Abstract
This article describes 20 years of research that investigated a second novel target for ribosomal antibiotics, the biogenesis of the two subunits. Over that period, we have examined the effect of 52 different antibiotics on ribosomal subunit formation in six different microorganisms. Most of the antimicrobials we have studied are specific, preventing the formation of only the subunit to which they bind. A few interesting exceptions have also been observed. Forty-one research publications and a book chapter have resulted from this investigation. This review will describe the methodology we used and the fit of our results to a hypothetical model. The model predicts that inhibition of subunit assembly and translation are equivalent targets for most of the antibiotics we have investigated.
Introduction
There is currently a worldwide threat to infectious disease management and therapy.1 , 2 One of the most pressing medical issues today is the continuing rise in antibiotic-resistant microorganisms.3–5 MDR microorganisms are of particular concern.6 , 7 Nosocomial infection rates with resistant organisms continue to increase.8 For some types of infections, resistance is so prevalent that older abandoned drugs must be used to stop the spread of resistant organisms.9 Many currently used drugs are chemically modified versions of prior antibiotics, which show limited efficacy for short periods of time.10 Clearly, new antimicrobial agents need to be found, not based on current drugs, but new compounds with enhanced effectiveness.11–15 An example is linezolid, which was synthesized as an anticancer compound, then abandoned and later resurrected as a novel antimicrobial.16 Natural resistance to these types of chemically synthesized molecules is generally very low.
In addition, new antibiotic targets must be identified, so that additional antimicrobials can be developed.17–21 These targets must be novel, so that development of resistance should be uncommon.22 A number of unique targets have been identified and more are being searched for using genomic computer analysis23 , 24 and structure-based antibiotic design.25 , 26
Bacterial ribosome biogenesis
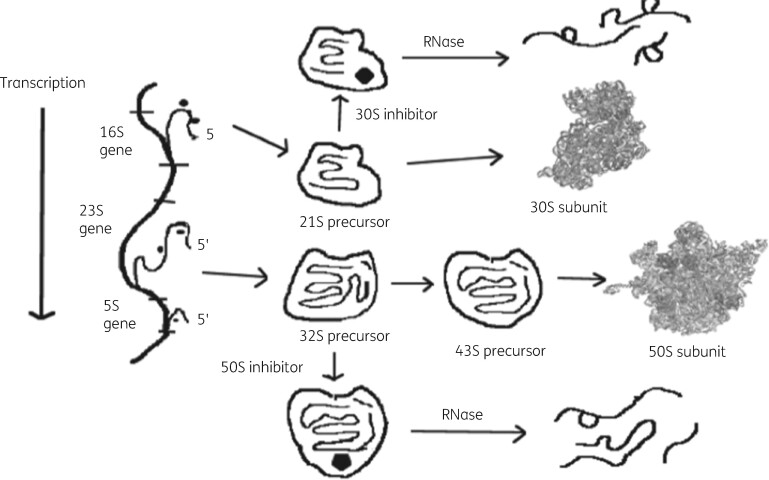
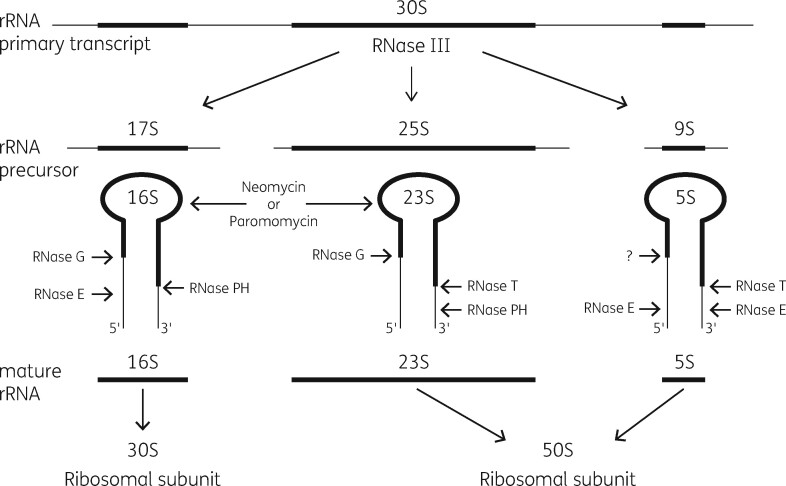
The bacterial ribosome is the most frequent antibiotic target in microorganisms.27 This organelle is the site of cellular protein biosynthesis in all living organisms. It functions in mRNA translation in a similar fashion in all cells.28 Its three RNA molecules and numerous ribosomal proteins associate in a complex fashion to generate the smaller 30S subunit and the larger 50S subunit.29 , 30 Biogenesis involves the transcription of one single precursor rRNA molecule, which is processed into a precursor 16S rRNA molecule and into 23S and 5S precursor sequences.31 Ribosomal proteins add to these molecules during transcription and generate a 21S precursor structure for the 30S subunit and a 32S precursor for the 50S subunit, which contains both 23S and 5S sequences. A conformational change in the 21S precursor allows the remaining 30S subunit proteins to associate to generate the completed 30S structure. Genesis of the 50S subunit goes through a second intermediate structure of 43S, which then matures with the addition of the remaining 50S proteins.32 , 33 Formation of both particles involves the assistance of a large collection of chaperone proteins with specific functions in the maturation process.34 A set of unique ribonuclease (RNase) enzymes is also necessary for maturation of the rRNA molecules.35 , 36 Figure 1 shows the broad details of this process in the absence and presence of antibiotics.
Figure 1.
A model for ribosomal subunit assembly inhibition by antibiotics. 16S rRNA transcription followed by protein addition gives a 21S precursor particle, which matures to give 30S subunits. 23S and 5S rRNA transcription is followed by formation of both 32S and 43S intermediates, which lead to 50S particle formation. In the presence of antibiotics, assembly stalls at the first defined intermediate particle that can bind the appropriate antibiotic. RNases degrade the stalled intermediate particle reducing net subunit formation. Reproduced with permission from Bentham Science Publishers from reference 92.
Characteristics of the ribosomal antibiotics investigated
Essentially all antibiotics that target the ribosome bind to the rRNA sequences without direct protein involvement.3 , 37–39 rRNA is a very old molecule in evolutionary terms and ribosome-targeting compounds are believed to have evolved with the primitive ribosome.40–42 Almost all ribosomal antibiotics interfere with the process of protein biosynthesis through a variety of mechanisms.27 , 37 These include inhibition of peptide bond formation, protein exit from the 50S subunit tunnel, transfer RNA (tRNA) binding, subunit association and disassociation and turnover, as examples. In addition, the biogenesis of the two subunits has been described as a potential target for antibiotic inhibition.43–47 Current investigations of ribosomal antibiotic targets and binding characteristics have been achieved by two important methods. The precise features of the binding site interactions have been established by X-ray diffraction analysis of ribosome crystals with a bound antibiotic.48 Also, high-resolution cryo-electron microscopy has aided in the identification of antibiotic binding sites in the native ribosome.49
Our studies on the potential of ribosomal antimicrobial agents to disrupt subunit biogenesis included a wide variety of 50S subunit-specific compounds. Macrolide antimicrobials tested included erythromycin, azithromycin, clarithromycin and related compounds.50 , 51 They all have a specific binding site in domain V of the 23S rRNA and require the adenosine at position 2058 (A2058) for association. They inhibit translation by blocking the 50S exit tunnel in a nascent peptide sequence-specific fashion.52–54
The ketolide antimicrobials were also studied.55 They are third-generation derivatives of the macrolides, in which the 3-cladinose sugar is replaced by a ketone group. The 6-O position is methylated and there is an aliphatic or aromatic extension from the carbamate at C11/C12 in the macrolactone ring. They bind to domain V and also to domain II in 23S rRNA, which increases their binding affinity about 10-fold. A comparison of the difference from the macrolides in 50S subunit interactions has been described.56
There are other antibiotics that also target the large subunit in the domain V region of the peptidyltransferase centre (PTC). These include the 16-membered-ring macrolides, the lincosamides and the streptogramin A and B compounds (MLSB). The 16-carbon macrolactone ring compounds, such as spiramycin and tylosin, and the lincosamides, including clindamycin and lincomycin, have overlapping binding sites and also associate with A2058 and adjacent nucleotides.57 , 58 The streptogramin B family, including pristinamycin IA and virginiamycin S, also have a domain V binding site.59 All of these translational inhibitors bind in the PTC and function to block peptide exit from the 50S subunit. Crystallographic analysis has revealed the precise 23S rRNA interactions between these antimicrobial agents.60 The overlap of the binding sites in the PTC is clearly shown in the work of Schwarz et al.61 and Tu et al.62
Chloramphenicol and the streptogramin A compounds act differently from the macrolides and inhibit the peptidyltransferase function of the subunit, thus preventing peptide bond formation. The binding interactions of these antibiotics are also well established. Tereshchenkov et al.63 have investigated this RNA association by X-ray structure analysis.
Four additional novel antimicrobials that bind to 23S rRNA in the PTC were also investigated. Evernimicin, a natural product from Micromonospora species binds to 23S rRNA and interferes with aminoacyl-tRNA association with the 50S subunit.64 , 65 The binding interactions with the minor grooves of helix 89 (H89) and H91 in 23S rRNA were uncovered by cryo-electron microscopy.66
TAN-1057A is a natural product from Flexibacter species.67 , 68 It is a dipeptide and binds in the PTC and reduces chain elongation. It seems to function as an inhibitor of the peptidyltransferase activity of the large subunit.69 , 70
Linezolid is a synthetic oxazolidinone compound, not a natural product.71 It binds to the A site in the PTC region of 23S rRNA. It affects aminoacyl-tRNA binding to the 50S subunit.72 , 73 It overlaps the binding sites for chloramphenicol, clindamycin, tiamulin and streptogramin A compounds, as determined by crystallography of the 50S-bound drug.74 , 75
Retapamulin is a member of the pleuromutilin family of antimicrobials.76 It binds in the PTC of the 50S and affects tRNA binding to the 50S subunit.77–79 The specific binding site interactions for all of these compounds have been determined by high resolution X-ray diffusion analysis of ribosome crystals with a bound antibiotic48 and by cryo-electron microscopy.49
Three aminoglycoside antimicrobial agents that bind to the 30S subunit were also tested for effects on 30S subunit biogenesis.80 , 81 Hygromycin B interferes with the translocation step on the 30S subunit and affects A-site tRNA binding in the major groove of H44 in 16S rRNA. It restricts movement of the 16S rRNA by a conformational change in the helix.82 , 83 Neomycin and paromomycin are two other aminoglycoside molecules that have a specific association with the decoding centre of helix 44.84–86 They bind to A1492 and A1493 in 16S rRNA and stimulate mistranslation of mRNA. Neomycin binding differs slightly from paromomycin binding. During the course of our investigations it was discovered that aminoglycosides also have a binding site on H69 of 23S rRNA in the 50S subunit.87
Background studies
This research was initiated as a reinterpretation of the results described by Vester and Garrett.88 They proposed that erythromycin stimulated the degradation of 50S subunits in antibiotic-inhibited cells. We concluded that their findings could also be interpreted as resulting from erythromycin inhibition of 50S subunit assembly. Since then, we have examined the effect of 52 different antibiotic molecules on ribosomal subunit formation in six different microorganisms. Most of the molecules we have studied are specific and prevent the formation of only the subunit to which they bind. A few interesting exceptions have also been observed. This review will describe the methodology we used and the fit of our results to a hypothetical model. The model predicts that inhibition of subunit assembly and translation are equivalent targets for most of the antibiotics we have investigated. Some of this work has been reviewed previously.89–92
Figure 1 shows the model we have proposed.92 It predicts that the inhibitory antibiotics should bind to the rRNA in one of the intermediates in the assembly process and thus prevent subunit maturation. RNase enzymes would then degrade RNA in the stalled precursor molecule.
