Abstract
The epidemiologic study of pregnancy and birth outcomes may be hindered by several unique and challenging issues. Pregnancy is a time-limited period in which severe cohort attrition takes place between conception and birth and adverse outcomes are complex and multi-factorial. Biases span those familiar to epidemiologists: selection, confounding and information biases. Specific challenges include conditioning on potential intermediates, how to treat race/ethnicity, and influential windows of prolonged, seasonal and potentially time-varying exposures. Researchers studying perinatal outcomes should be cognizant of the potential pitfalls due to these factors and address their implications with respect to formulating questions of interest, choice of an appropriate analysis approach and interpretations of findings given assumptions. In this article, we catalogue some of the more important potential sources of bias in perinatal epidemiology that have more recently gained attention in the literature, provide the epidemiologic context behind each issue and propose practices for dealing with each issue to the extent possible.
Keywords: Perinatal epidemiology, fixed cohort bias, conditioning on intermediates, immortal time bias, selection bias
Key Messages
Various sources of bias exist in perinatal epidemiology studies spanning issues of selection bias, confounding and information bias.
Challenges such as fixed cohort bias, selection of live births, conditioning on potential intermediates, and issues arising from prolonged and potentially time-varying exposure can all lead to bias.
These challenges and the methods to address them are not always obvious and even if addressed there may still be implications for interpretability of findings.
Clear definitions of the question and target parameter(s) of interest, and awareness of these potential challenges, would benefit investigators.
Introduction
The gestational period is a critical time that can define the future health and quality of life both for the child and its family.1–3 It is, therefore, critically important to study and identify potential risk factors for adverse pregnancy, birth and—in general—perinatal outcomes. In utero exposures are potentially modifiable factors that can contribute to or ameliorate risk of adverse perinatal outcomes and their study can provide valuable information for the protection of fetal and maternal health. Observational studies aiming to assess potential adverse health effects of such exposures can generate information relevant to possible interventions that may mitigate the risk of such outcomes. However, methodological and analytic challenges can hinder interpretability of results from such studies and, consequently, muddy actionable conclusions. Epidemiologists and clinical researchers newer to perinatal epidemiologic research may be less familiar with some of these special cases of bias. In this paper, we attempt to highlight some of these issues by providing the epidemiologic context and suggesting approaches to address them, when possible. Perinatal epidemiology studies are subject to a variety of potential biases hampering all observational studies, such as general confounding, or more specific forms like confounding by indication,4,5 selection bias including cases of loss to follow-up,6,7 ascertainment8 and information bias.9 In this paper, we try to focus on some more specific cases of bias often encountered in perinatal epidemiology that have gained attention in the last decade or typically remain inadequately addressed by approaches that are considered the norm in the overall perinatal epidemiology literature. We highlight limitations in some commonly used approaches to study perinatal outcomes, and provide recommendations for good practices in dealing with each of these issues to the extent possible.
Fixed cohort bias
Birth registry datasets include all births that took place within specific time periods (e.g. 1 March 2000–30 November 2019). Because live births can occur from the extremes of peri-viability at 20 weeks to the post-term after 42 weeks, as Figure 1 displays in the yellow rectangle, fixed cohorts tend to include a higher proportion of longer gestations at the beginning and a higher proportion of preterm births at the end of the study period.10 Ideally, we would include the full conception dates as depicted in the pink rectangle, including both the shorter gestations at the beginning and the longer gestations at end of the study period. The fixed nature of birth registry data forces researchers to incorrectly omit these gestations from the at-risk population. Bias can, thus, result in both cohort and case–control studies,11 especially when exposure is seasonal or happens to occur very near the beginning or end of the study period. Fixed cohort bias may be avoided in several ways. First, if the exposure has no temporal pattern, aligning the start and end dates (i.e. start = 1 March 2000 and end = 28 February 2019) can eliminate the bias. Second, if exposure has a temporal component, investigators can restrict the study population to pregnancies with conception dates 22 weeks before the beginning of data collection and 44 weeks prior to the end of data collection. Third, these associations can be evaluated in cohort studies that, ideally, begin follow-up near conception, but this presents logistical and financial difficulty.
Figure 1 .
