Why was the cohort set up?
Innovations in the understanding of childhood cancer biology, treatment and supportive care have dramatically improved cure rates such that 84% of newly diagnosed children and adolescents in high-income countries are expected to survive beyond 5-years from diagnosis.1 As a result, the number of childhood cancer survivors living in the USA surpassed 420 000 in 20131 and is anticipated to approach 500 000 by 2020.2 The medical success manifest by this growing population has been realized at a cost, however, as many survivors experience adverse health outcomes related to cancer and its treatment.3–6 In addition to chronic morbidity, survivors experience premature mortality compared to age- and sex-matched controls.7–10 Current understanding of late health outcomes in adult survivors of childhood cancer has largely been the result of a number of cohort studies that have been instrumental in identifying, quantifying and characterizing cancer treatment-related health risks.11–17 Health outcomes research findings have had a major influence on changes in paediatric cancer therapy and have informed health-screening strategies of childhood cancer survivors. However, in the early years of these studies, many were limited by dependence upon self-reported outcomes or registry data, smaller population sizes and restriction to single disease subsets and/or limited follow-up duration. To address these limitations, some have evolved to include periodic, prospective physical assessments extending beyond the period of cancer centre follow-up for single disease groups,11 nested subsets within the larger cohort14, and more recently for all survivors,12,17 in an effort to gain a better understanding of the true prevalence of disease and contributing risk factors. To this end, St. Jude Children’s Research Hospital (SJCRH) established in 2007 the prospective St. Jude Lifetime (SJLIFE) cohort, open to survivors of all paediatric cancer subtypes representing the spectrum of paediatric, adolescent and young adult cancers, as well as frequency-matched community controls, including collection of comprehensive treatment data on all participants, provision of protocol-based medical assessments, assessment of patient-reported outcomes, validation of self-reported medical events, performance of periodic longitudinal evaluations and collection of biologic specimens.18 Longitudinal, systematic medical assessments facilitate elucidation of the pathophysiology of cancer treatment-related morbidity, identification of biomarkers of subclinical organ dysfunction and characterization of high-risk survivor groups who may benefit from interventions to preserve health.
At its inception in 2007, SJLIFE was an institutionally funded resource designed to study adults previously treated for childhood cancer at SJCRH who had survived ≥10 years from cancer diagnosis. In 2015, SJLIFE received extramural support from the National Institutes of Health (CA195547) to expand enrollment to include individuals of any age treated at SJCRH who had survived ≥5 years from cancer diagnosis in order to facilitate: (i) detection of asymptomatic and premorbid conditions; (ii) discovery of early predictors of adverse outcomes; and (iii) to collectively provide the basis for impactful and targeted interventions. These changes significantly increased the number of eligible survivors and aligned eligibility requirements with existing observational cohorts of childhood cancer survivors, expanding the opportunity for novel research initiatives, including replication and validation of previous findings. The study was reviewed by the SJCRH Institutional Review Board and ethical approval was obtained on 25 April 2007. Details regarding study design, recruitment and participant characteristics of the original cohort have been previously published.18 This study is registered at ClinicalTrials.gov under the identifier NCT00760656.
Who is in the cohort?
The SJLIFE study utilizes a retrospective cohort study design with prospective follow-up and ongoing accrual of patients diagnosed and treated at SJCRH over five decades (1962–2012). Additional information regarding the SJLIFE cohort is available at https://sjlife.stjude.org/. St. Jude provides financial support for transportation, medical evaluations, meals, domiciliary care and monetary compensation for days of study participation. The original inclusion criteria, as established in 2007, were limited to adults ≥18 years of age at the time of evaluation who were treated at SJCRH and had lived ≥10 years from initial cancer diagnosis. In 2015, the criteria were expanded to include survivors of any age at the time of evaluation who had lived ≥5 years post diagnosis and who had been diagnosed through 30 June 2012. Table 1 shows the distribution of characteristics of the pool of survivors eligible for recruitment into the cohort prior to expansion and after expansion.
Table 1.
