Abstract
STUDY QUESTION
To what extent do characteristics of germline genome editing (GGE) determine whether the general public supports permitting the clinical use of GGE?
SUMMARY ANSWER
The risk that GGE would cause congenital abnormalities had the largest effect on support for allowing GGE, followed by effectiveness of GGE, while costs, the type of application (disease or enhancement) and the effect on child well-being had moderate effects.
WHAT IS KNOWN ALREADY
Scientific progress on GGE has increased the urgency of resolving whether and when clinical application of GGE may be ethically acceptable. Various expert bodies have suggested that the treatment characteristics will be key in determining whether GGE is acceptable. For example, GGE with substantial risks (e.g. 15% chance of a major congenital abnormality) may be acceptable to prevent a severe disease but not to enhance non-medical characteristics or traits of an otherwise healthy embryo (e.g. eye colour or perhaps in the future more complex traits, such as intelligence). While experts have called for public engagement, it is unclear whether and how much the public acceptability of GGE is affected by the treatment characteristics proposed by experts.
STUDY DESIGN, SIZE, DURATION
The vignette-based survey was disseminated in 2018 among 1857 members of the Dutch general public. An online research panel was used to recruit a sample representing the adult Dutch general public.
PARTICIPANTS/MATERIALS, SETTING, METHODS
A literature review identified the key treatment characteristics of GGE: the effect on the well-being of the future child, use for disease or enhancement, risks for the future child, effectiveness (here defined as the chance of a live birth, assuming that if the GGE was not successful, the embryo would not be transferred), cost and availability of alternative treatments/procedures to prevent the genetic disease or provide enhancement (i.e. preimplantation genetic testing (PGT)), respectively. For each treatment characteristic, 2–3 levels were defined to realistically represent GGE and its current alternatives, donor gametes and ICSI with PGT. Twelve vignettes were created by fractional factorial design. A multinominal logit model assessed how much each treatment characteristic affected participants’ choices.
MAIN RESULTS AND THE ROLE OF CHANCE
The 1136 respondents (response rate 61%) were representative of the Dutch adult population in several demographics. Respondents were between 18 and 89 years of age. When no alternative treatment/procedure is available, the risk that GGE would cause (other) congenital abnormalities had the largest effect on whether the Dutch public supported allowing GGE (coefficient = −3.07), followed by effectiveness (coefficient = 2.03). Costs (covered by national insurance, coefficient = −1.14), the type of application (disease or enhancement; coefficient = −1.07), and the effect on child well-being (coefficient = 0.97) had similar effects on whether GGE should be allowed. If an alternative treatment/procedure (e.g. PGT) was available, participants were not categorically opposed to GGE, however, they were strongly opposed to using GGE for enhancement (coefficient = −3.37). The general acceptability of GGE was higher than participants’ willingness to personally use it (P < 0.001). When participants considered whether they would personally use GGE, the type of application (disease or enhancement) was more important, whereas effectiveness and costs (covered by national insurance) were less important than when they considered whether GGE should be allowed. Participants who were male, younger and had lower incomes were more likely to allow GGE when no alternative treatment/procedure is available.
LIMITATIONS, REASONS FOR CAUTION
Some (e.g. ethnic, religious) minorities were not well represented. To limit complexity, not all characteristics of GGE could be included (e.g. out-of-pocket costs), therefore, the views gathered from the vignettes reflect only the choices presented to the respondents. The non-included characteristics could be connected to and alter the importance of the studied characteristics. This would affect how closely the reported coefficients reflect ‘real-life’ importance.
WIDER IMPLICATIONS OF THE FINDINGS
This study is the first to quantify the substantial impact of GGE’s effectiveness, costs (covered by national insurance), and effect on child well-being on whether the public considered GGE acceptable. In general, the participants were strikingly risk-averse, in that they weighed the risks of GGE more heavily than its benefits. Furthermore, although only a single study in one country, the results suggests that—if sufficiently safe and effective—the public may approve of using GGE (presumably combined with PGT) instead of solely PGT to prevent passing on a disease. The reported public views can serve as input for future consideration of the ethics and governance of GGE.
STUDY FUNDING/COMPETING INTEREST(S)
Young Academy of the Royal Dutch Academy of Sciences (UPS/RB/745), Alliance Grant of the Amsterdam Reproduction and Development Research Institute (2017–170116) and National Institutes of Health Intramural Research Programme. No competing interests.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: gene editing; preimplantation diagnosis; bioethics; public opinion; surveys and questionnaires; germline genome editing; genetic enhancement; genetic disease, inborn; genetic techniques
Introduction
In November 2018, a Chinese scientist claimed he had created the first genome-edited babies. The scientist had attempted to edit the C-C motif chemokine receptor 5 (CCR5) gene of several human embryos to introduce HIV resistance (Regalado, 2019). Three implanted embryos resulted in live births. This is an example of germline genome editing (GGE): directly modifying the DNA of embryos or germ cells, thereby introducing heritable changes. GGE is a form of germline gene therapy (GGT; the term ‘gene therapy’ is not meant to imply that any such experimental therapies will have therapeutic benefits).
Beyond this case, scientific progress has also been moving closer to clinical applications of GGE (Smith et al., 2012). Several studies have reported successful GGE on human embryos without implantation (e.g. Liang et al., 2015). Furthermore, progress is being made in animal research, including in non-human primates (Ishii, 2015). Although increasingly successful, GGE is still considered insufficiently safe and effective for clinical application (NASEM, 2017). The scientific community thus overwhelmingly condemned the Chinese scientist’s actions for violating research regulations and ethical norms, some even calling for a temporary global moratorium or ban (Adelman et al., 2019; Lander et al., 2019; Botkin, 2020). However, scientists expect that safety and effectiveness will improve, making clinical use of GGE feasible in the foreseeable future (Smith et al., 2012; NASEM, 2017). GGE, however, raises various ethical questions (generally, GGE raises more ethical concern than somatic gene therapy, which does not result in heritable changes). The recent scientific developments have increased the urgency to resolve whether and when the potential clinical application of GGE may be considered ethically acceptable (NASEM, 2017; Ormond et al., 2017; Howard et al., 2018; NCOB, 2018; Andorno et al., 2020).
Various influential bodies have concluded that clinical application of GGE may be acceptable under certain conditions (NASEM, 2017; Ormond et al., 2017; de Wert et al., 2018; NCOB, 2018). In addition to two consensus criteria, namely safety and effectiveness (Baltimore et al., 2015; NASEM, 2017), several other conditions for clinical use have been proposed. Some have argued that GGT may only be used when no alternative treatment is available to prevent the disease (Green, 2008; NASEM, 2017). Other proposed criteria include that GGE should be sufficiently affordable (de Wert et al., 2018), used to prevent diseases (not for enhancement, which raises additional ethical concerns (NASEM, 2017; Knoppers et al., 2018)), and contribute significantly to the future child’s well-being (Smith et al., 2012; Knoppers et al., 2018). Notably, these conditions do not cover all ethical questions that GGE raises, including concerns about justice and eugenics (van Dijke et al., 2018; Andorno et al., 2020).