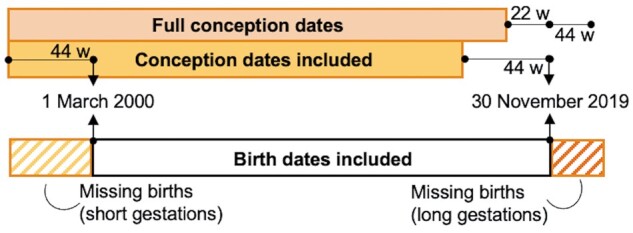
Fixed cohort bias. Black shows the available birth cohort data, with births spanning 1 2000– 30 November 2019. Yellow indicates the conception dates included in the birth cohort. Pink indicates the conception dates we would like to include to prevent fixed-cohort bias. At the beginning of the study period, longer gestations are disproportionately represented, and at the end only shorter gestations are included, as birth must take place prior to 30 November 2019, the end of available birth record data
Live-birth bias
Fewer than half of fertilized eggs will result in a live birth,12,13 representing extreme cohort attrition over the gestational period and leading to a selection of only live births into analyses of adverse birth and developmental outcomes. Under certain conditions, this selection can lead to biased results. Liew et al.14 and Raz et al.15 have both discussed this potential bias, scenarios for its occurrence and implications for study results. Briefly, two possible mechanisms for live-birth bias are conditioning on a collider and depletion of susceptibles.15 For live-birth bias with a collider type structure two conditions need to hold: (i) the exposure of interest is related to pregnancy loss and (ii) a second variable exists, separate from the exposure of interest, that is related both to pregnancy loss and the outcome of interest. This creates a non-causal path between exposure A and outcome Y via the—by definition—conditioned pregnancy loss and the unmeasured common cause(s) U (Figure 2a). The second type of live-birth bias occurs when the exposure of interest preferentially results in fetal loss of those fetuses that are more susceptible to the outcome of interest or early pregnancy loss among pregnancies that would have resulted in such susceptible fetuses. These mechanisms could be addressed in the statistical analysis if there were available data on pregnancy loss in the study population or, for the first mechanism, on all potential common causes between pregnancy loss and the outcome of interest. However, accurate data on pregnancy loss, including in the weeks after implantation, are only available in select small studies with detailed information on daily hormonal levels,16 and no study can guarantee complete information on all potential common causes of pregnancy loss and outcome of interest. To date, to the best of our knowledge, no method exists to correct for live-birth bias in the absence of this information, and often—e.g. when studying effects of environmental exposures on neurodevelopmental outcomes—the outcome can only be observed in live births. Whereas live-birth bias likely results in underestimation of harmful effects, more studies on pregnancy loss are required to help identify common causes of pregnancy loss and adverse birth outcomes. However, a large number of conceptions do not result in a viable gestation and it is not straightforward—at least to date—to successfully differentiate causes of early pregnancy loss at the population level or even between infertility and failure to implant. Therefore, because not all pregnancies are clinically detectable or recognized, it remains impossible to measure common causes between early pregnancy loss and the outcome of interest, regardless of study design. Given these issues, it is possible that even with additional information on common causes of fetal loss (which realistically can be measured) and adverse health outcomes and use of advanced methods that account for survivor bias, it may not be feasible to adequately control for live-birth bias. Finally, attempting to correct for live-birth bias by defining the target population as all conceptions rather than live births is not necessarily always meaningful or relevant; a well-defined research question and identification of the at-risk population, however, always are.17
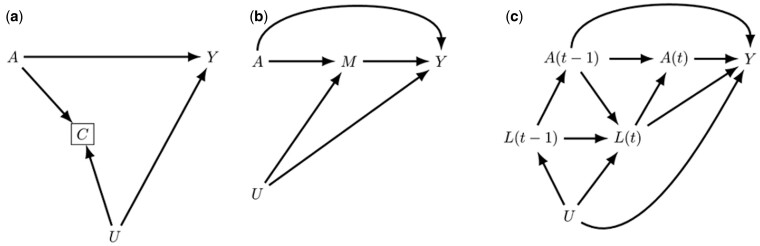
Figure 2 .
(a) Directed acyclic graph depicting potential live birth bias: an exposure of interest A is a cause of both a birth outcome of interest Y and pregnancy loss C, with the latter two also sharing an unmeasured common cause U. Live birth datasets are by definition conditioned on C creating a biased path from the exposure to outcome of interest via collider C and U. (b) Potential for bias by controlling for intermediates in the exposure–outcome relationship. A commonly controlled for mediator M for an exposure A and outcome of interest Y is gestational age. If gestational age and the outcome of interest share an unmeasured common cause U, then controlling for M will induce a biased path from A to Y via collider M and U. In addition, the effect of A on Y via M that may be of interest is also controlled for. (c) Example of time varying confounder L(t) for the relationship of an exposure A(t) and outcome of interest Y. If the confounder is also affected by past exposure and shares an unmeasured common cause U with Y, traditional regression methods cannot account for this type of confounder and use of estimation approaches such as g-methods is recommended
Conditioning on intermediates
