Abstract
Aims
It is well-established that endothelial dysfunction promotes activation of vascular smooth muscle cell (VSMC). Whether decreased accumulation of VSMCs affects endothelial regeneration and functions in arteriovenous graft (AVG) remodelling has not been studied. We sought to identify mechanisms by which the Notch ligand, Jagged1, in VSMCs regulates endothelial cell (EC) functions in AVGs.
Methods and results
AVGs were created in transgenic mice bearing VSMC-specific knockout (KO) or overexpression of Jagged1. VSMC migration, EC regeneration, and its barrier functions as well as AVG remodelling were evaluated. Jagged1 expression was induced in VSMCs of neointima in the AVGs. Jagged1 KO in VSMCs inhibited the accumulation of extracellular matrix as well as VSMC migration. Fewer α-SMA-positive VSMCs were found in AVGs created in VSMC-specific Jagged1 KO mice (VSMCJagged1 KO mice) vs. in WT mice. Decreased VSMCs in AVGs were associated with deterioration of EC functions. In AVGs created in transgenic mice bearing Jagged1 KO in VSMCs exhibited delayed EC regeneration and impaired EC barrier function. Barrier dysfunction of ECs increased inflammatory cell infiltration and dysregulation of AVG remodelling and arterialization. The increased expression of IL-1β in macrophages was associated with expression of adhesion markers in ECs in AVGs created in VSMCJagged1 KO mice. In contrast, AVGs created in mice with overexpression of Jagged1 in VSMCs exhibited improved EC regeneration plus decreased macrophage infiltration. This led to AVG remodelling and arterialization. In co-cultures of ECs and VSMCs, Jagged1 deficiency in VSMCs suppressed N-cadherin and integrin β3 expression in ECs. Inhibition of integrin β3 activation delayed EC spreading and migration. Notably, Jagged1 overexpression in VSMCs or treatment with recombinant Jagged1 stimulated the expression of N-cadherin and integrin β3 in ECs. Jagged1-induced responses were blocked by inhibition of Notch signalling.
Conclusions
Jagged1 expression in VSMCs maintains EC barrier functions and blocks infiltration of macrophages. These responses promote remodelling and arterialization of AVGs.
Keywords: Arteriovenous graft, Jagged1, Notch signalling pathway, Vascular smooth muscle cell, Endothelial cell regeneration
Graphical Abstract
Graphical Abstract.
Time for primary review: 37 days
1. Introduction
Patients with end-stage renal disease (ESRD) undergoing maintenance haemodialysis require vascular access for the dialysis procedure, with surgical creation of an arteriovenous fistula (AVF) or graft (AVG) being preferred over central venous catheters. However, both AVG and AVF are prone to thrombosis and failure. As many as 50% of AVGs may fail within 10 years of their creation costs for maintaining functional AVGs have been estimated near $2 billion per year.1,2 To reduce the loss of AVGs, new insights into mechanisms causing AVG failure are needed.
A potential mechanism causing reduced blood flow in AVGs is the development of a neointima near the arteriovenous anastomosis arising in part from the proliferation and hyperplasia of vascular smooth muscle cells (VSMC).3–6 In earlier experiments, we showed that the formation of a neointima proceeds from interactions among VSMCs, mediated at least in part by Notch signalling between neighbouring cells.7 The binding of Notch ligands (in mammals, designated as Delta-like or Jagged) with their receptors stimulates proteolytic cleavage of the extracellular domain of Notch. Subsequently, the Notch intracellular domain (NICD) is stimulated; it directly interacts with the RBP-Jκ transcription factor. However, if Notch is absent, RBP-Jκ will interact with corepressors that suppress transcriptional activity of targeted genes.8,9 Alternatively, that activation of Notch will displace corepressors from RBP-Jκ and induce the expression of the target genes, Hes1 and Hey.10 Thus, Notch signalling regulates VSMC differentiation: when Notch is suppressed, arterial VSMCs are severely reduced.11 Importantly, activated Notch signalling is also necessary for the development of VSMCs in arteries of the branchial arch.12 To identify the role of Notch in regulating vascular responses, Chang et al. have created a doxycycline-inducible system in Tie1-Cre/dnMAML mice. The expression of dominant negative MAML1 (dnMAML1), inhibits Notch signalling and reduces the thickness of arteries.13 We reported that Notch activation leads to endothelial mesenchymal transition and impairment of endothelial cell (EC) barrier function.7,9
Jagged1 is a Notch ligand that plays a key role in vascular haemostasis and remodelling. Mutation of Jagged1 in patients with the Alagille syndrome is characterized by major abnormalities in pulmonary arteries.14–16 In adults, Jagged1 is mainly expressed in ECs and this response maintains VSMCs in a quiescent condition.17 Jagged1 deletion in ECs reportedly decreases Notch activation in neighbouring VSMCs and produces defects in vascular walls.18,19 In the present study, we generated mice with VSMC-specific gain or loss of function of Jagged1 in order to study the role of Jagged1 on AVG remodelling. We hypothesized that Jagged1 expression in neointimal VSMCs would be required for EC regeneration, EC barrier functions, and AVG arterialization.
2. Methods
2.1 Animals
All studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Baylor College of Medicine, in Houston, TX, and performed in accordance with National Institutes of Health (NIH) guidelines. Mice were housed in an animal facility with a 12 h light/dark cycle. Jagged1f/f mice (strain, C57BL/6J, 010618) were purchased from Jackson Laboratories (Bar Harbor, ME). Jagged1 overexpression mice20 were kindly provided by Dr Xin (Baylor College of Medicine, Houston, TX); SMMHC-CreERT2 transgenic mice were obtained from Dr S. Offermanns (Max-Planck-Institute for Heart and Lung Research, Bad Nauheim, Germany) and used as described.7 To generate mice with knockout (KO) of Jagged1 in VSMCs, mice with a floxed Jagged1 allele were bred with SMMHC-ERCre mice. After a backcross, Jagged1f/f/SMMHC-ERCre+ (VSMCJagged1KO) mice were obtained. Male mice from VSMCJagged1KO and littermate controls mice were studied. Similar breeding strategy was used for VSMCJagged1OE transgenic mice. Jagged1 deletion was induced by Tamoxifen (2 mg/mouse/day i.p. for 5 days) 2 weeks before venous graft placement was performed.
2.2 Reagents and virus
Penicillin, streptomycin, DMEM, and FBS and the fluorescent-680 (Cat. number: A-10038) or 800 (Cat. number: A-32808) secondary antibodies for western blotting and all secondary antibodies for immunofluorescent staining were obtained from Invitrogen (Invitrogen Life Technologies; Carlsbad, CA). The γ-secretase Notch inhibitor, DAPT, was from Calbiochem (San Diego, CA) while human TGF-β1 and recombinant rat Jagged1 protein were obtained from R&D (Minneapolis, MN). Antibodies against Notch 1-ICD (Cat. number: 4147) and Jagged1 (Cat. number: 70109) were from Cell Signaling Biotechnology (Cell Signaling, Danvers, MA). While antibodies against calponin (Cat. number: C2687), α-SMA-FITC (Cat. number: F3777), α-SMA (Cat. number: A5228), and β-actin (Cat. number: A5441) were from Sigma-Aldrich (Louis, MO). The FSP-1 antibody (Cat. number: A5114) was from DAKO (Carpentaria, MA). Antibodies against PCNA (Cat. number: sc-7907), Jagged1 (Cat. number: sc-6011), Hes1 (Cat. number: sc-166410), ICAM (Cat. number: sc-71303), integrin-β3 (Cat. number: sc-6626), IL-1β (Cat. number: sc-12742), and GAPDH antibodies (Cat. number: sc-32233) were from Santa Cruz Biotechnology (Santa Cruz, CA). While an antibody against Hey2 (Cat. number: AB5716) and Cyclin D1 (Cat. number: 05-362) were from Millipore (Billerica, MA). Antibodies against vWF (Cat. number: ab6994), PCNA (Cat. number: ab92552), rabbit anti-α-SMA (Cat. number: ab5694), GFP (Cat. number: ab6556), F4/80 (Cat. number: ab6640), E-selectin (Cat. number: ab18981), Ki67 (Cat. number: ab16667) were from Abcam (Cambridge, MA). CD45 antibody (Cat. number: 553076), and VE-cadherin (Cat. number: 550548), and N-cadherin (Cat. number: 610920) were from BD Pharmingen (San Jose, CA). Antibodies against PECAM (CD31) (Cat. number: DIA-310-M) and Mac2 (Cat. number: CL8942AP) were from Cedarlane (Burlington, NC), Control rabbit IgG (Cat. number: S-5000) was from Vector Labs (Burlingame, CA). The detailed information about antibodies used in this study was listed in Supplementary material online, Table S1. 4′,6-diamidino-2-phenylindole (DAPI) was from Southern Biotech (Birmingham, AL). The full-length, Jagged-1 adenovirus was kindly provided by Dr M.J. Post (Maastricht University, Netherlands). The packaging vectors for lentivirus were purchased from Addgene (Cambridge, MA). The Lentivirus for KO Jagged1 was constructed into CRIPR-Caspase9 vector by following the protocol.21 The lentivirus-infected cells were selected by puromycin treatment.
