Abstract
Aims
Vericiguat reduced the primary composite outcome of cardiovascular death or heart failure (HF) hospitalization in patients with worsening HF with reduced ejection fraction (HFrEF) and a lower limit of baseline estimated glomerular filtration rate (eGFR) of 15 mL/min/1.73 m2. We evaluated the relationship between the efficacy of vericiguat and baseline and subsequent changes in renal function.
Methods and results
In VICTORIA, core laboratory serum creatinine was measured at baseline (n = 4956) and weeks 16, 32, and 48. Worsening renal function (WRF), defined as an increase ≥0.3 mg/dL in creatinine from baseline to week 16, was assessed via a Cox model with respect to subsequent primary events. Mean age was 69 years, 24% were female, and mean baseline eGFR was 61 mL/min/1.73 m2. During 48 weeks of treatment, the trajectories in eGFR and creatinine with vericiguat were similar to placebo (P = 0.50 and 0.18). The beneficial effects of vericiguat on the primary outcome were not influenced by baseline eGFR (interaction P = 0.48). WRF occurred in 15% of patients and was associated with worse outcomes (adjusted hazard ratio 1.28, 95% confidence interval 1.11–1.47; P < 0.001), but the beneficial effects of vericiguat on the primary outcome were similar in patients with or without WRF (interaction P = 0.76).
Conclusion
Renal function trajectories were similar between vericiguat‐ and placebo‐treated patients and the beneficial effects of vericiguat on the primary outcome were consistent across the full range of eGFR and irrespective of WRF.
Keywords: Heart failure, Heart failure with reduced ejection fraction, Renal function, Estimated glomerular filtration rate
The left panel shows no differences in the change in creatinine (P = 0.18) between the vericiguat and placebo groups, as evaluated by the interaction between treatment and study visit in the model. The right panel shows a natural cubic spline plot showing that the treatment effect of vericiguat on the primary outcome was similar across the full range of estimated glomerular filtration rate (eGFR) (P = 0.23).
Introduction
Renal dysfunction, worsening renal function, and hyperkalaemia are frequently present in patients with heart failure (HF) with reduced ejection fraction (HFrEF), especially those with severe HF.1, 2 Antagonists of the renin–angiotensin–aldosterone system (RAAS) are the cornerstone of the treatment of patients with HFrEF and angiotensin‐converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), angiotensin receptor–neprilysin inhibitors (ARNI), and mineralocorticoid receptor antagonists (MRAs) have been shown to reduce (cardiovascular) death and/or HF hospitalization in patients with HFrEF.3, 4 However, the use of these agents requires monitoring of renal function and electrolytes and may be hampered by poor renal function and/or high serum potassium concentrations.1 In a large European registry in patients with HF, both initiation and up‐titration of ACE inhibitors and ARBs were considerably less frequent in those with a lower estimated glomerular filtration rate (eGFR), higher N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), and serum potassium concentrations.5, 6 This presents a major challenge since these patients have higher risks of cardiovascular death and hospitalizations for HF and may have even greater need for these treatments. Accordingly, an unmet need exists for effective therapies in patients with severe HFrEF and advanced chronic kidney disease that do not adversely influence renal function and/or potassium concentrations.
Vericiguat, a soluble guanylate cyclase stimulator, reduced the primary composite outcome of cardiovascular death or HF hospitalization in patients with worsening HFrEF from 37.8 to 33.6 events per 100 patient‐years [hazard ratio (HR) 0.90, 95% confidence interval (CI) 0.82–0.98].7, 8, 9 The VICTORIA trial included patients with severe HFrEF and a very high primary event rate. An eGFR of 15 mL/min/1.73 m2 was permitted in VICTORIA and is a lower threshold than prior randomized clinical HF trials with RAAS inhibitors.10, 11, 12, 13, 14 Therefore, this drug might be of potential benefit to patients with severe HF irrespective of their baseline renal function without the need to down‐titrate or interrupt therapy if worsening renal function occurs. It could be argued that vericiguat might improve renal perfusion based on its potential to improve endothelial and myocardial function. On the other hand, vericiguat's modest blood pressure lowering effects might impair renal perfusion. However, the effects of vericiguat on renal function have not yet been established in a large cohort of patients with severe HF and it also remains unknown whether the beneficial effects of vericiguat are seen both in patients with and without renal dysfunction. The objective of this study was to evaluate the relationships between the effects of vericiguat and both baseline and subsequent sequential changes in renal function in patients with severe HFrEF.
Methods
Study and patients
The design, baseline characteristics, and main results of the VICTORIA trial (NCT02861534) (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) have been published elsewhere.7, 8, 9 In brief, VICTORIA included 5050 patients with HF with a reduced left ventricular ejection fraction (LVEF <45%) and elevated natriuretic peptide concentrations who recently experienced an episode of worsening of HF. Patients were randomly assigned in a 1:1 ratio to receive vericiguat or placebo. The primary outcome of VICTORIA was the composite endpoint of time to cardiovascular death or first HF hospitalization. Secondary outcomes included time to all‐cause death or first HF hospitalization and time to cardiovascular death. All patients provided written informed consent and the study protocol was approved by ethics committees and review boards at participating sites.
Laboratory measurements and definitions
Core laboratory serum creatinine, sodium, and potassium were measured at baseline and weeks 16, 32, and 48. eGFR was calculated using the Modification of Diet in Renal Disease formula [eGFR = 175 × (SCr)–1.154 × (age) –0.203 × 0.742 (if female) × 1.212 (if black)]. Worsening renal function was defined as an increase ≥0.3 mg/dL in creatinine from baseline to week 16, as this is the most commonly used definition of worsening renal function in chronic HF.15 Any potential effect on renal function was hypothesized to be evident by 16 weeks. In addition, week 16 was chosen as a landmark in order to retain a large sample of patients for the analyses due to the high early event rate.
