Abstract
Aims:
A complete metabolic response (CMR) on early post-treatment 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) is a positive prognostic factor for cervical cancer patients treated with definitive chemoradiation, but long-term outcomes of this group of patients are unknown. Patterns of failure and risk subgroups are identified.
Materials and methods:
Patients who received curative-intent chemoradiation from 1998 to 2018 for International Federation of Gynecology and Obstetrics (FIGO) stage IB1–IVA cervical cancer and had a CMR on post-treatment FDG-PET within 5 months of treatment completion were included. Cox proportional hazards models determined factors associated with locoregional and distant failure. Kaplan–Meier estimates of freedom from any recurrence (FFR) of patient subgroups were compared with Log-rank tests.
Results:
There were 402 patients with a CMR after chemoradiation on FDG-PET. Initial T stage was T1 (38%)/T2 (40%)/T3 (20%)/T4 (2%); initial FDG-avid nodal status was no nodes (50%)/pelvic lymph nodes (40%)/pelvic and para-aortic lymph nodes (10%). After a median follow-up of 6 years, 109 (27%) recurred. The pattern of recurrence was locoregional (27%), distant (61%) or both (12%). No factors were associated with locoregional failure. Distant recurrence was more likely in patients with T3–4 lesions (hazard ratio = 2.4, 95% confidence interval 1.5–3.8) and involvement of pelvic (hazard ratio = 1.6, 95% confidence interval 1.0–2.7) or para-aortic lymph nodes (hazard ratio = 2.7, 95% confidence interval 1.4–5.0) at diagnosis. The 5-year FFR rates for T1–2 patients with no nodes, pelvic nodes alone or para-aortic nodes at diagnosis were 85, 76 and 62%, respectively (P = 0.04, none versus para-aortic nodes). The 5-year FFR for T3–4 patients with no nodes, pelvic nodes alone or para-aortic nodes at diagnosis were 68, 56 and 25%, respectively (P = 0.09, none versus para-aortic nodes).
Conclusions:
T3–4 tumours and para-aortic nodal involvement at diagnosis are poor prognostic factors, even after a CMR following chemoradiation.
Keywords: Cervical cancer, chemoradiation, complete metabolic response, FDG-PET, prognosis, recurrence
Introduction
Locally advanced cervical cancer is treated with definitive chemoradiation therapy. At initial staging, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) is widely used to define metabolically active tissues, specifically the primary tumour and involved lymph nodes, which can then be targeted during treatment planning [1]. Following chemoradiation, the extent of residual FDG-uptake in the primary tumour and involved lymph nodes on post-treatment FDG-PET is highly prognostic of overall survival [2]. About 70% of patients will have a complete metabolic response (CMR), but 23% of these responders eventually experience cervical cancer recurrence [3].
Previous efforts to further define prognostic factors associated with recurrence in this subset of patients have shown mixed results. Beriwal et al. [4] reported on 112 cervical cancer patients with a CMR after chemoradiation, but only 11 (10%) had recurred after a short median follow-up of 15 months. Only initial tumour size was associated with local tumour recurrence [4]. Onal et al. [5] published on 122 patients with a CMR after chemoradiation and a median follow-up of 29 months. There were 25 (21%) recurrences, which were associated with tumour size ≥ 5 cm, International Federation of Gynecology and Obstetrics (FIGO) stage ≥ IIB and pelvic and/or para-aortic nodal metastasis at diagnosis [5].
In this study, we examined the patterns of failure and risk factors for recurrence in a larger patient cohort with longer clinical follow-up than in the previous reports. Our goal was to define low-risk and high-risk subgroups of patients among those who had a CMR after curative chemoradiation, which could help to determine the necessity of subsequent follow-up imaging and/or identify patients who may benefit from additional adjuvant therapies.
Materials and Methods
Patients and Initial Treatment
Cervical cancer patients treated at a tertiary academic medical centre by definitive external beam radiation with brachytherapy boost and concurrent chemotherapy were identified in a prospectively maintained database (by a single physician, author PWG) from March 1998 to November 2018. All patients had a complete pre-treatment work-up, including history and physical examination, examination under anaesthesia, cervical tumour biopsy, pelvic computed tomography (CT) or magnetic resonance imaging (MRI) and FDG-PET. Patients were treated with external beam radiation to the pelvis to 50.4 Gy in 28 daily fractions and received either low or high dose rate brachytherapy boost to the cervix, as previously described [6]. Para-aortic nodal regions up to the renal veins were treated if para-aortic nodes were involved on staging FDG-PET. Concurrent weekly cisplatin 40 mg/m2 was prescribed to all patients. Patients who did not complete radiation treatment or did not have FDG-PET staging and follow-up scans were excluded.
