Abstract
STUDY QUESTION
What demographic and baseline characteristics are predictive of adherence to reproductive medicine clinical trial protocols, live birth or participation in genetic studies?
SUMMARY ANSWER
Race, BMI and lower income are associated with likelihood of non-adherent to reproductive medicine clinical trial protocols, while race influences collection of biological samples and non-adherent to study protocols is associated with lower probability of live birth.
WHAT IS KNOWN ALREADY
Although aspects of adherence to study protocol have previously been evaluated as individual factors in infertile women, the factors that affect overall non-adherent to study protocol have not been previously evaluated.
STUDY DESIGN, SIZE, DURATION
A secondary data analysis of 1650 participants from two prospective multicenter, double-blind controlled studies was carried out: Pregnancy in Polycystic Ovary Syndrome II (PPCOS II) and Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS).
PARTICIPANTS/MATERIALS, SETTING, METHODS
The participants were women aged 18–40 years old with either polycystic ovary syndrome (PCOS) with ovulatory dysfunction in combination with either hyperandrogenemia and/or polycystic ovarian morphology (PPCOS II), or regular ovulatory cycles with unexplained infertility (AMIGOS). The study was carried out in 14 clinical sites in the USA. Non-adherence to clinical trial protocol was chosen as the primary outcome for this analysis. To evaluate whether demographic and baseline characteristics were predictive of adherence to study protocols, live birth or participation in blood sampling for DNA and repository, and pregnancy registry, these putative factors were compared between the outcome measures. Logistic regression was used to establish a prediction model using the putative predictors introduced above.
MAIN RESULTS AND THE ROLE OF CHANCE
Women who self-identified as African American or Asian and those with higher BMI and lower household income were less likely to adhere to protocol. Non-adherence to the study protocol was associated with a lower probability of live birth (odds ratio: 0.180, 95% CI: 0.120, 0.272, P < 0.001). African Americans or Asians were less likely to participate in optional study DNA collection compared to Whites. Participants who were African American or with high annual income or from the Southwest sites or had PCOS were less likely to participate in the blood repository studies.
LIMITATIONS, REASONS FOR CAUTION
Race and ethnicity were self-reported and such self-classification to strict race and ethnicity may not always be representative of a whole racial or ethnic group. This study included two US multicenter trials and therefore the findings may not be extrapolated to international trials.
WIDER IMPLICATIONS OF THE FINDINGS
Identification of populations with low participation is an important initial step, as further investigation can develop specific measures to improve adherence to study protocols and participation in biospecimen banking and thereby extend the representativeness of reproductive medicine clinical trial findings.
STUDY FUNDING/COMPETING INTEREST(S)
Supported by NIH Eunice Kennedy Shriver NICHD Grants: U10 HD39005, U10 HD38992, U10 HD27049, U10 HD38998, U10 HD055942, HD055944, U10 HD055936, U10HD055925, PPCOSII: U10 HD27049, U10 HD38992, U10 HD055925, U10 HD39005, U10 HD38998, U10 HD055936, U10 HD055942, U10 HD055944; Clinical Reproductive Endocrine Scientist Training Program (CREST): R25HD075737. Outside this study, M.P.D. received NIH/NIHCD research grant and R.S.L. received research grant from Ferring and was consultant for Bayer, Kindex, Odega, Millendo and AbbVie.
TRIAL REGISTRATION NUMBER
ClinicalTrials.gov number: NCT00719186; NCT01044862
Keywords: adherence to study protocol, non-adherence, medication compliance, dropout, race, ethnicity, live birth, biospecimen banking, randomized controlled trial, pregnancy registry
Introduction
Randomized controlled trials (RCTs) have been universally accepted as the gold standard for research design for evaluating the effectiveness of medications, technologies and protocols (Sibbald and Roland, 1998). The results of such trials are therefore important to guide management and decision-making and in developing consensus guidelines. The success of a clinical trial is dependent on several factors, including the ability to recruit and retain patients (Walters et al., 2017), as well as collect and analyze data. The high percentage of recruitment and retention failures in some clinical trials (Walters et al., 2017) affects their reliability as well as the internal and external validity of the outcome of such trials (Shiovitz et al., 2016). Improved adherence to clinical trial protocols enables reliable research results and helps identify effective therapies for various diseases. Moreover, accurate data collection and analysis is feasible only if study participants attend visits, follow directions and adhere to the medication and testing regimen.
Although several studies in other specialties, such as cervical cancer and hypertension research, have examined various strategies to improve retention and adherence to clinical trials in general (Grant and DePew, 1999; Shumaker et al., 2000; Bailey et al., 2004), only a few have evaluated factors that predict retention and adherence in clinical trials including race, ethnicity, socio-economic status and sex, and these have had varying results (Moore, 1997; Gorelick et al., 1998; Bowen et al., 2000). Non-adherence to study protocol in trials involving infertile women may include failure to attend critical study visits, take the assigned medication (McGovern et al., 2008) maintain a prescribed schedule of timed intercourse (Pagidas et al., 2010), and continue participation and follow-up (Kuang et al., 2015). Identifying factors that predict overall non-adherence to study protocol, and the effect of non-adherence upon the primary study outcomes, may help to underscore the importance of adherence, and lead to development of targeted strategies to facilitate adherence to protocol.
Race, ethnicity, BMI, insurance coverage and history of smoking or alcohol use previously have been shown to be predictors of study retention and dropout in Reproductive Medicine Network (RMNs) trials involving women with infertility (Kuang et al., 2015). While medication adherence (McGovern et al., 2008) and intercourse compliance (Pagidas et al., 2010) as individual factors did not affect pregnancy rates, the effect of a composite non-adherence to a study protocol, that includes all these factors, on the outcome of interventional trials in infertile women has not been previously evaluated.
Understanding the role of genetic factors in disease occurrence and the prediction of infertility treatment outcome are essential in advancing personalized medicine. To that end, a biospecimen and data bank that contains samples representative of the study population contributes to the accuracy of future research (Krawetz et al., 2011). The success of such biobanks depends on successfully recruiting participants willing to provide the appropriate and complete biological samples. Understanding factors that are associated with providing samples for bio banking may be important in increasing uptake in a wider and more representative population that serves as a resource of biological samples for future studies. It has been shown that among other factors, African American women are less likely to enroll in cancer genetic registries than are White women (Moorman et al., 2004), although there are no such studies in reproductive medicine research.
Factors that predict adherence to study protocol and participation in biospecimen banking in reproductive medicine clinical trials may be entirely different from similar studies in the general population, because patients with infertility may be considered more motivated than the general population. We therefore performed a secondary analysis of two RMN trials to evaluate whether baseline characteristics, such as race/ethnicity, socio-economic factors and duration of infertility, were predictive of adherence to study protocol, and whether adherence to protocol is associated with live birth outcomes. We also evaluated factors that are associated with optional participation in biobank and pregnancy registry studies.
Materials and methods
Study design and trials included
The data were derived from two concurrent trials carried out by the National Institute of Child Health and Human Development Cooperative Reproductive Medicine Network: Pregnancy in Polycystic Ovary Syndrome II (PPCOS II) and Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) (Legro et al., 2014; Diamond et al., 2015). Institutional Review Board approval was obtained at each study site prior to initiation of the primary trials and participants underwent the written informed consent process.
The PPCOS II trial (Legro et al., 2014) was a multicenter, randomized, controlled, double-blind clinical trial conducted at 11 clinical sites across the USA (ClinicalTrials.gov number: NCT00719186). The purpose of the trial was to determine live birth rates after clomiphene citrate or letrozole ovulation induction for up to five treatment cycles in 750 infertile women with polycystic ovary syndrome (PCOS). Women aged 18–40 years with PCOS and evidence of a normal uterine cavity and at least one patent fallopian tube, with a partner whose sperm concentration was at least 14 million/ml with some motile sperm within the previous year, were included in the study. Couples with other causes of infertility were excluded.