2.3 Mouse model of venous graft
All animal protocols were approved by IACUC of Baylor College of Medicine. Wild-type C57/B6, VSMCJagged1KO, and VSMCJagged1OE mice. AVG was performed under dissecting microscope (Leica MZ6; Leica, Germany) as previously described.22 In brief, rodent III combo, that each millilitre contains ketamine (37.5 mg), xylazine (1.9 mg), and acepromazine (0.37 mg), was used to anesthetize mice at dose of 3 ml/kg body weight by intraperitoneal injection. The right common carotid artery of a male mouse was surgically exposed to place a cuff on both ends of the artery. The ends were then everted over the cuff and ligated with an 8.0 silk suture. Vena cava from donor mice was grafted by ‘sleeving’ ends of the vein over the artery cuff and secured with 8.0 silk sutures. After 4 weeks, mice with AVGs were anesthetized by intraperitoneal injection of rodent III combo with same dose as above and euthanized by perfusing the left ventricle with PBS and 10% formalin for 10 min (to maintain the endothelium and morphology of the AVG). AVGs were collected and slides from 0.5 to 1 mm of the anastomosis were prepared for haematoxylin/eosin staining. The AVG was dissected and vessel wall thickness measured as the area of the vessel minus that of the lumen using NIS-Elements BR 3.0 program. The personnel conducting various examination and calculations were blinded to the mouse genotyping background. This mouse model does not cause mortality.
2.4 Bone marrow transplantation
Mice were anesthetized by rodent III combo, that each millilitre contains ketamine (37.5 mg), xylazine (1.9 mg), and acepromazine (0.37 mg), at dose of 3 ml/kg body weight by intraperitoneal injection. Bone marrow cells were obtained from mouse tibias and femurs of GFP transgenic mice (Jackson lab, 00329). The mice were euthanized by overdose injection of rodent III combo (9 ml/kg body weight) by intraperitoneal injection. Bone marrow transplantation was performed by injecting 5×106 BM cells into the lateral tail vein of lethally irradiated (1100 rads) mice recipients.23,24
2.5 Ex vivo culture of mouse common carotid arteries
Mouse common carotid arteries were collected from WT and Jagged1 KO mice and assayed.25 Mice were anesthetized by rodent III combo, that each millilitre contains ketamine (37.5 mg), xylazine (1.9 mg), and acepromazine (0.37 mg), at dose of 3 ml/kg body weight by intraperitoneal injection and euthanized by perfusing the left ventricle with PBS. The adventitial layer was removed under dissect microscope. Then the arteries were opened longitudinally and cut transversely into small (0.2 cm) segments. These segments contained the media layer were seeded onto 12-well plates with luminal surface facing down and cultured individually in the DMEM media. AdGFP or AdJagged1 with dose of 2×106 plaque forming units to infect cells in 6-well plate were added and the treatment and culture media were replaced every other day. Outgrowth of the VSMCs was recorded.
2.6 Histology and immunohistochemistry
For histological analyses, mice were perfused through the left ventricle and slides of AVGs were prepared as described.7 Immunohistochemical staining and H&E-stained sections from AVGs of five mice in each group were examined by a pathologist who was masked to treatment. For double immunofluorescence staining, primary antibodies were added followed by fluorescent secondary antibodies; DAPI was used to stain nuclear DNA. To capture images, the Nikon Eclipse 80i fluorescence microscope (Melville, NY) was used. The negative controls were either addition of isotype-matched IgG or PBST. The areas of positive signal were measured using the NIS-Elements BR 3.0 program. Images (× 400) from each section were analysed in a blinded manner and quantified using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). Measurements were performed by three investigators, all blinded to the clinical information.
2.7 Real-time RT–PCR
Total RNAs from control vein or from AVGs were isolated using the RNeasy kit (Qiagen, Valencia, CA). Real-time RT–PCR was performed using Opticon real-time RT–PCR machine (MJ Research, Waltham, MA). The specificity of real-time RT–PCR was confirmed using agarose gel electrophoresis and melting-curve analysis.
2.8 Western-blot analysis
The protein content of cell extracts prepared in RIPA buffer, was determined using the Bradford protein assay kit (BioRad, Hercules, CA). About 30 μg of proteins were separated by SDS–PAGE and after transferring to nitrocellulose membranes, immunoblots were probed separately with various primary antibodies. Subsequently, the immunoblots were blocked with 5% skimmed milk in TBS. Fluorescently labelled secondary antibodies were detected by the Odyssey Infrared Imaging System (LI-COR, Inc, Lincoln, NE).
2.9 Mouse EC isolation
Since the amount of ECs from mouse arteries was not sufficient for proposed experiments, we isolated ECs from mouse kidneys. The method used to isolate mouse ECs was adapted from that described by Jackson with Modification.26 Mice were anesthetized by rodent III combo, that each millilitre contains ketamine (37.5 mg), xylazine (1.9 mg), and acepromazine (0.37 mg), at dose of 3 ml/kg body weight by intraperitoneal injection and euthanized by perfusing the left ventricle with PBS. We used anti-PECAM-1 and ICAM-2 conjugated Dynabeads (Invitrogen, Carlsbad, CA) to immunomagnetically separate the ECs from the other cells present in the tissue.27 The purified ECs were confirmed by CD31 and VE-cadherin immunostaining. Over 90% of these cells were positive for EC markers.
2.10 Co-culture of EC with VSMC
Direct contact-co-culture of VSMC and EC. The co-culture was done as previously reported.28 Briefly, co-culture was established by plating cells on the two sides of a 10 µm thick porous polyethylene terephthalate (PET) membrane (Falcon cell culture inserts; Becton Dickinson, Lincoln Park, NJ). The bottom side of the insert (with membrane of diameter of 24 mm with 0.4 µm pores) was coated with 1% gelatin. ECs were first seeded onto the bottom side of the insert at a density of 2×106 cells/cm2. After allowing 2 h for adherence, the insert with the EC side down was inserted into a 6-well plate containing the EGCF medium, and the opposite side of the membrane (the inner side of the insert) was seeded with identical ECs (EC/EC) or VSMCs (EC/VSMC) at a density of 2×105 cells/cm2. ECs and VSMCs were maintained in their respective media until confluence. The cells were further incubated for 24 h prior to the experiment.
2.11 En face analysis of AVG
We analysed the endothelium in AVGs using an en face technique with immunostaining, as described previously.29 AVG segments were cut longitudinally, mounted on glass slides with endothelium facing upward, and air dried for 1–2 h. AVGs were incubated with antibodies against VE-cadherin followed by immunofluorescently labelled secondary antibodies. The ratio of endothelial marker-positive cells to total cells was calculated; DAPI was used to stain nuclei.
2.12 Evans blue examination of endothelial barrier function of AVGs
At the end of the experiment, 50 µl of 5% Evans blue diluted in saline was injected into the tail vein, and 10 min later, the mice were euthanized. AVGs were removed, washed with PBS, and fixed for 5 min in 10% neutral buffered formalin.29 AVGs were photographed with the vena cava used as control. Evans blue accumulated extracellular matrix following disruption of the endothelium.
2.13 Statistical analysis
All data were assessed for normal distribution. All the data were presented as the mean ±SD. Comparisons between groups were analysed using t-test or ANOVA as appropriate; P < 0.05 was considered to be statistically significant.