Many scholars have argued for public engagement (NASEM, 2017; de Wert et al., 2018; NCOB, 2018; McCaughey et al., 2019; Andorno et al., 2020). Including the general public may improve the quality of governance decisions, encourage democratic deliberation about technologies with societal implications and improve public trust in science (Srinivas, 2017; NCOB, 2018).
Several studies have since investigated public views. GGE acceptability varies considerably by case and between studies, ranging from 8% to 72% (Blendon et al., 2016; Funk and Hefferon, 2018; Delhove et al., 2020). These studies suggest that—at least for a significant part of the general public—the acceptability of GGE depends on certain conditions (Delhove et al., 2020). The characteristics of GGT that affect acceptability among the general public seem similar to those identified by experts, including GGT's safety (Robillard et al., 2014; Wang et al., 2017), effectiveness (Kalfoglou et al., 2005), costs (Xiang et al., 2015; Wang et al., 2017) and whether alternative treatments are available (Hendriks et al., 2018). Furthermore, acceptability depends on whether GGE is used for disease prevention or enhancement of specific characteristics/traits (Hendriks et al., 2018) and how much GGE improves the future child’s well-being (Funk et al., 2016).
The existing literature, however, does not provide a comprehensive overview of the extent to which several treatment characteristics influence the acceptability of GGE. Several studies report importance ratings of GGT treatment characteristic(s) (e.g. reporting that safety is important (Wang et al., 2017)). However, how these importance ratings translate into the acceptability of GGE with certain characteristics is unclear. Other studies do examine how a treatment characteristic affects the acceptability of GGE (e.g. reporting that GGE used for diseases is more acceptable than for enhancement (Scheufele et al., 2017; McCaughey et al., 2019)), but have significant limitations. The main impediment to understanding public views on actual cases is that existing studies have not taken into account that actual cases of GGE comprise various characteristics—both positive and negative (Delhove et al., 2020). To determine the acceptability of a potential application of GGE, one needs to consider the relative importance of these characteristics and the trade-offs between them. For example, substantial risks may be acceptable if the prevented disease is severe, but not for less severe conditions. Disease applications may be more acceptable generally, yet enhancements with large benefits (e.g. longevity) may be more acceptable than preventing trivial diseases (e.g. inclination towards ingrown toenails). To our knowledge, the simultaneous effects of multiple treatment characteristics on public acceptability of GGE have not been assessed.
This study examined the extent to which key treatment characteristics of GGE determine whether the Dutch general public supports permitting clinical use. As a secondary aim, the study explored whether the effects of the treatment characteristics changed if an alternative treatment would be available, or participants considered willingness to use instead of acceptability. Additionally, the study considered how much safer, more effective or cheaper GGE should be, to counterbalance the negative effect of enhancement applications on acceptability. Finally, the study explored the perceived importance of potential societal effects and ethical arguments for or against GGE.
Materials and methods
A vignette-based survey was developed, adhering to the International Society for Pharmacoeconomics and Outcomes Research criteria (Bridges et al., 2011).
Selecting treatment characteristics and their levels
A systematic literature review and a public survey with open-ended questions were conducted to identify reasons (n = 189) for or against clinical use of GGE which could be included as treatment characteristics in this study (Hendriks et al., 2018; van Dijke et al., 2018). However, vignettes with more than six characteristics are too complex (Ryan and Gerard, 2003), so the number of characteristics was limited using four strategies. First, reasons were excluded that were difficult to transform into treatment characteristics with quantifiable levels (e.g. GGE is too unnatural). Second, the most frequently reported reasons in the systematic review and qualitative study were shortlisted. Third, the relative importance of the shortlisted characteristics was reviewed based on comparative importance ratings from empirical studies and recommendations in conceptual papers (Supplementary Table SI). Finally, the treatment characteristics with the largest hypothesized impact on GGE acceptability were selected. Additionally, cost (covered by national health insurance, like most reproductive technologies in the Netherlands) was selected despite its mixed importance ratings, considering its importance for other reproductive treatments (Hendriks et al., 2019). The final six characteristics included: (i) type of application (modifying an affected embryo to prevent the future child from having a disease or enhancing an embryo to provide the future child with a desirable characteristic); (ii) effect of preventing the index disease or introducing the desirable characteristic on the future child’s well-being; (iii) risk that the reproductive technology would cause major abnormalities (e.g. though off-target effects); (iv) effectiveness (i.e. chance of a pregnancy that results in a live birth); (v) costs covered by national health insurance; and (vi) availability of alternative treatments to prevent the future child from having the disease or alternative procedures to provide a desirable characteristic (e.g. preimplantation genetic testing (PGT); Supplementary Table SII).
For effectiveness (characteristic iv), instead of narrow definitions of GGE effectiveness sometimes referred to in the literature (e.g. successful modification of cells or embryos), a broader definition was adapted: the chance of a live birth following the procedure (Duffy et al., 2020) (assuming that if the modification would not be successful, the embryo would not be transferred). This allowed for comparison with PGT and referred to the most meaningful outcome for patients. Corresponding to the literature, alternative treatments/procedures (characteristic vi) were framed as options leading to genetic parenthood (NASEM, 2017). However, to avoid misconceptions (Andorno et al., 2020), the vignettes in which no alternative treatment/procedure was available for couples’ hypothetical embryos, noted that couples could still forgo genetic parenthood and use adoption or gamete donation to have child without the disease or with the desirable characteristic. Finally, throughout the paper, the word ‘procedure’ is used to refer to enhancement, and ‘treatment’ to refer to the prevention of a disease. We note that the Dutch survey used the Dutch word ‘behandeling’ to refer to the prevention of a disease or the introduction of a desirable characteristic. While commonly translated to ‘treatment’, ‘behandeling’ is not necessarily connected to a medical condition. For example, it is commonly used to refer to cosmetic or wellness procedures.
For each treatment characteristic, 2–3 levels were defined to realistically represent GGE and its current alternatives, donor gametes and ICSI with PGT (Supplementary Table SII). As GGE is still in a preclinical stage of development, GGE levels were defined by expert judgement. Risk, effectiveness, and treatment costs were presumed to be evaluated relative to an alternative therapy, if available (Cavaliere, 2017; NCOB, 2018). Thus, when an alternative treatment/procedure was available, risks, effectiveness and costs were described relative to the alternative (e.g. GGE is more, equally or less expensive than the alternative). When no alternative treatment/procedure was available, these characteristics were described using absolute numbers (e.g. €5000, €10 000 or €20 000).
Survey design
The six treatment characteristics and their 2–3 levels resulted in 216 [23*33] possible hypothetical treatments. A fractional factorial design drew an efficient sample of 12 vignettes (Ryan and Gerard, 2003). For each vignette, participants were asked whether Dutch couples should be allowed to use GGE and whether they would personally use GGE if the described scenario would apply to them (Fig. 1).
Figure 1.
Sample question from the survey. The combination of the description of the case (‘scenario’) and the questions about this case is referred to as a ‘vignette’.
