following long-term administration of typical and atypical antipsychotic drugs. Neuropsychopharmacology 27:143–151. [DOI] [PubMed] [Google Scholar]
- Anderson G, Maes M (2013) Schizophrenia: linking prenatal infection to cytokines, the tryptophan catabolite (TRYCAT) pathway, NMDA receptor hypofunction, neurodevelopment and neuroprogression. Prog Neuropsychopharmacol Biol Psychiatry 42:5–19. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS (1998) “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 24:203–218. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R (2008) The role of the cerebellum in schizophrenia. Biol Psychiatry 64:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Gee JC (2004) Geodesic estimation for large deformation anatomical shape averaging and interpolation. Neuroimage 23 Suppl 1:S139–S150. [DOI] [PubMed] [Google Scholar]
- Berger GE, Bartholomeusz CF, Wood SJ, Ang A, Phillips LJ, Proffitt T, Brewer WJ, Smith DJ, Nelson B, Lin A, Borgwardt S, Velakoulis D, Yung AR, McGorry PD, Pantelis C (2017) Ventricular volumes across stages of schizophrenia and other psychoses. Aust N Z J Psychiatry 51:1041–1051. [DOI] [PubMed] [Google Scholar]
- Bikovsky L, Hadar R, Soto-Montenegro ML, Klein J, Weiner I, Desco M, Pascau J, Winter C, Hamani C (2016) Deep brain stimulation improves behavior and modulates neural circuits in a rodent model of schizophrenia. Exp Neurol 283:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bioque M, García-Bueno B, Macdowell KS, Meseguer A, Saiz PA, Parellada M, Gonzalez-Pinto A, Rodriguez-Jimenez R, Lobo A, Leza JC, Bernardo M; FLAMM-PEPs study—Centro de Investigacio´n Biome´dica en Red de Salud Mental (2013) Peripheral endocannabinoid system dysregulation in first-episode psychosis. Neuropsychopharmacology 38:2568–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MA, Bonoldi I, Kalk N, Turkheimer F, McGuire P, de Paola V, Howes OD (2016) Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry 173:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghammer P, Aanerud J, Gjedde A (2009) Data-driven intensity normalization of PET group comparison studies is superior to global mean normalization. Neuroimage 46:981–988. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ (2010) Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167:261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casquero-Veiga M, García-García D, MacDowell KS, Pérez-Caballero L, Torres-Sánchez S, Fraguas D, Berrocoso E, Leza JC, Arango C, Desco M, Soto-Montenegro ML (2019) Risperidone administered during adolescence induced metabolic, anatomical and inflammatory/oxidative changes in adult brain: a PET and MRI study in the maternal immune stimulation animal model. Eur Neuropsychopharmacol 29:880–896. [DOI] [PubMed] [Google Scholar]
- Casquero-Veiga M, Romero-Miguel D, MacDowell KS, Torres-Sanchez S, Garcia-Partida JA, Lamanna-Rama N, Gómez-Rangel V, Romero-Miranda A, Berrocoso E, Leza JC, Arango C, Desco M, Soto-Montenegro ML (2021) Omega-3 fatty acids during adolescence prevent schizophrenia-related behavioural deficits: neurophysiological evidences from the prenatal viral infection with PolyI:C. Eur Neuropsychopharmacol 46:14–27. [DOI] [PubMed] [Google Scholar]
- Chaudhry IB, Hallak J, Husain N, Minhas F, Stirling J, Richardson P, Dursun S, Dunn G, Deakin B (2012) Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacol 26:1185–1193. [DOI] [PubMed] [Google Scholar]
- Chaves C, Marque CR, Trzesniak C, Machado de Sousa JP, Zuardi AW, Crippa JA, Dursun SM, Hallak JE (2009) Glutamate-N-methyl-D-aspartate receptor modulation and minocycline for the treatment of patients with schizophrenia: an update. Braz J Med Biol Res 42:1002–1014. [DOI] [PubMed] [Google Scholar]
- Chaves C, de Marque CR, Wichert-Ana L, Maia-de-Oliveira JP, Itikawa EN, Crippa JA, Zuardi AW, Todd KG, Baker GB, Dursun SM, Hallak JE (2010) Functional neuroimaging of minocycline’s effect in a patient with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 34:550–552. [DOI] [PubMed] [Google Scholar]