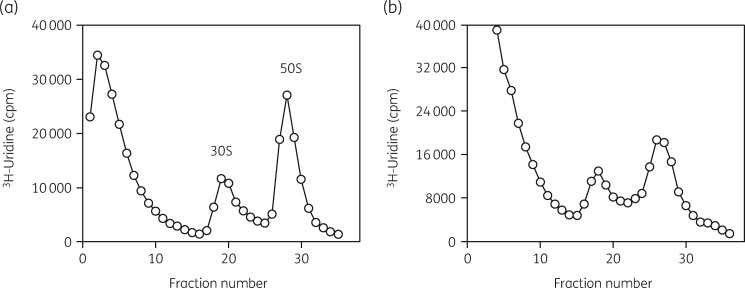
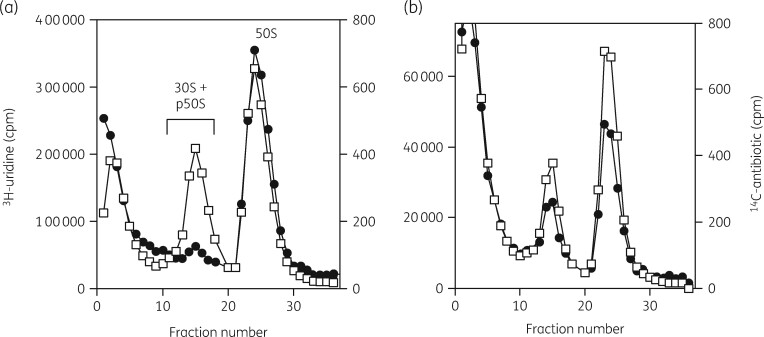
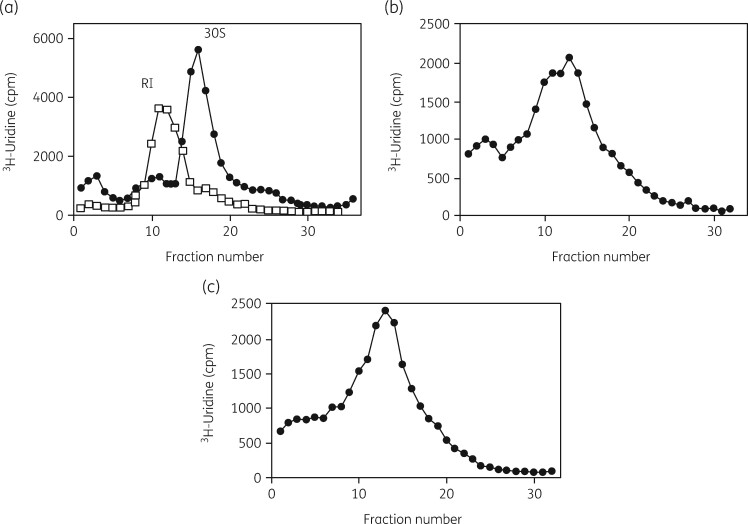
A very important part of the methodology used in these studies was an examination of the status of both subunits by sucrose density gradient ultracentrifugation of [3H]-uridine-labelled rRNA.93 This procedure allowed quantitative analysis of the amount of each subunit present, the binding distribution of radiolabelled antibiotics in the cells, the isolation of subunits and precursor particles, the separation of 16S and 23S rRNAs and the collection of small RNA oligonucleotides from the top of the gradient fractions. Typical gradient profiles are shown in Figure 2. rRNA status was examined by gel electrophoresis and northern blot analysis with specific DNA probes. Ribosomal proteins were separated and identified by 2D PAGE and by MALDI-TOF MS. 3H-labelled aminoglycosides were synthesized by a reductive methylation procedure.94
Figure 2.
Sucrose gradient profiles of [3H]-uridine-labelled ribosomal subunits from H. influenzae cells grown with and without azithromycin. (a) Gradient profile of labelled subunits from untreated cells. (b) Gradient profile of subunits from azithromycin-treated cells. Reproduced with permission from Bentham Science Publishers Ltd from reference 118.
In our initial investigation, we found that erythromycin and azithromycin specifically reduced the amounts of 50S subunits in Escherichia coli cells. Importantly, we also showed that erythromycin did not stimulate the breakdown of pre-existing 50S subunits.95 We then examined two Gram-positive microorganisms, Staphylococcus aureus and Bacillus subtilis. In both organisms, 50S subunit biogenesis was specifically impaired by erythromycin, azithromycin and clarithromycin.96 We subsequently showed that protein synthesis and 50S subunit formation were impaired to the same extent, suggesting that translation and 50S biogenesis were equivalent targets in S. aureus.97 The macrolides azithromycin and clarithromycin were examined in more detail.98 The relationship between the inhibition of translation and large subunit biogenesis was concentration dependent and revealed IC50 values of 0.15 μg/mL for clarithromycin and 5 μg/mL for azithromycin. This suggested that clarithromycin would be the preferred antibiotic for S. aureus infections.
Antibiotic concentration effects on 50S biogenesis
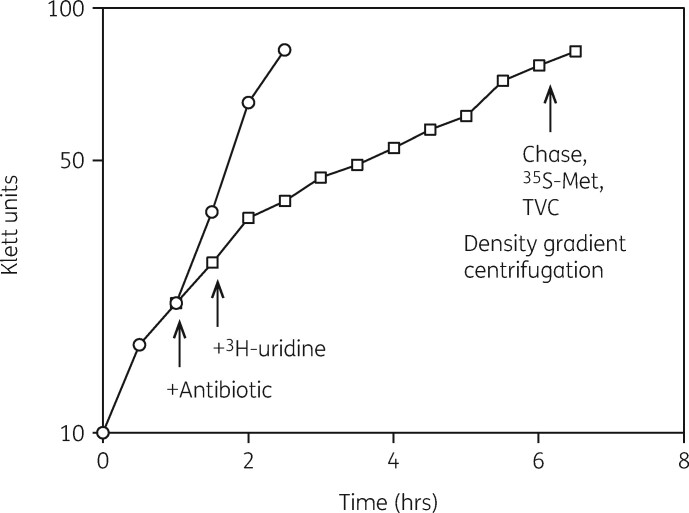
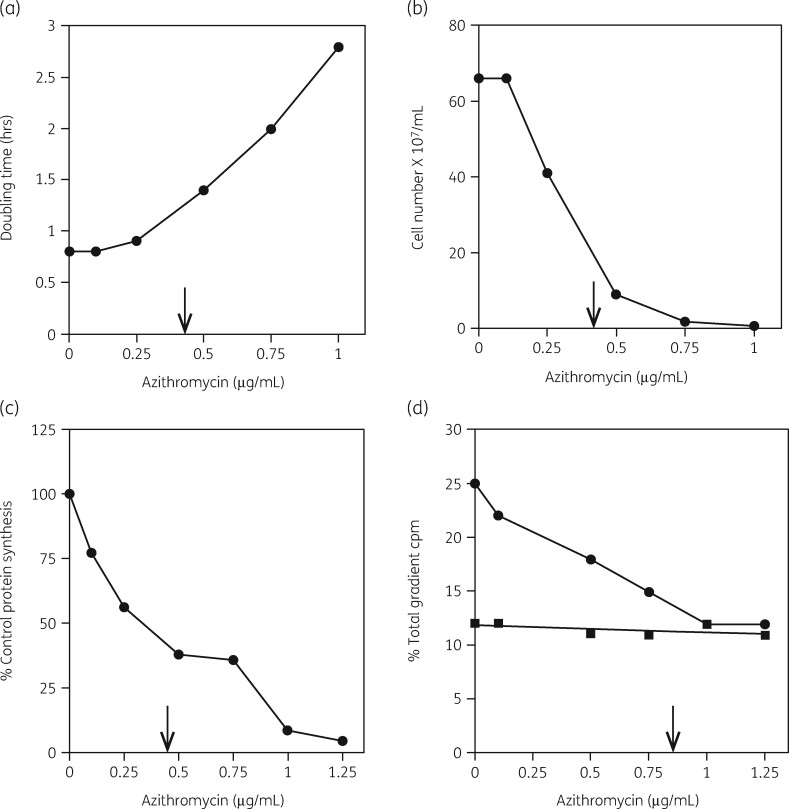
We then developed three different experimental approaches to examine features of the model in more detail.93 First, we applied a four-part assay that measured the inhibitory influence of the antibiotic on cellular growth rate, viability and protein synthesis. The amounts of 30S and 50S subunits were also measured as a function of antibiotic concentration. This protocol is illustrated in Figure 3. Figure 4 shows the equivalence of the IC50 values for azithromycin inhibition of the growth rate, viable cell numbers and protein synthesis rates in Haemophilus influenzae cells. The IC50 measured for the effect of azithromycin was 0.4 μg/mL (Table 1). The relative amounts of the two subunits in the cells were also determined. Figure 2 shows the inhibitory effect of azithromycin on 50S subunit levels. The control shows the expected 2:1 ratio of 23S to 16S rRNA in the two subunits (Figure 2a). Figure 2(b) shows the inhibitory effect of azithromycin on 50S subunit levels. Azithromycin resulted in the reduction of 50S subunits by 60% with little effect on 30S subunits levels. The additional 3H-labelled RNA in the top fractions of the gradient were 23S rRNA oligonucleotides generated by RNase activity.
Figure 3.
Representative four-part assay experiment. Cells growing with (open squares) and without (open circles) an antibiotic were labelled with [3H]-uridine and after two cell doublings the isotope was chased from the cells. [35S]-Methionine was added to measure protein synthesis rates and cell samples were diluted to measure total viable cell numbers (TVC). Growth rates were determined by the increase in cell density (Klett units). Cell lysates were centrifuged through sucrose density gradients and subunit amounts were measured. Reproduced with permission from Bentham Science Publishers Ltd from reference 91.
Figure 4.
Azithromycin inhibition of cell growth, cell numbers, protein synthesis and 30S and 50S subunit formation in H. influenzae cells. (a) Azithromycin inhibition of cell growth rate. (b) Azithromycin inhibition of viable cell number. (c) Azithromycin inhibition of protein synthesis rate. (d) Azithromycin inhibition of 50S subunit amounts (closed circles) and 30S subunit amounts (closed squares). IC50 values are indicated by arrows. Reproduced with permission from Bentham Science Publishers Ltd from reference 118.
Table 1.
IC50 values (mg/L) for antibiotic inhibition of protein synthesis and 50S subunit formation in different microorganisms
| Organism | Antibiotic | Protein synthesis | 50S synthesis | References |
|---|---|---|---|---|
| S. aureus | ||||
| erythromycin | 0.17 | 0.36 | 92 | |
| clarithromycin | 0.075 | 0.15 | 92 | |
| azithromycin | 2.5 | 5.0 | 92 | |
| telithromycin | 0.04 | 0.08 | 92 | |
| cethromycin | 0.022 | 0.035 | 92 | |
| solithromycin | 0.04 | 0.065 | 115 | |
| evernimicin | 0.03 | 0.4 | 92 | |
| TAN1057A | 4.5 | 9.0 | 92 | |
| linezolid | 0.3 | 0.6 | 92 | |
| retapamulin | 0.05 | 0.27 | 112 | |
| neomycin | 1.25 | 3.0 | 92 | |
| paromomycin | 3.0 | 2.75 | 92 | |
| S. pneumoniae | ||||
| telithromycin | 0.0075 | 0.015 | 114 | |
| cethromycin | 0.0025 | 0.005 | 113 | |
| solithromycin | 0.0075 | 0.015 | 115 | |
| H. influenzae | ||||
| erythromycin | 1.5 | 8.0 | 92 | |
| azithromycin | 0.4 | 0.8 | 92 | |
| clarithromycin | 5.6 | 9.0 | 92 | |
| fluorithromycin | 6.0 | 8.0 | 92 | |
| roxithromycin | 9.0 | 12.5 | 92 | |
| telithromycin | 1.25 | 2.5 | 92 | |
| cethromycin | 1.25 | 2.5 | 92 | |
| solithromycin | 0.125 | 0.23 | 115 | |
| E. coli | ||||
| hygromycin B | 16 | 65 | 133 | |
| neomycin | 3.6 | 7.2 | 92 | |
| paromomycin | 3.2 | 6.4 | 92 |
Table 2 shows the inhibitory effect of 18 different antibiotics on the number of viable cells and the relative protein synthesis rate in S. aureus. S. aureus was chosen as the test organism for these studies because of its importance as a human pathogen and the unique features of its binding association with 50S subunit antibiotics.99 For each antimicrobial tested, the amounts of 50S subunits were substantially reduced and the 30S levels remained essentially constant (Table 2). The difference with the three drugs shown in bold in Table 2 will be discussed subsequently.
Table 2.
Antibiotic effects on growth rates, cell viability, protein synthesis and 30S and 50S subunit formation in S. aureus cells
| Proportion of control (%) |
Proportion of total cpm (%) |
|||||
|---|---|---|---|---|---|---|
| Antibiotic | Concentration (mg/L) | cell number | protein synthesis | 30S | 50S | References |
| None | 0.0 | 100 | 100 | 19 | 38 | 91 |
| Erythromycin | 0.5 | 56 | 30 | 22 | 19 | 101 |
| Clarithromycin | 0.2 | 33 | 18 | 20 | 25 | 102 |
| Azithromycin | 0.5 | 80 | 79 | 17 | 33 | 101 |
| Telithromycin | 0.02 | 84 | 70 | 17 | 29 | 91 |
| Cethromycin | 0.02 | 96 | 48 | 17 | 20 | 91 |
| Tylosin | 0.5 | 64 | 85 | 21 | 25 | 105 |
| Spiramycin | 10 | 24 | 13 | 16 | 21 | 105 |
| Lincomycin | 0.2 | 64 | 73 | 19 | 21 | 105 |
| Clindamycin | 0.1 | 48 | 53 | 22 | 16 | 105 |
| Pristinamycin IA | 0.75 | 84 | 87 | 20 | 28 | 105 |
| Virginiamycin S | 10 | 48 | 40 | 21 | 33 | 105 |
| Virginiamycin M1 | 2.0 | 22 | 12 | 14 | 33 | 105 |
| CP36926 | 0.5 | 23 | 46 | 13 | 26 | 105 |
| Chloramphenicol | 20 | 19 | 14 | 13 | 26 | 105 |
| Evernimicin | 0.16 | 52 | 19 | 22 | 30 | 108 |
| TAN1057A | 6.0 | 72 | 42 | 18 | 21 | 109 |
| Linezolid | 0.5 | 52 | 23 | 19 | 21 | 111 |
| Retapamulin | 0.02 | 50 | 44 | 19 | 21 | 112 |
Values listed are from four-part assay experiments as described in the text. Growth rates, viable cell numbers, [35S]-methionine incorporation rates and [3H]-uridine incorporation into 30S and 50S subunits were measured in S. aureus cells growing with and without each antibiotic at the concentration listed. (Republished with permission from Bentham Science Publishers Ltd from reference 91).
