Abstract
Background
Late Stanford type A aortic dissections (TAADs) are a very rare complication after transcatheter aortic valve implantation (TAVI). Surgery is the treatment of choice, but perioperative mortality (25%) and neurological complications (18%) remain high.
Case summary
An 85-year-old male patient presented with acute chest pain 5 months after a transfemoral Evolut R 34 mm transcatheter heart valve (THV) implantation. On multi-slice computed tomography (MSCT) a TAAD was found with a 7 mm primary entry at the supra-annular aortic edge of the THV expanding to the innominate artery without re-entry. Due to extensive comorbidities including two bypass operations in the history, the Heart Team declined surgery. Within 6 months of watchful waiting the maximal aortic diameter (MAD) increased from 57 to 62 mm. The decision was made to perform an endovascular closure of the inflow to the false lumen by implanting a 25 mm Amplatzer™ Cribriform Septal Occluder. MSCT 4 weeks after occlusion showed the false lumen almost completely filled with thrombus, MSCT 3 months later showed a MAD reduction to 55 mm with shrinkage of the false lumen.
Discussion
Presumably, the late TAAD was caused by the supra-annular edge of the Evolut-stent. Because of the extreme risk surgical repair was not an option and a stent graft would have occluded the vein grafts. This case shows that in absence of any other treatment options endovascular closure of the entry to the false lumen can be successfully performed in a TAAD after TAVI.
Keywords: Stanford type A aortic dissection, TAVI, Self-expanding transcatheter heart valve, Case report
Learning points
Late Stanford type A aortic dissection (TAAD) is a rare vascular complication after transcatheter aortic valve implantation (TAVI) with self-expanding transcatheter heart valve (THV).
Surgery remains the treatment of choice for post-TAVI TAAD but if it is not feasible, endovascular approach including stent graft or closure of dissection entry could be an option.
Endovascular closure of the entry to the false lumen is a therapeutic option.
Choice of the closure device is crucial, the THV-stent can be used to secure a stable device position.
Introduction
Left untreated, a Stanford type A aortic dissection (TAAD) has a very poor prognosis, with a mortality rate of 1%/h up to 48 h and a 30-day mortality of 90%.1,2 Type A aortic dissection is a rare complication after transcatheter aortic valve implantation (TAVI) with a reported incidence of 0.6–1.9%.3 Treatment options for post-TAVI aortic dissections occurring peri-procedurally have been discussed by Langer et al.4 Surgery is the treatment of choice, but perioperative mortality (25%) and neurological complications (18%) remain high.5 We describe a case with TAAD which occurred late after TAVI and is treated by endovascular closure of the false lumen.
Timeline
| 12 January 2020 | Transcatheter valve implantation (Evolut R 34 mm, Medtronic) |
| 8 March 2020 | Non-ST-elevation myocardial infarction with percutaneous coronary intervention of vein graft to right coronary artery (dual antiplatelet therapy with Ticagrelor) |