AMIGOS (Diamond et al., 2015) was a multicenter, randomized, controlled, double-blind clinical trial conducted at 12 clinical sites across the USA (ClinicalTrials.gov number: NCT01044862). The purpose of the trial was to determine live birth rates after treatment with up to four cycles of letrozole versus clomiphene citrate versus gonadotrophin, with hCG triggering of ovulation in conjunction with IUI, in 900 couples with unexplained infertility. Women were 18–40 years of age with regular ovulatory menstrual cycles, had a normal uterine cavity with at least one patent fallopian tube, and a male partner with a semen specimen with a minimum of 5 million total motile sperm/ml.
Study variables
The primary objective was to determine the predictive factors for adherence to study protocol and therefore non-adherence to clinical trial protocol was chosen as the primary outcome for this analysis. We defined non-adherence as not being compliant to at least one of the following: not taking study medications as directed; not attending two or more study visits; not adhering to the schedule of timed intercourse (PPCOS II) or IUI (AMIGOS); or dropping out before study completion.
Medication compliance was determined prospectively during the trials by inspecting the returned medication bottles and comparing to expected pill or number of vials of recommended gonadotrophin remaining when these were returned. Participants who missed two or more study visits were considered non-compliant. Intercourse compliance in the PPCOSII was determined from participants’ written, prospective diaries, which were reviewed by study staff. Intercourse frequency of 2–3/week was recommended in the study protocol and was considered as intercourse compliant, and a frequency of ≤1/week as non-compliant. Failure to keep an intercourse diary with entries of intercourse frequency was considered non-compliant. Participants who discontinued the trial before achieving live birth or before the completion of the trial protocol and follow-up were considered to have dropped out.
When data were missing for any of the four variables despite known attendance at the study visit, these variables were considered as adherent.
All couples completed several psychological instruments at the time of screening including the Primary Care Evaluation of Mental Disorders (PRIME-MD) and total fertility-related quality of life (FertiQoL). The PRIME-MD was used for diagnosis of major psychiatric disorder and psychiatric syndrome (Spitzer et al., 1994, 2000). FertiQoL is an assessment of infertility on the quality of life and is scored as 0–100 (Boivin et al., 2011; Santoro et al., 2016).
Putative factors associated with adherence included certain data collected at the screening or baseline visit: demographic and baseline characteristics, such as race/ethnicity, age, prior parity, BMI, educational status, annual household income, insurance coverage, smoking, prior infertility treatment, duration of infertility, FertQoL score and PRIME-MD. Covariates were study type (PPCOS II or AMIGOS) and study sites.
Participants self-reported race as Black (African American), White, Asian, American Indian or Native Alaskan Americans, or Native Hawaiian or Pacific Islander and Mixed Race. Any participant who reported more than one race was considered Mixed Race. Ethnicity was reported as Hispanic or Non-Hispanic. Insurance status was classified into Managed Care Plan or Health Maintenance Organization (HMO), Other Private Insurance, Medicaid, Medicare or self-pay/uninsured. Educational status was classified as high school graduate or less, college graduate or some college, or graduate degree. There were 14 study sites, which were categorized into five geographic regions: Northeast, Midwest, Southwest, Southeast and West.
Biospecimen banking and pregnancy registry
To determine if baseline and demographic data predicted participation in optional biospecimen banking and pregnancy registry, we evaluated three outcome measures: participants agreed to collection of study DNA; participants consented to store blood in the biospecimen repository; and participants agreed to take part in the Pregnancy Registry. The Pregnancy Registry was established as a distinct protocol to collect the pregnancy and birth outcomes, and this required a separate consent. It included information regarding pregnancy loss, neonatal morbidity and mortality, and fetal anomalies as well as infants’ developmental milestones at annual intervals up to 3 years. We also reviewed whether there were differences in the reasons for refusal to provide biological samples among demographic characteristics. The reasons for refusal included: too much blood to be drawn; worried about future use of biological specimens, worried about access to health information by insurance companies and other reasons.
Statistical analysis
To evaluate whether demographic and baseline characteristics were predictive of adherence to study protocols, live birth or participation in genetic studies, the putative factors were compared between outcome measures.
Student’s t-test, χ2 or Fisher’s exact tests were performed to compare outcome measures to the putative predictors depending on the data type (continuous or categorical) and distribution (normal or not) of a predictor. Logistic regression was used to establish a prediction model using the putative predictors introduced above. Variables were introduced into a multivariate logistic regression analysis in a stepwise fashion, with a P-value of <0.10 to enter and P-value of <0.05 to stay. Prior parity and PRIME-MD had 20% missing values. We performed missing data imputation and introduced them in the logistic regression models.
We present tables with odds ratios (ORs) and the corresponding 95% CIs for the predictors for adjusted logistic regression analysis. SAS 9.4 (Cary, NC, USA) was used for all the analyses.
Results
All 1650 female participants from the AMIGOS (n = 900) and PPCOS II (n = 750) trials were included in the study (Fig. 1). Of these, 1188 participants (72%) met the definition for adherence to study protocol and 462 (28%) were non-adherent to some aspect of the protocol. Of the 462 non-adherent participants, 312 participants (67.53%) dropped out before study completion; 115 couples (24.89%) were non-compliant with intercourse schedule but none of the patients missed an appointment for IUI; 19 women (4.11%) were non-compliant with medication; and 16 women (3.46%) were non-compliant with study visits.
Figure 1.
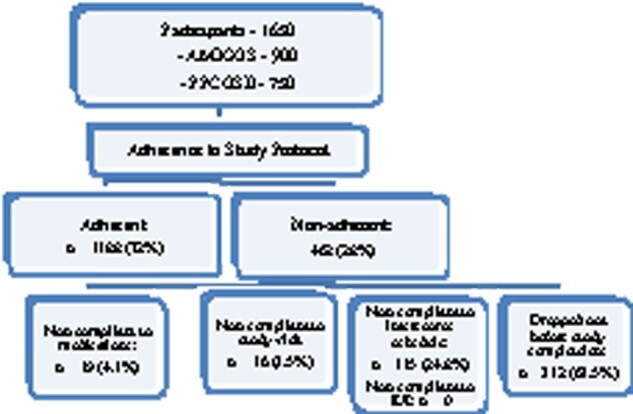
Flowchart of participants in the study of adherence in reproductive medicine clinical trials. AMIGOS, Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation; PPCOS II, Pregnancy in Polycystic Ovary Syndrome II.
Descriptive summary
Baseline characteristics for adherence to the study protocol overall, and in the different trials, is presented in Table I. Non-adherence to study protocol was significantly different between racial groups, with differences more specific to the PPCOS II participants (P < 0.001). African American women as well as Asians were more likely to be non-adherent. In the PPCOS II study, 82.9% were White, 7.6% were African American and 5.9% were Asian among those who were adherent to study protocol, whereas 69.1% were White, 16.2% were Black and 8.9% were Asian among those who were non-adherent.
Table I.