A correlation was made that connected the inhibitory influence of the antimicrobial on the four parameters, based on their similar IC50 values. This protocol was used to examine 50S ribosomal subunit formation in four different microorganisms, two Gram-positive and two Gram-negative species, as Table 1 shows. By this protocol, an IC50 value for the inhibition of 50S formation and protein synthesis could be calculated. For most of these compounds, the values were the same, indicating that assembly inhibition and translational inhibition were equivalent targets. The apparent 2-fold difference between IC50 values is due to the fact that at the IC50 for assembly inhibition, the cells have only 50% of the subunits compared with control cells. At this concentration, the activity in protein synthesis by the remaining ribosomes is also inhibited by 50%, giving the apparent 2-fold difference as observed. Normal translation of mRNA by the remaining 50% of the 50S subunits at the IC50 should be possible with the excess of 30S subunits available. Even translation of polycistronic mRNAs containing sequences coding for both 30S and 50S ribosomal subunit proteins should still occur.
The four-part assay protocol was extended to include other important human microbial pathogens. MRSA is a serious cause of human disease. MRSA strains were examined by the same methodology.100 A specific inhibitory effect on 50S assembly was found in MRSA cells treated with three macrolides (erythromycin, azithromycin and clarithromycin), the ketolide solithromycin and the pleuromutilin compound retapamulin.
A series of structure–function experiments was also conducted to examine the relative inhibitory effects of nine structurally related macrolides on 50S synthesis.101 Six 11,12-carbonate macrolides were examined in a similar fashion.102 The results identified key structural differences in the macrolides related to their IC50 values. Two sets of similar assays were conducted with related ketolide antimicrobials and these results showed their superior inhibitory qualities compared with the effectiveness of the macrolides examined.103 , 104
Several members of the MLSB family were examined in a similar fashion.105 These included spiramycin and tylosin as representatives of the 16-membered-ring macrolides and the lincosamides, lincomycin and clindamycin. Streptogramin B compounds examined were pristinamycin IA, virginiamycin S and CP37277. Finally, two streptogramin A antibiotics were studied, virginiamycin M1 and CP36926. As Table 2 shows, all of the 16-membered-ring macrolides, the two lincosamides and the three streptogramin B compounds showed similar results. They all reduced cell viability and protein synthesis to some degree at the concentration used. Importantly, they all showed a specific inhibitory effect on 50S subunit biogenesis, with essentially no effect on 30S subunit formation. In contrast, the two streptogramin A antibiotics and chloramphenicol (as a control), showed a proportional reduction in the formation of both subunits, as expected for drugs inhibiting the peptidyltransferase activity of the 50S subunit.
Two publications from the laboratory of Tenson have suggested that ribosomal subunit assembly inhibition by erythromycin was only a consequence of the generalized inhibition of translation by this antibiotic.106 , 107 They compared it with the inhibition of translation by chloramphenicol, a known peptidyltransferase inhibitor and concluded that both drugs functioned in the same fashion.
We agree with their findings about chloramphenicol, which showed that the synthesis of both subunits was affected equally by translational inhibition, as expected for a peptidyltransferase inhibitor. In fact, we published results 9 years prior to their work, showing that chloramphenicol and also two streptogramin A antibiotics reduced the formation of both subunits to the same extent, as expected (Table 2 in bold). Surprisingly, our results were not referenced in either of their manuscripts.
The primary difference in their conclusion about erythromycin is largely explained by the drug concentration that they used (100 μg/mL). At this concentration, translational inhibition is the dominant effect of this drug and specific effects on 50S subunit formation would have been masked. Measurements of growth rates, viable cell numbers and protein synthesis rates would have revealed this effect. A comprehensive review article by Maguire examines this difference objectively.43
Four novel antimicrobials were also examined by this protocol. Evernimicin has a specific 50S subunit binding site in domain V of 23S rRNA. It was examined for inhibitory effects on translation and 50S formation in S. aureus.108 There was a dose-dependent reduction in both protein synthesis rates and 50S subunit biogenesis with IC50 values of 0.03 μg/mL and 0.4 μg/mL, respectively. 30S subunit formation was unaffected by the drug. This is an unusual finding and indicates that translation was more susceptible to evernimicin inhibition compared with 50S biogenesis. The synthesis rate of the large subunit was reduced by 7-fold at a concentration of 0.32 μg/mL. Specific reductions in 50S subunit formation were found in two erm(C) strains and in two MRSA strains.
TAN1057A is a natural product from a Flexibacter species. It also has a specific binding site on the large subunit.109 The rate of 50S subunit formation was reduced to 25% of the control rate at the IC50 (4 μg/mL) with no small subunit impairment. Translation and 50S subunit inhibition were affected equivalently. TAN1057A showed a post-antibiotic effect (PAE) with a significant lag in 50S resynthesis and recovery of full translational capacity.
Linezolid is an unusual antibiotic because it is a synthetic oxazolidinone molecule, not a natural product.110 It has a specific large-subunit binding site in domain V of 23S rRNA. It inhibited protein synthesis and 50S subunit assembly with an equivalent IC50 of 0.3 μg/mL.111 A pulse-and-chase assay showed a concentration-dependent inhibition of the rate of 50S synthesis, with no effect on 30S synthesis. It also revealed a specific PAE, with 2 h required for the resynthesis to 50S subunit amounts found in the control.
The fourth novel antimicrobial tested was a pleuromutilin compound, retapamulin. It shows specific 50S binding by an induced-fit mechanism.79 It was examined in both an MSSA strain and an MRSA strain.112 As expected, 50S formation was specifically reduced in both strains, with IC50 values of 27 ng/mL and 20 ng/mL, respectively. The rate of 50S synthesis was specifically reduced by 40% at 8 ng/mL. An examination of 23S rRNA turnover revealed a significant loss of 23S rRNA with 16S rRNA levels unaffected.
This assay protocol was extended to examine 50S subunit assembly in two other important human pathogens, Streptococcus pneumoniae and Streptococcus pyogenes. Three prescription ketolides were tested: telithromycin, cethromycin and solithromycin.113–115 All three antimicrobials were far superior to any macrolide tested, as Table 1 shows. Erythromycin-resistant strains of S. pneumoniae and S. pyogenes were also susceptible to the ketolides, indicating an important role for these drugs in antibiotic-resistant human infections.116 , 117
The Gram-negative pathogen H. influenzae was examined to test for 50S subunit inhibition in this important microorganism. As seen with the Gram-positive organisms tested, this microbe also showed specific 50S subunit assembly interference by five different macrolides with no effect on 30S assembly.118 , 119 In contrast, the ketolides telithromycin and cethromycin showed no target specificity, reducing 50S and 30S biogenesis to the same extent (Table 1).120 The reason remains to be determined.
It is significant that most of the inhibitors we examined bind to 23S rRNA in the PTC, using many of the same nucleotides for the association. Indeed, many of their binding sites overlap and affect translation in a similar fashion, targeting aminoacyl-tRNA, peptidyl-tRNA or the exit tunnel. The streptogramin A compounds and chloramphenicol, which inhibit the function of the peptidyltransferase directly, behaved differently. This predicts that many of these drugs could also bind to the 32S precursor particle, as erythromycin and azithromycin do (Figure 5).
Figure 5.
Sucrose gradient profiles of ribosomal subunits from cells grown with [3H]-uridine- and [14C]-labelled antibiotics. (a) Profile showing [3H]-uridine-labelled subunits (open squares) and [14C]-erythromycin (closed circles) bound to both 50S subunits and to a 32S precursor particle. (b) Profile showing [3H]-uridine-labelled subunits (open squares) and [14C]-azithromycin (closed circles) bound to both 50S subunits and to a 32S precursor particle. Reproduced with permission from Bentham Science Publishers Ltd from reference 92.
Kinetics of ribosomal subunit formation
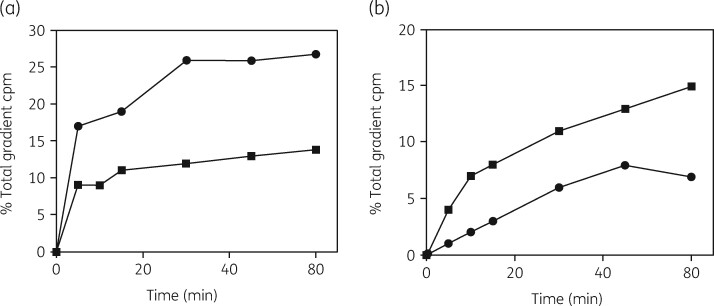
The second protocol developed was a pulse-and-chase labelling procedure for examining rRNA entering the subunit assembly pathway. This methodology was used to examine the kinetics of the assembly process in four different microorganisms. Figure 6 shows the results of azithromycin inhibition of 30S and 50S subunit biogenesis in H. influenzae cells.118 The uninhibited control showed the kinetics expected, with a 2-fold difference in the assembly time and the amounts of the two subunits (Figure 6a). The specific reduction in the rate of 50S subunit assembly by azithromycin is apparent (Figure 6b). The formation of the 30S subunit was affected to a lesser extent.
Figure 6.
Kinetics of subunit formation in H. influenzae cells growing with and without azithromycin. (a) Formation of 30S subunits (closed squares) and 50S subunits (closed circles) in the absence of an antibiotic. (b) Formation of 30S subunits (closed squares) and 50S subunits (closed circles) in cells growing with azithromycin at 0.3 μg/mL. Reproduced with permission from Bentham Science Publishers Ltd from reference 118.
Table 3 is a summary of the results obtained by examining five macrolides, three ketolides, linezolid, TAN1057A and the three aminoglycosides hygromycin B, neomycin and paromomycin. In most cases, the antibiotics reduced the rate of 50S subunit synthesis significantly but 30S formation was also affected to a lesser degree, reflecting the link between 30S and 50S biogenesis.
Table 3.
Relative rates of ribosomal subunit synthesis in antibiotic-inhibited cells
| Synthesis time (min) |
|||||
|---|---|---|---|---|---|
| Organism | Antibiotic | Concentration (mg/L) | 30S | 50S | References |
| S. aureus | |||||
| none | 0 | 10 | 20 | 92 | |
| solithromycin | 0.04 | 40 | 240 | 115 | |
| evernimicin | 0.32 | 10 | 240 | 92 | |
| TAN1057A | 5.0 | 10 | 120 | 29 | |
| linezolid | 0.5 | 20 | 60 | 92 | |
| neomycin | 3.0 | 120 | 240 | 92 | |
| paromomycin | 3.0 | 180 | 360 | 92 | |
| S. pneumoniae | |||||
| none | 0 | 60 | 120 | 113 | |
| cethromycin | 0.002 | 180 | 180 | 113 | |
| telithromycin | 0.008 | 90 | 180 | 114 | |
| solithromycin | 0.008 | 60 | 60 | 115 | |
| H. influenzae | |||||
| none | 0 | 15 | 30 | 92 | |
| erythromycin | 2.0 | 100 | 240 | 92 | |
| azithromycin | 0.3 | 60 | 200 | 92 | |
| clarithromycin | 5.0 | 30 | 60 | 92 | |
| fluorithromycin | 5.0 | 60 | 120 | 92 | |
| roxithromycin | 6.0 | 60 | 120 | 92 | |
| solithromycin | 0.125 | 45 | 200 | 115 | |
| E. coli | |||||
| none (37°C) | 0 | 7.5 | 15 | 25 | |
| hygromycin B | 50 | 60 | 120 | 94 | |
| erythromycin | 75 | 7.5 | 90 | 25 | |
| none (27°C) | 0 | 15 | 30 | 97 | |
| neomycin (27°C) | 4.0 | 30 | 75 | 97 | |
| paromomycin (27°C) | 4.0 | 30 | 30 | 97 | |
PAE
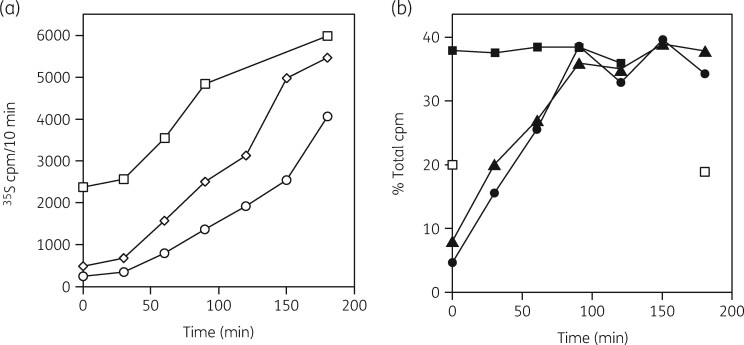
The PAE is the time needed to recover normal, uninhibited growth after antibiotic removal.121 , 122 We asked if resynthesis of the 50S subunit after antibiotic removal could explain the PAE for ribosomal antibiotics.123 In the third assay, the four parameters were measured after drug removal from growing cells. Figure 7 shows the 50S subunit synthesis rates in the control cells and in erythromycin- or clarithromycin-inhibited S. aureus cells after antibiotic removal. A significant lag in the recovery time was seen for the antibiotic-inhibited cells. Protein synthesis rates were significantly reduced and required about 3 h to achieve the control rate. Two hours were needed to achieve the control levels of 50S subunits. Importantly, 30S subunit levels were unchanged. An investigation of the PAE in S. aureus was also conducted with linezolid111 and TAN1057A109 with similar results. These results suggested that resynthesis of 50S subunits was a limiting factor in recovery from macrolide inhibition. This recovery time was dependent on the time for resumption of normal protein synthesis rates, as expected.