Characteristics of eligible SJLIFE survivors prior to and after expansion
| Characteristic | Prior to expansion (2007–14), n (%) |
After expansion (2015 to present), n (%) |
P-Value |
|---|---|---|---|
| Total population | 4895 | 8192 | |
| Sex | |||
| Female | 2236 (45.7) | 3774 (46.1) | 0.39 |
| Male | 2659 (54.3) | 4418 (53.9) | |
| Race | |||
| White | 4086 (83.5) | 6499 (79.3) | <0.001 |
| Black | 722 (14.8) | 1381 (16.9) | |
| Other | 87 (1.7) | 312 (3.8) | |
| Ethnicity | |||
| Hispanic | 123 (2.5) | 360 (4.4) | <0.001 |
| Non-Hispanic | 4772 (97.5) | 7832 (95.6) | |
| Diagnosis | |||
| Acute lymphoblastic leukaemia | 1526 (31.2) | 2230 (27.2) | <0.001 |
| Acute myeloid leukaemia | 178 (3.6) | 359 (4.4) | |
| Other leukaemia | 92 (1.9) | 194 (2.4) | |
| Hodgkin lymphoma | 564 (11.5) | 738 (9.0) | |
| Non-Hodgkin lymphoma | 377 (7.7) | 504 (6.1) | |
| Central nervous system malignancy | 533 (10.9) | 1431 (17.5) | |
| Wilms tumor | 324 (6.6) | 464 (5.7) | |
| Neuroblastoma | 218 (4.5) | 376 (4.6) | |
| Retinoblastoma | 142 (2.9) | 442 (5.4) | |
| Germ cell tumor | 122 (2.5) | 178 (2.2) | |
| Liver malignancies | 42 (0.9) | 63 (0.8) | |
| Osteosarcoma | 177 (3.6) | 248 (3.0) | |
| Rhabdomyosarcoma | 174 (3.5) | 252 (3.1) | |
| Ewing sarcoma family of tumors | 136 (2.8) | 196 (2.4) | |
| Non-rhabdomyosarcoma soft tissue sarcoma | 146 (3.0) | 232 (2.8) | |
| Other malignancies | 144 (2.9) | 285 (3.4) | |
| Age at diagnosis (years) | |||
| <1 | 306 (6.3) | 723 (8.8) | <0.001 |
| 1–4 | 1521 (31.1) | 2783 (34.0) | |
| 5–9 | 1173 (24.0) | 1861 (22.7) | |
| 10–14 | 1095 (22.3) | 1606 (19.6) | |
| 15–19 | 761 (15.5) | 1152 (14.1) | |
| 20+ | 39 (0.8) | 67 (0.8) | |
| Birth decade | |||
| 1940–49 | 10 (0.2) | 10 (0.1) | <0.001 |
| 1950–59 | 111 (2.3) | 113 (1.4) | |
| 1960–69 | 637 (13.0) | 648 (7.9) | |
| 1970–79 | 1494 (30.5) | 1531 (18.7) | |
| 1980–89 | 1838 (37.5) | 2033 (24.8) | |
| 1990–99 | 805 (16.5) | 2208 (27.0) | |
| 2000–09 | 0 (0.0) | 1564 (19.1) | |
| 2010+ | 0 (0.0) | 85 (1.0) | |
| Current age (years) | |||
| 5–9 | 0 (0.0) | 158 (1.9) | <0.001 |
| 10–19 | 0 (0.0) | 1665 (20.3) | |
| 20–29 | 955 (19.5) | 2227 (27.2) | |
| 30–39 | 1859 (38.0) | 2019 (24.7) | |
| 40–49 | 1402 (28.6) | 1432 (17.5) | |
| 50–59 | 580 (11.9) | 590 (7.2) | |
| 60+ | 99 (2.0) | 101 (1.2) |
To be eligible for participation in SJLIFE, individuals must have: (i) a history of childhood cancer diagnosed and have been treated at SJCRH between 1962 and 2012 and (ii) have survived ≥5 years from diagnosis. Whereas SJLIFE does not specify age at cancer diagnosis, SJCRH generally restricts acceptance to children <25 years of age at the time of cancer diagnosis. Recruitment utilizes a centralized process: (i) eligible survivors (through 30 June 2012) were identified via predetermined queries of medical record data in the SJCRH Clinical Research Informatics System; (ii) personalized invitation letters that provide mechanisms to respond electronically or via mail are sent to the survivor’s last known address; (iii) if there is no response after 2 weeks, a study interviewer calls to determine the survivor’s level of interest in participating; (iv) those interested in participating are transferred to a visit coordinator who describes the study and schedules the survivor for an on-campus visit (average duration of 3–4 days). If participants cannot be reached via voice call, electronic communications (i.e. email and text messaging) are attempted. To minimize loss to follow-up (both for original recruitment and for subsequent visits), the study team uses a combination of tracing procedures (e.g. patient and family contact information, postal forwarding addresses and internet queries) to locate participants, and verifies participant addresses at each point of contact (e.g. at the time of on-campus visit and follow-up phone calls).
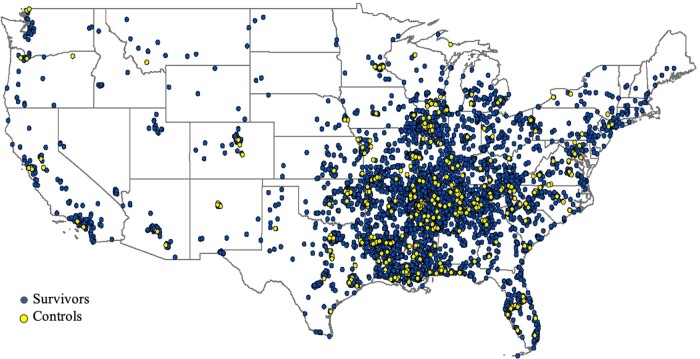
To facilitate estimates of risk associated with cancer and its treatment, SJLIFE is recruiting age-, sex- and race-frequency matched individuals without a history of childhood cancer to complete the same clinical assessment as survivors. We refer to these individuals as ‘community controls’ since they are being recruited through a variety of approaches from the same general geographic area as the SJLIFE survivor population (Figure 1). To achieve the desired geographic distribution, we offer the SJLIFE survivor participant the opportunity to identify a friend or non-first degree relative to accompany them to their on-campus visit and enrol as a community control. Additional community controls are recruited from the Memphis area through advertisements. As of 12 March 2020, 736 (target accrual of 1250) community controls have completed on-campus evaluations. The geographical distribution of eligible survivor and control participants is shown in Figure 1.