Follow-up and Outcomes
Patients were followed with clinical examinations about every 2 months for the first 6 months, every 3 months for the next 2 years and then every 6 months. FDG-PET was typically ordered 3 months after the completion of treatment and then as indicated by clinical examination or symptoms. Patients had a CMR if pre-treatment FDG-avid disease had post-treatment FDG uptake that was less than or equal to blood pool activity and there were no new sites of disease. Patients whose disease subsequently recurred were restaged at that time with another FDG-PET. Locoregional failure was defined as within the initial radiation field, and distant failure was outside the initial radiation field. The time to event was determined from each patient’s pre-treatment PET scan. This retrospective analysis was approved by our institutional Human Research Protection Office with waiver of informed consent (IRB# 201911195).
Statistical Analysis
Fisher’s exact test was used to compare categorical data and the non-parametric Mann–Whitney U test was used for continuous variables. Freedom from any recurrence (FFR) was shown with Kaplan–Meier analyses. Statistical significance was calculated by Log-rank test. Cox regression analysis was carried out for both univariable and multivariable modelling of locoregional and distant failure. Factors significant on univariable analysis (P < 0.1) were entered in a backward-conditional multivariable model. Final significance was defined as P ≤ 0.05, and all tests were two-tailed. Statistical analyses were carried out in SPSS, version 23 (IBM, Armonk, NY, USA).
Results
The median time from the completion of therapy to post-treatment FDG-PET was 2.9 months (range 1.0–5.0). Of a total of 542 patients completing curative chemoradiation, there were 402 (74%) with a CMR after chemoradiation on FDG-PET (16% imaged with PET alone and 84% PET/CT). Table 1 shows the baseline clinical characteristics of these patients. The median age was 49 years (23–86 years); initial T stage was T1 (38%)/T2 (40%)/T3 (20%)/T4 (2%); the median cervical metabolic tumour volume [7] was 31 ml (1–347 ml); initial FDG-avid nodal status was involvement of no nodes (50%)/pelvic lymph nodes (40%)/pelvic and para-aortic lymph nodes (10%); and histology was squamous (84%)/non-squamous (16%). The median treatment length was 48 days (range 35–97).
Table 1:
Baseline clinical and initial treatment characteristics of patients that had a complete metabolic response on early post-treatment FDG-PET, stratified by those that subsequently had disease recurrence.
| Baseline factors | Any Recurrence n=109 | No Recurrence n=293 | P value |
|---|---|---|---|
| Median Age | 47 (26–77) | 49 (23–86) | 0.27 |
| Race | 0.32 | ||
| Caucasian | 76 (26%) | 214 (74%) | |
| African American | 32 (32%) | 69 (68%) | |
| Asian | 0 (0%) | 6 (100%) | |
| Hispanic | 1 (20%) | 4 (80%) | |
| Histology | 0.52 | ||
| Squamous | 93 (27%) | 246 (73%) | |
| Adenosquamous | 3 (43%) | 4 (57%) | |
| Adenocarcinoma | 13 (23%) | 43 (77%) | |
| FIGO 2009 (T stage) | 0.03 | ||
| IB1 | 8 (17%) | 38 (83%) | |
| IB2 | 24 (22%) | 85 (78%) | |
| IIA1 | 1 (33%) | 2 (67%) | |
| IIA2 | 0 (0%) | 1 (100%) | |
| IIB | 39 (25%) | 117 (75%) | |
| IIIA | 3 (60%) | 2 (40%) | |
| IIIB | 31 (41%) | 45 (59%) | |
| IVA | 3 (50%) | 3 (50%) | |
| Median metabolic tumor volume (mL)* | 37 (1–198) | 30 (2–346) | 0.10 |
| Cervix SUVmax** | 12 (3.4–50) | 13 (2.1–60) | 0.20 |
| PET Lymph Nodes | 0.10 | ||
| None | 44 (24%) | 143 (76%) | |
| Pelvic | 47 (28%) | 122 (72%) | |
| Pelvic and Para-aortic | 18 (39%) | 28 (61%) | |
| Brachytherapy | 0.80 | ||
| LDR | 12 (26%) | 35 (74%) | |
| HDR | 97 (27%) | 258 (73%) | |
| EBRT planning | 0.002 | ||
| 2D | 49 (37%) | 83 (63%) | |
| IMRT | 60 (22%) | 210 (78%) | |
| Median Treatment Days | 50 (39–90) | 48 (35–97) | 0.008 |
Abbreviations: FIGO-International Federation of Gynecology and Obstetrics; PET- positron emission tomography; LDR- low dose rate; HDR- high dose rate; EBRT- external beam radiation therapy; 2D- 2 dimensional; IMRT- intensity modulated radiation therapy
Calculated from the FDG-avid cervical tumor primary, with 40% maximum standard uptake value as the volume threshold. Data incomplete. 293 pts with PET volume data.
