Abstract
Aims
To determine the risk of fracture associated with direct oral anticoagulants (DOACs) compared with vitamin K antagonists (VKAs) in patients with non-valvular atrial fibrillation (NVAF), accounting for cumulative duration of use.
Methods and results
Using Quebec administrative healthcare databases, we formed a cohort of all patients aged 40 years or older newly diagnosed with NVAF, who filled a first prescription for DOACs or VKAs between 2011 and 2014. Exposure was modelled as a time-varying variable whereby patients were considered unexposed up to 180 days of cumulative duration of use (to account for a biologically meaningful exposure) and exposed thereafter. The final cohort included 10 306 new users of DOACs and 15 357 new users of VKAs. After propensity score-based fine stratification and weighting, use of DOACs for 180 days or greater was associated with a 35% decreased risk of fracture [crude incidence rates 7.5 vs. 15.3 per 1000 person-years; adjusted hazard ratio (HR) 0.65, 95% confidence interval (CI) 0.46–0.91] compared to VKA duration ≥180 days. Direct oral anticoagulants use was also associated with a lower risk of hip fracture (HR 0.51, 95% CI 0.31–0.86) compared with VKAs. There was no difference in the rate of fracture for shorter duration of use (HR 1.10; 95% CI 0.79–1.53). The risk was not modified by age, sex, chronic kidney disease, osteoporosis, history of fracture or falls.
Conclusion
Prolonged use of DOACs is associated with a lower risk of fracture compared with VKAs. These findings support the first-line recommendation for DOACs in patients with NVAF.
Keywords: Atrial fibrillation, Oral anticoagulants, Fracture, Cohort study
Introduction
Atrial fibrillation is the most common cardiac arrhythmia and its prevalence increases with advancing age.1,2 Oral anticoagulants (OACs), including vitamin K antagonists (VKAs) and direct oral antagonists (DOACs), are usually given lifelong to prevent stroke in patients with non-valvular atrial fibrillation (NVAF).3 The prolonged use of VKAs is a concern, given their potential detrimental effect on bone metabolism. Vitamin K antagonists produce their anticoagulant effect by interfering with the gamma-carboxylation of glutamic acid residues on clotting factors II, VII, IX, and X. Vitamin K antagonists can also interfere with the biosynthesis of gamma-carboxyglutamic acid proteins in the bone, including osteocalcin and other bone matrix proteins, thus potentially affecting bone metabolism.4,5
Several studies6–13 have investigated the risk of fracture associated with VKAs compared with no use, with conflicting results. Recently, four studies specifically assessed the risk of fracture associated with DOACs compared with VKAs in patients with NVAF.14–17 Findings were overall consistent with a decreased risk of fracture with DOACs compared with VKAs but varied from a small decreased risk to a strong protective association. One potential explanation for these discrepancies is the definition of exposure, although differences in design, analyses and control for confounding may also have contributed to these differences. Exposure was defined starting from the first prescription in two studies,15,16 whereas the analysis was restricted to patients with 6 months and 3 months of continuous use before cohort entry in the two other studies.14,17 The latter approach may be more appropriate because the action of VKAs on bone metabolism suggests a progressive effect that needs to be reflected in the exposure definition to properly estimate the association with the risk of fracture. However, no study has included all patients initiating OACs and explored the potential duration-response relation between OACs use and the risk of fracture, accounting for cumulative duration of use.
Oral anticoagulants are predominantly prescribed long term to an elderly population at high risk of fracture. Notably, fracture is associated with diminished quality of life and excess mortality among the elderly.18–20 Therefore, the objective of this population-based cohort study was to assess the risk of fracture associated with DOACs compared with VKAs among patients with NVAF, using a relevant time-window of exposure accounting for cumulative duration of use.
Methods
Data source
We conducted a population-based cohort study by linking three computerized healthcare databases from the Canadian province of Québec: the Régie de l’assurance maladie du Québec (RAMQ), the Maintenance et exploitation des données pour l’étude de la clientèle hospitalière (MED-ECHO), and the Institut de la statistique du Québec (ISQ), described in details elsewhere.21–23 The RAMQ is responsible for administering the universal health care services, serving around 8 million people covered by the Québec Health Insurance Plan.24 RAMQ databases contain patients information on demographics, medical services [coded using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) or enhanced version of ICD-10 for Canada ICD-10-CA], and dispensed outpatient medication prescriptions for those covered by the Public Prescription Drug Insurance Plan. This prescription plan covers all individuals 65 years of age and older, welfare recipients, and all Québec residents who do not have a private medication insurance programme (around 45% of the Québec population). MED-ECHO maintains records of all Québec hospital stays including date and type of admission and discharge, primary and secondary diagnoses (coded using ICD-10-CA), and procedure codes.24 Finally, ISQ contains date and cause of death. Data from these databases were linked using the individual's health insurance number, a unique number acquired at birth or at the time of residency. These databases have been used previously for population-based studies on NVAF,23 OACs,21,25 and drug-induced fracture risk,26 and the data quality has been well documented.27,28 The Research Ethical Committee of the Jewish General Hospital, Montreal, Canada approved the protocol and waived the need for obtaining inform consent.