Figure 7.
(a) Relative rates of protein synthesis for untreated cells (open squares) and for erythromycin- (open diamonds) and clarithromycin (open circles)-treated cells following antibiotic removal. (b) Kinetics of 50S ribosomal subunit reformation. The percentages of the total gradient [3H]-uracil radioactivity in the 30S and 50S subunit regions of the sucrose gradients are shown for untreated cells (closed squares) and for cells recovering from erythromycin (closed triangles) and clarithromycin (closed circles) treatments. 30S subunit levels at 0 and 180 min are also shown (open squares). Reproduced from reference 123 (American Society for Microbiology, all rights reserved 1999).
Dual antibiotic interference with 50S assembly
We also tested the idea that antibiotic inhibition of 50S assembly could be enhanced by the inclusion of other antibiotics. Azithromycin inhibition of 50S assembly in S. aureus cells was tested alone and with the addition of rifampicin, an RNA polymerase inhibitor, and/or co-treatment with ciprofloxacin, a DNA replication inhibitor.124–126
The two antibiotics paired with azithromycin substantially reduced the rates of protein synthesis and 30S and 50S assembly compared with azithromycin alone (Table 4).127 The combinations all had a greater effect on 50S subunit formation and a lesser effect on 30S formation, as expected for inhibition by a 50S subunit-specific antibiotic. The relative amounts of 23S and 16S rRNA were also examined. The combinations resulted in major reductions in 23S rRNA levels compared with azithromycin alone, with lesser effects on 16S rRNA amounts. Dual-drug treatment also resulted in the enhanced accumulation of small RNA oligonucleotides derived from the degradation of 23S rRNA species, examined by northern hybridization tests. Dual-drug treatments should be effective in reducing the selection of antibiotic-resistant microorganisms.
Table 4.
Effect of antibiotic combinations on cell viability, protein synthesis and ribosomal subunit biogenesis in S. aureus cells
| Antibiotic | Cell viabilitya | Protein synthesisb | 30S subunit synthesisc | 50S subunit synthesisd |
|---|---|---|---|---|
| Control | 100 | 100 | 16.5 | 28.4 |
| AZM | 92 | 41.3 | 14.0 | 21.5 |
| RIF | 66 | 89.5 | 4.4 | 6.0 |
| CIP | 1.8 | 50.8 | 11.4 | 21.5 |
| AZM/RIF | 24 | 19.8 | 11.4 | 14.1 |
| RIF/CIP | 5.0 | 12.6 | 14.7 | 20.1 |
| AZM/CIP | 0.5 | 37.0 | 11.5 | 13.5 |
| AZM/RIF/CIP | 7.5 | 19.4 | 11.4 | 15.5 |
AZM, azithromycin; RIF, rifampicin; CIP, ciprofloxacin. Adapted from reference 127.
Percentage of control cfu/mL.
Percentage of control protein synthesis rate.
Percentage of total 30S.
Percentage of total 50S.
Erm methyltransferase target
An important mechanism for resistance to 50S subunit-binding antibiotics is induction of the gene for an erythromycin methyltransferase enzyme.128 , 129 The enzyme specifically adds one or two methyl groups to A2058 in domain V of the 23S rRNA.130 This modification results in resistance to all of the MLSB antibiotics we have described. Adenosine 2058 is an essential nucleotide for the binding of each of these inhibitors and methylation reduces the drug binding at least 50-fold. Gene expression is induced by the presence of erythromycin or other MLSB antibiotics but not by ketolides. Curiously, 23S rRNA is a good substrate for the enzyme in vitro but 50S subunits cannot be methylated. This finding suggested that the erythromycin-stalled 32S precursor particle might be the natural substrate for the methylating enzyme activity. Binding of [14C]-erythromycin and [14C]-azithromycin to 50S subunits and to a 32S precursor particle in E. coli cells was suggestive of this possibility (Figure 5).
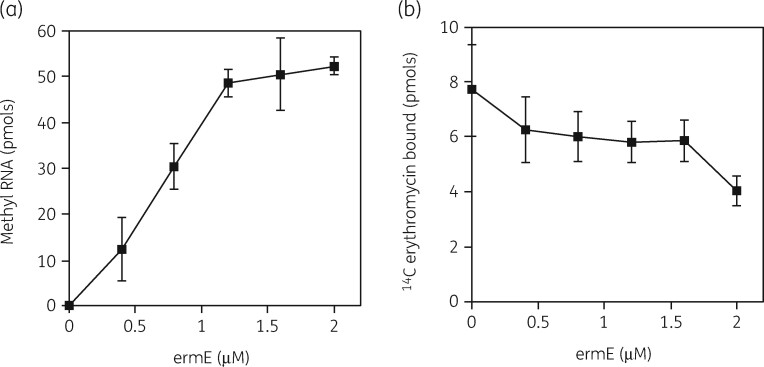
Our initial work was conducted in S. aureus cells containing an inducible erm(C) gene.131 Erm gene expression by erythromycin revealed that the reduction in 50S subunit formation was paralleled by an increased binding of [14C]-erythromycin. An S1 nuclease protection assay showed that after erm(C) induction, protection of a 23S rRNA sequence could be detected with RNA from both 50S and 30S subunit regions of sucrose gradients, indicating the presence of 23S rRNA in the 32S precursor particle. Methylation of these RNAs by an Erm methyltransferase enzyme assay was also demonstrated. In addition, this assay showed that 50S subunits from uninduced cells could not be methylated but that 23S rRNA sequences from the 32S precursor particle were a substrate and induction stimulated this activity 2.5-fold. These results suggested that the natural substrate for the enzyme was the stalled 32S precursor particle generated by erythromycin binding.
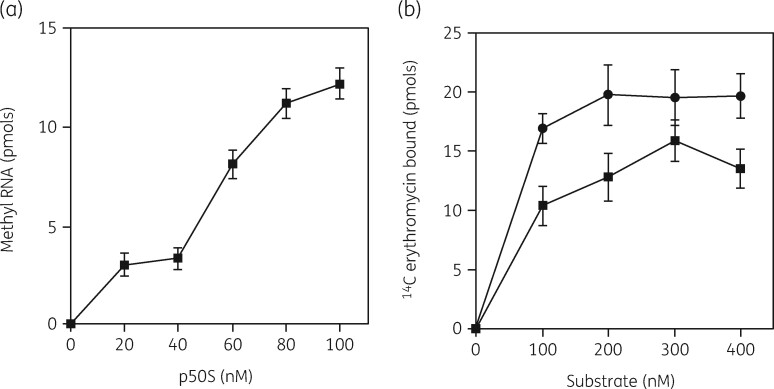
Subsequent studies were conducted with a purified ErmE methyltransferase.132 The enzyme showed a K M of 60 nM for methylation of the precursor 23S rRNA (Figure 8a). 14C-labelled erythromycin binding to the precursor and to 50S subunits showed similar saturation curves with K D values of 77 and 55 nM (Figure 8b). This was strong evidence that inhibition of 50S assembly and translation were equivalent targets, the same conclusion reached from prior studies in vivo.97
Figure 8.
(a) Substrate saturation curves for ErmE methyltransferase activity with p50S (closed squares). (b) Binding of [14C]-erythromycin to 50S subunits (closed circles) or to the 50S precursor (p) particle (closed squares). Bars indicate SEM with a mean of three experiments. Reprinted with permission from Taylor and Francis from reference 132.
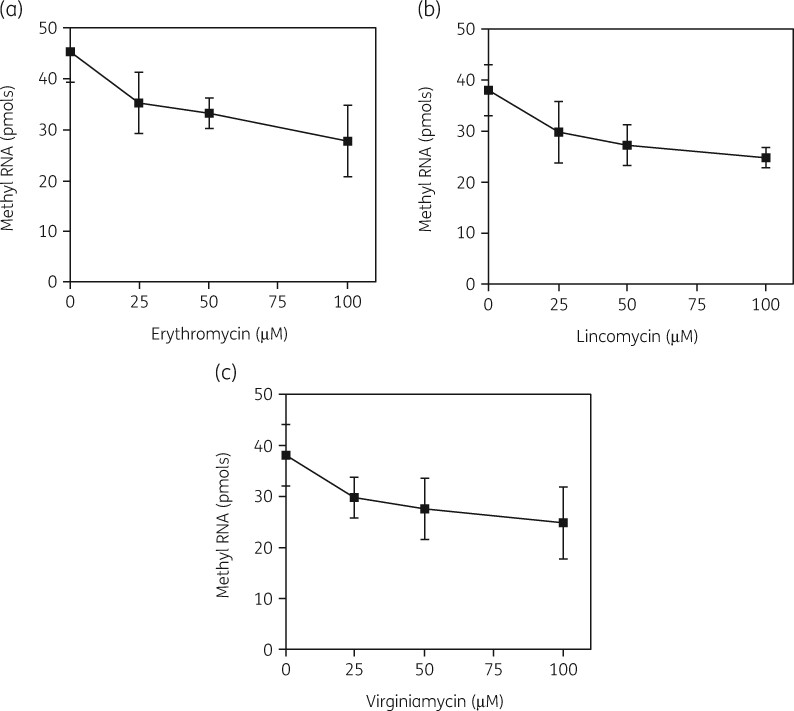
The model also predicted competition between erythromycin binding to the precursor and binding of the enzyme. As Figures 9(a) and (b) show, the displacement of bound erythromycin by increasing amounts of the Erm enzyme was found, with a K D value for the enzyme of 1 μM and a K i for erythromycin of 2 μM. Conversely, substrate methylation by the enzyme was reduced with increasing amounts of the drug (Figure 10a). In support of the competition model, both lincomycin and virginiamycin S also reduced the enzyme activity, as predicted for these drugs, which have the same 23S rRNA binding site (Figure 10b and c). The K i values for these three antibiotics were 99, 132 and 108 μM respectively.
Figure 9.
Competition between the ErmE methyltransferase and erythromycin for the 50S subunit precursor. (a) [3H]-methylation of the 50S precursor particle by increasing ErmE methyltransferase in the presence of erythromycin at 100 μM. (b) Competition between ErmE methyltransferase and [14C]-erythromycin. Loss of erythromycin was measured by a filter assay. Bars represent SEM. Reproduced with permission from Taylor and Francis from reference 132.
Figure 10.
Concentration-dependent inhibition of ErmE methyltransferase activity on the precursor particle by MLSB antibiotics. (a) Inhibition of [3H]-methyl RNA formation by erythromycin. (b) Inhibition of [3H]-methyl RNA formation by lincomycin. (c) Inhibition of [3H]-methyl RNA formation by virginiamycin S. Bars indicate SEM. Reproduced with permission from Taylor and Francis from reference 132.
The identity of the 50S ribosomal proteins in the stalled 32S precursor particle was investigated. Genome searching identified putative genes for S. aureus ribosomal proteins. The search predicted 21 small-subunit proteins and 36 large-subunit proteins. The protein identity was confirmed by both MALDI-TOF MS analysis and by 2D gel electrophoresis. These methods were used to identify the ribosomal protein content of the 32S precursor. Comparison with the known ribosomal protein composition in E. coli cells revealed a close identity, with 19 of 23 E. coli protein analogues identified.28 , 29 This was the first identification of the 30S and 50S ribosomal protein composition of S. aureus cells. The results clearly showed that the stalled 50S subunit precursor particle was the natural substrate for the methyltransferase enzyme activity and that this was generated by erythromycin stalling of 50S subunit biogenesis.
