Abstract
Chronic kidney disease (CKD) is highly prevalent in patients with chronic heart failure (CHF) and increases the risk of overall and cardiovascular (CV) mortality. Despite evidence supporting the effectiveness of angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin receptor blockers, and mineralocorticoid receptor antagonists in decreasing mortality in patients with CHF, CKD hampers the optimization of standard pharmacologic therapy for heart failure. Therefore, other treatment options are needed to optimize treatment outcomes in CHF patients with CKD. The first-in-class angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, has a complementary activity that counteracts the potential unwanted long-term effects of over-activation of the renin–angiotensin–aldosterone system. Sacubitril/valsartan reduced the risk of CV mortality compared to standard therapy with an ACE-I in patients with heart failure with reduced ejection fraction (HFrEF) in the PARADIGM-HF trial and has been shown to be safe and effective in a broad range of HFrEF patients. However, data on the efficacy and tolerability of sacubitril/valsartan in patients with more advanced CKD are limited. This review discusses the evidence for the role of sacubitril/valsartan in providing additional renal benefit in patients with HFrEF. Data from clinical trials and real-world experience in patients with HFrEF and advanced CKD support the benefits of dual angiotensin/neprilysin inhibition across the breadth of kidney disease stages, including patients with significant renal impairment that was not reported in the pivotal PARADIGM-HF trial, and suggests a central role for the cardiac benefits of sacubitril/valsartan in nephroprotection.
Keywords: Sacubitril/valsartan, Chronic heart failure, Chronic kidney disease, Renoprotection, Renal function
Introduction
Chronic kidney disease (CKD) is highly prevalent in patients with chronic heart failure (CHF) and imposes a considerable unfavourable prognostic burden in terms of global and cardiovascular (CV) mortality.1 Data from key CHF trials show prevalence rates of CKD between 32% and 50%, and deteriorating renal function has been observed in approximately 30% of patients with heart failure (HF).2 Furthermore, CKD is a risk factor for both CV sequelae and death.3
CKD hampers the optimization of standard pharmacologic therapy for HF and leads to underuse of renin–angiotensin–aldosterone system (RAAS) inhibitors and aldosterone antagonists (mineralocorticoid receptor antagonists; MRAs), despite evidence of the effectiveness of angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin receptor blockers (ARBs), and MRA in decreasing mortality in CHF.4,5 Therefore, other treatment options are needed to optimize treatment outcomes in CHF patients with CKD.
Sacubitril/valsartan is the first-in-class of angiotensin receptor-neprilysin inhibitors (ARNIs).6 Sacubitril is a prodrug that is metabolized into an active inhibitor of the endopeptidase neprilysin, the key enzyme responsible for degrading natriuretic and other vasoactive peptides. However, N-terminal pro-B-type natriuretic peptide (NT-proBNP), which is a marker of HF events in patients with HF with reduced ejection fraction (HFrEF),7 is not a substrate for sacubitril.
The PARADIGM-HF trial showed that sacubitril/valsartan reduced the risk of CV mortality compared to standard therapy with an ACE-I in patients with HFrEF [hazard ratio, 0.80; 95% confidence interval (CI), 0.71–0.89; P < 0.001].8 The relative risk reduction in CV events with sacubitril/valsartan was similar in patients with and without CKD at baseline [stratified by ≥estimated glomerular filtration rate (eGFR) 60 mL/min/1.73 m2 or <eGFR 60 mL/min/1.73 m2, and excluding those with eGFR <30 mL/min/1.73 m2], and the renal safety profile and rate of decline of GFR with sacubitril/valsartan were more favourable than that of enalapril.3
More recently, the PIONEER-HF trial,7 the TRANSITION study,9 and the TITRATION study3 have shown that sacubitril/valsartan is safe and effective in a broad range of HFrEF patients, extending to patients with mid-range, borderline, or mildly reduced ejection fraction.10 However, patients with an eGFR <30 mL/min/1.73 m2 were excluded from these trials, and thus data on the efficacy and tolerability of sacubitril/valsartan in patients with advanced CKD are limited.
Sacubitril/valsartan has subsequently become regarded as an evidence-based and guideline-recommended disease-modifying therapy for patients with HFrEF5,11,12 with an established role in routine clinical practice.13
Furthermore, evidence from large randomized controlled trials (RCTs) has shown that sacubitril/valsartan is superior to renin–angiotensin system inhibitors in preserving renal function in patients with HFrEF3,10 and has a beneficial role on eGFR, compared with standard optimal medical therapy, in the ‘real-world’ setting.14
Several meta-analyses have shown that combined neprilysin-RAAS inhibition with sacubitril/valsartan or omapatrilat may have beneficial effects on renal function in HF compared with RAAS inhibition alone.15,16 However, data on the role of sacubitril/valsartan in the preservation of renal function in patients with HFrEF are limited.