Study population
Within a cohort of patients with an incident inpatient or outpatient diagnosis of atrial fibrillation between 1 January 2010 and 31 March 2014, we identified all patients at least 40 years of age, who filled a first prescription for a DOAC (dabigatran, rivaroxaban, or apixaban) or VKA from 1 January 2011 (when the first DOAC, dabigatran, was approved for NVAF in Québec) to 31 March 2014. Cohort entry was defined as the date of the first OAC prescription. We excluded patients dispensed DOACs or VKAs in the year before the atrial fibrillation diagnosis to maximize the inclusion of new users, and patients with a first prescription for both VKAs and DOACs on the same day. To select patients with NVAF only, we excluded patients with any mention of valvular mitral or aortic heart disease, or valvular repair before cohort entry. We also excluded patients with hyperthyroidism, renal transplant or dialysis, and patients with history of bone tumours before cohort entry. Finally, all cohort members were required to have RAMQ medication coverage for at least 1 year before their initial prescription. All cohort members were followed until the occurrence of the study outcome (described below), the patient’s RAMQ deregistration, death, or end of study period (31 March 2014), whichever occurred earlier.
Exposure
Exposure to DOACs and VKAs was modelled as a time-varying variable whereby patients could move from a period of non-exposure to a period of exposure. As such, patients were considered unexposed up to 180 days (6 months) of cumulative duration of use (to account for a biologically meaningful exposure) and exposed thereafter. We defined cumulative duration by summing the duration of each dispensed prescription with a grace period of 100% of the last prescription duration. Patients who switched from DOACs to VKAs (or vice versa) during follow-up were classified in a separate category (multiple use) at the time of switching for the remainder of the follow-up. Thus, for each individual, each day of follow-up was classified into one of the following mutually exclusive exposure categories: (i) VKA duration <180 days; (ii) VKA duration ≥180 days; (iii) DOAC duration <180 days; (iv) DOAC duration ≥180 days; and (v) multiple use. VKA duration ≥180 days served as the reference category.
Outcome
The primary outcome was a hospitalization with a diagnosis of fracture (admission or primary diagnosis), a composite of hip fracture, vertebral fracture, upper extremity fracture (humerus, forearm, or wrist fracture), and osteoporosis with pathologic fracture. Secondary outcomes considered the components of the primary outcome separately. Outcomes were identified using ICD-10 codes (Supplementary material online, Table S1).
Propensity score estimation and stratification
We estimated the propensity score (PS) of DOAC initiation vs. VKA initiation using multivariate logistic regression. The covariates included in the PS were age (in categories), sex, osteoporosis, diabetes mellitus, rheumatoid arthritis, hypertension, congestive heart failure, anaemia, inflammatory bowel disease, chronic liver disease, chronic kidney disease (CKD), respiratory disease (chronic obstructive pulmonary disease or asthma), epilepsy, Parkinson's disease, dementia, depression, stroke or transient ischaemic attack, cancer, history of fractures, history of falls, predisposition to falls, complications of alcohol abuse, and time from NVAF diagnosis to treatment initiation. Comorbidities were measured anytime before cohort entry. We also included the following drugs (measured in the year before cohort entry): angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta blockers, loop diuretics, thiazide diuretics, statins, thiazolidinediones, proton pump inhibitors (PPIs), systemic glucocorticoids, aromatase inhibitors, gonadotropin-releasing hormone (GnRH) agonists or GnRH antagonists, hormone replacement therapy, antidepressants (selective serotonin reuptake inhibitors or tricyclic antidepressants), antipsychotics, bisphosphonates, vitamin D, and calcitonin. We excluded patients in the non-overlapping range of PS distribution (i.e. 2.5% trimming in both sides). Next, we used a PS-based fine-stratification and weighting approach to further control for potential confounding.29 We created 100 strata based on the PS distribution of the entire cohort. VKA users were then weighted proportionally to the distribution of DOAC users in the corresponding PS stratum.29 Standardized differences were used to assess the covariate balance between treatment groups, with meaningful imbalances set at values greater than 10%.30
Statistical analyses
The crude incidence rate of fractures in each exposure group was estimated based on a Poisson distribution. We used weighted Cox proportional hazards models with robust sandwich variance estimation31 to estimate the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of fracture associated with DOACs compared with VKAs. We also plotted the weighted cumulative incidence curve of fracture for new users of DOACs and VKAs over the follow-up time.
In secondary analyses, we assessed the association between DOACs and each type of fracture separately. We also performed stratified analyses to assess whether the risk varies by sex, age (≤75 years vs. >75 years), CKD, osteoporosis, history of fracture, history of fracture or falls, and any risk factor (i.e. history of fracture, falls, predisposition to falls, and osteoporosis).
