I read with great interest the recent article by Salvalaggio and colleagues in Brain, ‘Post- stroke deficit prediction from lesion and indirect structural and functional disconnection’ (Salvalaggio et al., 2020). In this article, the authors evaluated the relative value of lesion location and lesion-associated networks in predicting behavioural deficits in a sample of 132 individuals with new-onset stroke. The authors compared three different approaches for estimating the impact of the lesion in disrupting a broader network of structures that extend beyond the anatomical boundaries of the lesion: one relying on each subject’s own functional connectivity MRI data, a second that relied on white matter tractography data from healthy individuals to infer structural disconnection, and a third that relied on functional connectivity data from healthy individuals to infer which functional networks were disrupted by the lesion, a method referred to as lesion network mapping (Boes et al., 2015). The results of this analysis were fairly conclusive—each method performed reasonably well with the exception of lesion network mapping, which explained the smallest amount of variance in behavioural outcomes across all but one domain, visual. This is an important and timely study that was rigorously conducted. The results raise important questions about the utility of the lesion network mapping approach and the numerous studies that have been published to date using this approach. The purpose of this letter is to review some potentially important differences between the original lesion network mapping study and its subsequent use, as well as offer a perspective on where lesion network mapping goes from here.
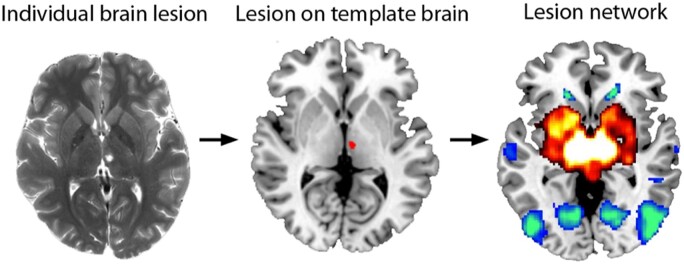
Lesion network mapping is simple in design. The 3D volume of a brain lesion is first mapped onto a reference brain. The location of the lesion is used to ‘seed’ a lesion-associated network derived from a large cohort of healthy subjects using resting state functional connectivity MRI, which relies on correlated patterns of spontaneous blood oxygen level-dependent (BOLD) signal. This allows the identification of a broader network of regions that are functionally connected to the lesion site (Fig. 1). The resulting networks can then be analysed in relation to symptoms, often by comparing the distribution of networks derived from lesions associated with a syndrome under investigation versus comparison lesions not associated with the same syndrome. Lesion network mapping provides information about what the likely connectivity pattern of the lesioned site was prior to the lesion onset, which is different and potentially complementary information relative to studies that evaluate altered patterns of functional imaging within the brains of individuals with focal brain lesions.
Figure 1.
Lesion network mapping. Lesion network mapping involves three steps: (i) a brain lesion from a patient scan acquired clinically or for research is mapped onto a template brain; (ii) the lesion volume is used as a seed region of interest for a resting state functional connectivity MRI analysis that uses normative data; and (iii) the lesion-associated networks can then be analysed, such as comparing network results in relation to the presence or absence of a symptom being investigated.
The first application of lesion network mapping was in 23 patients who experienced visual hallucinations following small lesions of the brainstem or thalamus, known as peduncular hallucinosis (Boes et al., 2015). We demonstrated that the location of 22 of the 23 lesions was negatively correlated with a lateral extrastriate region that had previously been implicated in visual hallucinations. An example from the first case in the series analysed this way is shown in Fig. 1. These findings supported the hypothesis that lesion network mapping could be used to identify a cortical region implicated in symptom expression based on shared connectivity to the subcortical lesion sites. The results also presented an interesting hypothesis regarding a possible mechanism by which small subcortical lesions may influence network dynamics in remote cortical regions, which has since received some support (Geddes et al., 2016). In the same article we evaluated whether lesion network mapping had broader applicability beyond peduncular hallucinosis by evaluating subcortical lesions that caused expressive aphasia, central post-stroke pain, and auditory hallucinations. In each case, lesion network mapping linked small, anatomically disparate subcortical lesions to regions of the cerebral cortex previously implicated in symptom expression.