This narrative review discusses the evidence for the role of sacubitril/valsartan in providing additional renal benefit in patients with HFrEF.
Pathophysiology of cardiorenal interaction in chronic heart failure
CHF and CKD share several etiologic risk factors, including ‘traditional’ CV risk factors such as age, gender, hypertension, dyslipidaemia, and diabetes.4 As well as aetiological risk factors, there is an interaction of respective pathophysiologies. In CHF, structural or functional abnormalities that affect the cardiac cycle impair the ability of the heart to maintain tissue perfusion adequate to meet metabolic requirements and to accommodate venous return.4 The deterioration of renal function in CHF is further accelerated by the presence of type 2 diabetes mellitus.3
In CHF, impaired cardiac function, together with reduced cardiac output, decreases renal blood flow and the perfusion gradient, exacerbating renal haemodynamic changes.17 Several compensatory mechanisms follow as the failing heart attempts to maintain adequate function, including increased cardiac output, ventricular remodelling, and activation of neurohormonal systems to augment mean arterial pressure.18 While the pathophysiology of HF with preserved ejection fraction (HFpEF) is more heterogeneous compared to HFrEF,19 the increases in serum creatinine are largely similar in both HFpEF and HFrEF, and thus the mechanisms leading to renal impairment may be similar.17
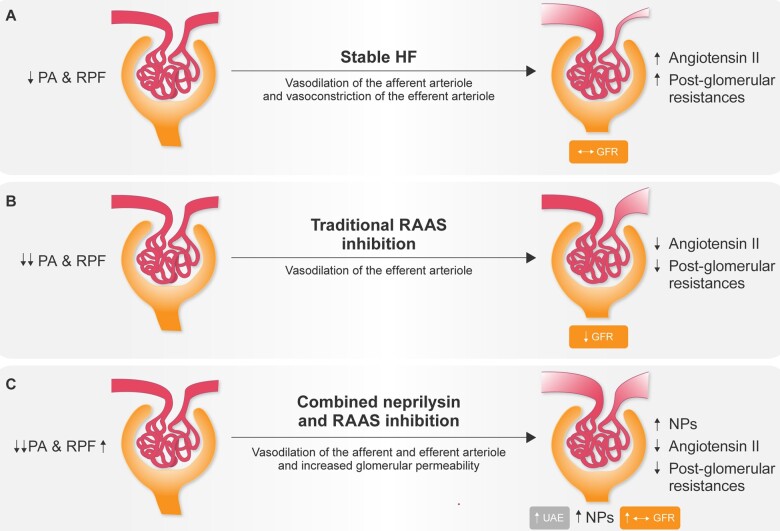
The neurohormonal adaptive mechanisms that regulate renal perfusion in CHF are illustrated in Figure 1.18 In patients with stable HF (Figure 1A), the decrease in renal perfusion leads to adaptive mechanisms through activation of the RAAS, which induces a predominant angiotensin II-mediated vasoconstriction of the efferent arteriole with a secondary increase in post-glomerular resistance and, consequently, increased intra-glomerular pressure. The increase in post-glomerular resistance increases the intracapillary hydraulic pressure even though kidney perfusion is decreased secondary to a decrease in systemic blood pressure (BP). Accordingly, the proportion of renal plasma flow that is ultra-filtered through the glomerular barrier increases, enabling the maintenance of GFR despite decreased kidney perfusion.
Figure 1.
Renal adaptive mechanisms to renal hypoperfusion in chronic heart failure. (A) Adaptive mechanisms in stable heart failure. (B) Effects of renin–angiotensin system inhibition on adaptive mechanisms to renal hypoperfusion. (C) Effects of combined neprilysin and renin–angiotensin system inhibition on adaptive mechanisms to renal hypoperfusion. GFR, glomerular filtration rate; NPs, natriuretic peptides; PA, arterial pressure; RPF, renal plasma flow; UAE, urinary albumin excretion. Modified from Di Tano et al.,18 2018. Reproduced with permission from Il Pensiero Scientifico Editore.
RAAS inhibition by ACE-I or ARBs in the presence of the greatly reduced renal blood flow counteracts renal auto-regulation, decreasing intra-glomerular pressure by preventing angiotensin II-induced predominant vasoconstriction of the efferent arteriole (Figure 1B), contributing to a decrease in intracapillary hydraulic pressure and, consequently, filtration fraction and GFR, which therefore becomes BP dependent.