- Cierpka M, Wolf ND, Kubera KM, Schmitgen MM, Vasic N, Frasch K, Wolf RC (2017) Cerebellar contributions to persistent auditory verbal hallucinations in patients with schizophrenia. Cerebellum 16:964–972. [DOI] [PubMed] [Google Scholar]
- Collste K, Plavén-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A, Amini N, Aeinehband S, Erhardt S, Halldin C, Flyckt L, Farde L, Cervenka S; Karolinska Schizophrenia Project (KaSP) consortium (2017) Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C]PBR28. Mol Psychiatry 22:850–856. [DOI] [PubMed] [Google Scholar]
- Correll CU, Brevig T, Brain C (2019) Patient characteristics, burden and pharmacotherapy of treatment-resistant schizophrenia: results from a survey of 204 US psychiatrists. BMC Psychiatry 19:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, Kim PK, Ford CN, Higgs C, Hayes LN, Schretlen DJ, Dannals RF, Kassiou M, Sawa A, Pomper MG (2016) In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 6:e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Hua Y, Keep RF, Novakovic N, Fei Z, Xi G (2019) Minocycline attenuates brain injury and iron overload after intracerebral hemorrhage in aged female rats. Neurobiol Dis 126:76–84. [DOI] [PubMed] [Google Scholar]
- Das NP, Ratty AK (1987) Studies on the effects of the narcotic alkaloids, cocaine, morphine, and codeine on nonenzymatic lipid peroxidation in rat brain mitochondria. Biochem Med Metab Biol 37:258–264. [DOI] [PubMed] [Google Scholar]
- Deakin B, et al. ; BeneMin Study team . (2018) The benefit of minocycline on negative symptoms of schizophrenia in patients with recent-onset psychosis (BeneMin): a randomised, double-blind, placebo-controlled trial. Lancet Psychiatry 5:885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Picker LJ, Morrens M, Chance SA, Boche D (2017) Microglia and brain plasticity in acute psychosis and schizophrenia illness course: a meta-review. Front Psychiatry 8:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase MA, Zalesky A, O’keefe G, Laskaris L, Baune BT, Weickert CS, Olver J, McGorry PD, Amminger GP, Nelson B, Scott AM, Hickie I, Banati R, Turkheimer F, Yaqub M, Everall IP, Pantelis C, Cropley V (2017) PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry 7:e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Luo C, Guell X, Wang Y, He H, Duan M, Eickhoff SB, Yao D (2020) Compression of cerebellar functional gradients in schizophrenia. Schizophr Bull 46:1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC (2009) Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 50:1801–1807. [DOI] [PubMed] [Google Scholar]
- Dubol M, Trichard C, Leroy C, Sandu AL, Rahim M, Granger B, Tzavara ET, Karila L, Martinot JL, Artiges E (2018) Dopamine transporter and reward anticipation in a dimensional perspective: a multimodal brain imaging study. Neuropsychopharmacology 43:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebdrup BH, Glenthøj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson OB, Lublin H, Skimminge A, Baaré W (2010) Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci 35:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Ishima T, Kunitachi S, Hagiwara H, Zhang L, Iyo M, Hashimoto K (2008) Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the antibiotic drug minocycline. Prog Neuropsychopharmacol Biol Psychiatry 32:336–339. [DOI] [PubMed] [Google Scholar]
- Garrido-Mesa N, Zarzuelo A, Gálvez J (2013a) What is behind the non-antibiotic properties of minocycline? Pharmacol Res 67:18–30. [DOI] [PubMed] [Google Scholar]