Finally, we performed six sensitivity analyses to assess the robustness of the results. First, we repeated the primary analysis using a grace period of 90 days. Second, we performed a modified intention-to-treat analysis, in which patients who switched from DOACs to VKAs (or vice versa) remained in their current exposure category until the end of follow-up. Third, we changed the exposure threshold to 90 days of cumulative duration. Fourth, we repeated the primary analysis using an intention-to-treat approach, where patients were considered exposed to their initially prescribed OAC during the entire follow-up irrespective of treatment switch or discontinuation. Fifth, we used a stricter outcome definition by excluding fractures with a record of traffic accidents or accident fall from higher than standing height on the same date, so as to exclude potentially non-osteoporotic fractures. Sixth, we accounted for competing risk due to death using the subdistribution model proposed by Fine and Gray.32 Finally, in a post hoc analysis, we repeated the primary analysis using inverse probability of treatment weights (IPTW) to balance covariates between exposure groups. All analyses were conducted with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
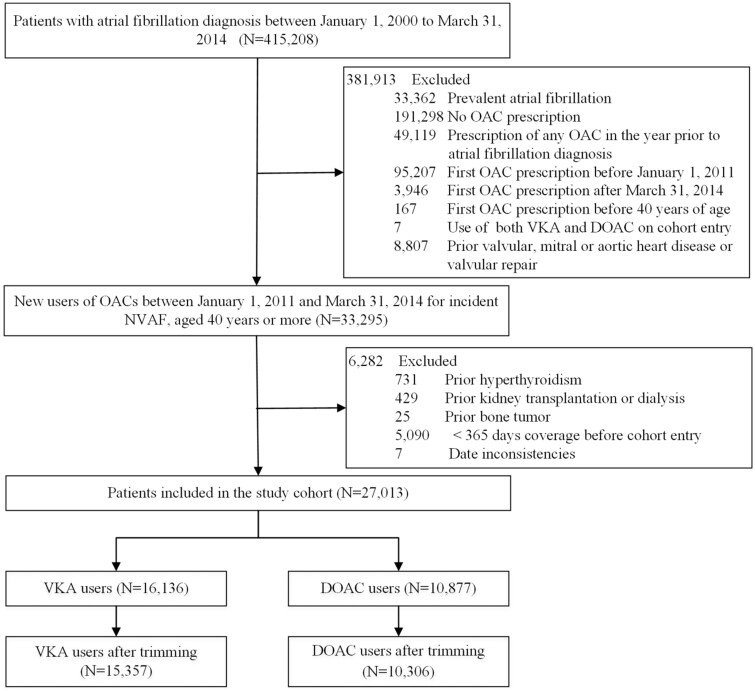
The final cohort included 10 306 (40.2%) DOACs initiators and 15 357 (59.8%) VKAs initiators (Figure 1). Before PS stratification and weighting, new users of DOACs were younger, less likely to have diabetes mellitus, hypertension, CKD, respiratory disease, dementia, or stroke; and less likely to have used statins, PPIs or loop diuretics (Table 1). After weighting, the PS model c-statistic was 0.54 and covariates distribution achieved excellent balance, with the absolute standardized mean differences less than 0.05 for all covariates.
Figure 1.
Flowchart describing cohort definition of new users of oral anticoagulants following incident non-valvular atrial fibrillation.
Table 1.
Baseline characteristics of patients with NAVF initiating treatment with DOACs vs. VKAs, before and after propensity score-based fine-stratification and weightinga
| Characteristic | Before propensity score weighting |
After propensity score weighting |
||||
|---|---|---|---|---|---|---|
| VKA | DOAC | Std diff | VKA | DOAC | Std Diff | |
| Number of patients | 15 357 | 10 306 | 15 357 | 10 306 | ||
| Demographic characteristics | ||||||
| Age, mean (SD) | 78.0 (8.9) | 75.6 (8.9) | 0.263 | 75.8 (9.0) | 75.6 (8.9) | 0.015 |
| 40–49 | 90 (0.6) | 90 (0.9) | 0.034 | 137 (0.9) | 90 (0.9) | 0.002 |
| 50–59 | 384 (2.5) | 371 (3.6) | 0.064 | 558 (3.6) | 371 (3.6) | 0.002 |
| 60–69 | 2103 (13.7) | 1928 (18.7) | 0.136 | 2878 (18.7) | 1928 (18.7) | 0.001 |
| 70–79 | 5564 (36.2) | 4280 (41.5) | 0.109 | 6365 (41.4) | 4280 (41.5) | 0.002 |
| 80–85 | 4104 (26.7) | 2324 (22.5) | 0.097 | 3459 (22.5) | 2324 (22.5) | 0.001 |
| >85 | 3112 (20.3) | 1313 (12.7) | 0.204 | 1959 (12.8) | 1313 (12.7) | 0.001 |
| Sex, female | 7850 (51.1) | 5125 (49.7) | 0.028 | 7618 (49.6) | 5125 (49.7) | 0.002 |
| Time from NVAF diagnosis to treatment initiation, in years mean (SD) | 0.68 (1.93) | 0.79 (1.97) | 0.054 | 0.83 (2.07) | 0.79 (1.97) | 0.023 |
| Comorbidities | ||||||
| Osteoporosis | 2384 (15.5) | 1525 (14.8) | 0.020 | 2275 (14.8) | 1525 (14.8) | 0.000 |
| Diabetes mellitus | 5115 (33.3) | 2748 (26.7) | 0.145 | 4091 (26.6) | 2748 (26.7) | 0.000 |
| Rheumatoid arthritis | 424 (2.8) | 249 (2.4) | 0.022 | 382 (2.5) | 249 (2.4) | 0.005 |
| Hypertension | 11 545 (75.2) | 7059 (68.5) | 0.149 | 10 510 (68.4) | 7059 (68.5) | 0.001 |
| Congestive heart failure | 4098 (26.7) | 1867 (18.1) | 0.207 | 2796 (18.2) | 1867 (18.1) | 0.002 |
