Abstract
BACKGROUND
Virtual reality (VR) allows for presurgical planning. Intraoperatively, augmented reality (AR) enables integration of segmented anatomic information with neuronavigation into the microsurgical scene to provide guidance without workflow disruption. Combining VR and AR solutions may help guide microsurgical technique to improve safety, efficiency, and ergonomics.
OBJECTIVE
To describe a VR/AR platform that provides VR planning and intraoperative guidance via microscope ocular injection of a comprehensive AR overlay of patient-specific 360°/3D anatomic model aligned and synchronized with neuronavigation.
METHODS
Custom 360° models from preoperative imaging of 49 patients were utilized for preoperative planning using a VR-based surgical rehearsal platform. Each model was imported to SyncAR, the platform's intraoperative counterpart, which was coregistered with Medtronic StealthStation S8 and Zeiss or Leica microscope. The model was injected into the microscope oculars and referenced throughout by adjusting overlay opacity. For anatomic shifts or misalignment, the overlay was reregistered via manual realignment with known landmarks.
RESULTS
No SyncAR-related complications occurred. SyncAR contributed positively to the 3D understanding of patient-specific anatomy and ability to operate. Preoperative planning and intraoperative AR with 360° models allowed for more precise craniotomy planning and execution. SyncAR was useful for guiding dissection, identifying critical structures including hidden anatomy, understanding regional anatomy, and facilitating resection. Manual realignment was performed in 48/49 surgeries. Gross total resection was achieved in 34/40 surgeries. All aneurysm clipping and microvascular decompression procedures were completed without complications.
CONCLUSION
SyncAR combined with VR planning has potential to enhance surgical performance by providing critical information in a user-friendly, continuously available, heads-up display format.
Keywords: Virtual reality, Surgical planning, Augmented reality, Navigation, Microscopic surgery, Craniotomy
ABBREVIATIONS
- AR
augmented reality
- SRP
SuRgical Planner
- SyncAR
SynchronizAR
- VR
Virtual Reality
- XR
extended reality
Development in virtual and augmented reality (VR/AR) has aimed to improve visualization and navigation techniques in neurosurgery.1-3 VR has shown promise as a surgical planning tool for improving efficiency and outcomes.1,4-8 AR innovations have enabled the integration of navigation information displayed in 2D or 3D with the surgical scene.9-12 Overlay of visual information onto the operative field eliminates the need for external monitors, reducing context switching and associated cognitive load and performance errors.13,14 Additionally, ergonomics may improve when surgeons do not need to shift attention between the operative field and monitor(s).
We describe our early experience with an extended reality (XR) technology that provides preoperative planning in VR and enhanced intraoperative guidance with a comprehensive AR overlay of patient-specific 360°/3D model injected into the microscope oculars for image-guided microsurgery. The interactive 360°XR renderings are created from volumetric data from different imaging modalities and fused using the VR-based SuRgical Planner (SRP). A virtual craniotomy along with surgical corridor and trajectory can be planned. The model can be imported to SynchronizAR (SyncAR) and utilized in conjunction with microscope and navigation for enhanced guidance via AR overlay with the microscope view. SyncAR injects the 360°XR model synchronized with neuronavigation into the microscope oculars. Combining VR and AR solutions may help guide microsurgical technique without workflow disruption and improve safety, efficiency, and ergonomics. Utilization of the 360°XR model to enhance planning and guidance was recently demonstrated for clinoidal meningioma resection.15 This multicenter observational study presents our initial experience with the platform for various intracranial pathologies. To our knowledge, this is the largest series utilizing an XR platform for preoperative VR planning and intraoperative guidance with AR overlay injected into the microscope oculars with patient-specific models available in 3D at every stage.
METHODS
A retrospective case series of microsurgical procedures involving FDA-cleared SRP v7.8.2 and SyncAR v3.8.0 (Surgical Theater, Cleveland, Ohio) performed by 7 neurosurgeons at 5 institutions from March to October 2020 (n = 49). All were attendings with an average of 3.8 ± 1.6 and 1.8 ± 1.6 yr of experience with VR for surgical planning and AR for endoscopic surgery. None had prior experience with SyncAR. Craniotomies for various pathologies were included (Tables 1 and 2). Patients were informed about their surgery with written consent obtained. Ethical approval was not required since no identifiable information was collected.