| 15 July 2020 | Patient presented with acute-onset chest pain caused by type A aortic dissection at the edge of the transcatheter heart valve. Surgery was declined |
| 7 December 2020 | Multi-slice computed tomography (MSCT): increase of maximal aorta diameter (MAD) from 57 to 62 mm |
| 8 December 2020 | Endovascular closure of the entry using an Amplatzer™ Cribriform Septal Occluder (Abbott) |
| 4 January 2021 | First follow-up MSCT: false lumen almost completely filled by thrombus. MAD remained 62 mm |
| 31 March 2021 | Second follow-up MSCT: reduction of MAD to 55 mm, shrinkage of the false lumen by half |
Case presentation
An 85-year-old patient was admitted to the emergency department with acute-onset chest pain that awoke him from sleep. He was haemodynamically stable with normal blood pressure without site difference, regular heart rate, gentle systolic murmur at Erb’s point, no respiratory compromise. Electrocardiogram showed sinus rhythm with pacemaker-induced ventricular stimulation. He was on dual antiplatelet therapy with Aspirin and Ticagrelor, Torasemide 10 mg/day, Bisoprolol 1.25 mg/day, Amlodipine 10 mg/day, Candesartan 8 mg/day, and Atorvastatin 40 mg/day. Abnormal laboratory tests included haemoglobin 8.2 g/dL, leucocytes 14 000/nL, lactate dehydrogenase 356 U/I, Troponin 311 ng/L, N-terminal prohormone of brain natriuretic peptide 1713 pg/mL, and a glomerular filtration rate of 40 mL/min. Comorbidities were renal failure, arterial hypertension, diabetes mellitus, carotid artery stenosis, and normocytic normochromic anaemia of unknown cause. He had a history of two bypass surgeries (1987/2006). In January 2020, he presented with symptomatic severe aortic stenosis. Multi-slice computed tomography (MSCT) revealed a calcified tricuspid aortic valve and a normal-sized ascending aorta (Figure 1A and B). Due to high risk of re-re-reoperation [log. EuroSCORE 26.37, EuroSCORE II 14.07, Society of Thoracic Surgeons Score (STS Score) 5.51] the Heart Team decided for TAVI. A self-expanding transcatheter heart valve (THV) (Evolut R 34 mm, Medtronic) was implanted transfemorally. Final root shot revealed a small paravalvular leak with mild aortic regurgitation, the ascending aorta was without pathological findings (Figure 2). Because of total atrioventricular block a pacemaker was implanted. In March 2020, the patient had a non-ST segment elevation myocardial infarction with percutaneous coronary intervention of the vein graft to right coronary artery (RCA). Dual antiplatelet therapy with Ticagrelor was started.
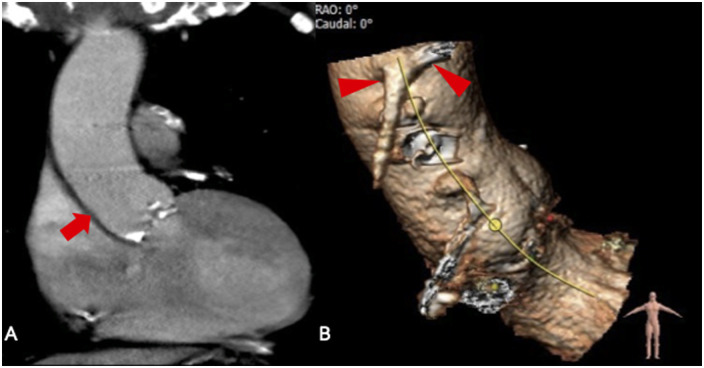
Figure 1.
(A) Multi-slice computed tomography of the calcified aortic valve and a normal-sized ascending aorta before transcatheter aortic valve implantation (arrow). (B) Arrowheads indicate the ostia of the patent vein grafts to right coronary artery and left circumflex artery.
Figure 2.
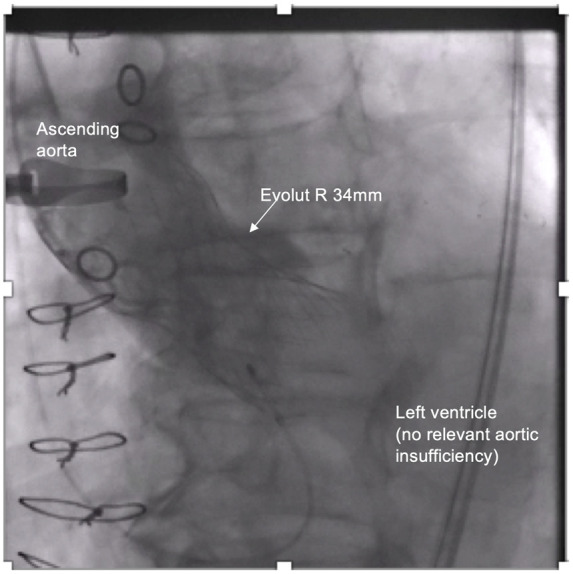
Final root shot revealed a small paravalvular leak with mild aortic regurgitation and without pathological findings of the ascending aorta.