Statistical analysis
Continuous variables are described using medians (25th, 75th percentiles) and tested for trends across eGFR levels using Spearman correlations. Categorical variables are presented as frequencies (%) and tested for trends using trend or Cochran–Mantel–Haenszel tests, where appropriate. Safety assessments, including syncope, hyperkalaemia, premature treatment discontinuation, and worsening renal function by week 16, are described by eGFR group and treatment arm.
To assess the effect of treatment on changes in electrolytes over time, a linear mixed model was used so that all available data were utilized in the modelling process. The model was fit with treatment arm, baseline laboratory values, visit, and the interaction between treatment arm and visit as covariates. The value of the electrolyte at each follow‐up time point was used as the outcome. Repeated measurements within patients were taken into account using an unstructured correlation matrix. Mean [standard deviation (SD)] of electrolytes at baseline and follow‐up visits were calculated by treatment arm, and the difference between vericiguat and placebo (95% CI) at each follow‐up visit was derived from the model. In addition to the mean (SD) and difference, the interaction P‐value from each model is presented to indicate if there was a significant difference in trajectories between treatment arms over follow‐up.
Incidence rates by eGFR level and treatment arm were calculated as the number of events per 100 patient‐years of follow‐up. A Cox proportional hazards model was used to assess if the effect of vericiguat on the primary and secondary outcomes differed by eGFR level. The model included treatment arm, eGFR, and their interaction. The proportional hazards assumption was checked for each variable using weighted Schoenfeld residuals, and no major violations were found. The HR with 95% CI comparing vericiguat with placebo within each eGFR level and the interaction P‐value are presented.
As a sensitivity analysis, the interaction between eGFR and randomized treatment with respect to the primary outcome was performed with continuous eGFR. The relationship between eGFR and time to cardiovascular death or HF hospitalization was non‐linear, as assessed by a natural cubic spline with knots at the 5th, 35th, 65th and 95th percentiles, and was included as a spline in the interaction model. The predicted risk of the primary outcome at 1 year was plotted by randomized treatment over all values of eGFR.
Finally, a landmark analysis was performed to determine the relationship between worsening renal function by week 16 and subsequent outcomes, and to assess treatment modification by worsening renal function status on subsequent outcomes. Worsening renal function was defined as an increase ≥0.3 mg/dL in serum creatinine from baseline to week 16 and was determined for all patients who had survived to that point and had week 16 laboratory data available. The relationship between worsening renal function and outcomes was evaluated using a Cox proportional hazards model that included treatment, baseline creatinine, and time from last HF hospitalization or intravenous diuretics to the week 16 lab date. The interaction between worsening renal function and treatment was also assessed in a second model. Finally, additional adjustment variables [age, albumin, beta‐blocker use, bilirubin, chloride, haemoglobin, duration of HF, NT‐proBNP, New York Heart Association (NYHA) class, systolic blood pressure, race/region, urate, history of anaemia, myocardial infarction, and peripheral artery disease] were added to these models to reduce the potential for bias induced by landmarking at week 16. HRs with 95% CIs and P‐values are presented.
All analyses were performed by the Duke Clinical Research Institute (Durham, NC, USA) using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
From the 5050 patients randomized in VICTORIA, 4956 patients with baseline renal function measurements available were included in the present study. Baseline characteristics according to eGFR ≤30 (n = 507), 30–60 (n = 2130), and >60 mL/min/1.73 m2 (n = 2319) are presented in Table 1. Baseline characteristics by eGFR ≤30 and >30 mL/min/1.73m2 are provided in online supplementary Table S1 . Patients in lower eGFR groups were older, more often female, white and from Western Europe, had slightly higher LVEF and NYHA class, more comorbidities, more frequent history of cardiovascular disease, and higher NT‐proBNP concentrations (all P < 0.001). Patients with an eGFR ≤30 mL/min/1.73 m2 less often received ACE inhibitors, ARBs, and MRAs than those with an eGFR 30–60 and >60 mL/min/1.73 m2 (P < 0.001); use of sacubitril/valsartan and beta‐blockers was similar between groups. Safety outcomes by treatment and eGFR category (eGFR ≤30, 30–60, and >60 mL/min/1.73m2) are presented in Table 2. Safety outcomes by treatment and eGFR category ≤30 and >30 mL/min/1.73m2 are presented in online supplementary Table S2 . Although hyperkalaemia, treatment discontinuations, and worsening renal function were more prevalent in patients with an eGFR <30 mL/min/1.73 m2, no differences were apparent between those on vericiguat vs. those on placebo.
Table 1.