Inhibition of neprilysin enhances the bioavailability of natriuretic peptides; concomitant inhibition of the angiotensin II type-1 receptor and neprilysin inhibition further reduces systemic BP and kidney perfusion pressure, inducing preferential vasorelaxation of the pre-glomerular arteriole and relative vasoconstriction of the post-glomerular arteriole (Figure 1C). The consequent decrease in pre-glomerular resistance and increase in post-glomerular resistance contributes to increasing intracapillary hydraulic pressure, despite a decrease in the renal perfusion pressure, increasing the filtration fraction and GFR. The increased intracapillary hydraulic pressure possibly combined with a direct effect of ARNIs on the glomerular barrier may contribute to increased albumin ultrafiltration which, in combination with possible attenuation of tubular protein reabsorption, may lead to the clinically modest albuminuria which may be observed after starting ARNI treatment.3,20
Over the last two decades, several large RCTs have shown that targeting neurohumoral imbalances of the RAAS, the natriuretic peptide system, and the sympathetic nervous system provides incremental benefit and is cost-effective in terms of survival and quality of life. Specifically, ACE-I (which target the angiotensin-converting enzyme), ARBs (which target the angiotensin receptor), beta-blockers (BB; beta-adrenergic receptor antagonists), MRA (which target the mineralocorticoid receptor), and more recently ARNIs, inhibitors of both the angiotensin-II receptor and the endopeptidase neprilysin, have all been shown to be effective in HFrEF and are recommended unless contraindicated or not tolerated.5,11 Furthermore, current treatment guidelines for the treatment of HF recommend that ARNI should replace ACE-I or ARB in patients with New York Heart Association class II or III HFrEF who tolerate an ACE-I or ARB in order to further reduce morbidity and mortality.5,11
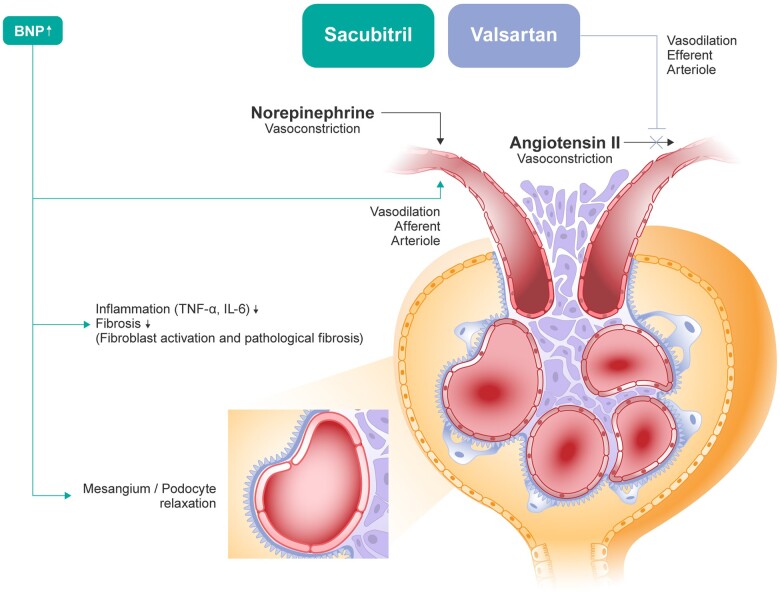
In PARADIGM-HF, sacubitril/valsartan provided greater renal protection as compared to enalapril despite slightly lower BP values. Furthermore, analysis of the relationship between renal effects and CV and renal outcomes in PARADIGM-HF showed that the modest increase in urine albumin excretion associated with ARNI treatment was not associated with a higher risk of renal endpoints,3 suggesting that the rise in the urinary albumin/creatinine ratio over time is likely mediated by an increase in glomerular permeability, a mechanism that does not lead to a progressive reduction of renal function. This was further demonstrated by the beneficial effect of sacubitril/valsartan therapy on the risk for HF hospitalization of CV mortality compared with enalapril.3 Therefore, the J-curve phenomenon has been somewhat challenged by the distinctive dual-acting mechanism of action of sacubitril/valsartan (Figure 2) in inhibiting both neprilysin and angiotensin II.21 This is deemed to be due to specific pathophysiological changes taking place under sacubitril/valsartan treatment at the glomerular level, which, taken together, may lead to preservation of renal function despite a substantial reduction in systemic BP.
Figure 2.