The issue of conditioning on intermediates is also prominent in perinatal epidemiology, as investigators often want to condition on intermediate variables that may be important drivers of an association of interest. The issue first gained attention with the birth weight ‘paradox’, as Hernández-Díaz et al.18 showed that conditioning on birth weight—a potential intermediate for the effect of smoking on infant mortality—induces collider bias leading to an inverse association between smoking during pregnancy and infant mortality among the low-birth-weight infants. Conditioning on gestational age carries the same implications and could also lead to collider bias, an issue that has been identified in the literature for some time.19 Researchers often conceptualize gestational age as a confounder, even though it can be a potential mediator of the exposure–outcome relationship of interest, if the exposure affects timing of birth in addition to the outcome of interest.20 This appears to be the case for certain environmental exposures, such as air pollution, as well as social exposures, such as psychosocial stress. Although the attention paid to the issue of overadjustment and potential for collider bias has begun to shift common practice in perinatal epidemiology, many recent studies still employ some kind of conditioning either through restriction, standardization of the outcome or model adjustment for gestational age in their primary analysis, without being clear on the rationale for doing so.21–28 In general, researchers must consider the question of interest and assumptions needed before deciding whether conditioning on an intermediate is necessary. Such an adjustment may remove some of the effect of interest or introduce potential for collider bias by opening the pathway between exposure A, intermediate M, unmeasured common cause(s) U and the outcome Y (Figure 2b).29 If a total effect is of interest, then it is important not to condition on intermediates. If a specific pathway is of interest (e.g. effects not mediated through particular mediators) then this should be explicitly stated and conditioning is perhaps warranted, but additional assumptions are required: the effect of interest is essentially a ‘direct effect’ requiring no unmeasured mediator–outcome confounding among other assumptions. This assumption is unlikely to hold when gestational age is the mediator.29–31 It should also be noted that the issue of conditioning on a potential intermediate (and potential collider) is problematic when causal effects are the parameters of interest. Clinician researchers often restrict populations to specific subgroups of interest, for example very preterm (28–32 weeks) or extremely preterm (28–32 weeks) or extremely preterm (<28 weeks) neonates, in order to identify prognostic factors that best predict risk of an outcome of interest in these specific subgroups. If prediction rather than causation is the target, then such restrictions are not necessarily problematic, and estimated associations are still of value.32
Assessing critical windows of exposure
The time-varying nature of some exposures over the duration of pregnancy presents investigators with further complexity. The typical approach of using exposures averaged over the pregnancy fails to account for the variability in exposures and implicitly assumes a constant effect of exposure throughout the duration of pregnancy. This results in potential for bias if the effect of exposure differs over its duration,33 and can also miss an association if the critical window of exposure is short. Pregnancy-average exposure also fails to account for differences in duration of exposure (or cumulative exposure) in the case where events such as preterm birth or spontaneous abortion affect the duration of pregnancy. Another common approach relies on trimester-specific average exposure, but this also fails to account for variability in exposure within trimesters and can potentially lead to bias if exposures during different trimesters correlate with each other, but are modelled separately.34 For example, if environmental chemical exposures in the first trimester of pregnancy increase risk of congenital birth defects, an approach modelling only second trimester exposure may also yield an effect even though second trimester exposures are outside the critical window and do not increase risk of the outcome. More flexible approaches to modelling the lag-response are advised in the cases of time-varying exposures during pregnancy, such as distributed lag models, which have been applied in studies of prenatal environmental exposures such as air pollution and temperature and perinatal outcomes.35–37
Season of conception as a confounder
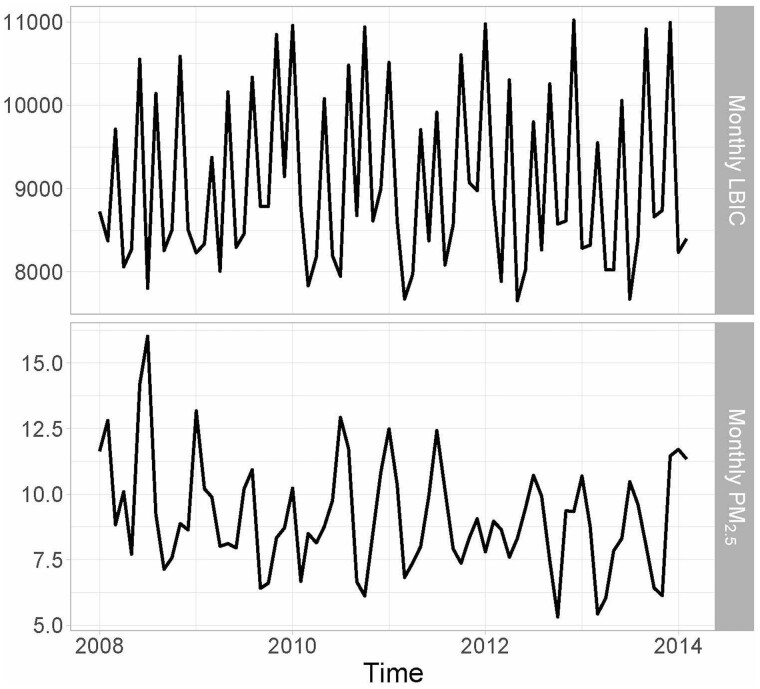
Environmental, behavioural, biological and cultural factors may drive seasonality in birth counts and outcomes, with no single factor explaining all seasonal variation. In Figure 3, we show an example from New York City where exposure and outcome both exhibit seasonality. A recent preconception cohort study of North American and Norwegian women noted seasonality in initiation of pregnancy attempts and fecundability. They found peak fecundability in November, with the strongest seasonality seen at lower latitudes in North America, possibly reflecting the role of environmental factors.38 A study using birth registry data from Atlanta, GA found college-educated individuals more likely to conceive in summer, which could help explain improved outcomes among spring births in this subpopulation.39 Similarly, in Norway, maternal smoking, sociodemographic factors and misclassification of gestational duration accounted for the majority of observed seasonality in preterm birth.40 Conversely, a within-mother study in the Northeastern USA found that seasonality in pregnancy weight gain and influenza—not necessarily sociodemographic selection into conception month—appeared to drive fluctuations in gestational length and birth weight.41 This study also found that conception rate across the year contributed to 22% of seasonality in gestation length. Regardless of cause, these seasonal differences in the distribution of gestational age among fetuses at-risk can confound the exposure–gestational age relationship if exposure contains seasonality. When this circumstance is of concern, using a fetuses-at-risk approach40 or including fixed effects for season of conception or a smooth term for time can help reduce bias. Control for season of conception is preferable to season of birth, which could be an intermediate variable.