3. Results
3.1 Jagged1 is expressed in VSMCs of AVGs
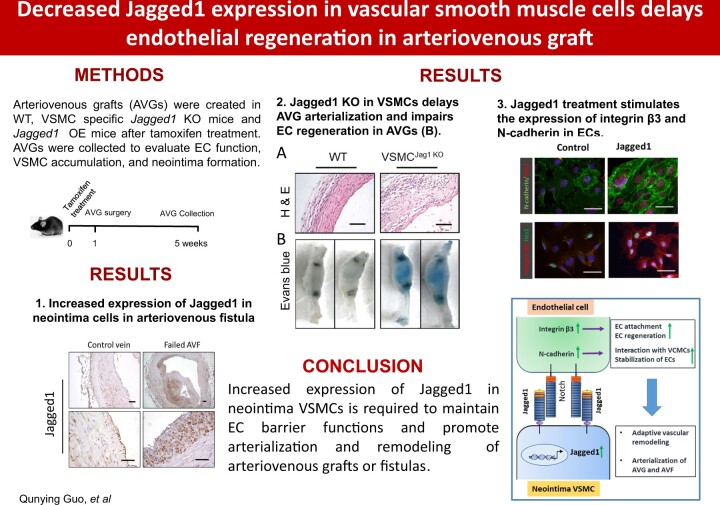
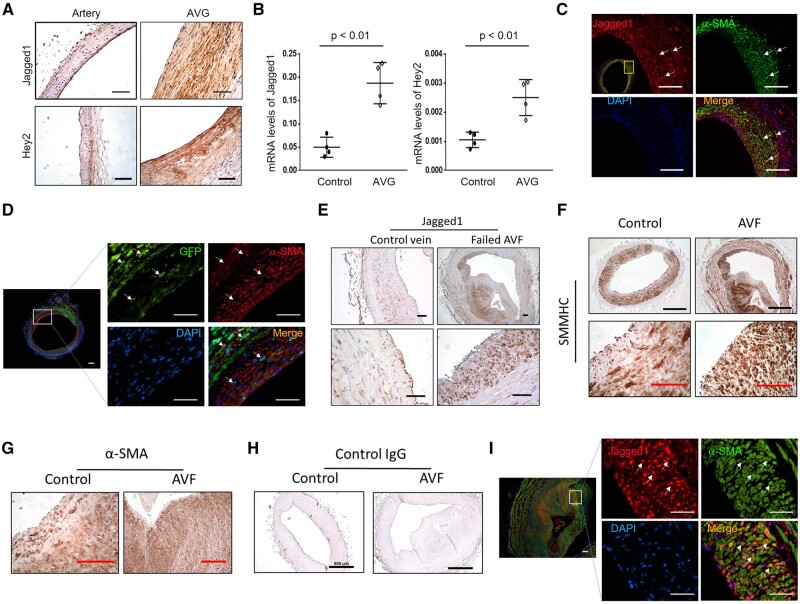
Compared to the common carotid artery Jagged1 is expressed in ECs; but in AV grafts, there was increased expression of Jagged1 and the Notch downstream target Hey2 in the neointima (Figure 1A). In addition, mRNA levels of Jagged1 and the Notch target, Hey2, were upregulated in VSMCs of AV grafts vs. results found in control arteries. These results confirm the activation of Notch signalling in VSMCs (Figure 1B). To determine which type of cells in the neointima express Jagged1, double immunofluorescent staining was performed. The results uncovered that most of the Jagged1 positive cells in the neointima were co-stained with VSMC marker α-SMA in AVGs (white arrows pointed cells; Figure 1C).
Figure 1.
Jagged1 expression is increased in neointima cells. (A) The expression Jagged1 and Hey2 in common carotid artery and in 1 month AVGs was determined by immunohistochemistry staining. (B) mRNA levels of Jagged1 and Hey2 from common carotid artery and AVGs were determined by real time RT–PCR. The Ct values for Jagged1 in control arteries and AVGs are 26.6 ± 1.26 and 24.9 ± 1.71. The Ct values for Hey2 in control and AVGs are 31.6 ± 2.41 and 30.2 ± 2.35 (n = 4; *, P < 0.05 compared with artery, paired t-test was used for statistical analysis). (C) The expression of Jagged1 and α-SMA were determined in 1 month AVGs by double fluorescent immunostaining. (D) The expression of GFP and α-SMA were determined by double immunostaining in AVGs that were created in VSMCGFP mice. (E) Representative images of Jagged1 expression in AVF from ESRD patients. (F–H) Neointima cells in AVFs from ESRD patients are characterized by immunostaining. Representative images of immunostaining of VSMC markers, SMMHC (F) and α-SMA (G), and control rabbit IgG were shown (H) (n = 6; black scale bars, 500 µm; red bars, 100 µm). (I) Representative imagines of co-immunostaining of Jagged1 and α-SMA in patient AVFs (n = 6; scale bars are 50 µm in all panels).
The origin of the neointima of VSMCs in AVGs was determined by transgenic reporter mice and by bone marrow transplantation. Firstly, we used the VSMC reporter mice, mTmG/SHHMC-ERCre+ mice (VSMCGFP), to label and track VSMCs (see Supplementary material online, Figure S1A). After tamoxifen treatment, the VSMCs in the common carotid arteries became GFP positive and co-stained with α-SMA (see Supplementary material online, Figure S1B). In AVGs created in VSMCGFP mice, > 90% of neointimal VSMCs were originated from GFP-labelled VSMCs (Figure 1D). Consistently, in AVGs that were created in WT mice with bone marrow from GFP transgenic mice, bone marrow-derived GFP positive cells did not contribute to α-SMA+ VSMCs in the neointima (see Supplementary material online, Figure S2).
A substantial increase in the expression of Jagged1 was also found in neointima cells that were present in AV fistulas of ESRD patients. In control veins, few Jagged1-positive signals were present (Figure 1E). The cells from the neointima and wall of the vein in AVFs were positive stained for VSMC markers SMMHC and α-SMA (Figure 1F and G). Control anti-rabbit antibodies did not show positive signals (Figure 1H). These Jagged1 positive cells in the neointima were co-stained with α-SMA (Figure 1I).
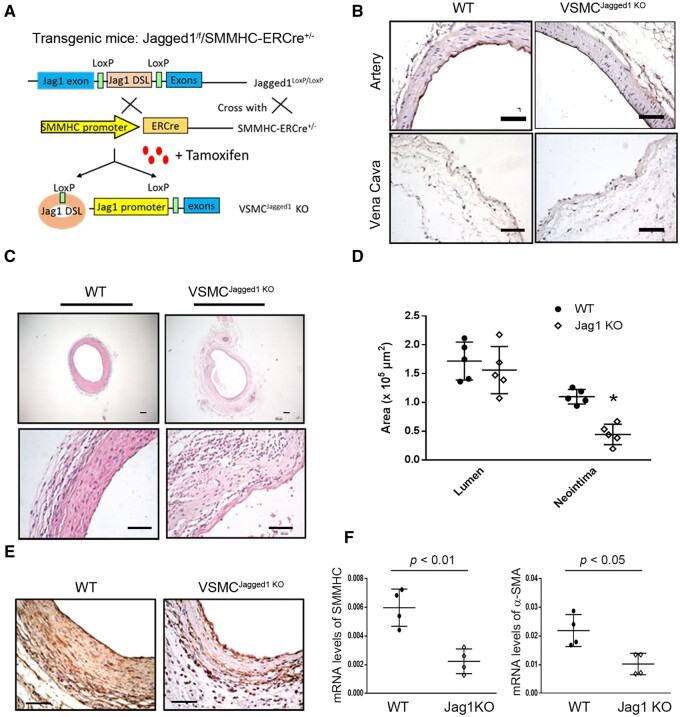
3.2 KO of Jagged1 in VSMCs impairs vascular remodelling in AVGs
Since deficiency of Jagged1 is embryonic lethal, we created conditionally inducible Jagged1 KO mice by breeding Jagged1flox/flox with SMMHC-ERCre mice (Figure 2A). Following tamoxifen treatment (i.p., 40 mg/kg, bw, for five constitutive days), Jagged1 expression in VSMCs of Jagged1f/f/SMMHC-ERCre+ mice (i.e., VSMCJagged1KO mice) was eliminated (Figure 2B). There were no vascular structure changes between WT and Jagged1 KO mice (Figure 2B). Two weeks after tamoxifen treatment, we created AVGs in VSMCJagged1KO mice in order to identify if KO of Jagged1 affects vascular remodelling. KO of Jagged1 in VSMCs dramatically decreased neointima formation and inhibited vascular remodelling in AVGs. In contrast, AVGs created in WT mice (VSMCJagged1+/+ mice) led to VSMC accumulation and neointima formation (Figure 2C). The area of neointima was significantly smaller in VSMCJagged1KO mice vs. results in control (VSMCJagged1+/+) mice, while the lumen area had no significant changes between the two groups (Figure 2D). There was significant decreased expression of α-SMA in AVGs that had been created in VSMCJagged1KO mice (Figure 2E). The expression of mRNAs of α-SMA and SMMHC was decreased in AVGs in VSMCJagged1KO mice vs. results in control (VSMCJagged1+/+) mice (Figure 2F). These findings highlight our result that KO of Jagged1 in VSMCs suppresses VSMC accumulation and the arterialization of the AVGs.