TABLE 1.
Pathologies of Included Cases
| Pathology | # of cases (%) |
|---|---|
| Acoustic neuroma | 3 (6.1%) |
| Astrocytoma | 1 (2%) |
| Aneurysm | 4 (8.2%) |
| Middle cerebral artery (MCA) | 3 (6.1%) |
| Internal carotid artery (ICA) | 1 (2%) |
| Brain Metastasis | 4 (8.2%) |
| Brain tissue necrosis | 1 (2%) |
| Carcinoma | 2 (4.1%) |
| Cavernoma | 4 (8.2%) |
| Chiari malformation | 1 (2%) |
| Facial spasms | 1 (2%) |
| Glioma | 8 (16.3%) |
| Glioblastoma | 7 (14.3%) |
| Meningioma | 12 (24.5%) |
| Metastatic adenocarcinoma | 2 (4.1%) |
| Neoplasm | 2 (4.1%) |
| Neurocytoma | 1 (2%) |
| Temporal lobe mass | 1 (2%) |
| Trigeminal neuralgia | 2 (4.1%) |
| Total | 49 |
TABLE 2.
Surgical Approach of Included Cases
| Surgical approach | # of cases (%) |
|---|---|
| Clinoidal | 1 (2%) |
| Frontal | 8 (16.3%) |
| Frontoparietal | 4 (8.2%) |
| Frontotemporal | 6 (12.2%) |
| Minipterional | 3 (6.1%) |
| Occipital | 6 (12.2%) |
| Parietal | 3 (6.1%) |
| Posterior Fossa | 1 (2%) |
| Pterional | 2 (4.1%) |
| Retrolabyrinthine | 1 (2%) |
| Retromastoid | 2 (4.1%) |
| Retrosigmoid | 4 (8.2%) |
| Suboccipital | 2 (4.1%) |
| Supraorbital | 2 (4.1%) |
| Temporal | 3 (6.1%) |
| Translabyrinthine | 1 (2%) |
Preoperative Planning/Rehearsal
A 360°XR model from each patient's preoperative imaging imported as DICOM files was created using the SRP (Table 3). Scans were ≤1 mm in slice thickness in all planes. Multiple modalities were automatically volume rendered and fused as layers in the same space based on slice thickness. Each layer (up to 8/case) represents a scan or an extraction of a particular scan. DTI was postprocessed by NordicBrainEx (NordicNeuroLab, Bergen, Norway). Color and opacity were adjusted accordingly to display structures of interest that existed within the selected voxel range. Manual segmentation was also performed to highlight relevant structures (3-9 min/segmentation; Table 3). The models were created by either a clinical specialist or the surgeon who provided guidance and final approval.
TABLE 3.
Imaging Modalities Included and Segmented Anatomy Shown During Cases
| Anatomy shown | Modality used | # of cases |
|---|---|---|
| Tumor | T1 MRI pre/postcontrast | 36 |
| Cavernoma | T1 MRI pre/postcontrast | 3 |
| Optic nerve | T1 MRI pre/postcontrast, T2 MRI | 6 |
| Spinal cord | T1 MRI pre/postcontrast | 1 |
| Ventricles | T1 MRI postcontrast, T2 MRI | 1 |
| Wernicke's area | T1 MRI precontrast | 2 |
| Broca's area | T1 MRI precontrast | 1 |
| Cranial nerves | T2 FIESTA/CISS MRI | 6 |
| Trigeminal nerve | 2 | |
| Facial nerve | 3 | |
| Vestibulocochlear nerve | 1 | |
| Venous structures | CTA, T1 MRI postcontrast | 10 |
| Sigmoid sinus | 1 | |
| Frontal sinus | 1 | |
| Superior sagittal sinus | 4 | |
| Confluence of sinus | 1 | |
| Transverse sinus | 3 | |
| Bony structures | CT/CTA | 5 |
| Skull base | 1 | |
| Burr hole location | 3 | |
| Sphenoid bone | 1 | |
| Clinoid bone | 1 | |
| Arterial structures | MRA, CTA, angiogram | 31 |
| Aneurysm | 4 | |
| Compressing artery | 3 | |
| Basilar artery | 4 | |
| Posterior cerebellar artery (PICA) | 2 | |
| Internal carotid artery (ICA) | 8 | |
| Middle cerebral artery (MCA) | 7 | |
| Posterior cerebral artery (PCA) | 1 | |
| Anterior cerebral artery (ACA) | 2 | |
| White matter tracts | DTI | 11 |
| Arcuate fasciculus | 2 | |
| Corticospinal tract | 6 | |
| Optic radiation | 3 |
CISS, constructive interference in steady-state; CT, computed tomography; CTA, CT angiogram; DTI, diffusion tensor imaging; MRA, magnetic resonance angiogram; MRI, magnetic resonance imaging; FIESTA, fast-imaging employing steady-state acquisition.