Figure 3 .
Monthly particulate matter with a diameter of ⩽2.5 μm (PM2.5) concentrations (μg/m3) and monthly live birth identified conceptions (LBIC) in New York City (2008–14)
Race/ethnicity as a confounding variable
In the USA, stark and unchanging racial/ethnic disparities exist in maternal and infant morbidity and mortality.42,43 Such disparities also appear to occur in other countries, such as the UK,44 but are less-often studied. Therefore, careful consideration of race/ethnicity is warranted in perinatal epidemiology. Researchers should conceive of race/ethnicity as integrating social position, material resources, culture, experiences of racism at the inter personal level (e.g. being followed while shopping) and structural racism (e.g. discriminatory lending practices), rather than conceptualizing race/ethnicity as a biologic variable that causes adverse perinatal outcomes.45 Unfortunately, many studies mechanically include maternal race/ethnicity as a variable in regression models without stating the purpose.46 In the clinical setting, race/ethnicity has been incorporated into prediction algorithms in ways that may perpetuate racism and racial disparities in birth outcomes. For example, the Vaginal Birth after Cesarean (VBAC) algorithm provides a measure of risk of attempting vaginal delivery after a cesarean section, and takes as an input patient race, predicting lower vaginal delivery success rates for Hispanic and Black women.47 Critical Race Theory’s praxis challenges researchers to use research questions, methods and interpretation to focus on structural forces that drive inequities, rather than individual/interpersonal mechanisms.48 Depending on the goal of the analysis, adjusting for race/ethnicity may or may not make sense and can perpetuate a harmful narrative around the biological basis of racial differences. Explicitly stating what the researchers believe race/ethnicity represents in their analysis can also aid analytic decisions and interpretation. Adjustment in regression models, if race/ethnicity is not actually a confounder, can mask important racially-patterned differences in exposure–effect relationships. Treating race/ethnicity as a confounder may emphasize behavioural and biologic explanations for associations, whereas instead stratifying by race/ethnicity to evaluate effect modification can focus findings on structural factors, such as transgenerational poverty and racial residential segregation.49,50 For example, after controlling for many facets of socio-economic status, Braveman et al. still found large racial disparities in preterm birth comparing non-Hispanic Black and White mothers.51 Boyd et al. provide concrete recommendations for researchers publishing on racial health inequities.52 The extreme Black–White disparities in birth outcomes that persist today justify a careful treatment of race in theoretical frameworks, models and interpretation in order to inform mechanistic interpretations as well as policy to begin closing the racial gap in perinatal health.48
Immortal time bias
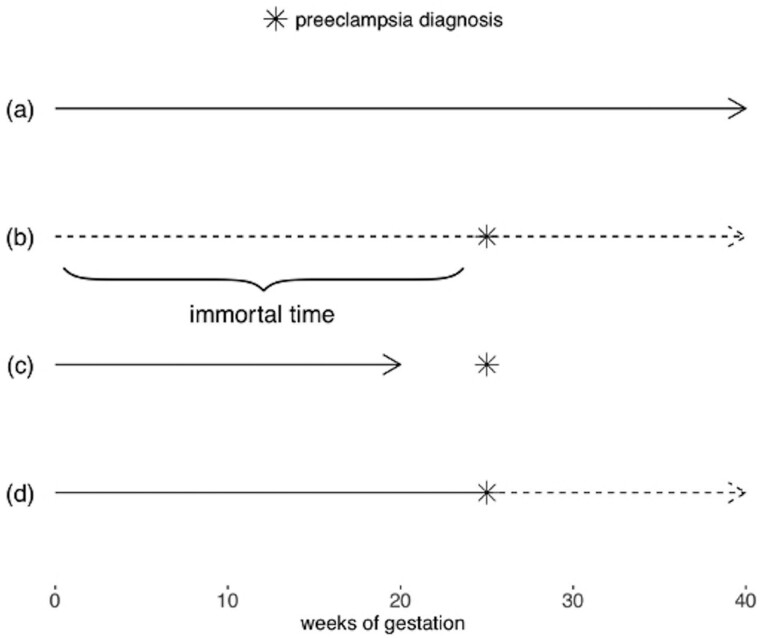
When an exposure is time-varying, people can move in and out of exposure states during the course of the study. If the exposure status of this person-time is inaccurately attributed or misclassified, immortal time bias can be introduced.53 When studying pregnancy outcomes, this can occur if exposure states change over the course of gestation, but are represented in the analysis as though they are fixed at baseline. A classic example of immortal time bias in perinatal epidemiology studies is the relationship between pregnancy complications and stillbirth.54 Understanding whether pregnancy complications, such as preeclampsia, increase the risk of stillbirth is an important clinical and public health question. However, these complications often do not manifest until later in pregnancy. Therefore, pregnancies that survive longer than others will have greater opportunity to have the ‘exposure’ diagnosed before the outcome occurs. If the time before diagnosis is not considered unexposed time, it becomes immortal time, because by design the pregnancy cannot end during this time. Had the pregnancy ended in stillbirth before diagnosis, the exposure state would be misclassified, because the exposure had not occurred yet (Figure 4). This can introduce bias by artificially inflating the risk of stillbirth in the unexposed group, because some portion of those pregnancies are likely to have been diagnosed with complications had they lasted long enough. Similarly, the risk of stillbirth can be underestimated in the exposed group, because the pregnancies had to last long enough to be exposed. Issues of immortal time bias also tend to arise in studies of spontaneous