Inhibitors of 30S ribosomal subunit formation
Our model predicted that 30S subunit formation should be a target for antimicrobials that bind to 16S rRNA. Aminoglycoside compounds target the 30S subunit and impair the translational functions of the particle.80 , 81 Hygromycin B is an unusual aminoglycoside that contains four amino sugar rings instead of the three rings found in most other aminoglycosides.82 , 83 This compound was examined for effects on protein synthesis and ribosomal subunit assembly in E. coli.133 The IC50 value for protein synthesis inhibition was 16 μg/mL (Table 2). Ribosomal subunit assembly was impaired, with both subunits affected, but with a greater effect on the 30S subunit, with an IC50 of 65 μg/mL. In pulse-and-chase labelling studies, the assembly of both subunits was inhibited 8-fold compared with the control (Table 3). The status of 23S and 16S rRNAs was examined by a gel-on-a-chip procedure. 16S rRNA amounts were reduced by half at 100 μg/mL and the presence of a larger precursor species was found. RNase fragmentation of 16S rRNA resulted in the appearance of small 16S rRNA oligonucleotides, as assayed by northern blot analysis. 23S rRNA levels were reduced less. Binding of this antimicrobial agent to the 30S subunit reduced the levels of this subunit substantially but also caused some impairment of 50S formation.
The effect of this antibiotic on 50S subunit synthesis can now be understood by the report that aminoglycosides have a distinct binding site on helix 69 of 23S rRNA in the 50S subunit.87 The binding affinities of these drugs may well be different between the two subunits. The possible effect of aminoglycosides on 50S subunit assembly was not investigated further.
We also examined the two related aminoglycosides, neomycin and paromomycin, in both E. coli and S. aureus. These compounds differ only by the presence of an amino group on the C′6 position in neomycin or by a hydroxyl group in paromomycin. Both antibiotics have been shown to bind to 16S rRNA in the 30S subunit, thereby stimulating mistranslation of mRNA.85 , 86 In E. coli, paromomycin and neomycin inhibited translation, with IC50 values of 3.2 and 3.6 μg/mL (Table 2).134 Pulse-and-chase labelling assays revealed a 50% reduction of 30S subunits in cells treated with either antibiotic, with a smaller effect on the rates of 50S formation (Table 3). There was a concentration-dependent decline in the amounts of both subunits with either antibiotic. The presence of a 21S precursor particle increased with increasing drug treatment. This work suggested that 30S subunit formation was impaired, generating the observed 21S precursor. It is also significant that 50S subunit formation was also reduced but to a lesser degree.
Similar results were obtained when these two compounds were examined in S. aureus cells.135 The IC50 values for the inhibitory effects of both drugs on growth rate, cell viability, protein synthesis rates and 30S subunit amounts were comparable. The only significant difference was the more substantial reduction in the rates of subunit formation in S. aureus cells, which were only 20% of control rates compared with 50% of the controls at the same antibiotic concentrations in E. coli (Table 3).
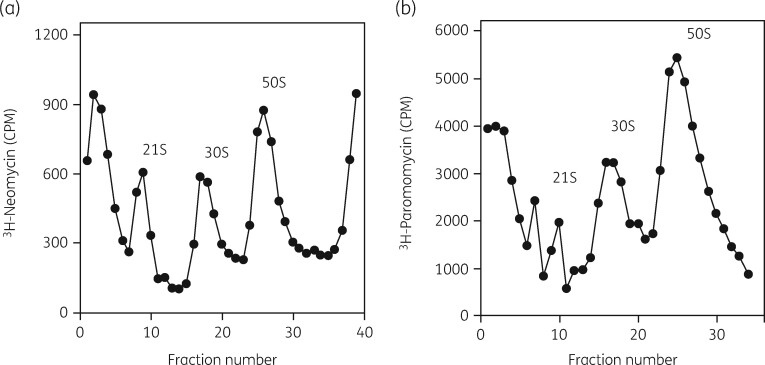
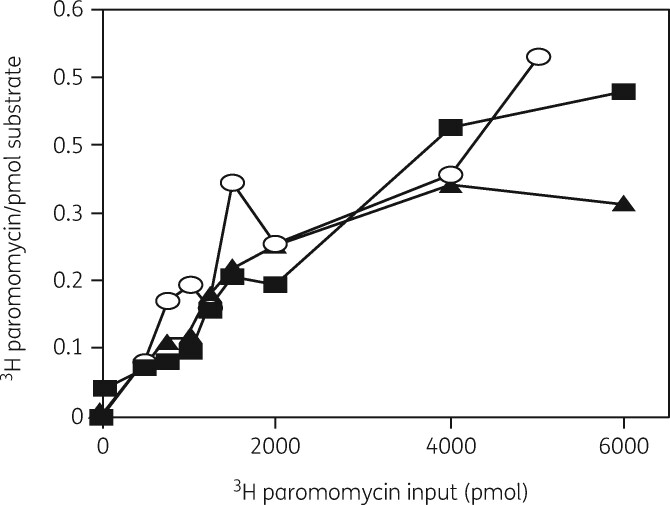
The interactions of these two drugs with the 30S subunit were investigated in more detail.136 Cells grown in the presence of either [3H]-neomycin or [3H]-paromomycin showed binding of the antibiotics to a 21S precursor particle and to 30S and 50S subunits, as expected (Figure 11). The isolated subunits and the precursor all showed equivalent binding of [3H]-paromomycin, with similar affinities, showing a K D of 40 μM and a binding stoichiometry of 0.5 moles/mole of particle (Figure 12). As with erythromycin binding to the 50S precursor, this was evidence that translation and 30S subunit assembly were equivalent targets.
Figure 11.
Aminoglycoside binding to a 30S subunit assembly intermediate. (a) Sucrose gradient profile of a lysate from cells grown in the presence of [3H]-neomycin. (b) Sucrose gradient profile of a lysate from cells grown in the presence of [3H]-paromomycin. Reproduced with permission from Springer Nature from reference 136.
Figure 12.
Binding of [3H]-paromomycin to 30S and 50S subunits and the 21S precursor particle. A filter-binding assay with increasing amounts of [3H]-paromomycin was used to measure the binding interaction. [3H]-paromomycin binding to 30S subunits (filled squares), 50S subunits (filled triangles) and to the 21S precursor (open circles). Reproduced with permission from Springer Nature, reference 136.
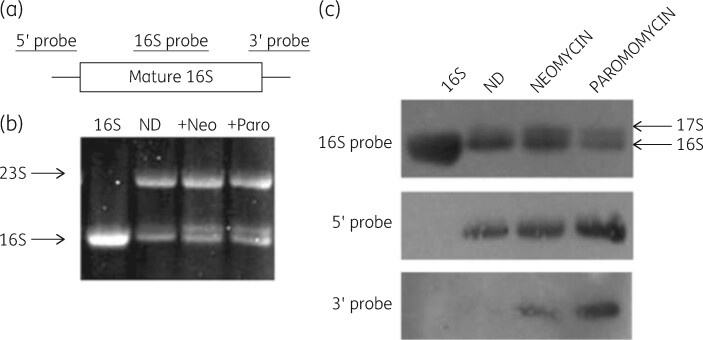
Hybridization with a DNA probe for 16S rRNA revealed binding to 30S subunit rRNA and to rRNA sequences from the 21S precursor region of sucrose gradients. This analysis indicated the presence of a significant 17S precursor RNA molecule in antibiotic-treated cells (Figure 13) and this molecule had both 5′ and 3′ extensions, characteristics of the natural precursor to 16S rRNA (Figure 13). The ribosomal protein content of the 21S precursor particle was determined. Gel electrophoresis analysis revealed 12 of the 21 small-subunit proteins in the precursor. This protein content compared well with the nine small-subunit proteins found in the natural 21S precursor particle.137 The reconstitution of 30S subunits from isolated 16S rRNA and total proteins was tested to examine aminoglycoside impairment of the assembly process.138 Reconstitution of complete 30S subunits was accomplished in the absence of the drugs and a 21S precursor particle was generated by incubation of the mixture at 4°C, as expected (Figure 14). Both aminoglycosides inhibited total reconstitution and generated the expected 21S intermediate particle. This was the first demonstration that the aminoglycoside inhibition of 30S assembly in vivo could be duplicated in vitro and significantly strengthened the support for our model.
Figure 13.
Identification of precursor forms of 16S rRNA in antibiotic-treated cells. (a) Location of the hybridization probes used. (b) Gel pattern of rRNA from control and drug-treated cells. (c) Northern blot of rRNA from antibiotic-treated cells. Top: hybridization with internal 16S rRNA-specific probe. Middle: hybridization with a 5′ precursor sequence-specific probe. Bottom: hybridization with a 3′ precursor sequence-specific probe. ND, control. Reproduced with permission from Springer Nature, reference 136.
Figure 14.
Inhibition of 30S assembly in vitro by aminoglycosides. Sucrose gradient sedimentation profiles of subunits reconstituted with [3H]-uridine-labelled 16S rRNA. (a) Reconstitution of 30S subunits (filled circles) by incubation at 42°C and reconstitution of an RI particle (open squares) by incubation at 4°C. (b) Reconstitution in the presence of neomycin at 0.4 μg/mL. (c) Reconstitution in the presence of paromomycin at 0.4 μg/mL. Reproduced with permission from Springer Nature, reference 136.
RNase as a target for assembly inhibition
Our model predicts that RNases responsible for precursor RNA maturation and RNA turnover should be additional drug targets.92 The 17S precursor to 16S rRNA is processed by RNases III, E, G and PH. The 25S precursor to 23S rRNA is also processed by RNases III, G, PH and T (Figure 15).139–141 This prediction was examined by the analysis of strains of E. coli containing mutated forms of these genes. Our initial study was conducted in an RNase E mutant.142 Erythromycin slowed the rate of subunit synthesis of WT cells by 50% but reduced this rate to 30% in the mutant strain. 30S subunit formation was unaffected. [14C]-Erythromycin binding to both 50S subunits and to the 32S precursor particle was demonstrated both in the cells and in vitro, with a similar binding stoichiometry. The mutant cells showed a greater amount of 23S rRNA in the 32S precursor particle relative to WT cells by hybridization analysis. Accumulation of 23S rRNA oligonucleotides was seen in both strains.
Figure 15.
Bacterial rRNA processing pathways. The major rRNA processing enzymes are indicated. The binding of both aminoglycosides to 16S and 23S rRNA is also shown. Modified with permission from John Wiley and Sons from reference 141.
This study was expanded to examine azithromycin inhibition of 50S subunit formation in a WT strain and in several mutants.143 Cells with defects in RNases I, II, III, E and PNPase and others with multiple RNase mutations were examined. Azithromycin treatment reduced the viable cell numbers and protein synthesis rates in all mutants tested relative to WT cells. Analysis of 50S particle formation showed normal amounts of both subunits in all strains grown in the absence of azithromycin. Antibiotic treatment reduced 50S subunit amounts to a greater extent in the mutants relative to the control, with the loss of 50S subunits by 55% to 65% in some mutant cells. The most affected were an RNase II strain and a triple mutant with defects in RNase I, II and PNPase.
The distribution and relative amounts of 16S and 23S rRNA were examined by northern blot analysis with sequence-specific DNA probes. Each strain grown with azithromycin showed the accumulation of RNA oligonucleotides in the top gradient regions, indicative of RNA degradation by RNase. 23S rRNA was found in the 32S precursor region in all of the treated cells, with substantially more in the mutant strains. In a PAE experiment, the mutations also affected the rates of recovery after azithromycin removal. WT cells and mutants in RNase I, E and II and PNPase recovered in 2 h. The rates of recovery in mutants with mutations in RNases II, III, E and PNPase were significantly longer, requiring between 2 and 4 h to reach WT levels. 30S subunit levels remained constant throughout the recovery period. These results suggested an important role for several RNAses in 50S biogenesis, which could be impaired by gene mutations.
We also tested the effects of aminoglycoside antibiotics for assembly defects in these mutants.144 Neomycin and paromomycin were tested for enhanced inhibitory effects in the same set of RNase mutants examined previously. Growth with either aminoglycoside reduced the growth rate and cell viability of each mutant strain. Mutants affected in RNases III, E, G and PH were the most susceptible and were examined further. The relative amounts of both subunits were reduced after drug treatment, with 50S levels reduced by 10% to 60% of controls and 30S reduced by 35% and 75% of the control amounts.
