Abstract
Focal adhesion kinase (FAK) has been implicated in cellular processes that control cell adhesion, migration, cell cycle progression, and apoptosis. FRNK (FAK-related nonkinase) is the autonomously expressed, noncatalytic C-terminal portion of FAK. When ectopically expressed in cells, FRNK has been shown to act as a negative regulator of FAK activity, inhibiting cell spreading, migration, and cell cycle progression. The mechanisms that regulate FRNK expression during embryonic development and the functional role of FRNK in normal cell homeostasis remain poorly understood. Herein we show that FRNK expression in chicken cells is directed by an alternative promoter residing within an intron of FAK, positioned 3′ of the exon encoding sequences for the catalytic domain and 5′ of the exon encoding sequences for the C-terminal domain of FAK (e.g., FRNK). Using probes specific for FRNK, we show that FRNK expression occurs early in chicken embryogenesis, being readily detected at day 3, 6, or 9. Late in embryogenesis, at day 18, FRNK is expressed in a tissue-specific manner, predominately in lung and intestine cells. Western blot analysis of mouse tissues with a FAK-specific antibody revealed the expression of FRNK in the mouse lung. Reverse transcriptase PCR analysis of mouse lung RNA revealed the presence of spliced FRNK mRNAs containing 5′ untranslated sequences derived from a positionally conserved exon present in the mouse genome. FAK is the first example of a tyrosine kinase regulated by a domain under the control of an alternative intronic promoter. It is also the first example of a focal adhesion-associated protein regulated by such a mechanism and thus represents a novel means for the modulation of cell adhesion signaling.
Cell adhesion to the extracellular matrix (ECM) is vital for functional and structural integrity of cells and tissues (15, 18). A primary mediator of cell-matrix interactions is the integrin family of adhesion receptors (25, 26, 48). Integrin receptors are a family of transmembrane glycoproteins consisting of individual alpha and beta subunits that combine to form heterodimers with unique ECM specificities. The extracellular portion of each heterodimer specifically engages one or more ECM proteins, while the intracellular portion is coupled to the actin cytoskeleton of the cell via cytoplasmic plaque proteins. In adherent cells in culture, integrins are clustered at points of cell-matrix contact known as focal adhesions (FAs) (5, 6). Although points of cell-matrix contact in tissues are less prominent, the structural components are the same, and thus FAs provide a valuable model for studying cell-matrix adhesion. During morphogenesis, cell-matrix interactions are dynamic. Likewise, as cells migrate in culture, FAs are constantly turned over in order to allow cells to move forward (30). De novo FAs form at the leading edge of the cell as old FAs are broken down at the rear, with central FAs presumably being the most stable. Thus, understanding the mechanisms by which FAs are formed and subsequently broken down is essential to understanding cell migration. These mechanisms remain elusive.
The clustering of integrins in response to cell adhesion, or by cross-linking with antibodies, triggers actin stress fiber formation and recruitment of cytoplasmic plaque proteins (31, 32). A number of well-characterized cytoplasmic plaque proteins localize to FAs, lending a complex architecture to these adhesive sites (5). Some of these cytoplasmic plaque proteins, such as vinculin, talin, and α-actinin, provide structural integrity to the FA, while others serve as signaling proteins which are phosphorylated in response to FA formation. Among these signaling proteins are paxillin, tensin, p130CAS, and the protein tyrosine kinase designated focal adhesion kinase (FAK).
FAK is a nonreceptor protein tyrosine kinase that has been shown to be one of the major signaling components of FAs (19, 38, 43, 45). FA formation triggers FAK autophosphorylation and activation, leading to recruitment of SH2 and SH3 domain-containing kinases such as Src and Fyn (10, 53) and subsequent phosphorylation of other cytoplasmic plaque proteins such as paxillin, tensin, and p130CAS (28, 39, 40, 45). These events are dependent upon FAK’s ability to autophosphorylate on Tyr 397, which in turn serves as a binding site for Src and/or Fyn (7, 46). A second, truncated isoform of FAK has been identified in a number of cell types and characterized. This noncatalytic isoform, called FAK-related nonkinase (FRNK), is identical in sequence to the C-terminal domain of FAK at both nucleotide and amino acid levels (44). FRNK-specific cDNAs are characterized by long 5′noncoding leader sequences (5′ leader sequences), indicating that expression of FRNK is mediated by alternative splicing or transcription of FRNK-specific sequences (44).
The C-terminal domain of FAK, and therefore FRNK, contains binding sites for several FA signaling proteins including the adapter proteins p130CAS (20, 36, 37), Grb2 (47), paxillin (23), Graf (the GTPase regulator associated with FAK) (24), and phosphatidylinositol 3-kinase (2, 8, 9, 17). The C-terminal domain also contains a functional domain (focal adhesion targeting domain) that is necessary and sufficient to target proteins to FAs (22). When overexpressed in chicken embryo fibroblasts (CEF), FRNK acts as an inhibitor of FAK activation (39). Phosphorylation of FAK itself is decreased, as is phosphorylation of at least two other FA-associated proteins, paxillin and tensin. FRNK-overexpressing cells spread more slowly when plated on fibronectin (39, 40).
In this report we present evidence that FRNK expression is directed by an alternative promoter residing within an intron of FAK positioned 3′ of the exon encoding sequences for the catalytic domain and 5′ of the exon encoding sequences for the C-terminal domain of FAK. FRNK expression occurs early in chick embryogenesis, being readily detected at days 3, 6 or 9. Late in embryogenesis, at day 18, FRNK is expressed in a tissue-specific manner, predominately in cells of lung and intestine. This differential regulation of FRNK in embryonic tissues may play an important role in modulating FAK activity during development. Western blot analysis of mouse tissues with a FAK-specific antibody revealed the expression of FRNK in mouse lung. Reverse transcriptase PCR (RT-PCR) analysis of mouse lung RNA revealed the presence of spliced FRNK mRNAs containing 5′ untranslated sequences derived from a positionally conserved exon present in the mouse genome. FAK is the first example of a tyrosine kinase regulated by a domain under the control of an alternative intronic promoter. It is also the first example of an FA-associated protein regulated in this manner, and thus represents a novel mechanism by which cell-adhesion signaling can be regulated.
MATERIALS AND METHODS
Cell culture.
Primary CEF were prepared and cultured as described previously (3). The chicken B-cell lymphoma cell line BK3A (kindly provided by T. Bender, Department of Microbiology, University of Virginia) was maintained in Dulbecco’s modified Eagle’s medium containing 5% fetal calf serum, 1% chick serum, 10% tryptose phospate broth, and 50 μM β-mercaptoethanol.
Cloning of genomic sequences.
A 301-bp DraI-BglII fragment of the FRNK leader sequence (nucleotides +45 to +346) (44) was gel purified (Qiagen) and labeled with [α-32P]dCTP by random priming. This probe was used to screen a chicken genomic library derived from adult chicken liver DNA and cloned into lambda phage EMBL-3 (Clontech). The phage library was plated onto host strain Escherichia coli K803, and plaques were screened for hybridization to the FRNK leader probe by standard procedures. Positive colonies were plaque purified and rescreened with a 272-bp AccI-ScaI DNA fragment containing sequences encoding amino acid residues 552 to 642 of the catalytic domain of FAK, labeled in the same manner as the FRNK leader probe. DNA was isolated from phage exhibiting strong hybridization to probes derived from the FRNK leader and the FAK catalytic domain. Restriction enzyme digestion, Southern blotting, and sequence analysis were performed to identify the location of intron and exon sequences within these clones. DNA sequencing was carried out with a PE-ABI Prism 377 automated DNA sequencer (Applied Biosystems, Foster City, Calif.), using an ABI PRISM dye terminator cycle sequencing Ready Reaction kit (Perkin-Elmer).