Renal mechanisms of sacubitril/valsartan. ANP, atrial natriuretic peptide. Adapted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Current Heart Failure Reports. Tersalvi et al., 2020.21
Dual angiotensin-neprilysin inhibition in chronic heart failure
Dual angiotensin-neprilysin inhibition by the ARNI sacubitril/valsartan inhibits degradation of endogenous natriuretic peptides, in addition to other vasoactive peptides, including atrial natriuretic peptide (ANP), angiotensin II, bradykinin, adrenomedullin, endothelin, brain (B-type) natriuretic peptide (BNP), and C-type natriuretic peptide,6,22 increasing the levels of these substances. Activation of neprilysin increases intracellular cGMP, leading in turn to vasodilatation, natriuresis and diuresis, inhibition of cardiac fibrosis and hypertrophy, and inhibition of the RAAS.6,22,23 These effects are mediated by the natriuretic peptide receptors (NPRs), NPR-A, and NPR-B, whereas the primary role of NPR-C is to bind and internalize natriuretic peptides, clearing them from circulation,23,24 although they may also mediate anti-fibrotic effects.
The simultaneous blockade of the angiotensin II type 1 receptor by sacubitril/valsartan also counteracts the potential long-term harmful effects of RAAS over-activation (i.e. increased BP and sodium and water retention)23 that may result from neprilysin inhibition. There is also evidence that increased activity of natriuretic peptides exerts direct antioxidant, anti-inflammatory, and anti-fibrotic effects in experimental models, and that sacubitril/valsartan may prevent fibrosis and reduce the oxidative stress, apoptosis, and mitochondrial damage observed in the kidney and heart tissues of animal models of cardio-renal syndrome.25,26
Increased renal perfusion because of sacubitril/valsartan-related improvement of cardiac function may partly explain the effects of sacubitril/valsartan on kidney function in an HF population. As NPRs and neprilysin are expressed in the kidney as well as the myocardium,27 neprilysin inhibition is postulated to increase the bioavailability of renal natriuretic peptides and contribute to the preservation of renal function. It has also been shown that drugs that provide natriuretic peptide system augmentation assist in the preservation of renal function and improve GFR.27 There is further evidence that sacubitril mainly acts by enhancing ANP instead of BNP,28 suggesting that the benefit of neprilysin inhibition may be mediated, at least in part, by increased ANP concentrations.
Evidence for a renal protective role in chronic heart failure
The two largest studies of sacubitril/valsartan in patients with HF, the PARADIGM-HF and PARAGON-HF trials, demonstrated positive renal outcomes with sacubitril/valsartan, compared with ACE-I (enalapril)3,8 or ARB (valsartan).10,29 In PARADIGM-HF in patients with HFrEF, 33% of patients had CKD at baseline.3 In addition to improving CV outcomes,8 sacubitril/valsartan was associated with a slower rate of decrease in eGFR, compared with enalapril (Table 1). During follow-up, the decrease in eGFR was −1.61 mL/min/1.73 m2/year with sacubitril/valsartan (95% CI, −1.77 to −1.44 mL/min/1.73 m2/year) vs. −2.04 mL/min/1.73 m2/year with enalapril (95% CI, −2.21 to −1.88 mL/min/1.73 m2/year; P < 0.001), despite a greater increase in urinary albumin/creatinine ratio with sacubitril/valsartan (1.20 mg/mmol vs. 0.90 mg/mmol, P < 0.001).3
Table 1.
Effect of sacubitril/valsartan on glomerular filtration rate in patients with heart failure
| Comparator | Patient population | Subgroups | No. | Estimated glomerular filtration rate (mL/min/1.73 m2) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sacubitril/valsartan |
Comparator |
|||||||||
| Baseline | Reduction at the end of the study | Reduction per year | Baseline | Reduction at the end of the study | Reduction per year | |||||
| PARAMOUNT-HF Voors et al. 201520 | Valsartan | Patients with HFpEF, 42% with CKD, 38% with diabetes | All | 301 | 66.5 ± 19 | −1.5* | −2.2‡ | 64.3 ± 21 | −5.2 | −7.5‡ |
| PARADIGM-HF Damman et al. 20183 | Enalapril | Patients with HFrEF, 33% with CKD, 45% with diabetes | All | 8399 | 70 ± 20 | −7.8 | −1.61† | 70 ± 20 | −10.2 | −2.04 |
| No CKD | 5654 | −1.98§ | −2.29 | |||||||
| CKD | 2745 | −0.80† | −1.55 | |||||||
| PARADIGM-HF (secondary analysis) Packer et al., 201830 | No Diabetes | 4615 | −1.0† | −1.3 | ||||||
| Diabetes | 3784 | −1.7† | −2.3 | |||||||
| PARAGON-HF McCausland et al 202029 | Valsartan | Patients with HFpEF, 47% with CKD, 43% with diabetes | All | 4822 | 63 ± 19 | −7.7‡ | −2.0† | 62 ± 19 | −10.1‡ | −2.7 |
Data from randomized controlled trials vs. angiotensin receptor blocker (valsartan) or angiotensin-converting enzyme inhibitor (enalapril).