On current admission an acute coronary syndrome was suspected. Transthoracic echocardiography showed left ventricular function of 42% with anteroseptal hypokinesia, competent THV with mild paravalvular leak, and bilateral small pleural effusions. Coronary angiography was performed through the right femoral artery and ruled out coronary obstructions. All bypasses were patent. To complete evaluation of chest pain, a transoesophageal echocardiography (TOE) was performed and revealed a TAAD. Multi-slice computed tomography confirmed TAAD with the primary tear at the aortic edge of the THV extending to the innominate artery without re-entry, maximal aortic diameter (MAD) of 57 mm. Supra-aortic and coronary arteries were not involved. Due to the high risk of re-re-reoperation (log. EuroSCORE 26.37, EuroSCORE II 14.07, STS Score 5.51) surgery was refused by Heart Team. After stabilization with rigorous blood pressure control the patient was discharged home.
Multi-slice computed tomography 6 months later showed MAD increase to 62 mm. Facing a pending aortic perforation a surgical approach was re-discussed with heart surgery team from another hospital (second opinion), but was again refused by the surgeons.
Driven by the urgent treatment request of the patient we strived for endovascular closure of the false lumen to limit perfusion and induce thrombosis. The patient was comprehensively informed about potential procedure-related complications, in particular death by aortic rupture, device embolization, and stroke. He gave his written informed consent.
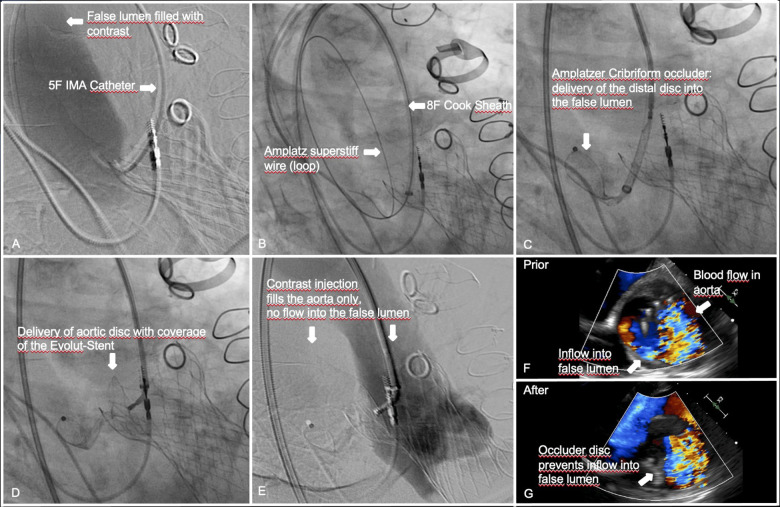
Coronary angiography revealed patent vein grafts to RCA and left circumflex artery, a patent left internal mammary artery (LIMA)—jump to left anterior descending artery/first diagonal and right internal mammary artery (RIMA) T graft to obtuse marginal. The primary tear size was 7 mm in MSCT (Figure 3). We aimed to implant a 25 mm Amplatzer™ Cribriform Septal Occluder transfemorally under general anaesthesia with TOE and fluoroscopic guidance, using the aortic stent edge of the THV as an anchor. The false lumen was cannulated using an internal mammary (IMA) diagnostic catheter (Video 1A) and a soft J-tip Terumo® polymer wire, which was exchanged for an Amplatz® superstiff wire. An 8 Fr Cook® sheath was advanced into the false lumen (Video 1B). The distal disc of the device was unfolded within the false lumen and pulled towards the entry (Video 2A). It did not expand completely but kept a stable position. The proximal disc was then placed into the aorta with half of the disc covering the stent edge of the THV to achieve stability of the device (Video 2B). In TOE valve function was uncompromised, blood flow to the false lumen was significantly reduced (Video 3A). After successful tug test, the occluder was released. A root shot confirmed the TOE result (Video 3B). Procedural steps are summarised in Figure 4. Dual antiplatelet therapy was continued. Rigorous blood pressure treatment with diuretic, AT2-blocker, calcium channel blocker, and beta-blocker revealed 24 h ambulatory measurements of 130/70 mmHg on average. First follow-up MSCT at Week 4 showed the device anchored at the stent of the Evolut valve and the false lumen almost completely filled with thrombus (Figure 5A–C), the second MSCT 3 months later showed a reduction of MAD to 55 mm due to shrinkage of the thrombosed false lumen (Figure 5D and E). The patient was well without symptoms.