Baseline characteristics of participants in a study of adherence to reproductive medicine clinical trial protocols.
| Variables | All subjects |
P-value | AMIGOS |
P-value | PPCOS II |
P-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Adherent | Non-adherent | Adherent | Non-adherent | Adherent | Non-adherent | ||||
| (n = 1188) | (n = 462) | (n = 722) | (n = 178) | (n = 466) | (n = 284) | ||||
| Race | 0.002 | 0.001 | 0.601 | ||||||
| White | 975/1188 (82.07) | 337/462 (72.94) | 599/722 (82.96) | 123/178 (69.10) | 376/466 (80.69) | 214/284 (75.35) | |||
| Black or African American | 111/1188 (9.34) | 73/462 (15.80) | 55/722 (7.62) | 29/178 (16.29) | 56/466 (12.02) | 44/284 (15.49) | |||
| Asian | 56/1188 (4.71) | 27/462 (5.84) | 43/722 (5.96) | 16/178 (8.99) | 13/466 (2.79) | 11/284 (3.87) | |||
| American Indian or Alaska Native | 12/1188 (1.01) | 5/462 (1.08) | 8/722 (1.11) | 2/178 (1.12) | 4/466 (0.86) | 3/284 (1.06) | |||
| Native Hawaiian or Pacific Islander | 1/1188 (0.08) | 1/462 (0.22) | 0/722 (0.00) | 0/178 (0.00) | 1/466 (0.21) | 1/284 (0.35) | |||
| Mixed race | 33/1188 (2.78) | 19/462 (4.11) | 17/722 (2.35) | 8/178 (4.49) | 16/466 (3.43) | 11/284 (3.87) | |||
| Ethnic group | 0.057 | 0.496 | 0.370 | ||||||
| Not Hispanic or Latino | 1040/1188 (87.54) | 388/462 (83.98) | 649/722 (89.89) | 157/178 (88.20) | 391/466 (83.91) | 231/284 (81.34) | |||
| Hispanic or Latino | 148/1188 (12.46) | 74/462 (16.02) | 73/722 (10.11) | 21/178 (11.80) | 75/466 (16.09) | 53/284 (18.66) | |||
| Age, years (total n) | 30.86 ± 4.40 (1188) | 30.22 ± 4.93 (462) | 0.015 | 32.14 ± 4.14 (722) | 32.42 ± 4.67 (178) | 0.472 | 28.87 ± 4.05 (466) | 28.84 ± 4.58 (284) | 0.923 |
| BMI, kg/m2 (total n) | 29.79 ± 8.36 (1188) | 32.90 ± 9.78 (462) | <0.001 | 26.88 ± 6.50 (722) | 27.12 ± 6.72 (178) | 0.671 | 34.30 ± 8.91 (466) | 36.53 ± 9.65 (284) | 0.001 |
| Prior parity | 249/1188 (20.96) | 90/462 (19.48) | 0.057 | 149/722 (20.64) | 38/178 (21.35) | 0.496 | 100/466 (21.46) | 52/284 (18.31) | 0.370 |
| Insurance coverage | <0.001 | 0.001 | 0.382 | ||||||
| Managed Care Plan or HMO | 773/1188 (65.07) | 267/462 (57.79) | 495/722 (68.56) | 115/178 (64.61) | 278/466 (59.66) | 152/284 (53.52) | |||
| Other Private Insurance | 231/1188 (19.44) | 72/462 (15.58) | 167/722 (23.13) | 31/178 (17.42) | 64/466 (13.73) | 41/284 (14.44) | |||
| Medicaid | 24/1188 (2.02) | 25/462 (5.41) | 5/722 (0.69) | 7/178 (3.93) | 19/466 (4.08) | 18/284 (6.34) | |||
| Medicare | 6/1188 (0.51) | 4/462 (0.87) | 3/722 (0.42) | 1/178 (0.56) | 3/466 (0.64) | 3/284 (1.06) | |||
| Self-pay/uninsured | 154/1188 (12.96) | 94/462 (20.35) | 52/722 (7.20) | 24/178 (13.48) | 102/466 (21.89) | 70/284 (24.65) | |||
| Education | <0.001 | 0.869 | 0.019 | ||||||
| High school graduate or less | 155/1188 (13.05) | 91/462 (19.70) | 57/722 (7.89) | 16/178 (8.99) | 98/466 (21.03) | 75/284 (26.41) | |||
| College graduate or some college | 778/1188 (65.49) | 302/462 (65.37) | 474/722 (65.65) | 115/178 (64.61) | 304/466 (65.24) | 187/284 (65.85) | |||
| Graduate degree | 255/1188 (21.46) | 69/462 (14.94) | 191/722 (26.45) | 47/178 (26.40) | 64/466 (13.73) | 22/284 (7.75) | |||
| Income | <0.001 | 0.002 | 0.009 | ||||||
| <US$50 000 | 279/995 (28.04) | 173/390 (44.36) | 107/592 (18.07) | 45/150 (30.00) | 172/403 (42.68) | 128/240 (53.33) | |||
| ≥US$50 000 | 716/995 (71.96) | 217/390 (55.64) | 485/592 (81.93) | 105/150 (70.00) | 231/403 (57.32) | 112/240 (46.67) | |||
| History of smoking | 0.007 | 0.160 | 0.243 | ||||||
| Never smoked | 765/1188 (64.39) | 261/462 (56.49) | 485/722 (67.17) | 108/178 (60.67) | 280/466 (60.09) | 153/284 (53.87) | |||
| Current smoking | 118/1188 (9.93) | 64/462 (13.85) | 52/722 (7.20) | 19/178 (10.67) | 66/466 (14.16) | 45/284 (15.85) | |||
| Quit smoking | 305/1188 (25.67) | 137/462 (29.65) | 185/722 (25.62) | 51/178 (28.65) | 120/466 (25.75) | 86/284 (30.28) | |||
| Duration of infertility | 0.044 | 0.431 | 0.389 | ||||||
| <2 years | 440/1164 (37.80) | 148/442 (33.48) | 265/715 (37.06) | 56/176 (31.82) | 175/449 (38.98) | 92/266 (34.59) | |||
| 2–4 years | 481/1164 (41.32) | 177/442 (40.05) | 333/715 (46.57) | 89/176 (50.57) | 148/449 (32.96) | 88/266 (33.08) | |||
| >4 years | 243/1164 (20.88) | 117/442 (26.47) | 117/715 (16.36) | 31/176 (17.61) | 126/449 (28.06) | 86/266 (32.33) | |||
| History of alcohol use | |||||||||
| Never used | 136/1188 (11.45) | 62/462 (13.42) | 0.247 | 78/722 (10.80) | 28/178 (15.73) | 0.114 | 58/466 (12.45) | 34/284 (11.97) | 0.792 |
| Current alcohol use | 831/1188 (69.95) | 304/462 (65.80) | 542/722 (75.07) | 121/178 (67.98) | 289/466 (62.02) | 183/284 (64.44) | |||
| Quit alcohol use | 221/1188 (18.60) | 96/462 (20.78) | 102/722 (14.13) | 29/178 (16.29) | 119/466 (25.54) | 67/284 (23.59) | |||
| History of prior infertility treatment | 657/1188 (55.30) | 258/462 (55.84) | 0.843 | 404/722 (55.96) | 95/178 (53.37) | 0.556 | 253/466 (54.29) | 163/284 (57.39) | 0.449 |
| Total FertiQol score | 75.82 ± 13.41 (1158) | 72.46 ± 15.11 (440) | <0.001 | 77.07 ± 12.70 (699) | 77.01 ± 13.33 (166) | 0.956 | 73.93 ± 14.24 (459) | 69.71 ± 15.48 (274) | <0.001 |
| PRIME-MD | 0.449 | 0.250 | 0.528 | ||||||
| Major depressive syndrome | 11/59 (18.64) | 11/33 (33.33) | 3/23 (13.04) | 0/0 (0.00) | 8/36 (22.22) | 11/32 (34.38) | |||
| Other depressive syndrome | 32/59 (54.24) | 14/33 (42.42) | 13/23 (56.52) | 0/0 (0.00) | 19/36 (52.78) | 14/32 (43.75) | |||
| Panic syndrome | 9/59 (15.25) | 5/33 (15.15) | 5/23 (21.74) | 0/0 (0.00) | 4/36 (11.11) | 5/32 (15.63) | |||
| Other anxiety syndrome | 7/59 (11.86) | 3/33 (9.09) | 2/23 (8.70) | 1/1 (100.00) | 5/36 (13.89) | 2/32 (6.25) | |||
| Psychiatric syndrome | 71/1151 (6.17) | 40/441 (9.07) | 0.042 | 28/693 (4.04) | 1/164 (0.61) | 0.028 | 43/458 (9.39) | 39/277 (14.08) | 0.054 |
AMIGOS, Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation; FertiQol, total fertility-related quality of life; PPCOS II, Pregnancy in Polycystic Ovary Syndrome II; PRIME-MD, Primary Care Evaluation of Mental Disorders.