Promoter-luciferase plasmid constructs.
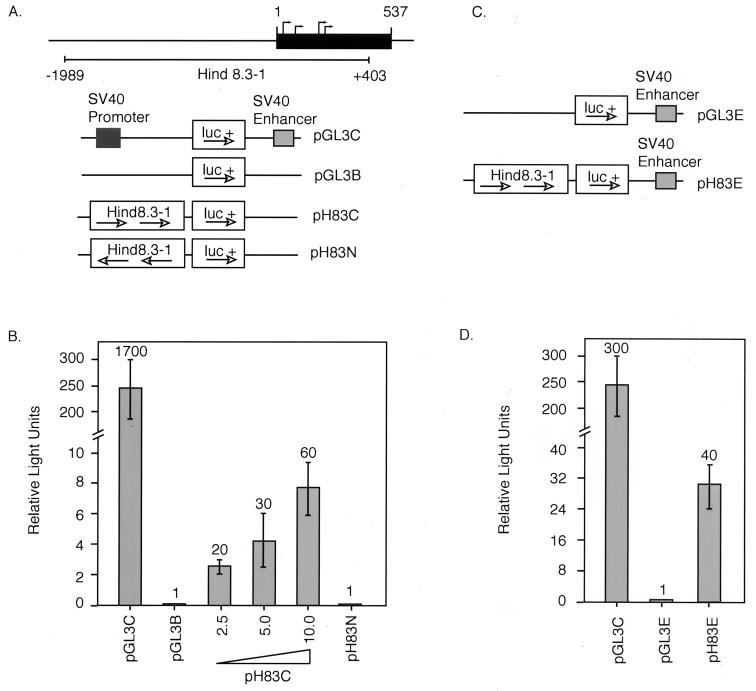
A 2.4-kbp HindIII fragment spanning nucleotides −1989 to +403 of the FRNK genomic sequence (Hind8.3-1) was cloned directly into the HindIII sites of the pGL3B and the pGL3E luciferase reporter vectors (Promega). The orientation of Hind8.3-1 within each pGL3 vector was determined by digestion with BglII and confirmed by sequence analysis. pH83C and pH83N denote Hind8.3-1 in pGL3B in the correct and incorrect orientations, respectively. pH83E is Hind8.3-1 in pGL3E in the correct orientation.
Cell transfections and luciferase assays.
For CEF transfections, cells were plated in complete growth medium at a density of 8 × 105 cells per 60-mm-diameter dish the day before transfection. A total of 10 μg of test DNA (or test DNA plus pGL3B to show dose dependence) was transfected per 60-mm-diameter dish by the calcium phosphate precipitation method. Medium was replaced on the cells within 8 to 12 h of transfection, and cells were lysed 48 h after transfection in pGL3 reporter lysis buffer (Promega). For BK3A transfections, cells were subcultured at a density of 5 × 105 cells per ml the day before transfection. For each transfection, 10 μg of test DNA and 750 μl of cells (at 1.25 × 107 cells/ml) were electroporated at 960 μF and 350 V in growth medium. Electroporated cells were then transferred to 25-cm2 flasks containing 10 ml of growth medium, cultured for 48 hours, and lysed in pGL3 reporter lysis buffer (Promega) 48 h later. For all transfections, 300 or 500 ng of cytomegalovirus–β-galactosidase was cotransfected with test DNA. Luciferase activity for all lysates was quantitated in a luminometer using Promega’s luciferase assay system. β-Galactosidase activity of each lysate was measured by standard procedures. Luciferase activity measured for each lysate was normalized to the relative β-galactosidase activity of that lysate to give relative light units.
RNase protection assay.
FRNK- and FAK-specific probes for RNase protection were generated by using synthetic oligonucleotide primers to PCR-amplify a region of the FRNK genomic sequence spanning nucleotides −324 to +387 (Fig. 1B) and a region from the FAK cDNA sequence spanning from +875 to +1227 (Fig. 1A). The PCR products were subsequently cloned into the pCR-Script SK+ plasmid by using a pCR-Script Amp SK+ cloning kit (Promega), and [α-32P]CTP-labeled RNA probes were generated by using T3 and T7 RNA polymerases (Promega). The glyceraldehyde-3-phosphate (GAD) probe was transcribed from a pGEM3 vector (Promega) containing a 1-kb PstI GAD fragment (11). Total RNA was isolated from cells and tissues by using RNeasy columns (Qiagen), and poly(A) RNA was isolated from total RNA by using Oligotex resin (Qiagen). Fifty-microgram samples of total RNA were hybridized to 2 × 105 cpm of 32P-labeled antisense RNA probe in 30 μl of 40 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (pH 6.4)–0.4 M NaCl–1 mM EDTA–80% formamide for 12 to 18 h at 50°C. Samples were digested with RNase T1 (2 μg/ml) in 10 mM Tris-HCl (pH 7.5)–0.3 M NaCl–5 mM EDTA for 30 min at room temperature. Protected fragments were resolved on a 6% denaturing polyacrylamide-urea gel. Dried gels were autoradiographed for 1 to 7 days at −70°C with intensifying screens.
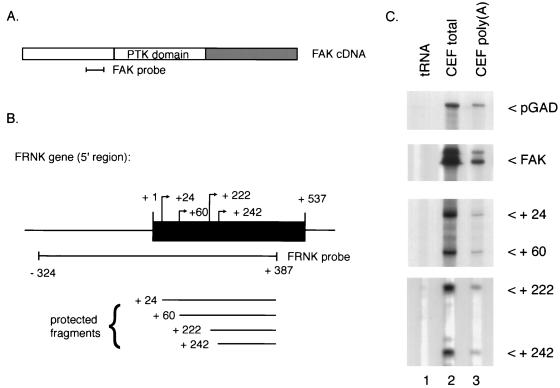
FIG. 1.
RNase protection analysis of FRNK-specific RNAs. (A) Position of the FAK-specific probe, a 352-bp fragment corresponding to nucleotides 875 through 1227 of the chicken FAK cDNA. PTK, protein tyrosine kinase. (B) Position of the FRNK-specific probe, a genomic 711-bp probe which spans the 5′ region of the unique FRNK leader exon. Also indicated are the approximate map positions of the 5′ ends of the RNase-resistant fragments protected by the FRNK-specific probe (C). (C) Segments of a sequencing gel showing the positions of labeled RNase-resistant products obtained after incubation of either control RNA (tRNA), total CEF RNA (CEF total), or poly(A)-enriched CEF RNA [CEF poly(A)] with a probe to GAD, FAK, or FRNK. The numbers in the lower two panels denote relative position of the 5′ end of the protected fragment as determined by mobility relative to known standard fragments. Exposure times are 2 days for FAK and FRNK and 4 h for pGAD. RNA amounts subjected to RNase protection were 50 μg for FAK and FRNK and 1 μg for pGAD.
Cell lysis, immunoprecipitation, and immunoblotting.