P = 0.002 vs. comparator;
P ≤ 0.001 vs. comparator;
calculated;
P < 0.05.
Neprilysin inhibition had an incremental benefit on renal function in diabetic patients with HFrEF in PARADIGM-HF. Patients treated with sacubitril/valsartan had a slower rate of decline of eGFR compared with enalapril recipients (a difference of 0.6 vs. 0.3 mL/min/1.73 m2 per year in patients with vs. without diabetes; P = 0.038 for the interaction).3 The effect was independent of treatment effect on the course of HF or changes in glycated haemoglobin. Furthermore, in PARADIGM-HF, sacubitril/valsartan caused less hyperkalaemia than enalapril and reduced the use of loop diuretics,8,31,32 with which there is a dose-dependent association with impaired survival outcomes in patients with advanced HF.33
In PARAGON-HF in patients with HFpEF, in which approximately 45–50% of patients had CKD or diabetes at baseline, worsening renal function occurred in 1.4% of patients in the sacubitril/valsartan group, compared with 2.7% in the valsartan group (hazard ratio, 0.50; 95% CI, 0.33–0.77).10 During follow-up, the decrease in eGFR was −2.0 mL/min/1.73 m2/year with sacubitril/valsartan (95% CI, −2.2 to −1.9 mL/min/1.73 m2/year) vs. −2.7 mL/min/1.73 m2/year with valsartan (95% CI, −2.8 to −2.5 mL/min/1.73 m2/year; P < 0.001)29 (Table 1). PARAGON-HF also showed that sacubitril/valsartan was effective in reducing the primary outcome (hospitalizations for HF and death from CV causes) in patients with renal impairment or diabetes.10 In the PIONEER-HF trial,7 which investigated sacubitril/valsartan vs. enalapril in hospitalized patients with acute decompensated HFrEF, in which renal perfusion is further compromised, sacubitril/valsartan also showed a good safety profile in terms of worsening renal function and decrease in eGFR.
A recent systematic review and meta-analysis of 10 RCTs underscores the protective role of sacubitril/valsartan on the kidney in terms of a lower risk of worsening renal function in HF and other conditions.34 The meta-analysis included a total of 16 456 patients across the indications of HFrEF, HFpEF, hypertension, and CKD, although most data were available for patients with HF. The analysis showed a 30% lower risk of renal events and progressive decline of eGFR compared to patients treated with RAAS inhibitors (ACE-I and ARBs) alone (pooled odds ratio 0.70, 95% CI 0.57–0.85; P < 0.001). Risk reduction was greater in older patients and patients with HFpEF.
Another meta-analysis16 investigated the effects of sacubitril/valsartan in 3460 patients with HF and CKD enrolled in three RCTs; PARADIGM-HF (vs. enalapril in patients with HFrEF),3 PARAMOUNT (vs. valsartan in patients with HFpEF),20 and the United Kingdom Heart and Renal Protection-III (HARP-III) trial (vs. irbesartan in patients with HF and CKD).35 Sacubitril/valsartan significantly increased eGFR compared with RAAS inhibitors (mean difference, 1.90; P = 0.02) and was more effective in reducing BP and NT-proBNP, suggesting CV and renal benefits over RAAS inhibition alone in patients with HF and CKD. There was no between-group difference in urinary albumin/creatinine ratio.
Insights into the favourable renal effects of sacubitril/valsartan in the real-world HFrEF population are available from three recent studies.13,14,25 In a study in 54 consecutive outpatients followed for 12 months, over half of the patients were aged ≥65 years, and 53.7% had CKD at baseline.14 Compared with historical controls who received optimal medical therapy, renal function improved during the 12 months of follow-up (P < 0.001 for improvement in eGFR vs. controls). There was no interaction between eGFR trend and systolic BP or baseline left ventricular ejection fraction (LVEF). Meanwhile, systolic BP decreased (P = 0.014) and LVEF slightly increased (P < 0.001). There was a greater benefit in subjects aged <65 years and in patients with CKD (P = 0.009). A statistically (P = 0.009), but not clinically, significant increase in serum potassium was also found, regardless of age and CKD.