An RNase III strain and one with mutations in RNases I, II, PNPase and PH were affected the most. Antibiotic treatment reduced the amounts of 16S and 23S rRNA significantly. Gel electrophoresis was used to examine the RNA species status in each strain. A small amount of the 17S precursor to the 16S rRNA was found in WT cells and the amount was greatly increased in the mutants by 10- to 30-fold. A 23S rRNA precursor molecule was not detected. Northern blot hybridization analysis with 16S-specific DNA probes revealed extensive 16S rRNA fragmentation in each strain grown with either antibiotic (Figure 16). Mutants defective in RNases III, E and PH showed the largest extent of 16S rRNA fragmentation. These mutant strains also showed a similar extent of 23S rRNA fragmentation. These results extended our previous observation of enhanced inhibition of 50S synthesis and RNA degradation by azithromycin in these same RNase mutants.143
Figure 16.
Northern blot analysis of 16S rRNA fragmentation for WT and RNase mutant E. coli cells. RNA was isolated from cells grown without and with antibiotics. RNA sequences were identified by hybridization with a 16S DNA probe. C, control; N, neomycin; P, paromomycin. Oligonucleotide sizes are shown. Reproduced with permission from Springer Nature from reference 144.
The previous results with the RNase mutants suggested that RNase activity could be a target for novel drugs. In a review by Stokes and Brown,45 the possibility that small chemical modulators of ribosome biogenesis could be exploited as subunit assembly inhibitors was described. Inhibiting the RNases needed for processing the 16S and 23S precursor molecules should affect subunit formation.145 The compound vanadyl ribonucleoside complex (VRC) has been shown to be a generalized inhibitor of RNase activity.146 It has been used to enhance the stability of RNA species during isolation from cells.
VRC effects were examined in S. aureus. 147 The RNase inhibitor reduced the amounts of both subunits when used alone and enhanced this reduction when used in combination with azithromycin or paromomycin. The inhibitor reduced cell viability in both MSSA and MRSA strains when used alone or in combination. The amounts of 50S subunits were reduced by 33% to 50% in the VRC-treated cells. The formation of 30S subunits was less affected (Table 5). Significantly, protein synthesis rates were unaffected in cells growing with VRC, suggesting that the inhibitory effect was restricted to rRNA processing. In these cells, VRC reduced the rate of synthesis of both subunits to the same extent, with a 50% reduction at 5 mM VRC. RNA analysis revealed reductions in the amounts of both 16S and 23S rRNAs, with 23S rRNA affected to a greater extent. Both antibiotics enhanced the loss of intact RNA. Fragmentation of the RNA species was observed by northern hybridization analysis with increases in oligonucleotides derived from both RNAs.
Table 5.
Comparison of VRC effects on S. aureus and E. coli cells
| Organism | Cell viabilitya | 30S subunitsb | 50S subunitsb | Protein synthesisc | 30S synthesisd | 50S synthesisd |
|---|---|---|---|---|---|---|
| E. coli control | 100 | 23.8 | 41.2 | 100 | 60 | 60 |
| E. coli + VRC | 86.7 | 17.8 | 22.7 | 150 | 240 | 240 |
| S. aureus (MSSA) | 100 | 21.7 | 41.6 | 100 | 60 | 60 |
| S. aureus (MSSA) + VRC | 5.3 | 14.6 | 24.5 | 116 | 90 | 120 |
| S. aureus (MRSA) | 100 | 17.5 | 47.9 | 100 | 60 | 60 |
| S. aureus (MRSA) + VRC | 1.6 | 17.0 | 33.0 | 200 | 120 | 120 |
Percentage reduction in cfu for E. coli or S. aureus cells treated with 5 mM VRC.
Percentage of total 30S and 50S subunit amounts.
Relative protein synthesis rates for control and VRC-treated cells.
Subunit synthesis times (min) for control and VRC-treated cells.
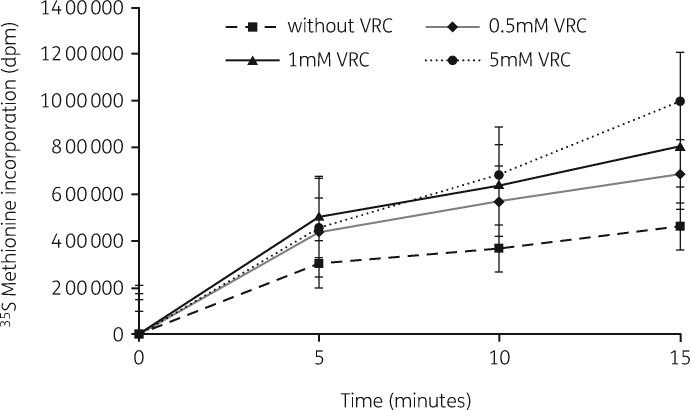
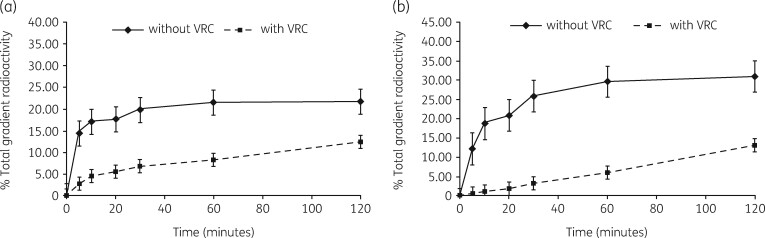
We also examined this molecule to see if it could stimulate the antibiotic inhibition of subunit assembly in E. coli cells.148 Protein synthesis rates were unaffected, similar to the finding in S. aureus (Figure 17). Decreases in both subunit amounts and assembly times were observed and the reduction was enhanced in cells grown with both VRC and either antibiotic (Figure 18). In every case, VRC treatment resulted in an increase in small RNA species in cells grown with both azithromycin and VRC (Figure 19). A comparison of VRC effects in the two organisms is shown in Table 5. This compound unlinks translational inhibition from subunit formation and suggests that both processes are targets for antibiotic effects. A search for other RNase inhibitors could be fruitful.
Figure 17.
Protein synthesis rates in E. coli cells growing without VRC (filled squares) or with VRC at 0.5 mM (closed diamond), 1 mM (closed triangle) or 5 mM (closed circle) measured by [35S]-methionine incorporation into proteins. Reproduced with permission from Springer Nature from reference 148.
Figure 18.
Ribosomal subunit synthesis rates in E. coli measured by [3H]-uridine pulse-and-chase labelling assays. (a) Rates of formation of 30S subunits in cells growing with (filled squares) or without (filled diamonds) 5 mM VRC. (b) Rates of formation of 50S subunits in cells growing with (filled squares) or without (filled diamonds) 5 mM VRC. Reproduced with permission from Springer Nature from reference 148.
Figure 19.
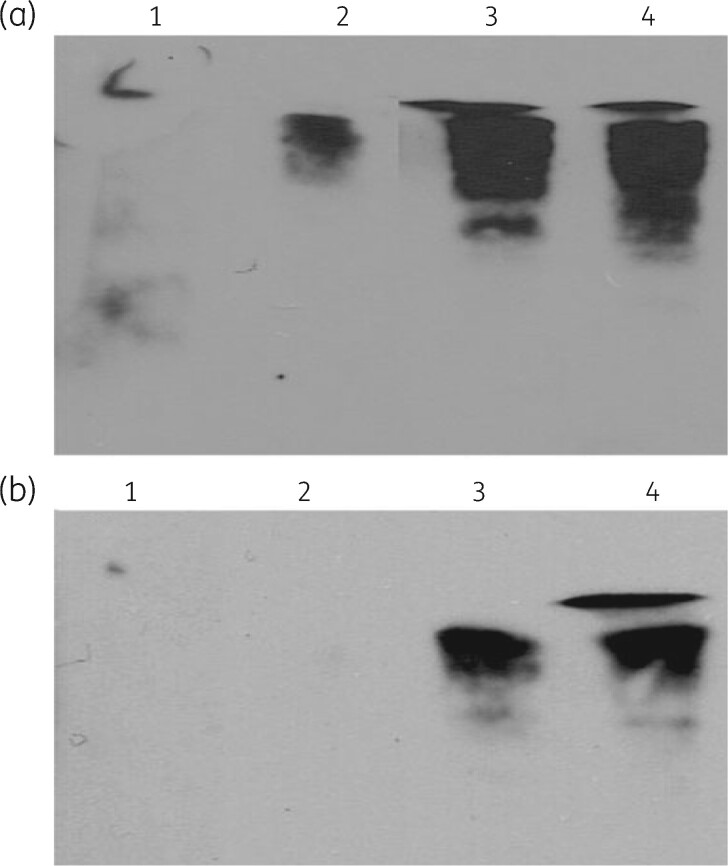
Northern hybridization analysis of rRNA fragmentation. RNA was isolated from cells grown in the presence of 10 μg/mL antibiotics and/or 5 mM VRC. The top of the gel containing the intact 16S and 23S rRNA was removed in order to allow probe hybridization to only rRNA fragments. (a) 16S rRNA hybridization. The samples are: Lane 1, RNA from control cells; Lane 2 RNA from cells grown with 5 mM VRC; Lane 3, RNA from cells grown with paromomycin and VRC; Lane 4, RNA from cells grown with azithromycin and VRC. (b) 23S rRNA hybridization, lanes as in (a). Reproduced with permission from Springer Nature from reference 148.
Summary
All of these experiments were conducted to test various aspects of a model describing the inhibitory effects of many different antimicrobial agents on bacterial ribosomal subunit biogenesis. All of the predictions of the model were fulfilled. The important findings follow.
All of the compounds investigated reduced the synthesis rates of one or both subunits in six microorganisms, four Gram-positive and two Gram-negative species.
Increasing concentrations of each drug examined reduced the amounts of one or both subunits specifically. Cellular growth rates, cell viability and protein synthesis rates were reduced proportionally.
Translation and subunit formation were equivalent targets for most drugs examined.
Antibiotic binding to both subunit precursors (21S and 32S) was demonstrated both in cells and in vitro.
An examination of the PAE with four different antibiotics revealed that the time required for 50S subunit resynthesis was a limiting factor in recovery.
The substrate for the Erm methyltransferase was identified and characterized as a 50S subunit precursor particle.
Aminoglycoside inhibition of 30S subunit formation was demonstrated both in cells and with in vitro reconstitution assays.
Cellular RNase activity was an inhibitory target. Inhibition of these enzyme functions by small molecules such as VRC occurred without an effect on translation.
Acknowledgements
I am very grateful to the nine graduate students and seven research technicians who generated the data and results in this work over a 20 year period. I also appreciate financial support from Abbott Laboratories, Aventis Pharma, Cempra Pharmaceuticals, GSK, Gilead Sciences, Pfizer Pharmaceuticals, Schering-Plough and the NIH.
Transparency declarations
None to declare.