Chicken embryos were harvested after 18 days of gestation. Organs were removed, rinsed in calcium- and magnesium-free phosphate-buffered saline (CMF-PBS; 137 mM Nacl, 2.7 mM KCL, 8.0 mM, Na2 HpO4 · 7H2O, 1.4 mM KH2PO4 [pH 7.2]), and immediately frozen in liquid nitrogen. Whole embryos were harvested after 3, 6, and 9 days of gestation, rinsed in CMF-PBS, and frozen in liquid nitrogen. Protein extracts were prepared by homogenizing tissues in supplemented radioimmunoprecipitation (S-RIPA) lysis buffer (29) with a small homogenizer (Kontes, Vineland, N.J.) and 1 ml of buffer per 0.1 g of tissue. Whole embryo extracts were prepared in the same manner, using 2 ml of buffer per 0.1 g. CEF extracts and extracts of FRNK-overexpressing CEF were obtained by lysing 100-mm-diameter plates of cells in 1 ml of S-RIPA buffer (39). All lysates were cleared by centrifugation at 4°C for 10 min, and the supernatants were collected into a 1.5-ml microcentrifuge tube. Protein concentrations were determined by the bicinchoninic assay (Pierce, Rockford, Ill.).
For immunoprecipitations, extracts from chicken tissue (1 mg), chicken whole embryos (3 mg), or CEF (1 mg) were incubated with a 1:1,000 dilution of antibody BC3 overnight at 4°C, using constant rotation. BC3 is a rabbit polyclonal antibody raised against a bacterial TrpE fusion protein containing residues 651 to 1020 of the chicken FAK C-terminal domain (29). Immune complexes were recovered by using protein A-Sepharose beads (1:1 slurry; Pharmacia) followed by a 2-h incubation 4°C with rotation. Immune complexes were collected by centrifugation, solubilized in sodium dodecyl sulfate sample buffer, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. After the transfer, membranes were divided such that the upper half was probed for FAK and the lower half was probed for FRNK. For FAK detection, the upper membrane was blocked for 1 h at room temperature in 5% (wt/vol) instant nonfat dry milk–TBST (Tris-buffered saline [TBS] containing Tween 20) (29), followed by an overnight 4°C incubation in fresh blotting solution containing antibody BC3 diluted 1:1,000. The lower half of the membrane was probed for FRNK by the method described above except that Tween 20 was absent for the blotting solution. The membranes were then incubated for 1 h at room temperature with the secondary antibody (donkey anti-rabbit immunoglobulin G; Amersham, Arlington Heights, Ill.) linked to horseradish peroxidase in 5% (wt/vol) instant nonfat dry milk–TBST for FAK or 5% milk–TBS for FRNK, at a 1:1000 dilution. Horseradish peroxidase-antibody binding was visualized with enhanced chemiluminescence as instructed by the manufacturer (Amersham).
Immunoblotting and immunoprecipitation procedures for FAK and FRNK in mouse cell lines and adult tissues were identical to those for chicken samples, with the following modifications. Tissues were ground under liquid nitrogen prior to lysis in S-RIPA buffer. Lysates, 0.5 mg of protein, from tissues, whole embryos, or cultured cells were subjected to immunoprecipitation with a 1:100 dilution of FAK C-20 (Santa Cruz Biotechnology). Immunoblots were probed with FAK C-20 at a 1:1,000 dilution. FAK C-20 was raised against FAK C-terminal sequences of human origin which are identical to their corresponding mouse sequences.
RT-PCR of mouse FRNK leader.
Primer 8b1 (TCATCAGACCCTCCAGAG) was used to generate a first-strand cDNA from 250 ng of mouse lung poly(A) RNA (Ambion), using the protocol described by Gibco BRL for the Superscript II reverse transcriptase enzyme. This first-strand cDNA was amplified by PCR using primers P3 (GAGTAATTCTGGGTGGTT) and P6 (GTAGCCTGTCTTCTGGAT). PCR conditions for this and subsequent reactions are as follows: denature at 95°C for 1 min, anneal at 57°C for 1 min, and elongate at 72°C for 1 min (30 cycles). PCRs were carried out with the Pfu Turbo polymerase (Stratagene) and GeneAmp PCR core reagents (Perkin-Elmer). Transcripts in the range of 100 to 500 bp were gel purified with Qiaex II (Qiagen) and subjected to a secondary amplification with primer P6 and nested primer P5 (CCATCTGTTTGCCAAGGG). A prominent PCR product of approximately 320 bp was gel purified and reamplified with nested primers P22 (TTGCTGCACCTTCTCCTC) and P23 (TAGGGAATAGGAGGGCTG). This tertiary nested amplification gave a single prominent PCR product which was directly sequenced with primers P22 and P23.
RESULTS
Genomic organization of FRNK sequences.
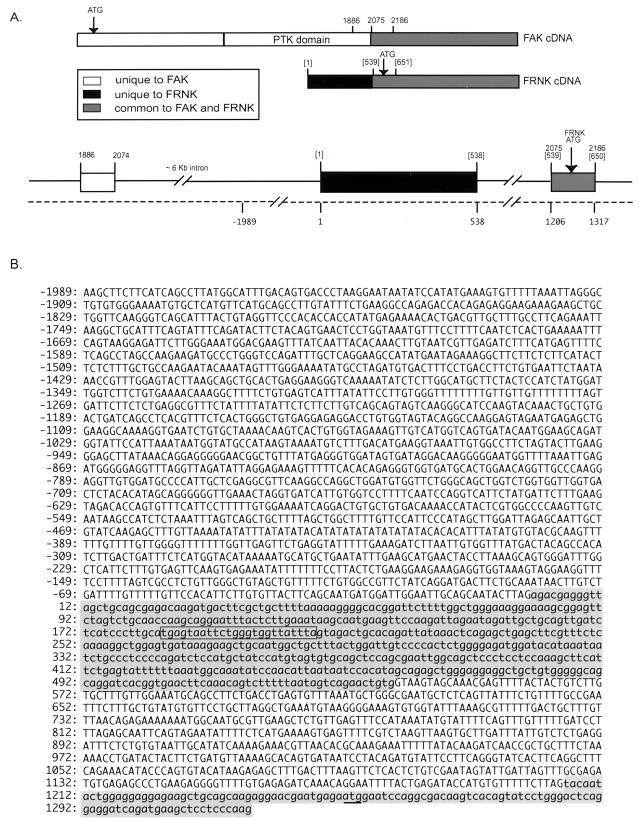
To determine whether FRNK expression is regulated by an autonomous promoter or by splicing of a larger FAK-specific RNA transcript, we cloned the genomic sequences encoding the 5′ noncoding leader sequences present in FRNK cDNAs (FRNK leader sequence). To determine where the FRNK leader sequence maps within the FAK locus, an [α-32P]dCTP-labeled probe specific for the leader sequence was used to screen a chicken genomic library. Two phage clones which hybridized strongly to a labeled leader probe were characterized further by restriction enzyme digestion and DNA sequence analysis. As shown in Fig. 2A, the 5′ FRNK leader sequence is encoded by a single exon which resides within a large intron separating the 3′-most exon of the catalytic domain of FAK and the exon encoding the 5′-most region of the C terminus (common to FAK and FRNK). Since all of the previously defined leader sequence resides within this exon, it is likely that this exon encodes for the complete 5′ leader sequence (see below).