Student’s t-test and χ2 or Fisher’s exact tests were performed for continuous or categorical variables, respectively.
Data are presented as mean ± SD, unless otherwise stated.
Values in parentheses represent percentages unless otherwise stated.
Women who were non-adherent were younger than those who were adherent (P = 0.015), although there were no specific differences in age between those who were adherent and non-adherent in the PPCOS II and AMIGOS trials. Those who were non-adherent to protocol had a higher BMI than those who were adherent to protocol; this was more pronounced in the AMIGOS trial where BMI was higher in the non-adherent group (P = 0.001). Women who were high school graduate or less were more likely to be non-adherent to study protocol (P < 0.001); this difference is most pronounced in the AMIGOS study (P = 0.019). Differences in income were consistent in both studies, and women with low income (US$50 000) were more likely to be non-adherent in both the PPCOS II (P = 0.002) and AMIGOS (P = 0.009) trials.
Overall, current smokers (P = 0.007) and women with longer duration of infertility (P = 0.044) were more likely to be non-adherent, although there were no specific differences between those who were adherent or non-adherent in both the PPCOS II and AMIGOS trials. Women with lower total FertilQol score, and a higher proportion of patients with a psychiatric syndrome, were non-adherent to the study protocol.
Predictors for adherence to study protocol
The data were pooled and variables including race, ethnicity, education, age, BMI, insurance, income, smoking history, prior infertility treatment, duration of infertility and baseline FertiQol total score were introduced to a multivariate logistic regression analysis in a stepwise fashion to determine predictive probability of non-adherence to study protocol (Table II). Self-identified African American (P < 0.005) and Asian (P < 0.003) participants were more likely to be non-adherent to the study protocol compared with Whites. Ethnicity was not associated with non-adherence to study protocol and therefore did not enter the final model. A higher BMI, assessed as a continuous variable, was associated with non-adherence to study protocol (P = 0.041). Women with lower annual household income of <US$50 000 were also less adherent to the study protocol (P = 0.004). Participants in the PPCOSII trial were more likely to be non-adherent to protocol compared with the AMIGOS trial (P < 0.001). There was a trend for women who were current smokers to be non-adherent (P = 0.063), but the difference was not significant, although women who quit smoking were more likely to be non-adherent to study protocol than women who never smoked (P = 0.020).
Table II.
Adjusted odds ratios for predictive variables and participants not adherent to protocols (with covariates of study type and site region added into the model).
| Variables | Not adherent to protocols |
|
|---|---|---|
| Odds ratio (95% CI) | P-value | |
| Race | ||
| White | 1 | |
| Black or African American | 1.747 (1.184, 2.577) | 0.005 |
| Asian | 2.359 (1.343, 4.146) | 0.003 |
| American Indian or Alaska Native | 1.520 (0.433, 5.337) | 0.514 |
| Native Hawaiian or Pacific Islander | 2.229 (0.138, 36.060) | 0.573 |
| Mixed race | 1.599 (0.865, 2.954) | 0.134 |
| BMI (kg/m2) | 1.016 (1.001, 1.032) | 0.041 |
| Income | ||
| <US$50 000 | 1 | |
| ≥US$50 000 | 0.674 (0.516, 0.882) | 0.004 |
| History of smoking | ||
| Never smoked | 1 | |
| Current smoking | 1.453 (0.980, 2.155) | 0.063 |
| Quit smoking | 1.401 (1.056, 1.860) | 0.020 |
| Study type | ||
| AMIGOS | 1 | |
| PPCOSII | 1.819 (1.373, 2.411) | <0.001 |
Non-adherence to study protocol and probability of live birth
We evaluated differences in baseline characteristics between women who had live birth and those who did not (Supplementary Table SI). There were differences between women who had live birth and those who did not in baseline characteristics and protocol adherence. In this dataset, older age (P = 0.001), higher BMI (P < 0.001), low annual income (<US$50 000) (P = 0.032), history of smoking (P = 0.014), longer duration of infertility (P < 0.001), history of prior infertility treatment (P = 0.009) and non-adherence to protocols (P < 0.001) were less likely to result in live birth.
The adjusted ORs for predictive factors for live birth are presented in Table III. Non-adherence to study protocol resulted in a lower probability of live birth (OR: 0.180, 95% CI: 0.120, 0.272, P < 0.001). Moreover, participants who were older, quit smoking, and those with a longer duration of infertility of 2–4 years or over 4 years, as well as those who had a prior pregnancy loss, had a lower probability of having a live birth. Participants who had gonadotrophin treatment had a higher probability of live birth compared to clomiphene treatment (0.009).
Table III.
Adjusted odds ratios for predictive variables and participants who had live birth (with covariates of study type and site region added into the model).
| Variables | Live birth |
|
|---|---|---|
| Odds ratio (95% CI) | P-value | |
| Age | 0.930 (0.901, 0.961) | <0.001 |
| History of smoking | ||
| Never smoked | 1 | |
| Current smoking | 0.843 (0.522, 1.362) | 0.484 |
| Quit smoking | 1.379 (1.029, 1.847) | 0.031 |
| Duration of infertility | ||
| <2 years | 1 | |
| 2–4 years | 0.564 (0.421, 0.756) | <0.001 |
| >4 years | 0.534 (0.367, 0.776) | 0.001 |
| Prior pregnancy loss | ||
| Yes | 1 | |
| No | 0.626 (0.463, 0.847) | 0.002 |
| Adherence to study | ||
| Yes | 1 | |
| No | 0.180 (0.120, 0.272) | <0.001 |
| Treatment | ||
| Clomiphene | 1 | |
| Letrozole | 1.098 (0.816, 1.479) | 0.536 |
| Gonadotropin | 1.624 (1.131, 2.330) | 0.009 |
Since many of the factors that are associated with live birth may also be associated with adherence to study protocol, we ran an additional logistic regression analysis by adding the interaction between adherence and each of the other factors into the live birth model. All variables were introduced into a multivariable logistic regression analysis in a stepwise fashion with a P-value <0.10 to enter and P-value of <0.05 to stay. The results showed that none of the interaction variables were statistically significant (Supplementary Table SII).
Participation in ancillary protocols (biospecimen banking and pregnancy registry)
Study DNA collection
In total, 86.3% (1350/1565) participants agreed to study DNA (blood sample) collection and 13.7% (215/1565) participants did not agree. Race, BMI, prior parity, insurance coverage and history of smoking were related to whether participants agreed to the study DNA collection or not (Supplementary Table SIII). Adjusted ORs showed that only race and the study type were predictive of participation in study DNA collection (Table IV). More specifically, African Americans (P < 0.001), Asians (P = 0.016) or Native Hawaiian/Pacific Islanders (P = 0.032) were less likely to participate in study DNA collection when compared with Whites. Participants with a higher BMI (OR: 1.051, 95% CI: 1.013, 1.062; P = 0.004) were more likely to agree to study DNA collection but when study type and geographic regions were added as covariates, it was no longer significant. Participants in the PPCOSII study (P < 0.001) were more likely to agree to participate.
Table IV.