Figure 2.
Jagged1 KO in VSMCs suppresses vascular remodelling in AVGs. (A) The schematic for generating VSMC-specific Jagged1 KO mice. (B) Jagged1 expression was determined in control vein and arteries from WT and VSMCJagged1 KO mice by immunostaining. (C and D) Representative images of H&E staining (C) and the areas of the lumen and neointima from of the 1 month AVGs created in VSMCJagged1+/+ and VSMCJagged1 KO mice (D) (n = 5; two sample t-test was used for statistical analysis; scale bars are 50 µm in all panels). (E) Representative images of the immunostaining of α-SMA in AVGs from WT and VSMCJagged1 KO mice. (F) The expression of VSMC markers were determined by RT–PCR in AVGs from WT and VSMCJagged1 KO mice. The Ct values for SMMHC in AVGs created in WT and Jagged1 KO mice are 29.7 ± 1.52 and 31.1 ± 2.6). The Ct values for α‐SMA in AVGs created in WT and Jagged1 KO mice are 27.91 ± 2.38 and 28.6 ± 2.65) (n = 4, *, P < 0.05 compared with results of WT; t-test was used for statistical analysis).
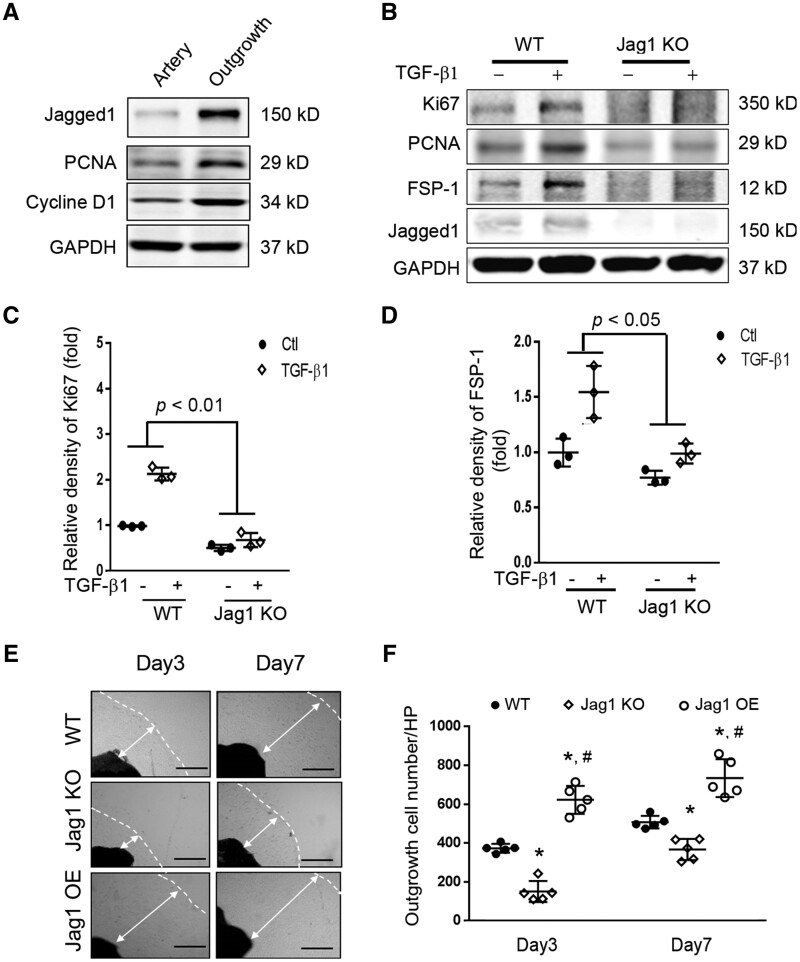
3.3 Jagged1 KO inhibits VSMC proliferation and migration
To determine the function of Jagged1 in VSMCs, we compared expression levels of Jagged1 in quiescent vs. proliferating VSMCs. Specifically, Jagged1 was undetectable in quiescent, arterial VSMC but there was increased Jagged1 expression in activated VSMCs (Figure 3A). In identifying potential mediators of Jagged1 expression in VSMCs, we treated these cells with TGF-β1 (2 ng/ml): the treatment stimulated Jagged1 expression and upregulated the proliferation markers, PCNA and Ki67 (Figure 3B and C). While Jagged1 KO in VSMCs did not significantly inhibit VSMC proliferation; only mild inhibition was uncovered in VSMCs with KO of Jagged1 vs. results in WT cells (Figure 3B and C). Notably, Jagged1 KO suppressed TGF-β1-stimulated pro-growth responses and inhibited the expression of fibroblast-specific protein 1 (FSP-1) in VSMCs (Figure 3B and D). Furthermore, seeding the artery segments induced outgrowth of VSMCs that are positive for VSMC markers (α-SMA and calponin) (see Supplementary material online, Figure S3). Jagged1 KO also suppressed VSMC outgrowth and its migration (Figure 3E and F). Jagged1 overexpression rescued VSMC migration (Figure 3E and F).
Figure 3.
Expression of Jagged1 promoted SMC phenotype switch from a quiescent to the synthetic status: (A) Common carotid arteries were dissected, half of the arteries were cultured, and half were kept for later use. After 7 days, proteins from the artery and outgrowth SMCs were isolated and the western blot was performed (n = 3), depicting gain of Jagged1 expression and increasing expression of PCNA and Cyclin D1 in the outgrowth SMCs. (B) VSMCs from WT and VSMCJagged1 KO mice were treated with or without 2 ng/ml TGF-β1 for 24 hrs. The expressions of Ki67, PCNA, and FSP-1 were detected by western blot. (C and D) Quantitation analysing the densities of Ki67 and FSP-1. Representative data from three experiments. Two-way ANOVA with an interaction between treatment and Jagged1 expression levels was used to test the expression of Ki67 and FSP-1 changes among the two groups. The effects of TGF-β1 on the expression of Ki67 and FSP1 were different in Jagged1 KO VSMCs compared to WT cells treated with TGF-β1. (E) Artery segments from WT, Jagged1 KO mice, and Jagged1 OE mice were seeded onto 24-well plates ex vivo and photographs were taken at indicated time points. Representative pictures are presented. The dashed lines indicate the edge of the cells, and the double arrowed lines show the distance of outgrowth cells (scale bars are 200 µm). (F) The cell numbers in panel (E) were counted and summarized (*, P < 0.05 vs. WT; #, P < 0.05 vs. Jagged1 KO; one-way ANOVA was used for statistical analysis for day 3 or day 7 separately, n = 5).
3.4 In VSMCs, Jagged1 deficiency is associated with inflammation in AVGs due to delayed EC regeneration
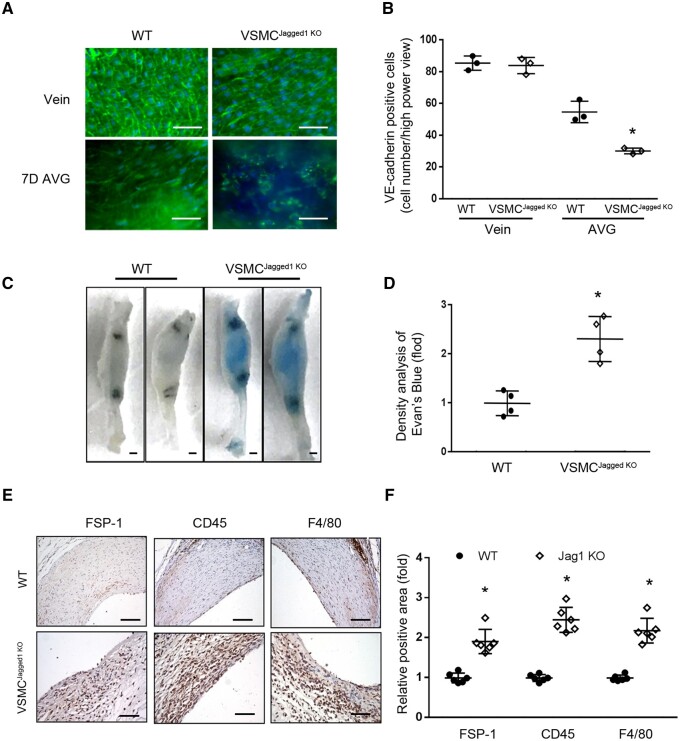
Cellular events such as EC regeneration and infiltration of inflammatory macrophages are involved in AVG remodelling.23 Using en face analyses, we found that loss of Jagged1 in VSMCs impaired the integrity of the endothelium of AVGs. The endothelial layer in AVGs that were created in WT mice showed there was an intact VE-cadherin+ EC network. In contrast, there were decreased VE-cadherin signals due to the decreased numbers of ECs that were randomly distributed in AVGs created in VSMCJagged1KO mice (Figure 4A and B). After 1 month, AVGs created in VSMCJagged1KO mice exhibited increased accumulation of Evans blue dye. In contrast, there was only faint staining of blue dye in AVGs from WT mice (Figure 4C and D), indicating the presence of endothelial leakage in AVGs created in VSMCJagged1KO mice. These results demonstrate that a deficiency of Jagged1 in VSMCs delays EC regeneration while impairing EC barrier functions.