FIG. 2.
The FRNK leader sequence is encoded by a single exon that resides within an intron of the FAK or FRNK gene. (A) Schematic representation of the FAK and FRNK cDNAs and the region of chicken genomic DNA encoding the 5′ FRNK leader sequence. Open boxes denote coding sequences unique to FAK; shaded boxes denote coding sequences common to FAK and FRNK; black boxes denote the 5′ leader sequence of FRNK previously determined by sequence analysis of FRNK cDNAs (44). Locations of the intron/exon boundaries are denoted by numbers which correspond to the previously published nucleotide sequences of FAK (43); the bracketed numbers denote nucleotide sequences specific to FRNK cDNAs (44). Numbers below the dashed line denote sequences present in the chicken genome. PTK, protein tyrosine kinase. (B) Nucleotide sequence of a segment of the chicken genome containing the 5′ region of the FRNK gene extending from −1989 to +403 (+1 indicates the first nucleotide of the previously published FRNK cDNA sequence). The FRNK translational start ATG codon is underlined.
FRNK mRNAs differ in their 5′ termini.
To determine the transcriptional start site(s) of the FRNK RNA transcripts, RNase protection experiments were carried out with both total and poly(A) CEF RNAs. RNA probes specific for the FRNK leader sequence (spanning nucleotides −324 to +387 of the FRNK genomic clone [Fig. 1B, where +1 represents the first nucleotide of the published FRNK cDNA]), FAK (nucleotides 875 to 1227 of FAK, encoding amino acids 253 to 369), and GAD were hybridized to either total CEF RNA or poly(A) RNA and subjected to digestion by RNase T1 to remove single-stranded, unhybridized probe. Analysis of the protected fragments on a denaturing polyacrylamide sequencing gel revealed the protection of four major fragments with the FRNK-specific probe (Fig. 1C). Comparison of the size of each protected fragment with sizes of DNA fragments of a DNA sequencing ladder analyzed in parallel indicated 5′ termini at positions +24, +60, +222, and +242 relative to the FRNK cDNA sequence (Fig. 1B). Since the same pattern of protected RNAs was obtained with either total RNA or poly(A) RNA (Fig. 1C), it is likely that all of the protected RNA species observed are derived from cytoplasmic FRNK mRNAs. The pattern of protected FRNK RNAs is most consistent with a transcriptional initiation process that results in several preferred sites of RNA initiation, although at this time we cannot rule out the possibility that some of these RNAs are generated by an as yet uncharacterized 5′ processing mechanism.
Unexpectedly, an abundant, protected RNA species containing nucleotides +1 to +23 of the published FRNK cDNA sequence was not observed. This may be due to the fact that the initial FRNK cDNA was cloned from a chicken embryo cDNA library, and thus the transcript giving rise to the initial cDNA may not be abundant in CEF RNA or may be present at levels so low as to be undetectable in our analysis.
FRNK expression during chick embryogenesis.
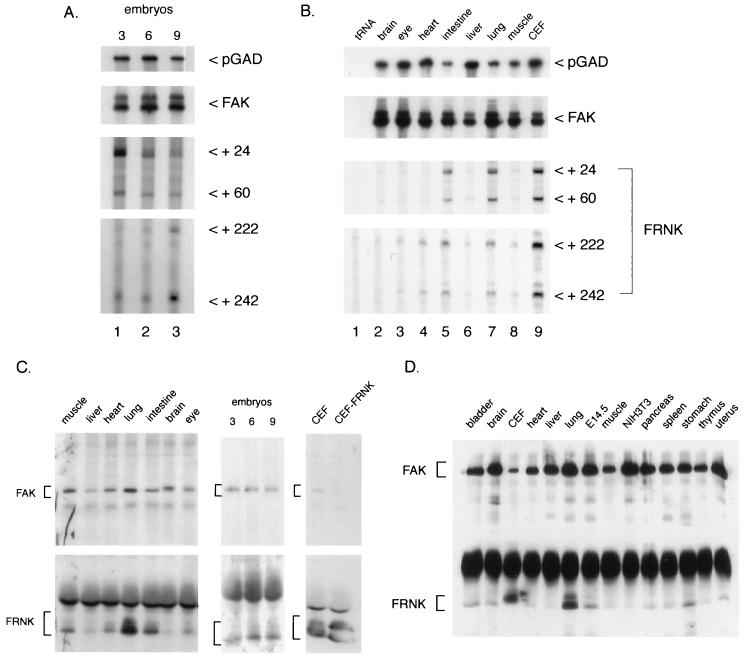
To more fully characterize the pattern of FRNK expression during chicken embryogenesis, RNase protection and Western blotting were used to measure FRNK RNA and protein levels. Total RNA was prepared from whole chicken embryos harvested at day 3, 6, or 9 and hybridized to an RNA probe specific for FAK, FRNK, or GAD, respectively (Fig. 3A). The four major FRNK transcripts were readily detected in RNA prepared from 3-, 6-, or 9-day embryos although there appeared to be a slight increase in the recovery of the +24 species at day 3 relative to days 6 and 9. Conversely, in RNA prepared from day 9 embryos, there appeared to be an increase in the +242 RNA species. The relative levels of FAK and GAD protected RNAs were constant in each of the RNA samples (Fig. 3A).
FIG. 3.
FRNK expression during chicken embryogenesis. RNA samples were prepared from either cultured CEF, whole chicken embryos (day 3, 6, or 9), or tissues isolated from day 18 chicken embryos as described in Materials and Methods. Individual RNA samples were hybridized to FAK-, FRNK-, and GAD-specific RNA probes and analyzed as described in the legend to Fig. 1. (A) RNA from day 3 (lane 1), day 6 (lane 2), and day 9 (lane 3) whole chicken embryos. Exposure times for the autoradiograms were 24 h for FAK, 1 week for FRNK, and 4 h for pGAD. (B) RNAs from 18-day-old chicken embryo brain (lane 2), eye (lane 3), heart (lane 4), intestine (lane 5), liver (lane 6), lung (lane 7), and muscle (lane 8). Exposure times were 2 days for FAK and FRNK and 3 h for pGAD. (C) Expression of FAK and FRNK proteins in chicken tissues and whole embryos. One milligram individual tissues or cultured cells and 3 mg (whole embryos) of cell extracts were immunoprecipitated with the FAK/FRNK-specific antibody BC3 as described in Materials and Methods. Detection of FAK and FRNK with BC3 was optimized by separating upper and lower halves of the membrane and immunoblotting each portion of the membrane as described in Materials and Methods. CEF ectopically expressing (CEF-FRNK) were used as positive controls for FRNK expression. The positions of FAK- and FRNK-specific proteins are indicated by brackets. The slower-migrating form of FAK present in brain is likely encoded by an alternatively spliced FAK transcript identified by sequence analysis of brain cDNAs (4). (D) Expression of FAK and FRNK proteins in murine cells and tissues. One-half milligram of lysate protein from each cell and tissue type was immunoprecipitated and immunoblotted with a polyclonal antibody raised against murine FAK sequences, FAK C-20 (see Materials and Methods).