Baseline characteristics by estimated glomerular filtration rate category
| Characteristic | Overall (n = 4956) | eGFR ≤30 mL/min/1.73 m2 (n = 507) | 30 < eGFR ≤60 mL/min/1.73 m2 (n = 2130) | eGFR >60 mL/min/1.73 m2 (n = 2319) | P‐value |
|---|---|---|---|---|---|
| Age, years | 69 (60, 76) | 75 (67, 80) | 72 (65, 79) | 64 (55, 71) | <0.001 |
| Female sex | 1189 (24.0%) | 151 (29.8%) | 540 (25.4%) | 498 (21.5%) | <0.001 |
| Race | <0.001 | ||||
| Asian | 1126 (22.7%) | 104 (20.5%) | 462 (21.7%) | 560 (24.2%) | |
| Black | 244 (4.9%) | 10 (2.0%) | 66 (3.1%) | 168 (7.2%) | |
| White | 3159 (63.8%) | 365 (72.0%) | 1448 (68.0%) | 1346 (58.1%) | |
| Other | 426 (8.6%) | 28 (5.5%) | 154 (7.2%) | 244 (10.5%) | |
| Region | <0.001 | ||||
| Asia Pacific | 1173 (23.7%) | 112 (22.1%) | 492 (23.1%) | 569 (24.5%) | |
| Eastern Europe | 1670 (33.7%) | 164 (32.3%) | 656 (30.8%) | 850 (36.7%) | |
| Latin and South America | 717 (14.5%) | 41 (8.1%) | 274 (12.9%) | 402 (17.3%) | |
| North America | 537 (10.8%) | 52 (10.3%) | 248 (11.6%) | 237 (10.2%) | |
| Western Europe | 859 (17.3%) | 138 (27.2%) | 460 (21.6%) | 261 (11.3%) | |
| Worsening HF event | 0.122 | ||||
| HF hospitalization 3–6 months | 846 (17.1%) | 87 (17.2%) | 365 (17.1%) | 394 (17.0%) | |
| HF hospitalization within 3 months | 3316 (66.9%) | 355 (70.0%) | 1426 (66.9%) | 1535 (66.2%) | |
| IV diuretic for HF (without hospitalization) within 3 months | 794 (16.0%) | 65 (12.8%) | 339 (15.9%) | 390 (16.8%) | |
| BMI, kg/m2 | 27 (24, 31) | 27 (24, 31) | 27 (24, 31) | 27 (24, 31) | 0.455 |
| Ejection fraction, % | 30 (23, 35) | 30 (25, 36) | 30 (24, 35) | 28 (21, 35) | <0.001 |
| Ejection fraction ≤40% | 4580 (92.6%) | 469 (93.4%) | 1955 (92.0%) | 2156 (93.1%) | 0.589 |
| NYHA class | <0.001 | ||||
| I/II | 2930 (59.1%) | 259 (51.1%) | 1213 (56.9%) | 1458 (62.9%) | |
| III/IV | 2026 (40.9%) | 248 (48.9%) | 917 (43.1%) | 861 (37.1%) | |
| Medical history | |||||
| Diabetes | 2331 (47.0%) | 328 (64.7%) | 1068 (50.1%) | 935 (40.3%) | <0.001 |
| Hypertension | 3925 (79.2%) | 453 (89.3%) | 1776 (83.4%) | 1696 (73.1%) | <0.001 |
| Hyperlipidaemia | 2843 (57.4%) | 355 (70.0%) | 1315 (61.7%) | 1173 (50.6%) | <0.001 |
| Anaemia | 1053 (21.2%) | 207 (40.8%) | 548 (25.7%) | 298 (12.9%) | <0.001 |
| CAD | 2815 (56.8%) | 362 (71.4%) | 1322 (62.1%) | 1131 (48.8%) | <0.001 |
| History of MI | 2090 (42.2%) | 273 (53.8%) | 972 (45.6%) | 845 (36.4%) | <0.001 |
| History of stroke | 569 (11.5%) | 80 (15.8%) | 274 (12.9%) | 215 (9.3%) | <0.001 |
| Prior PCI | 1652 (33.3%) | 214 (42.2%) | 766 (36.0%) | 672 (29.0%) | <0.001 |
| Tobacco use | 2922 (59.0%) | 276 (54.4%) | 1265 (59.4%) | 1381 (59.6%) | 0.109 |
| Vitals | |||||
| SBP, mmHg | 119 (109, 131) | 120 (109, 135) | 119 (109, 131) | 118 (108, 130) | 0.017 |
| DBP, mmHg | 72 (65, 80) | 70 (62, 79) | 71 (63, 78) | 74 (67, 82) | <0.001 |
| Pulse, bpm | 72 (64, 81) | 70 (62, 79) | 70 (63, 80) | 73 (65, 83) | <0.001 |
| SOC medications and devices | |||||
| ACE or ARB | 3641 (73.5%) | 289 (57.0%) | 1510 (70.9%) | 1842 (79.4%) | <0.001 |
| Beta‐blocker | 4614 (93.1%) | 473 (93.3%) | 1978 (92.9%) | 2163 (93.3%) | 0.796 |
| Sacubitril/valsartan | 717 (14.5%) | 61 (12.0%) | 348 (16.3%) | 308 (13.3%) | 0.355 |
| MRA | 3487 (70.4%) | 227 (44.8%) | 1411 (66.2%) | 1849 (79.7%) | <0.001 |
| Diuretics | 4812 (97.1%) | 497 (98.0%) | 2086 (97.9%) | 2229 (96.1%) | <0.001 |
| Biventricular pacemaker | 724 (14.6%) | 88 (17.4%) | 391 (18.4%) | 245 (10.6%) | <0.001 |
| ICD | 1370 (27.6%) | 156 (30.8%) | 697 (32.7%) | 517 (22.3%) | <0.001 |
| Labs | |||||
| NT‐proBNP | 2817 (1554, 5329) | 5323 (2790, 10 624) | 3316 (1769, 5930) | 2253 (1296, 3960) | <0.001 |
| BNP | 745 (456, 1343) | 917 (594, 1700) | 754 (476, 1405) | 704 (410, 1207) | <0.001 |
| Creatinine | 1.2 (0.9, 1.6) | 2.4 (2.2, 2.8) | 1.4 (1.3, 1.7) | 0.9 (0.8, 1.0) | <0.001 |
| Sodium | 140 (138, 142) | 140 (138, 142) | 140 (138, 142) | 140 (138, 142) | 0.355 |
| Potassium | 4.5 (4.2, 4.8) | 4.6 (4.2, 5.0) | 4.5 (4.2, 4.9) | 4.4 (4.1, 4.7) | <0.001 |
| Randomized arm | 0.590 | ||||
| Placebo | 2482 (50.1%) | 246 (48.5%) | 1070 (50.2%) | 1166 (50.3%) | |
| Vericiguat | 2474 (49.9%) | 261 (51.5%) | 1060 (49.8%) | 1153 (49.7%) |
Continuous variables are presented as median (25th, 75th percentiles) and categorical variables as frequencies (%). Tests for trend across eGFR strata were performed for all baseline characteristics.
ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; CAD, coronary artery disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; IV, intravenous; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonists NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SOC, standard of care.
Table 2.
Safety outcomes by treatment and estimated glomerular filtration rate category
| eGFR ≤30 mL/min/1.73 m2 | 30 < eGFR ≤60 mL/min/1.73 m2 | eGFR >60 mL/min/1.73 m2 | Overall (n = 4956) | ||||
|---|---|---|---|---|---|---|---|
| Vericiguat (n = 261) | Placebo (n = 246) | Vericiguat (n = 1060) | Placebo (n = 1070) | Vericiguat (n = 1153) | Placebo (n = 1166) | ||
| Syncope | 11 (4.2) | 10 (4.1) | 48 (4.5) | 38 (3.6) | 41 (3.6) | 37 (3.2) | 185 (3.7) |
| Symptomatic hypotension | 29 (11.1) | 22 (8.9) | 109 (10.3) | 98 (9.2) | 86 (7.5) | 72 (6.2) | 416 (8.4) |
| Hyperkalaemia | 21 (8.0) | 25 (10.2) | 71 (6.7) | 84 (7.9) | 29 (2.5) | 39 (3.3) | 269 (5.4) |
| Treatment discontinuation | 144 (55.2) | 138 (56.1) | 436 (41.1) | 435 (40.7) | 371 (32.2) | 368 (31.6) | 1892 (38.2) |
| Reason for discontinuation | |||||||
| Adverse event | 30 (20.8) | 32 (23.2) | 83 (19.0) | 75 (17.2) | 59 (15.9) | 50 (13.6) | 329 (17.4) |
| Death | 55 (38.2) | 57 ((41.3) | 156 (35.8) | 173 (39.8) | 141 (38.0) | 149 (40.5) | 731 (38.6) |
| Lost to follow‐up | 2 (1.4) | 2 (1.4) | 2 (0.5) | 2 (0.5) | 4 (1.1) | 7 (1.9) | 19 (1.0) |
| Non‐compliance with study drug | 2 (1.4) | 6 (4.3) | 23 (5.3) | 24 (5.5) | 22 (5.9) | 19 (5.2) | 96 (5.1) |
| Physician decision | 30 (20.8) | 22 (15.9) | 78 (17.9) | 70 (16.1) | 65 (17.5) | 62 (16.8) | 327 (17.3) |
| Protocol deviation | 1 (0.7) | 1 (0.7) | 4 (0.9) | 1 (0.2) | 3 (0.8) | 0 (0.0) | 10 (0.5) |
| Withdrawal by patient | 24 (16.7) | 18 (13.0) | 90 (20.6) | 90 (20.7) | 77 (20.8) | 81 (22.0) | 380 (20.1) |
| Worsening renal function by 16 weeks | 47/210 (22.4) | 35/184 (19.0) | 183/892 (20.5) | 173/921 (18.8) | 116/1016 (11.4) | 92/1041 (8.8) | 646/4264 (15.2) |
Data presented as n (%).
eGFR, estimated glomerular filtration rate.
The trajectories of serum creatinine and eGFR over time in the vericiguat and placebo groups are presented in Figure 1 and Table 3. During 48 weeks of treatment, the mean decrease in eGFR was approximately 1 mL/min/1.73 m2 in both groups. A minor drop in eGFR in the first 16 weeks was observed in both groups without a between group difference over the first 48 weeks of treatment (P = 0.50). Similarly, the trajectory of serum creatinine during the first 48 weeks of treatment was similar in vericiguat‐ and placebo‐treated patients (P = 0.18). Serum sodium and potassium levels at baseline, and weeks 16, 32, and 48 in the vericiguat and placebo groups are shown in Table 3 and Figure 2. There was a significant interaction between visit and treatment for sodium (Table 2) (P = 0.045); however, this difference was deemed clinically negligible. There were no significant differences between the two treatment groups at individual time points on serum potassium concentrations and no significant difference between the potassium trajectories of the treatment groups (P = 0.68).
Figure 1.
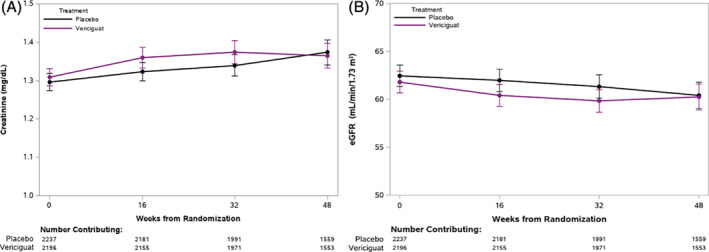
Change in serum creatinine (A) and estimated glomerular filtration rate (eGFR, B) over time in vericiguat‐ and placebo‐treated patients was assessed with a linear mixed model. This figure shows no differences in the change in creatinine (P = 0.18) and eGFR (P = 0.50) between the vericiguat and placebo groups, as evaluated by the interaction between treatment and study visit in the model.