Calculated from the FDG-avid cervical tumor primary. Data incomplete. 318 patients with cervix SUVmax data.
After a median follow-up of 6 years (range 0.5–20), 109 (27%) patients had cancer recurrence. The pattern of first recurrence was locoregional (27%), distant (61%) or both (12%). All cervix recurrences were biopsy-proven. There were 37 (34%) patients with recurrences diagnosed by clinical examination and imaging alone. Table 2 shows the patterns of recurrence by initial FDG-avid nodal status. The predominant risk of recurrence in patients with initial pelvic or para-aortic nodes was distant failure outside the radiation field. Isolated para-aortic failure in patients irradiated to the pelvis alone was rare (2%). No baseline or treatment factors were significantly associated with cervix or pelvic failure (see Supplementary Tables S1 and S2). Table 3 shows that distant recurrence was more likely in patients with T3–4 lesions (hazard ratio = 2.4, 95% confidence interval 1.5–3.8) and involvement of pelvic (hazard ratio = 1.6, 95% confidence interval 1.0–2.7) or para-aortic lymph nodes (hazard ratio = 2.7, 95% confidence interval 1.4–5.0) at diagnosis. Similar results were found in the Cox model for any recurrence (Table 4).
Table 2:
Sites of failure in patients with CMR stratified by initial FDG-avid lymph node status
| Pre-treatment FDG-avid lymph nodes | Failure in cervix alone | Failure in pelvic lymph nodes alone | Failure in para-aortic nodes alone | Locoregional failure inside radiation field alone * | Distant failure outside radiation field alone | Both locoregional and distant failure |
|---|---|---|---|---|---|---|
| None (n =187) | 13 (7%) | 3 (2%) | 3 (2%) | 16 (9%) | 21 (11%) | 7 (4%) |
| Pelvic only (n=169) | 6 (4%) | 4 (2%) | 3 (2%) | 10 (6%) | 33 (20%) | 4 (2%) |
| Pelvic and para-aortic (n=46) | 0 (0%) | 2 (4%) | 0 (0%) | 3 (7%) | 13 (28%) | 2 (4%) |
Radiation field included the pelvis for all patients. The para-aortic region was only irradiated in patients with pre-treatment FDG-avid para-aortic lymph nodes.
Table 3:
Univariable and multivariable Cox proportional hazard models for distant failure after CMR on early post-treatment FDG-PET.
| Baseline factors | Univariable HR (95% CI) | P value | Multivariable HR (95% CI) | P value |
|---|---|---|---|---|
| Age | 1.00 (0.98–1.01) | 0.57 | ||
| T stage | ||||
| I-II | Ref | Ref | ||
| III-IVA | 2.53 (1.61–3.97) | <0.001 | 2.42 (1.53–3.82) | <0.001 |
| MTV* | 1.00 (0.99–1.01) | 0.93 | ||
| Cervix SUVmax** | 1.00 (0.97–1.03) | 0.86 | ||
| PET lymph nodes | ||||
| None | Ref | Ref | ||
| Pelvic | 1.61 (0.98–2.62) | 0.06 | 1.63 (1.00–2.66) | 0.05 |
| Pelvic and PA | 2.93 (1.57–5.50) | 0.001 | 2.65 (1.41–4.98) | <0.001 |
| Histology | ||||
| Squamous | Ref | |||
| Non-squamous | 0.85 (0.44–1.65) | 0.63 | ||
| Brachytherapy | ||||
| HDR | Ref | |||
| LDR | 0.73 (0.36–1.47) | 0.37 | ||
| EBRT planning | ||||
| IMRT | Ref | |||
| 2D EBRT | 1.41 (0.90–2.21) | 0.13 | ||
| Treatment length | 1.03 (1.003–1.05) | 0.02 | NS, removed from model |
Abbreviations: as in Table 1; MTV- metabolic tumor volume; Ref- reference value; HR- hazard ratio; CI- confidence interval; NS- not statistically significant
Table 4:
Univariable and multivariable Cox proportional hazard models for any failure after CMR on early post-treatment FDG-PET.