Each patient's 360°VR model was utilized for case review and/or planning either outside the operating room on the SRP or inside on SyncAR prior to scrubbing in. Different scan combinations were analyzed. The selective clipping and opacity control tools were utilized to visualize specific structures. Surgeons were able to manipulate the models in real time to view the patient's anatomy from different angles using touchscreen controls on the Surgical Theater systems or VR headsets with controllers. Virtual drills were used to remove bony anatomy and simulate craniotomies. One or more surgical trajectories and corridors were simulated. When appropriate, 3D markers were placed within the model and referenced during surgery. For aneurysms, appropriate clips were selected along with clip angle planned on the model.
Intraoperative Navigation
Each model with planned craniotomy was imported to SyncAR, which was integrated and coregistered with StealthStation S8 (Medtronic, Dublin, Ireland) and a microscope (Zeiss Kinevo 900, Oberjoken, Germany, or Leica M530 OH-6, Wetzler, Germany). Patient registration and microscope integration were performed in standard fashion. The model was injected into the microscope oculars and referenced throughout by adjusting overlay opacity using a foot switch. SyncAR connectivity to both navigation and microscope allowed for automated tracking of the navigation probe by displaying a synchronized virtual probe. When the microscope was repositioned, the AR overlay automatically scaled and aligned with the surgical field. To verify overlay accuracy, alignment of the virtual probe with its physical counterpart was visually confirmed by the surgeon. SyncAR accuracy is limited by StealthStation's accuracy. Templates of surgical plans were superimposed onto the patient. Manual realignment with anatomic landmarks was performed to reregister the overlay when there was an anatomic shift or misalignment. Surgeons provided feedback immediately after each case.
RESULTS
Average patient age was 58 ± 15 yr (20-84 yr), and 69% were female. Gross total resection was achieved in 34 of 40 surgeries (85%). All aneurysm clipping and microvascular decompression procedures were completed without complications (Videos 1 and 2). The 360°XR models consisting of 1 to 2 (37% of cases), 3 to 5 (43%), and 6 to 8 layers (20%) took approximately 10, 25, and 50 min, respectively, to build. The models were utilized for VR planning for 96% of surgeries. Average planning time was 9.5 ± 10.2 min (median = 5 min). The surgeons did not have any issues integrating the SRP and SyncAR into their workflow and found both easy to operate. No intraoperative complications were encountered, nor obstacles introduced related to SyncAR. Hands-free manipulation of AR overlay opacity was easy to use. All surgeons agreed the models contributed positively to their 3D understanding of patient-specific anatomy and ability to operate. Surgeons utilized the models and virtual tools to map out lesion borders, plan corridors and trajectories, and place 3D markers to highlight burr hole location and specific structures such as critical vessels, sinuses, and nerves. The increased anatomic information obtained from evaluating the models may have led to changes in the surgical approach of 2 meningioma cases, resulting in gross total resection in 1 and a greater degree of resection in the other. Surgeons felt rehearsal performed in the operative position helped them to preoperatively predict the line of sight.