In a real-world study in 108 patients with HFrEF, eGFR values increased significantly (73.8 vs. 61.2 mL/min/1.73 m2, P < 0.05) in sacubitril/valsartan recipients, compared to the control arm.13 There were also greater improvements in LVEF with sacubitril/valsartan (42.4% vs. 34.2%, P < 0.05), while values of NT-proBNP, systolic and diastolic BP, and uricaemia also decreased to a greater extent in the sacubitril/valsartan arm (P < 0.05).
Additionally, 932 patients with HFrEF were treated at an HF referral centre (466 with sacubitril/valsartan and 466 with standard HF treatment without ARNI).25 Sacubitril/valsartan was more effective than standard HF treatment in reducing CV deaths or hospitalizations for HF in patients with significant renal insufficiency at baseline, reducing these endpoints by 28% in patients with severe renal impairment (GFR <30 mL/min/1.73 m2).25
Finally, renal outcomes with the SGLT2 inhibitors dapagliflozin and empagliflozin have been assessed in large CV outcomes trials in which a proportion of patients were being treated with sacubitril/valsartan. In the DAPA-HF trial, the dapagliflozin vs. placebo hazard ratio was 0.74 (95% CI: 0.65–0.85; P < 0.0001) for the primary composite endpoint (CV death or a worsening HF event) and had a consistent effect if background therapy included either an MRA or sacubitril/valsartan.36 In the EMPEROR trial which studied cardiac and renal outcomes with empagliflozin, the hazard ratio for the primary outcome (death from CV causes or hospitalization for HF) was 0.64 (95% CI 0.45–0.89) vs. 0.77 (95% CI 0.66 to 0.90) among patients who were not on therapy with sacubitril/valsartan.37 A small study in 108 patients with type 2 diabetes and HFrEF treated with sacubitril/valsartan and empagliflozin further reported that the combination appeared to be safe considering renal function.38 Thus, for both SGLT2 inhibitors, benefits were seen in patients treated with sacubitril–valsartan for HF.
Implications for clinical practice
The complex interconnected functions of the heart and kidneys in regulating fluid and electrolyte homeostasis are markedly altered in HF,4 in which CKD is a frequent concomitant condition and an independent risk factor for CV disease.2,3 Maintaining the necessary balance between vasoconstricting, sodium-retaining, systems, and processes involved in vasodilatation and natriuresis to ensure circulatory integrity becomes difficult as HF progresses and enhanced sodium and water retention adds to the debilitating symptoms of cardiac stress and myocardial damage associated with HF.27 Therefore, CHF therapies that allow the kidney to maintain glomerular and tubular function will help to prevent congestion and contribute to delaying disease progression and improving HF-related morbidity and mortality. PARADIGM-HF showed that sacubitril/valsartan provides CV protection in HFrEF that is superior to traditional RAAS inhibition,8 and data from PARADIGM-HF that first suggested superior renal protection with sacubitril/valsartan in HFrEF3 has since been confirmed in patients with CKD at baseline and in diabetic patients.3,10 Furthermore, PARADIGM-HF showed that sacubitril/valsartan causes less hyperkalaemia than standard therapy with ACE-I,8,31,32 suggesting that neprilysin inhibition attenuates the risk of hyperkalaemia and facilitates the prescribing of a concomitant MRA and the option of reducing the use of loop diuretics in patient management.
Conclusions
Natriuretic peptides deliver several direct biological effects on the kidney, affecting both the glomerulus, through improvements in renal blood flow and GFR while inhibiting renin release, and the tubule, by decreasing sodium reabsorption. Thus, there is a net benefit in terms of natriuresis, diuresis, and preservation of renal function. These mechanisms, taken together, help to explain ARNI-induced preservation of the residual renal function in patients with HFrEF. Although the long-term renal effects of dual angiotensin-neprilysin inhibition in HFrEF remain to be fully elucidated, the available evidence suggests that sacubitril/valsartan is a valid choice of therapy and might replace ACE-I and ARBs in HFrEF patients at renal risk, at least in those with eGFR >30 mL/min/1.73 m2. Full implementation of the current standard of care has the potential to improve outcomes in HFrEF.
Acknowledgements
We thank Ray Hill, an independent medical writer, who provided medical writing support on behalf of Health Publishing & Services Srl. This unconditional support was provided by Novartis Farma SpA.
Conflict of interest: R.P., C.B., and P.P.F. have received consulting and speaker fees from Novartis Farma.
Data Availability
No new data were generated or analysed in support of this research.