| Baseline factors | Univariable HR (95% CI) | P value | Multivariable HR (95% CI) | P value |
|---|---|---|---|---|
| Age | 0.99 (0.98–1.01) | 0.40 | ||
| T stage | ||||
| I-II | Ref | Ref | ||
| III-IVA | 2.16 (1.46–3.22) | <0.001 | 2.09 (1.40–3.11) | <0.001 |
| MTV* | 1.00 (0.99–1.01) | 0.94 | ||
| Cervix SUVmax** | 1.00 (0.97–1.02) | 0.70 | ||
| PET lymph nodes | ||||
| None | Ref | Ref | ||
| Pelvic | 1.28 (0.85–1.93) | 0.24 | 1.29 (0.86–1.95) | 0.22 |
| Pelvic and PA | 2.21 (1.28–3.83) | 0.005 | 2.03 (1.17–3.52) | 0.01 |
| Histology | ||||
| Squamous | Ref | |||
| Non-squamous | 1.04 (0.61–1.76) | 0.90 | ||
| Brachytherapy | ||||
| HDR | Ref | |||
| LDR | 0.67 (0.36–1.23) | 0.20 | ||
| EBRT planning | ||||
| IMRT | Ref | |||
| 2D EBRT | 1.29 (0.87–1.90) | 0.21 | ||
| Treatment length | 1.03 (1.005–1.05) | 0.01 | NS, removed from model |
Figure 1 shows that the 5-year FFR rates for T1–2 patients with no nodes, pelvic nodes alone or para-aortic nodes at diagnosis were 85, 76 and 62%, respectively (P = 0.04 between no nodes and para-aortic nodes). The 5-year FFR rates for T3–4 patients with no nodes, pelvic nodes alone or para-aortic nodes at diagnosis were 68, 56 and 25%, respectively (P = 0.09 between no nodes and para-aortic nodes).
Fig 1.
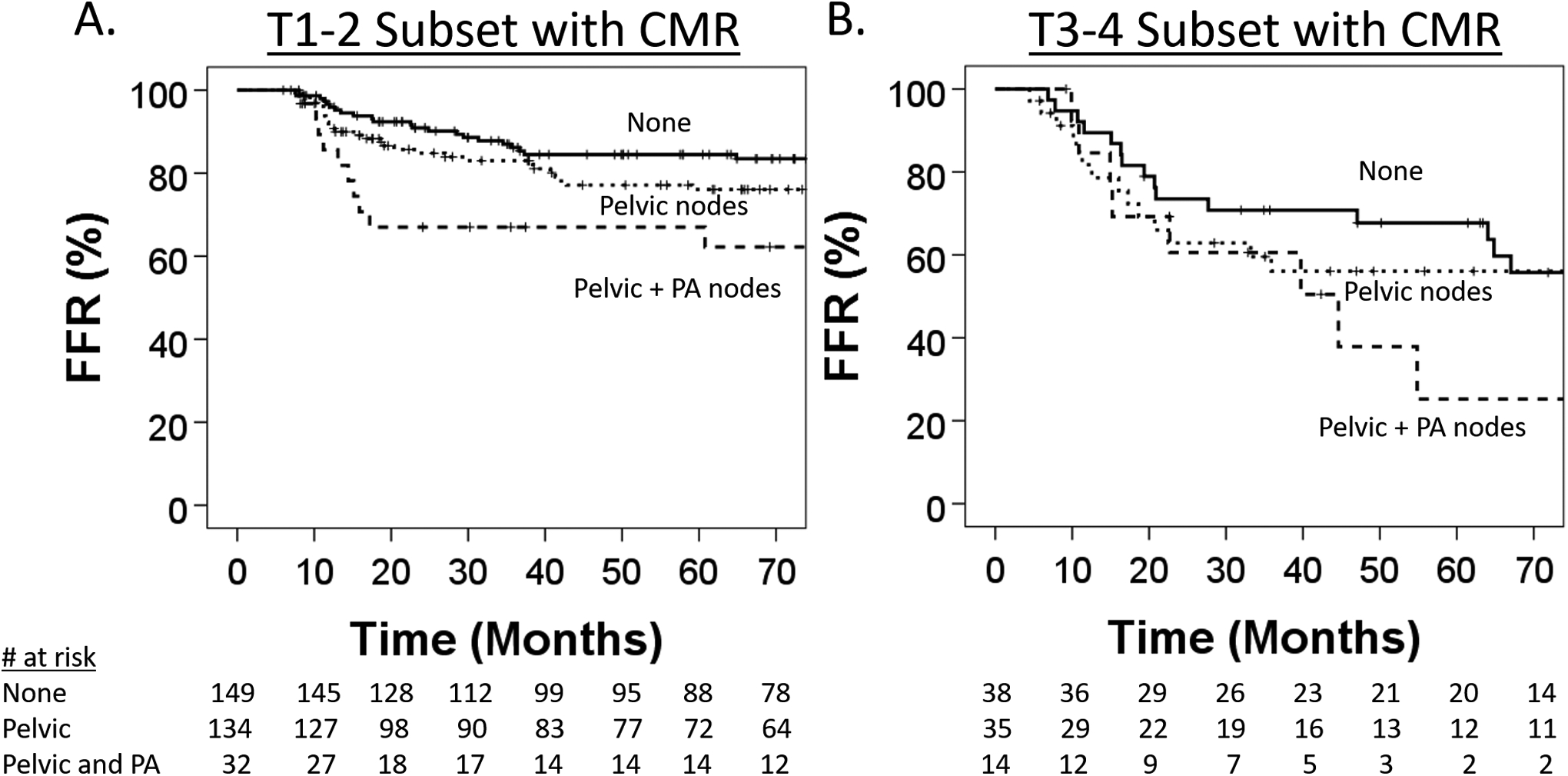
(A) Kaplan–Meier estimates of freedom from recurrence (FFR) in the subset of T1–2 patients with a complete metabolic response (CMR) after curative chemoradiation stratified by pre-treatment fluorodeoxyglucose (FDG)-avid lymph nodes. (B) FFR in the subset of T3–4 patients with a CMR after curative chemoradiation stratified by pre-treatment FDG-avid lymph nodes.
Discussion