abortion and preterm birth, in which the outcome of interest is a function of gestational age.55–57 Insufficient consideration of immortal time can result in large biases, potentially reversing the direction of association; it is, therefore, crucial to account for when studying time-varying exposures in the pregnancy context. Several analysis and design choices can mitigate bias due to immortal time. In prospective cohort designs, for instance in pregnancy cohorts where women are enrolled either before they become pregnant or early in pregnancy, immortal time bias can be addressed by design. Say, for example, that a pregnancy cohort enrols women during their first trimester. They will then be followed until the pregnancy ends, either in birth or pregnancy loss. To avoid issues of immortal time bias, researchers should either define the exposed and unexposed groups based on their exposure status at enrollment (following the target trial approach,58 in which alignment of eligibility, exposure assignment and follow-up time in an observational study mimics a randomized trial) or create a ‘landmark’ time during which exposure status is determined. A landmark time is a window during which exposure is measured which is early in the follow-up time. Then any births or pregnancy losses that occur during that time are considered censored and those participants are excluded from the subsequent analysis. When the exposure window is pregnancy, the landmark period is often the first 20 weeks of gestation, which corresponds to the earliest week of gestation in which both fetal deaths and live births tend to be recorded in birth records. Exclusion of pregnancy loss before 20 weeks, which is often necessary in birth outcome studies, is therefore built into the design (although this exclusion will result in live-birth bias if the target population is all conceptions or when the target population is live births and there is an unmeasured common cause of pregnancy loss and the outcome of interest). In the example of pregnancy complications and stillbirth, the follow-up time could begin at the time of screening or diagnosis, ensuring that all pregnancies included in the analysis have the opportunity to be diagnosed with the complication of interest. Most importantly, the landmark period must be the same for both exposed and unexposed individuals; excluding the time before exposure only for exposed individuals will induce bias.59 Furthermore, the results may be sensitive to the time period selected as the landmark time and shorter times are more likely to induce bias as there is more potential for exposure misclassification after the landmark period has ended. For additional discussion of the tradeoffs in choosing a landmark time see Mi et al.59 It is also possible to evaluate time-varying exposures in pregnancy cohorts, although it is critical to include person-time before exposure as unexposed in the analysis stage. This can be done by estimating hazard ratios using a Cox model and modeling the exposure as a time-varying covariate. This approach accurately represents the time spent in both exposed and unexposed states and eliminates immortal time bias. However, care should be taken if there is a time-varying confounder (L(t)) that is also affected by prior exposure (A(t − 1)), thus creating potential for collider bias via unmeasured common causes U if L(t) is adjusted for using standard methods (Figure 2c). In this situation, g-methods or other approaches that account for this type of confounding are required.53,60,61 Furthermore, relying on a Cox model alone does not allow for estimation of survival curves or estimation of parameters on the additive scale, compared with other approaches for survival analyses.62 In retrospective cohort designs, like those that use administrative birth records, it is often easier to induce immortal time bias by mistake, because the follow-up is already completed and so the full experience of exposures can be observed at study initiation. Introduction of immortal time bias can be avoided by utilizing similar analytic approaches to those described above. Say, for example, that the outcome of interest is preterm birth and the exposure is ≥5days of poor air quality, defined as levels of fine particulate matter >20 μg/m3. The gestational age at birth and the birth date is used to calculate the week of conception, which is considered the ‘enrollment’ time. Then the time during gestation until a mother has experienced 5 days of poor air quality should be classified as unexposed, and time after as exposed, using a Cox proportional hazard model with time-varying covariates. More details, additional considerations and further examples of immortal person-time are available elsewhere.53,58,63
Figure 4 .