The survey introduction explained how GGE would work and current alternatives. Furthermore, the survey listed several ethical arguments for or against clinical use of GGE that were derived from the literature, which could not be transformed into treatment characteristics (e.g. GGE is too unnatural). Participants were requested to rate the importance of these arguments using a Likert scale. Finally, data on sociodemographic characteristics, engagement with biotechnology, trust in institutions, beliefs about nature and nurture and the impact of genetic modification were collected (Singer et al., 1998; Gaskell et al., 2003, 2006).
A science education expert edited the survey to lower its reading level. The survey was pilot tested and subsequently adapted to further improve understandability. Twenty-two cognitive interviews were conducted with a convenience sample of the general public until three iterative interviews revealed no new issues. See Supplementary data for the English translation of the survey.
Data collection
Public acceptability of prenatal gene therapy in the Netherlands is similar to other European Economic Area countries and the USA (Gaskell et al., 2017). However, as limited public education has been a major limitation of previous studies (Blendon et al., 2016), the Dutch public is interesting as they have the highest familiarity with gene therapy in the European Union (Gaskell et al., 2006).
A sample of 1857 members of the general public, matching specified demographic characteristics (i.e. gender, age, education, household composition and region) of the Dutch adult population, was drawn from the online Flycatcher panel. Panel members (>10 000) are invited to participate in ∼10 surveys annually. The survey was disseminated in January 2018. One reminder was sent. Participants received points upon completion (approximate monetary value of €3).
Analysis
Analyses were performed using SPSS 24 (Armonk, NY: IBM Corp) and R (version 3.1.2; http://www.r-project.org).
For the background variables, the proportions or measures of central tendency and variability were calculated.
The primary outcome was whether GGE should be allowed when no alternative is available. A main-effects multinominal logit model was used to determine how much each treatment characteristic and its levels affected participants’ choices. All treatment characteristics were initially included as categorical variables. Risks, effectiveness and costs were evaluated as continuous variables after confirming a linear relationship (determination was based on the Akaike information criterion). The output of the multinominal logit models included mean coefficients and their SDs, presented as 95% CIs.
The required amount of improvement in other treatment characteristics that would counterbalance the reduced acceptability of using GGE for enhancement (instead of for disease prevention) was calculated (i.e. marginal rate of substitution (MRS)). Of note, calculating improvements of other characteristics that would counterbalance the negative effect of enhancement on acceptability does not imply that the reasons why enhancement is less acceptable are related to these other characteristics. The MRS was calculated by dividing the difference in the importance scores between the highest and lowest treatment characteristic levels by the importance of GGE for enhancement, modelled as a continuous variable. The median and 95% CIs of the MRS were estimated through Monte Carlo sampling and expressed as percentages (Berg, 2004). CIs were based on the Krinsky Robb method adjusted for class probabilities. Child well-being was excluded as this was a binary, categorical variable for which this analysis could yield no meaningful outcomes (i.e. it would yield a percentage of a large effect, as opposed to, e.g. a percentage live birth rate).
Pre-planned multivariable analyses explored the associations of age, income and gender with choices. Additional analyses explored the associations of the sociodemographic variables, the attitudes towards science, and the ethical arguments with the outcomes whether GGE should be allowed and willingness to use (when no alternative is available). These associations were only evaluated further when, based on univariate statistics, the variables were associated with preference at a P-value <0.15 to avoid overfitting.
Sample size calculations indicated that 280 participants were required for the main analyses. Larger samples allow for detecting effects of participants’ sociodemographics.
Throughout the manuscript, participants’ responses on whether Dutch couples should be allowed to use GGE, are referred to as public ‘acceptability’ of GGE. ‘Acceptability’ is thus a descriptive term—describing the survey results—but also has a normative component, since participants drew normative judgements about GGE cases. Normative reasoning by the public, may, however, be significantly different in nature than that by academics (Bærøe, 2020).
Ethical approval
Public surveys are exempt from ethics committee review in the Netherlands.
Results
Participants and their attitudes
The survey was completed by 1136 participants (response rate 61%). Table I presents the participants’ sociodemographic characteristics and their attitudes towards science. Of the participants, 28% had a serious hereditary or genetic condition themselves, or had a family member or acquaintance with such a condition. The participants were representative of the adult Dutch general public regarding gender, region, educational level and household composition, but were older (P = 0.01, representing an 8-year difference in mean age). Figures 2 and 3 present participants’ views on the importance of arguments for and against GGE. The most important arguments were the possibility of eradicating diseases (for) and the possibility of GGE being misused for profit (against).
Table I.
Sociodemographic characteristics of participants in a survey of the acceptability of germline genome editing and their attitudes towards science.
| Proportion (%) | ||
|---|---|---|
| Sociodemographic characteristics | ||
| Male gender | 576/1136 (50.7%) | |
| Age (year) | 18–44 | 419/1136 (36.9%) |
| 44–65 | 445/1136 (39.2%) | |
| >65 | 272/1136 (23.9%) | |
| Western ethnic backgrounda | 1115/1136 (98.2%) | |
| Education level | Low | 335/1136 (29.5%) |
| Middle | 499/1136 (43.9%) | |
| High | 302/1136 (26.6%) | |
| Having children | 740/1136 (65.1%) | |
| Income | Minimum (less than €11 000) | 67/1136 (5.9%) |
| Below average (between €11 000 and €23 000) | 229/1136 (20.1%) | |
| Modal (between €23 000 and €34 000) | 239/1136 (21.0%) | |
| Between 1 and 2 times modal (between €34 000 and €56 000) | 221/1136 (19.5%) | |
| Two times modal or more (€56 000 or more) | 112/1136 (9.9%) | |
| Do not know/do not want to say | 268/1136 (23.6%) | |
| Type of religion | None | 606/1114 (54.4%) |
| Roman Catholic | 240/1114 (21.5%) | |
| Protestant | 183/1114 (16.4%) | |
| Other, including Islam, Hinduism, Buddhism | 85/1114 (7.6%) | |
| Importance of religion (mean ± SD; maximum importance is 10) | 2.3 ± 3.2 | |
| Political preferenceb | Left (1–4) | 353/1036 (34.1%) |
| Middle (5–6) | 362/1036 (34.9%) | |
| Right (7–10) | 321/1036 (31.0%) | |
| Political preferenceb | Progressive (1–4) | 360/1053 (34.2%) |
| Middle (5–6) | 373/1053 (35.4%) | |
| Conservative (7–10) | 320/1053 (30.4%) | |
| The participant, or a family member or acquaintance of the participant has a serious hereditary or genetic condition | 308/1100 (28.0%) | |
| Self-reported knowledge about genetics | No knowledge | 246/1136 (21.7%) |
| Limited knowledge | 726/1136 (63.9%) | |
| A fair amount of knowledge | 134/1136 (11.8%) | |
| A lot of knowledge | 30/1136 (2.6%) | |
| Attitudes towards science | ||
| Engagement with biotechnologyc (mean ± SD, maximum score is 1) | 0.37 ± 0.28 | |
| Nature versus nurture beliefs | Heredity and genes determine the behaviour of a person as much as the environment and society in which a person grows up | 706/1136 (62.1%) |
| The environment and society in which a person grows up determine the behaviour of a person most | 271/1136 (23.9%) | |
| Heredity and genes determine the behaviour of a person most | 159/1136 (14.0%) | |
| Trust in institutions | Physicians who are monitoring the health implications | 915/1136 (80.5%) |
| University scientists who are developing treatments | 776/1136 (68.3%) | |
| Government institutions (e.g. National Institute for Public Health and the Environment) that are monitoring the health implications | 649/1136 (57.1%) | |
| Ethics committees advising on the moral aspects | 591/1136 (52.0%) | |
| The Dutch government in making regulations on the techniques | 479/1136 (42.2%) | |
| The European Commission in making regulations on the techniques | 374/1136 (32.9%) | |
| Media that are reporting on the techniques | 203/1136 (17.9%) | |
| Scientists in industry who are developing treatments | 195/1136 (17.2%) | |
| Spiritual/religious leaders advising on the moral aspects | 147/1136 (12.9%) | |
| Expected future impact of gene editing (in general) | Don’t know | 393/1136 (34.6%) |
| It will improve people's lives | 359/1136 (31.6%) | |
| It will worsen people's lives | 202/1136 (17.8%) | |
| It will not affect people’s lives | 182/1136 (16.0%) | |
Persons were defined as having a non-western ethnic background if they were born in a non-western country or at least one parent was born in a non-western country (https://www.cbs.nl/en-gb/our-services/methods/definitions? tab=m#id=migration-background).