Factors associated with ancillary study participation: biospecimen banking and pregnancy register.
| Adjusted odds ratios for predictive variables and participants agreed to blood sampling for DNA (with covariates of treatment protocol and site region added into the model). | ||
|---|---|---|
| Variables | Agreed to the study DNA collection |
|
| Odds ratio (95% CI) | P-value | |
| Race | ||
| White | 1 | |
| Black or African American | 0.284 (0.173, 0.467) | <0.001 |
| Asian | 0.420 (0.216, 0.852) | 0.016 |
| American Indian or Alaska Native | 0.462 (0.096, 2.221) | 0.335 |
| Native Hawaiian or Pacific Islander | 0.046 (0.003, 0.766) | 0.032 |
| Mixed race | 0.817 (0.311, 2.145) | 0.681 |
| Age | 1.044 (1.001, 1.089) | 0.043 |
| Study type | ||
| AMIGOS | ||
| PPCOSII | 3.349 (2.152, 5.212) | <0.001 |
|
Adjusted odds ratios for predictive variables and participants agreed to take part in blood repository collection or blood storage for future use (with covariates of study type and site region added into the model). | ||
|---|---|---|
| Variables | Agreed to take part in blood repository collection or blood storage for future use |
|
| Odds ratio (95% CI) | P-value | |
| Race | ||
| White | 1 | |
| Black or African American | 0.308 (0.189, 0.503) | <0.001 |
| Asian | 0.726 (0.358, 1.474) | 0.376 |
| American Indian or Alaska Native | 0.489 (0.100, 2.401) | 0.378 |
| Native Hawaiian or Pacific Islander | 0.093 (0.005, 1.597) | 0.102 |
| Mixed race | 0.874 (0.357, 2.143) | 0.769 |
| BMI | 1.027 (1.002, 1.052) | 0.031 |
| Income | ||
| <$50 000 | 1 | |
| ≥$50 000 | 1.520 (1.038, 2.227) | 0.032 |
| Region | ||
| Northeast | 1 | |
| Midwest | 1.111 (0.683, 1.808) | 0.190 |
| Southeast | 0.321 (0.184, 0.561) | 0.052 |
| Southwest | 1.750 (0.917, 3.340) | 0.003 |
| West | 0.585 (0.359, 0.953) | 0.784 |
| Study type | ||
| AMIGOS | 1 | |
| PPCOSII | 2.056 (1.360, 3.110) | 0.001 |
| Adjusted odds ratios for predictive variables and participants agreed to take part in pregnancy registry (with covariates of study type and site region added into the model). | ||
|---|---|---|
| Variables | Agreed to take part in pregnancy registry |
|
| Odds ratio (95% CI) | P-value | |
| Duration of infertility | ||
| <2 years | 1 | |
| 2–4 years | 0.551 (0.369, 0.822) | 0.003 |
| >4 years | 0.740 (0.440, 1.247) | 0.259 |
| Study type | ||
| AMIGOS | 1 | |
| PPCOSII | 1.547 (1.072, 2.233) | 0.020 |
Blood repository collection or blood storage for future use
A total of 86.8% (1433/1650) participants agreed to blood repository collection and blood storage for future use, while 13.2% (217/1650) participants did not agree. Race, age, insurance coverage and income were associated with whether participants agreed to participate in blood repository or blood storage or the future (Supplementary Table SIII). Adjusted ORs showed that race, study type and geographic region of the study were predictive of whether a participant agreed for blood repository (Table IV). Participants who were older (OR: 1.039 95% CI: 1.001, 1.078, P = 0.043) were more likely to agree to take part in blood repository collection, but when covariates of study type and site regions were added into the model, this finding was no longer significant and therefore was not included in the final model. Participants who were African American (P < 0.001), increasing BMI (P = 0.031) or with high annual income (>US$50 000) (P = 0.032) or were from the Southwestern region (P < 0.003) or in the PPCOSII Study (P = 0.001) were less likely to participate in the blood repository studies.
Pregnancy registry
In total, 47% (277/589) of women agreed to participate in the pregnancy registry and 53% (312/589) participants (53%) did not agree. Age and duration of infertility were associated with participants agreeing to take part in the pregnancy registry (Supplementary Table SIII). The adjusted ORs, controlling for study site, region and study type, showed that duration of infertility and study type were predictive of participation in the pregnancy registry (Table IV). Participants who had 2–4 years duration of infertility (P = 0.003), and in the AMIGOS trial (P = 0.020) were less likely to participate in pregnancy registry.
Reasons for not participating in biospecimen banking and pregnancy registry
We evaluated whether there were any differences among baseline characteristics associated with non-participation in genetic/repository collection. The reasons for refusal included too much blood to be drawn, worry about future use of biological samples, worry about access of health information by insurance companies and other reasons. Multivariate analysis showed that participants who were African American, Asian or American Indian or Alaska Native were more likely to refuse to provide biological samples because too much blood was to be drawn (Supplementary Table SIV), whilst participants with higher BMI were more likely to refuse to provide biological samples because they were worried about future use of biological samples (Supplementary Table SIV). In this analysis, there were no differences among groups regarding likelihood to refuse to provide biological samples because of concerns regarding access of health information by insurance companies or other reasons.
We evaluated whether reasons for participation in the pregnancy registry varied by baseline characteristics. The reasons for refusal included: does not want dysmorphology examination of their baby, worried about future use of biological samples, does not want to provide infant saliva sample, time commitment and other reasons. Participants with a high total FertiQol score were less likely to be worried about future use of biological specimens (OR: 0.948 95% CI: 0.911, 0.987, P = 0.009) and those who were not adherent to study protocol were less likely to use time commitment as a reason for refusal to participate in pregnancy registry (OR: 0.096 95% CI: 0.013, 0.708, P = 0.022). However, adjusted ORs did not show any differences between participant groups regarding the other reasons for not participating in the pregnancy registry.
Discussion
In this study, we have shown that African Americans and Asians were less likely to be adherent to study protocols and also less likely to agree to collection of biological samples for DNA or repository. Participants with a higher BMI or those with lower income were also more likely to have a non-adherent event. Moreover, participants who were non-adherent to study protocol had a dramatically lower probability of live birth. Hispanics were equally likely to be adherent to study protocols as non-Hispanic participants.
It is ironic and extra challenging that the very populations one wants to learn more from to increase representativeness are less likely to collaborate with the researchers (Harris et al., 1996; Arnold et al., 2014). Less participation and adherence to study protocols imply less data, leading to fewer breakthroughs applicable to those populations. It is therefore of utmost importance to make every effort to understand how to improve the representativeness of such groups in clinical trials.
Race is a well-known predictor of adherence to research study protocols in other non-reproductive medicine trials (Ford et al., 2003; Murthy et al., 2004; Douketis et al., 2005). In this study, African Americans (70% more likely) and Asians (twice as likely) were more likely to be non-adherent to study protocol. Participants who did not complete the prescribed medications were considered non-compliant. This is consistent with previous studies showing that African Americans may be over 80% less likely to adhere to cardiac or hypertensive medications (Douglas et al., 2002; Charles et al., 2003).
We can only postulate potential reasons for lack of medication adherence, maintenance of scheduled intercourse or study visit non-compliance. Lack of motivation may be a reason for non-adherence in other areas of medicine, but it would be expected that this may be less prevalent in patients who are infertile and therefore usually highly motivated to conceive. Lack of appropriate education about the study, and participants’ educational status, their ability to understand the study protocol and rationale for medication compliance, and the importance of attending study visits, are important factors. However, in this study, educational status was not a significant predictive factor for study non-adherence, although women of a lower-income status were more non-adherent to study protocol.