References
- 1.Chaudhary AS. A review of global initiatives to fight antibiotic resistance and recent antibiotics’ discovery. Acta Pharm Sin B 2016; 6: 552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theuretzbacher U. Antibiotic innovation for future public health needs. Clin Microbiol Infect 2017; 23: 713–7. [DOI] [PubMed] [Google Scholar]
- 3.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 2010; 74: 417–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felden B, Cattoir V. Bacterial adaptation to antibiotics through regulatory RNAs. Antimicrob Agents Chemother 2018; 62: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strachan CR, Davies J. The whys and wherefores of antibiotic resistance. Cold Spring Harbor Perspect Med 2017; 7: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giedraitien A, Vitkauskien A, Naginien R et al. Resistance mechanisms of clinically important bacteria. Medicina (Kaunas) 2011; 47: 137–46. [PubMed] [Google Scholar]
- 7.Frieri M, Kumar K, Boutin A. Antibiotic resistance. J Infect Public Health 2017; 10: 369–78. [DOI] [PubMed] [Google Scholar]
- 8.Xia J, Gao J, Tang W. Nosocomial infection and its molecular mechanisms of antibiotic resistance. BioSci Trends 2016; 10: 14–21. [DOI] [PubMed] [Google Scholar]
- 9.Arenz S, Wilson DN. Blast from the past: reassessing forgotten translation inhibitors, antibiotic selectivity, and resistance mechanisms to aid drug development. Mol Cell 2016; 61: 3–14. [DOI] [PubMed] [Google Scholar]
- 10.Dinos PG. The macrolide antibiotic renaissance. Br J Pharmacol 2017; 174: 2967–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matzov D, Bashan A, Yonath A. A bright future for antibiotics? Annu Rev Biochem 2017; 86: 567–83. [DOI] [PubMed] [Google Scholar]
- 12.Oldfield E, Feng X. Resistance-resistant antibiotics. Trends Pharmacol Sci 2014; 35: 664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolain JM, Abat C, Jimeno M-T et al. Do we need new antibiotics? Clin Microbiol Infect 2016; 22: 408–15. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes P, Martens E. Antibiotics in late clinical development. Biochem Pharmacol 2017; 133: 152–63. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard SC. A much-needed boost for the dwindling antibiotic pipeline. Mol Cell 2018; 70: 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemett D, Markham A. Linezolid. Drugs 2000; 59: 815–27. [DOI] [PubMed] [Google Scholar]
- 17.Champney WS. New Antibiotic Targets. Humana Press, 2008. [Google Scholar]
- 18.Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol 2010; 8: 423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver LL. Appropriate targets for antibacterial drugs. Cold Spring Harbor Perspect Med 2016; 6: a030239.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alanjary M, Kronmiller B, Adamek M et al. The Antibiotic Resistant Target Seeker (ARTS), an exploration engine for antibiotic cluster prioritization and novel drug target discovery. Nucl Acids Res 2017; 45: 42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monserrat-Martinez AM, Gambin Y, Sierecki E. Thinking outside the bug: molecular targets and strategies to overcome antibiotic resistance. Int J Mol Sci 2019; 20: 1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gajdács M. The concept of an ideal antibiotic: implications for drug design. Molecules 2019; 24: 892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wecke T, Mascher T. Antibiotic research in the age of omics: from expression profiles to interspecies communication. J Antimicrob Chemother 2011; 66: 2689–704. [DOI] [PubMed] [Google Scholar]
- 24.Fields FR, Lee SW, McConnell MJ. Using bacterial genomes and essential genes for the development of new antibiotics. Biochem Pharmacol 2017; 134: 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staker BL, Buchko GW, Myler PJ. Recent contributions of structure-based drug design to the development of antibacterial compounds. Curr Opin Microbiol 2015; 27: 133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neville N, Ji Z. Approaches to the structure-based design of antivirulence drugs: therapeutics for the post-antibiotic era. Molecules 2019; 24: 378–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez D. Inhibitors of Protein Biosynthesis. Springer, 1979. [DOI] [PubMed] [Google Scholar]
- 28.Kaczanowska M, Rydén-Aulin M. Ribosome biogenesis and the translation process in Escherichia coli. Microbiol Mol Biol Rev 2007; 71: 477–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nomura M. Regulation of ribosome biosynthesis in Escherichia coli and Saccharomyces cerevisiae: diversity and common principles. J Bacteriol 1999; 181: 6857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shajani Z, Sykes MT, Williamson JR. Assembly of bacterial ribosomes. Annu Rev Biochem 2011; 80: 501–26. [DOI] [PubMed] [Google Scholar]
- 31.Ginsburg D, Steitz JA. The 30S ribosomal precursor RNA from Escherichia coli. A primary transcript containing 23S, 16S and 5S sequences. J Biol Chem 1975; 250: 5647–54. [PubMed] [Google Scholar]
- 32.Davis JH, Williamson JR. Structure and dynamics of bacterial ribosome biogenesis. Philos Trans R Soc B 2017; 372: 20160181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandt F, Etchells SA, Oritz JO. The native 3D organization of bacterial polysomes. Cell 2009; 136: 261–71. [DOI] [PubMed] [Google Scholar]
- 34.Karbstein K. Chaperoning ribosome assembly. J Cell Biol 2010; 189: 11–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Britton RA. Role of GTPases in bacterial ribosome assembly. Annu Rev Microbiol 2009; 63: 155–76. [DOI] [PubMed] [Google Scholar]
- 36.Deutscher MP. How bacterial cells keep ribonucleases under control. FEMS Microbiol Rev 2015; 39: 350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCoy LS, Xie Y, Tor Y. Antibiotics that target protein synthesis. Wiley Interdiscip Rev RNA 2011; 2: 209–32. [DOI] [PubMed] [Google Scholar]
- 38.Wilson DN. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat Rev Microbiol 2014; 12: 35–48. [DOI] [PubMed] [Google Scholar]
- 39.Greulich P, Dolezal J, Scott M et al. Predicting the dynamics of bacterial growth inhibition by ribosome-targeting antibiotics. Phys Biol 2017; 14: 065005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgs PG, Lehman N. The RNA world: molecular cooperation at the origins of life. Nat Rev Genet 2015; 16: 7–17. [DOI] [PubMed] [Google Scholar]
- 41.Pressman A, Blanco C, Chen IA. The RNA world as a model system to study the origin of life. Curr Biol 2015; 25: R953–63. [DOI] [PubMed] [Google Scholar]
- 42.Robertson MP, Joyce GF. The origins of the RNA world. Cold Spring Harb Perspect Biol 2012; 4: a003608.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maguire BA. Inhibition of bacterial ribosome assembly: a suitable drug target? Microbiol Mol Biol Rev 2009; 73: 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanchard SC, Cooperman B, Wilson DM. Probing translation using small molecule inhibitors. Chem Biol 2010; 17: 633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stokes JM, Brown ED. Chemical modulators of ribosome biogenesis as biological probes. Nat Chem Biol 2015; 11: 924–32. [DOI] [PubMed] [Google Scholar]
- 46.Stokes JM, Davis JH, Mangat CS et al. Discovery of a small molecule that inhibits bacterial ribosome biogenesis. Elife 2014; 3: e03574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thakrala D, Tae HS. Discovery of a structurally unique small molecule that inhibits protein synthesis. Yale J Biol Med 2017; 90: 35–43. [PMC free article] [PubMed] [Google Scholar]
- 48.Noeske J, Wasserman MR, Terry DS et al. High-resolution structure of the Escherichia coli ribosome. Nat Struct Mol Biol 2015; 22: 336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Razi A, Britton RA, Ortega J. The impact of recent improvements in cryo-electron microscopy technology on the understanding of bacterial ribosome assembly. Nucl Acids Res 2017; 45: 1027–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omura S. Macrolide Antibiotics. Academic Press, 1984. [Google Scholar]
- 51.Bryskier A, Bergogne-Berezin E. Macrolides. In: Bryskier A, ed. Antimicrobial Agents: Antibacterials and Antifungals. ASM Press, 2005; 475–526. [Google Scholar]
- 52.Starosta AL, Karpenko VV, Shishkina AV et al. Interplay between the ribosomal tunnel, nascent chain, and macrolides influences drug inhibition. Chem Biol 2010; 17: 504–14. [DOI] [PubMed] [Google Scholar]
- 53.Svetlov MS, Vazquez-Laslop N, Mankin AS. Kinetics of drug–ribosome interactions defines the cidality of macrolide antibiotics. Proc Natl Acad Sci USA 2017; 114: 13673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vázquez-Laslop N, Mankin AS. How macrolide antibiotics work. Trends Biochem Sci 2018; 43: 668–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bryskier A. Ketolides. In: Bryskier A, ed. Antimicrobial Agents: Antibacterials and Antifungals . ASM Press, 2005; 527–69. [Google Scholar]
- 56.Douthwaite S, Champney WS. Structures of ketolides and macrolides determine their mode of interaction with the ribosomal target site. J Antimicrob Chemother 2001; 48 Suppl T1: 1–8. [DOI] [PubMed] [Google Scholar]
- 57.Bryskier A. Lincosamides. In: Bryskier A, ed. Antimicrobial Agents: Antibacterials and Antifungals. ASM Press, 2005; 592–603. [Google Scholar]
- 58.Bryskier A, Streptogramins. In: Bryskier A, ed. Antimicrobial Agents: Antibacterials and Antifungals. ASM Press, 2005; 570–91. [Google Scholar]
- 59.Roberts MC. Update on macrolide, lincosamide, streptogramin, ketolide, and oxazolidinone resistance genes. FEMS Microbiol Lett 2008; 282: 147–59. [DOI] [PubMed] [Google Scholar]
- 60.Matzov D, Zohar E, Benhamou RI et al. Structural insights of lincosamides targeting the ribosome of Staphylococcus aureus. Nucl Acids Res 2017; 45: 10284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwarz S, Shen J, Kadlec K et al. Lincosamides, streptogramins, phenicols, and pleuromutilins: mode of action and mechanisms of resistance. Cold Spring Harb Perspect Med 2016; 6: a027037.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tu D, Blaha G, Moore PB et al. Structures of MLSB antibiotics bound to mutated large ribosomal subunits provide a structural explanation for resistance. Cell 2005; 121: 257–70. [DOI] [PubMed] [Google Scholar]
- 63.Tereshchenkov AG, Dobosz-Bartoszek M, Osterman IA et al. Binding and action of amino-acid analogs of chloramphenicol upon the bacterial ribosome. J Mol Biol 2018; 430: 842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakashio S, Iwasawa H, Dun F et al. Everninomicin, a new oligosaccharide antibiotic: its antimicrobial activity, post-antibiotic effect and synergistic bactericidal activity. Drugs Exp Clin Res 1995; 21: 7–16. [PubMed] [Google Scholar]
- 65.Belova L, Tenson T, Xiong L et al. A novel site of antibiotic action in the ribosome: interaction of evernimicin with the large ribosomal subunit. Proc Natl Acad Sci USA 2001; 98: 3726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arenz S, Juette MF, Graf M. Structures of the orthosomycin antibiotics avilamycin and evernimicin in complex with the bacterial 70S ribosome. Proc Natl Acad Sci USA 2016; 113: 7527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katayama N, Fukusumi S, Funabashi Y et al. TAN-1057 A-D, new antibiotics with potent antibacterial activity against methicillin resistant Staphylococcus aureus. J Antibiot 1993; 46: 606–13. [DOI] [PubMed] [Google Scholar]
- 68.Williams RM, Yuan C, Lee VJ et al. Synthesis and evaluation of TAN-1057 A/B analogs. J Antibiot 1998; 51: 189–201. [DOI] [PubMed] [Google Scholar]
- 69.Limburg E, Gahlmann R, Kroll H-P. Ribosomal alterations contribute to bacterial resistance against the dipeptide antibiotic TAN-1057. Antimicrob Agents Chemother 2000; 48: 619–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boddeker N, Bahador G, Gibbs CJ et al. Characterization of a novel antibacterial agent that inhibits bacterial translation. RNA 2002; 8: 1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bryskier AB. Oxazolidinones. In: Bryskier A, ed. Antimicrobial Agents: Antibacterials and Antifungals. ASM Press, 2005; 604–30. [Google Scholar]
- 72.Lin AH, Murray RW, Vidmar TJ et al. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob Agents Chemother 1997; 41: 2127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilson DM, Schluenze F, Harms JM et al. The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Proc Natl Acad Sci USA 2008; 105: 13339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skripkin E, McConnell TS, DeVito J. Oxazolidinones that overcome ribosome-based linezolid resistance. Antimicrob Agents Chemother 2008; 52: 3550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Long KS, Vester B. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother 2012; 56: 603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hodgin LA, Hogenauer G. The mode of action of pleuromutilin derivatives. Eur J Biochem 1974; 47: 527–33. [DOI] [PubMed] [Google Scholar]
- 77.Dornhelm P, Hogenauer G. The effects of tiamulin, a semisynthetic pleuromutilin derivative, on bacterial polypeptide chain initiation. Eur J Biochem 1978; 91: 465–73. [DOI] [PubMed] [Google Scholar]
- 78.Hogenauer G, Ruf C. Ribosomal binding region for the antibiotic tiamulin: stoichiometry, subunit location, and affinity for various analogs. Antimicrob Agents Chemother 1981; 19: 260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davidovich C, Bashan A, Auerbach-Nevo T et al. Induced-fit tightens pleuromutilins binding to ribosomes and remote interactions enable their selectivity. Proc Natl Acad Sci USA 2007; 104: 4291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Veyssier P, Bryskier A. Aminocyclitol aminoglycosides. In: Bryskier A, ed. Antimicrobial Agents: Antibacterials and Antifungals. ASM Press, 2005; 453–69. [Google Scholar]