| Anaemia | 4003 (26.1) | 1783 (17.3) | 0.214 | 2647 (17.2) | 1783 (17.3) | 0.002 |
| Inflammatory bowel disease | 252 (1.6) | 130 (1.3) | 0.032 | 186 (1.2) | 130 (1.3) | 0.004 |
| Chronic liver disease | 641 (4.2) | 331 (3.2) | 0.051 | 517 (3.4) | 331 (3.2) | 0.009 |
| Chronic kidney disease | 3139 (20.4) | 972 (9.4) | 0.313 | 1489 (9.7) | 972 (9.4) | 0.009 |
| Respiratory disease (COPD or asthma) | 4571 (29.8) | 2626 (25.5) | 0.096 | 3938 (25.6) | 2626 (25.5) | 0.004 |
| Epilepsy | 299 (1.9) | 155 (1.5) | 0.034 | 244 (1.6) | 155 (1.5) | 0.007 |
| Parkinson's disease | 255 (1.7) | 126 (1.2) | 0.037 | 194 (1.3) | 126 (1.2) | 0.003 |
| Dementia | 1743 (11.3) | 759 (7.4) | 0.137 | 1149 (7.5) | 759 (7.4) | 0.005 |
| Depression | 1198 (7.8) | 761 (7.4) | 0.016 | 1144 (7.5) | 761 (7.4) | 0.003 |
| Stroke/TIA | 2099 (13.7) | 998 (9.7) | 0.124 | 1559 (10.2) | 998 (9.7) | 0.016 |
| Cancer | 3269 (21.3) | 2031 (19.7) | 0.039 | 3072 (20.0) | 2031 (19.7) | 0.007 |
| History of fracture | 1634 (10.6) | 918 (8.9) | 0.058 | 1395 (9.1) | 918 (8.9) | 0.006 |
| History of falls | 650 (4.2) | 258 (2.5) | 0.096 | 394 (2.6) | 258 (2.5) | 0.004 |
| Predisposition to falls | 3239 (21.1) | 1705 (16.5) | 0.117 | 2600 (16.9) | 1705 (16.5) | 0.010 |
| Complications of alcohol abuse | 700 (4.6) | 402 (3.9) | 0.033 | 598 (3.9) | 402 (3.9) | 0.000 |
| Concomitant drugs | ||||||
| ACEIs or ARBs | 10 437 (68.0) | 6443 (62.5) | 0.115 | 9584 (62.4) | 6443 (62.5) | 0.002 |
| Beta blockers | 11 061 (72.0) | 7396 (71.8) | 0.006 | 11 005 (71.7) | 7396 (71.8) | 0.002 |
| Statins | 9510 (61.9) | 5880 (57.1) | 0.099 | 8711 (56.7) | 5880 (57.1) | 0.007 |
| Loop diuretics | 4519 (29.4) | 1960 (19.0) | 0.245 | 2934 (19.1) | 1960 (19.0) | 0.002 |
| Thiazide diuretics | 5290 (34.4) | 3394 (32.9) | 0.032 | 4983 (32.4) | 3394 (32.9) | 0.010 |
| Thiazolidinediones | 239 (1.6) | 91 (0.9) | 0.061 | 146 (0.9) | 91 (0.9) | 0.007 |
| Proton pump inhibitors | 8479 (55.2) | 4903 (47.6) | 0.153 | 7288 (47.5) | 4903 (47.6) | 0.002 |
| Systemic glucocorticoids | 2517 (16.4) | 1403 (13.6) | 0.078 | 2091 (13.6) | 1403 (13.6) | 0.000 |
| Aromatase inhibitors | 144 (0.9) | 87 (0.8) | 0.010 | 131 (0.9) | 87 (0.8) | 0.001 |
| GnRH agonists or antagonists | 154 (1.0) | 88 (0.9) | 0.016 | 138 (0.9) | 88 (0.9) | 0.005 |
| Hormone replacement therapyb | 617 (7.9) | 482 (9.4) | 0.055 | 723 (9.5) | 482 (9.4) | 0.003 |
| Antidepressants (SSRIs or TCAs) | 1997 (13.0) | 1187 (11.5) | 0.045 | 1798 (11.7) | 1187 (11.5) | 0.006 |
| Antipsychotics | 1170 (7.6) | 627 (6.1) | 0.061 | 961 (6.3) | 627 (6.1) | 0.007 |
| Bisphosphonates | 2661 (17.3) | 1512 (14.7) | 0.073 | 2281 (14.9) | 1512 (14.7) | 0.005 |
| Vitamin D | 4804 (31.3) | 2899 (28.1) | 0.069 | 4292 (27.9) | 2899 (28.1) | 0.004 |
| Calcitonin | 184 (1.2) | 92 (0.9) | 0.030 | 133 (0.9) | 92 (0.9) | 0.003 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; GnRH, gonadotropin-releasing hormone; NVAF, non-valvular atrial fibrillation; SD, standard deviation; SSRI, selective serotonin reuptake inhibitor; Std diff, standardized difference; TCA, tricyclic antidepressant; TIA, transient ischaemic attack; VKA, vitamin-K antagonist.
Values are presented as number (%), unless otherwise specified.
Percentage in women.
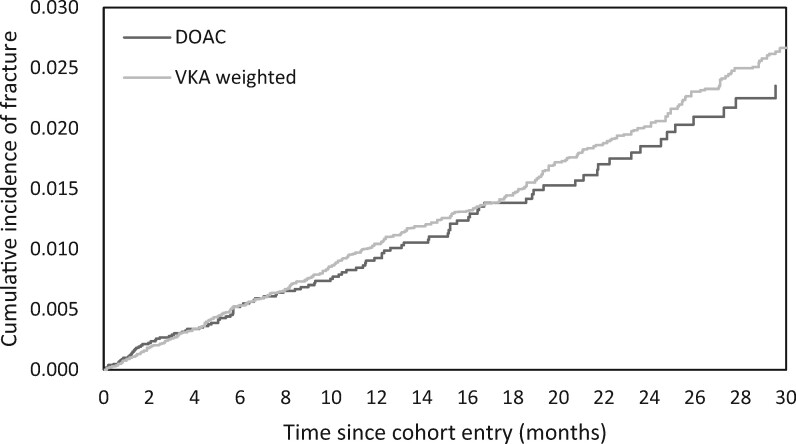
Overall, 464 fractures occurred during 35 252 person-years of follow-up in the entire cohort, resulting in a crude incidence rate of 13.2 (12.0–14.4) per 1000 person-years. As shown in the PS-weighted cumulative incidence curve (Figure 2), the incidence of fractures between new DOAC users and VKA users began to diverge around 6–8 months after cohort entry.