Participants were asked to place themselves on a ‘political’ scale of 0–10 (left-right and progressive-conservative), which were grouped into categories.
A composite measure for engagement with biotechnology was created by adding (i) the frequency of discussing biotechnology, (ii) the willingness to read articles or watch TV shows on biotechnology and (iii) the willingness to participate in biotechnology debates (Gaskell et al., 2003). The composite variable was divided by 3 to get a score between 0 and 1, and described by mean and SD.
Figure 2.
Importance of arguments for clinical use of GGE. Mean important scores are displayed in the white boxes on the bars. *A child that is conceived from the sperm and egg cell of his/her intended parents. The child thus has the genes and hereditary characteristics of his/her intended parents. GGE, germline genome editing.
Figure 3.
Importance of arguments against clinical use of GGE. Mean importance scores are displayed in the white boxes on the bars.
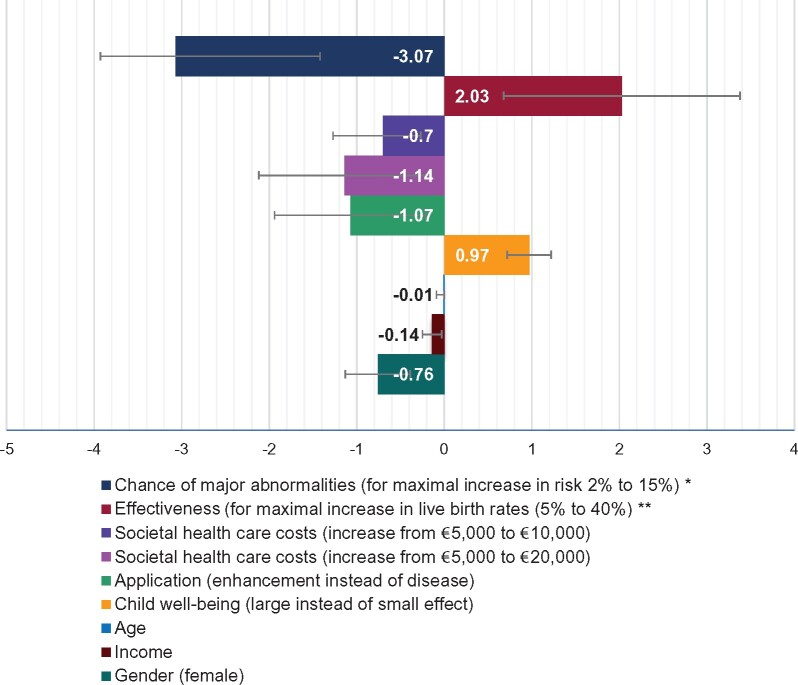
The effect of the treatment characteristics on GGE acceptability when no alternative treatment/procedure is available
Figure 4 uses coefficients to display the effect of each treatment characteristic on acceptability of GGE when no alternative treatment/procedure is available. The larger the coefficient, the larger the effect of this treatment characteristic on whether participants thought GGE should be allowed. If treatment characteristics increased the acceptance of GGE, their coefficients are positive. Negative coefficients reflect that treatment characteristics decreased the acceptance of GGE. The absolute values of the coefficients have no direct interpretation (Hauber et al., 2016).
Figure 4.
The effect of the treatment characteristics on whether participants thought GGE should be allowed in the Netherlands, when no alternative treatments/procedures are available. Model parameters: 2 log-likelihood = −542; Pseudo R2 = 0.591; consistent Akaike Info Criterion = 1183. *A translation of the linear variable. Coefficient per per cent increase in child safety −0.24 [CI: −0.37 to −0.10]. **A translation of the linear variable coefficient per per cent increase in effectiveness 0.06 [CI: 0.02 to 0.10].
All treatment characteristics affected whether participants thought GGE should be allowed in the Netherlands. Participants were more likely to allow GGE when it was used to prevent diseases, it substantially benefitted child well-being, risks of causing congenital malformations were low, success rates were high and costs (covered by health insurance) were low.
Within their presumably realistic ranges, safety had the largest effect on whether participants thought GGE should be allowed, followed by effectiveness. Costs (covered by health insurance), the type of application (disease or enhancement), and the effect on child well-being had similar, more moderate, effects on whether participants thought GGE should be allowed. Participants who were male, younger and had lower incomes were more likely to allow GGE.
The effect of the treatment characteristics when considering willingness to use GGE or when an alternative treatment/procedure is available
There was a very strong positive relationship between participants’ views on allowing GGE and their willingness to use GGE (Phi-coefficient 0.71–0.82; Supplementary Table SIII). However, GGE acceptability was higher than participants’ willingness to personally use it (P < 0.001). All treatment characteristics affected participants’ willingness to use GGE if no alternative was available (Fig. 5a). Males and participants with lower incomes were more willing to use GGE. As compared to their effect on whether GGE should be allowed, the type of application (disease or enhancement) seemed to affect willingness to use GGE more, whereas effectiveness and costs (covered by national insurance) seemed to have less of an effect.
Figure 5.