- Garrido-Mesa N, Zarzuelo A, Gálvez J (2013b) Minocycline: far beyond an antibiotic. Br J Pharmacol 169:337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasull-Camos J, Soto-Montenegro ML, Casquero-Veiga M, Desco M, Artigas F, Castañé A (2017) Differential patterns of subcortical activity evoked by glial GLT-1 blockade in prelimbic and infralimbic cortex: relationship to antidepressant-like effects in rats. Int J Neuropsychopharmacol 20:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR (2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 156:117–154. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Feldon J, Riva MA, Schedlowski M, Meyer U (2016) Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia. Transl Psychiatry 6:e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadar R, Soto-Montenegro ML, Götz T, Wieske F, Sohr R, Desco M, Hamani C, Weiner I, Pascau J, Winter C (2015) Using a maternal immune stimulation model of schizophrenia to study behavioral and neurobiological alterations over the developmental course. Schizophr Res 166:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadar R, Bikovski L, Soto-Montenegro ML, Schimke J, Maier P, Ewing S, Voget M, Wieske F, Götz T, Desco M, Hamani C, Pascau J, Weiner I, Winter C (2018) Early neuromodulation prevents the development of brain and behavioral abnormalities in a rodent model of schizophrenia. Mol Psychiatry 23:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukvik UK, Hartberg CB, Agartz I (2013) Schizophrenia–what does structural MRI show? Tidsskr Nor Laegeforen 133:850–853. [DOI] [PubMed] [Google Scholar]
- Horga G, Fernández-Egea E, Mané A, Font M, Schatz KC, Falcon C, Lomeña F, Bernardo M, Parellada E (2014) Brain metabolism during hallucination-like auditory stimulation in schizophrenia. PLoS One 9:e84987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DH, Kwon KW, Gu BM, Choi JS, Jang JH, Kwon JS (2008) Structural abnormalities of the right inferior colliculus in schizophrenia. Psychiatry Res 164:160–165. [DOI] [PubMed] [Google Scholar]
- Kelly DL, et al. (2015) Adjunctive minocycline in clozapine-treated schizophrenia patients with persistent symptoms. J Clin Psychopharmacol 35:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G, Meyer JH, Wilson AA, Houle S, Mizrahi R (2015) Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr Bull 41:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaie-Ardakani MR, Mirshafiee O, Farokhnia M, Tajdini M, Hosseini SM, Modabbernia A, Rezaei F, Salehi B, Yekehtaz H, Ashrafi M, Tabrizi M, Akhondzadeh S (2014) Minocycline add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized double-blind placebo-controlled study. Psychiatry Res 215:540–546. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim JH, Son YD, Joo YH, Lee SY, Kim HK, Woo MK (2017) Altered interregional correlations between serotonin transporter availability and cerebral glucose metabolism in schizophrenia: a high-resolution PET study using [11C]DASB and [18F]FDG. Schizophr Res 182:55–65. [DOI] [PubMed] [Google Scholar]
- Konradi C, Heckers S (2001) Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol Psychiatry 50:729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak A, Steullet P, Cabungcal JH, Werge T, Ingason A, Cuenod M, Do KQ (2013) Redox dysregulation in the pathophysiology of schizophrenia and bipolar disorder: insights from animal models. Antioxid Redox Signal 18:1428–1443. [DOI] [PubMed] [Google Scholar]
- Kumar V, Singh BK, Chauhan AK, Singh D, Patel DK, Singh C (2016) Minocycline rescues from zinc-induced nigrostriatal dopaminergic neurodegeneration: biochemical and molecular interventions. Mol Neurobiol 53:2761–2777. [DOI] [PubMed] [Google Scholar]
- Lee SM, Yune TY, Kim SJ, Kim YC, Oh YJ, Markelonis GJ, Oh TH (2004) Minocycline inhibits apoptotic cell death via attenuation of TNF-alpha expression following iNOS/NO induction by lipopolysaccharide in neuron/glia co-cultures. J Neurochem 91:568–578. [DOI] [PubMed] [Google Scholar]
- Levine J, Cholestoy A, Zimmerman J (1996) Possible antidepressant effect of minocycline. Am J Psychiatry 153:582. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Levi U, Braw Y, Cohen H (2007) Minocycline, a second-generation tetracycline, as a neuroprotective agent in an animal model of schizophrenia. Brain Res 1154:154–162. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Mendlovich S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G, Fennig S, Treves I, Kron S (2010) A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry 71:138–149. [DOI] [PubMed] [Google Scholar]