Figure 2.
Weighted cumulative incidence curve of fracture events among new users of direct oral anticoagulants and vitamin-K antagonists.
Table 2 shows the crude and adjusted HRs of all the comparisons with respect to primary and secondary outcomes. Compared with VKA duration ≥ 180 days, use of DOACs for 180 days or greater was associated with a 35% decreased risk of fracture (crude incidence rates 7.5 vs. 15.3 per 1000 person-years; adjusted HR 0.65, 95% CI 0.46–0.91). When assessing the risk associated with types of fracture, DOACs duration ≥ 180 days was associated with a decreased rate of hip fracture (crude incidence rates 3.2 vs. 8.6 per 1000 person-years; adjusted HR 0.51, 95% CI 0.31–0.86) and osteoporosis with pathologic fracture (crude incidence rates 0.4 vs. 1.8 per 1000 person-years; adjusted HR 0.24, 95% CI 0.06–1.00) compared with VKAs. There was no association with upper extremity fracture and vertebral fracture. There was no difference in the rate of fracture for shorter duration of use of DOACs (<180 days) compared with the same short duration of use of VKAs (HR 1.10; 95% CI 0.79–1.53).
Table 2.
Crude and adjusted hazard ratios for the association between DOACs and the risk of fracture
| Exposure | Events | Person-years | Incidence rate a | Crude HR | Adjusted HRb (95% CI) |
|---|---|---|---|---|---|
| All fracture | |||||
| VKA (≥180 days) | 183 | 11 930 | 15.3 | 1.00 (Ref) | 1.00 (Reference) |
| DOAC (≥180 days) | 43 | 5709 | 7.5 | 0.49 | 0.65 (0.46–0.91) |
| VKA (<180 days) | 126 | 8001 | 15.7 | 1.00 | 0.79 (0.52–1.19) |
| DOAC (<180 days) | 55 | 4862 | 11.3 | 0.71 | 0.86 (0.52–1.43) |
| Multiple use | 57 | 4749 | 12.0 | 0.78 | 0.73 (0.52–1.03) |
| Hip fracture | |||||
| VKA (≥180 days) | 103 | 11 930 | 8.6 | 1.00 (Ref) | 1.00 (Reference) |
| DOAC (≥180 days) | 18 | 5709 | 3.2 | 0.36 | 0.51 (0.31–0.86) |
| VKA (<180 days) | 66 | 8001 | 8.2 | 0.98 | 0.90 (0.52–1.55) |
| DOAC (<180 days) | 24 | 4862 | 4.9 | 0.59 | 0.80 (0.40–1.62) |
| Multiple use | 34 | 4749 | 7.2 | 0.84 | 0.82 (0.53–1.27) |
| Upper extremity fracture | |||||
| VKA (≥180 days) | 27 | 11 930 | 2.3 | 1.00 (Ref) | 1.00 (Reference) |
| DOAC (≥180 days) | 16 | 5709 | 2.8 | 1.24 | 1.37 (0.73–2.57) |
| VKA (<180 days) | 26 | 8001 | 3.2 | 1.76 | 1.04 (0.42–2.58) |
| DOAC (<180 days) | 17 | 4862 | 3.5 | 1.90 | 1.95 (0.68–5.55) |
| Multiple use | 10 | 4749 | 2.1 | 0.96 | 1.05 (0.52–2.11) |
| Vertebral fracture | |||||
| VKA (≥180 days) | 32 | 11 930 | 2.7 | 1.00 (Ref) | 1.00 (Reference) |
| DOAC (≥180 days) | 7 | 5709 | 1.2 | 0.46 | 0.62 (0.27–1.45) |
| VKA (<180 days) | 21 | 8001 | 2.6 | 0.76 | 0.51 (0.15–1.82) |
| DOAC (<180 days) | 10 | 4862 | 2.1 | 0.59 | 0.52 (0.15–1.77) |
| Multiple use | 6 | 4749 | 1.3 | 0.46 | 0.34 (0.12–1.01) |
| Other fracturec | |||||
| VKA (≥180 days) | 21 | 11 930 | 1.8 | 1.00 (Ref) | 1.00 (Reference) |
| DOAC (≥180 days) | <5 d | – | 0.4 | 0.21 | 0.24 (0.06–1.00) |
| VKA (<180 days) | 13 | 8001 | 1.6 | 0.45 | 0.25 (0.06–1.11) |
| DOAC (<180 days) | <5 d | – | 0.8 | 0.22 | 0.22 (0.03–1.60) |
| Multiple use | 7 | 4749 | 1.5 | 0.71 | 0.41 (0.13–1.31) |
CI, confidence interval; DOAC, direct oral anticoagulant; HR, hazard ratio; VKA, vitamin-K antagonist.
Per 1000 person-years.
Propensity score fine stratification and weighting was used for adjustment.
Osteoporosis with pathological fracture, defined as ICD-10 code M80.
Cells with less than five events were suppressed owing to privacy restrictions.