Figure 4.
Jagged1 KO in VSMCs delays the EC regeneration and increases the inflammation. (A and B). Representative images of en face analysis of 7 day AVGs created in VSMCJagged1 KO mice and WT mice with VE-cadherin immunofluorescent staining (A). The number of VE-cadherin positive cells in AVGs were counted and analysed (B). (The data are represented as means ± SD; n = 3. *, P < 0.05 vs. results from AVGs created in WT mice; t-test was used for statistical analysis). (C and D). Jagged1 KO in VSMCs induces endothelial barrier dysfunction in AVGs. Before collecting AVGs, Evans blue was administrated intravenously to mice, followed by washing with PBS. The AVGs were dissected, and photographs were obtained (C). The intensity of blue staining was analysed (D). The data are represented as means ± SD; n = 4; *, P < 0.05 vs. WT control; t-test was used for statistical analysis. (E and F). The expression of inflammatory markers were determined by immunostaining in 1 month AVGs created in WT and VSMCJagged1KO mice (E). The density analysis were shown in (F). (n = 6, *, P < 0.05 vs. WT controls; scale bars in panels (A), (E), and (D) are 50 µm, in panel (C) are 1 mm; t-test were used for FSP-1, Mann–Whitney U test was used for CD45 and F4/80).
As expected from the demonstrated leakage in the endothelium, we found increased infiltration of CD45+ monocytes and F4/80+ macrophages in 1 month AVGs in Jagged1 KO mice vs. results from VSMCJagged1+/+ mice (Figure 4E and F). FSP-1 promotes cell migration and expresses in different type of cells. There were increased FSP-1+ signals in AVGs created in VSMCJagged1KO mice (Figure 4E and F). IgG control staining did not show positive signals (see Supplementary material online, Figure S4A). There FSP-1+ signals co-stained with macrophage marker Mac2 (see Supplementary material online, Figure S4B).
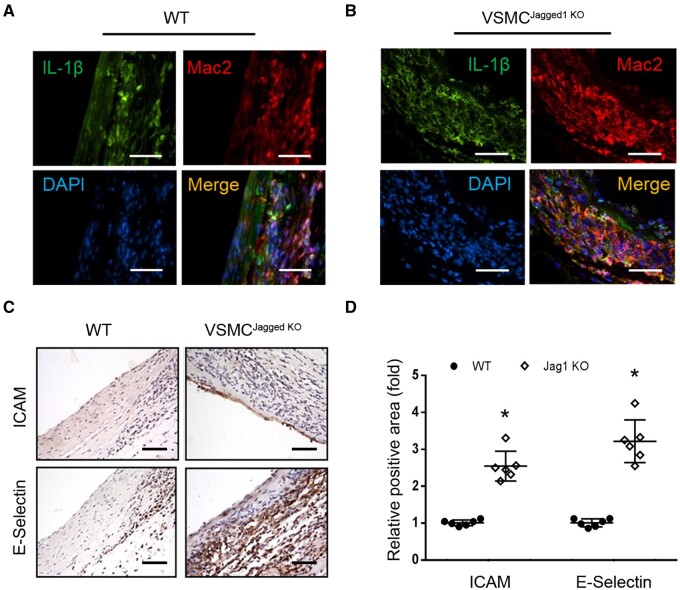
The infiltrated Mac2+ macrophages expressed cytokine, IL-1β; Increased IL-1β signals were detected in macrophages in AVGs that were created in VSMCJagged1KO mice vs. results from WT mice (Figure 5A and B). There also were increases in ICAM and E-selectin expressions in ECs of AVGs created in VSMCJagged1KO mice (Figure 5C and D). These results demonstrate that a deficiency of Jagged1 in VSMCs resulted in infiltration of macrophages that produce IL-1β, associated with increased expression of adhesion molecules in ECs in AVGs.
Figure 5.
Increased expression of adhesion molecules in ECs is associated with macrophage-produced IL-1β. (A and B). Representative images of immunostaining of IL-1β and Mac2 in AVGs creased in WT and VSMCJagged1KOmice. (C and D). The expression of ICAM and E-selectin were determined by immunostaining in AVGs that were created in WT and VSMCJagged1KOmice. (D) The density analysis of the positive signals was shown in (C). (n = 6, *, P < 0.05; scale bars are 50 μm. Mann–Whitney U test was used for statistical analysis).
3.5 Factors affecting endothelial functions in AVGs include integrin β3 and N-cadherin
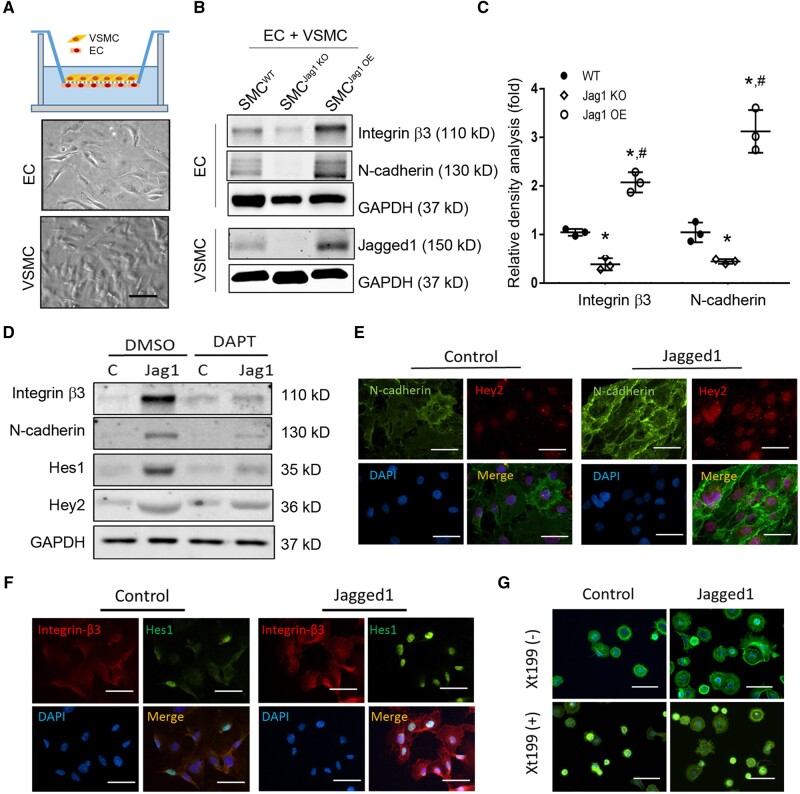
Defective EC functions uncovered in Figure 4 indicate that Jagged1 in VSMCs might increase the expression of integrin β3 and N-cadherin and enhance ECs functions. Co-culture experiments between ECs and VSMCs were utilized to determine how Jagged1 in VSMCs could affect EC functions. In these co-culture experiments, mouse kidney ECs were seeded on the reverse side of transwell inserts, while mouse VSMCs from mice were seeded on the other side of the membrane (Figure 6A). When co-culturing with VSMCs lacking Jagged1, there was decreased expression of N-cadherin and integrin β3 in ECs (Figure 6B and C). In contrast, overexpression of Jagged1 in VSMCs rescued the expression of N-cadherin and integrin β3 in ECs (Figure 6B and C). To specify the role of Jagged1 in regulating responses in ECs, we used recombinant Jagged1 to treat ECs. Coating of recombinant Jagged1 dose-dependently stimulated the expression of N-cadherin, VE-cadherin, and integrin β3 in ECs (see Supplementary material online, Figure S6). ECs seeded on recombinant Jagged1-coated plate also showed increased expressions of N-cadherin and integrin β3 (Figure 6D) plus higher levels of the Notch targets, Hes1 and Hey2 vs. results in untreated controls (Figure 6D). Jagged1 treatment-induced expression of Hes1 and Hey2 was blocked when ECs were treated with the Notch inhibitor, DAPT (Figure 6D). Double immunofluorescence analysis showed that treatment of recombinant Jagged1 increased the expression of N-cadherin and integrin β3 in ECs as well as the expression of Hes1 and Hey2 (Figure 6E and F). Finally, EC-attachment measurements were evaluated. Jagged1-treated ECs were dispersed more rapidly compared to control ECs. These responses disappeared when ECs were pre-treated with the integrin β3 inhibitor Xt199 (Figure 6G). Consequently, Jagged1 expression in VSMCs led to improvements in EC functions via stimulation of N-cadherin and integrin β3 expression in ECs.