The effect of the treatment characteristics on willingness to use GGE when no alternatives are available and on GGE acceptability when alternative treatments/procedures are available. (a) The effect of the treatment characteristics on whether participants would genetically modify their own embryo, when no alternative treatments/procedures are available. Model parameters: 2 log-likelihood = −542; Pseudo R2 = 0.591; consistent Akaike Info Criterion = 1183. If the 95% CI does not cross zero, the effect is significant at P < 0.05. *A translation of the linear variable. Coefficient per per cent increase in child safety −0.21 [CI: −0.35 to −0.06]. **A translation of the linear variable. Coefficient per per cent increase in effectiveness 0.03 [CI: 0.01 to 0.05]. (b) The effect of the treatment characteristics on whether participants thought GGE should be allowed in the Netherlands, when alternative treatments/procedures are available. Model parameters: 2 log-likelihood = −637; Pseudo R2 = 0.328; consistent Akaike Info Criterion = 1352. If the 95% CI does not cross zero, the effect is significant at P < 0.05.
When the vignette described that an alternative treatment/procedure was available (and safety, effectiveness and costs of GGE were described relative to the alternative), the effect of the treatment characteristics on the participants’ support for GGE was different (Fig. 5b). Specifically, when an alternative procedure was available, participants were strongly opposed to using GGE for enhancement. The effect of the type of application (disease or enhancement) was so dominant to participants’ (dis)approval of GGE, that the other treatment characteristics had only a limited effect. Only the effect on child well-being and effectiveness (compared to the alternative) also significantly impacted participants’ choices. Supplementary Fig. SI displays the willingness to use GGE when an alternative treatment/procedure was available.
Associations with sociodemographic characteristics, attitudes towards science or ethical arguments
Sociodemographic characteristics, attitudes towards science or the value participants attached to ethical arguments (Figs 2 and 3) were not associated with allowing GGE or willingness to use GGE, with one exception. Participants who considered uncertain long-term societal consequences important were less likely to approve of GGE (coefficient 1.08, P < 0.01) or use GGE (coefficient 1.13, P < 0.01) when there was no alternative. As few participants had non-Western ethnic backgrounds, the effect of ethnicity was not assessed.
The necessary improvements in safety, effectiveness or costs to compensate for the negative effect of enhancement
If two cases of GGE were identical in all characteristics (i.e. similar safety, effectiveness, costs, and effect on child well-being), but one prevented a disease and the other introduced an enhancement, the disease case would be more acceptable. However, MRS analysis showed that if, for example, the costs of the enhancement would be €13 950 lower, both cases would be equally acceptable (95% CI: €8400–19 500). Similarly, a 15.6% higher success rate or a 4.5% lower risk of major abnormalities would offset the reduced acceptability of GGE being used for enhancement instead of for disease prevention (95% CI: 10.5–20.6% and 2.10–6.8%, respectively). Notably, while acceptability may increase after compensating for the negative effect of enhancement, this does not necessarily mean a GGE case would be considered above the threshold of acceptability.
Discussion
The results demonstrate that when no alternative for GGE was available, risks had the largest effect on whether the public supported allowing GGE, followed by effectiveness. Costs, the type of application and the effect on child well-being had similar, more moderate effects on whether GGE should be allowed.
Strengths and limitations
To our knowledge, the simultaneous effects of multiple treatment characteristics on public acceptability of GGE have not previously been assessed. Existing literature focuses on importance ratings (e.g. Wang et al., 2017) or on how individual treatment characteristics affect acceptability (e.g. Scheufele et al., 2017). This study provides a more comprehensive and nuanced analysis of the effect that various treatment characteristics can have on GGE acceptability.
To enable investigating public views on a complex topic like GGE, several strategies were employed: providing information about GGE, using concrete vignettes (instead of abstract trade-off questions), employing a science education expert and pilot-testing. While survey participants’ understanding of complicated concepts is difficult to assess, the overlap between participant responses and expert views was encouraging. Furthermore, 87% of participants rated the survey’s difficulty as easy to neutral.
This study’s participants were representative of the (Dutch) general public in multiple demographics, unlike many of the existing studies (e.g. Weisberg et al., 2017; McCaughey et al., 2019). Despite a good response rate (61%), some (e.g. ethnic, religious) minorities were underrepresented. Further research should assess additional perspectives. The latter may also include views of couples who are trying to conceive without passing on a genetic disorder as their willingness to use GGE may differ from that of the general public who are imagining being in this situation.
To limit vignette complexity, only six treatment characteristics were included, consistent with accepted limitations of this methodology (Ryan and Gerard, 2003). However, the characteristics that were not included (e.g. out-of-pocket costs) may be connected to and alter the importance of the studied characteristics. This would affect how closely the reported coefficients reflect ‘real-life’ importance. Furthermore, as GGE is still being developed, the realistic ranges of levels of the characteristics were based on expert judgement, with some being further from current possibilities than others. Most notably, the combination of enhancement and GGE having a large effect on child well-being seems theoretically possible (e.g. with longevity as the enhancement) but far removed from current abilities and more uncertain and dependent on contextual factors (NCOB, 2018).
If alternatives to GGE were available, the risks, effectiveness and costs levels were described relative to the alternative treatment/procedure, instead of using absolute numbers. While consistent with the literature (Cavaliere, 2017; NCOB, 2018), this limited the comparability of the scenarios and thereby assessment of the effect of alternatives being available.
Finally, the vignettes were selected using a theoretically efficient fractional factorial design. This design did not account for the dominance of the type of application in scenarios where an alternative was available, limiting detection of smaller effects of the other treatment characteristics.
Findings in the context of the literature
The acceptability (9–47%) and willingness to use GGE (6–38%) in this study’s hypothetical scenarios were comparable to acceptability ranges in previous studies on GGE 8–72% (Blendon et al., 2016; Funk and Hefferon, 2018). GGE acceptability was higher than willingness to use although the two were correlated. While this was not previously assessed for GGE, similar results are reported for paediatric vaccines (Hadisoemarto and Castro, 2013).
The effect on child well-being, the type of application, safety, effectiveness and costs all significantly affected GGE acceptability when no alternative was available. This validated their selection based on importance in the literature.
When no alternatives were available, risk of congenital abnormalities most affected acceptability and willingness to use GGE, a finding which corresponds to previous studies on GGT (Rabino, 2006; Wang et al., 2017) and some experts’ views (Smith et al., 2012). Still, participants were strikingly risk-averse (Tversky and Kahneman, 1981) in that they weighed the risks of GGE more heavily than its benefits. For example, GGE use was more acceptable when it would cure a severe (rather than minor) disease. However, this positive effect was roughly nullified if GGE would increase the risk of a congenital abnormality by just 4% (extracted from Fig. 5). This was true even though major abnormalities and severe diseases were described as having similar effects on well-being. The appropriate policy implications of this risk aversion should be considered.
GGE’s relative safety compared to available alternative treatments/procedures did not impact GGE approval. Based on the cognitive interviews, participants may have assumed that current treatments are low-risk (indeed, available data about ICSI with PGT is reassuring (Heijligers et al., 2018)), such that increased safety will have little marginal utility. Moreover, participants may trust clinicians not to propose risky therapies when low-risk alternatives exist, such that when GGE was listed as riskier than the alternative, they presumed it not to be high-risk. Although safety may have had a small effect, the dominance of the type of application in the analysis prevented the detection of such effects. Further studies could compare GGE with riskier potential alternative therapies.