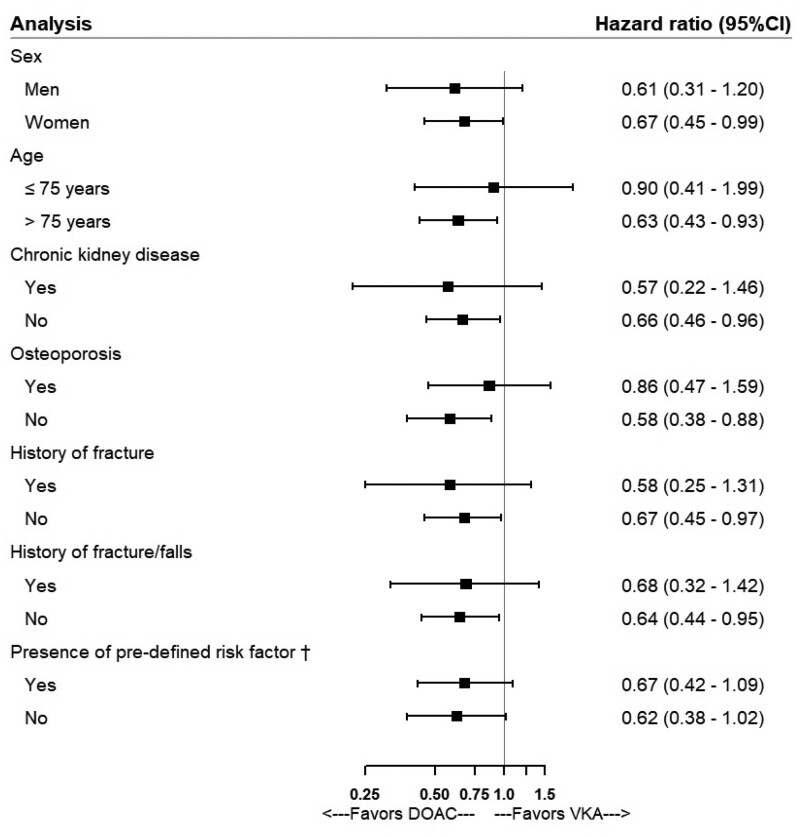
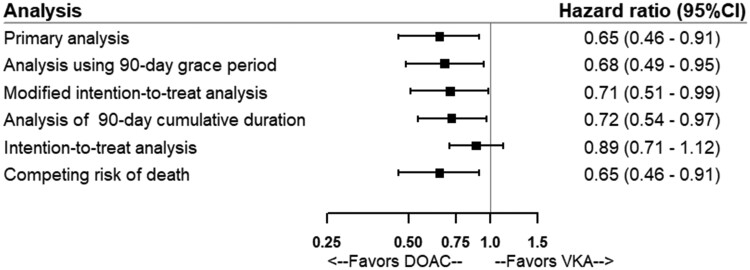
The stratified analyses showed that the association was not modified by sex, age, CKD, osteoporosis, history of fracture, history of fracture or falls, and history of fracture/falls/predisposition to falls/osteoporosis (Figure 3 and Supplementary material online, Tables S2–S8). The sensitivity analyses yielded results similar to those of the primary analysis, except in the intention-to-treat analysis where the association was not statistically significant and the point estimate was closer to the null (Figure 4 and Supplementary material online, Table S9). There was no record of traffic accidents or fall from higher than standing height on the same day as the fracture event therefore the last sensitivity analysis was not performed. Results of the primary analyses remained virtually unchanged in the post hoc sensitivity analysis using IPTW (data not shown).
Figure 3.
Forest plot showing adjusted hazard ratios and 95% confidence intervals in stratified analyses for the association between direct oral anticoagulants and the risk of fracture. †any risk factor: history of fall, prior fracture, osteoporosis or predisposition to falls.
Figure 4.
Forest plot showing adjusted hazard ratios and 95% confidence intervals from the results of the primary analysis and sensitivity analyses to assess the risk of fracture associated with direct oral anticoagulants.
Discussion
In this population-based cohort study, prolonged use of DOACs (≥180 days) was associated with a 35% decreased risk of fracture compared with prolonged use of VKAs. A similar decreased risk was observed for the association with hip fracture. There was no difference in the risk of fracture for shorter duration of use of DOACs compared with VKAs. No apparent effect modifier was detected for the relative effect and the results were robust in multiple sensitivity analyses.
The potential increased risk of fracture with VKAs is coherent with the mechanism of action of VKAs. Exposure to VKAs can decrease vitamin K levels directly by inhibiting vitamin K epoxide reductase in vitamin K cycle. Moreover, the limited vitamin-K intake of patients on VKA treatment may also contribute to lowering vitamin K levels.33 Vitamin K deficiency can in turn reduce the carboxylated form of osteocalcin, leading to osteoporosis and fragility fracture.