Figure 1.
Geographical distribution of survivors and controls in the St. Jude Lifetime Cohort in the conterminous USA. Note that participants from Alaska (n = 4), Hawaii (n = 6) and International (n = 68) participants are not shown
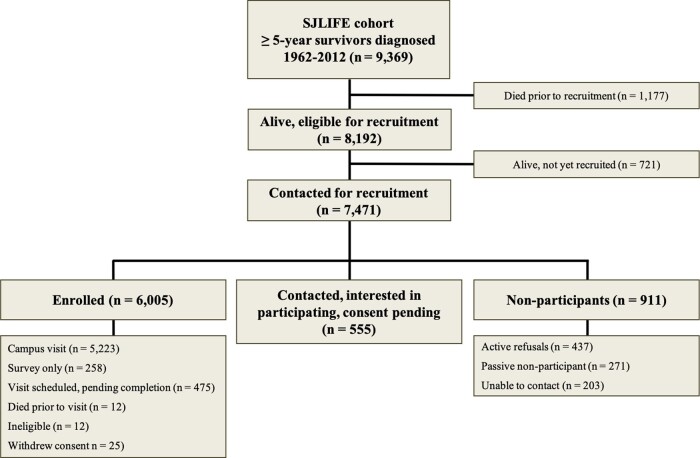
The following categories are used to classify participants: (i) ‘eligible participants’ are individuals meeting eligibility criteria and alive at the time of recruitment; (ii) ‘enrolled participants’ are those who have signed consent to participate; (iii) ‘confirmed interest participants’ have been contacted and are interested, but have not yet signed consent to participate; (iv) ‘non-participants’ have declined participation, failed to respond after initially indicating interest or were not able to be contacted. As of 12 March 2020, 73.3% (6005/8192) of all living, eligible survivors have enrolled and 80.1% have enrolled or confirmed their interest in participating. Among those approached for study participation, 80.3% (6005/7471) have been successfully enrolled, 87.8% have enrolled or have confirmed interest in participating and 94.9% of those enrolled have elected to complete an on-campus assessment (Figure 2). Differences between participants and non-participants are detailed in Supplementary Table 1, available as Supplementary data at IJE online.
Figure 2.
Participant flow for recruitment of survivors into the St. Jude Lifetime Cohort. Of the 9369 survivors, 8192 were eligible for recruitment, with 5223 completing a campus assessment as of 12 March 2020. Response rate is defined as those who have completed a campus visit (n = 5223)/those contacted for recruitment (n = 7471) = 69.9%
How often have they been followed up?
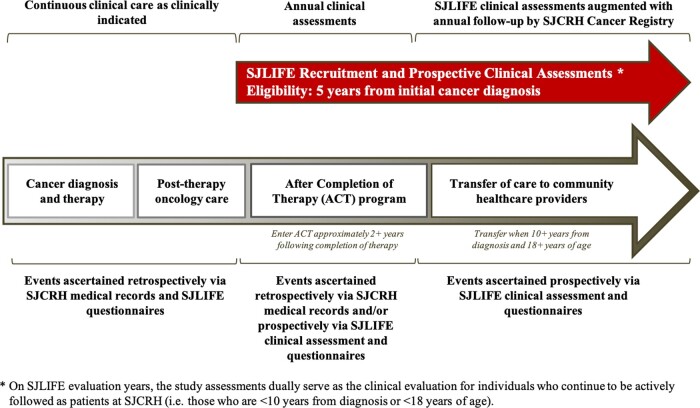
The timing and sequence of participant data acquisition for events occurring prior to and after study enrollment are reflected in Figure 3. During and following treatment of paediatric cancer, remission status and treatment-related toxicities are routinely monitored by the primary oncology team and/or the long-term follow-up (after completion of therapy) clinic until the survivor is ≥10 years from diagnosis and ≥18 years of age, whichever occurs later. Data abstracted from medical records for all participants include demographics, cumulative chemotherapy doses, radiation fields and cumulative doses, information on surgical interventions, primary cancer recurrences and subsequent neoplasms, and acute and late organ-specific toxicity.
Figure 3.