Effectiveness has only been reported as having a substantial impact on support for allowing GGT in qualitative data (in which effectiveness was not clearly defined, making comparison difficult) (Lewis et al., 1997; Kalfoglou et al., 2005; Hendriks et al., 2018). However, the present study’s finding aligns with public views on other reproductive therapies (Hendriks et al., 2017) and general drug approval processes. While incorporating an effectiveness threshold could limit reproductive autonomy, such restrictions may protect patients from therapies with unfavourable risk-benefit ratios, and could be justified by public health insurance prioritizing cost-effective therapies (Riggan et al., 2019). Interestingly, participants’ support for allowing GGE increased if GGE was more effective than alternative treatments, providing an opening for couples to use GGE to increase the number of available embryos after PGT. Around 2771 PGT cycles are annually registered by ESHRE for intended parents who are carriers of genetic disorders (including PGT for chromosome abnormalities, sexing for X-linked disease and single-gene disorders from 71 centres (De Rycke et al., 2017)). Potential use of GGE by these couples could substantially expand potential users beyond the small number of couples who are unable to create disease-free embryos (Viotti et al., 2019) (which some experts proposed as a limit (Green, 2008; NASEM, 2017)).
GGE costs covered by public health insurance had a surprisingly large effect on whether participants supported allowing GGE, considering that in other studies the costs of GGT had mixed importance and insurance coverage was not always specified (Xiang et al., 2015; Wang et al., 2017). The results, however, resemble those for other reproductive treatments (Hendriks et al., 2019) and may be understood in the context of a finite national healthcare budget and distributive justice concerns (Wellcome Trust, 2005; Hui et al., 2009). Insurance-covered costs affected willingness to use less than acceptability. This might be because people are more likely to consider the treatment coverage’s burden on the national healthcare budget when considering population-level introduction than when considering whether they would use such a treatment themselves. Self-interest may also play a role accepting costly treatments for themselves.
GGE acceptability was affected by the type of application (enhancement or disease) and the effect it would have on child well-being. Participants considered enhancements applications less acceptable than disease applications, even if the effect of both on the well-being of the child would be the same. An enhancement with a large effect on child well-being was considered almost as acceptable as a disease application with a small effect on child well-being. Participants thus disfavoured enhancement, instead of merely judging both application types by their benefits (and risks), as some experts proposed (Green, 2008). Enhancement may be less acceptable in and of itself because of public concerns about its societal effects or ethics (e.g. justice) (Hendriks et al., 2018). When alternative procedures would be available that could provide couples with embryos with a desirable characteristic (e.g. PGT, theoretically), participants seemed to support expert statements that rule out using GGE for enhancement (NASEM, 2017; Ormond et al., 2017). In the absence of alternative procedures, enhancement applications were still significantly less acceptable; however, this effect was not overwhelming relative to other undesirable treatment characteristics. This might indicate that, in this context, the public is slightly more tolerant of enhancement. Future research may explore this further.
The effect of GGE on child well-being was previously reported to be a key argument for GGT (Robillard et al., 2014; Hendriks et al., 2018). Furthermore, previous studies compared the acceptability of using GGE to prevent different diseases (e.g. HIV or a neuromuscular disease (Hendriks et al., 2018)). However, because specific diseases differ in multiple ways, such comparisons do not reveal how the effect of GGE on child well-being influences GGE acceptability. While these previous studies did suggest that the effect on child well-being would likely influence GGE acceptability, to our knowledge, this study was the first to directly test—and confirm—this.
Similar to previous studies on GGT, being male was substantially associated with GGE acceptance (Criger and Fekken, 2013; Weisberg et al., 2017) and being young was slightly associated with GGE acceptance (Weisberg et al., 2017). The result that low-income participants more frequently accept GGE contrasts with a Chinese study on GGT (Wang et al., 2017). Surprisingly, considering various previously reported associations (e.g. Wellcome Trust, 2005; Weisberg et al., 2017), other background variables had no effects in this study. This may relate to these previously reported effects being application- and context-dependent (Wellcome Trust, 2005; Scheufele et al., 2017). Alternatively, by using detailed vignettes about GGE, this study may have evaded some confounders, for example, that trust in institutions increases acceptance of biotechnologies by decreasing perceived risks and increasing perceived benefits (Siegrist, 2000). Uncertainty about societal consequences affected GGE acceptability more than specific potential societal consequences, corresponding to a low tolerance for uncertain long-term consequences of biotechnologies among the European public (Pardo et al., 2002). Despite the importance participants attached to some of the other potential societal effects and ethical arguments for or against GGE (Figs 2 and 3), the importance scores of these ethical arguments had surprisingly limited effects on GGE acceptability in the vignettes. Further research may provide deeper insight into public views on these broader ethical arguments.
Implications
This study suggests that public support for allowing GGE is partially based on its risk-benefit profile as compared to an alternative treatment baseline, supporting previous qualitative findings on GGT (Wellcome Trust, 2005). This suggests that the general public conceptualizes GGE in a way that is consistent with several expert and committee position statements on GGE or GGT (Green, 2008; Smith et al., 2012; NASEM, 2017; Ormond et al., 2017). This in and of itself, as well as the relative importance of the different treatment characteristics to the public, provides input for future consideration of GGE ethics and policy. Additionally, areas in which participants diverged from expert views may justify further consideration and study, such as participants’ increased approval of GGE when it would be more effective than current treatments. Finally, the results can inform the research agenda for developing GGE applications. Specifically, the increased risks, effectiveness and cost thresholds for accepting enhancement may help to determine when, if ever, the technology is sufficiently advanced to consider enhancement applications. Generally, GGE policy should take into consideration both expert perspectives including rigorous normative analysis and—given the societal interest—public views on the ethics of GGE.
Several areas require further research. First, cultural differences, patients’ views, and the effects of other treatment characteristics on GGE acceptability should be explored. Second, further analysis should clarify whether using donor gametes or adoption (not leading to genetic parenthood) is a ‘reasonable alternative’ to GGE. Whereas the dominant view is that these are not ‘reasonable alternatives’ (NASEM, 2017), conceptual bioethics papers (e.g. Hyun and Osborn, 2017) and an empirical paper (Hendriks et al., 2019) have challenged this. Finally, this study may serve as an example of the merits of the more comprehensive and nuanced public engagement necessary for other high-impact emerging technologies (Riggan et al., 2019).
Supplementary Material
Acknowledgements
We thank the participants, Flycatcher for disseminating the survey and Dr Robin Lovell-Badge and Ms Karin Janssen for their critical review of the study design.
Authors’ roles
I.D. contributed to the data collection, analysis and manuscript drafting. M.W. contributed to the study design, analysis and manuscript drafting. B.E.B. contributed to critical discussion. A.L.B., L.H., R.V. and S.R. contributed to the study design and critical discussion. S.H. contributed to all components of the study.
Funding
Young Academy of the Royal Dutch Academy of Sciences (UPS/RB/745), Alliance Grant of the Amsterdam Reproduction and Development Research Institute (I.D., 2017–170116) and National Institutes of Health Intramural Research Programme (S.H.).