Figure 6.
Jagged1 in VSMCs regulates EC adhesion function by enhance expression of Integrin β3 and N-cadherin in ECs. (A) Schematic diagram of the co-culture with a Boyden chamber. Mouse ECs were seeded onto the inverted side of the membrane containing 0.4 m pores configured at a density of 1.6×106 pores/cm2. While mouse SMCs were cultured in the opposite side of the membrane. (B) Western-blot analysis of EC after co-cultured with WT, Jagged1 KO, or Jagged1 OE VSMCs. (C) The density analysis of the expression of integrin β3 and N-cadherin (n = 3, *, P < 0.05 compared with controls; #, P < 0.05 compared with Jagged1 KO; one-way ANOVA was used for statistical analysis). (D) Notch signalling-dependent expression of integrin β3 and N-cadherin. ECs were cultured on recombinant rat Jagged1 (10 mg/ml)-coated plate in the presence or absence of 10 µM DAPT (inhibitor of Notch signalling), the expression of Notch targeted genes, integrin β3 and N-cadherin was determined by western blotting. Representative data from three repeated experiments were shown. (E and F). ECs were cultured on Jagged1-coated cover slip for 24 h, the co-expression of N-cadherin (E) and integrin β3 (F) with Hey2 and Hes1 were determined by immunofluorescent staining. (G) Spreading assay of ECs. ECs were stained with FITC-phalloidin to visualize F-actin (green) and DAPI to indicate nuclei (blue). Representative images in panel (E–G) from three repeated experiments were presented. The scale bars are 50 µm.
3.6 Overexpression of Jagged1 stimulates EC regeneration leading to remodelling and arterialization of AV grafts
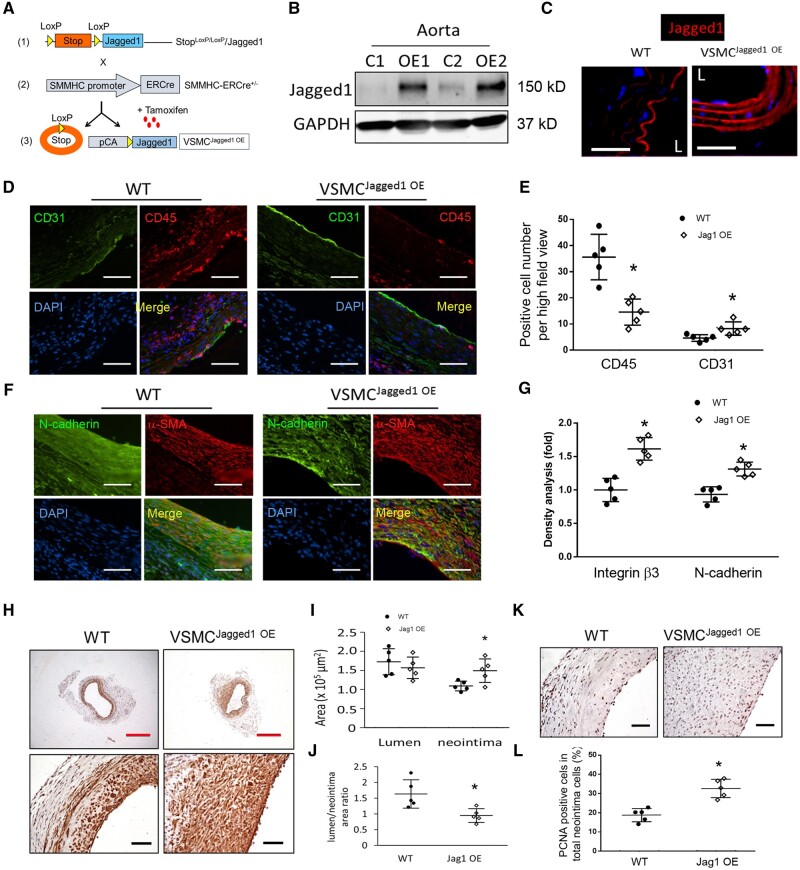
In order to determine if overexpression of Jagged1 in VSMCs promotes maturation of AVGs, we generated mice (Stopf/f-Jagged1/SMMHC-ERCre+ mice; abbreviated VSMCJagged1OE mice). These mice overexpress Jagged1 in VSMCs and this response is specifically induced in VSMCs following tamoxifen treatment (Figure 7A). Indeed, the expression of Jagged1 in aortas of VSMCJagged1OE mice was substantially increased vs. results in tamoxifen-treated WT mice (VSMCJagged1+/+ mice) (Figure 7B). Immunostaining demonstrated that Jagged1 expression was majorly expressed in ECs in aorta from WT mice; and more Jagged1 was found in the media cells in aorta from VSMCJagged1 OE mice (Figure 7C). There were no morphological and structural changes of arteries and veins in WT compared to VSMCJagged1OE mice.
Figure 7.
Overexpression of Jagged1 promotes AVG arterialization. (A) Schematic of generation of VSMC-specific overexpression of Jagged1 transgenic mice. (B) Western blotting analysis of Jagged1 expression in aorta from WT and VSMCJagged1 OE mice (n = 3). (C) Representative images of immunofluorescent staining of the Jagged1 (red) in artery (L, lumen). (D and E) Representative images of immunostaining and densitometric analysis of CD31 and CD45 in 2 week AVGs created in WT and VSMCJagged1 OE mice (*, P < 0.05 vs. WT, n = 5; t-test was used for statistical analysis). (F and G) Representative images of immunostaining and densitometric analysis of N-cadherin and integrin β3 in AVGs created in WT and VSMCJagged1 OE mice (*, P < 0.05 vs. WT, n = 5; t-test was used for statistical analysis). (H–J) Representative images of immunostaining of α-SMA from 1 month AVGs created in WT and Jagged1 OE mice were shown (H). The areas of neointima and lumen (I) as well as their ratio (J) were measured and calculated (*, P < 0.05 vs. WT, n = 5; t-test was used for statistical analysis). (K and L) The numbers of PCNA positive cells in neointima of the AVGs created in WT and VSMCJagged1 OE mice were counted and calculated (*, P < 0.05 vs. WT, n = 5; t-test was used for statistical analysis). The scale bars (with white and black colours) in panel (D), (F), (H), and (K) are 50 µm; the scale bars with red colour in panel (H) are 500 µm.
To determine if Jagged1 overexpression in VSMCs enhances EC regeneration in vivo, AVGs were created in VSMCJagged1+/+ and VSMCJagged1OE mice. Fourteen days later, the CD31+ signals were stronger and there were more ECs in the lumen surface in AVGs created in VSMCJagged1OE mice vs. results in AVGs from WT mice (Figure 7D). Consistently, AVGs created in VSMCJagged1OE mice exhibited decreases in the infiltration of CD45 inflammatory cells in AVGs (Figure 7D). A similar observation was made by using another EC marker vWF: more vWF positive cells and less CD45 inflammatory cells were found in AVGs from VSMCJagged1 OE mice vs. in WT mice (see Supplementary material online, Figure S6). These results indicate that Jagged1 expression in VSMCs, promotes EC regeneration and decreases inflammation. Furthermore, Jagged1 overexpression in VSMCs increased the levels of N-cadherin and integrin β3 in both endothelial and neointimal cells of AVGs (Figure 7F and G). Immunostaining and morphologic analyses revealed that increased Jagged1 in VSMCs acts to promote neointimal formation; this does not change the lumen areas of AVGs (Figure 7H and I). Consistently, we found more PCNA+ proliferation of VSMCs in neointimas of AVGs created in VSMCJagged1OE mice vs. that in wild-type mice (Figure 7K and L). Thus, increased Jagged1 expression in VSMCs improves AVG arterialization and remodelling.