Timing and sequence of participant data acquisition for events prior to and after study enrollment in the St. Jude Lifetime Cohort
The health and vital status of potential participants are monitored by the St. Jude Cancer Registry and supplemented by National Death Index (NDI) searches. The NDI provides a centralized database of death record information ascertained by state vital statistics offices. The SJLIFE study team periodically submits a request to NDI for vital statistics on study participants known to have died or without known contact since the last query. The NDI then returns a list of probabilistic matches to the SJLIFE team using a standardized algorithm.19 The results are then processed by the SJLIFE team via the Center for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) data linkage process.20 The combined findings of both NDI and NPCR are then centrally reviewed and a final match status assigned. The Cancer Registry is an established SJCRH resource designed to maintain systematic, life-time annual follow-up for all SJCRH patients, irrespective of participation in SJLIFE. Yearly attempts to contact all prior patients, with the primary purpose of assessing vital status and cancer status (no evidence of disease, recurrence, subsequent primary cancers), are made via written or verbal communication with patients, registries, emergency contacts, newspapers, vital status sites and local providers, with a goal follow-up rate of 90–95%. Delinquent cases remain in follow-up until contacted or confirmation of death is obtained. Following provision of informed consent, SJLIFE participants (both in the original and expanded cohorts) are invited to return to SJCRH at least once every 5 years for protocol-based medical evaluations and assessments of neurocognitive function, physical performance status and patient-reported outcomes. Permission for release of medical records for designated facilities up to 1 year from the campus visit is requested at each evaluation to validate interim, survivor-reported medical events, in particular those relating to the most recent campus visit (e.g. follow-up biopsy and breast cancer diagnosis after a screening imaging abnormality identified during a SJLIFE visit). In addition, two study-team members have the sole responsibility of following-up on health events identified during SJLIFE evaluations and those self-reported between campus visits, and their contact supplements the annual contact by the Cancer Registry. When new interval events are reported, additional permission to seek medical records is obtained on a case-by-case basis. Records are routinely requested for events involving imaging, tissue biopsies, surgical and diagnostic procedures, or interventions specifically impacting gradable conditions (e.g. skin biopsies, outside breast imaging) and/or clinical management (e.g. suspected cancer recurrence, chronic kidney disease, chronic hepatitis C). With the exception of vital status, interval events not coinciding with a campus visit (e.g. a self-reported myocardial infarction occurring 3 years after the last SJLIFE campus assessment) are censored from research analyses until the next subsequent campus visit occurs for uniform cross-sectional validation of events.
As of 12 March 2020, 51.3% (2680/5223) of survivors who had completed a baseline clinical assessment have returned for one or more subsequent follow-up assessments. Supplementary Table 2, available as Supplementary data at IJE online, describes baseline characteristics as well as providing a comparison between those who have returned for a subsequent visit and those who declined additional visits. Table 2 describes grade 3–4 chronic conditions by organ system prevalent at baseline.
Table 2.
Modified CTCAE21 defined chronic condition prevalence in individuals who have completed a baseline SJLIFE assessment (n = 5223)
| Organ system | Grade 3–4 chronic condition, n (%) |
|---|---|
| Cardiovascular | 376 (7.2) |
| Endocrine | 867 (16.6) |
| Gastrointestinal | 663 (12.7) |
| Musculoskeletal | 538 (10.3) |
| Neurological | 470 (9.0) |
| Pulmonary | 292 (5.6) |
| Renal | 178 (3.4) |
| Subsequent neoplasms | 193 (3.7) |
What has been measured?
Two levels of participation are offered in SJLIFE and have been applied to both the original and expanded cohorts: (i) an on-campus, comprehensive health evaluation (including health questionnaires and direct assessment) or (ii) comprehensive health questionnaires only, that are completed by mail or phone interview (Table 3). Detailed methodology on data collection, including medical record abstraction, questionnaires and medical assessments have been previously published for the original cohort.18 On campus study participation involves travel to the SJCRH campus for a comprehensive assessment averaging 3–4 days, during which biologic specimens (e.g. blood, urine) are collected; metabolic, cognitive and neuromuscular functional status are systematically evaluated; and screening of organ function is performed (Supplementary Table 3, available as Supplementary data at IJE online). These uniform assessments (Table 3) include identification and grading of chronic conditions using modified Common Terminology Criteria for Adverse Events (CTCAE) criteria,21 assessment of patient-reported outcomes, whole genome and exome sequencing, and linkage to the NDI for mortality follow-up. Relevant findings are summarized on a survivorship care plan and mailed to the survivor after the on-campus assessment, with direct phone contact by a study nurse or healthcare provider for critical findings that should be followed up by the participant’s community providers (e.g. suspicious lesions found on breast mammography). On-campus assessments are funded by institutional and extramural support. No study-related costs are submitted to healthcare insurance agencies.
Table 3.