Conflict of interest
None.
Disclaimer
The views expressed are the authors’ own and do not reflect those of the National Institutes of Health, the Department of Health and Human Services or the US government.
References
- Adelman B, Albright C, Andrews L, Annas G, Appelbaum PS, Azam U, Barrett D, Bennett J, Burns JW, Callahan D et al. Clinical Germline gene editing letter to U.S. Department of Health and Human Services. American Society of Gene and Cell Therapy, 2019. https://www.asgct.org/global/documents/clinical-germline-gene-editing-letter.aspx. [Google Scholar]
- Andorno R, Baylis F, Darnovsky M, Dickenson D, Haker H, Hasson K, Lowthorp L, Annas GJ, Bourgain C, Drabiak K et al. Geneva statement on heritable human genome editing: the need for course correction. Trends Biotechnol 2020;38:351–354. [DOI] [PubMed] [Google Scholar]
- Bærøe K. Incommensurable processes of reasoning and implications for empirical and normative bioethics. AJOB Empir Bioeth 2020;11:2–4. [DOI] [PubMed] [Google Scholar]
- Baltimore D, Berg P, Botchan M, Carroll D, Charo RA, Church G, Corn JE, Daley GQ, Doudna JA, Fenner M. A prudent path forward for genomic engineering and germline gene modification. Science 2015;348:36–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg BA. Markov Chain Monte Carlo Simulations and Their Statistical Analysis. Singapore: World Scientific Publishing Company, 2004. [Google Scholar]
- Blendon RJ, Gorski MT, Benson JM. The public and the gene-editing revolution. N Engl J Med 2016;374:1406–1411. [DOI] [PubMed] [Google Scholar]
- Botkin JR. The case for banning heritable genome editing. Genet Med 2020;22:487–489. [DOI] [PubMed] [Google Scholar]
- Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, Johnson FR, Mauskopf J. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health 2011;14:403–413. [DOI] [PubMed] [Google Scholar]
- Cavaliere G. Genome editing and assisted reproduction: curing embryos, society or prospective parents? Med Health Care Philos 2017;21:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S, Donovan PJ, Douglas T. et al. Genome editing technologies and human germline genetic modification: The Hinxton Group Consensus Statement. Am J Bioeth 2015;15:42–47. [DOI] [PubMed] [Google Scholar]
- Criger B, Fekken CG. Human germline engineering: a study of attitudes among Canadian university students and the American Public. Int J Humanit Soc Sci 2013;3:148–159. [Google Scholar]
- CritchleyC, NicolD, BruceG, WalsheJ, TreleavenT, Tuch B. Predicting public attitudes toward gene editing of germlines: the impact of moral and hereditary concern in human and animal applications. Frontiers in Genetics 2019;9:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crne-HladnikH, PeklajC, KosmeljK, HladnikA, Javornik B. Assessment of Slovene secondary school students' attitudes to biotechnology in terms of usefulness, moral acceptability and risk perception. Public Understanding of Science 2009;18:747–758. [Google Scholar]
- De Rycke M, Goossens V, Kokkali G, Meijer-Hoogeveen M, Coonen E, Moutou C. ESHRE PGD Consortium data collection XIV–XV: cycles from January 2011 to December 2012 with pregnancy follow-up to October 2013†. Hum Reprod 2017;32:1974–1994. [DOI] [PubMed] [Google Scholar]
- de Wert G, Heindryckx B, Pennings G, Clarke A, Eichenlaub-Ritter U, van El C, Forzano F, Goddijn M, Howard HC, Radojkovic D et al. Responsible innovation in human germline gene editing. Background document to the recommendations of ESHG and ESHRE. Hum Reprod Open 2018;2018:hox024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhove J, Osenk I, Prichard I, Donnelley M. Public acceptability of gene therapy and gene editing for human use: a systematic review. Hum Gene Ther 2020;31:20–46. [DOI] [PubMed] [Google Scholar]
- Duffy J, Al Ahwany H, Bhattacharya S, Collura B, Curtis C, Evers J, Farquharson R, Franik S, Giudice L, Khalaf Y et al. Developing a core outcome set for future infertility research: an international consensus development study. Hum Reprod 2020;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FriedmannT, JonlinEC, KingNMP, TorbettBE, WivelNA, KanedaY, Sadelain M. ASGCT and JSGT Joint Position Statement on Human Genomic Editing. Mol Ther 2015;23:1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C, Hefferon M. Public Views of Gene Editing for Babies Depend on How It Would Be Used. Pew Research Center, 2018. https://www.pewresearch.org/science/2018/07/26/public-views-of-gene-editing-for-babies-depend-on-how-it-would-be-used/. [Google Scholar]
- Funk C, Kennedy B, Sciupac E. US public wary of biomedical technologies to ‘enhance’human abilities. Pew Research Center 2016:1–131. https://www.pewresearch.org/science/2016/07/26/u-s-public-wary-of-biomedical-technologies-to-enhance-human-abilities/. [Google Scholar]
- Gaskell G, Allansdottir A, Allum N, Corchero C, Fischler C, Hampel J, Jackson J, Kronberger N, Mejlgaard N, Revuelta G. Europeans and biotechnology in 2005: patterns and trends. Final Rep Eurobarometer 2006;64: 1–88. [Google Scholar]
- Gaskell G, Allum N, Stares S. Europeans and Biotechnology in 2002: Eurobarometer 58.0. Brussels: European Commission, 2003. [Google Scholar]
- Gaskell G, Bard I, Allansdottir A, Da Cunha RV, Eduard P, Hampel J, Hildt E, Hofmaier C, Kronberger N, Laursen S. Public views on gene editing and its uses. Nat Biotechnol 2017;35:1021. [DOI] [PubMed] [Google Scholar]
- Green RM. Babies by Design: The Ethics of Genetic Choice. New Haven and London: Yale University Press, 2008. [Google Scholar]
- Hadisoemarto PF, Castro MC. Public acceptance and willingness-to-pay for a future dengue vaccine: a community-based survey in Bandung, Indonesia. PLoS Negl Trop Dis 2013;7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, IJzerman MJ, Bridges JF. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health 2016;19:300–315. [DOI] [PubMed] [Google Scholar]
- Heijligers M, van Montfoort A, Meijer-Hoogeveen M, Broekmans F, Bouman K, Homminga I, Dreesen J, Paulussen A, Engelen J, Coonen E et al. Perinatal follow-up of children born after preimplantation genetic diagnosis between 1995 and 2014. J Assist Reprod Genet 2018;35:1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks S, Giesbertz N, Bredenoord A, Repping S. Reasons for being in favour of or against genome modification: a survey of the Dutch general public. Hum Reprod Open 2018;2018:hoy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks S, van Wely M, D'Hooghe TM, Meissner A, Mol F, Peeraer K, Repping S, Dancet EAF. The relative importance of genetic parenthood. Reprod Biomed Online 2019;39:103–110. [DOI] [PubMed] [Google Scholar]