Different types of beliefs, including behavioral beliefs, control beliefs and normative beliefs, may influence compliance to medications in the African American population. Although patients may be fully knowledgeable about the benefits of compliance with medications, the side effects of medications, daily stresses of life related to limited financial resources, neighborhood violence and distrust of medical professionals are potential barriers to medication compliance (Lukoschek, 2003; Fongwa et al., 2008; Lewis et al., 2010). Although we found that participants with a lower income were less adherent to study protocol, one would have expected that financial constraints would not be a barrier to medication compliance, since patients did not have to pay for medications in this study. However, it is conceivable that daily stresses related to issues such as limited financial resource in general, as previously described, are a barrier to medication and study protocol compliance. Some participants may experience hardships that may interfere with showing up for research appointments or being compliant with medication. It is therefore important that staff members are focused and insistent in addressing adherence problems, and identifying and addressing potential underlying factors for non-adherence (Zweben et al., 2009).
Since non-adherence to study protocol was associated with negative outcome, it is important to address the modifiable factors that might increase compliance in study protocols in the future. Although intention-to-treat analysis may partially address this problem statistically, inclusion of a wide spectrum of participants is important for obtaining the best answers to research questions. Thus, targeting behavioral and normative beliefs may improve adherence. It has been suggested that approval and support from family and friends may be associated with medication adherence (Lewis et al., 2010). It may therefore be important to screen individuals for their available social support systems and educate them to use these systems. Moreover, educating patients regarding managing their daily stresses may improve compliance. The development and maintenance of trust with research participants is essential in maintaining commitment and ensuring a vested interest in the study. In this study, participants with lower income, but not the less educated, were less adherent, which suggests a class barrier that is often trust related. Building trust will include making the study as smooth and enjoyable as possible and having a welcoming and respectful staff as well as having a nonjudgmental and accepting attitude toward participants (Zweben et al., 2009).
Blood repository or storage for future DNA is an important resource for personalized medicine. Understanding factors that may affect participation in ancillary protocol enhancements, such as biospecimen repositories, especially in women who are already part of an ongoing trial, is important. African Americans in this reproductive medicine clinical trial were less likely to participate in study DNA collection as well as blood repository collection or blood storage, which is consistent with studies in other areas of medicine (Moorman et al., 2004).
General mistrust in the medical establishment and research trials (Corbie-Smith et al., 2002) arises because of previous abuses, such as the Tuskegee study (Brandt, 1978), or concerns that biomaterial could be exploited without consent, as in the case of Henrietta Lacks, or that DNA testing may result in racist eugenic conclusions. Moreover, lack of understanding of research and concerns relating to the relevance of such trials are factors that may contribute to non-participation (Harris et al., 1996) and therefore adversely affect biospecimen storage trials. Recruiting participants who have previously taken part in another trial, and therefore are acquainted with research and receptive to being part of research studies (Moorman et al., 2004), should improve participation in biospecimen banking. These measures are likely to minimize factors such as mistrust of research or researchers’ motives. Our study included women who had already agreed to be part of a general infertility research trial and were offered participation in the ancillary studies, thus the barrier of mistrust of research organizations may not be a reason for refusal to participate. In this study, African Americans were reluctant to take part in genetic studies because they were worried about too much blood being drawn. It may therefore be important to educate patients specifically about bio bank research as well as about the negligible amount of blood that will be drawn. Kiviniemi et al. (2013) demonstrated that a culturally appropriate focused educational program about the nature, methods and process of research and bio banking can reduce negative associations with research participation and therefore increase participation in research.
It is striking that although African Americans were less likely to be adherent to study protocol and to participate in ancillary studies, ethnicity was not predictive of non-adherence to study protocol or participation in genetic studies. We can speculate that since Hispanics have also been found to be non-adherent to medication in other areas of medicine (Sudano and Baker, 2001; Diaz et al., 2005; Poon et al., 2009) and also less likely to participate in genetic studies (Glenn et al., 2012), it is possible that differences in acculturation and culturally specific health beliefs in our population may account for this dichotomy. For example, it has been postulated that limited English proficiency and communication barriers may be more important in Hispanics (Sudano and Baker, 2001) in contrast to any entrenched cultural beliefs in African Americans. Recruitment of particicipants and implementation of study design should therefore take into consideration differences in cultural beliefs and behaviors.
This study was a secondary analysis of a multicenter RCT and the data on protocol adherence was collected prospectively, thus ensuring reliability of these factors. Medication bottles were inspected at each visit to determine medication compliance and intercourse diaries were inspected for intercourse compliance, which represent objective assessments of adherence factors. However, we did not assess reasons for non-adherence to the study protocol, such as ‘non-voluntary’ non-adherence due to blizzards or hurricanes, versus a voluntary decision. The extremely low OR for live birth in participants who were non-adherent to study protocol should be interpreted with caution because a large proportion consisted of dropouts, hence were considered not pregnant. This assumption may be flawed since the reason for dropout may not be related to outcome of the trial. However, it is essential to evaluate this since dropout is indeed a cause of ‘no live birth’, although it may be argued that this is misleading since participants who dropped out did not attempt conception. Moreover, although race and ethnicity are self-reported, self-classification to strict race and ethnicity may not always be reliable or representative of a whole racial or ethnic group. This study included two US multicenter trials and therefore the findings may not be extrapolated to international trials. However, its strength lies in the fact that the study was performed in 14 different study sites encompassing five geographic regions of the USA. Nevertheless, this study is an important contribution because it identifies factors that influence non-adherence, which can be addressed with study design or incentives so that reproductive clinical studies can be made more inclusive and thus more generalizable.
Although several studies in other specialties have had low levels of retention of about 31–64% (Slymen et al., 1996; Douketis et al., 2005), other studies have been able to report high rates of retention of approximately 85–90% (Stevens et al., 2001; Bailey et al., 2004; Warner et al., 2013). Several of the studies with high rates of retention have adopted various approaches, including planning and dedication, continuing engagement, flexibility and sensitivity, to ensure that this is achievable (Nicholson et al., 2011; Warner et al., 2013).
Hence, several strategies have been suggested to improve compliance to study protocol and prevention of study dropouts (Shumaker et al., 2000; Strunk et al., 2002; Loftin et al., 2005). Efforts to increase adherence to clinical trial protocols should be balanced by ethical issues of autonomy, privacy and best interest (Rand and Sevick, 2000). Research focusing on factors that improve adherence should be an integral part of future randomized controlled clinical trials in order to fully elucidate potential problems for non-adherence to protocols (Strunk et al., 2002). This will help to incorporate strategies to anticipate and identify problems with adherence early to ensure improved retention in clinical trials. Screening out participants, during the enrollment phase, who might not commit to the duration of the trial in the enrollment phase may decrease dropouts, although this might affect recruitment and exclude heterogeneous populations (Shumaker et al., 2000). Once enrolled, tracking of behaviors associated with non-adherence will identify potential dropouts early in order to enact strategies to address potential problems, including rewarding and recognition of participants. Training of staff to improve listening skills and establishment of optimal rapport can prevent further slippage to prevent progression to non-adherence (Grant and DePew, 1999).