Study measures and clinical assessments for the SJLIFE cohort
| Evaluation | Domain | Measures |
|---|---|---|
| Comprehensive health questionnairea | Health outcomes and status | Medical service utilization, medication use, current and past health problems, reproductive status and pregnancies |
| Social and demographic factors | Marital status, living arrangements, academic achievement, employment status, insurance access, income and financial hardship | |
| Health behaviours | Tobacco use, alcohol intake, substance use, physical activity, sedentary behaviour, sun exposure behaviours, participation in health screening, and use of complementary and alternative medicine | |
| Psychosocial constructs | Health perceptions, motivation for behaviour change, body image/weight concerns, perceived stress, cancer impact, post-traumatic distress, social desirability, depression, anxiety and somatization | |
| Men’s/women’s health | Fertility, onset of puberty, sexual development, relationship/marital satisfaction and sexual health/functioning. History of testosterone therapy, sperm banking and erectile dysfunction for male survivors and controls | |
| Quality of life | Pediatric Quality of Life Inventory (PedsQL) for children/parents22; Medical Outcomes Study 36-Item Short Form Health Survey (SF-36) for adults23,24 | |
| Dietary intake | Block Food Frequency Questionnaire25 | |
| Neurocognitive assessment | Global intelligence | Wechsler Abbreviated Scale of Intelligence-Second Edition26 |
| Reading and mathematical academic skills | Woodcock Johnson Tests of Achievement27 | |
| Processing speed | Wechsler Intelligence Scale for Children,28 Wechsler Adult Intelligence Scale29 | |
| Sustained attention | Conner’s Continuous Performance Test30 | |
| Memory | California Verbal Learning Test31 | |
| Executive functions | Cognitive flexibility, fluency, planning and organization; Delis–Kaplan Executive Function System32 | |
| Patient report | Executive function using the Behavior Rating Inventory of Executive Function;33,34 attention and memory skills using the Childhood Cancer Survivor Study Neurocognitive Questionnaire35 for adults and the Behavioral Assessment Scale for Children36 | |
| Patient-reported fatigue | Multidimensional Fatigue Scale22 for children and parents or the Functional Assessment of Chronic Illness Therapy (FACIT) Fatigue Scale37 for adults | |
| Blood analysis | General organ function | Complete blood count w/differential, comprehensive metabolic panel, lipid panel, c-reactive protein high sensitivity, haemoglobin A1c, insulin level, 25 hydroxy-vitamin D, insulin-like growth factor-1, thyroxine free, thyroid stimulating hormone, oestradiol level, testosterone total, follicle stimulating hormone assay, luteinizing hormone, cortisol, ferritin, cystatin C assay, urinalysis, random urine creatinine, random urine protein, random urine calcium, hepatitis B core antibody IgG and IgM, hepatitis B surface antigen, hepatitis B surface antibody, hepatitis C antibody, human immunodeficiency virus 1/2 antibody/antigen with positive reflex testing |
| Echocardiogram | Cardiac function | Left ventricular volume, mass, ejection fraction, E velocity, A velocity, E/A ratio, isovolumetric relaxation time (IVRT), deceleration time, medial annulus velocity, E/Em, estimation of filling pressures, and evaluation of the pulmonary veins, right atrial pressure and estimation of right ventricular systolic pressure |
| Pulmonary function testing | Respiratory muscle strength and gas exchange | Forced vital capacity, forced expiratory volume, and peak expiratory flow |
| Cardiac biomarker testing | Diagnosis/prognosis of heart failure | N-Terminal pro-brain natriuretic peptide (NT pro-BNP), troponin-T |
| Neuromuscular functional assessment | Muscle strength and flexibility | Knee extension and dorsiflexion strength, hand-grip strength, ankle dorsiflexion active and passive range of motion, sit and reach test |
| Exercise stress test | Cardio-respiratory fitness using a modified Bruce protocol | |
| Aerobic capacity | Peak VO2 estimated with the Duke Activity Status Index (DASI),38 six-minute walk test39 | |
| Mobility | Timed Up and Go40 | |
| Anthropometrics | Height, weight, body mass index, waist and hip circumference | |
| Body composition | Percent body fat, lean body mass, skeletal muscle mass and bone mineral content from dual X-ray absorptiometry (DXA) scans, lumbar bone mineral density using quantitative computed tomography (QCT) | |
| Balance | Berg Balance Measure41 | |
| Overall physical performance | Physical performance test (PPT)42 | |
| Fertility | Semen analysis for male participants | |
| Vision | Ophthalmology exam | Visual acuity testing, refraction testing, retinoscopy, ocular pressure, examination under mydriasis, fundus photography to screen for hypertensive retinopathy |
| Hearing | Audiology exam | Speech audiometry, tympanometry, otoacoustic emissions testing |
| Cancer screening | Subsequent neoplasms | Colonoscopy, breast mammogram and magnetic resonance imaging |
| Psychosocial assessment | Social adjustment | Comprehensive assessment by licensed social worker, assistance with referrals to community providers and resources for ongoing care as needed |
| Medical record abstraction | Cancer-related treatment exposures | Cumulative doses for 22 specific chemotherapeutic agents [actinomycin-D, carmustine, bleomycin, busulfan, carboplatin, chlorambucil, cis-platinum, lomustine, cyclophosphamide (oral, intravenous), cytosine arabinoside (intravenous, intramuscular, intrathecal, subcutaneous), daunorubicin, doxorubicin, idarubicin, ifosfamide, melphalan, methotrexate (intravenous, intramuscular, intrathecal), busulfan, nitrogen mustard, procarbazine, thiotepa, etoposide (oral, intravenous), tenopiside], surgical procedures, and radiation treatment fields, dose and energy source |
| Radiation dosimetry | Site-specific radiation dose reconstruction | Cranial, neck, chest, abdomen, pelvis, arm and leg |
| Chronic condition assessment | Morbidity | Assessed using modified CTCAE21 |
| DNA collection | Genetics | Whole genome and exome sequencing |
| Mortality | Mortality | Linkage to the NDI, all cause and cause-specific mortality |
Modified versions of questionnaires are administered to participants who are <18 years of age; for participants 11–18 years of age, parents and participants complete the questionnaires; for participants <11 years of age, parents answer the questions on behalf of the child.