Improving Safety
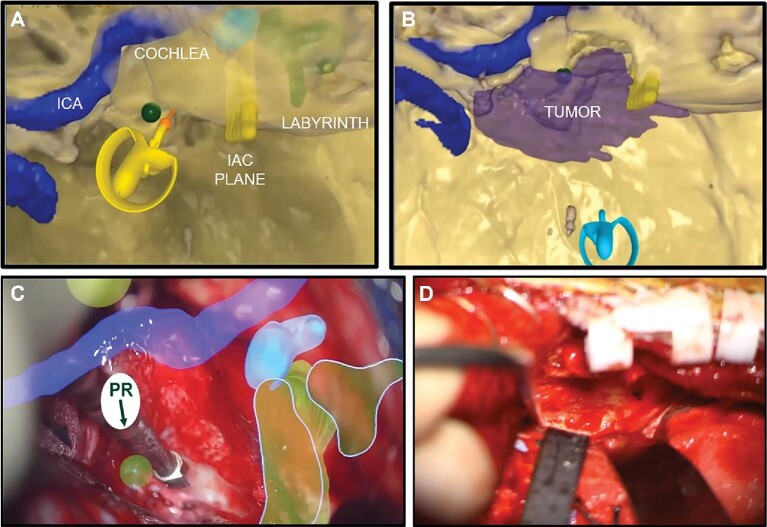
Surgeons reported SyncAR was useful for guiding dissection and identifying and preserving critical structures including hidden anatomy. They also stated the overlay aided in their decision to either proceed quickly or with caution. For a partially coiled ruptured MCA aneurysm, the overlay helped define its anatomy and made visible the patent portion and one of the M2 branches arising from the back that was difficult to visualize. For an ICA aneurysm, SyncAR helped identify the dome, which was hidden due to the swollen, edematous brain, and facilitated quicker identification of the A1 to A2 segments located bilaterally. For a petrotentorial meningioma, the model was utilized for preoperative planning to create a template with relevant structures highlighted and markers placed to set drilling boundaries (Figure 1). Overlay of the template showing the ICA and cochlea was helpful for guiding drilling in real time.
FIGURE 1.
Preoperative planning with SRP and intraoperative visualization with SyncAR for anterior petrosectomy of a petrotentorial meningioma. A, Preoperative VR rehearsal using virtual drill on 360° model with highlighted critical structures and 3D circles marking the boundaries for drilling. B, The segmented tumor is made visible to show adequate exposure for resection after drilling. C, AR image with HUD to guide drilling in real time. D, Microscopic image without AR overlay for a similar case. ICA, internal carotid artery; IAC, internal acoustic canal; PR, petrous ridge.
Precise Execution
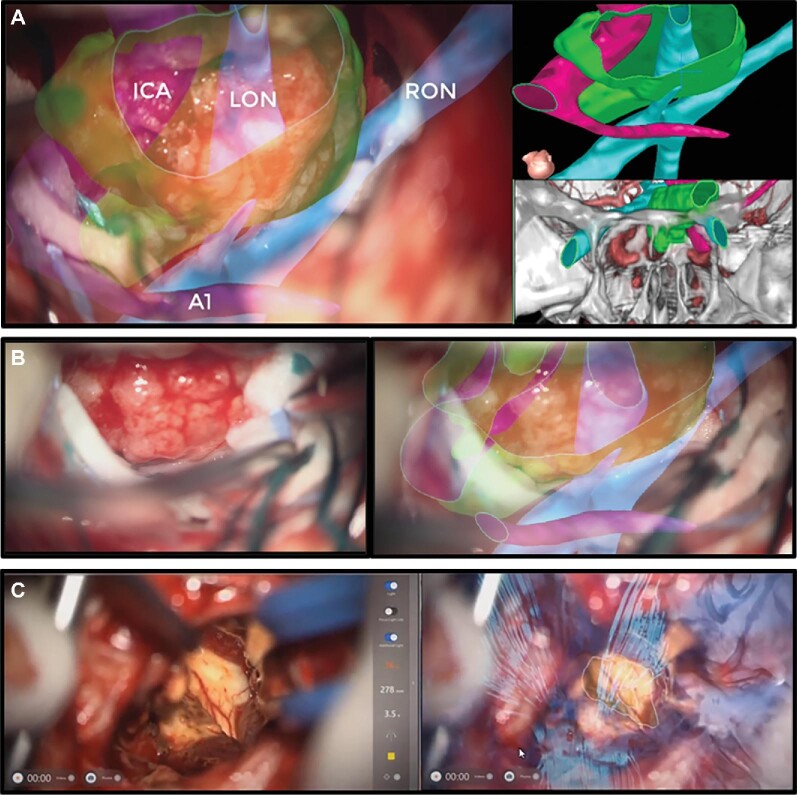
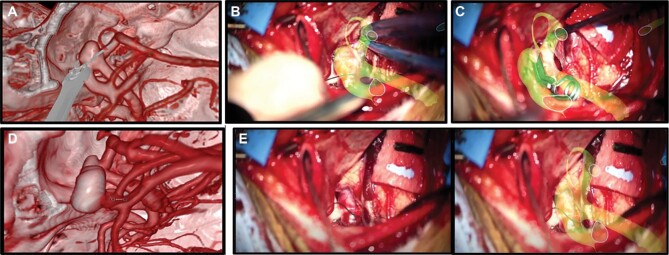
Surgeons felt SyncAR supported better visualization of lesion borders and interface with the brain. Figure 2 shows an overlay of a clinoidal meningioma encasing the optic nerve and ICA. In a glioblastoma case, SyncAR was used to visualize its borders, its invasion of the splenium of the corpus callosum, and the ventrally displaced corticospinal tract (Figure 2B). The improved visualization aided in achieving gross total resection while preserving critical structures. SyncAR also facilitated the extradural anterior clinoidectomy for clinoidal meningioma