Since 2015, the lesion network mapping approach has been applied by a number of investigators evaluating how the network organization of the brain may help to explain symptoms associated with focal brain lesions (Fischer, 2016; Sutterer et al., 2016; Darby et al., 2017, 2018a, b; Fasano et al., 2017; Joutsa et al., 2018, 2019; Cohen et al., 2019; Corp et al., 2019; Ferguson et al., 2019; Padmanabhan et al., 2019; Kim et al., 2020; Phillipi et al., 2020). A number of interesting and original contributions have come from these efforts and may have contributed, in part, to a renewed enthusiasm for the lesion approach in neurology and cognitive neuroscience in recent years. However, there has been some evolution in how the lesion network method is applied relative to the original study, in ways that may be problematic and require validation. A primary concern that I have had involves the use of large lesions, such as those from large vessel ischaemic strokes. These large lesions tend to be heavily represented in most lesion studies, including the Salvalaggio study. Large lesions are problematic for lesion network mapping on two accounts. First, these lesions span both grey and white matter, and the grey matter within the lesion volume contributes preferentially to the BOLD signal used to generate candidate networks, yet the white matter is often the source of the strongest associations with deficits in lesion symptom mapping studies (Corbetta et al., 2015; Griffis et al., 2017). Lesion network mapping is quite limited in how it can account for disrupted white matter contributing to deficits. Second, large lesions that are used to ‘seed’ functional connectivity analyses rely on the average BOLD signal derived from the lesion volume. It is not clear that meaningful information is gleaned from the average BOLD signal derived from large regions that contain multiple discrete parcels of functionally heterogeneous tissue combined with the white matter BOLD signal. This signal averaging likely contributes to low dimensionality of the lesion-associated network data observed by Salvalaggio and colleagues (2020). A final concern relates to the higher sensitivity and lower specificity of lesion-associated networks relative to lesion symptom mapping. The whole-brain connectivity pattern resulting from each analysis presents many opportunities to highlight network findings that fit with the existing literature, but determining which, if any, of the regions within the brain-wide network are causally related to the symptoms being studied requires validation with other methods. These issues likely contributed to the underwhelming predictions of behavioural deficits from lesion network mapping by Salvalaggio et al., which aligns with some of my own observations from work currently in progress that has led to more cautious and judicious use of lesion network mapping while attempting to better understand its limitations.
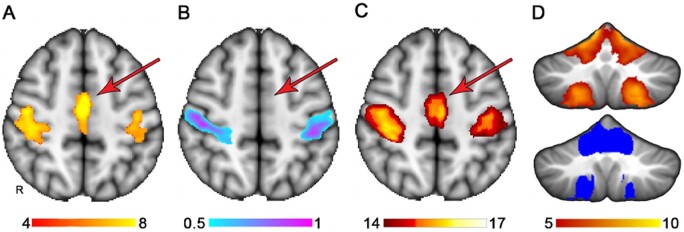
The findings of Salvalaggio et al. provide a robust and welcomed call to action that should propel new efforts to refine and improve lesion network mapping to more optimally integrate the lesion method with connectome data. For this effort I think the future is bright. A variation of lesion network mapping that avoids some of the limitations listed above involves using lesion location information to first identify regions maximally associated with impairment. The results of the traditional lesion symptom mapping approach can then be used to seed either functional connectivity or tractography network analyses to place the findings within the context of a broader functional network. The resulting network can be further refined by evaluating which nodes significantly discriminate case versus comparison groups based on connectivity strength (Albazron et al., 2019). While it remains to be seen whether this approach can predict behavioural deficits above and beyond those made by lesion location alone, there is evidence that this approach can be used to augment lesion symptom mapping in a meaningful way. Figure 2 demonstrates an illustrative example of this where where lesion network mapping extends lesion localization to functionally related brain regions that lack adequate lesion coverage. Here, significant lesion symptom mapping results were used to ‘seed’ the network. Regions identified using this approach do not provide the same level of causal evidence that lesion symptom mapping does, but the results nevertheless present an unambiguous anatomical hypothesis that could be tested in subsequent studies that have lesion coverage of these regions.