References
- 1.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW.. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 2004;109:1004–1009. [DOI] [PubMed] [Google Scholar]
- 2.Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL.. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 2014;35:455–469. [DOI] [PubMed] [Google Scholar]
- 3.Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, Zile MR, Packer M, Desai AS, Solomon SD, McMurray JJV.. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail 2018;6:489–498. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW.. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:e391–e479. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 6.Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP.. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol 2010;50:401–414. [DOI] [PubMed] [Google Scholar]
- 7.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, Pioneer-Hf I.. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 8.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 9.Wachter R, Senni M, Belohlavek J, Straburzynska-Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bøhmer E, Pouleur A-C, Mueller C, Tribouilloy C, Lonn E, A L Buraiki J, Gniot J, Mozheiko M, Lelonek M, Noè A, Schwende H, Bao W, Butylin D, Pascual-Figal D; TRANSITION Investigators. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail 2019;21:998–1007. [DOI] [PubMed] [Google Scholar]
- 10.Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, McCague K, Abbas CA, Rocha R, Mitchell GF; for the EVALUATE-HF Investigators. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2019;322:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C; WRITING COMMITTEE MEMBERS. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016;134:e282–e293. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham JW, Vaduganathan M, Claggett BL, Zile MR, Anand IS, Packer M, Zannad F, Lam CSP, Janssens S, Jhund PS, Kober L, Rouleau J, Shah SJ, Chopra VK, Shi VC, Lefkowitz MP, Prescott MF, Pfeffer MA, McMurray JJV, Solomon SD.. Effects of sacubitril/valsartan on N-terminal pro-B-type natriuretic peptide in heart failure with preserved ejection fraction. JACC Heart Fail 2020;8:372–381. [DOI] [PubMed] [Google Scholar]
- 13.Mazza A, Townsend DM, Torin G, Schiavon L, Camerotto A, Rigatelli G, Cuppini S, Minuz P, Rubello D.. The role of sacubitril/valsartan in the treatment of chronic heart failure with reduced ejection fraction in hypertensive patients with comorbidities: from clinical trials to real-world settings. Biomed Pharmacother 2020;130:110596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spannella F, Marini M, Giulietti F, Rosettani G, Francioni M, Perna GP, Sarzani R.. Renal effects of sacubitril/valsartan in heart failure with reduced ejection fraction: a real life 1-year follow-up study. Intern Emerg Med 2019;14:1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodey F, Hopper I, Krum H.. Neprilysin inhibitors preserve renal function in heart failure. Int J Cardiol 2015;179:329–330. [DOI] [PubMed] [Google Scholar]
- 16.Kang H, Zhang J, Zhang X, Qin G, Wang K, Deng Z, Fang Y, Chen G.. Effects of sacubitril/valsartan in patients with heart failure and chronic kidney disease: a meta-analysis. Eur J Pharmacol 2020;884:173444. [DOI] [PubMed] [Google Scholar]
- 17.Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, Lerma EV, Mezue K, Molitch M, Mullens W, Ronco C, Tang WHW, McCullough PA; American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 2019;139:e840–e878. [DOI] [PubMed] [Google Scholar]
- 18.Di Tano G, Di Lenarda A, Gabrielli D, Aspromonte N, De Maria R, Frigerio M, Iacoviello M, Mortara A, Murrone A, Nardi F, Oliva F, Pontremoli R, Scherillo M, Senni M, Urbinati S, Gulizia MM.. [ANMCO position paper on sacubitril/valsartan in the management of patients with heart failure] [Italian]. G Ital Cardiol (Rome) 2018;19:568–590. [DOI] [PubMed] [Google Scholar]
- 19.Borlaug BA.The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2014;11:507–515. [DOI] [PubMed] [Google Scholar]
- 20.Voors AA, Gori M, Liu LC, Claggett B, Zile MR, Pieske B, McMurray JJ, Packer M, Shi V, Lefkowitz MP, Solomon SD; PARAMOUNT Investigators. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2015;17:510–517. [DOI] [PubMed] [Google Scholar]
- 21.Tersalvi G, Dauw J., Martens P., Mullens W.. Impact of sacubitril-valsartan on markers of glomerular function. Curr Heart Fail Rep 2020;17:145–152. [DOI] [PubMed] [Google Scholar]