resection by helping identify the optic foramen. Surgeons reported that craniotomy or burr hole planning coupled with intraoperative AR overlay of the template allowed for more precise execution to minimize size and optimize placement. Using SyncAR, the planned burr holes and craniotomies were duplicated. Aneurysm clips were placed as rehearsed with exact clip and orientation. Figure 3 illustrates the aneurysm case workflow.
FIGURE 2.
Tumor resection with SyncAR. A, AR image with HUD of left clinoidal meningioma encasing optic nerve and left ICA. Reference image on lower right quadrant for regional orientation. B, Microscopic images without and with AR overlay from the same case. C, Microscopic images without and with AR overlay of right parietal glioblastoma with 3D tractography. ICA, internal carotid artery; LON, left optic nerve; RON, right optic nerve.
FIGURE 3.
VR/AR workflow of aneurysm clipping procedure. Sequential images for A, preoperative VR rehearsal; B, AR guidance; C, clip placement; and D, postoperative clip position. Preoperative rehearsal and AR guidance led to correct selection and placement of clip in single attempt. E, Microscopic images without and with AR overlay.
Surgeons and the System
SyncAR’s automatic clipping feature was useful for maintaining excellent depth perception and accurate spatial orientation. When the microscope's focal point was adjusted, the AR image selectively removed obscuring anatomy to show only relevant structures at each depth level.
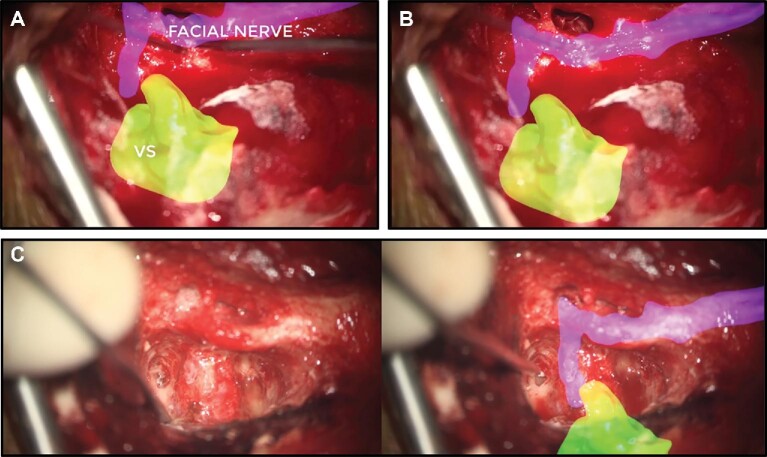
The surgeons found the real-time reregistration of SyncAR valuable when there was an anatomic shift or misalignment. The manual realignment of the overlay was performed in 48 of 49 cases (98%). The accurate visualization of a vestibular schwannoma relative to the facial nerve provided by SyncAR following realignment may have helped achieve near gross total resection (99.5%; Figure 4). In a trigeminal neuralgia case, reregistration to align the trigeminal nerve and artery compressing it showed compression occurred further anterior than the surgical corridor. This information led to readjustment of the approach, and effective decompression was achieved.
FIGURE 4.