Table 3.
Change in creatinine, estimated glomerular filtration rate, sodium, and potassium by treatment arm over trial follow‐up
| Vericiguat mean (SE) | Placebo mean (SE) | Difference estimatea(95% CI) | SliceP‐valueb | InteractionP‐valuec | |
|---|---|---|---|---|---|
| Creatinine | 0.18 | ||||
| Baseline | 1.31 (0.012) | 1.30 (0.011) | – | ||
| Week 16 | 1.36 (0.014) | 1.32 (0.012) | 0.026 (0.004 to 0.047) | 0.018 | |
| Week 32 | 1.37 (0.015) | 1.34 (0.014) | 0.021 (−0.006 to 0.049) | 0.13 | |
| Week 48 | 1.36 (0.016) | 1.37 (0.017) | 0.002 (−0.027 to 0.032) | 0.88 | |
| eGFR | 0.50 | ||||
| Baseline | 61.82 (0.572) | 62.47 (0.569) | – | ||
| Week 16 | 60.42 (0.585) | 61.99 (0.598) | −1.013 (−1.900 to −0.126) | 0.025 | |
| Week 32 | 59.84 (0.598) | 61.35 (0.617) | −0.816 (−1.785 to 0.153) | 0.10 | |
| Week 48 | 60.26 (0.686) | 60.42 (0.698) | −0.400 (−1.494 to 0.695) | 0.47 | |
| Sodium | 0.045 | ||||
| Baseline | 139.97 (0.073) | 139.92 (0.071) | – | ||
| Week 16 | 140.09 (0.071) | 140.00 (0.072) | 0.055 (−0.117 to 0.226) | 0.53 | |
| Week 32 | 140.09 (0.072) | 140.20 (0.070) | −0.092 (−0.266 to 0.081) | 0.30 | |
| Week 48 | 140.19 (0.080) | 140.08 (0.077) | 0.153 (−0.037 to 0.343) | 0.11 | |
| Potassium | 0.68 | ||||
| Baseline | 4.49 (0.011) | 4.51 (0.011) | – | ||
| Week 16 | 4.44 (0.012) | 4.48 (0.011) | −0.025 (−0.054 to 0.003) | 0.08 | |
| Week 32 | 4.46 (0.012) | 4.50 (0.012) | −0.031 (−0.061 to −0.001) | 0.043 | |
| Week 48 | 4.48 (0.013) | 4.50 (0.013) | −0.015 (−0.048 to 0.019) | 0.39 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; SE, standard error.
Estimate obtained from a mixed model fit with treatment, time, their interaction and the baseline lab value.
Slice P‐value is for the comparison of vericiguat to placebo at each time point.
Interaction P‐value for the interaction between treatment and time.
Figure 2.
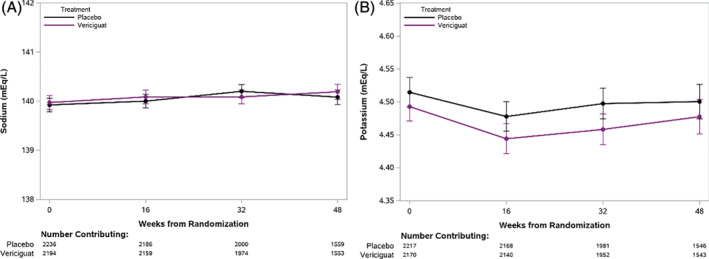
Trajectories of serum sodium (A) and potassium (B) over time in vericiguat‐ and placebo‐treated patients. Differences in trajectory between vericiguat and placebo groups were evaluated with a linear mixed model. This figure shows a small but significant change in sodium (P = 0.045) but no difference in change for potassium (P = 0.68) between vericiguat and placebo groups, as assessed via the interaction between treatment and study visit.
The clinical event rate and treatment effect of vericiguat in patients in the three strata of eGFR are shown in Table 4. Patients with eGFR ≤30 mL/min/1.73 m2 had much higher rates of cardiovascular death and HF hospitalizations compared with those with eGFR 30–60 and >60 mL/min/1.73m2. Online supplementary Figure S1 shows Kaplan–Meier survival curves of patients with eGFR 30–60 and >60 mL/min/1.73 m2. Although vericiguat did not show a statistically significant benefit in the separate subgroups of eGFR ≤30 and >60 mL/min/1.73 m2, the relative reduction of the primary endpoint by vericiguat was independent of baseline eGFR (interaction P‐value = 0.17). The clinical event rate and treatment effect of vericiguat in patients in the two strata of eGFR (≤30 and >30 mL/min/1.73 m2) are shown in online supplementary Table S3 . Again, the relative reduction of the primary endpoint by vericiguat was independent of baseline eGFR (interaction P‐value = 0.14). Figure 3 shows that the treatment effect of vericiguat on the primary outcome was similar across the full range of eGFR (P = 0.23).
Table 4.