- Leza JC, García-Bueno B, Bioque M, Arango C, Parellada M, Do K, O’Donnell P, Bernardo M (2015) Inflammation in schizophrenia: a question of balance. Neurosci Biobehav Rev 55:612–626. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA (2009) Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci 4:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipner E, Murphy SK, Ellman LM (2019) Prenatal maternal stress and the cascade of risk to schizophrenia spectrum disorders in offspring. Curr Psychiatry Rep 21:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Guo X, Wu R, Ou J, Zheng Y, Zhang B, Xie L, Zhang L, Yang L, Yang S, Yang J, Ruan Y, Zeng Y, Xu X, Zhao J (2014) Minocycline supplementation for treatment of negative symptoms in early-phase schizophrenia: a double blind, randomized, controlled trial. Schizophr Res 153:169–176. [DOI] [PubMed] [Google Scholar]
- MacDowell KS, García-Bueno B, Madrigal JL, Parellada M, Arango C, Micó JA, Leza JC (2013) Risperidone normalizes increased inflammatory parameters and restores anti-inflammatory pathways in a model of neuroinflammation. Int J Neuropsychopharmacol 16:121–135. [DOI] [PubMed] [Google Scholar]
- MacDowell KS, Munarriz-Cuezva E, Caso JR, Madrigal JL, Zabala A, Meana JJ, García-Bueno B, Leza JC (2017) Paliperidone reverts Toll-like receptor 3 signaling pathway activation and cognitive deficits in a maternal immune activation mouse model of schizophrenia. Neuropharmacology 116:196–207. [DOI] [PubMed] [Google Scholar]
- Massana G, Salgado-Pineda P, Junqué C, Pérez M, Baeza I, Pons A, Massana J, Navarro V, Blanch J, Morer A, Mercader JM, Bernardo M (2005) Volume changes in gray matter in first-episode neuroleptic-naive schizophrenic patients treated with risperidone. J Clin Psychopharmacol 25:111–117. [DOI] [PubMed] [Google Scholar]
- Mattei D, Djodari-Irani A, Hadar R, Pelz A, de Cossío LF, Goetz T, Matyash M, Kettenmann H, Winter C, Wolf SA (2014) Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun 38:175–184. [DOI] [PubMed] [Google Scholar]
- Millan MJ, et al. (2016) Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov 15:485–515. [DOI] [PubMed] [Google Scholar]
- Moberget T, et al. (2018) Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: a multisite mega-analysis of 983 patients and 1349 healthy controls. Mol Psychiatry 23:1512–1520. [DOI] [PubMed] [Google Scholar]
- Moller M, Swanepoel T, Harvey BH (2015) Neurodevelopmental animal models reveal the convergent role of neurotransmitter systems, inflammation, and oxidative stress as biomarkers of schizophrenia: implications for novel drug development. ACS Chem Neurosci 6:987–1016. [DOI] [PubMed] [Google Scholar]
- Muller N (2018) Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull 44:973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez E, Benito C, Pazos MR, Barbachano A, Fajardo O, González S, Tolón RM, Romero J (2004) Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse 53:208–213. [DOI] [PubMed] [Google Scholar]
- Okada N, et al. (2016) Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry 21:1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Aracil-Fernández A, García-Gutiérrez MS, Navarrete F, Manzanares J (2011) Deletion of CB2 cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology 36:1489–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae CU, Marks DM, Han C, Patkar AA (2008) Does minocycline have antidepressant effect? Biomed Pharmacother 62:308–311. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2008) The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press. [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I (2011) Abnormal trajectories of neurodevelopment and behavior following in utero insult in the rat. Biol Psychiatry 70:842–851. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I (2012) Tracing the development of psychosis and its prevention: what can be learned from animal models. Neuropharmacology 62:1273–1289. [DOI] [PubMed] [Google Scholar]