To examine the tissue distribution of FRNK RNAs, RNA was prepared from tissues isolated from late-stage (18-day) chicken embryos. Again, using RNA probes specific for FRNK, FAK, and GAD, the pattern of RNase-resistant products was examined. As shown in Fig. 3B, FRNK RNAs were expressed at low levels in the brain (lane 2), eye (lane 3), heart (lane 4), liver (lane 6), and muscle (lane 8). In contrast, FRNK RNA expression was highest in the intestine (lane 5) and lung (lane 7), approaching the levels expressed in explanted cultured fibroblasts. As previously reported (54) and shown in Fig. 3B, FAK expression is relatively constant in all of the tissues examined. Together, these results show that FRNK and FAK mRNAs are expressed early in embryogenesis; however following organogenesis, there appears to be differential expression of FRNK and FAK RNAs, with FRNK RNA being highly expressed in the lung and intestine.
To determine whether FRNK protein expression correlates with the observed expression of FRNK RNAs, cell lysates were prepared from day 18 chicken embryo tissues, immunoprecipitated, and blotted with polyclonal antibody BC3, which recognizes both FAK and FRNK. As shown in Fig. 3C (top panel), FAK protein was readily detected in skeletal muscle, liver, heart, lung, intestine, brain, and eye, as well as in day 3, 6, and 9 chicken embryos and cultured CEF. In contrast, as shown in Fig. 3C (bottom panel), FRNK protein expression was highest in lysates from lung and intestine. An appreciable level of FRNK protein also was detected in skeletal muscle. Lysates from day 3, 6, and 9 chicken embryos, as well as cultured CEF, expressed detectable levels of FRNK. In some tissue and cell lysates, the FRNK protein appears to migrate as a doublet, likely due to serine phosphorylation (41). These data confirm that the tissue distribution of FRNK protein is similar to that observed for FRNK RNA (Fig. 3B), suggesting that FRNK expression patterns are regulated at the RNA level.
The expression of FRNK protein in tissues other than chicken tissues has not been demonstrated, which has led to some skepticism as to the existence of FRNK in nonavian species. For this reason, we carried out an analysis of FRNK expression in adult murine and embryonic tissues. As shown in Fig. 3D, FRNK protein expression is readily detected in the lung, with lower levels detected in the stomach. FRNK was also observed in lysates of whole murine embryos (lane E14.5) but was not readily detected in murine NIH 3T3 fibroblasts (Fig. 3D).
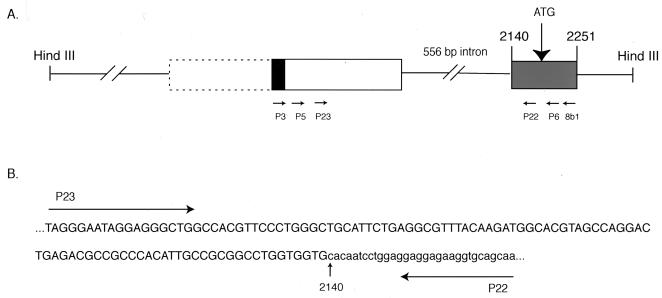
To show that the mouse FRNK immunoreactive band in lung is derived from an independent mRNA similar to that in chicken (as opposed to being a product of FAK protease cleavage), we set out to identify FRNK-specific mRNA sequences in murine lung by RT-PCR. To this end, we used sequences obtained from a mouse genomic clone isolated from a 129SVJ library (Stratagene) which is analogous to the genomic region published here for the chicken (data not shown). In the mouse, the first FRNK-encoding exon containing the FRNK ATG is conserved; it is 112 bp long, and the FRNK start ATG is intact. We sequenced 5′ of this 112-bp exon in the mouse genomic clone and found 24 nucleotides conserved from the chicken leader sequence (shown in Fig. 4A and 2B). We hypothesized that these conserved base pairs were part of the leader exon of murine FRNK and therefore designed primers to this region to amplify the mouse FRNK leader by RT-PCR (Fig. 4A).
FIG. 4.
RT-PCR analysis of mouse lung RNA. (A) Schematic representation of an approximately 2.9-kb HindIII fragment of mouse genomic DNA containing the 5′ region of the FRNK-encoding portion of the murine FAK/FRNK gene. Boxes represent exons; solid lines represent introns. Numbers above boxes denote corresponding nucleotides of the murine FAK cDNA (19). The black box represents 24 nucleotides conserved from chicken leader exon (corresponding to boxed nucleotides in Fig. 2B). PCR primers used for RT-PCR analysis are indicated by arrows. The dashed box indicates the approximate boundaries of the putative 5′ portion of the mouse FRNK leader exon. (B) Sequence of a representative RT-PCR product derived from nested amplification of mouse lung RNA. The sequence of the putative untranslated mouse FRNK leader sequences are capitalized; mouse FRNK-encoding sequences are in lowercase.
Primer 8b1 was used for first-strand cDNA synthesis from poly(A) lung RNA, and subsequent nested amplifications of the first-strand cDNA were performed with P3, P5, P6, P22, and P23 (see Materials and Methods). Nested PCR yielded an approximately 140-bp DNA fragment which upon sequence analysis yielded the sequence shown in Fig. 4B. Comparing the sequence of this RT-PCR product with the mouse genomic sequence reveals that a 556-bp intron separates the murine FRNK untranslated leader exon from the first coding exon of mouse FRNK, similar to the splicing pattern seen for chicken FRNK. Sequence analysis of DNAs derived from PCR amplification of first-strand cDNAs by using different combinations of nested primers yielded the same splice junction. These data support the expression of FRNK mRNA and protein in murine tissues and show that the elevated expression of a spliced FRNK mRNA in lung is evolutionarily conserved between the chicken and the mouse.
DNA sequences proximal to the 5′ FRNK leader exhibit promoter activity.
To delineate the possible mechanism(s) that govern differential FRNK expression, we tested whether sequences proximal to the putative start sites of FRNK RNA transcription exhibited promoter activity. A HindIII restriction fragment derived from a genomic clone and containing nucleotides −1989 to +403 (Hind8.3-1) was cloned into the luciferase reporter vector pGL3B (Fig. 5A) in both correct and incorrect orientations (pH83C and pH83N, respectively). These constructs were tested for the ability to drive luciferase expression following transfection into cultured CEF. As shown in Fig. 5B, cells transfected with the plasmid pH83C (2.5 to 10 μg per transfection) showed a dose-dependent transactivation of luciferase expression. Luciferase activity in pH83C lysates was 60-fold higher than in lysates expressing a control construct (pGL3B). Lysates derived from cells transfected with pH83N exhibited background levels of luciferase activity (10 μg of each construct was used per transfection unless otherwise indicated).
FIG. 5.
Activation of luciferase expression by DNA sequences 5′ of the FRNK unique leader exon. (A) pGL3 luciferase reporter constructs. A 2.4-kbp HindIII fragment (Hind8.3-1) which spans 1,989 bp upstream of the FRNK leader exon and includes 403 bp of the FRNK leader exon was cloned into the pGL3B vector in both correct (pH83C) and incorrect (pH83N) orientations. The pGL3C vector containing the SV40 promoter and enhancer was used as a positive control. (B) CEF were transfected with each of the pGL3 constructs depicted in panel A, and cell lysates were assayed for luciferase activity as described in Materials and Methods (10 μg of each construct was used per transfection unless otherwise indicated). β-Galactosidase activity of each lysate was measured, and luciferase activity for each lysate was normalized to the relative β-galactosidase activity of that lysate to give relative light units. Numbers denote fold activation over the pGL3B vector control. Error bars denote standard deviations from the means of three separate transfections. (C) SV40 enhancer-containing pGL3 luciferase reporter constructs. (D) CEF were transfected as for panel B with SV40 enhancer pGL3 constructs shown in panel C. Numbers denote fold activation over the pGL3E vector control. Error bars denote standard deviations from the means of three separate transfection events.