Illustration of immortal time where exposure is diagnosis of preeclampsia and the outcome is stillbirth. The star represents time of preeclampsia diagnosis and the arrow indicates when stillbirth occurs. Solid lines represent unexposed person-time and dashed lines exposed person-time. In (a), the mother is not diagnosed with preeclampsia for the duration of pregnancy. In (b), the mother is diagnosed, and the time before diagnosis occurs is the immortal time, because if she had experienced a stillbirth prior to the diagnosis, as in (c), she would have been in the unexposed, or no preeclampsia, group. Therefore, pregnant woman (b) could not have experienced a stillbirth prior to her preeclampsia diagnosis while maintaining her status in the exposed group. The time before exposure occurs should be considered unexposed, whereas the time after exposure should be counted as exposed person-time, as in (d)
Conclusion
The importance of studying perinatal health is matched by its unique challenges. Issues such as the extreme cohort attrition that occurs between conception and birth, the delimited nature of pregnancy duration, and accessing exposures and outcomes in mother––child dyads rather than the typical single unit of observation give rise to special cases of bias in perinatal epidemiology studies. For example, adjusting analyses for live-birth bias arising because of the cohort attrition between conception and birth can be infeasible in many studies and complicated, at best, in others; even after adjustment, the estimated parameter may not have a direct link to the real-world outcomes. Other examples, such as correction for fixed-cohort bias, a consequence of the time delimited nature of duration of pregnancy, have more straightforward solutions. With this paper, we attempted to (i) provide an overview of some special cases of bias that epidemiologists and clinician researchers newer to perinatal epidemiologic research may not be fully aware of; and (ii) introduce methods to work through them to the extent possible. These issues, as well as a variety of other biases, render perinatal epidemiology an especially challenging field to infer causal effects of potential modifiable exposures of interest; observational studies of perinatal outcomes will always have limitations. Although the way to address these biases may not always be obvious or even entirely possible, increased familiarity with the potential problems enables a clearer discussion of study limitations and additional opportunities for sensitivity analyses to assess the extent of potential biases. In general, precisely stating the question to be answered by the analysis up front, discussing the biological or psychosocial plausibility underlying a relationship of interest, as well as explicitly stating the assumptions required for identifiability of the target parameter intended to answer this question, as has been recommended for causal inference in epidemiology,64 should clarify analysis goals and the most important sources of bias to consider.
Funding
This work was supported by the Niational Institute of Environmental Health Sciences (grant numbers R00 ES027511, R00 ES027023, P30 ES009089, R01 ES029943).
Conflict of interest
None declared.
References
- 1.Saigal S, Feeny D, Rosenbaum P, Furlong W, Burrows E, Stoskopf B. Self-perceived health status and health-related quality of life of extremely low-birth-weight infants at adolescence. JAMA 1996;276:453–59. [PubMed] [Google Scholar]
- 2.Zwicker JG, Harris SR. Quality of life of formerly preterm and very low birth weight infants from preschool age to adulthood: a systematic review. Pediatrics 2008;121:e366–76. [DOI] [PubMed] [Google Scholar]
- 3.Baumann N, Bartmann P, Wolke D. Health-related quality of life into adulthood after very preterm birth. Pediatrics 2016;137:e20153148. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell AA, Gilboa SM, Werler MM et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol 2011;205:51-e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph KS, Mehrabadi A, Lisonkova S. Confounding by indication and related concepts. Curr Epidemiol Rep 2014;1:1–8. [Google Scholar]
- 6.Hille ETM, Elbertse L, Gravenhorst JB, Brand R, Verloove-Vanhorick SP others. Nonresponse bias in a follow-up study of 19-year-old adolescents born as preterm infants. Pediatrics 2005;116:e662–66. [DOI] [PubMed] [Google Scholar]
- 7.Wolke D, Söhne B, Ohrt B, Riegel K. Follow-up of preterm children: important to document dropouts. Lancet 1995;345:447. [DOI] [PubMed] [Google Scholar]
- 8.Howards PP, Johnson CY, Honein MA, Flanders WD. Adjusting for bias due to incomplete case ascertainment in case-control studies of birth defects. Am J Epidemiol 2015;181:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kesmodel US. Information bias in epidemiological studies with a special focus on obstetrics and gynecology. Acta Obstet Gynecol Scand 2018;97:417–23. [DOI] [PubMed] [Google Scholar]