To conclude, African Americans, Asians and participants with higher BMI and lower income were more likely to be non-adherent to study protocol in reproductive medicine clinical trials. Moreover, African Americans and Asians were less likely to agree to collection of biological samples for DNA or repository. It is essential that these populations are targeted with specific measures to improve participation and thereby extend the representativeness of reproductive medicine clinical trial findings. Further studies are required to evaluate specific reasons for non-adherence to clinical trials in reproductive medicine.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data Availability
The data underlying this article are available in FAIRsharing.org: NICHD DASH; NICHD Data and Specimen hub; DOI: https//doi.org/10.25504/FAIRsharing.dYS140
Supplementary Material
Acknowledgements
We wish to thank the study staff at each site and all the women who participated in the RMN study. In addition to the authors, other members of the National Institute of Child Health and Human Development (NICHD) Reproductive Medicine Network were as follows: Pennsylvania State University College of Medicine, Hershey: C. Bartlebaugh, W. Dodson, S. Estes, C. Gnatuk, J. Ober; University of Texas Health Science Center at San Antonio: R. Brzyski, C. Easton, A. Hernandez, M. Leija, D. Pierce, R. Robinson; Wayne State University: A. Awonuga, L. Cedo, A. Cline, K. Collins, S. Krawetz, E. Puscheck, M. Singh, M. Yoscovits; University of Pennsylvania: K. Barnhart, C. Coutifaris, K. Lecks, L. Martino, R. Marunich, P. Snyder; University of Colorado: R. Alvero, A. Comfort, M. Crow, W. Schlaff; University of Vermont: P. Casson, A. Hohmann, S. Mallette; University of Michigan: G. Christman, D. Ohl, M. Ringbloom, J. Tang; University of Alabama Birmingham: G. Wright Bates, S. Mason; Carolinas Medical Center: N. DiMaria, R. Usadi; University of Oklahoma Health Sciences Center, Oklahoma City: Karl Hansen; Virginia Commonwealth University: R. Lucidi, M. Rhea; Stanford University Medical Center: V. Baker, K. Turner; Urology, SUNY Upstate Medical University, Syracuse, New York: J. Trussell; Yale University: D. DelBasso, H. Huang, Y. Li, R. Makuch, P. Patrizio, L. Sakai, L. Scahill, H. Taylor, T. Thomas, S. Tsang, Q. Yan, M. Zhang; Ligand Core Laboratory University of Virginia Center for Research in Reproduction, Charlottesville, Virginia: D. Haisenleder; Eunice Kennedy Shriver National Institute of Child Health and Human Development; C. Lamar, L. DePaolo; Advisory Board: D. Guzick (Chair), A. Herring, J. Bruce Redmond, M. Thomas, P. Turek, J. Wactawski-Wende; Data and Safety Monitoring Board: R. Rebar (Chair), P. Cato, V. Dukic, V. Lewis, P. Schlegel, F. Witter.
Authors’ roles
L.E.: participated in conception and study design, manuscript drafting and critical discussion, final approval of manuscript. F.S.: participated in study conception and design, analysis, critical discussion, final approval of manuscript. R.S.L.: participated in study conception and design, execution, manuscript drafting and critical discussion, final approval of manuscript. M.P.D.: participated in study conception and design, execution and critical discussion, final approval of manuscript. H.Z.: participated in study conception and design and execution, analysis, manuscript drafting, critical discussion, final approval of manuscript. N.S.: participated in study conception and design, execution, manuscript drafting and critical discussion, final approval of manuscript.
Funding
Supported by National Institute of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grants: U10 HD39005, U10 HD38992, U10 HD27049, U10 HD38998, U10 HD055942, HD055944, U10 HD055936, U10HD055925, PPCOSII: U10 HD27049, U10 HD38992, U10 HD055925, U10 HD39005, U10 HD38998, U10 HD055936, U10 HD055942, U10 HD055944; Clinical Reproductive Endocrine Scientist Training Program (CREST): R25HD075737.
Conflict of interest
Outside this study, M.P.D. received NIH/NIHCD research grant and R.S.L. received research grant from Ferring and was consultant for Bayer, Kindex, Odega, Millendo and AbbVie.
Contributor Information
Lawrence Engmann, Department of Obstetrics and Gynecology, University of Connecticut School of Medicine, Farmington, CT, USA.
Fangbai Sun, Department of Biostatistics, Yale University School of Public Health, New Haven, CT, USA.
Richard S Legro, Department of Obstetrics and Gynecology, Penn State College of Medicine, Hershey, PA, USA.
Michael P Diamond, Department of Obstetrics and Gynecology, Augusta University, Augusta, GA, USA.
Heping Zhang, Department of Biostatistics, Yale University School of Public Health, New Haven, CT, USA.
Nanette Santoro, Department of Obstetrics and Gynecology, University of Colorado School of Medicine, Aurora, CO, USA.
Reproductive Medicine Network:
C Bartlebaugh, W Dodson, S Estes, J Ober, R Brzyski, C Easton, A Hernandez, M Leija, D Pierce, R Robinson, A Awonuga, L Cedo, A Cline, K Collins, S Krawetz, E Puscheck, M Singh, M Yoscovits, K Barnhart, C Coutifaris, K Lecks, L Martino, R Marunich, P Snyder, R Alvero, A Comfort, M Crow, W Schlaff, P Casson, A Hohmann, S Mallette, G Christman, D Ohl, M Ringbloom, J Tang, G Wright Bates, S Mason, N DiMaria, R Usadi, R Lucidi, M Rhea, V Baker, K Turner, J Trussell, D DelBasso, H Huang, Y Li, R Makuch, P Patrizio, L Sakai, L Scahill, H Taylor, T Thomas, S Tsang, Q Yan, M Zhang, D Haisenleder, C Lamar, L DePaolo, A Herring, J Bruce Redmond, M Thomas, P Turek, J Wactawski-Wende, R Rebar, P Cato, V Dukic, V Lewis, P Schlegel, and F Witter
References
- Arnold KB, Hermos JA, Anderson KB, Minasian L, Tangen CM, Probstfield JF, Cook ED. Retention of Black and White participants in the selenium and vitamin E cancer prevention trial (SWOG-coordinated intergroup study S0000). Cancer Epidemiol Biomarkers Prev 2014;23:2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JM, Bieniasz ME, Kmak D, Brenner DE, Ruffin MT. Recruitment and retention of economically underserved women to a cervical cancer prevention trial. Appl Nurs Res 2004;17:55–60. [DOI] [PubMed] [Google Scholar]
- Boivin J, Takefman J, Braverman A. The Fertility Quality of Life (FertiQoL) tool: development and general psychometric properties. Fertil Steril 2011;96:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen D, Raczynski J, George V, Feng Z, Fouad M. The role of participation in the Women’s Health Trial: feasibility study in minority populations. Prev Med 2000;31:474–480. [DOI] [PubMed] [Google Scholar]
- Brandt AM. Racism and research: the case of the Tuskegee Syphilis Study. Hastings Cent Rep 1978;8:21–29. [PubMed] [Google Scholar]
- Charles H, Good CB, Hanusa BH, Chang CC, Whittle J. Racial differences in adherence to cardiac medications. J Natl Med Assoc 2003;95:17–27. [PMC free article] [PubMed] [Google Scholar]
- Corbie-Smith G, Thomas SB, St George DM. Distrust, race, and research. Arch Intern Med 2002;162:2458–2463. [DOI] [PubMed] [Google Scholar]
- Diamond MP, Legro R, Coutifaris C, Alvero R, Robinson RD, Casson P, Christman G, Ager J, Huang H, Hansen KR et al. Letrozole, gonadotropins and clomiphene citrate for unexplained infertility. N Engl J Med 2015;373:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz E, Woods SW, Rosenheck RA. Effects of ethnicity on psychotropic medications adherence. Community Ment Health J 2005;41:521–537. [DOI] [PubMed] [Google Scholar]
- Douglas JG, Ferdinand KC, Bakris GL, Sowers JR. Barriers to blood pressure control in African Americans. Overcoming obstacles is challenging, but target goals can be attained. Postgrad Med 2002;112:51–52. [DOI] [PubMed] [Google Scholar]
- Douketis J, Macie C, Thabane L, Williamson D. Systematic review of longterm weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes 2005;29:1153–1167. [DOI] [PubMed] [Google Scholar]