To date, four studies have assessed the risk of fracture associated with DOACs compared with VKAs.14–17 Although all studies suggested that DOACs were associated with a decreased fracture risk compared with VKAs, the point estimates differed greatly.14–17 Among differences in methods used, exposure definition varied between studies. Two studies considered patients exposed from the date of OAC initiation, and the follow-up was censored at the time of drug discontinuation in one study,15 or considered them exposed during the entire follow-up.16 However, the biological effect of VKAs on bone metabolism is cumulative and chronic, and thus likely delayed. Although an analysis stratified by duration of use (less and more than 1 year) was additionally performed in one study, the shorter use category still included some very short-term users of OACs.15 The two other studies restricted cohort eligibility to patients who had been continuously exposed to OACs for 3 and 6 months, respectively.14,17 Although limiting the cohort to patients with a minimum duration of continuous use before cohort entry is relevant given the mechanism of action of VKAs on bone metabolism, this restriction does not permit to assess the risk in patients with shorter duration of use and does not consider patients who reach a biologically meaningful cumulative duration of VKAs at any time after OAC initiation. In our study, we included all patients initiating an OAC and considered them unexposed up to 180 days of cumulative duration of use and exposed thereafter. This approach also allowed to examine the risk of fractures with shorter duration of use, showing no difference between VKAs and DOACs. Additionally, we carefully took into account CKD, as renal function is an important factor affecting the initiation of DOACs or VKAs34,35 and a strong association between moderate to severe CKD and hip fracture risk has been established.36,37 We excluded patients with kidney transplant or dialysis at cohort entry, and included CKD as one of the confounding factors. Although the severity of CKD could not be accounted for, the results remained consistent when the cohort was restricted to patients without prior CKD. Finally, we assessed the risk associated with different fracture sites and showed a decreased risk of hip fracture with prolonged use of DOACs compared with VKAs. This finding is clinically relevant, as hip fracture is generally considered the most serious type of osteoporotic fracture.38
The choice of OACs for NVAF patients is based on careful evaluation of the risk-benefit trade-off. Direct oral anticoagulants have been recommended over warfarin in eligible patients with NVAF in latest guidelines, as DOACs have several advantages, including a lower risk of bleeding without compromised effectiveness, a rapid onset of action, few drug interactions, with no need for laboratory monitoring.39,40 OACs are usually prescribed to the elderly, who are also at high risk of osteoporosis and fracture. Considering the morbidity and mortality associated with fractures among elderly patients,19 the impact of OACs on bone metabolism deserves specific attention from health professionals. Our study showing a clinical benefit of DOACs compared with VKAs regarding bone health further supports the recommendation for DOACs as the first choice for anticoagulant therapy. Additionally, monitoring of bone mineral density may be necessary for elderly patients while on VKA treatment. Conversely, fracture risk may not be a concern with shorter duration of VKA therapy, for example in patients planning for cardioversion, or for prevention or treatment of venous thromboembolism.
This study has several strengths. First, we adopted a new-user design with an active comparator, thus minimizing the potential biases resulting from confounding by indication and inclusion of prevalent users. Second, we used PS-based methods to further control confounding and took into account several important confounders. Third, we defined exposure in a time-varying manner using a biologically meaningful time window for exposure. This study also has some limitations. First, we defined exposure based on prescriptions dispensed, which may result in exposure misclassification, as adherence could vary. Second, ICD-10 codes used to identify fracture events do not have enough granularity to differentiate osteoporotic fracture from traumatic fracture. To overcome this limitation, we only included in our definition fracture sites most likely to be osteoporotic fracture, and we identified fractures recorded as an inpatient primary diagnosis or admission diagnosis. We had plan to perform a sensitivity analysis excluding fractures accompanied with a record of traffic accidents or accident falls from higher than standing height; no patient was found with such record, suggesting that the potential inclusion of traumatic fractures was minimal. Finally, residual confounding remains possible given the observational nature of our study. Some potential confounders were not measured, such as body mass index, smoking status, and diet. Moreover, although all known baseline characteristics were well balanced after PS-based fine-stratification and weighting, some unknown confounders may not have been accounted for.
Overall, compared with VKA, prolonged use of DOACs (≥180 days) is associated with a lower risk of fracture, including hip fracture. These findings further support the first-line recommendation for DOACs in patients with NVAF, especially for elderly patients initiating lifelong anticoagulation therapy.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Supplementary Material
Acknowledgements
Dr Christel Renoux is the recipient of a Chercheur Boursier salary award from the Fonds de Recherche du Québec—Santé. Dr Samy Suissa is the recipient of the James McGill Chair. We thank Dr Chunli Song who provided suggestions for the clinical interpretation of this study.
Funding
This study was supported thanks to infrastructure funding from the Canadian Institutes of Health Research and the Canadian Foundation for Innovation.
Conflict of interest: S.S. has received research grants from, or participated in advisory board meetings or as speaker for, AstraZeneca, Bayer, Bristol-Myers-Squibb, Boehringer-Ingelheim and Novartis. All other authors have no conflicts of interest to disclose.
References
- 1.Wolf PA, Benhamin EJ, Belanger AJ, Kannel WB, Levy D, D'Agostino RB.. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J 1996;131:790–795. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE.. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K, Vardas P; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 4.Tufano A, Coppola A, Contaldi P, Franchini M, Minno GD.. Oral anticoagulant drugs and the risk of osteoporosis: new anticoagulants better than old? Semin Thromb Hemost 2015;41:382–388. [DOI] [PubMed] [Google Scholar]
- 5.Vermeer C.Vitamin K: the effect on health beyond coagulation—an overview. Food Nutr Res 2012;56: 5329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gage BF, Birman-Deych E, Radford MJ, Nilasena DS, Binder EF.. Risk of osteoporotic fracture in elderly patients taking warfarin: results from the National Registry of Atrial Fibrillation 2. Arch Intern Med 2006;166:241–246. [DOI] [PubMed] [Google Scholar]