- 22.Langenickel TH, Dole WP.. Angiotensin receptor-neprilysin inhibition with LCZ696: a novel approach for the treatment of heart failure. Drug Discov Today Ther Strateg 2012;9:e131–e139. [Google Scholar]
- 23.Levin ER, Gardner DG, Samson WK.. Natriuretic peptides. N Engl J Med 1998;339:321–328. [DOI] [PubMed] [Google Scholar]
- 24.Gardner DG, Chen S, Glenn DJ, Grigsby CL.. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension 2007;49:419–426. [DOI] [PubMed] [Google Scholar]
- 25.Chang HY, Feng AN, Fong MC, Hsueh CW, Lai WT, Huang KC, Chong E, Chen CN, Chang HC, Yin WH.. Sacubitril/valsartan in heart failure with reduced ejection fraction patients: real world experience on advanced chronic kidney disease, hypotension, and dose escalation. J Cardiol 2019;74:372–380. [DOI] [PubMed] [Google Scholar]
- 26.Jing W, Vaziri ND, Nunes A, Suematsu Y, Farzaneh T, Khazaeli M, Moradi H.. LCZ696 (sacubitril/valsartan) ameliorates oxidative stress, inflammation, fibrosis and improves renal function beyond angiotensin receptor blockade in CKD. Am J Transl Res 2017;9:5473–5484. [PMC free article] [PubMed] [Google Scholar]
- 27.Tanase DM, Radu S, Al SS, Baroi GL, Florida CC, Turliuc MD, Ouatu A, Floria M.. Natriuretic peptides in heart failure with preserved left ventricular ejection fraction: from molecular evidences to clinical implications. Int J Mol Sci 2019;20:2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim NE, McCarthy CP, Shrestha S, Gaggin HK, Mukai R, Szymonifka J, Apple FS, Burnett JC Jr, Iyer S, Januzzi JL Jr. Effect of neprilysin inhibition on various natriuretic peptide assays. J Am Coll Cardiol 2019;73:1273–1284. [DOI] [PubMed] [Google Scholar]
- 29.McCausland FR, Lefkowitz MP, Claggett B, Anavekar NS, Senni M, Gori M, Jhund PS, McGrath MM, Packer M, Shi V, Van Veldhuisen DJ, Zannad F, Comin-Colet J, Pfeffer MA, McMurray JJV, Solomon SD.. Angiotensin-neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation 2020;142:1236–1245. [DOI] [PubMed] [Google Scholar]
- 30.Packer M, Claggett B, Lefkowitz MP, McMurray JJV, Rouleau JL, Solomon SD, Zile MR.. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: a secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol 2018;6:547–554. [DOI] [PubMed] [Google Scholar]
- 31.Vardeny O, Claggett B, Kachadourian J, Desai AS, Packer M, Rouleau J, Zile MR, Swedberg K, Lefkowitz M, Shi V, McMurray JJV, Solomon SD.. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM-HF trial. Eur J Heart Fail 2019;21:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai AS, Vardeny O, Claggett B, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Zile MR, Lefkowitz M, Shi V, Solomon SD.. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: A secondary analysis of the PARADIGM-HF Trial. JAMA Cardiol 2017;2:79–85. [DOI] [PubMed] [Google Scholar]
- 33.Eshaghian S, Horwich TB, Fonarow GC.. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 2006;97:1759–1764. [DOI] [PubMed] [Google Scholar]
- 34.Spannella F, Giulietti F, Filipponi A, Sarzani R.. Effect of sacubitril/valsartan on renal function: a systematic review and meta‐analysis of randomized controlled trials. ESC Heart Failure 2020;7:3487–3496. 10.1002/ehf2.13002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haynes R, Judge PK, Staplin N, Herrington WG, Storey BC, Bethel A, Bowman L, Brunskill N, Cockwell P, Hill M, Kalra PA, McMurray JJV, Taal M, Wheeler DC, Landray MJ, Baigent C.. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation 2018;138:1505–1514. [DOI] [PubMed] [Google Scholar]
- 36.Docherty KF, Jhund PS, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, DeMets DL, Sabatine MS, Bengtsson O, Sjöstrand M, Langkilde AM, Desai AS, Diez M, Howlett JG, Katova T, Ljungman CEA, O’Meara E, Petrie MC, Schou M, Verma S, Vinh PN, Solomon SD, McMurray JJV.. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur Heart J 2020;41:2379–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Bohm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; Investigators EM-RT. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 38.Espriella R, Bayés‐genís A, Morillas H, Bravo R, Vidal V, Núñez E, Santas E, Miñana G, Sanchis J, Fácila L, Torres F, Górriz J L, Valle A, Núñez J.. Renal function dynamics following co‐administration of sacubitril/valsartan and empagliflozin in patients with heart failure and type 2 diabetes. ESC Heart Failure 2020;7:3792–3800. 10.1002/ehf2.12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.