- Qurashi I, Chaudhry IB, Khoso AB, Farooque S, Lane S, Husain MO, Chu S, Sarginson J, Hamarani M, Naqvi HA, Razzaque B, Minhas FA, Yung AR, Deakin JFW, Husain N (2017) A randomised, double-blind, placebo-controlled trial of minocycline and/or omega-3 fatty acids added to treatment as usual for at-risk mental states (NAYAB): study protocol. Trials 18:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Munoz M, Sánchez-Blázquez P, Callado LF, Meana JJ, Garzón-Niño J (2017) Schizophrenia and depression, two poles of endocannabinoid system deregulation. Transl Psychiatry 7:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Miguel D, Lamanna-Rama N, Casquero-Veiga M, Gómez-Rangel V, Desco M, Soto-Montenegro ML (2021) Minocycline in neurodegenerative and psychiatric diseases: an update. Eur J Neurol 28:1056–1081. [DOI] [PubMed] [Google Scholar]
- Sakata H, Niizuma K, Yoshioka H, Kim GS, Jung JE, Katsu M, Narasimhan P, Maier CM, Nishiyama Y, Chan PH (2012) Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci 32:3462–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Perez A, Llansola M, Cauli O, Felipo V (2005) Modulation of NMDA receptors in the cerebellum. II. Signaling pathways and physiological modulators regulating NMDA receptor function. Cerebellum 4:162–170. [DOI] [PubMed] [Google Scholar]
- Sathyanesan A, Zhou J, Scafidi J, Heck DH, Sillitoe RV, Gallo V (2019) Emerging connections between cerebellar development, behaviour and complex brain disorders. Nat Rev Neurosci 20:298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Koschel J, Zink M, Bauer M, Sommer C, Frank J, Treutlein J, Schulze T, Schneider-Axmann T, Parlapani E, Rietschel M, Falkai P, Henn FA (2010) Gene expression of NMDA receptor subunits in the cerebellum of elderly patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 260:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seethalakshmi R, Parkar SR, Nair N, Pandit AG (2006) Increased thalamostriatal-mesiotemporal glucose uptake with symptom remission in schizophrenia–a case report. J Ect 22:74–75. [DOI] [PubMed] [Google Scholar]
- Sengupta P (2013) The laboratory rat: relating its age with human’s. Int J Prev Med 4:624–630. [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK (2000) Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 57:1033–1038. [DOI] [PubMed] [Google Scholar]
- Soto-Montenegro ML, Pascau J, Desco M (2014) Response to deep brain stimulation in the lateral hypothalamic area in a rat model of obesity: in vivo assessment of brain glucose metabolism. Mol Imaging Biol 16:830–837. [DOI] [PubMed] [Google Scholar]
- Stegmayer K, Horn H, Federspiel A, Razavi N, Bracht T, Laimböck K, Strik W, Dierks T, Wiest R, Müller TJ, Walther S (2014) Ventral striatum gray matter density reduction in patients with schizophrenia and psychotic emotional dysregulation. Neuroimage Clin 4:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Shigemi H, Tanaka Y, Yamauchi T, Ueda T, Iwasaki H (2015) Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways. Biochem Biophys Rep 4:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar PM, Abdul F, Maes M, Binu VS, Venkatasubramanian G, Kutty BM, Debnath M (2020) Maternal immune activation causes schizophrenia-like behaviors in the offspring through activation of immune-inflammatory, oxidative and apoptotic pathways, and lowered antioxidant defenses and neuroprotection. Mol Neurobiol 57:4345–4361. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT (1992) Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 49:522–530. [DOI] [PubMed] [Google Scholar]
- Tang J, Chen Q, Guo J, Yang L, Tao Y, Li L, Miao H, Feng H, Chen Z, Zhu G (2016) Minocycline attenuates neonatal germinal-matrix-hemorrhage-induced neuroinflammation and brain edema by activating cannabinoid receptor 2. Mol Neurobiol 53:1935–1948. [DOI] [PubMed] [Google Scholar]
- Templeton GF (2011) A two-step approach for transforming continuous variables to normal: implications and recommendations for IS research. CAIS 28:41–58. [Google Scholar]