What has it found? Key findings and publications
Since the inception of the SJLIFE study, 120 manuscripts have been published featuring results of late health outcomes. These publications have yielded a number of important findings based on analyses of on-campus participants, including clinically ascertained and validated prevalence6 and cumulative burden estimates4 for cancer treatment-related organ dysfunction, and documentation of novel late health outcomes.43–47
Prior to SJLIFE, prospective clinical ascertainment of a large population of childhood cancer survivors to determine prevalence of chronic conditions had not been performed. Hudson et al. reported the prevalence of chronic conditions and the proportion associated with treatment exposures in the first 1713 adult SJLIFE survivors who had completed a baseline assessment. Pulmonary, auditory, endocrine, reproductive, cardiac and neurocognitive adverse health outcomes were common, with the cumulative prevalence of chronic conditions by organ-specific outcomes by age 50.6
Bhatka et al.4 subsequently expanded upon these findings by applying the mean cumulative count method,48 which estimates the mean number of recurrent or multiple health events occurring in a cohort over time in the presence of competing risk events, in order to describe the unique patterns and excess cumulative burden of chronic health conditions experienced by childhood cancer survivors compared with community controls. By age 50 years, survivors experienced, on average, 17.1 grade 1–5 and 4.7 grade 3–5 chronic health conditions compared with 9.2 and 2.3, respectively, among community controls.
Most recently, Wang et al.49 utilized whole genome and exome sequencing to assess the contribution of germline genetic abnormalities to subsequent neoplasm risk in survivors assessed for mutations in cancer predisposition genes. Pathogenic/likely pathogenic mutations were identified in 5.8% of survivors and were associated with increased risk of breast cancer and sarcoma in irradiated survivors and for developing any subsequent neoplasm, breast cancer, non-melanoma skin cancer and two or more histologically distinct subsequent neoplasms among non-irradiated survivors.
What are the main strengths and weaknesses?
Through systematic, prospective medical assessments and longitudinal follow-up, the SJLIFE cohort provides the opportunity to accurately characterize contributions of treatment, behavioural, genetic and social factors to the health status of childhood cancer survivors. A major strength of SJLIFE is the ability to perform direct and longitudinal medical assessments on a large cohort of prospectively monitored childhood cancer survivors. Robust characterization of health outcomes and cancer-, cancer treatment- and health behaviour-related exposures provide an unparalleled opportunity to identify novel associations and to refine our understanding of the effects of cancer and its treatment on the health of aging survivors.
The SJLIFE study must be interpreted in the context of a number of limitations. Notably, SJLIFE is a single-institution study, therefore late effects and detection may be biased by institutional practices, whereas population demographics and ethnicity may not be generalizable to the remainder of the USA. In addition, because it was not established until 2007, many survivors treated in earlier decades were no longer living at study onset, introducing the potential for survival bias. Furthermore, while participation rates in survivorship research are typically high, it remains possible that survivors with the highest burden of late effects are more likely to decline participation. However, access to treatment records for eligible non-participants and regular linkage to NDI records well-positions SJLIFE to better characterize these biases moving forward. Lastly, as the SJLIFE demographics are reflective of the surrounding population at large, the study is currently limited in its ability to perform robust analyses on minority populations, therefore application of the findings should be considered in the context of one’s surrounding demographics.
Can I get hold of the data? Where can I find out more?