Figure 2.
Extension of lesion localization to connected brain areas. (A) The somatomotor network derived from a previously published atlas of functional connectivity data derived from normal healthy adults (Smith et al., 2009). (B) A lesion symptom map derived from somatomotor network ‘lesion load’ values from A used as simulated behavioural data, where the success or failure of the lesion symptom map could be judged based on the similarity to A. (C) A potential use of lesion network mapping in extending the lesion symptom mapping findings in B to other functionally related brain regions, in this case identifying the medial node of the somatomotor network despite inadequate lesion coverage in this region, evident by the absence of findings in B. (D) Similarly, lesion network mapping extends the functional network to the cerebellum somatomotor network even though the cerebellum had no lesion coverage in this analysis. The results from lesion network mapping are on top and the somatomotor network of the cerebellum from a published atlas is shown below for reference (Buckner, 2011). The colour bars denote voxel-wise Z-scores for the somatomotor network (A), the strength of association of lesion location with somatomotor network lesion load based on multivariate lesion symptom mapping, which is output as arbitrary units from 0 to 1, thresholded at 0.5 to display the strongest findings (Pustina et al., 2018). And voxel-wise Z-scores reflecting strength of connectivity with the regions denoted in B.
Other promising approaches remain to be tested, such as mapping lesion locations onto highly sampled multimodal template brains that have been preparcellated in an individualized and data-driven way to identify discrete network parcels and white matter tracts. Here, the connectivity pattern of each discrete parcel of grey matter and white matter within the lesion could be considered individually and aggregated at a group level, as opposed to an ‘average’ signal within the lesion where important and discriminating features of the networks in which the lesion is embedded are lost. This is especially problematic with larger lesions.
The relative merit and validity of these approaches should ultimately be tested using a similar approach of Salvalaggio et al. by evaluating the amount of variance that can be explained across multiple domains in large and well-characterized samples. This will be increasingly feasible as large shared lesion repositories with behavioural data are being developed (Liew et al., 2020). It is likely that a combination of approaches that incorporate both lesion location and lesion-associated networks, measured directly and indirectly, can be leveraged in unique combinations to optimize predictions in different domains. Large-scale lesion studies like that of Salvalaggio and colleagues will be essential to the development and improvement of these methods with time and provide the foundation upon which clinical tools can eventually be developed that inform prognosis or guide rehabilitation strategies.
Data availability
The data that support the illustrative example in Fig. 2 are available from the corresponding author, upon reasonable request.
Acknowledgements
I thank Fatimah Albazron and Joel Bruss for their assistance in preparing Fig. 2 and Kai Hwang, Daniel Tranel, Ralph Adolphs, and Nick Trapp for helpful comments on an earlier draft of this letter.
Funding
This work was supported by the National Institute of Health, R01NS114405 and R21MH120441.
Competing interests
The author reports no competing interests.