The National Comprehensive Cancer Network guidelines recommend a 3–6 month post-treatment surveillance FDG-PET after definitive chemoradiation for cervical cancer. Patients who have a CMR after definitive radiation on early post-treatment FDG-PET have a good prognosis compared with patients who have only a partial metabolic response or progressive disease [8]. However, the crude recurrence rate still approaches 30% in this large series of over 400 patients with a CMR, indicating that the predictive power of FDG-PET may be limited by its insensitivity for detection of microscopic disease. Pre-treatment T-stage and metabolically active pelvic and/or para-aortic lymph nodes were independently associated with risk of recurrence after a CMR, ranging from 15 to 75% in different subsets of patients. The pattern of failure was mostly distant, and no factors tested predicted locoregional failure. This study identified high-risk patients with a CMR who might benefit from either adjuvant therapy or more intensive surveillance, both of which are active areas of research.
In contrast to prior studies [4,5], we did not show initial tumour volume or the maximum standardised uptake value to predict local failure in patients with a CMR. Notably, 88% of the patients in our series received 6 weekly fractions of HDR intracavitary brachytherapy, starting usually within 2 weeks of treatment interdigitated with external beam radiation. The dose delivered to at least 90% of the primary tumour was often over 100 Gy (equivalent dose in 2 Gy fractions) [9], which is an ablative dose. This differs from the previous studies in which external beam radiation was given upfront and then followed by a brachytherapy boost, which was prescribed to a lower dose. Tumour volume changes after external beam radiation but prior to brachytherapy have been shown to predict tumour sensitivity to radiation [10]. In the Pittsburgh cohort [4], interstitial brachytherapy was used if patients had a poor response to external beam radiation (about 25% of their patients), which could also change the patterns of recurrence.