- 7.Woo C, Chang LL, Ewing SK, Bauer DC; for the Osteoporotic Fractures in Men Study Group. Single-point assessment of warfarin use and risk of osteoporosis in elderly men. J Am Geriatr Soc 2008;56:1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misra D, Zhang Y, Peloquin C, Choi HK, Kiel DP, Neogi T.. Incident long-term warfarin use and risk of osteoporotic fractures: propensity-score matched cohort of elders with new onset atrial fibrillation. Osteoporos Int 2014;25:1677–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucenteforte E, Bettiol A, Lombardi N, Mugelli A, Vannacci A.. Risk of bone fractures among users of oral anticoagulants: an administrative database cohort study. Eur J Intern Med 2017;44:e30–e31. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, Honda Y, Jun I.. Long-term oral anticoagulation therapy and the risk of hip fracture in patients with previous hemispheric infarction and nonrheumatic atrial fibrillation. Cerebrovasc Dis 2010;29:73–78. [DOI] [PubMed] [Google Scholar]
- 11.Fiordellisi W, White K, Schweizer M.. A systematic review and meta-analysis of the association between vitamin K antagonist use and fracture. J Gen Intern Med 2019;34:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu ZC, Zhou LY, Shen L, Zhang C, Pu J, Lin HW, Liu XY.. Non-vitamin K antagonist oral anticoagulants vs. warfarin at risk of fractures: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol 2018;9:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veronese N, Bano G, Bertozzo G, Granziera S, Solmi M, Manzato E, Sergi G, Cohen AT, Correll CU.. Vitamin K antagonists' use and fracture risk: results from a systematic review and meta-analysis. J Thromb Haemost 2015;13:1665–1675. [DOI] [PubMed] [Google Scholar]
- 14.Binding C, Bjerring Olesen J, Abrahamsen B, Staerk L, Gislason G, Nissen Bonde A.. Osteoporotic fractures in patients with atrial fibrillation treated with conventional versus direct anticoagulants. J Am Coll Cardiol 2019;74:2150–2158. [DOI] [PubMed] [Google Scholar]
- 15.Lau WC, Chan EW, Cheung CL, Sing CW, Man KK, Lip GY, Siu CW, Lam JK, Lee AC, Wong IC.. Association between dabigatran vs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA 2017;317:1151–1158. [DOI] [PubMed] [Google Scholar]
- 16.Lutsey PL, Norby FL, Ensrud KE, MacLehose RF, Diem SJ, Chen LY, Alonso A.. Association of anticoagulant therapy with risk of fracture among patients with atrial fibrillation. JAMA Intern Med 2020;180:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang HK, Liu PP, Hsu JY, Lin SM, Peng CC, Wang JH, Loh CH.. Fracture risks among patients with atrial fibrillation receiving different oral anticoagulants: a real-world nationwide cohort study. Eur Heart J 2020;41:1100–1108. [DOI] [PubMed] [Google Scholar]
- 18.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA.. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 1999;353:878–882. [DOI] [PubMed] [Google Scholar]
- 19.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR.. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009;301:513–521. [DOI] [PubMed] [Google Scholar]
- 20.Alexiou KI, Roushias A, Varitimidis SE, Malizos KN.. Quality of life and psychological consequences in elderly patients after a hip fracture: a review. Clin Interv Aging 2018;13:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douros A, Azoulay L, Yin H, Suissa S, Renoux C.. Non-vitamin K antagonist oral anticoagulants and risk of serious liver injury. J Am Coll Cardiol 2018;71:1105–1113. [DOI] [PubMed] [Google Scholar]
- 22.Renoux C, Coulombe J, Suissa S.. Long-term vitamin K antagonists treatment patterns of non-valvular atrial fibrillation (NVAF): a population-based cohort study. BMC Cardiovasc Disord 2016;16:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renoux C, Coulombe J, Suissa S.. Revisiting sex differences in outcomes in non-valvular atrial fibrillation: a population-based cohort study. Eur Heart J 2017;38:1473–1479. [DOI] [PubMed] [Google Scholar]
- 24.Régie de l’assurance maladiedu Québec (RAMQ). http://www.ramq.gouv.qc.ca/fr/donnees-et-statistiques/donnees-sur-demande/Pages/modalites-et-domaines-de-valeurs.aspx (4 December 2019). [Google Scholar]
- 25.Douros A, Renoux C, Yin H, Filion KB, Suissa S, Azoulay L.. Concomitant use of direct oral anticoagulants with antiplatelet agents and the risk of major bleeding in patients with nonvalvular atrial fibrillation. Am J Med 2019;132:191–199.e12. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez AV, Coulombe J, Ernst P, Suissa S.. Long-term use of inhaled corticosteroids in COPD and the risk of fracture. Chest 2018;153:321–328. [DOI] [PubMed] [Google Scholar]
- 27.Tamblyn R, Lavoie G, Petrella L, Monette J.. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol 1995;48:999–1009. [DOI] [PubMed] [Google Scholar]
- 28.Wilchesky M, Tamblyn RM, Huang A.. Validation of diagnostic codes within medical services claims. J Clin Epidemiol 2004;57:131–141. [DOI] [PubMed] [Google Scholar]
- 29.Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF.. A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology 2017;28:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC.Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai RJ, Franklin JM.. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ 2019;367:l5657. [DOI] [PubMed] [Google Scholar]
- 32.Austin PC, Fine JP.. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017;36:4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usman N, Qaseem A, Jayaraj JS, Fathima N, Janapala RN.. Drug-induced reduction of gamma carboxylation in osteocalcin: what is the fallback? Cureus 2019;11:e5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aarnio E, Huupponen R, Korhonen MJ.. Important factors affecting the choice of an oral anticoagulant may be missed in database studies. J Intern Med 2018;283:214–215. [DOI] [PubMed] [Google Scholar]
- 35.Douros A, Renoux C, Coulombe J, Suissa S.. Patterns of long-term use of non-vitamin K antagonist oral anticoagulants for non-valvular atrial fibrillation: quebec observational study. Pharmacoepidemiol Drug Saf 2017;26:1546–1554. [DOI] [PubMed] [Google Scholar]
- 36.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017;7:1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR; Osteoporotic Fractures Research Group. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 2007;167:133–139. [DOI] [PubMed] [Google Scholar]
- 38.Keene GS, Parker MJ, Pryor GA.. Mortality and morbidity after hip fractures. BMJ 1993;307:1248–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW.. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–132. [DOI] [PubMed] [Google Scholar]
- 40.Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbüchel H, Lip GYH, Weitz J, Fauchier L, Lane D, Boriani G, Goette A, Keegan R, MacFadyen R, Chiang C-E, Joung B, Shimizu W; ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330–1393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.