References
- Albazron FM, Bruss J, Jones RM, Yock TI, Pulsifer MB, Cohen AL, et al. Pediatric postoperative cerebellar cognitive affective syndrome follows outflow pathway lesions. Neurology 2019; 93: e1561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axer M, Amunts K, Grassel D, Palm C, Dammers J, Axer H, et al. A novel approach to the human connectome: ultra-high resolution mapping of fiber tracts in the brain. Neuroimage 2011; 54: 1091–101. [DOI] [PubMed] [Google Scholar]
- Boes AD, Prasad S, Liu H, Liu Q, Pascual-Leone A, Caviness VS Jr, et al. Network localization of neurological symptoms from focal brain lesions. Brain 2015; 138: 3061–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT, The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106: 2322–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AL, Soussand L, Corrow SL, Martinaud O, Barton JJS, Fox MD. Looking beyond the face area: lesion network mapping of prosopagnosia. Brain 2019; 142: 3975–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Ramsey L, Callejas A, Baldassarre A, Hacker CD, Siegel JS, et al. Common behavioral clusters and subcortical anatomy in stroke. Neuron 2015; 85: 927–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corp DT, Joutsa J, Darby RR, Delnooz CCS, van de Warrenburg BPC, Cooke D, et al. Network localization of cervical dystonia based on causal brain lesions. Brain 2019; 142: 1660–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby RR, Horn A, Cushman F, Fox MD. Lesion network localization of criminal behavior. Proc Natl Acad Sci USA 2018. a; 115: 601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby RR, Joutsa J, Burke MJ, Fox MD. Lesion network localization of free will. Proc Natl Acad Sci USA 2018. b; 115: 10792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby RR, Laganiere S, Pascual-Leone A, Prasad S, Fox MD. Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain 2017; 140: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol 2017; 81: 129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MA, Lim C, Cooke D, Darby RR, Wu O, Rost NS, et al. A human memory circuit derived from brain lesions causing amnesia. Nat Commun 2019; 10: 3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DB, Boes AD, Demertzi A, Evrard HC, Laureys S, Edlow BL, et al. A human brain network derived from coma-causing brainstem lesions. Neurology 2016; 87: 2427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes MR, Tie Y, Gabrieli JD, McGinnis SM, Golby AJ, Whitfield-Gabrieli S. Altered functional connectivity in lesional peduncular hallucinosis with REM sleep behavior disorder. Cortex 2016; 74: 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, et al. Precision functional mapping of individual human brains. Neuron 2017; 95: 791–807.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis JC, Nenert R, Allendorfer JB, Szaflarski JP. Damage to white matter bottlenecks contributes to language impairments after left hemispheric stroke. Neuroimage Clin 2017; 14: 552–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsa J, Horn A, Hsu J, Fox MD. Localizing Parkinsonism based on focal brain lesions. Brain 2018; 141: 2445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsa J, Shih LC, Fox MD. Mapping Holmes tremor circuit using the human brain connectome. Ann Neurol 2019; 86: 812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NY, Hsu J, Talmasov D, Joutsa J, Soussand L, Ona W, et al. Lesions causing hallucinations localize to one common brain network. Mol Psychiatry 2020. doi: 10.1038/s41380-019-0565-3. [DOI] [PubMed] [Google Scholar]
- Liew SL, Zavaliangos-Petropulu A, Jahanshad N, Lang CE, Hayward KS, Lohse KR, et al. The ENIGMA Stroke Recovery Working Group: big data neuroimaging to study brain-behavior relationships after stroke. Hum Brain Mapp 2020. Advance Access published on April 20, 2020. doi: 10.1002/hbm.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan JL, Cooke D, Joutsa J, Siddiqi SH, Ferguson M, Darby RR, et al. A human depression circuit derived from focal brain lesions . Biol Psychiatry 2019; 86: 749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipi C, Boes A, Albazron FM, Bruss J, Deifelt Streese C, Rudrauf D, et al. Lesion network mapping demonstrates that mind-wandering is associated with the default mode network. Neurosci Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustina D, Avants B, Faseyitan OK, Medaglia JD, Coslett HB. Improved accuracy of lesion to symptom mapping with multivariate sparse canonical correlations. Neuropsychologia 2018; 115: 154–66. [DOI] [PubMed] [Google Scholar]
- Salvalaggio A, De Filippo De Grazia M, Zorzi M, Thiebaut de Schotten M, Corbetta M. Post- stroke deficit prediction from lesion and indirect structural and functional disconnection. Brain 2020; 143: 2173–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 2009; 106: 13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterer MJ, Bruss J, Boes A, Bechara A, Tranel D. Canceled connections: lesion-derived network mapping helps explain differences in performance on a complex decision-making task. Cortex 2016; 78: 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the illustrative example in Fig. 2 are available from the corresponding author, upon reasonable request.