Tumour histology also did not impact the prognosis after a CMR in this cohort. Onal et al. [5] found that patients with squamous histology had a worse overall survival compared with those with adenocarcinoma after a CMR, but this was not significant after accounting for tumour size and stage. The negative predictive value of FDG-PET for detecting lymph node metastasis in early stage cervical cancer is only 78% for squamous versus 92% for adenocarcinoma histology [11]. A CMR theoretically could be more predictive of lower recurrence risk for adenocarcinoma histology, but a larger cohort may be needed to discover this effect.
The 5-year risk of any recurrence after a CMR in this study was stratified from 15 to 75%, depending on the pre-treatment T and N stage. These results could help to select only high-risk patients for future studies testing adjuvant therapy. Adjuvant gemcitabine and cisplatin after concurrent chemoradiation improved overall survival over concurrent chemoradiation alone for locally advanced cervical cancer patients in a prospective randomised trial [12], but utilisation in standard practice has been limited because of concerns of overtreatment and toxicity. Another recently reported trial testing three cycles of adjuvant carboplatin and paclitaxel failed to accrue and was thus underpowered to show any survival differences [13]. The OUTBACK trial (Clinicaltrials.gov ID: NCT01414608) tested four cycles of adjuvant carboplatin and paclitaxel and we await early results to be reported. In contrast to cytotoxic chemotherapy, adjuvant immunotherapy after chemoradiation in locally advanced cervical cancer patients seemed to be tolerable in a phase I study [14]. Response rates in metastatic cervical cancer have been low when given in a non-selected patient population, yet most patients with an immune response against tumours have had sustained progression-free survival [15]. Identifying both imaging and immune biomarkers for patients who benefit from immunotherapy is a secondary objective of an ongoing randomised phase II multi-institutional trial of concurrent or adjuvant pembroluzimab for locally advanced cervical cancer (NCT02635360).
Post-treatment circulating blood biomarkers, such as squamous cell carcinoma antigen [16,17], the circulating neutrophil-to-lymphocyte ratio [18] and circulating tumour cells [19] or human papillomavirus (HPV) DNA [20], are prognostic of disease-free survival in cervical cancer patients. In addition to detecting residual microscopic disease in patients with a CMR, these liquid biopsies could also give an early readout of whether adjuvant systemic therapy is going to be effective. For example, detectable circulating tumour DNA (ctDNA) after definitive chemoradiation was predictive of response to adjuvant immunotherapy in locally advanced lung cancer [21]. Recurrence rates were low in patients who had no detectable ctDNA whether they received adjuvant immunotherapy or not. If detectable ctDNA increased early during adjuvant immunotherapy, recurrence rates were higher than if ctDNA levels decreased. These blood biomarkers need to be evaluated in a prospective trial, which would be costly if tested in every locally advanced cervical cancer patient. The positive predictive value of these tests could be enriched by only evaluating patients with an intermediate-to-high risk of recurrence, such as those identified in this study.
Finally, the role of continued surveillance FDG-PET after a CMR is controversial because of the cost of scans. We and others have shown that surveillance FDG-PET scans detect asymptomatic metastasis early, which has translated to increased overall survival in retrospective series [22,23]. The efficacy of radical local therapy, such as surgery or ablative radiation, to extend survival in oligometastatic disease has been shown prospectively [24]. However, this study proves that 3-month post-treatment FDG-PET alone cannot be used to direct surveillance intensity or give cervical cancer patients confidence in their prognosis. Therefore, alternative strategies for surveillance should be explored in prospective randomised studies. For example, our group recently showed that something as simple as post-treatment HPV DNA clearance from a vaginal swab sample was independently associated with better recurrence-free and overall survival [25]. Distant recurrence is the most common pattern of failure in this subset of patients, but continued FDG-PET surveillance may not necessarily be the only option. Future prospective work could focus on the cost-effectiveness of obtaining surveillance FDG-PET versus CT or MRI in patients with high risk of progression after a CMR, based on this study.
In conclusion, this retrospective study’s strengths are that nearly all cervical cancer patients at our institution received an early post-treatment FDG-PET in the study period and we had adequate follow-up time to capture recurrences after a CMR. However, some clinics outside of the USA and in much of the developing world do not routinely carry out FDG-PET scans due to constrained resources, which limits the generalisability of this study. Most recurrences occurred outside the radiation field, indicating that better systemic therapies are needed. Patients with either T3–4 disease or FDG-avid para-aortic lymph nodes pre-treatment are at high risk of recurrence even with a CMR. Both imaging and circulating biomarkers of initial treatment response will probably be important for current and future studies of adjuvant therapy. Our results indicate that continued clinical follow-up is necessary despite a CMR after definitive chemoradiation therapy.