Clinical outcomes by treatment and baseline estimated glomerular filtration rate
| Clinical outcome | Kaplan–Meier event rate at 1 year | Kaplan–Meier event rate at 2 years | HR (95% CI) | Interaction P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Vericiguat(%) | Placebo(%) | ARR [%] (95% CI) | Vericiguat(%) | Placebo(%) | ARR [%] (95% CI) | |||
| HF hospitalization or CV death | 0.17 | |||||||
| eGFR ≤30 mL/min/1.73 m2 | 47.98 | 47.88 | 0.10 (−9.09–9.28) | 70.40 | 62.51 | 7.89 (−3.91–19.69) | 1.06 (0.84–1.35) | |
| 30 < eGFR ≤60 mL/min/1.73 m2 | 33.65 | 37.65 | −4.00 (−8.29–0.28) | 45.06 | 52.05 | −6.99 (−12.37 to −1.60) | 0.83 (0.72–0.95) | |
| eGFR>60 mL/min/1.73 m2 | 25.25 | 28.90 | −3.65 (−7.44–0.15) | 36.68 | 38.42 | −1.75 (−6.75–3.26) | 0.93 (0.80–1.08) | |
| HF hospitalization or all‐cause death | 0.22 | |||||||
| eGFR ≤30 mL/min/1.73 m2 | 50.32 | 52.18 | −1.86 (−10.86–7.15) | 72.88 | 67.82 | 5.06 (−5.90–16.02) | 1.01 (0.80–1.26) | |
| 30 < eGFR ≤60 mL/min/1.73 m2 | 35.13 | 39.07 | −3.94 (−8.23–0.35) | 46.86 | 54.23 | −7.37 (−12.70 to −2.04) | 0.83 (0.73–0.95) | |
| eGFR >60 mL/min/1.73 m2 | 26.57 | 29.63 | −3.06 (−6.89–0.77) | 38.86 | 40.23 | −1.38 (−6.43–3.67) | 0.95 (0.83–1.10) | |
| CV death | 0.67 | |||||||
| eGFR ≤30 mL/min/1.73 m2 | 17.88 | 23.64 | −5.76 (−13.26–1.73) | 37.56 | 36.27 | 1.28 (−10.43–13.00) | 0.90 (0.64–1.26) | |
| 30 < eGFR ≤60 mL/min/1.73 m2 | 13.13 | 14.22 | −1.09 (−4.21–2.02) | 23.05 | 25.80 | −2.75 (−7.52–2.02) | 0.87 (0.71–1.06) | |
| eGFR >60 mL/min/1.73 m2 | 10.49 | 10.68 | −0.18 (−2.82–2.46) | 16.96 | 18.65 | −1.69 (−5.76–2.38) | 0.99 (0.80–1.24) | |
ARR, absolute risk reduction; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio.
Figure 3.
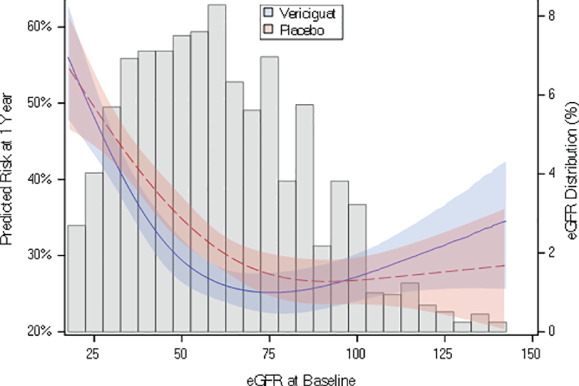
Natural cubic spline plot depicting the interaction between estimated glomerular filtration rate (eGFR) and randomized treatment with respect to the primary outcome The predicted risk of the primary outcome at 1 year was plotted by randomized treatment over all values of eGFR.
Worsening renal function occurred in 15% of evaluable patients and was associated with a higher risk of cardiovascular death and HF hospitalization, both in unadjusted and adjusted analyses (Table 5). However, the beneficial effects of vericiguat on the primary outcome were similar in patients with or without worsening renal function (interaction P‐value = 0.76).
Table 5.
Association between worsening renal function by week 16 and subsequent clinical outcomes
| Subsequent clinical outcome | WRF rateb (events) | No WRF rateb (events) | Unadjusteda | Adjusted | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |||
| HFH or CV deathc | 48.4% (249) | 30.0% (973) | 1.41 (1.22–1.62) | <0.001 | 1.28 (1.11–1.47) | <0.001 |
| HFH or all‐cause deathd | 50.8% (261) | 31.7% (1030) | 1.40 (1.22–1.60) | <0.001 | 1.24 (1.08–1.43) | 0.002 |
| CV deathe | 16.3% (107) | 10.5% (393) | 1.35 (1.08–1.67) | 0.007 | 1.25 (1.01–1.56) | 0.044 |
CI, confidence interval; CV, cardiovascular; HFH, heart failure hospitalization; HR, hazard ratio; WRF, worsening renal function.
All models include baseline creatinine and time from prior hospitalization or intravenous diuretics to week 16 visit date (categorized).
Rates computed as the number of events per 100 patient‐years of follow‐up.
Adjusted for worsening heart failure event, angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker use, bilirubin, chloride, hemoglobin, duration of heart failure, gamma‐glutamyl transferase, N‐terminal pro‐brain natriuretic peptide, New York Heart Association class, pulse, QT interval corrected with Fridericia's formula, urate and history of: myocardial infarction, peripheral artery disease, percutaneous coronary intervention.
Adjusted for worsening heart failure event, angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker use, albumin, bilirubin, chloride, hemoglobin, duration of heart failure, gamma‐glutamyl transferase, N‐terminal pro‐brain natriuretic peptide, New York Heart Association class, pulse, QT interval corrected with Fridericia's formula, urate and history of: hyperlipidemia, myocardial infarction, peripheral artery disease.
Adjusted for age, albumin, beta‐blocker use, bilirubin, chloride, hemoglobin, duration of heart failure, N‐terminal pro‐brain natriuretic peptide, New York Heart Association class, systolic blood pressure, race/region, urate and history of: anemia, myocardial infarction, peripheral artery disease.