To test the ability of simian virus 40 (SV40) enhancer to stimulate the promoter activity found within the sequences present in pH83C, we cloned this fragment into the pGL3E reporter vector to generate pH83E (Fig. 5C) and measured the ability of pH83E to drive luciferase expression as described above. Lysates from CEF expressing pH83E showed a level of luciferase activity approximately 40-fold higher than that in lysates expressing the control empty vector, pGL3E (Fig. 5D). Therefore, transactivation via the sequences present in pH83C was not substantially influenced by the presence of the SV40 enhancer, suggesting that pH83C may contain both positive and negative cis-acting regulatory elements. Thus, the dose-dependent, orientation-dependent transactivation of luciferase expression by pH83C argues for the presence of a promoter sequence within the portion of the chicken genome as defined by nucleotides −1989 to +403 of the FAK locus.
FRNK promoter activity is cell type specific.
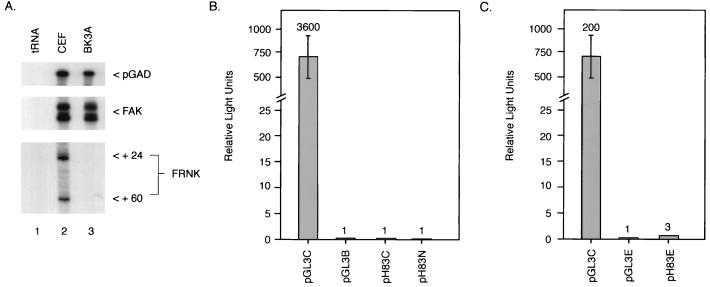
The differential expression of FRNK RNAs in tissues of the late-stage embryos suggested the possibility that the promoter-like elements controlling FRNK expression are regulated by tissue-specific factors. Previous studies had indicated that in lymphoid cells or organs enriched with hematopoietic cells, FRNK protein expression was undetectable (39a). Thus, we examined FRNK RNA expression and pH83C transactivation of luciferase expression in a chicken B-cell lymphoid tumor cell line, BK3A. As expected, using the RNase protection assay, FRNK RNA expression was undetectable in RNA prepared from BK3A cells. In contrast, FAK expression was readily observed (Fig. 6A). To determine whether pH83C drives luciferase expression in the BK3A cells, pH83C, pH83N, and pH83E were transfected into the B-cell lymphoma cell line by electroporation. Cells were harvested after 48 h, and lysates were assayed for luciferase activity. As shown in Fig. 6B, pH83C and pH83N failed to stimulate luciferase expression above background levels in BK3A cells. Lysates from cells expressing the SV40 enhancer linked to the FRNK sequences (pH83E) exhibited approximately a threefold increase in luciferase activity compared to lysates expressing the basic pGL3E (Fig. 6C). These data indicate that the SV40 enhancer is capable of only a slight augmentation of the low basal activity exhibited in the BK3A cells transfected with sequences between −1989 and +403 and suggest that the regulation of transcription within this region may be attenuated by as yet undefined tissue-specific elements and/or factors.
FIG. 6.
Cell-type-specific activation of FRNK expression. (A) RNase protection of total RNA from CEF and B-cell lymphoma cell line BK3A shows lack of FRNK expression in BK3A cells. RNase protection experiments were carried out with RNA purified from CEF and BK3A cells, using probes specific for FAK and FRNK as depicted in Fig. 1. (B) Hind8.3-1 fails to drive luciferase activity in BK3A cells. Transfections were carried out as for Fig. 5. Numbers denote fold activation over pGL3B. (C) The SV40 enhancer does not significantly enable Hind8.3-1 to drive luciferase expression in BK3A cells. Numbers above error bars represent fold activation over pGL3E; error bars denote standard deviations from the means of three separate transfection events.
DISCUSSION
In this report we show that expression of FRNK, the autonomously expressed C-terminal domain of FAK, is regulated by DNA sequences located between the exon encoding the C-terminal portion of the catalytic domain and the first exon encoding the C-terminal noncatalytic domain of FAK. Four putative transcription initiation sites, each residing within sequences originally identified as the 5′ noncoding leader of FRNK (44), have been mapped by RNase protection. FRNK-specific RNA is readily detected in cultured 10-day-old CEF as well as cells derived from early-stage embryos (e.g., days 3, 6, and 9). However, analysis of FRNK-specific RNA in tissues harvested from an 18-day embryo indicates that the expression of FRNK RNA becomes regulated in a tissue-specific fashion late in development, being expressed at high levels in the lung and intestine but at low levels in other organs, including the spleen, heart, and brain. A 2.4-kbp HindIII DNA fragment containing sequences 5′ and 3′ to the putative sites of RNA transcription fused to the luciferase gene (pH83C) activates luciferase expression when transiently expressed in CEF. In a cell line devoid of FRNK, the pre-B-cell lymphoma BK3A, pH83C does not drive luciferase expression. Thus, these results are consistent with regulation of FRNK-specific promoter elements in a tissue-specific fashion in chickens.
The identification of FRNK-specific transcriptional regulatory elements within an intron of FAK indicates that FRNK is a gene within a gene. In recent years, several examples of alternative promoters that direct the transcription of multiple RNA transcripts from a single gene have been described, thus establishing this as a novel and distinct paradigm in eukaryotic gene regulation (1). Prominent examples of a gene within a gene include telokin, inducible cyclic AMP early repressor (ICER), and calspermin. Telokin is transcribed from a smooth muscle cell-specific alternative promoter located within an intron of the smooth muscle myosin light-chain kinase (MLCK) gene (13, 21). Telokin dimers are proposed to modulate the rate of myosin phosphorylation by MLCK by direct or indirect inhibition of the active site of MLCK (49, 51). An alternative promoter embedded within the gene encoding the cyclic AMP-responsive element modulator (CREM) leads to autonomous expression of its C-terminal domain, called ICER (33). ICER appears to act as a transcriptional repressor of CREM. ICER expression is tissue specific, being predominately expressed in neuroendocrine tissues. A third example is the male germ cell-specific calmodulin binding protein calspermin, which is transcribed from an intronic promoter within the calcium/calmodulin-dependent protein kinase (CaM kinase) IV gene (52).
Unlike the genes for MLCK and CaM kinase IV, which both encode CaM kinases, the FAK gene is the first example of a protein tyrosine kinase gene in which an embedded intronic promoter regulates the autonomous expression of a noncatalytic domain. Interestingly, PYK2, a protein tyrosine kinase structurally related to FAK, appears to be regulated in a similar manner. A subset of cells expressing PYK2 also express the C-terminal domain of PYK2, termed PRNK (55). Like FRNK, PRNK cDNA clones contain a unique untranslated leader sequence not found in PYK2 cDNAs, indicating that PRNK may have a unique untranslated exon and be regulated by an embedded intronic promoter element. The function of PRNK is not yet known.