4. Discussion
Neointima formation is a major determinant of the utility of AV grafts. Notch signalling influences neointima formation and vascular remodelling. One of the Notch ligands, Jagged1, is critical for determining neointimal formation during vascular remodelling. Jagged1 is expressed principally in ECs of normal vessels.30 Interestingly, we uncovered a dramatic increase in Jagged1 in VSMCs in neointima cells during vascular remodelling. Specific KO of Jagged1 in VSMCs suppresses EC regeneration in AVGs, which is related to the decreased expression of N-cadherin and integrin β3 in ECs. Besides responses to the genetic manipulation of Jagged1, treatment with DAPT, the Notch inhibitor, also blocked the Jagged1-induced expression of N-cadherin and integrin β3 in ECs. Delayed EC regeneration in AVGs created in VSMCJagged1 KO mice led to infiltration of inflammatory cells into AVGs. Moreover, the macrophages in AVGs produce more cytokine IL-1β, which is associated with increased inflammatory responses in ECs in AVGs created in VSMCJagged1 KO mice (i.e., upregulated expression of ICAM and E-selectin). In contrast, Jagged1 overexpression in VSMCs enhances EC regeneration and increases EC attachment. These responses prevent the inflammation related to dysfunctions of ECs. In addition, we found that increased Jagged1 expression in VSMCs promotes VSMC proliferation and migration leading to arterialization of veins in AVGs. We conclude that Jagged1 expression in neointimal VSMCs are important for maintaining EC regeneration and AVG remodelling.
One novel finding of this study is that Jagged1 expression in VSMCs enhances EC integrity and stability through activation of Notch1 in ECs. It has been reported that reducing Notch signalling in ECs can destabilize cellular interactions.31 We uncovered a dramatic increase in Jagged1 in VSMCs during vascular remodelling, which induced Notch signalling enhances EC regeneration through upregulating two molecules, N-cadherin and integrin β3. N-cadherin is a major cadherin that links ECs with VSMCs. In our AVG model, a newly formed endothelium gradually covers the denuded vascular walls.32 This newly formed endothelium is vulnerable to stresses arising from the arterial blood pressure but this is combatted by VSMCs that are underneath. Thus, the induction of N-cadherin expression by Jagged1 in VSMC promotes EC and VSMC interaction, and helps stabilize newly regenerated ECs. Therefore, it is not surprising that specific KO of Jagged1 in VSMCs suppresses EC regeneration in AVGs. In addition, Jagged1 overexpression in VSMCs or in response to Jagged1 treatment stimulates expression of integrin β3 in ECs. This is relevant because integrin β3 can enhance the attachment of ECs to exposed basement membranes, also increase EC regeneration in AVGs. This finding is consistent with our previous reports that KO of integrin β3 inhibits EC regeneration in AVGs.33 Moreover, loss of Jagged1 in VSMCs impairs the barrier function of ECs, enhancing the infiltration of inflammatory cells. Infiltration of macrophages in AVGs produce cytokines, including IL-1β, increases levels of ICAM and E-selectin in ECs in AVGs created in VSMCJagged1 KO mice; both molecules are active in recruiting monocytes and promote inflammation reaction in ECs. Thus, deficiency of Jagged1 in VSMCs impair EC regeneration and induces inflammatory responses deteriorating the vascular remodelling of AVGs.
ECs from different organs present different function and structures.34,35 This heterogeneity may lead to different outcomes in response to specific microenvironment stimuli. Kidney ECs, which are fenestrated, are different from continuous ECs in the lung and artery.36 While Notch signalling is a conserved signalling pathway, different types of ECs show Notch activation after exposure to Notch ligands.37–39 Thus, the results generated from mouse kidney ECs, such as Jagged1-induced Notch activation and expression of Notch downstream targets (N-cadherin and integrin β3), could be observed from ECs derived from other organs, including the arteries.
Another important finding of this study is that expression of Jagged1 in VSMC during AVG remodelling stimulates VSMC migration and proliferation. Indeed, Jagged1 losses in VSMCs during development are associated with abnormalities of arterial structures that include thin vascular walls and reduced VSMC accumulation and maturation.40,41 While in adult mice, Jagged1 expression in mature VSMCs is significantly decreased and undetectable in arterial media, our study uncovered that VSMCs re-express Jagged1 during vascular remodelling. During this process, VSMCs need Jagged1 to proliferate and migrate to form neointima in veins of AVG, adapting to the arterial blood pressure and against sustained mechanical stretch. Thus, KO of Jagged1 in VSMCs dramatically decreased neointima formation and inhibited vascular remodelling in our AVG model. However, overexpression of Jagged1 in VSMCs successfully rescued this defect. Therefore, we conclude that Jagged1 expression in VSMC is required for arterialization and neointima formation of AVGs. Consistently, other investigators have shown that increased Notch signalling pathway promotes the proliferation and migration of VSMCs, and thereby contributes to the progression of vascular remodelling.42 Yang et al.43 reported that a loss of Notch activity decreases VSMC proliferation and the expression of PDGFRβ and Jagged1. Caolo et al.44 found that Jagged1 expression in VSMCs can regulate arterial maturation by improving VSMC proliferation and differentiation during vascular injury. Interestingly, Wu et al.45 have shown that endothelial Jagged1 is required to maintain VSMC contractile phenotype. Downregulated expression of Jagged1 in ECs enhances VSMC proliferation and promotes neointima formation.45 These results demonstrate that control Jagged1 expression in both ECs and VSMCs are required for an adaptive vascular remodelling.
In conclusion, we have defined a new set of responses of Jagged1 in VSMCs. It mediates cellular processes that are necessary for vascular remodelling in AVGs. Specifically, we demonstrate that Jagged1 expression in VSMCs is required for EC regeneration, the VSMC migration, accumulation, and arterialization of AVGs.
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by American Heart Association grants 5GRNT25700209 and R01-DK095867 (to J.C.), National Institutes of Health grants R37DK37175, China Scholarship Fund 201606385023, and generous support from Dr and Mrs Harold Selzman.
Supplementary Material
Translational perspective
The vein is subjected to arterial environment immediately after AVG or arteriovenous fistula surgery. ECs in the vein of the AVGs and arteriovenous fistulae are denuded and regenerated later. Deficiency of Jagged1 expression in VSMCs inhibits VSMC migration, proliferation, and interferes with arterialization of vein of the AVGs. These responses also delay EC regeneration and EC barrier function with increased inflammation leading to failed vascular remodelling of AVGs. Thus, a strategy to regulate Jagged1 expression will improve AVG arterialization and function.