Supplementary Material
Highlights:
Complete metabolic response (CMR) on 3-month PET has non-homogeneous cervical cancer control rates
Distant recurrence is the most common pattern of failure
Initial T and N stage independently stratify risk of distant recurrence after CMR
Acknowledgements
P.W. Grigsby is supported by NIH R21 CA223799-01. J.K. Schwarz is supported by NIH R01 CA181745. S. Markovina is supported by NIH K08 CA237822. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest with this work.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clon.2021.01.010.
References
- [1].Lin AJ, Dehdashti F, Grigsby PW. Molecular imaging for radiotherapy planning and response assessment for cervical cancer. Semin Nucl Med 2019;49(6):493–500. doi: 10.1053/j.semnuclmed.2019.06.009. [DOI] [PubMed] [Google Scholar]
- [2].Schwarz JK, Siegel BA, Dehdashti F, Grigsby P. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA 2007;298(19):2289–95. doi: 10.1001/jama.298.19.2289. [DOI] [PubMed] [Google Scholar]
- [3].Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Metabolic response on post-therapy FDG-PET predicts patterns of failure after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2012;83(1):185–90. doi: 10.1016/j.ijrobp.2011.05.053. [DOI] [PubMed] [Google Scholar]
- [4].Beriwal S, Kannan N, Sukumvanich P, Richard SD, Kelley JL, Edwards RP et al. Complete metabolic response after definitive radiation therapy for cervical cancer: patterns and factors predicting for recurrence. Gynecol Oncol 2012;127(2):303–6. doi: 10.1016/j.ygyno.2012.08.006. [DOI] [PubMed] [Google Scholar]
- [5].Onal C, Reyhan M, Guler OC, Yapar AF. Treatment outcomes of patients with cervical cancer with complete metabolic responses after definitive chemoradiotherapy. Eur J Nucl Med Mol Imaging 2014;41(7):1336–42. doi: 10.1007/s00259-014-2719-5. [DOI] [PubMed] [Google Scholar]
- [6].Lin AJ, Kidd E, Dehdashti F, Siegel BA, Mutic S, Thaker PH et al. Intensity modulated radiation therapy and image-guided adapted brachytherapy for cervix cancer. Int J Radiat Oncol Biol Phys 2019;103(5):1088–97. doi: 10.1016/j.ijrobp.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Olsen JR, Esthappan J, Dewees T, Narra VR, Dehdashti F, Siegel BA et al. Tumor volume and subvolume concordance between FDG-PET/CT and diffusion-weighted MRI for squamous cell carcinoma of the cervix. J Magn Reson Imaging 2013;37(2):431–4. doi: 10.1002/jmri.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim YJ, Han S, Kim YS, Nam JH. Prognostic value of post-treatment 18F-fluorodeoxyglucose positron emission tomography in uterine cervical cancer patients treated with radiotherapy: a systematic review and meta-analysis. J Gynecol Oncol 2019;30(5):1–13. doi: 10.3802/jgo.2019.30.e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dyk P, Jiang N, Sun B, Dewees TA, Fowler KJ, Narra V et al. Cervical gross tumor volume dose predicts local control using magnetic resonance imaging/diffusion-weighted imaging guided high-dose-rate and positron emission tomography/computed tomography guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2014;90(4):794–801. doi: 10.1016/j.ijrobp.2014.07.039. [DOI] [PubMed] [Google Scholar]
- [10].Schernberg A, Bockel S, Annede P, Fumagalli I, Escande A, Mignot F et al. Tumor shrinkage during chemoradiation in locally advanced cervical cancer patients: prognostic significance, and impact for image-guided adaptive brachytherapy. Int J Radiat Oncol Biol Phys 2018;102(2):362–72. doi: 10.1016/j.ijrobp.2018.06.014. [DOI] [PubMed] [Google Scholar]
- [11].Lin AJ, Wright JD, Dehdashti F, Siegel BA, Markovina S, Schwarz J et al. Impact of tumor histology on detection of pelvic and para-aortic nodal metastasis with 18 F-fluorodeoxyglucose–positron emission tomography in stage IB cervical cancer. Int J Gynecol Cancer 2019;29:1351–4. doi: 10.1136/ijgc-2019-000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dueñas-González A, Zarbá JJ, Patel F, Alcedo JC, Beslija S, Casanova L et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol 2011;29(13):1678–85. doi: 10.1200/JCO.2009.25.9663. [DOI] [PubMed] [Google Scholar]