Discussion
In patients with severe HFrEF and a high risk of cardiovascular events, renal function trajectories were similar between vericiguat‐ and placebo‐treated patients. In addition, vericiguat reduced the primary composite endpoint of cardiovascular death or HF hospitalization across the full range of baseline eGFR, from 15 mL/min/1.73 m2 and higher. Worsening renal function was associated with worse outcomes, but the beneficial effects of vericiguat were similar in patients with and without worsening renal function.
One of the unique characteristics of VICTORIA was the inclusion of patients with eGFR <30 mL/min/1.73 m2. All pivotal HF trials with RAAS inhibitors only included patients with an eGFR >30 mL/min/1.73 m2 or with serum creatinine concentrations <221–265 µmol/L.10, 11, 12, 13, 14 A recent trial on the effects of empagliflozin in patients with chronic HF included patients with an eGFR >20 mL/min/1.73m2.16 Patients in VICTORIA with an eGFR <30 mL/min/1.73 m2 were sicker, with more comorbidities, higher NYHA class, higher NT‐proBNP, and worse clinical outcomes. The finding that a lower baseline eGFR is associated with worse outcomes confirms results shown in many other studies,15 but extends these findings to eGFR levels between 15–30 mL/min/1.73 m2. As expected, the use of ACE inhibitors, ARBs, and MRAs was much lower in patients with an eGFR <30 mL/min/1.73 m2, while beta‐blocker use was similar in patients with impaired and normal renal function.5, 6 These data emphasize that the highest‐risk patients receive the lowest percentage of some of the main lifesaving therapies. Therefore, despite major advances in the treatment of patients with HFrEF, there is still a clinical need for novel therapies especially for patients with severe HFrEF with an eGFR <30 mL/min/1.73 m2 and/or hyperkalaemia.
In this study in patients with severe HF and a recent worsening HF event, mean eGFR showed a decrease of approximately 1 mL/min/1.73 m2 during 48 weeks of treatment. Although the slope of decline in eGFR was slightly greater during the first 16 weeks in patients taking vericiguat, this minor treatment difference disappeared after 48 weeks. Also, the development of worsening renal function was not different between groups.
This analysis further addressed whether the beneficial effects of vericiguat on the primary endpoint of cardiovascular death or HF hospitalization were maintained across the full spectrum of eGFR, ranging from very low to high levels. In the eGFR <30 mL/min/1.73 m2 subgroup, patients on vericiguat had numerically higher primary event rates compared with placebo, but this difference was not statistically significant. On the other end of the spectrum, patients in the eGFR >60 mL/min/1.73 m2 subgroup also did not show a statistically significant reduction of the primary endpoint on vericiguat. A statistically significant reduction of the primary endpoint on vericiguat was observed only in the eGFR 30–60 mL/min/1.73 m2 subgroup. However, there was no significant interaction between baseline eGFR and the overall reduction of the primary endpoint by vericiguat. Similarly, the subpopulation treatment effect pattern plot (STEPP) analysis showed that the treatment effect of vericiguat on the primary outcome was similar across the eight subpopulations. STEPP analysis was specifically developed to investigate the heterogeneity of treatment effects on survival outcomes across values of a (continuously measured) covariate, such as serum creatinine or eGFR.17 From these analyses, we confirm no significant treatment heterogeneity across the full range of eGFR. Finally, the beneficial effects of vericiguat on the primary endpoint were similar both in those patients who developed worsening renal function and those who did not. These data indicate a similar effect of vericiguat across the full range of eGFR, where neither those with lower nor higher eGFR have a greater benefit.
In contrast to the pivotal randomized clinical trials with inhibitors of the RAAS,10, 11, 12, 13, 14 VICTORIA did not exclude patients with hyperkalaemia. In addition, there was no signal that vericiguat either increased or decreased serum potassium levels. Therefore, vericiguat can likely be safely provided to patients with elevated serum potassium concentrations, including those with hyperkalaemia in whom RAAS blockers are contraindicated.
This study has both strengths and limitations. The main strength of this analysis is the large contemporary patient population including patients ranging from very low to normal eGFR. In addition, the collection of samples for analyses across multiple time points of the study is a particular strength. Although values for the 94 missing eGFR values at randomization were not imputed, there were no substantial differences between those patients and the overall cohort. The current study is a post‐hoc analysis; however, an analysis on the effects of vericiguat in patients with an eGFR below and above 30 mL/min/1.73 m2 was pre‐specified.
In conclusion, in patients with severe HF and a recent episode of worsening HF, renal function trajectories were similar between patients treated with vericiguat vs. placebo. In addition, the development of worsening renal function was not different between groups. The beneficial effects of vericiguat on the primary outcome of VICTORIA were consistent across the full range of eGFR irrespective of worsening renal function. Therefore, vericiguat might be of benefit in patients with severe HF irrespective of their baseline renal function, and this study indicates that there is likely no need to down‐titrate or stop vericiguat when worsening renal function or hyperkalaemia occurs.
Supporting information
Appendix S1. Supporting information
Acknowledgements
The authors are pleased to acknowledge Elizabeth Cook, BA of the Duke Clinical Research Institute for her editorial assistance and preparation of the manuscript submission.
Funding
This work and the VICTORIA trial was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and Bayer AG, Wuppertal, Germany.