The evidence presented above indicate that the majority of FRNK mRNAs contain 5′ ends (defined by RNase protection) which map to a region (the FRNK noncoding exon) previously identified by sequence analysis of cDNA clones isolated from a chicken embryo library. We have been unable to convincingly demonstrate RNA transcripts whose 5′ ends map at or 5′ to the previously identified start of the FRNK noncoding leader sequence (44). However we cannot rule out the possibility that such transcripts are present in low abundance and hence undetectable or are expressed in tissues not subject to RNase protection analysis. RNase protection studies reveal that the relative abundances of the four major FRNK RNA species are similar in FRNK-expressing cells and tissues examined, and thus these species most likely represent initiation of transcription at multiple sites.
Numerous examples of promoters that utilize multiple start sites have previously been described (27), but the mechanism by which imprecise transcription initiation is directed is still poorly understood. RNA polymerase transcripts derived from multiple start sites are generally regulated by a distinct class of promoters that lack the classic TFIID recognition element, the TATA box. Many TATA-less promoters still initiate transcription from a single site within a loosely conserved cis-acting element known as an initiator (50). However, studies of the mouse thymidylate synthase promoter suggest that initiators are not present in TATA-less promoters with multiple initiation sites (14), suggesting that novel cis-acting elements direct RNA polymerase II transcription in promoters with multiple start sites. The only conserved element that has thus far been identified in promoters with multiple starts is a protein binding sequence known as MED-1 (multiple start site element downstream 1) (27). The FRNK gene is characteristic of other genes transcribed at multiple initiation sites in that it lacks conserved positioning of classic TATA and initiator sequences relative to the start sites, and it contains the MED-1 sequence (GCTCCC) downstream of the initiation window (see below). However, the initiation window for FRNK spans about 220 bp, which is over twice the size of windows in genes in which MED-1 has been characterized. Also, MED-1 lies at position +361 relative to the first start site of the FRNK gene, while in promoters with windows of initiation of 100 bp or less, MED-1 lies between +60 and +145. These discrepancies indicate that MED-1 may be important in genes with windows of initiation larger than those initially analyzed. Studies are in progress to define the functional relevance of the putative FRNK MED-1 as well as define other elements that might contribute to the tissue-specific regulation of FRNK expression.
In contrast to the FAK RNA message, which is ubiquitously expressed, the FRNK RNA message is detected in only a subset of cells and tissues. Therefore, while FAK is likely to be under the control of a constitutively active promoter, the FRNK regulatory region tested here appears to contain tissue-specific regulatory elements. Although many TATA-less, CAAT-less promoters with multiple start sites have been identified in ubiquitously expressed housekeeping genes, such as Rb (16) and HPRT (42), other examples, such as the Wilms’ tumor gene (WT1) promoter (12), appear to be regulated in a tissue-restricted fashion. However, studies on the WT1 promoter reveal that its core promoter is promiscuous, showing activity in all cell lines tested whether or not they express WT1, indicating the involvement of other regulatory elements not present in the core promoter. Because we have identified a region that is capable of directing transcription of an FRNK reporter construct in a cell-type-specific manner, analogous to the expression of FRNK itself, we speculate that this region must contain a core promoter and possibly other cis-acting transcriptional regulators.
Lung and intestine cells show the highest levels of FRNK RNA and protein expression, indicating that FRNK may predominate in cells of mesenchymal origin. Proteins which exhibit a pattern of expression similar to that of FRNK, such as the transcriptional activator HFH-8 (35) and the vacuolar H+ ATPase (34), have been found to localize to the mesoderm during early embryogenesis, and to mesenchymal cells of the lung and intestine later in development and in adulthood. Lung and intestine are organs that undergo extensive branching morphogenesis during development and continue to maintain high levels of epithelial-mesenchymal cell interactions in adulthood. Therefore, FRNK may play a role in mesenchymal-epithelial cell interactions of the lung and intestine both during branching morphogenesis in embryos and on into adulthood.
As shown in Fig. 3D, we have detected significant expression of FRNK protein in the adult murine lung. In addition, FRNK-specific mRNAs from the adult mouse lung that contain 5′ noncoding sequences fused to FRNK coding sequences have been identified. Mapping of these sequences within a mouse genomic DNA fragment indicates that the 5′ FRNK noncoding sequences are positionally conserved in the mouse and chicken genomes (Fig. 4). These data strongly argue that FRNK expression is not limited to avian species and likely represents a conserved mechanism for the regulation of FRNK expression in rodents and perhaps humans.
ACKNOWLEDGMENTS
We thank T. Bender, D. Engel, M. Jelenik, A. Ma, A. Richardson, J. Slack, A. Sutherland, J. Taylor, S. Weed, and W. Xiong for helpful discussion. M. Macklem, C. Stoker, and J. Havens provided technical support.
This work was supported by DHHS grant CA40042 and CA29243 and grant 4491 from the Council for Tobacco Research, Inc. J.L. was supported by a fellowship from the Medical Research Council of Canada.
REFERENCES
- 1.Ayoubi T A Y, Van de Ven W J M. Regulation of gene expression by alternative promoters. FASEB J. 1996;10:453–460. [PubMed] [Google Scholar]
- 2.Bachelot C, Rameh L, Parsons T, Cantley L C. Association of phosphatidylinositol 3-kinase, via the SH2 domains of p85, with focal adhesion kinase in polyoma middle T-transformed fibroblasts. Biochim Biophys Acta. 1996;1311:45–52. doi: 10.1016/0167-4889(95)00176-x. [DOI] [PubMed] [Google Scholar]
- 3.Bryant D, Parsons J T. Amino acid alterations within a highly conserved region of the rous sarcoma virus src gene product pp60src inactivate tyrosine protein kinase activity. Mol Cell Biol. 1984;4:862–866. doi: 10.1128/mcb.4.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgaya F, Girault J. Cloning of focal adhesion kinase, pp125FAK, from rat brain reveals multiple transcripts with different patterns of expression. Mol Brain Res. 1996;37:63–73. doi: 10.1016/0169-328x(95)00273-u. [DOI] [PubMed] [Google Scholar]
- 5.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 6.Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1998;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 7.Calalb M B, Polte T R, Hanks S K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H-C, Guan J-L. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H-C, Appeddu P A, Isoda H, Guan J-L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 10.Cobb B S, Schaller M D, Leu T H, Parsons J T. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell Biol. 1994;14:147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dugaiczyk A, Haron J A, Stone E M, Dennison O E, Rothblum K N, Schwartz R J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983;22:1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- 12.Fraizer G C, Wu Y J, Hewitt S M, Maity T, Ton C C, Huff V, Saunders G F. Transcriptional regulation of the human Wilms’ tumor gene (WT1). Cell type-specific enhancer and promiscuous promoter. J Biol Chem. 1994;269:8892–900. [PubMed] [Google Scholar]
- 13.Gallager P J, Herring B P. The carboxyl terminus of the smooth muscle myosin light chain kinase is expressed as an independent protein, telokin. J Biol Chem. 1991;266:23945–52. [PMC free article] [PubMed] [Google Scholar]