- Fongwa MN, Evangelista LS, Hays RD, Martins DS, Elashoff D, Cowan MJ, Morisky DE. Adherence treatment factors in hypertensive African American women. Vasc Health Risk Manag 2008;4:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ME, Havstad SL, Tilley BC. Recruiting older African American men to a cancer screening trial (the AAMEN Project). Gerontologist 2003;43:27–35. [DOI] [PubMed] [Google Scholar]
- Glenn BA, Chawla N, Bastani R. Barriers to genetic testing for breast cancer risk among ethnic minority women: an exploratory study. Ethn Dis 2012;22:267–273. [PubMed] [Google Scholar]
- Gorelick PB, Harris Y, Burnett B, Bonecutter FJ. The recruitment triangle: reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African-American Antiplatelet Stroke Prevention Study (AAASPS). J Natl Med Assoc 1998;90:141–145. [PMC free article] [PubMed] [Google Scholar]
- Grant JS, DePew DD. Recruiting and retaining research participants for a clinical intervention study. J Neurosci Nurs 1999;31:357–362. [DOI] [PubMed] [Google Scholar]
- Harris Y, Gorelick PB, Samuels P, Bempong I. Why African Americans may not be participating in clinical trials. J Natl Med Assoc 1996;88:630–634. [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi MT, Saad-Harfouche FG, Ciupak GL, Davis W, Moysich K, Hargrave NC, Ambrosone CB, Walker C, Erwin DO. Pilot intervention outcomes of an educational program for biospecimen research participation. J Canc Educ 2013;28:52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawetz SA, Casson PR, Diamond MP, Zhang H, Legro RS, Schlaff WD, Coutifaris C, Brzyski RG, Christman GM, Santoro N et al. ; Reproductive Medicine Network. Establishing a biologic specimens repository for reproductive clinical trials: technical aspects. Syst Biol Reprod Med 2011;57:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang H, Jin S, Thomas T, Engmann L, Hansen KR, Coutifaris C, Casson P, Christman G, Alvero R, Santoro N et al. ; Reproductive Medicine Network. Predictors of participant retention in infertility treatment trials. Fertil Steril 2015;104:1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro R, Brzyski RG, Diamond MP, Coutifaris C, Schlaff W, Casson P, Christman G, Huang H, Yan Q, Alvero R, Haisenleder D et al. Letrozole versus clomiphene for infertility in polycystic ovary syndrome. N Engl J Med 2014;371:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LM, Askie P, Randleman S, Shelton-Dunston B. Medication adherence beliefs of community-dwelling hypertensive African Americans. J Cardiovasc Nurs 2010;25:199–206. [DOI] [PubMed] [Google Scholar]
- Loftin WA, Barnett SK, Bunn PS, Sullivan P. Recruitment and retention of rural African Americans in diabetes research: lessons learned. Diabetes Educ 2005;31:251–259. [DOI] [PubMed] [Google Scholar]
- Lukoschek P. African Americans' beliefs and attitudes regarding hypertension and its treatment: a qualitative study. J Health Care Poor Underserved 2003;14:566–587. [DOI] [PubMed] [Google Scholar]
- McGovern PG, Carson SA, Barnhart HX, Myers ER, Legro RS, Diamond MP, Carr BR, Schlaff WD, Coutifaris C, Cataldo NA et al. ; National Institute of Child Health and Human Development-Reproductive Medicine Network. Medication adherence and treatment success in the National Institute of Child Health and Human Development-Reproductive Medicine Network's Pregnancy in Polycystic Ovary Syndrome Trial. Fertil Steril 2008;90:1283–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ML. Recruitment and retention: Nursing research among low-income pregnant women. Appl Nurs Res 1997;10:152–158. [DOI] [PubMed] [Google Scholar]
- Moorman PG, Skinner CS, Evans JP, Newman B, Sorenson JR, Calingaert B, Susswein L, Crankshaw TS, Hoyo C, Schildkraut JM. Racial differences in enrolment in a cancer genetics registry. Cancer Epidemiol Biomarkers Prev 2004;13:1349–1354. [PubMed] [Google Scholar]
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004;291:2720–2726. [DOI] [PubMed] [Google Scholar]
- Nicholson LM, Schwirian PM, Klein EG, Skybo T, Murray-Johnson L, Eneli I, Boettner B, French GM, Groner JA. Recruitment and retention strategies in longitudinal clinical studies with low-income populations. Contemp Clin Trials 2011;32:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagidas K, Carson SA, McGovern PG, Barnhart HX, Myers ER, Legro RS, Diamond MP, Carr BR, Schlaff WD, Coutifaris C et al. ; National Institute of Child Health and Human Development-Reproductive Medicine Network. Intercourse compliance, ovulation, and treatment success in the National Institute of Child Health and Human Development-Reproductive Medicine Network's Pregnancy in Polycystic Ovary Syndrome (PPCOS) trial. Fertil Steril 2010;94:1444–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon I, Lal LS, Ford ME, Braun UK. Racial/ethnic disparities in medication use among veterans with hypertension and dementia: a national cohort study. Ann Pharmacother 2009;43:185–193. [DOI] [PubMed] [Google Scholar]
- Rand CS, Sevick MA. Ethics in adherence promotion and monitoring. Control Clin Trials 2000;21:241S–247S. [DOI] [PubMed] [Google Scholar]
- Santoro N, Eisenberg E, Trussell JC, Craig LB, Gracia C, Huang H, Alvero R, Casson P, Christman G, Coutifaris C et al. ; Reproductive Medicine Network Investigators. Fertility-related quality of life from two RCT cohorts with infertility: unexplained infertility and polycystic ovary syndrome. Hum Reprod 2016;31:2268–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiovitz TM, Bain EE, McCann DJ, Skolnick P, Laughren T, Hanina A, Burch D. Mitigating the effects of nonadherence in clinical trials. J Clin Pharmacol 2016;56:1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker SA, Dugan E, Bowen DJ. Enhancing adherence in randomized controlled clinical trials. Control Clin Trials 2000;21: 226S–232S. [DOI] [PubMed] [Google Scholar]
- Sibbald B, Roland M. Understanding controlled trials: why are randomised controlled trials important? Br Med J 1998;316:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slymen DJ, Drew JA, Elder JP, Williams SJ. Determinants of non-compliance and attrition in the elderly. Int J Epidemiol 1996;25:411–419. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Kroenke K, Hornyak R, McMurray J. Validity and utility of the PRIME-MD patient health questionnaire in assessment of 3000 obstetric-gynecologic patients: the PRIME-MD Patient Health Questionnaire Obstetrics-Gynecology Study. Am J Obstet Gynecol 2000;183:759–769. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV 3rd, Hahn SR, Brody D, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994;272:1749–1756. [PubMed] [Google Scholar]
- Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith WD, Milas NC, Mattfeldt-Beman M, Belden L, Bragg C. Long-term weight loss and changes in blood pressure: results of the trials of hypertension prevention, phase II. Ann Intern Med 2001;134:1. [DOI] [PubMed] [Google Scholar]
- Strunk RC, Bender B, Young DA, Sagel S, Glynn E, Caesar M, Lawhon C. Predictors of protocol adherence in a pediatric asthma clinical trial. J Allergy Clin Immunol 2002;110:596–602. [DOI] [PubMed] [Google Scholar]
- Sudano JJ Jr, Baker DW. Antihypertensive medication use in Hispanic adults: a comparison with Black adults and White adults. Med Care 2001;39:575–587. [DOI] [PubMed] [Google Scholar]
- Walters SJ, Bonacho Dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, Knox C, Nadin B, Rothwell J, Surtees M et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 2017;7:e015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner ET, Glasgow RE, Emmons KM, Bennett GG, Askew S, Rosner B, Colditz GA. Recruitment and retention of participants in a pragmatic randomized intervention trial at three community health clinics: results and lessons learned. BMC Public Health 2013;13:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweben A, Fucito LM, O'Malley SS. Effective strategies for maintaining research participation in clinical trials. Drug Inf J 2009;43:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in FAIRsharing.org: NICHD DASH; NICHD Data and Specimen hub; DOI: https//doi.org/10.25504/FAIRsharing.dYS140