Real-time reregistration via manual realignment for vestibular schwannoma resection. A, AR image with HUD demonstrating course of facial nerve through facial canal as seen via right translabrynthine approach showing navigation registration error of approximately 2 mm. B, Submillimeter accuracy of overlay after manual realignment with facial nerve. VS, vestibular schwannoma. C, Microscopic images without and with AR overlay.
All surgeons subjectively reported SyncAR helped them maintain their focus. With navigation synchronized with 3D depictions of anatomic and functional data, surgeons did not need to direct attention away from the operative field.
Illustrative Cases
Case 1
A 56-yr-old Japanese female with history of hypertension presented with an unruptured right MCA aneurysm. With no history of subarachnoid hemorrhage, she had a PHASES score 9, predicting a 4.3% 5-yr risk of rupture. The aneurysm morphology and regional vascular anatomy were closely examined in the 360°XR model from the patient's CTA with contrast and T1-weighted MRI MPRAGE (Video 1). Preoperative planning was also performed in VR to select the appropriate clip and rehearse placement to identify the optimal angle.
The patient underwent a right pterional craniotomy. The AR overlay injected into the Kinevo oculars helped verify regional anatomy including aneurysm orientation allowing for clip placement preparation. AR helped define the anterior temporal artery's location, which, after its takeoff from the MCA trifurcation, folded back upon itself and took a recurrent course along the deep aspect of the aneurysm neck. After having verified the overlay's accuracy by using the MCA’s main trunk and larger trifurcation branches, a #11 Sugita clip was placed along the neck with awareness that the artery's recurrent course would not also be occluded by the clip. With increased awareness provided by the overlay, the inferior jaw of the clip was used to sweep this branch out of the way and prevent it from becoming trapped against the neck. Using ICG angiography, flow was confirmed to the distal vessels at the MCA trifurcation.
The patient made a full recovery. Postoperative imaging verified appropriate clip placement and maintained blood flow through the parent and distal vessels.
Case 2
A 52-yr-old woman with a 10-yr history of right-sided trigeminal neuralgia presented for consideration of microvascular decompression. Her pain was localized to the V2 distribution on the right and became unbearable even though she was on pregabalin, carbamazepine and amitriptyline. Her imaging studies showed a compression at the right trigeminal root by the superior cerebellar artery.
During preoperative planning with the 360°VR model of the patient's CTA, a 13mm burr hole was made precisely at the junction of the transverse and sigmoid sinuses by drilling inside the skull outward (Video 2). With the patient anesthetized in a lateral position, the saved 360°XR template was projected as an AR overlay onto the patient's anatomy. The template burr hole was marked onto the patient's skin and used to guide incision placement. The overlay was used again to guide the bone opening. The template burr hole was duplicated in surgery, with the same size and placement as the one made in VR. The rest of the surgery proceeded in standard fashion. No restriction of movement was encountered because of the size of the opening.
The patient experienced pain relief immediately after surgery. At 3 mo, she had stopped taking amitriptyline and was actively weaning off the others without experiencing pain.
DISCUSSION
We present our early experience with a technology that provides both VR and AR solutions for enhancing preoperative planning and intraoperative guidance of various procedures. Preoperative planning with the Surgical Theater models was proven useful for optimizing the plan and led to improved safety and efficiency.5,16 For aneurysms, a 29% reduction in time per clip used was observed after planning with SRP.5 Intraoperatively, AR can provide improved visualization to guide microsurgical technique when the AR scene is injected into the oculars. SyncAR combines information from the microscope's focal point with information of the roll, pitch, and yaw rotations to project the 360° model directly onto the patient at the same point in space from matching point of view.
For various pathologies, preoperative planning with the patient-specific models enabled a better understanding of the operative field by allowing detailed evaluation of the patient's regional anatomy and their pathology's 3D relationship with surrounding anatomy from various vantage points. The model can interact with 3D tools to design an optimal approach. The ability to project the model with planned craniotomy directly on the patient provided a template for more precise execution during multiple stages of surgery including positioning, skin incision, arachnoid dissection, intracranial drilling, and lesion resection.