- Tian Y, Wu X, Guo S, Ma L, Huang W, Zhao X (2017) Minocycline attenuates sevoflurane-induced cell injury via activation of Nrf2. Int J Mol Med 39:869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utari A, Chonchaiya W, Rivera SM, Schneider A, Hagerman RJ, Faradz SM, Ethell IM, Nguyen DV (2010) Side effects of minocycline treatment in patients with fragile X syndrome and exploration of outcome measures. Am J Intellect Dev Disabil 115:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Hernandez PA, Sumiyoshi A, Nonaka H, Haga R, Aubert-Vásquez E, Ogawa T, Iturria-Medina Y, Riera JJ, Kawashima R (2011) An in vivo MRI template set for morphometry, tissue segmentation, and fMRI localization in rats. Front Neuroinform 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS (2008) Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 64:820–822. [DOI] [PubMed] [Google Scholar]
- van Os J, et al. (2014) Identifying gene-environment interactions in schizophrenia: contemporary challenges for integrated, large-scale investigations. Schizophr Bull 40:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger A, Stegmayr C, Galldiks N, Van Der Gucht A, Lohmann P, Stoffels G, Shah NJ, Fink GR, Eickhoff SB, Guedj E, Langen KJ (2018) Evaluation of factors influencing 18F-FET uptake in the brain. Neuroimage Clin 17:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S (2002) Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 22:1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeganeh-Doost P, Gruber O, Falkai P, Schmitt A (2011) The role of the cerebellum in schizophrenia: from cognition to molecular pathways. Clinics (Sao Paulo) 66 Suppl 1:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Shirayama Y, Iyo M, Hashimoto K (2007) Minocycline attenuates hyperlocomotion and prepulse inhibition deficits in mice after administration of the NMDA receptor antagonist dizocilpine. Neuropsychopharmacology 32:2004–2010. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhao J (2014) Profile of minocycline and its potential in the treatment of schizophrenia. Neuropsychiatr Dis Treat 10:1103–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zheng H, Wu R, Zhu F, Kosten TR, Zhang XY, Zhao J (2018) Minocycline adjunctive treatment to risperidone for negative symptoms in schizophrenia: association with pro-inflammatory cytokine levels. Prog Neuropsychopharmacol Biol Psychiatry 85:69–76. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zheng H, Wu R, Kosten TR, Zhang XY, Zhao J (2019) The effect of minocycline on amelioration of cognitive deficits and pro-inflammatory cytokines levels in patients with schizophrenia. Schizophr Res 212:92–98. [DOI] [PubMed] [Google Scholar]
- Zhao C, Zhu J, Liu X, Pu C, Lai Y, Chen L, Yu X, Hong N (2017) Structural and functional brain abnormalities in schizophrenia: a cross-sectional study at different stages of the disease. Prog Neuropsychopharmacol Biol Psychiatry 83:27–32. [DOI] [PubMed] [Google Scholar]
- Zheng W, Zhu XM, Zhang QE, Cheng G, Cai DB, He J, Ng CH, Ungvari GS, Peng XJ, Ning YP, Xiang YT (2019) Adjunctive minocycline for major mental disorders: a systematic review. J Psychopharmacol 33:1215–1226. [DOI] [PubMed] [Google Scholar]
- Zheng X, Liang Y, Kang A, Ma SJ, Xing L, Zhou YY, Dai C, Xie H, Xie L, Wang GJ, Hao HP (2014) Peripheral immunomodulation with ginsenoside Rg1 ameliorates neuroinflammation-induced behavioral deficits in rats. Neuroscience 256:210–222. [DOI] [PubMed] [Google Scholar]
- Zhu F, Zheng Y, Liu Y, Zhang X, Zhao J (2014a) Minocycline alleviates behavioral deficits and inhibits microglial activation in the offspring of pregnant mice after administration of polyriboinosinic-polyribocytidilic acid. Psychiatry Res 219:680–686. [DOI] [PubMed] [Google Scholar]
- Zhu F, Zheng Y, Ding YQ, Liu Y, Zhang X, Wu R, Guo X, Zhao J (2014b) Minocycline and risperidone prevent microglia activation and rescue behavioral deficits induced by neonatal intrahippocampal injection of lipopolysaccharide in rats. PLoS One 9:e93966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I (2003) Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology 169:308–313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.