Conflict of interest: A.A.V. reports research grants from Boehringer Ingelheim and Roche Diagnostics; consulting fees from Merck, Bayer, Amgen, AstraZeneca, Boehringer Ingelheim, Cytokinetics, Myokardia, Novartis, Servier, and Roche Diagnostics. A.F.H. reports research grants from Merck, AstraZeneca, Novartis, and Verily; honoraria from Merck, Bayer, Amgen, AstraZeneca, and Novartis. J.A.E. reports research grants from Bayer, Merck, Servier, Amgen Sanofi, Novartis, Cytokinetics, American Regent, and Applied Therapeutics; consulting fees from Bayer, Merck, Servier, Amgen, Sanofi, Novartis, Cytokinetics, American Regent, and Applied Therapeutics. J.B. reports consulting fees from Bayer, Merck, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, CVRx, G3 Pharmaceutical, Janssen, Luitpold, Medtronic, Novartis, Vifor, and Novo Nordisk. C.M.O'C. reports research funding from Merck; consulting fees from Bayer, Dey LP, and Bristol‐Myers Squibb Foundation. J.K. is an employee of Merck & Co., Inc. C.S.P.L. reports research grants from Bayer, National Medical Research Council of Singapore, Boston Scientific, Roche Diagnostic, Medtronic, Vifor Pharma, and AstraZeneca; consulting fees from Merck, Bayer, Boston Scientific, Roche Diagnostic, Vifor Pharma, AstraZeneca, Novartis, Amgen, Janssen Research & Development LLC, Menarini, Boehringer Ingelheim, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, Novo Nordisk, JanaCare, Biofourmis, Darma, Applied Therapeutics, MyoKardia, Cytokinetics, WebMD Global LLC, Radcliffe Group Ltd, and Corpus. In addition; she has a patent PCT/SG2016/050217 pending, and a patent 16/216929 pending and co‐founder & non‐executive director of eKo.ai. B.P. reports research grants from MSD, Bayer, and Servier; consulting fees from MSD, Bayer, Servier, Bristol‐Myers Squibb, MedScape, Daiichi‐Sankyo, and Novartis; non‐financial support from MSD, Bayer, and Novartis. L.R. is an employee of Bayer AG. P.P. reports research grants from Vifor Pharma Ltd, and Servier; consulting fees from MSD, Novartis, Vifor Pharma Ltd, Servier, BMS, Boehringer Ingelheim, Respicardia, AstraZencea, Cibiem, RenalGuardSolution, and Berlin Chemie. K.J.A. reports research grants from Merck and NIH. P.W.A. reports research grants from Merck, Bayer, Sanofi‐Aventis Recherche & Développement, Boehringer Ingelheim, and CSL Limited; consulting fees from Merck, Bayer, AstraZeneca, and Novartis. All other authors have nothing to disclose.
References
- 1.Rossignol P, Lainscak M, Crespo‐Leiro MG, Laroche C, Piepoli MF, Filippatos G, Rosano GMC, Savarese G, Anker SD, Seferovic PM, Ruschitzka F, Coats AJS, Mebazaa A, McDonagh T, Sahuquillo A, Penco M, Maggioni AP, Lund LH; Heart Failure Long‐Term Registry Investigators Group . Unravelling the interplay between hyperkalaemia, renin‐angiotensin‐aldosterone inhibitor use and clinical outcomes. Data from 9222 chronic heart failure patients of the ESC‐HFA‐EORP Heart Failure Long‐Term Registry. Eur J Heart Fail 2020;22:1378–1389. [DOI] [PubMed] [Google Scholar]
- 2.Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, Tang WHW, Skouri H, Verbrugge FH, Orso F, Hill L, Ural D, Lainscak M, Rossignol P, Metra M, Mebazaa A, Seferovic P, Ruschitzka F, Coats A. Evaluation of kidney function throughout the heart failure trajectory – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:584–603. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2016;68:1476–1488. [DOI] [PubMed] [Google Scholar]
- 5.Beusekamp JC, Tromp J, van der Wal HH, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Rossignol P, Zannad F, Voors AA, van der Meer P. Potassium and the use of renin‐angiotensin‐aldosterone system inhibitors in heart failure with reduced ejection fraction: data from BIOSTAT‐CHF. Eur J Heart Fail 2018;20:923–930. [DOI] [PubMed] [Google Scholar]
- 6.Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Ter Maaten JM, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Metra M, Zwinderman AH. Determinants and clinical outcome of uptitration of ACE‐inhibitors and beta‐blockers in patients with heart failure: a prospective European study. Eur Heart J 2017;38:1883–1890. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM; VICTORIA Study Group . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong PW, Roessig L, Patel MJ, Anstrom KJ, Butler J, Voors AA, Lam CSP, Ponikowski P, Temple T, Pieske B, Ezekowitz J, Hernandez AF, Koglin J, O'Connor CM. A multicenter, randomized, double‐blind, placebo‐controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA trial. JACC Heart Fail 2018;6:96–104. [DOI] [PubMed] [Google Scholar]
- 9.Pieske B, Patel MJ, Westerhout CM, Anstrom KJ, Butler J, Ezekowitz J, Hernandez AF, Koglin J, Lam CSP, Ponikowski P, Roessig L, Voors AA, O'Connor CM, Armstrong PW. Baseline features of the VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial. Eur J Heart Fail 2019;21:1596–1604. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN; SOLVD Investigators . Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 11.Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K; CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function intolerant to angiotensin‐converting‐enzyme inhibitors: the CHARM‐Alternative trial. Lancet 2003;362:772–776. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 13.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS‐HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21.21073363 [Google Scholar]
- 14.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 15.Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta‐analysis. Eur Heart J 2014;35:455–469. [DOI] [PubMed] [Google Scholar]
- 16.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 17.Yip WK, Bonetti M, Cole BF, Barcella W, Wang XV, Lazar A, Gelber RD. Subpopulation Treatment Effect Pattern Plot (STEPP) analysis for continuous, binary, and count outcomes. Clin Trials 2016;13:382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information