The AR overlay was intermittently introduced throughout each procedure to update the surgeon's perspective. Resultingly, a series of waypoints was created to help guide surgery. By providing a visual reminder of the critical structures’ locations, SyncAR can help reduce cognitive load and increase surgical efficiency. The live augmentation of the 360° renderings proved useful in progressively validating the mental model and awareness of relevant structures without needing to shift focus from the operative field. During rehearsal, segmented objects were specifically chosen and viewed separately or combined in a single comprehensive overlay during surgery. SyncAR provided visualization of lesion borders and anatomy not visible in the line of sight to optimize resection and critical structure preservation. For aneurysms, it allowed for verification of regional anatomy, including orientation of the neck, dome, and afferent and efferent vessels, which facilitated dissection for optimal clip placement.
SyncAR’s real-time reregistration was valuable to correct for navigational shifts or inaccuracies. This correction could be performed at any point if an identifiable structure is present. It was utilized in all but one case, which did not use SyncAR during navigation, and was deemed helpful in facilitating the cases’ success. While reregistration cannot solve brain shift since SyncAR is dependent on navigation, realignment with a known landmark may partially compensate for it. Furthermore, the platform was still useful for cranial tumors and aneurysms in planning the craniotomy and incision and navigating the trajectory for minimally invasive cases using NICO Myriad BrainPath device.
The safety and feasibility of other navigation-linked HUD platforms have been reported.10-12 Cabrilo et al described HUD’s use to guide AVM resection.10 They also reported on the usefulness of stereoscopic 3D image injection during craniotomy, subarachnoid dissection, and clip placement for aneurysms.11 AR was useful for clip positioning in 92.3% of cases. Mascitelli et al demonstrated HUD utility during multiple stages of surgery for 79 patients who underwent HUD-assisted surgery for various pathologies.12 These studies utilized Brainlab and Zeiss. In contrast, SyncAR was integrated with StealthStation and Zeiss or Leica to provide high-resolution renderings of anatomic and functional data fused in the same space. Additionally, SyncAR is available in 3D at every stage of surgery. Similar to the other platforms, SyncAR injects segmented structures into the microscope eyepiece, tracks movement and clips in to adjust to the microscope's focal point, and manually realigns to correct for navigational shifts. Overall, these platforms show promise in safely enhancing intraoperative visualization and navigation to improve surgical efficiency without workflow disruption. More outcome-based comparative studies are needed to validate efficacy.
Limitations
This is a pilot retrospective observational study without a comparative group. Specific utilization was not recorded. System accuracy was not measured, nor timing and source of inaccuracy recorded. Future studies should objectively evaluate utility and accuracy. Thorough analysis of SyncAR utility during specific stages of surgery should be performed. Surgical approach, craniotomy size, operative time, complication rates, extent of resection, and clinical outcome should be compared with a control group for each pathology type. For aneurysms, clip correction and occlusion rates should be measured.
CONCLUSION
Without disrupting workflow, valuable information was provided on demand in real time, which helped decrease cognitive load, validated spatial awareness, and supported intraoperative decision making. Based on our experience, SyncAR combined with preoperative VR planning has potential to enhance surgical safety and performance.
Funding
This study did not receive any funding or financial support.
Disclosures
Dr Robert Louis, Dr Gary Steinberg, Dr Christopher Duma, and Dr Walter Jean are consultants for Surgical Theater. Dr Louis and Dr Warren Selman have stock options in Surgical Theater. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
We would like to thank Alex Yefimov, Nick Quon, Tatiana Jansen, Diana Anthony, Aneil Srivastava, Carlton Johnson, Max Klepcha, Sean Copeland, Brady Culbreth, and Phuong Dang for assistance with 360° VR/AR case building, OR set up, and data collection.
Contributor Information
Robert G Louis, Pickup Family Neurosciences Institute, Hoag Memorial Hospital Presbyterian Newport Beach, Newport Beach, California, USA.
Gary K Steinberg, Department of Neurosurgery, Stanford University School of Medicine, Stanford, California, USA.
Christopher Duma, Pickup Family Neurosciences Institute, Hoag Memorial Hospital Presbyterian Newport Beach, Newport Beach, California, USA.
Gavin Britz, Department of Neurosurgery, Houston Methodist Hospital, Houston, Texas, USA.
Vivek Mehta, Pickup Family Neurosciences Institute, Hoag Memorial Hospital Presbyterian Newport Beach, Newport Beach, California, USA.
Jonathan Pace, Department of Neurosurgery, Allegheny Health Network, Pittsburgh, Pennsylvania, USA.