SJCRH has established the St. Jude Cloud (http://www.stjude.cloud/) to provide secure sharing and collaborative analysis of large and complex datasets to facilitate research addressing paediatric cancer and other rare diseases. The St. Jude Cloud, developed as a partnership between SJCRH, DNAnexus and Microsoft, is a secure cloud-based data-sharing and collaboration environment. Data are aggregated at the patient level, providing researchers access to an extensive public repository of paediatric cancer genomics data, accelerated data mining, analysis and visualization capabilities. Data requests can be made on the website and require a standard data-use agreement. The St. Jude Cloud provides genomic sequencing data to the global research community, while making complex computational analysis pipelines available through a collection of bioinformatics tools designed to help both experts and non-specialists interrogate genomic data. SJLIFE genomic data consisting of 5020 (4382 survivor and 638 community controls) whole genomes (30x) and whole exomes (100x) are currently available to the scientific community on the St. Jude Cloud.
Non-genomic SJLIFE data are posted to the Survivorship Portal on the St. Jude Cloud and include detailed information on: (i) cancer-related variables (diagnosis ICD-O-3 and ICCC-3), age at diagnosis, length of follow-up, and treatment including region and organ-specific radiation dosimetry, surgical procedures and medications including cumulative doses for individual chemotherapeutic agents, plus selected antibiotics and immune-suppressants; (ii) sociodemographic data (sex, race, ethnicity, education, income, employment) and health behaviours (e.g. smoking/tobacco use, drug use, alcohol consumption, physical activity and diet); and (iii) outcomes (modified CTCAE classification and severity grading for 190 medical and 18 neuropsychologic conditions, laboratory-based values (e.g. CBC, renal, liver, kidney, endocrine function) and procedural assessments (e.g. echocardiography, pulmonary function, dual-energy X-ray absorptiometry, etc.), data collected through physical function and neurocognitive assessments, and survivor-reported data obtained via the portfolio of SJLIFE questionnaires.
Profile in a nutshell
The St. Jude Lifetime Cohort was designed to establish a lifetime cohort of childhood cancer survivors in which to perform ongoing, prospective health outcomes assessments.
Childhood cancer survivors were recruited from 8192 survivors diagnosed between 1962 and 2012 with paediatric cancer and treated at St. Jude Children’s Research Hospital in Memphis, TN. Of the 8192 survivors, 6560 have agreed to participate and 5223 have completed a baseline clinical assessment.
Survivors are invited to return for follow-up assessment every 5 years. As of 12 March 2020, 51.3%% of survivors who have completed a baseline assessment have returned for one or more subsequent follow-up assessments.
Data collected includes detailed medical record abstraction of cancer-related therapeutic exposures (surgery, radiation, chemotherapy), identification and grading of chronic conditions using modified Common Terminology Criteria for Adverse Events (CTCAE) criteria, collection of biologic specimens (e.g. blood, urine), assessment of patient-reported outcomes, physical performance, neurocognitive function, whole genome and exome sequencing, and linkage to the National Death Index (NDI) for mortality follow-up.
Study details and data are available at http://sjlife.stjude.org/ and http://www.stjude.cloud/
Supplementary data
Supplementary data are available at IJE online.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health Cancer Center Support grant [5P30CA021765-33] and the St. Jude Lifetime Cohort Study Grant [U01 CA195547], and the American Lebanese Syrian Associated Charities. Registered at Clinicaltrials.gov (#NCT00760656). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
None declared.
Supplementary Material
References
- 1.Howlader N, Noone AM, Krapcho M et al. SEER Cancer Statistics Review, 1975–2013. http://seer.cancer.gov/csr/1975_2013/ (27 July 2020, date last accessed).
- 2.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014;14:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GT, Kawashima T, Leisenring W et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the Childhood Cancer Survivor Study. J Clin Oncol 2014;32:1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakta N, Liu Q, Ness KK et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 2017;390:2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson TM, Mostoufi-Moab S, Stratton KL et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970-99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 2018;19:1590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson MM, Ness KK, Gurney JG et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309:2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong GT, Chen Y, Yasui Y et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 2016;374:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebholz CE, Reulen RC, Toogood AA et al. Health care use of long-term survivors of childhood cancer: the British Childhood Cancer Survivor Study. J Clin Oncol 2011;29:4181–88. [DOI] [PubMed] [Google Scholar]
- 9.Reulen RC, Winter DL, Frobisher C et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA 2010;304:172–79. [DOI] [PubMed] [Google Scholar]
- 10.Richardson DP, Daly C, Sutradhar R et al. Hospitalization rates among survivors of young adult malignancies. J Clin Oncol 2015;33:2655–59. [DOI] [PubMed] [Google Scholar]