- [13].Tangjitgamol S, Tharavichitkul E, Tovanabutra C, Rongsriyam K, Asakij T, Paengchit K et al. A randomized controlled trial comparing concurrent chemoradiation versus concurrent chemoradiation followed by adjuvant chemotherapy in locally advanced cervical cancer patients: ACTLACC trial. J Gynecol Oncol 2019;30(4):1–13. doi: 10.3802/jgo.2019.30.e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mayadev JS, Enserro D, Lin YG, Da Silva DM, Lankes HA, Aghajanian C et al. Sequential ipilimumab after chemoradiotherapy in curative-intent treatment of patients with node-positive cervical cancer. JAMA Oncol 2019;6(1):92–9. doi: 10.1001/jamaoncol.2019.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dyer BA, Zamarin D, Eskandar RN, Mayadev JM. Role of immunotherapy in the management of locally advanced and recurrent/metastatic cervical cancer. JNCCN J Natl Compr Cancer Netw 2019;17(1):91–7. doi: 10.6004/jnccn.2018.7108. [DOI] [PubMed] [Google Scholar]
- [16].Markovina S, Wang S, Henke LE, Luke CJ, Pak SC, Dewees T et al. Serum squamous cell carcinoma antigen as an early indicator of response during therapy of cervical cancer. Br J Cancer 2018;118(1):72–8. doi: 10.1038/bjc.2017.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang W, Liu X, Hou X, Lian X, Liu Z, Shen J et al. Posttreatment squamous cell carcinoma antigen predicts treatment failure in patients with cervical squamous cell carcinoma treated with concurrent chemoradiotherapy. Gynecol Oncol 2019;155(2):224–8. doi: 10.1016/j.ygyno.2019.09.003. [DOI] [PubMed] [Google Scholar]
- [18].Lee HJ, Kim JM, Chin Y, Chong G, Park S, Lee Y et al. Prognostic value of hematological parameters in locally advanced cervical cancer patients treated with concurrent chemoradiotherapy. Anticancer Res 2020;40:451–8. doi: 10.21873/anticanres.13973. [DOI] [PubMed] [Google Scholar]
- [19].Wen YF, Cheng TT, Chen XL, Huang WJ, Peng HH, Zhou TC et al. Elevated circulating tumor cells and squamous cell carcinoma antigen levels predict poor survival for patients with locally advanced cervical cancer treated with radiotherapy. PLoS One 2018;13(10):1–13. doi: 10.1371/journal.pone.0204334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Han K, Leung E, Barbera L, Barnes E, Croke J, Di Grappa MA et al. Circulating human papillomavirus DNA as a biomarker of response in patients with locally advanced cervical cancer treated with definitive chemoradiation. JCO Precis Oncol 2018;(2):1–8. doi: 10.1200/po.18.00152. [DOI] [PubMed] [Google Scholar]
- [21].Moding EJ, Liu Y, Nabet BY, Chabon JJ, Chaudhuri AA, Hui AB et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nat Cancer 2020;1:176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brooks RA, Rader JS, Dehdashti F, Mutch DG, Powell MA, Thaker PH et al. Surveillance FDG-PET detection of asymptomatic recurrences in patients with cervical cancer. Gynecol Oncol 2009;112(1):104–9. doi: 10.1016/j.ygyno.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Peters PN, Pierson WE, Antonio C, Jocelyn CW. PET‑detected asymptomatic recurrence is associated with improved survival in recurrent cervical cancer. Abdom Radiol 2020. doi: 10.1007/s00261-020-02633-0. [DOI] [PubMed] [Google Scholar]
- [24].Palma D, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol 2020;38(25):2830–8. doi: 10.1101/2020.03.26.20044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Srivastava AJ, Contreras JA, Davis M, Markovina ST, Schwarz JK, Grigsby PW. Early posttherapy clearance of human papillomavirus and treatment response in cervical carcinoma. Cancer 2020;126:4168–76. doi: 10.1002/cncr.33040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