Warren Selman, Department of Neurosurgery, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA; Department of Neurosurgery, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA.
Walter C Jean, Department of Neurosurgery, George Washington University Hospital, Washington, District of Columbia, USA.
REFERENCES
- 1.Ferroli P, Tringali G, Acerbi Fet al. Advanced 3-dimensional planning in neurosurgery. Neurosurgery. 2013;72(Suppl_1):54-62. [DOI] [PubMed] [Google Scholar]
- 2.Fiani B, De Stefano F, Kondilis A, Covarrubias C, Reier L, Sarhadi K. Virtual reality in neurosurgery: “can you see it?”—a review of the current applications and future potential. World Neurosurg. 2020;141:291-298. [DOI] [PubMed] [Google Scholar]
- 3.Mikhail M, Mithani K, Ibrahim GM. Presurgical and intraoperative augmented reality in neuro-oncologic surgery: clinical experiences and limitations. World Neurosurg. 2019;128:268-276. [DOI] [PubMed] [Google Scholar]
- 4.Kin T, Nakatomi H, Shono Net al. Neurosurgical virtual reality simulation for brain tumor using high-definition computer graphics: a review of the literature. Neurol Med Chir (Tokyo). 2017;57(10):513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chugh AJ, Pace JR, Singer Jet al. Use of a surgical rehearsal platform and improvement in aneurysm clipping measures: results of a prospective, randomized trial. J Neurosurg. 2017;126(3):838-844. [DOI] [PubMed] [Google Scholar]
- 6.Kockro RA, Killeen T, Ayyad Aet al. Aneurysm surgery with preoperative three-dimensional planning in a virtual reality environment: technique and outcome analysis. World Neurosurg. 2016;96:489-499. [DOI] [PubMed] [Google Scholar]
- 7.Sun GC, Wang F, Chen XLet al. Impact of virtual and augmented reality based on intraoperative magnetic resonance imaging and functional neuronavigation in glioma surgery involving eloquent areas. World Neurosurg. 2016;96:375-382. [DOI] [PubMed] [Google Scholar]
- 8.Stadie AT, Kockro RA, Reisch Ret al. Virtual reality system for planning minimally invasive neurosurgery. J Neurosurg. 2008;108(2):382-394. [DOI] [PubMed] [Google Scholar]
- 9.Contreras Lopez WO, Navarro PA, Crispin S. Intraoperative clinical application of augmented reality in neurosurgery: a systematic review. Clin Neurol Neurosurg. 2019;177:6-11. [DOI] [PubMed] [Google Scholar]
- 10.Cabrilo I, Bijlenga P, Schaller K. Augmented reality in the surgery of cerebral arteriovenous malformations: technique assessment and considerations. Acta Neurochir. 2014;156(9):1769-1774. [DOI] [PubMed] [Google Scholar]
- 11.Cabrilo I, Bijlenga P, Schaller K. Augmented reality in the surgery of cerebral aneurysms: a technical report. Neurosurgery. 2014;10(Suppl 2):252-260; discussion 260-251. [DOI] [PubMed] [Google Scholar]
- 12.Mascitelli JR, Schlachter L, Chartrain AGet al. Navigation-linked heads-up display in intracranial surgery: early experience. Oper Neurosurg. 2018;15(2):184-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabbard JL, Mehra DG, Swan JE. Effects of AR display context switching and focal distance switching on human performance. IEEE Trans Vis Comput Graph. 2019;25(6):2228-2241. [DOI] [PubMed] [Google Scholar]
- 14.Herrlich M, Tavakol P, Black Det al. Instrument-mounted displays for reducing cognitive load during surgical navigation. Int J CARS. 2017;12(9):1599-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jean WC. Mini-Pterional craniotomy and extradural clinoidectomy for clinoid meningioma: optimization of exposure using augmented reality template: 2-dimensional operative video. Oper Neurosurg. 2020;20(1):E76. [DOI] [PubMed] [Google Scholar]
- 16.Bradley D, Willson T, Chang JBet al. Intraoperative three-dimensional virtual reality and computed tomographic guidance in temporomandibular joint arthroplasty of syndromic craniofacial dysostoses. Plast Reconstr Surg Glob Open. 2019;7(9):e2388. [DOI] [PMC free article] [PubMed] [Google Scholar]