- 81.Kotra LP, Haddad J, Mobashery S. Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob Agents Chemother 2000; 44: 3249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ditlev E, Brodersen DE, Clemons WM et al. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell 2000; 103: 1143–54. [DOI] [PubMed] [Google Scholar]
- 83.Borovinskay MA, Shoji S, Fredrick K et al. Structural basis for hygromycin B inhibition of protein biosynthesis. RNA 2008; 14: 1590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carter AP, Clemons WM, Ditlev E et al. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 2000; 407: 340–8. [DOI] [PubMed] [Google Scholar]
- 85.Schroeder R, Waldsich C, Wank H. Modulation of RNA function by aminoglycoside antibiotics. EMBO J 2000; 19: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fourmy D, Yoshizawa S, Puglisi JD. Paromomycin binding induces a local conformational change in the A-site of 16 s rRNA. J Mol Biol 1998; 277: 333–45. [DOI] [PubMed] [Google Scholar]
- 87.Borovinskaya MA, Pai RD, Zhang W et al. Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nat Struct Mol Biol 2007; 14: 727–32. [DOI] [PubMed] [Google Scholar]
- 88.Vester B, Garrett R. A plasmid-coded and site-directed mutation in Escherichia coli 23S RNA that confers resistance to erythromycin: implications for the mechanism of action of erythromycin. Biochimie 1987; 69: 891–900. [DOI] [PubMed] [Google Scholar]
- 89.Champney WS. Macrolide antibiotic inhibition of 50S ribosomal subunit formation in bacterial cells. Recent Res Dev Antimicrob Agents Chemother 1999; 3: 39–58. [Google Scholar]
- 90.Champney WS. Bacterial ribosomal subunit synthesis: a novel antibiotic target. Curr Drug Targets Infect Disord 2001; 1: 19–36. [DOI] [PubMed] [Google Scholar]
- 91.Champney WS. Bacterial ribosomal subunit assembly is an antibiotic target. Curr Top Med Chem 2003; 3: 929–47. [DOI] [PubMed] [Google Scholar]
- 92.Champney WS. The other target for ribosomal antibiotics: inhibition of bacterial ribosomal subunit formation. Infect Disord Drug Targets 2006; 6: 377–90. [DOI] [PubMed] [Google Scholar]
- 93.Champney WS. Three methods to assay inhibitors of ribosomal subunit assembly. In: Champney WS, ed . New Antibiotic Targets. Humana Press, 2008; 63–73. [DOI] [PubMed] [Google Scholar]
- 94.Champney WS. Reductive methods for isotopic labeling of antibiotics. Anal Biochem 1989; 181: 90–5. [DOI] [PubMed] [Google Scholar]
- 95.Chittum HS, Champney WS. Erythromycin inhibits the assembly of the large ribosomal subunit in growing Escherichia coli cells. Curr Microbiol 1995; 30: 273–9. [DOI] [PubMed] [Google Scholar]
- 96.Champney WS, Burdine R. Macrolide antibiotics inhibit 50S ribosomal subunit assembly in Bacillus subtilis and Staphylococcus aureus. Curr Microbiol 1995; 39: 2141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Champney WS, Burdine R. 50S ribosomal subunit synthesis and translation are equivalent targets for erythromycin inhibition in Staphylococcus aureus. Antimicrob Agents Chemother 1996; 40: 1301–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Champney WS, Burdine R. Azithromycin and clarithromycin inhibition of 50S ribosomal subunit formation in Staphylococcus aureus cells. Curr Microbiol 1998; 36: 119–23. [DOI] [PubMed] [Google Scholar]
- 99.Eyal Z, Matzov D, Krupkin M et al. Structural insights into species-specific features of the ribosome from the pathogen Staphylococcus aureus. Proc Natl Acad Sci USA 2015; 112: E5805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Champney WS, Burdine R. Macrolide antibiotic inhibition of translation and 50S ribosomal subunit assembly in methicillin-resistant Staphylococcus aureus cells. Microbial Drug Resist 1998; 4: 169–74. [DOI] [PubMed] [Google Scholar]
- 101.Champney WS, Tober CL, Burdine R. A comparison of the inhibition of translation and 50S ribosomal subunit formation in Staphylococcus aureus cells by nine different macrolide antibiotics. Curr Microbiol 1998; 37: 412–7. [DOI] [PubMed] [Google Scholar]
- 102.Champney WS, Tober CL. Superiority of 11,12 carbonate macrolide antibiotics as inhibitors of translation and 50S subunit formation in Staphylococcus aureus cells. Curr Microbiol 1999; 38: 342–8. [DOI] [PubMed] [Google Scholar]
- 103.Champney WS, Tober CL. Inhibition of translation and 50S ribosomal subunit formation in Staphylococcus aureus cells by 11 different ketolide antibiotics. Curr Microbiol 1998; 37: 418–25. [DOI] [PubMed] [Google Scholar]
- 104.Champney WS, Tober CL. Structure-activity relationships for six ketolide antibiotics. Curr Microbiol 2001; 42: 203–10. [DOI] [PubMed] [Google Scholar]
- 105.Champney WS, Tober CL. Specific inhibition of 50S ribosomal subunit formation in Staphylococcus aureus cells by 16-membered macrolide, lincosamide and streptogramin B antibiotics. Curr Microbiol 2000; 41: 126–35. [DOI] [PubMed] [Google Scholar]
- 106.Siibak T, Peil L, Xiong L et al. Erythromycin- and chloramphenicol-induced ribosomal assembly defects are secondary effects of protein synthesis inhibition. Antimicrob Agents Chemother 2009; 53: 563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siibak T, Peil L, Dönhöfer L et al. Antibiotic-induced ribosomal assembly defects result from changes in the synthesis of ribosomal proteins. Mol Microbiol 2011; 80: 54–67. [DOI] [PubMed] [Google Scholar]
- 108.Champney WS, Tober CL. Evernimicin (SCH27899) inhibits both translation and 50S ribosomal subunit formation in Staphylococcus aureus cells. Antimicrob Agents Chemother 2000; 44: 1413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Champney WS, Pelt J, Tober CL. TAN-1057A: a translational inhibitor with a specific inhibitory effect on 50S ribosomal subunit formation. Curr Microbiol 2001; 43: 340–5. [DOI] [PubMed] [Google Scholar]
- 110.Barbachyn MR, Ford CW. Oxazolidinone structure-activity relationships leading to linezolid. Angew Chem Int Ed Engl 2003; 42: 2010–23. [DOI] [PubMed] [Google Scholar]
- 111.Champney WS, Miller M. Linezolid is a specific inhibitor of 50S ribosomal subunit formation in Staphylococcus aureus cells. Curr Microbiol 2002; 44: 350–6. [DOI] [PubMed] [Google Scholar]
- 112.Champney WS, Rodgers WK. Retapamulin inhibition of translation and 50S ribosomal subunit formation in Staphylococcus aureus cells. Antimicrob Agents Chemother 2007; 51: 3385–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Champney WS, Pelt J. The ketolide antibiotic ABT-773 is a specific inhibitor of translation and 50S ribosomal subunit formation in Streptococcus pneumoniae cells. Curr Microbiol 2002; 45: 155–60. [DOI] [PubMed] [Google Scholar]
- 114.Champney WS, Pelt J. Telithromycin inhibition of protein synthesis and 50S ribosomal subunit formation in Streptococcus pneumoniae cells. Curr Microbiol 2002; 45: 328–33. [DOI] [PubMed] [Google Scholar]
- 115.Rodgers W, Frazier AD, Champney WS. Solithromycin inhibition of protein synthesis and ribosome biogenesis in Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae. Antimicrob Agents Chemother 2013; 57: 1632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Champney WS, Mentens N, Zurawick K. An examination of the differential sensitivity to ketolide antibiotics in ermB strains of Streptococcus pyogenes and Streptococcus pneumoniae. Curr Microbiol 2004; 49: 239–47. [DOI] [PubMed] [Google Scholar]
- 117.Mabe S, Champney WS. A comparison of a new oral streptogramin XRP 2868 with quinupristin-dalfopristin against antibiotic-resistant strains of Haemophilus influenzae, Staphylococcus aureus and Streptococcus pneumoniae. Curr Microbiol 2005; 51: 363–6. [DOI] [PubMed] [Google Scholar]
- 118.Champney WS, Miller M. Inhibition of 50S ribosomal subunit assembly in Haemophilus influenzae cells by azithromycin and erythromycin. Curr Microbiol 2002; 44: 418–24. [DOI] [PubMed] [Google Scholar]
- 119.Mabe S, Eller J, Champney WS. Structure-activity relationships for three macrolide antibiotics in Haemophilus influenzae. Curr Microbiol 2004; 49: 248–54. [DOI] [PubMed] [Google Scholar]
- 120.Champney WS, Tober CL. Preferential inhibition of protein synthesis by ketolide antibiotics in Haemophilus influenzae cells. Curr Microbiol 2003; 46: 103–8. [DOI] [PubMed] [Google Scholar]
- 121.MacKenzie FM, Gould IM. The post-antibiotic effect. J Antimicrob Chemother 1993; 32: 519–37. [DOI] [PubMed] [Google Scholar]
- 122.Isaksson B, Maller R, Nilsson LE et al. Postantibiotic effect of aminoglycosides on staphylococci. J Antimicrob Chemother 1993; 32: 215–22. [DOI] [PubMed] [Google Scholar]
- 123.Champney WS, Tober CL. A molecular investigation of the post-antibiotic effect of clarithromycin and erythromycin on Staphylococcus aureus cells. Antimicrob Agents Chemother 1999; 43: 1324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bryskier A. Ansamycins. In: Bryskier A, ed. Antimicrobial Agents: Antibacterials and Antifungals. ASM Press, 2005; 906–24. [Google Scholar]
- 125.Bryskier A, Fluoroquinolones. In: Bryskier A, ed. Antimicrobial Agents. Antibacterials and Antifungals. ASM Press, 2005; 668–88. [Google Scholar]
- 126.Sanders CC. Ciprofloxacin: in vitro activity, mechanism of action, and resistance. Rev Infect Dis 1988; 10: 516–27. [DOI] [PubMed] [Google Scholar]
- 127.Beach JM, Champney WS. An examination of the inhibitory effects of three antibiotics in combination on ribosome biosynthesis in Staphylococcus aureus. Arch Microbiol 2014; 196: 249–60. [DOI] [PubMed] [Google Scholar]
- 128.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother 1995; 39: 577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Denoya CD, Dubnau D. Site and substrate specificity of the ermC 23S rRNA methyltransferase. J Bacteriol 1987; 169: 3857–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Denoya CD, Dubnau D. Mono-and dimethylating activities and kinetic studies of the ermC 23 S rRNA methyltransferase. J Biol Chem 1989; 264: 2615–24. [PubMed] [Google Scholar]
- 131.Champney WS, Chittum H, Tober CL. A 50S ribosomal subunit precursor particle is a substrate for the ermC methyltransferase in Staphylococcus aureus cells. Curr Microbiol 2003; 46: 453–60. [DOI] [PubMed] [Google Scholar]
- 132.Pokkunuri I, Champney WS. Characteristics of a 50S ribosomal subunit precursor particle as a substrate for ermE methyltransferase activity and erythromycin binding in Staphylococcus aureus. RNA Biol 2007; 4: 147–53. [DOI] [PubMed] [Google Scholar]
- 133.McGaha S, Champney WS. Hygromycin B inhibition of protein synthesis and ribosome biogenesis in Escherichia coli. Antimicrob Agents Chemother 2007; 51: 591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mehta R, Champney WS. 30S ribosomal subunit assembly is a target for inhibition by aminoglycosides in Escherichia coli. Antimicrob Agents Chemother 2002; 46: 1546–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mehta R, Champney WS. Neomycin and paromomycin inhibit 30S ribosomal subunit assembly in Staphylococcus aureus. Curr Microbiol 2003; 47: 237–43. [DOI] [PubMed] [Google Scholar]
- 136.Foster C, Champney WS. Characterization of a 30S ribosomal subunit assembly intermediate found in Escherichia coli cells growing with neomycin or paromomycin. Arch Microbiol 2008; 189: 441–9. [DOI] [PubMed] [Google Scholar]
- 137.Nomura M. Assembly of bacterial ribosomes. Science 1973; 179: 863–73. [DOI] [PubMed] [Google Scholar]
- 138.Traub P, Mizushima S, Lowery CV et al. Reconstitution of ribosomes from subribosomal components. Methods Enzymol 1971; 20: 391–407. [Google Scholar]
- 139.Nicholson A. Function, mechanism and regulation of bacterial ribonucleases. FEMS Microbiol Rev 1999; 23: 371–90. [DOI] [PubMed] [Google Scholar]
- 140.Sulthana S, Basturea GN, Deutscher MP. Elucidation of pathways of ribosomal RNA degradation: an essential role for RNase E. RNA 2019; 22: 1163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Davies BW, Kohrer C, Jacob AI et al. Role of Escherichia coli YbeY, a highly conserved protein, in rRNA processing. Mol Microbiol 2010; 78: 506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Usary J, Champney WS. Erythromycin inhibition of 50S ribosomal subunit formation in Escherichia coli cells. Mol Microbiol 2001; 40: 951–62. [DOI] [PubMed] [Google Scholar]
- 143.Silvers JA, Champney WS. Accumulation and turnover of 23S ribosomal RNA in azithromycin-inhibited ribonuclease mutant strains of Escherichia coli. Arch Microbiol 2005; 184: 66–77. [DOI] [PubMed] [Google Scholar]
- 144.Frazier A, Champney WS. Impairment of ribosomal subunit synthesis in aminoglycoside treated ribonuclease mutants of Escherichia coli. Arch Microbiol 2012; 194: 1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bharat A, Blanchard JE, Brown ED. A high-throughput screen of the GTPase activity of Escherichia coli EngA to find an inhibitor of bacterial ribosome biogenesis. J Biomol Screen 2013; 18: 830–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Berger SL. Isolation of cytoplasmic RNA: ribonucleoside-vanadyl complexes. Methods Enzymol 1987; 152: 227–34. [DOI] [PubMed] [Google Scholar]
- 147.Frazier A, Champney WS. The vanadyl ribonucleoside complex inhibits ribosomal subunit formation in Staphylococcus aureus. J Antimicrob Chemother 2012; 67: 2152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Frazier A, Champney WS. Inhibition of ribosomal subunit synthesis in Escherichia coli by the vanadyl ribonucleoside complex. Curr Microbiol 2013; 67: 226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]