- 10.Strand LB, Barnett AG, Tong S. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol 2011;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnett AG. Time-dependent exposures and the fixed-cohort bias. Environ Health Perspect 2011;119:a422–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chard T. Frequency of Implantation and Early Pregnancy Loss in Natural Cycles in Bailliere Clinical Obstetrics and Gynaecology; Factors of Importance for Implantation, Seppala M. Ed Bailliere Tindall, London. 1991; [DOI] [PubMed] [Google Scholar]
- 13.Wilcox AJ, Weinberg CR, O'Connor JF et al. Incidence of early loss of pregnancy. N Engl J Med 1988;319:189–94. [DOI] [PubMed] [Google Scholar]
- 14.Liew Z, Olsen J, Cui X, Ritz B, Arah OA. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol 2015;44:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raz R, Kioumourtzoglou MA, Weisskopf MG. Live-birth bias and observed associations between air pollution and autism. Am J Epidemiol 2018;187:2292–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 1999;340:1796–99. [DOI] [PubMed] [Google Scholar]
- 17.Snowden JM, Bovbjerg ML, Dissanayake M, Basso O. The curse of the perinatal epidemiologist: inferring causation amidst selection. Curr Epidemiol Rep 2018;5:379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández-Díaz S, Schisterman EF, Hernán MA. The birth weight “paradox” uncovered? Am J Epidemiol 2006;164:1115–20. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol 2011;174:1062–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol 2017;217:366–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stokholm L, Juhl M, Talge NM, Gissler M, Obel C, Strandberg-Larsen K. Obstetric oxytocin exposure and ADHD and ASD among Danish and Finnish children. Int J Epidemiol 2020; 14 June. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Agier L, Basagaña X, Hernandez-Ferrer C et al. Association between the pregnancy exposome and fetal growth. Int J Epidemiol 2020;49:572–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao M, Scott K, Koupil I. Associations of perinatal characteristics with endometriosis: a nationwide birth cohort study. Int J Epidemiol 2020;49:537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bond TA, Karhunen V, Wielscher M et al. Exploring the role of genetic confounding in the association between maternal and offspring body mass index: evidence from three birth cohorts. Int J Epidemiol 2020;49:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardenas A, Lutz SM, Everson TM, Perron P, Bouchard L, Hivert M-F. Mediation by placental DNA methylation of the association of prenatal maternal smoking and birth weight. Am J Epidemiol 2019;188:1878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darling AM, Werler MM, Cantonwine DE, Fawzi WW, McElrath TF. Timing and amount of gestational weight gain in association with adverse birth outcomes. Epidemiology 2019;30:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Liang Q, Li C et al. Interaction of air pollutants and meteorological factors on birth weight in Shenzhen, China. Epidemiology 2019;30:S57–66. [DOI] [PubMed] [Google Scholar]
- 28.Casey JA, Goin DE, Rudolph KE et al. Unconventional natural gas development and adverse birth outcomes in Pennsylvania: the potential mediating role of antenatal anxiety and depression. Environ Res 2019;177:108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanderweele TJ, Mumford SL, Schisterman EF. Conditioning on intermediates in perinatal epidemiology. Epidemiology 2012;23:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacLehose RF, Kaufman JS. The wizards of odds. Epidemiology 2012;23:10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitcomb BW, Schisterman EF, Perkins NJ, Platt RW. Quantification of collider-stratification bias and the birthweight paradox. Paediatr Perinat Epidemiol 2009;23:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snowden JM, Basso O. Causal inference in studies of preterm babies: a simulation study. Bjog: Int J Obstet Gy 2018;125:686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasparrini A. Modeling exposure–lag–response associations with distributed lag non-linear models. Statist Med 2014;33:881–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson A, Chiu YHM, Hsu HHL, Wright RO, Wright RJ, Coull BA. Potential for bias when estimating critical windows for air pollution in children’s health. Am J Epidemiol 2017;186:1281–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Ye Y, Chen Y et al. Effects of prenatal exposure to air particulate matter on the risk of preterm birth and roles of maternal and cord blood LINE-1 methylation: a birth cohort study in Guangzhou, China. Environ Int 2019;133:105177. [DOI] [PubMed] [Google Scholar]
- 36.Martens DS, Plusquin M, Cox B, Nawrot TS. Early biological aging and fetal exposure to high and low ambient temperature: a birth cohort study. Environ Health Perspect 2019;127:117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheridan P, Ilango S, Bruckner TA, Wang Q, Basu R, Benmarhnia T. Ambient fine particulate matter and preterm birth in California: identification of critical exposure windows. Am J Epidemiol 2019;188:1608–15. [DOI] [PubMed] [Google Scholar]