- 14.Geng Y, Johnson L F. Lack of an initiator element is responsible for multiple transcriptional initiation sites of the TATA-less mouse thymidylate synthase promoter. Mol Cell Biol. 1993;13:4894–4903. doi: 10.1128/mcb.13.8.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George E L, Georges-Labousesse E N, Patel-King R S, Rayburn H, Hynes R O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 16.Gill R M, Hamel P A, Zhe J, Zacksenhaus E, Gallie B L, Philips R A. Characterization of the human RB1 promoter and of elements involved in transcriptional regulation. Cell Growth Differ. 1994;5:467–474. [PubMed] [Google Scholar]
- 17.Guinebault C, Payrastre B, Racaaud-Sultan C, Mazarguil H, Breton M, Mauco G, Plantavid M, Chap H. Integrin-dependent translocation of phophoinositide 3-kinase to the cytoskeleton of thrombin-activated platelets involves specific interactions of p85-alpha with actin filaments and focal adhesion kinase. J Cell Biol. 1995;129:831–842. doi: 10.1083/jcb.129.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumbiner B M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 19.Hanks S K, Calalb M B, Harper M C, Patel S K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harte M T, Hildebrand J D, Burnham M R, Bouton A H, Parsons J T. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- 21.Herring B P, Smith A F. Telokin expression is mediated by a smooth muscle cell-specific promoter. Am J Physiol. 1996;270:C1656–1665. doi: 10.1152/ajpcell.1996.270.6.C1656. [DOI] [PubMed] [Google Scholar]
- 22.Hildebrand J D, Schaller M D, Parsons J T. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildebrand J D, Schaller M D, Parsons J T. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein, binds to the carboxyl-terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildebrand J D, Taylor J M, Parsons J T. An SH3 domain-containing GTPase-activating protein for Rho and Cdc42 associates with focal adhesion kinase. Mol Cell Biol. 1996;16:3169–3178. doi: 10.1128/mcb.16.6.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hynes R O. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 26.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 27.Ince T A, Scotto K W. A conserved downstream element defines a new class of RNA polymerase II promoters. J Biol Chem. 1995;270:30249–30252. doi: 10.1074/jbc.270.51.30249. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan K B, Swedlow J R, Morgan D O, Varmus H E. c-Src enhances the spreading of src −/− fibroblasts on fibronectin by a kinase-independent mechanism. Genes Dev. 1995;9:1505–1517. doi: 10.1101/gad.9.12.1505. [DOI] [PubMed] [Google Scholar]
- 29.Lacoste J, Ma A, Parsons J T. Assay and purification of focal adhesion kinase. Methods Enzymol. 1998;298:89–102. doi: 10.1016/s0076-6879(98)98011-9. [DOI] [PubMed] [Google Scholar]
- 30.Lauffenburger D A, Horwitz A F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto S, Akiyama S K, Yamada K M. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267:887–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto S, Teramoto H, Coso O A, Gutkind J S, Burbelo P D, Akiyama S K, Yamada K M. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina C A, Foulkes N S, Lalli E, Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993;75:875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- 33a.Nolan, K., and J. T. Parsons. Unpublished data.
- 34.Numata M, Ohkuma S, Iseki S. Expression and localization of mRNA for the 16 KD subunit of V-ATPase in the rat embryo. J Histochem Cytochem. 1995;43:761–769. doi: 10.1177/43.8.7622839. [DOI] [PubMed] [Google Scholar]
- 35.Peterson R S, Lim L, Honggang Y, Heping Z, Overdier D G, Costa R H. The winged helix transcriptional activator HFH-8 is expressed in the mesoderm of the primitive streak stage of mouse embryos and its cellular derivatives. Mech Dev. 1997;69:53–69. doi: 10.1016/s0925-4773(97)00153-6. [DOI] [PubMed] [Google Scholar]
- 36.Polte T R, Hanks S K. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polte T R, Hanks S K. Complexes of focal adhesion kinase (FAK) and Crk-associated substrate (p130Cas) are elevated in cytoskeleton-associated fractions following adhesion and Src transformation. J Biol Chem. 1997;272:5501–5509. doi: 10.1074/jbc.272.9.5501. [DOI] [PubMed] [Google Scholar]
- 38.Richardson A, Parsons J T. Signal transduction through integrins: a central role for focal adhesion kinase? Bioessays. 1995;17:229–236. doi: 10.1002/bies.950170309. [DOI] [PubMed] [Google Scholar]
- 39.Richardson A, Parsons J T. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK. Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- 39a.Richardson, A., and J. T. Parsons. Unpublished data.
- 40.Richardson A, Malik R K, Hildebrand J D, Parsons J T. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson A, Shannon J D, Adams R B, Schaller M D, Parsons J T. Identification of integrin-stimulated sites of serine phosphorylation in FRNK, the separately expressed C-terminal domain of focal adhesion kinase: a potential role for protein kinase A. Biochem J. 1997;324:141–149. doi: 10.1042/bj3240141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rincon-Limas D E, Geske R S, Xue J J, Hsu C Y, Overbeek P A, Patel P I. 5′-flanking sequences of the human HPRT gene direct neuronal expression in the brain of transgenic mice. J Neurosci Res. 1994;38:259–267. doi: 10.1002/jnr.490380304. [DOI] [PubMed] [Google Scholar]
- 43.Schaller M D, Borgman C A, Cobb B C, Reynolds A B, Parsons J T. pp125FAK, a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaller M D, Borgman C A, Parsons J T. Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK. Mol Cell Biol. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaller M D, Parsons J T. Focal adhesion kinase and associated proteins. Curr Opin Cell Biol. 1994;6:705–710. doi: 10.1016/0955-0674(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 46.Schaller M D, Hildebrand J D, Shannon J D, Fox J W, Vines R R, Parsons J T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlaepfer D D, Hanks S K, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz M A, Schaller M D, Ginsberg M H. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 49.Shirinsky V P, Vorotnikow A V, Birukov K G, Nanaev A K, Collinge M, Lukas T J, Sellers J R, Watterson D M. A kinase-related protein stabilizes unphosphorylated smooth muscle myosin minifilaments in the presence of ATP. J Biol Chem. 1993;268:16578–16583. [PubMed] [Google Scholar]
- 50.Smale S T. Transcription initiation form TATA-less promoters within eukaryotic protein-coding genes. Biochim Biophys Acta. 1997;1351:73–83. doi: 10.1016/s0167-4781(96)00206-0. [DOI] [PubMed] [Google Scholar]
- 51.Sobieszek A, Andruchov O Y, Nieznanske K. Kinase-related protein (telokin) is phosphorylated by smooth-muscle myosin light-chain kinase and modulates the kinase activity. Biochem J. 1997;328:425–430. doi: 10.1042/bj3280425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Z, Sassone-Corsi P, Means A R. Calspermin gene transcription is regulated by two cyclic AMP response elements contained in an alternative promoter in the calmodulin kinase IV gene. Mol Cell Biol. 1995;15:561–571. doi: 10.1128/mcb.15.1.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas S M, Soriano P, Imamoto A. Specific and redundant roles of Src and Fyn in organizing the cytoskeleton. Nature. 1995;376:267–271. doi: 10.1038/376267a0. [DOI] [PubMed] [Google Scholar]
- 54.Turner C E, Schaller M D, Parsons J T. Tyrosine phosphorylation of the focal adhesion kinase pp125FAK during development: relation to paxillin. J Cell Sci. 1993;105:637–645. doi: 10.1242/jcs.105.3.637. [DOI] [PubMed] [Google Scholar]
- 55.Xiong W, Macklem M, Parsons J T. Expression and characterization of splice variants of PYK2, a focal adhesion kinase-related protein. J Cell Sci. 1998;111:1981–1991. doi: 10.1242/jcs.111.14.1981. [DOI] [PubMed] [Google Scholar]