- Hendriks S, Vliegenthart R, Repping S, Dancet E. Broad support for regulating the clinical implementation of future reproductive techniques. Hum Reprod 2017;33:39–46. [DOI] [PubMed] [Google Scholar]
- Holm S. Let us assume that gene editing is safe—the role of safety arguments in the gene editing debate. Camb Q Healthc Ethics 2019;28:100–111. [DOI] [PubMed] [Google Scholar]
- Howard HC, vn El C, Forzano F, Radojkovic D, Rial-Sebbag E, de Wert G, Borry P, Cornel MC. One small edit for humans, one giant edit for humankind? Points and questions to consider for a responsible way forward for gene editing in humans. Eur J Hum Genet 2018;26:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E, Chow K, Wu D, Liu A, Li Y. Opinion survey of the Hong Kong general public regarding genomic science and technology and their ethical and social implications. New Genet Soc 2009;28:381–400. [Google Scholar]
- Hyun I, Osborn C. Query the merits of embryo editing for reproductive research now. Nat Biotechnol 2017;35:1023–1025. [DOI] [PubMed] [Google Scholar]
- Ishii T. Germline genome-editing research and its socioethical implications. Trends Mol Med 2015;21:473–481. [DOI] [PubMed] [Google Scholar]
- Kalfoglou AL, Doksum T, Bernhardt B, Geller G, LeRoy L, Mathews DJ, Evans JH, Doukas DJ, Reame N, Scott J. Opinions about new reproductive genetic technologies: hopes and fears for our genetic future. Fertil Steril 2005;83:1612–1621. [DOI] [PubMed] [Google Scholar]
- Knoppers BM, Nguyenm MT, Noohi F, Kleiderman E. Human Genome Editing, Ethical and Policy Considerations. In Centre for Genomics and Policy (CGP) (ed). McGill University and Génome Québec Innovation Centre, Génome Québec, 2018. Montreal, Canada. [Google Scholar]
- Lander E, Baylis F, Zhang F, Charpentier E, Berg P. Adopt a moratorium on heritable genome editing. Nature 2019;567:165–168. [DOI] [PubMed] [Google Scholar]
- Lewis J, Driver R, Leach J, Wood-Robinson C. Opinions on and attitudes towards genetic engineering. acceptable limits: a discussion task. Working Paper 7 of the Young People’s Understanding of, and Attitudes to,“The New Genetics” Project. Centre for Studies in Science and Mathematics Education, University of Leeds, 1997.
- Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, Lv J, Xie X, Chen Y, Li Y. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell 2015;6:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughey, Budden DM, Sanfilippo PG, Gooden GEC, Fan L, Fenwick E, Rees G, MacGregor C, Si L, Chen C et al. A need for better understanding is the major determinant for public perceptions of human gene editing. Hum Gene Ther 2019;30:36–43. [DOI] [PubMed] [Google Scholar]
- NASEM. Human Genome Editing: Science, Ethics, and Governance. Washington, DC: National Academies of Sciences, Engineering, Medicine, 2017. [PubMed] [Google Scholar]
- NCOB. Genome Editing and Human Reproduction: Social and Ethical Issues. London, UK: Nuffield Council on Bioethics, 2018. [Google Scholar]
- Ormond KE, Mortlock DP, Scholes DT, Bombard Y, Brody LC, Faucett WA, Nanibaa’A G, Hercher L, Isasi R, Middleton A. Human germline genome editing. Am J Hum Genet 2017;101:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo R, Midden C, Miller JD. Attitudes toward biotechnology in the European Union. J Biotechnol 2002;98:9–24. [DOI] [PubMed] [Google Scholar]
- Rabino I. Research scientists surveyed on ethical issues in genetic medicine: a comparison of attitudes of US and European researchers. New Genet Soc 2006;25:325–342. [Google Scholar]
- Regalado A. China’s CRISPR babies: Read exclusive excerpts from the unseen original research. MIT Technol Rev 2019. https://www.technologyreview.com/2019/12/03/131752/chinas-crispr-babies-read-exclusive-excerpts-he-jiankui-paper/.
- Riggan KA, Sharp RR, Allyse M. Where will we draw the line? Public opinions of human gene editing. Qual Health Res 2019;29:1823–1835. [DOI] [PubMed] [Google Scholar]
- Robillard J, Roskams-Edris D, Kuzeljevic B, Illes J. Prevailing public perceptions of the ethics of gene therapy. Hum Gene Ther 2014;25:740–746. [DOI] [PubMed] [Google Scholar]
- Ryan M, Gerard K. Using discrete choice experiments to value health care programmes: current practice and future research reflections. Appl Health Econ Health Policy 2003;2:55–64. [PubMed] [Google Scholar]
- Scheufele DA, Xenos MA, Howell EL, Rose KM, Brossard D, Hardy BW. US attitudes on human genome editing. Science 2017;357:553–554. [DOI] [PubMed] [Google Scholar]
- Siegrist M. The influence of trust and perceptions of risks and benefits on the acceptance of gene technology. Risk Anal 2000;20:195–203. [DOI] [PubMed] [Google Scholar]
- Singer E, Corning A, Lamias M. Trends: genetic testing, engineering, and therapy: awareness and attitudes. Public Opin Q 1998;62:633–664. [Google Scholar]
- Smith KR, Chan S, Harris J. Human germline genetic modification: scientific and bioethical perspectives. Arch Med Res 2012;43:491–513. [DOI] [PubMed] [Google Scholar]
- Srinivas KR. Why public engagement matters in science. Trends Biotechnol 2017;35:281–283. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science 1981;211:453–458. [DOI] [PubMed] [Google Scholar]
- van Dijke I, Bosch L, Bredenoord A, Cornel M, Repping S, Hendriks S. The ethics of clinical applications of germline genome modification: a systematic review of reasons. Hum Reprod 2018;33:1777–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VassenaR, HeindryckxB, PecoR, PenningsG, RayaA, SermonK, Veiga A. Genome engineering through CRISPR/Cas9 technology in the human germline and pluripotent stem cells. Hum Reprod Update 2016;22:411–419. [DOI] [PubMed] [Google Scholar]
- Viotti M, Victor AR, Griffin DK, Groob JS, Brake AJ, Zouves CG, Barnes FL. Estimating demand for germline genome editing: an in vitro fertilization clinic perspective. CRISPR J 2019;2:304–315. [DOI] [PubMed] [Google Scholar]
- Wang JH, Wang R, Lee JH, Iao TW, Hu X, Wang YM, Tu LL, Mou Y, Zhu WL, He AY et al. Public attitudes toward gene therapy in China. Mol Ther Methods Clin Dev 2017;6:40–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SM, Badgio D, Chatterjee A. a crisPr new World: attitudes in the Public toward innovations in human genetic Modification. Front Public Health 2017;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellcome Trust. What Do People Think about Gene Therapy? London: University of Edinburgh, 2005. [Google Scholar]
- Xiang L, Xiao L, Gou Z, Li M, Zhang W, Wang H, Feng P. Survey of attitudes and ethical concerns related to gene therapy among medical students and postgraduates in China. Hum Gene Ther 2015;26:841–849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.