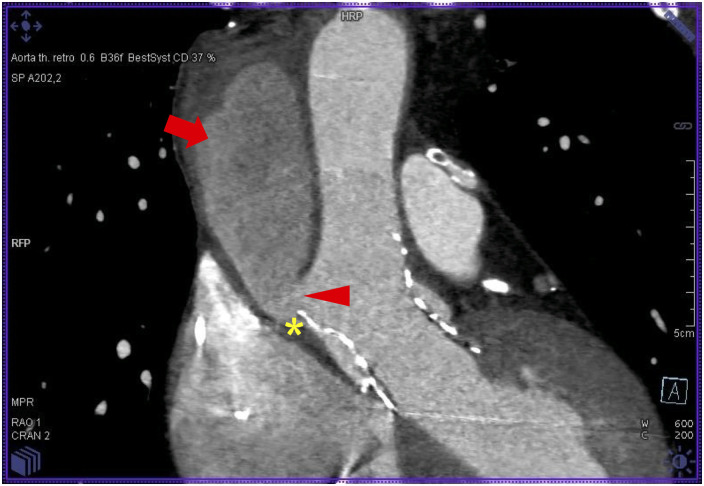
Figure 3.
Multi-slice computed tomography of the type A aortic dissection. Arrowhead indicates the primary tear (7 mm) at the supra-annular aortic edge of the transcatheter heart valve (*). Arrow indicates the perfused false lumen with a small thrombus formation in the upper part.
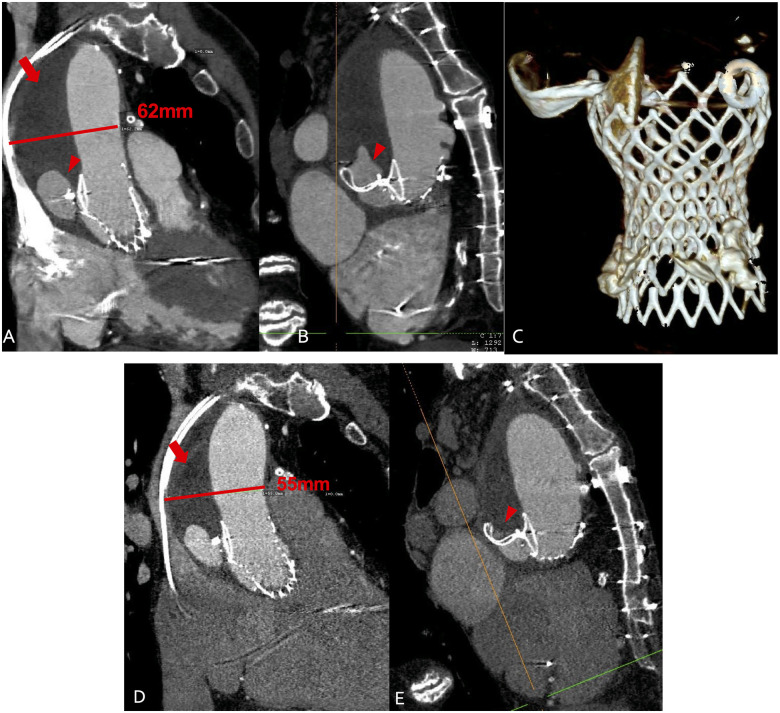
Figure 5.
(A–C) Multi-slice computed tomography 4 weeks after endovascular entry closure. Arrow indicates the false lumen now filled with thrombus. Arrowhead indicates a small residual perfusion. The aortic diameter is unchanged. (C) Three-dimensional reconstruction showing the Occluder fixed at the supra-annular nitinol frame of the Evolut valve. (D and E) Multi-slice computed tomography 12 weeks after endovascular closure. Arrow indicates the false lumen filled with thrombus. Arrowhead indicates the residual perfused lumen now reduced by half. The diameter of the aorta is reduced by 7–55 mm due to thrombus shrinkage.
Figure 4.
Step-by-step endovascular entry closure of a late type A aortic dissection after transcatheter heart valve implantation with an Amplatzer Cribriform PFO Occluder.
Patient perspective
After extensive discussion with the patient about the potential benefit of the procedure in terms of reducing the blood flow to the false lumen to induce thrombosis and, on the other hand, about the limited experience of percutaneous closure of a TAAD with the potential risk of fatal perforation and device embolization among others, the patient was absolutely willing to be treated and gave his written informed consent. Four weeks and 3 months after the procedure the patient felt well and was unaffected in his daily activities.