- 38.Wesselink AK, Wise LA, Hatch EE et al. Seasonal patterns in fecundability in North America and Denmark: a preconception cohort study. Hum Reprod 2020;35:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darrow LA, Strickland MJ, Klein M et al. Seasonality of birth and implications for temporal studies of preterm birth. Epidemiology 2009;20:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberg CR, Shi M, DeRoo LA, Basso O, Skjærven R. Season and preterm birth in Norway: a cautionary tale. Int J Epidemiol 2015;44:1068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Currie J, Schwandt H. Within-mother analysis of seasonal patterns in health at birth. Proc Natl Acad Sci 2013;110:12265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Admon LK, Winkelman TNA, Zivin K, Terplan M, Mhyre JM, Dalton VK. Racial and ethnic disparities in the incidence of severe maternal morbidity in the United States, 2012--2015. Obstet Gynecol 2018;132:1158–66. [DOI] [PubMed] [Google Scholar]
- 43.Others. American College of Obstetricians and Gynecologists and Racial and ethnic disparities in obstetrics and gynecology. ACOG Committee Opinion No. 649. Obs Gynecol 2015;126:e130–34. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Quigley MA, Macfarlane A, Jayaweera H, Kurinczuk JJ, Hollowell J. Ethnic differences in singleton preterm birth in England and Wales, 2006-12: Analysis of national routinely collected data. Paediatr Perinat Epidemiol 2019;33:449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burris HH, Lorch SA, Kirpalani H, Pursley DM, Elovitz MA, Clougherty JE. Racial disparities in preterm birth in USA: a biosensor of physical and social environmental exposures. Arch Dis Child 2019;104:931–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nuru-Jeter AM, Michaels EK, Thomas MD, Reeves AN, Thorpe RJ Jr, LaVeist TA. Relative roles of race versus socioeconomic position in studies of health inequalities: a matter of interpretation. Annu Rev Public Health 2018;39:169–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight-reconsidering the use of race correction in clinical algorithms. N Engl J Med 2020;383:874–82. [DOI] [PubMed] [Google Scholar]
- 48.Ford CL, Airhihenbuwa CO. The public health critical race methodology: praxis for antiracism research. Soc Sci Med 2010;71:1390–98. [DOI] [PubMed] [Google Scholar]
- 49.Ford CL, Airhihenbuwa CO. Critical race theory, race equity, and public health: toward antiracism praxis. Am J Public Health 2010;100:S30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kramer MR, Strahan AE, Preslar J et al. Changing the conversation: applying a health equity framework to maternal mortality reviews. Am J Obstet Gynecol 2019;221:609–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braveman PA, Heck K, Egerter S et al. The role of socioeconomic factors in black--white disparities in preterm birth. Am J Public Health 2015;105:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boyd RW, Lindo EG, Weeks LD, McLemore MR. On Racism: A New Standard For Publishing On Racial Health Inequities. July 2, 2020; doi:10.1377/hblog20200630.939347.
- 53.Platt RW, Hutcheon JA, Suissa S. Immortal Time Bias in Epidemiology. Curr Epidemiol Rep 2019;6:23–27. [Google Scholar]
- 54.Hutcheon JA, Kuret V, Joseph KS, Sabr Y, Lim K. Immortal time bias in the study of stillbirth risk factors: the example of gestational diabetes. Epidemiology 2013;24:787–90. [DOI] [PubMed] [Google Scholar]
- 55.Vazquez-Benitez G, Kharbanda EO, Naleway AL et al. Risk of preterm or small-for-gestational-age birth after influenza vaccination during pregnancy: caveats when conducting retrospective observational studies. Am J Epidemiol 2016;184:176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matok I, Azoulay L, Yin H, Suissa S. Immortal time bias in observational studies of drug effects in pregnancy. Birth Defects Res Part A Clin Mol Teratol 2014;100:658–62. [DOI] [PubMed] [Google Scholar]
- 57.Daniel S, Koren G, Lunenfeld E, Levy A. Immortal time bias in drug safety cohort studies: spontaneous abortion following nonsteroidal antiinflammatory drug exposure. Am J Obstet Gynecol 2015;212:307–e1. [DOI] [PubMed] [Google Scholar]
- 58.Hernán MA, Sauer BC, Hernández-D\’\Iaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol 2016;79:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mi X, Hammill BG, Curtis LH, Lai EC-C, Setoguchi S. Use of the landmark method to address immortal person-time bias in comparative effectiveness research: a simulation study. Statist Med 2016;35:4824–36. [DOI] [PubMed] [Google Scholar]
- 60.Robins JM, Hernán MA, Estimation of the causal effects of time-varying exposures. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G (eds) Longitud Data Anal. New York, NY: Chapman & Hall/CRC, 2009, pp. 553–99. [Google Scholar]
- 61.Keil AP, Edwards JK, Richardson DR, Naimi AI, Cole SR. The parametric g-formula for time-to-event data: towards intuition with a worked example. Epidemiology 2014;25:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hernán MA. The hazards of hazard ratios. Epidemiology 2010;21:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol 2008;167:492–99. [DOI] [PubMed] [Google Scholar]
- 64.Petersen ML, Laan M. V D. Causal models and learning from data: integrating causal modeling and statistical estimation. Epidemiology 2014;25:418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]