- 11.Berbis J, Michel G, Baruchel A et al. Cohort Profile: the French childhood cancer survivor study for leukaemia (LEA Cohort). Int J Epidemiol 2015;44:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geenen MM, Cardous-Ubbink MC, Kremer LC et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 2007;297:2705–15. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins MM, Lancashire ER, Winter DL et al. The British Childhood Cancer Survivor Study: objectives, methods, population structure, response rates and initial descriptive information. Pediatr Blood Cancer 2008;50:1018–25. [DOI] [PubMed] [Google Scholar]
- 14.Kuehni CE, Rueegg CS, Michel G et al. Cohort profile: the Swiss Childhood Cancer Survivor Study. Int J Epidemiol 2012;41:1553–64. [DOI] [PubMed] [Google Scholar]
- 15.Robison LL, Armstrong GT, Boice JD et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 2009;27:2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winther JF, Kenborg L, Byrne J et al. Childhood cancer survivor cohorts in Europe. Acta Oncol 2015;54:655–68. [DOI] [PubMed] [Google Scholar]
- 17.Sieswerda E, Mulder RL, van Dijk IW et al. The EKZ/AMC childhood cancer survivor cohort: methodology, clinical characteristics, and data availability. J Cancer Surviv 2013;7:439–54. [DOI] [PubMed] [Google Scholar]
- 18.Hudson MM, Ness KK, Nolan VG et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer 2011;56:825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fillenbaum GG, Burchett BM, Blazer DG. Identifying a national death index match. Am J Epidemiol 2009;170:515–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Registry Plus, a Suite of Publicly Available Software Programs for Collecting and Processing Cancer Registry Data. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, 2020. https://www.cdc.gov/cancer/npcr/ (6 October 2020, date last accessed); https://www.cdc.gov/cancer/npcr/tools/registryplus/lp.htm
- 21.Hudson MM, Ehrhardt MJ, Bhakta N et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev 2017;26:666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer 2002;94:2090–106. [DOI] [PubMed] [Google Scholar]
- 23.McHorney CA, Ware JE Jr, Ae R. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247–63. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 25.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology 1990;1:58–64. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Abbreviated Scale of Intelligence®—Second Edition (WASI®-II). San Antonio, TX: Pearson, 2011. [Google Scholar]
- 27.Woodcock RW, McGrew KS, Mather N, Schrank FA. Woodcock-Johnson III. Itasca, IL: Riverside Publishing, 2003. [Google Scholar]
- 28.Wechsler D. Wechsler Intelligence Scale for Children®—Fourth Edition (WISC®-IV). San Antonio, TX: Pearson, 2003. [Google Scholar]
- 29.Wechsler D. Wechsler Adult Intelligence Scale—Fourth Edition (WAIS-IV). San Antonio, TX: Pearson, 2008. [Google Scholar]
- 30.Conners CK. Conners' Continuous Performance Test II. Noth Tonawanda, NY: Multi-Health Systems, Inc., 2001. [Google Scholar]
- 31.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test®—Second Edition (CVLT® -II). San Antonio, TX: Pearson, 2000. [Google Scholar]
- 32.Delis DC, Kaplan E, Kramer JH. Delis–Kaplan Executive Function System™ (D-KEFS™). San Antonio, TX: Pearson, 2001. [Google Scholar]
- 33.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function® (BRIEF®). Odessa, FL: PAR, Inc., 2000. [Google Scholar]
- 34.Roth RM, Isquith PK, Gioia GA. Behavior Rating Inventory of Executive Function®–Adult Version (BRIEF®-a). Odessa, FL: PAR, Inc., 2005. [Google Scholar]
- 35.Kenzik KM, Huang IC, Brinkman TM et al. The Childhood Cancer Survivor Study-Neurocognitive Questionnaire (CCSS-NCQ) revised: item response analysis and concurrent validity. Neuropsychology 2015;29:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamphaus RW. Behavior Assessment System for Children, (BASC‐2). The encyclopedia of clinical psychology. 2014; 29:1–6.
- 37.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hlatky MA, Boineau RE, Higginbotham MB et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 1989;64:651–54. [DOI] [PubMed] [Google Scholar]
- 39.Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed) 1982;284:1607–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–48. [DOI] [PubMed] [Google Scholar]
- 41.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health 1992;83: S7–11. [PubMed] [Google Scholar]
- 42.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc 1990;38:1105–12. [DOI] [PubMed] [Google Scholar]
- 43.Chemaitilly W, Li Z, Huang S et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: a report from the St Jude Lifetime Cohort study. J Clin Oncol 2015;33:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chemaitilly W, Li Z, Krasin MJ et al. Premature ovarian insufficiency in childhood cancer survivors: a report from the St. Jude Lifetime Cohort. J Clin Endocrinol Metab 2017;102:2242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulrooney DA, Soliman EZ, Ehrhardt MJ et al. Electrocardiographic abnormalities and mortality in aging survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Am Heart J 2017;189:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ness KK, Krull KR, Jones KE et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol 2013;31:4496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nottage KA, Ness KK, Li C, Srivastava D, Robison LL, Hudson MM. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia: from the St. Jude Lifetime Cohort. Br J Haematol 2014;165:364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong H, Robison LL, Leisenring WM, Martin LJ, Armstrong GT, Yasui Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am J Epidemiol 2015;181:532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Wilson CL, Easton J et al. Genetic risk for subsequent neoplasms among long-term survivors of childhood cancer. J Clin Oncol 2018;36:2078–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.