Discussion
Type A aortic dissection is a rare complication after TAVI with a reported incidence of 0.6–1.9%, mostly occurring within the post-operative course;3 there is only one report of late occurrence 1 year after implantation of an Evolut THV.6 Presumably, late TAAD was caused by the aortic edge of the THV-stent. In that patient, the dissection repair was performed by open vascular surgery with a frozen elephant trunk.6
Considering its prohibitive risk, in our patient surgical repair was not an option, so we strived for endovascular solutions. Others have used a stent graft, which was shortened by removing the distal 3 stent rings not to compromise the THV.7 In our case, however, even a shortened stent graft would have occluded the vein grafts. The only percutaneous option was endovascular closure of the entry. Others have used a vascular plug for treatment of an abdominal aortic aneurysm8 or an Amplatzer Septal Occluder for closure of a traumatic pseudoaneurysm of the ascending aorta.9 We chose a 25 mm Amplatzer™ Cribriform Septal Occluder for two reasons: first, it has a small connecting waist (in contrast to Amplatzer Septal Occluder), which seemed suitable to not enlarge the dissection entry. Second, it consists of two uniformly sized discs with capability to guarantee a stable position by partially covering the supra-annular part of the Evolut stent. A 25 mm device was chosen because it covers the 7 mm entry but does not extend more than 12 mm into the THV, assuring not to compromise the leaflets. Transoesophageal echocardiography imaging quality was excellent, so we decided against other imaging modalities. The procedure was uneventful.
At follow-up we aimed for short-dated rigorous blood pressure control and repeated MSCT. Follow-up computed tomography (CT) at 4 weeks showed almost complete thrombosis of the false lumen and CT at 3 months showed a significant shrinkage of the false lumen. Patient is doing well.
To the best of our knowledge, this is the first description of an endovascular closure of a TAAD occurring late after TAVI.
Lead author biography

Dr Christina Brinkmann works as an interventional cardiologist. She is an operator and scientific researcher in the Structural Heart Program since 2019. She performs percutaneous coronary interventions since 2014.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Funding: None declared.
Supplementary Material
References
- 1.Bonser RS, Ranasinghe AM, Loubani M, Loubani M, Evans JD, Thalji NMA. et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol 2011;58:2455–2474. [DOI] [PubMed] [Google Scholar]
- 2.Chiappini B, Schepens M, Tan E, Dell' Amore A, Morshuis W, Dossche K. et al. Early and late outcomes of acute type A aortic dissection: analysis of risk factors in 487 consecutive patients. Eur Heart J 2005;26:180–186. [DOI] [PubMed] [Google Scholar]
- 3.Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H. et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2010;122:62–69. [DOI] [PubMed] [Google Scholar]
- 4.Langer NB, Hamid NB, Nazif TM, Khalique OK, Vahl TP, White J. et al. Injuries to the aorta, aortic annulus, and left ventricle during transcatheter aortic valve replacement. Circ Cardiovasc Interv 2017;10:e004735. [DOI] [PubMed] [Google Scholar]
- 5.ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 6.Pontious ME, Ashfaq A, Watson JJ, Song HK, Fuss C, Chadderdon SM. et al. Late type A dissection after transfemoral aortic valve replacement. JACC Case Rep 2020;2:877–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berfield KS, Sweet MP, McCabe JM, Reisman M, Mackensen B, Mokadam NA. et al. Endovascular repair for type A aortic dissection after transcatheter aortic valve replacement with a Medtronic CoreValve. Ann Thorac Surg 2015;100:1444–1446. [DOI] [PubMed] [Google Scholar]
- 8.Zander T, Baldi S, Rabellino M, Blasco O, Febles T, Wisniewska K. et al. Successful occlusion of a ruptured aortic aneurysm using the Amplatzer Vascular Plug: a technical note. Cardiovasc Intervent Radiol 2011;34(Suppl 2):136–141. [DOI] [PubMed] [Google Scholar]
- 9.Hussain J, Strumpf R, Ghandforoush A, Wheatley G, Sutherland J.. Transcatheter closure of recurrent aortic pseudoaneurysm previously treated by Amplatzer occlude device. J Vasc Surg 2010;52:196–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.