References
- 1.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation 1998;97:916–931. [DOI] [PubMed] [Google Scholar]
- 2.Baker AH, Yim AP, Wan S. Opportunities for gene therapy in preventing vein graft failure after coronary artery bypass surgery. Diabetes Obes Metab 2006;8:119–124. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 2013;127:143–152. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 2012;125:188–197. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association . Circulation 2011;123:933–944. [DOI] [PubMed] [Google Scholar]
- 6.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep 2009;57:1–134. [PubMed] [Google Scholar]
- 7.Liang M, Wang Y, Liang A, Mitch WE, Roy-Chaudhury P, Han G, Cheng J. Migration of smooth muscle cells from the arterial anastomosis of arteriovenous fistulas requires Notch activation to form neointima. Kidney Int 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dou G-R, Wang Y-C, Hu X-B, Hou L-H, Wang C-M, Xu J-F, Wang Y-S, Liang Y-M, Yao L-B, Yang A-G, Han H. RBP-J, the transcription factor downstream of Notch receptors, is essential for the maintenance of vascular homeostasis in adult mice. FASEB J 2008;22:1606–1617. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Liang A, Luo J, Liang M, Han G, Mitch WE, Cheng J. Blocking Notch in endothelial cells prevents arteriovenous fistula failure despite CKD. J Am Soc Nephrol 2014;25:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kageyama R, Ohtsuka T. The Notch-Hes pathway in mammalian neural development. Cell Res 1999;9:179–188. [DOI] [PubMed] [Google Scholar]
- 11.Chang AC, Fu Y, Garside VC, Niessen K, Chang L, Fuller M, Setiadi A, Smrz J, Kyle A, Minchinton A, Marra M, Hoodless PA, Karsan A. Notch initiates the endothelial-to-mesenchymal transition in the atrioventricular canal through autocrine activation of soluble guanylyl cyclase. Dev Cell 2011;21:288–300. [DOI] [PubMed] [Google Scholar]
- 12.High FA, Zhang M, Proweller A, Tu L, Parmacek MS, Pear WS, Epstein JA. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J Clin Invest 2007;117:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang L, Noseda M, Higginson M, Ly M, Patenaude A, Fuller M, Kyle AH, Minchinton AI, Puri MC, Dumont DJ, Karsan A. Differentiation of vascular smooth muscle cells from local precursors during embryonic and adult arteriogenesis requires Notch signaling. Proc Natl Acad Sci U S A 2012;109:6993–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, Costa T, Pierpont ME, Rand EB, Piccoli DA, Hood L, Spinner NB. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997;16:243–251. [DOI] [PubMed] [Google Scholar]
- 15.McElhinney DB, Krantz ID, Bason L, Piccoli DA, Emerick KM, Spinner NB, Goldmuntz E. Analysis of cardiovascular phenotype and genotype-phenotype correlation in individuals with a JAG1 mutation and/or Alagille syndrome. Circulation 2002;106:2567–2574. [DOI] [PubMed] [Google Scholar]
- 16.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet 1997;16:235–242. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Zhou Q, Huang L, Sun A, Wang K, Zou Y, Ge J. Ageing-exaggerated proliferation of vascular smooth muscle cells is related to attenuation of Jagged1 expression in endothelial cells. Cardiovasc Res 2008;77:800–808. [DOI] [PubMed] [Google Scholar]
- 18.High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci U S A 2008;105:1955–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann JJ, Briot A, Enciso J, Zovein AC, Ren S, Zhang ZW, Radtke F, Simons M, Wang Y, Iruela-Arispe ML. Endothelial deletion of murine Jag1 leads to valve calcification and congenital heart defects associated with Alagille syndrome. Development 2012;139:4449–4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Q, Zhang B, Zhang L, Dang T, Rowley D, Ittmann M, Xin L. Jagged1 upregulation in prostate epithelial cells promotes formation of reactive stroma in the Pten null mouse model for prostate cancer. Oncogene 2017;36:618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014;157:1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol 2007;27:1744–1751. [DOI] [PubMed] [Google Scholar]
- 23.Cheng J, Wang Y, Liang A, Jia L, Du J. FSP-1 silencing in bone marrow cells suppresses neointima formation in Vein Graft. Circ Res 2012;110:230–240. [DOI] [PubMed] [Google Scholar]
- 24.Liang M, Liang A, Wang Y, Jiang J, Cheng J. Smooth muscle cells from the anastomosed artery are the major precursors for neointima formation in both artery and vein grafts. Basic Res Cardiol 2014;109:431.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranjzad P, Salem HK, Kingston PA. Adenovirus-mediated gene transfer of fibromodulin inhibits neointimal hyperplasia in an organ culture model of human saphenous vein graft disease. Gene Ther 2009;16:1154–1162. [DOI] [PubMed] [Google Scholar]
- 26.Jackson CJ, Garbett PK, Nissen B, Schrieber L. Binding of human endothelium to Ulex europaeus I-coated Dynabeads: application to the isolation of microvascular endothelium. J Cell Sci 1990;96:257–262. [DOI] [PubMed] [Google Scholar]
- 27.McGinn S, Poronnik P, Gallery ED, Pollock CA. A method for the isolation of glomerular and tubulointerstitial endothelial cells and a comparison of characteristics with the human umbilical vein endothelial cell model. Nephrology 2004;9:229–237. [DOI] [PubMed] [Google Scholar]
- 28.Chiu JJ, Chen LJ, Lee PL, Lee CI, Lo LW, Usami S, Chien S. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood 2003;101:2667–2674. [DOI] [PubMed] [Google Scholar]
- 29.Liang A, Wang Y, Han G, Truong L, Cheng J. Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. Am J Physiol Renal Physiol 2013;304:F1413–F1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nus M, Martínez-Poveda B, MacGrogan D, Chevre R, D’Amato G, Sbroggio M, Rodríguez C, Martínez-González J, Andrés V, Hidalgo A, de la Pompa JL. Endothelial Jag1-RBPJ signalling promotes inflammatory leucocyte recruitment and atherosclerosis. Cardiovasc Res 2016;112:568.. [DOI] [PubMed] [Google Scholar]
- 31.Mack JJ, Mosqueiro TS, Archer BJ, Jones WM, Sunshine H, Faas GC, Briot A, Aragon RL, Su T, Romay MC, McDonald AI, Kuo CH, Lizama CO, Lane TF, Zovein AC, Fang Y, Tarling EJ, de Aguiar Vallim TQ, Navab M, Fogelman AM, Bouchard LS, Iruela-Arispe ML. NOTCH1 is a mechanosensor in adult arteries. Nat Commun 2017;8:1620.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Q, Zhang Z, Davison F, Hu Y. Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice. Circ Res 2003;93:e76–e86. [DOI] [PubMed] [Google Scholar]
- 33.Liang M, Wang Y, Liang A, Dong JF, Du J, Cheng J. Impaired integrin beta3 delays endothelial cell regeneration and contributes to arteriovenous graft failure in mice. Arterioscler Thromb Vasc Biol 2015;35:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 2007;100:174–190. [DOI] [PubMed] [Google Scholar]
- 35.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 2007;100:158–173. [DOI] [PubMed] [Google Scholar]
- 36.Williams IM, Wu JC. Generation of endothelial cells from human pluripotent stem cells. Arterioscler Thromb Vasc Biol 2019;39:1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen T, Margariti A, Kelaini S, Cochrane A, Guha ST, Hu Y, Stitt AW, Zhang L, Xu Q. MicroRNA-199b modulates vascular cell fate during iPS cell differentiation by targeting the Notch ligand Jagged1 and enhancing VEGF signaling. Stem Cells 2015;33:1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr BA, West XZ, Kim YW, Zhao Y, Tischenko M, Cull RM, Phares TW, Peng XD, Bernier-Latmani J, Petrova TV, Adams RH, Hay N, Naga Prasad SV, Byzova TV. Stability and function of adult vasculature is sustained by Akt/Jagged1 signalling axis in endothelium. Nat Commun 2016;7:10960.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon CH, Choi YE, Cha YR, Koh SJ, Choi JI, Kim TW, Woo SJ, Park YB, Chae IH, Kim HS. Diabetes-induced Jagged1 overexpression in endothelial cells causes retinal capillary regression in a murine model of diabetes mellitus: insights into diabetic retinopathy. Circulation 2016;134:233–247. [DOI] [PubMed] [Google Scholar]
- 40.Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development 2010;137:4061–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doi H, Iso T, Sato H, Yamazaki M, Matsui H, Tanaka T, Manabe I, Arai M, Nagai R, Kurabayashi M. Jagged1-selective Notch signaling induces smooth muscle differentiation via a RBP-Jkappa-dependent pathway. J Biol Chem 2006;281:28555–28564. [DOI] [PubMed] [Google Scholar]
- 42.Ozasa Y, Akazawa H, Qin Y, Tateno K, Ito K, Kudo-Sakamoto Y, Yano M, Yabumoto C, Naito AT, Oka T, Lee JK, Minamino T, Nagai T, Kobayashi Y, Komuro I. Notch activation mediates angiotensin II-induced vascular remodeling by promoting the proliferation and migration of vascular smooth muscle cells. Hypertens Res 2013;36:859–865. [DOI] [PubMed] [Google Scholar]
- 43.Yang K, Proweller A. Vascular smooth muscle Notch signals regulate endothelial cell sensitivity to angiogenic stimulation. J Biol Chem 2011;286:13741–13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caolo V, Schulten HM, Zhuang ZW, Murakami M, Wagenaar A, Verbruggen S, Molin DGM, Post MJ. Soluble Jagged-1 inhibits neointima formation by attenuating Notch-Herp2 signaling. Arterioscler Thromb Vasc Biol 2011;31:1059–1065. [DOI] [PubMed] [Google Scholar]
- 45.Wu X, Zou Y, Zhou Q, Huang L, Gong H, Sun A, Tateno K, Katsube K, Radtke F, Ge J, Minamino T, Komuro I. Role of Jagged1 in arterial lesions after vascular injury. Arterioscler Thromb Vasc Biol 2011;31:2000–2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.